1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and the third cause of cancer death worldwide [
1].
The choice of treatment in patients with hepatocellular carcinoma (HCC) is very complex since this neoplasm occurs in most cases in patients with chronic liver disease and other comorbidities. In the Western world, until recently, the therapeutic strategy was indicated exclusively by the stage (or substage) of the disease according to the Barcelona Clinic Liver Cancer (BCLC) scheme [
2].
However, liver stage can vary in the course of disease and sometimes therapeutic decisions can change considerably depending on effectiveness of specific therapy [
3].
Liver transplantation (LT) is the therapy for patients with nonmetastatic HCC (3) which achieves the greatest survival benefit for early-stage disease (BCLC-0 and A) with 5-year survival rates of about 80% [
4].
Nevertheless, only a small percentage of patients can be transplanted due to advanced tumor disease preventing organ allocation and the lack of systemic therapies that can effectively reduce or prevent tumor progression on the LT waiting list. In this regard, the guidelines recommend the use of Milan criteria (MC) in patients with HCC (a single tumour < 5 cm or 3 nodules < 3 cm) for inclusion on the liver transplant list [
5].
Since 1996, when the MCs were published, when clearly relapse rates were unacceptably high, several efforts have been made in subsequent years to broaden the criteria to allow more patients the best treatment [
6]. For example, these purely numerical and dimensional criteria do not always reflect the biology of the disease [
5]and for this reason, patients beyond MC could also benefit from a liver transplant [
6].
To increase the number of patients with HCC eligible for liver transplantation, other strategies have been used including the Toronto criteria [
7] (excluding patients with a poorly differentiated tumour or with evidence of extrahepatic disease or vascular invasion), the Ontario criteria (excluding patients with a total tumour volume > 145 cm^3 and alpha fetoprotein > 1000 ng/mL) or the University of California San Francisco criteria (excluding patients with a tumour size > 6.5 cm).
However, there is a considerably higher rate of waiting-list drop-out and survival in this population [
8].
For the reasons described above, the American Association for the Study of Liver Disease suggests that patients who do not meet the Milan criteria, can be considered for LT after successful downstaging according to the MC, which have been accepted by the United Network for Organ Sharing and provide a means of converting previously ineligible patients to liver transplantation [
9].
For a long time, the approaches used for pre-surgery downstaging were locoregional treatments i.e., ablation and transarterial therapies.
The first described cases of the use of neoadjuvant chemotherapy in primary liver tumors date from the 1970s. In that decade, Hermann described the use of adriamycin in six children with hepatoblastoma with lung metastases. Three patients underwent resection of the neoplasm after reduction of the initial mass. This chemotherapy regimen allowed resection of a previously unresectable hepatoblastoma and reduced the morbidity and mortality of an otherwise extensive operation [
10]. The development of immune checkpoint inhibitors (ICIs) has revolutionized cancer treatment, enabling the possibility to increase long-term survival in patients with metastatic disease and providing new therapeutic indications in early stages.
Currently, ICI is also being added as a therapeutic armamentarium for the treatment of unresectable or advanced HCC that is not amenable to curative or locoregional therapy.
The combination of atezolizumab and bevacizumab (IMbrave150 study) compared to sorafenib demonstrated superior response rates and overall survival (29.8% vs 12% and 19.8 vs 13.4 months, respectively; HR 0.66, 95% CI 0.52, 0.85; p=0.0009) [
11]
. Moreover, double ICI therapy with the combination of Durvalumab and Tremelimumab has received approval for the treatment of unresectable HCC [
12]
.
General Concepts of Immunotherapy
The cytotoxic immune checkpoint proteins T-lymphocyte-associated-4 (CTLA-4) and programmed cell death protein 1 (PD-1) are expressed on the surface of cytotoxic T cells, the ligands of which are CD80/CD86 and programmed death ligand 1 (PD-L1), respectively. The interaction of these proteins with their ligand prevents T cell activation, thus maintaining peripheral tolerance. This mechanism helps tumor cells escape cytotoxic T cell-mediated death [
13]. ICIs are immune checkpoint inhibitors that prevent this interaction, thus displaying an anti-tumor effect [
14]. The most frequent immune-related
adverse events (irAEs) involve the skin, endocrine tissue, liver, colon and lung [
15]. Liver events (hepatitis) occurs in 5%-10% (1%-2% grade 3) of patients treated in monotherapy and in 25%-30% (15% grade 3) in patients treated with combined anti-PD-(L)1 and anti-CTLA-4 therapy) [
15]. Immune-related hepatitis tends to present as an asymptomatic elevation in alanine or aspartate aminotransferase with or without elevation in bilirubin [
15]. The most typical morphological aspect of ICI damage is lobular hepatitis with necrosis, patchy or confluent. Liver damage caused is heterogeneous and involves lobular and periportal activity or sinusoidal histiocytosis, fibrin deposition, and central vein endothelitis by anti PD-(L)1 and anti-CTLA-4, respectively [
15].
Management of irAEs depends on the grade and includes the suspension of the drug or initiation of immunosuppressive therapy. Hepatitis usually resolves within four to six weeks after starting appropriate treatment [
15]. This delayed reaction could be due to the pharmacodynamics of the drug [
15].
Pharmacological effects and the likelihood of drug interactions are influenced by the half-life of the drug. The anti-PD1/PD-L1 agents (nivolumab, pembrolizumab, atezolizumab and durvalumab) have a half-life of approximately 3 to 4 weeks, whereas the anti-CTLA4 (ipilimumab) has a half-life of about 2 weeks [
16].
After organ transplantation, the induction of immune tolerance is fundamental for the survival of the transplant and therefore of the patient. The PD-1 and CTLA-4 signaling pathways are critical to immune tolerance after transplantation. Animal and human studies demonstrated that the PD-1/PDL-1 co-inhibitory pathway was influential in regulating transplant tolerance and rejection [
17]. PD-L1 and PD-1 are highly expressed in transplanted liver tissue, and PD-1/PD-L1 blockade leads to increased hepatic T cell proliferation until allostimulation. PD-1 was expressed in tissue T cells, PD-L1 was expressed in hepatocytes, bile duct cells, and along sinusoids [
18].
The considerable interest on the topic has in fact increased case reports, clinical cases and on-going studies in just 6 months [
19].
2. Methods
