Submitted:
04 June 2024
Posted:
06 June 2024
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. Reagents
1.2. Determination of Deacetylation Degree (DD, %) of CS
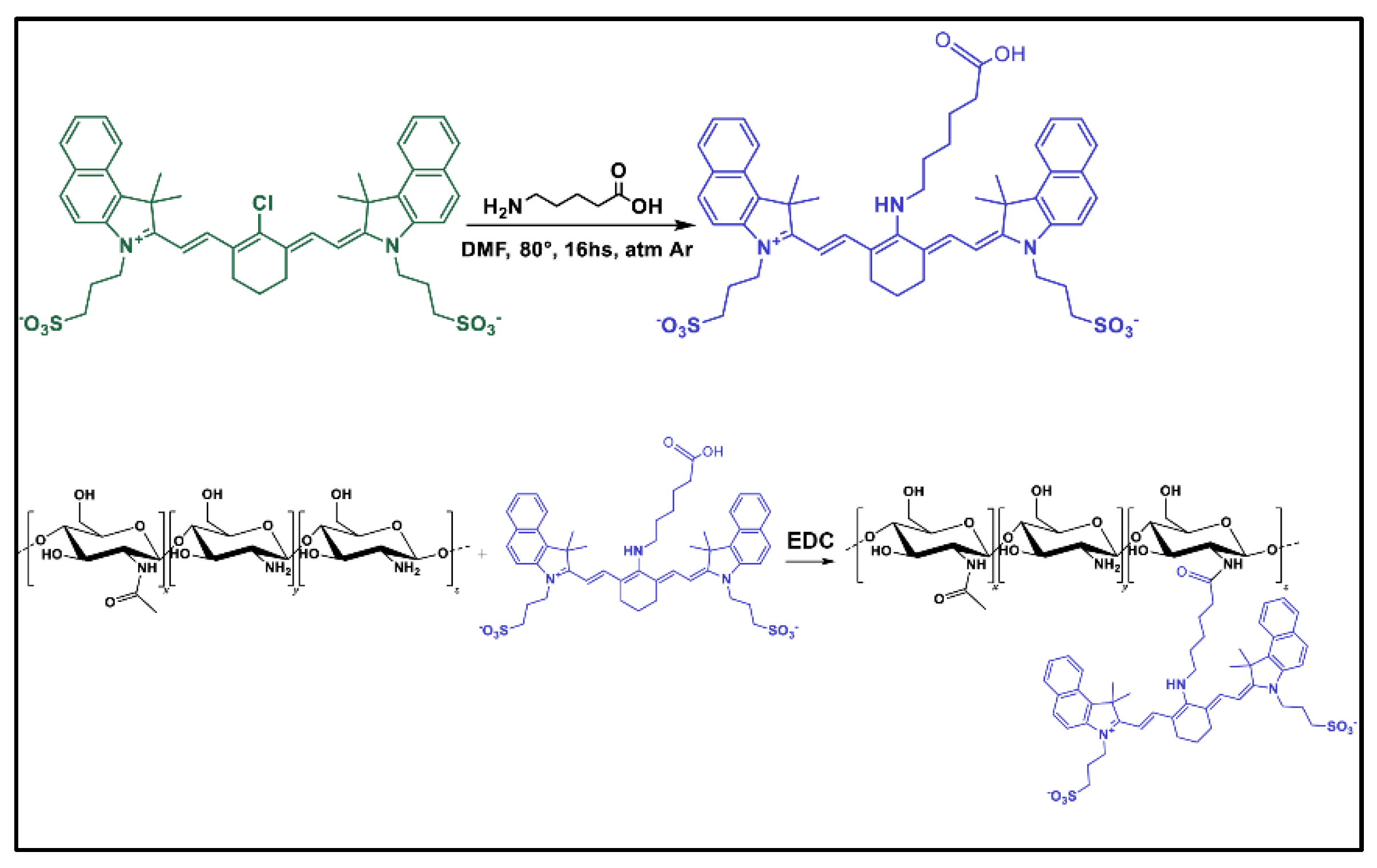
1.3. Synthesis of CNN Fluorescent Probe and CNN-Labelled CS
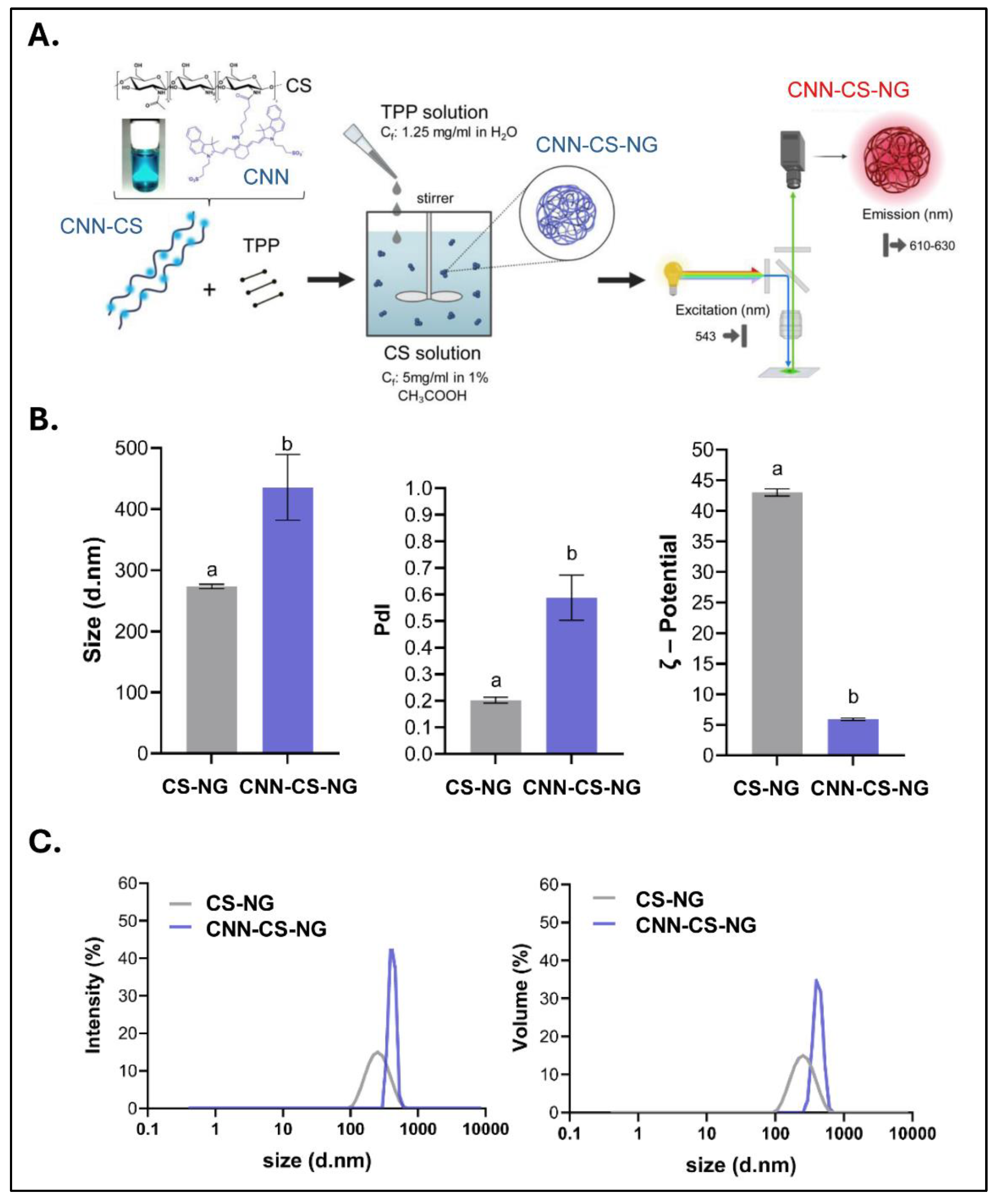
2.4. NG Generation
2.5. Characterizations of CCN-NG
2.5.1. Particle Size and ζ-Potential
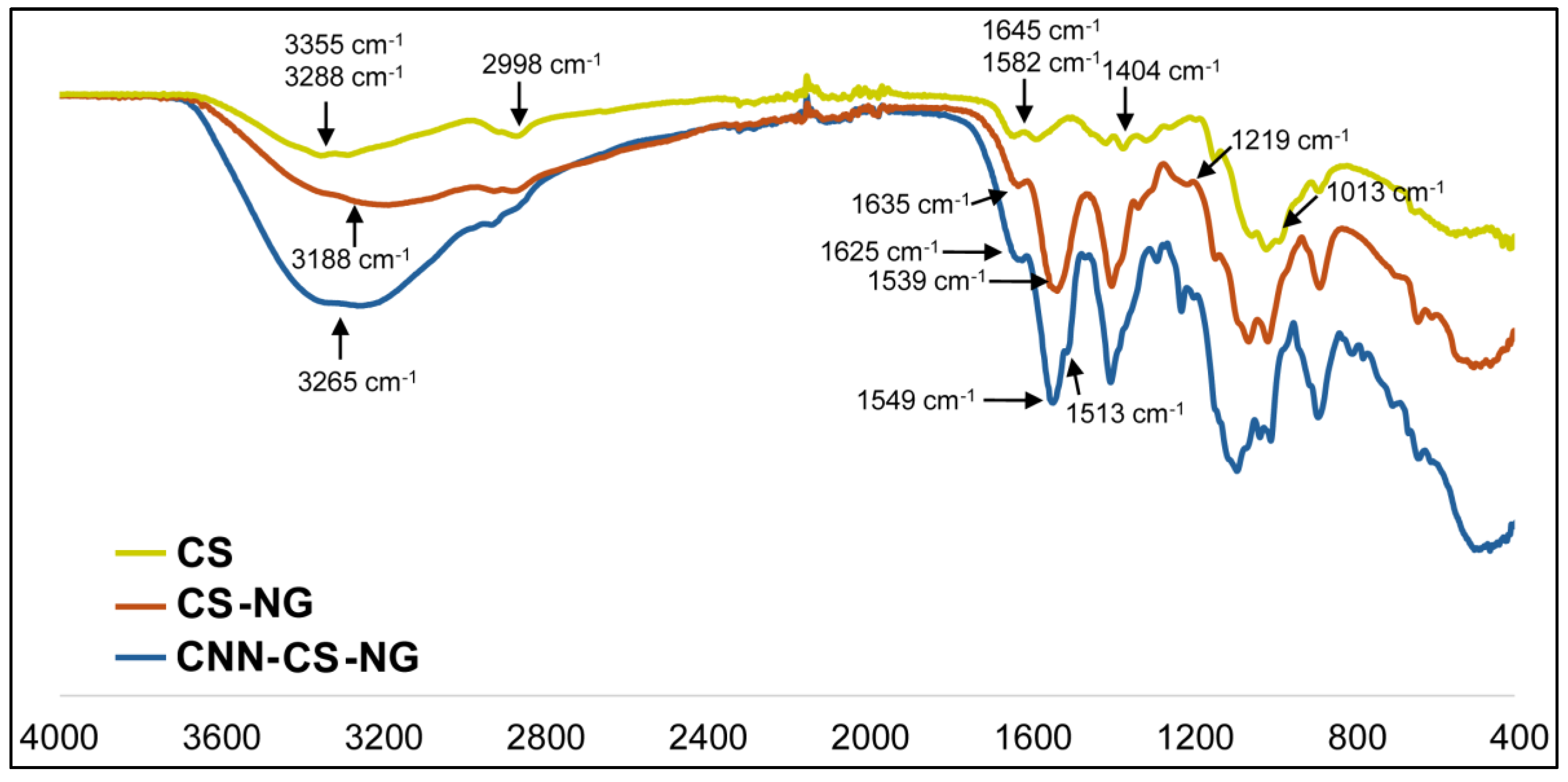
2.5.2. Fourier Transform Infrared Spectroscopy (FTIR)
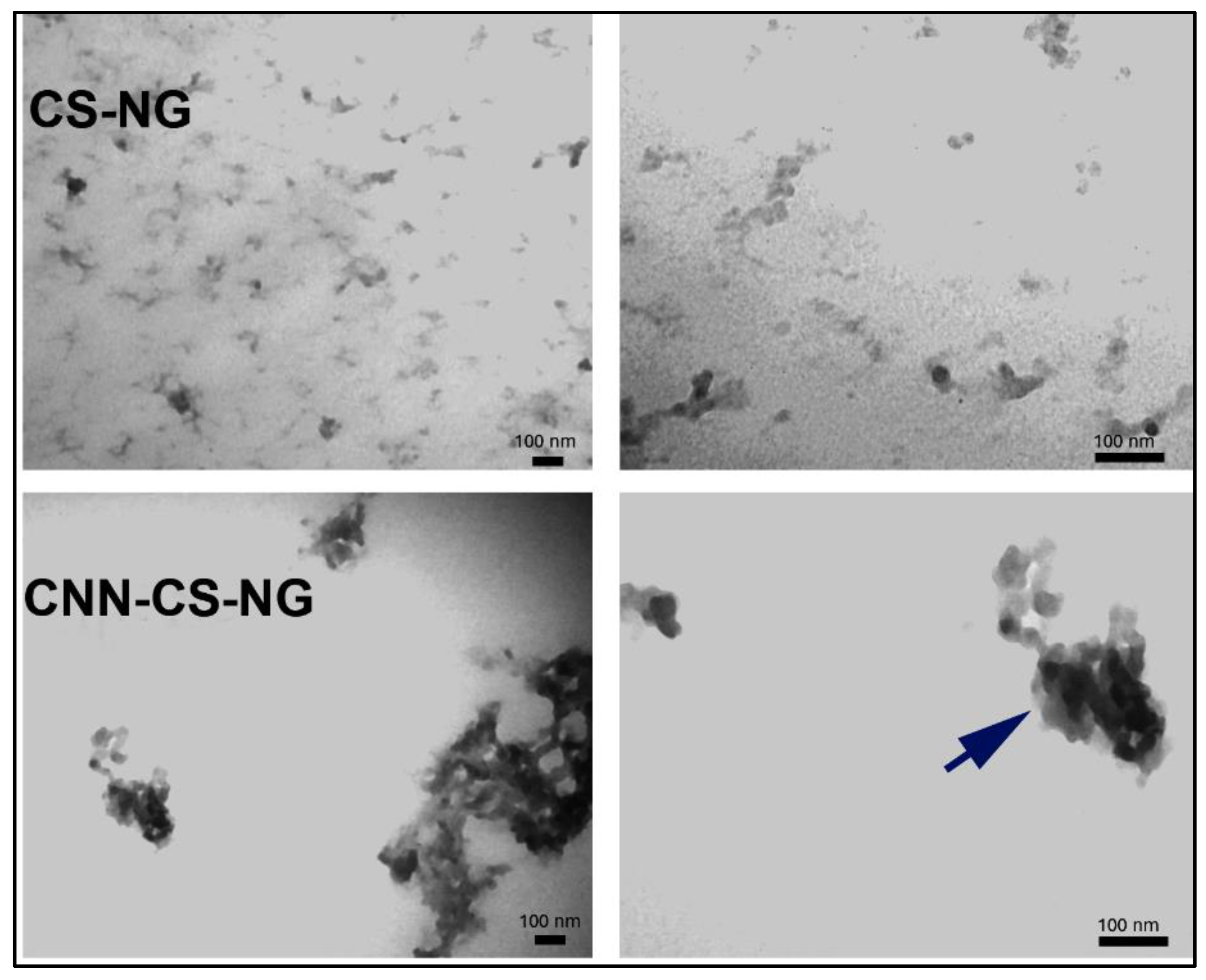
2.5.3. Transmission Electron Microscopy (TEM)
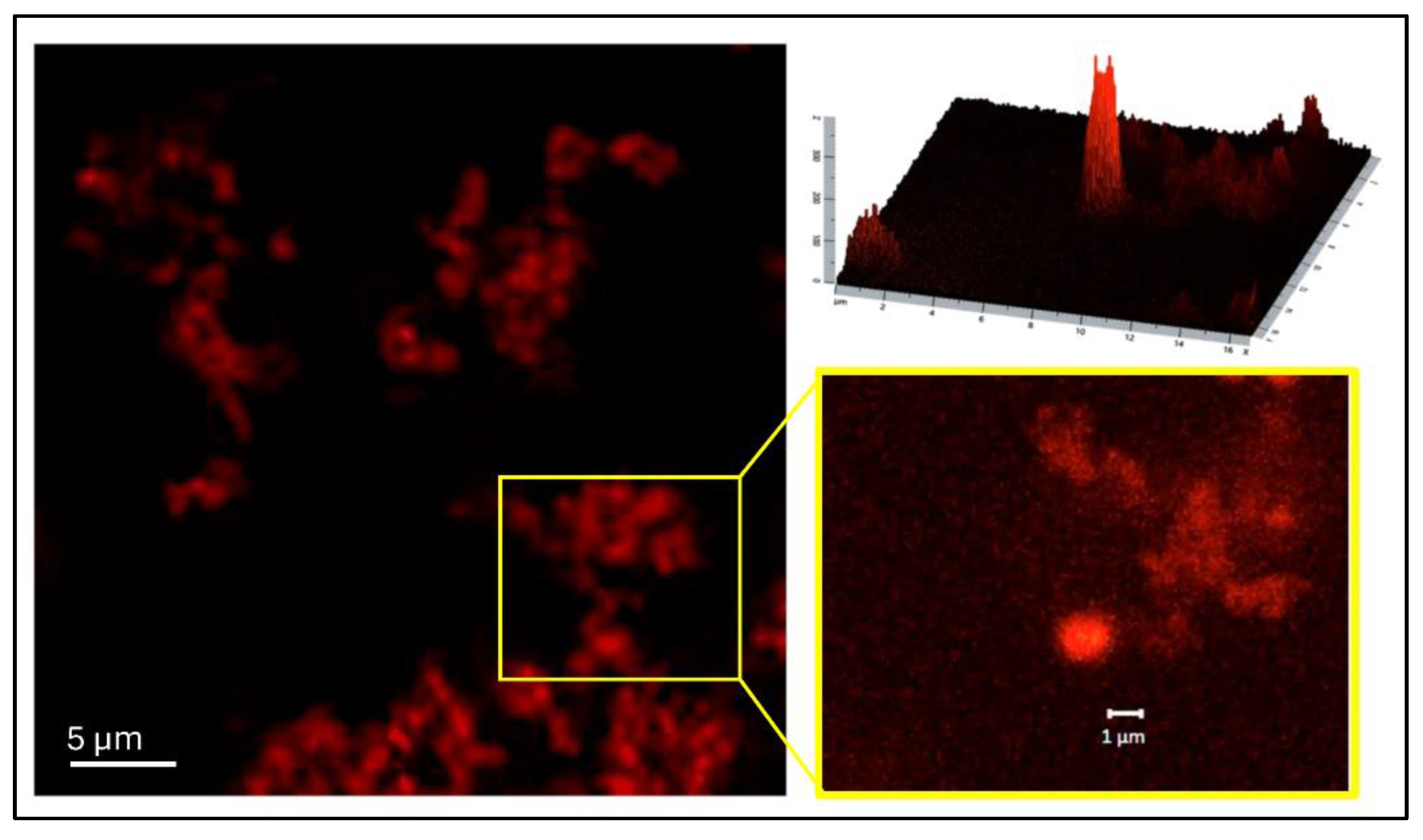
2.5.4. Confocal Laser Scanning Microscopy (CLSM)
2.6. In Vitro Studies
2.6.1. Cell Culture Conditions
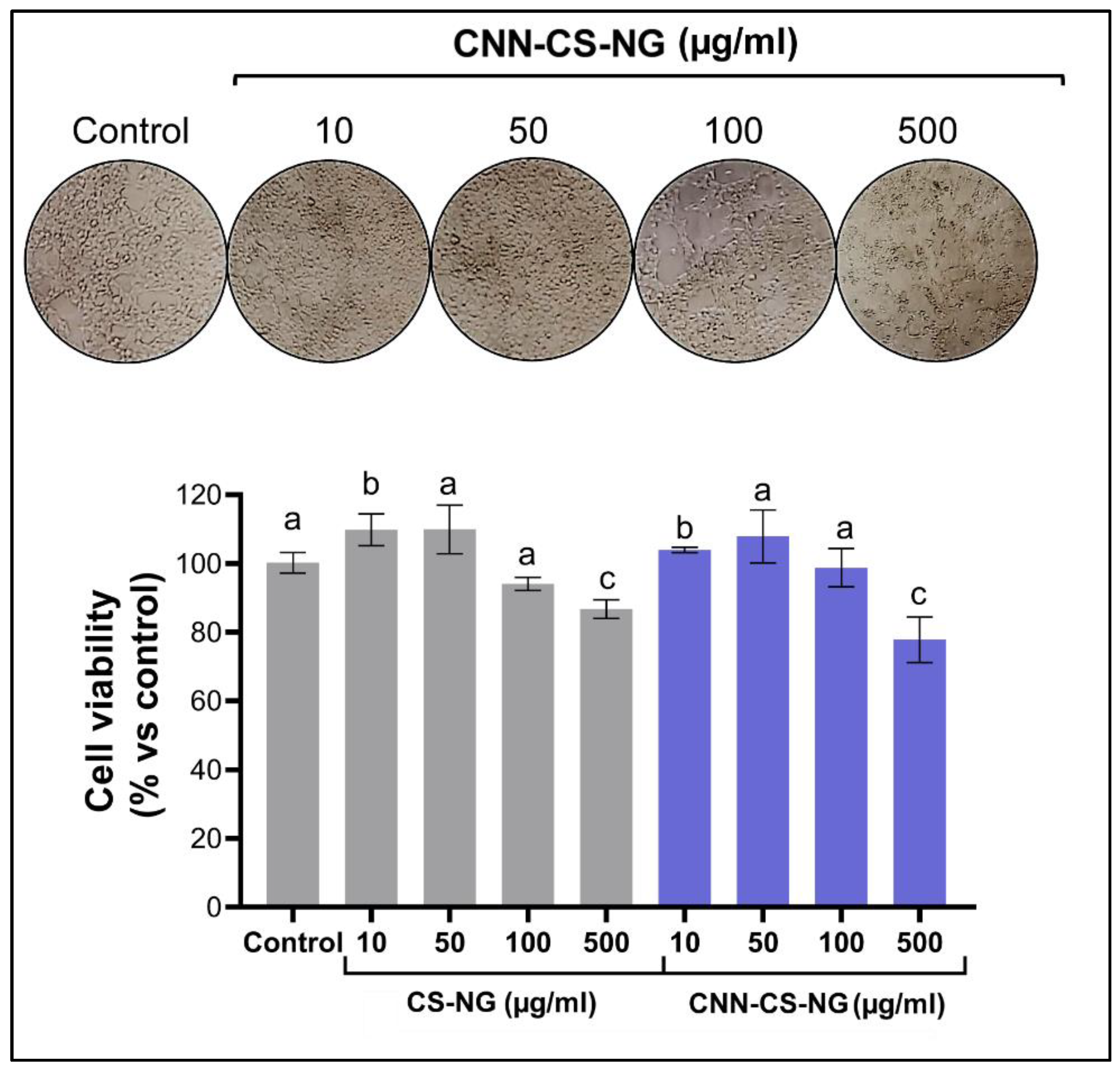
2.6.2. Cytotoxicity Assay
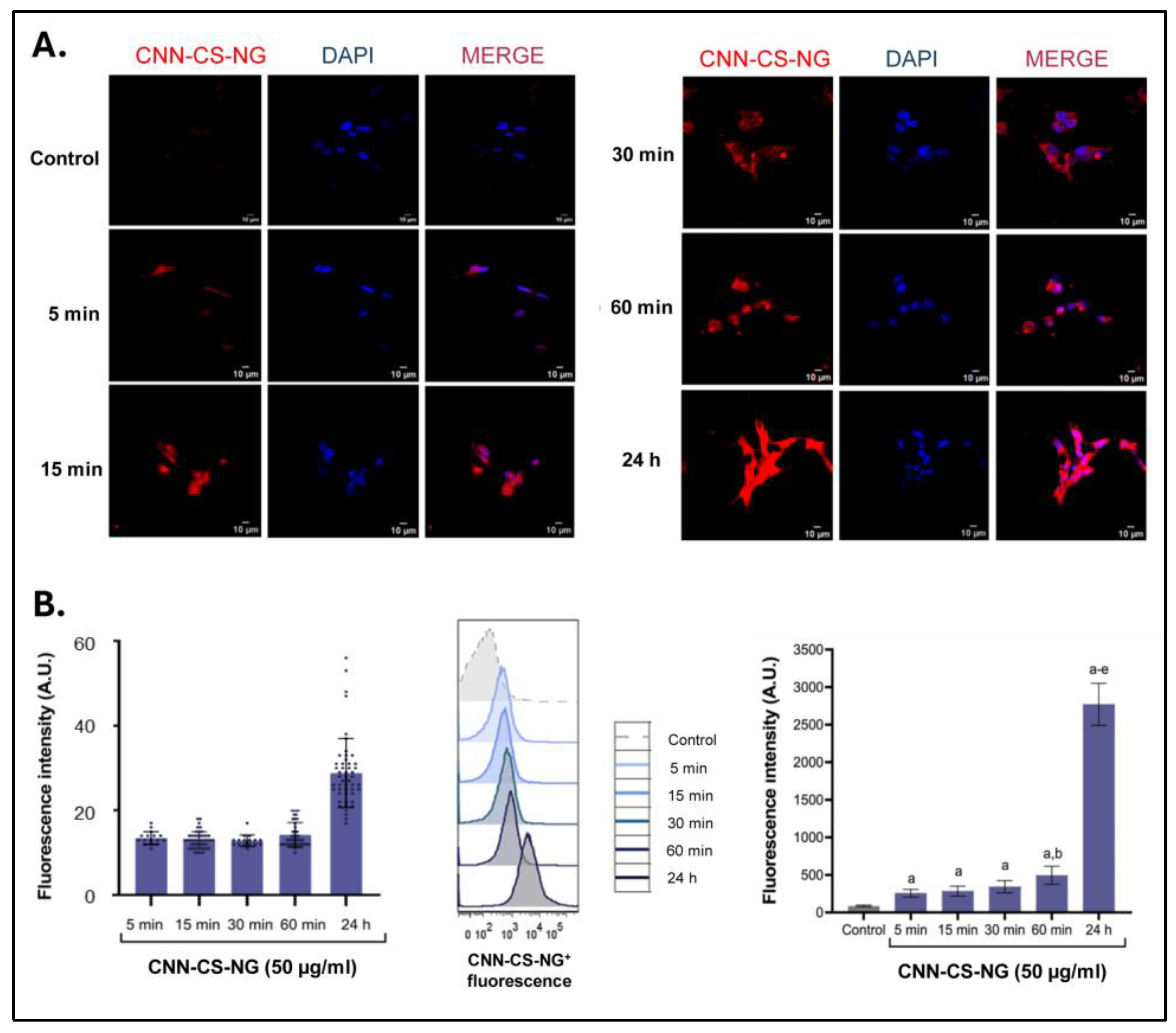
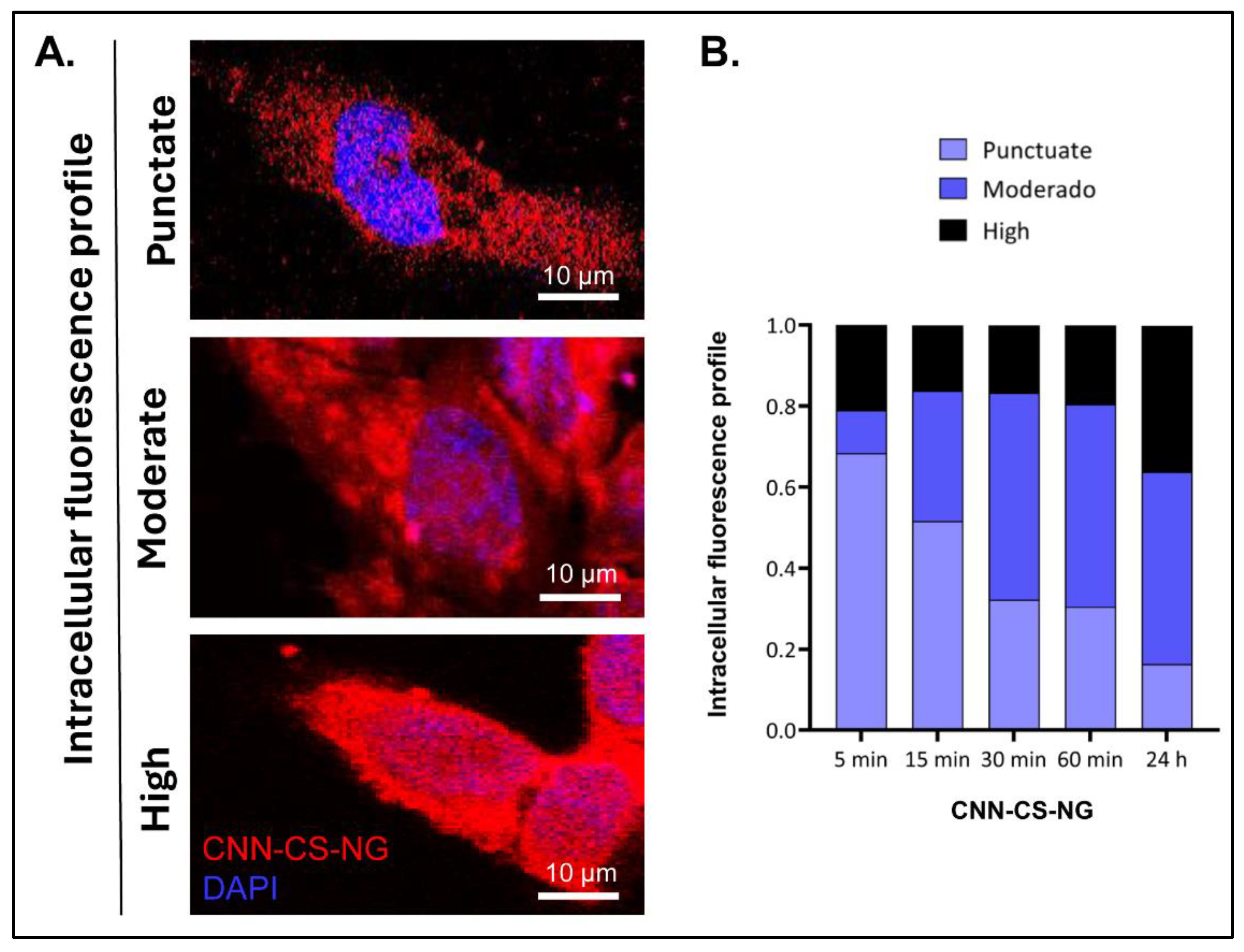
2.6.3. Cellular Uptake
- a)
- Fluorescence microscopy: cells were growth in Nunc™ 178599 Lab-Tek® Chamber Slide™ System, Glass, 16-Wells. CNN-CS-NG (50 µg/mL) were incubated in cells for 5, 15, 30, 60 min and 24h at culture conditions. After treatment, culture medium was removed, and cells were PBS-washed and fixed with 4% paraformaldehyde (PFA). After that, cells were incubated 10 min with DAPI for nuclei staining. [12]. Confocal images were acquired in a FV1000 Olympus confocal microscope (Olympus Inc., Japan). Same exposure times and camera settings were fixed for imaging under the same conditions. Disparities from different slides usually occur. For that reason, a non-specific signal, as an internal control for each sample, was considered: e.g., the average intensity of background signal outside of cells. Then, from the subtracted images, fluorescent mean intensity per cell can be measured by manually sketching out the cell boundaries. Also, the normalization of each cell’s intensity was considered by multiplying the area factor. Data were rendered as the fluorescence intensity average determined by employing ImageJ software (NIH). In each experiment (n = 3), 25 cells were analysed from at least 4 randomly chosen fields for each treatment. Digital images were optimized for contrast and brightness using Adobe Photoshop 7.0 Software.
- b)
- Flow cytometer: cells were growth in 12-well plates to be incubated then with CNN-CS-NG (50 µg/mL) for 5, 15, 30, 60 min and 24 h at culture conditions. After treatment, cells were washed with PBS, trypsinized, resuspended in PBS and immediately subjected to flow cytometry (FACS Aria Becton Dickinson). Mean fluorescence intensity of the cell population that internalized CNN-CS-NG was determined by using FlowJo v.10.7.2 software (TreeStar/BD Bioscience) [25].
2.7. In Vivo Studies
2.7.1. Animals
2.7.2. CNN-CS-NG Brain Uptake
- -
- Experimental groups: mice received an intraperitoneal (i.p.) injection with 200 μL solution containing 250 µg/mL or 1000 µg/mL of CNN-CS-NG.
- -
- Control group: mice were injected i.p. with 200 μL of distilled water.
2.8. Statistical Analysis
3. Results
3.1. NG Characterization
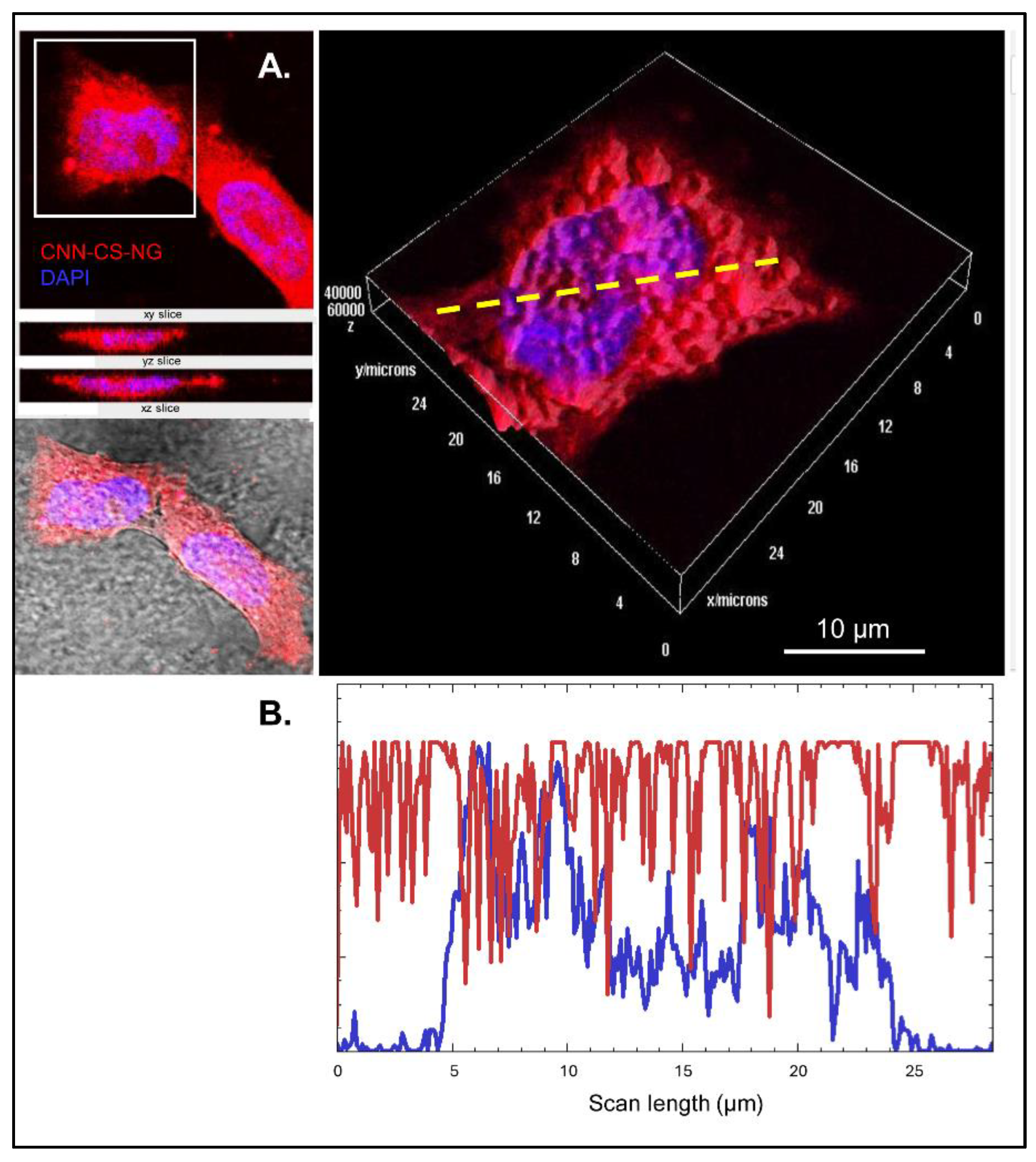
3.2. Biological Performance of CNN-CS-NG: Cellular Biocompatibility, Uptake, and Imaging
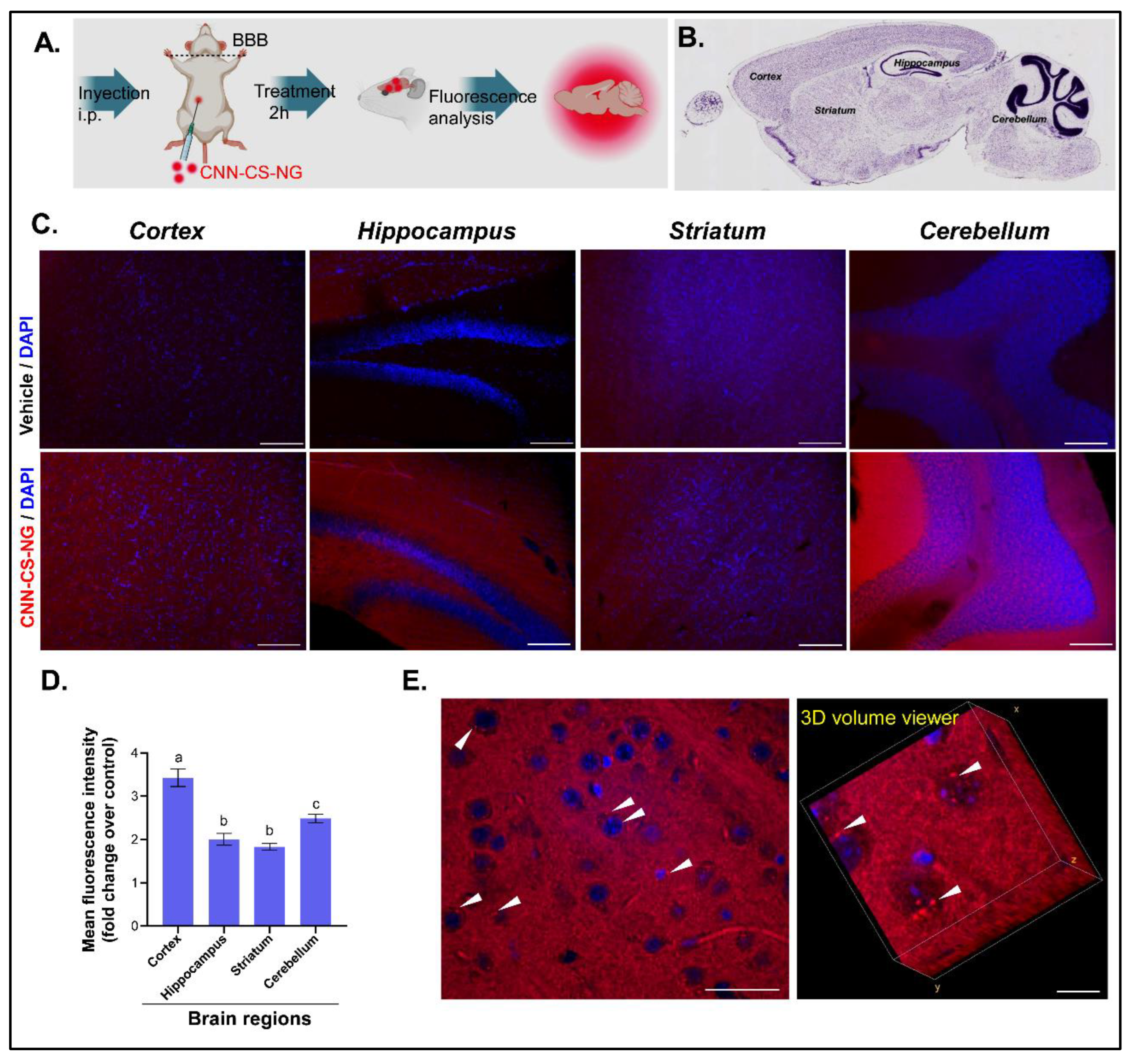
3.3. NG Reach the Mice Brains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Feigin, V.L.; Krishnamurthi, R. V.; Theadom, A.M.; et al.; Zaki, M.E. Global, Regional, and National Burden of Neurological Disorders during 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017, 16, 877–897. [CrossRef]
- Owolabi, M.; Leonardi, M.; Bassetti, C.; et al.; Servadei, F. Global Synergistic Actions to Improve Brain Health for Human Development. Nat Rev Neurol 2023, 19, 371–383. [CrossRef]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323.
- Pardridge, W.M. A Historical Review of Brain Drug Delivery. Pharmaceutics 2022, 14. [CrossRef]
- Li, J.; Zheng, M.; Shimoni, O.; Banks, W.A.; Bush, A.I.; Gamble, J.R.; Shi, B. Development of Novel Therapeutics Targeting the Blood–Brain Barrier: From Barrier to Carrier. Advanced Science 2021, 8, 2101090. [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of Clinical Drug Development Fails and How to Improve It? Acta Pharm Sin B 2022, 12, 3049–3062.
- Agrahari, V.; Kumar, P. Novel Approaches for Overcoming Biological Barriers. Pharmaceutics 2022, 14, 4–7. [CrossRef]
- Tan, Q.; Zhao, S.; Xu, T.; Wang, Q.; Lan, M.; Yan, L.; Chen, X. Getting Drugs to the Brain: Advances and Prospects of Organic Nanoparticle Delivery Systems for Assisting Drugs to Cross the Blood-Brain Barrier. J Mater Chem B 2022, 10, 9314–9333. [CrossRef]
- Sarkar, A.; Fatima, I.; Mohammad Sajid Jamal, Q.; Sayeed, U.; Kalim A. Khan, M.; Akhtar, S.; Amjad Kamal, M.; Farooqui, A.; Haris Siddiqui, M. Nanoparticles as a Carrier System for Drug Delivery Across Blood Brain Barrier. Curr Drug Metab 2017, 18, 129–137. [CrossRef]
- Khan, I.N.; Navaid, S.; Waqar, W.; Hussein, D.; Ullah, N.; Khan, M.U.A.; Hussain, Z.; Javed, A. Chitosan-Based Polymeric Nanoparticles as an Efficient Gene Delivery System to Cross Blood Brain Barrier: In Vitro and In Vivo Evaluations. Pharmaceuticals 2024, 17, 169. [CrossRef]
- Crini, G. Historical Review on Chitin and Chitosan Biopolymers. Environ Chem Lett 2019, 17, 1623–1643.
- Buosi, F.S.*; Alaimo, A.*; Di Santo, M.C.; Elías, F.; García Liñares, G.; Acebedo, S.L.; Castañeda Cataña, M.A.; Spagnuolo, C.C.; Lizarraga, L.; Martínez, K.D.; et al. Resveratrol Encapsulation in High Molecular Weight Chitosan-Based Nanogels for Applications in Ocular Treatments: Impact on Human ARPE-19 Culture Cells. Int J Biol Macromol 2020, 165, 804–821. [CrossRef]
- Silva Nieto, R.; Samaniego López, C.; Moretton, M.A.; Lizarraga, L.; Chiappetta, D.A.; Alaimo, A.; Pérez, O.E. Chitosan-Based Nanogels Designed for Betanin-Rich Beetroot Extract Transport: Physicochemical and Biological Aspects. Polymers (Basel) 2023, 15. [CrossRef]
- Algharib, S.A.; Dawood, A.; Zhou, K.; Chen, D.; Li, C.; Meng, K.; Zhang, A.; Luo, W.; Ahmed, S.; Huang, L.; et al. Preparation of Chitosan Nanoparticles by Ionotropic Gelation Technique: Effects of Formulation Parameters and in Vitro Characterization. J Mol Struct 2022, 1252, 132129. [CrossRef]
- Khalin, I.; Severi, C.; Heimburger, D.; Wehn, A.; Hellal, F.; Reisch, A.; Klymchenko, A.S.; Plesnila, N. Dynamic Tracing Using Ultra-Bright Labeling and Multi-Photon Microscopy Identifies Endothelial Uptake of Poloxamer 188 Coated Poly(Lactic-Co-Glycolic Acid) Nano-Carriers in Vivo. Nanomedicine 2022, 40, 102511. [CrossRef]
- Vargas-Nadal, G.; Köber, M.; Nsamela, A.; Terenziani, F.; Sissa, C.; Pescina, S.; Sonvico, F.; Gazzali, A.M.; Wahab, H.A.; Grisanti, L.; et al. Fluorescent Multifunctional Organic Nanoparticles for Drug Delivery and Bioimaging: A Tutorial Review. Pharmaceutics 2022, 14, 2498. [CrossRef]
- Samaniego Lopez, C.; Amparo, M.; Huvelle, L.; Uhrig, M.L.; Coluccio, F.; Spagnuolo, C.C. Electronic Supplementary Information (ESI) Recognition of Saccharides in the NIR Region with a Novel Fluorogenic Boronolectin: In Vitro and Live Cells Labeling; 2015;
- Bihari, P.; Vippola, M.; Schultes, S.; Praetner, M.; Khandoga, A.G.; Reichel, C.A.; Coester, C.; Tuomi, T.; Rehberg, M.; Krombach, F. Optimized Dispersion of Nanoparticles for Biological in Vitro and in Vivo Studies. Part Fibre Toxicol 2008, 5, 1–14. [CrossRef]
- Buosi, S.F.; Alaimo, A.; Di Santo, M.C.; Elías, F.; García, G.; Acebedo, S.L.; Alejandra, M.L.; Mayra Alejandra, Castañeda Spagnuolo, C.C.; Lizarraga, L.; Martínez, K.D.; et al. Resveratrol Encapsulation in High Molecular Weight Chitosan-Based Nanogels for Applications in Ocular Treatments : Impact on Human ARPE-19 Culture Cells. Int J Biol Macromol 2020, 165, 804–821. [CrossRef]
- Prudkin Silva, C.; Richmond, L.; Martínez, K.D.; Martínez, J.H.; Martínez, K.D.; Farías, M.E.; Pérez, O.E.O.E.; Leskow, F.C.; Pérez, O.E.O.E. Proposed Molecular Model for Electrostatic Interactions between Insulin and Chitosan. Nano-Complexation and Activity in Cultured Cells. Colloids Surf A Physicochem Eng Asp 2018, 537, 425–434. [CrossRef]
- Martin, E.R.; Gandawijaya, J.; Oguro-Ando, A. A Novel Method for Generating Glutamatergic SH-SY5Y Neuron-like Cells Utilizing B-27 Supplement. Front Pharmacol 2022, 13. [CrossRef]
- Martinez, J.H.; Alaimo, A.; Gorojod, R.M.; Porte Alcon, S.; Fuentes, F.; Coluccio Leskow, F.; Kotler, M.L. Drp-1 Dependent Mitochondrial Fragmentation and Protective Autophagy in Dopaminergic SH-SY5Y Cells Overexpressing Alpha-Synuclein. Molecular and Cellular Neuroscience 2018, 88, 107–117. [CrossRef]
- Boeneman, K.; Delehanty, J.B.; Blanco-Canosa, J.B.; Susumu, K.; Stewart, M.H.; Oh, E.; Huston, A.L.; Dawson, G.; Ingale, S.; Walters, R.; et al. Selecting Improved Peptidyl Motifs for Cytosolic Delivery of Disparate Protein and Nanoparticle Materials. ACS Nano 2013, 7, 3778–3796. [CrossRef]
- Martens, T.F.; Remaut, K.; Demeester, J.; De Smedt, S.C.; Braeckmans, K. Intracellular Delivery of Nanomaterials: How to Catch Endosomal Escape in the Act. Nano Today 2014, 9, 344–364. [CrossRef]
- Atabakhshi-Kashi, M.; Carril, M.; Mahdavi, H.; Parak, W.J.; Carrillo-Carrion, C.; Khajeh, K. In Vitro Cellular Uptake Studies of Self-Assembled Fluorinated Nanoparticles Labelled with Antibodies. Nanomaterials 2021, 11. [CrossRef]
- Berardino, B.G.; Ballarini, F.; Chertoff, M.; Igaz, L.M.; Cánepa, E.T. Nutritional Stress Timing Differentially Programs Cognitive Abilities in Young Adult Male Mice. Nutr Neurosci 2022, 25, 286–298. [CrossRef]
- Monge-Fuentes, V.; Biolchi Mayer, A.; Lima, M.R.; Geraldes, L.R.; Zanotto, L.N.; Moreira, K.G.; Martins, O.P.; Piva, H.L.; Felipe, M.S.S.; Amaral, A.C.; et al. Dopamine-Loaded Nanoparticle Systems Circumvent the Blood–Brain Barrier Restoring Motor Function in Mouse Model for Parkinson’s Disease. Sci Rep 2021, 11. [CrossRef]
- Paxinos, G.; Franklin, K.B.J. Paxinos and Franklin’s The Mouse Brain in Stereotaxic Coordinates. 1998, 4th Ed.
- Allen Institute for Brain Science Atlas Brain Map.
- Jiang, Y.; Fu, C.; Wu, S.; Liu, G.; Guo, J.; Su, Z. Determination of the Deacetylation Degree of Chitooligosaccharides. Mar Drugs 2017, 15. [CrossRef]
- Picone, C.S.F.; Cunha, R.L. Chitosan-Gellan Electrostatic Complexes: Influence of Preparation Conditions and Surfactant Presence. Carbohydr Polym 2013, 94, 695–703. [CrossRef]
- Khattab, T.A.; Kassem, N.F.; Adel, A.M.; Kamel, S. Optical Recognition of Ammonia and Amine Vapor Using “Turn-on” Fluorescent Chitosan Nanoparticles Imprinted on Cellulose Strips. J Fluoresc 2019, 29, 693–702. [CrossRef]
- Vimal, S.; Abdul Majeed, S.; Taju, G.; Nambi, K.S.N.; Sundar Raj, N.; Madan, N.; Farook, M.A.; Rajkumar, T.; Gopinath, D.; Sahul Hameed, A.S. Chitosan Tripolyphosphate (CS/TPP) Nanoparticles: Preparation, Characterization and Application for Gene Delivery in Shrimp. Acta Trop 2013, 128, 486–493. [CrossRef]
- Smith, S.A.; Selby, L.I.; Johnston, A.P.R.; Such, G.K. The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjug Chem 2019, 30, 263–272. [CrossRef]
- Cupic, K.I.; Rennick, J.J.; Johnston, A.P.R.; Such, G.K. Controlling Endosomal Escape Using Nanoparticle Composition: Current Progress and Future Perspectives. Nanomedicine (Lond) 2019, 14, 215–223. [CrossRef]
- Vermeulen, L.M.P.; Fraire, J.C.; Raes, L.; De Meester, E.; De Keulenaer, S.; Van Nieuwerburgh, F.; De Smedt, S.; Remaut, K.; Braeckmans, K. Photothermally Triggered Endosomal Escape and Its Influence on Transfection Efficiency of Gold-Functionalized JetPEI/PDNA Nanoparticles. Int J Mol Sci 2018, 19. [CrossRef]
- Zhu, J.Y.; Lei, Q.; Yang, B.; Jia, H.Z.; Qiu, W.X.; Wang, X.; Zeng, X.; Zhuo, R.X.; Feng, J.; Zhang, X.Z. Efficient Nuclear Drug Translocation and Improved Drug Efficacy Mediated by Acidity-Responsive Boronate-Linked Dextran/Cholesterol Nanoassembly. Biomaterials 2015, 52, 281–290. [CrossRef]
- Carton, F.; Malatesta, M. Assessing the Interactions between Nanoparticles and Biological Barriers in Vitro: A New Challenge for Microscopy Techniques in Nanomedicine; 2022; Vol. 66;.
- Álamo, P.; Pallarès, V.; Céspedes, M.V.; Falgàs, A.; Sanchez, J.M.; Serna, N.; Sánchez-garcía, L.; Voltà-duràn, E.; Morris, G.A.; Sánchez-chardi, A.; et al. Fluorescent Dye Labeling Changes the Biodistribution of Tumor-targeted Nanoparticles. Pharmaceutics 2020, 12, 1–18. [CrossRef]
- Marcelo, G.A.; Galhano, J.; Oliveira, E. Applications of Cyanine-Nanoparticle Systems in Science: Health and Environmental Perspectives. Dyes and Pigments 2022, 208. [CrossRef]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Türeli, N.G. Key for Crossing the BBB with Nanoparticles: The Rational Design. Beilstein Journal of Nanotechnology 2020, 11, 866–883.
- Jiang, L.Q.; Wang, T.Y.; Webster, T.J.; Duan, H.J.; Qiu, J.Y.; Zhao, Z.M.; Yin, X.X.; Zheng, C.L. Intracellular Disposition of Chitosan Nanoparticles in Macrophages: Intracellular Uptake, Exocytosis, and Intercellular Transport. Int J Nanomedicine 2017, 12, 6383–6398. [CrossRef]
- Ortega, E.; Blanco, S.; Ruiz, A.; Peinado, M.Á.; Peralta, S.; Morales, M.E. Lipid Nanoparticles for the Transport of Drugs like Dopamine through the Blood-Brain Barrier. Journal of Nanoparticle Research 2021, 23. [CrossRef]
- ŞEKO, I.; SAHÍN, A.; TONBUL, H.; ÇAPAN, Y. Brain-Targeted Nanoparticles to Overcome the Blood-Brain Barrier. Journal of Pharmaceutical Technolgy 2020, 1, 26–40. [CrossRef]
- Nance, E.A.; Woodworth, G.F.; Sailor, K.A.; Shih, T.Y.; Xu, Q.; Swaminathan, G.; Xiang, D.; Eberhart, C.; Hanes, J. A Dense Poly(Ethylene Glycol) Coating Improves Penetration of Large Polymeric Nanoparticles within Brain Tissue. Sci Transl Med 2012, 4. [CrossRef]
- Papadia, K.; Markoutsa, E.; Antimisiaris, S.G. How Do the Physicochemical Properties of Nanoliposomes Affect Their Interactions with the HCMEC/D3 Cellular Model of the BBB? Int J Pharm 2016, 509, 431–438. [CrossRef]
- Di Santo, M.C.; Alaimo, A.; Dominguez Rubio, A.; De Matteo, R.; Pérez, O.E. Biocompatibility Analysis of High Molecular Weight Chitosan Obtained from Pleoticus Muelleri Shrimps . Evaluation in Prokaryotic and Eukaryotic Cells . Biochem Biophys Rep 2020, 24, 100842. [CrossRef]
- Bhattamisra, S.K.; Shak, A.T.; Xi, L.W.; Safian, N.H.; Choudhury, H.; Lim, W.M.; Shahzad, N.; Alhakamy, N.A.; Anwer, M.K.; Radhakrishnan, A.K.; et al. Nose to Brain Delivery of Rotigotine Loaded Chitosan Nanoparticles in Human SH-SY5Y Neuroblastoma Cells and Animal Model of Parkinson’s Disease. Int J Pharm 2020, 579. [CrossRef]
- Salgado, A.J.; Oliveira, J.M.; Pirraco, R.P.; Pereira, V.H.; Fraga, J.S.; Marques, A.P.; Neves, N.M.; Mano, J.F.; Reis, R.L.; Sousa, N. Carboxymethylchitosan/Poly(Amidoamine) Dendrimer Nanoparticles in Central Nervous Systems-Regenerative Medicine: Effects on Neuron/Glial Cell Viability and Internalization Efficiency. Macromol Biosci 2010, 10, 1130–1140. [CrossRef]
- Del Prado-Audelo, M.L.; Magaña, J.J.; Mejía-Contreras, B.A.; Borbolla-Jiménez, F. V.; Giraldo-Gomez, D.M.; Piña-Barba, M.C.; Quintanar-Guerrero, D.; Leyva-Gómez, G. In Vitro Cell Uptake Evaluation of Curcumin-Loaded PCL/F68 Nanoparticles for Potential Application in Neuronal Diseases. J Drug Deliv Sci Technol 2019, 52, 905–914. [CrossRef]
- Monsalve, Y.; Tosi, G.; Ruozi, B.; Belletti, D.; Vilella, A.; Zoli, M.; Vandelli, M.A.; Forni, F.; López, B.L.; Sierra, L. PEG-g-Chitosan Nanoparticles Functionalized with the Monoclonal Antibody OX26 for Brain Drug Targeting. Nanomedicine 2015, 10, 1735–1750. [CrossRef]
- Kenesei, K.; Murali, K.; Czéh, Á.; Piella, J.; Puntes, V.; Madarász, E. Enhanced Detection with Spectral Imaging Fluorescence Microscopy Reveals Tissue- and Cell-Type-Specific Compartmentalization of Surface-Modified Polystyrene Nanoparticles. J Nanobiotechnology 2016, 14. [CrossRef]
- Kaur, S.; Manhas, P.; Swami, A.; Bhandari, R.; Sharma, K.K.; Jain, R.; Kumar, R.; Pandey, S.K.; Kuhad, A.; Sharma, R.K.; et al. Bioengineered PLGA-Chitosan Nanoparticles for Brain Targeted Intranasal Delivery of Antiepileptic TRH Analogues. Chemical Engineering Journal 2018, 346, 630–639. [CrossRef]
- Ma, F.; Yang, L.; Sun, Z.; Chen, J.; Rui, X.; Glass, Z.; Xu, Q. Neurotransmitter-Derived Lipidoids (NT-Lipidoids) for Enhanced Brain Delivery through Intravenous Injection. Sci Adv . 2020, 6, eabb4429. [CrossRef]
- Sharma, H.S.; Kreuter, J.; Latronico, T.; of Bari Aldo Moro, U.; Himakarnika Alluri, I.; Serrano Sponton, L.; Sponton, S.L. In-Vivo Time Course of Organ Uptake and Blood-Brain-Barrier Permeation of Poly(L-Lactide) and Poly(Perfluorodecyl Acrylate) Nanoparticles with Different Surface Properties in Unharmed and Brain-Traumatized Rats. Front Neurol . 2023, 6, 994877.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




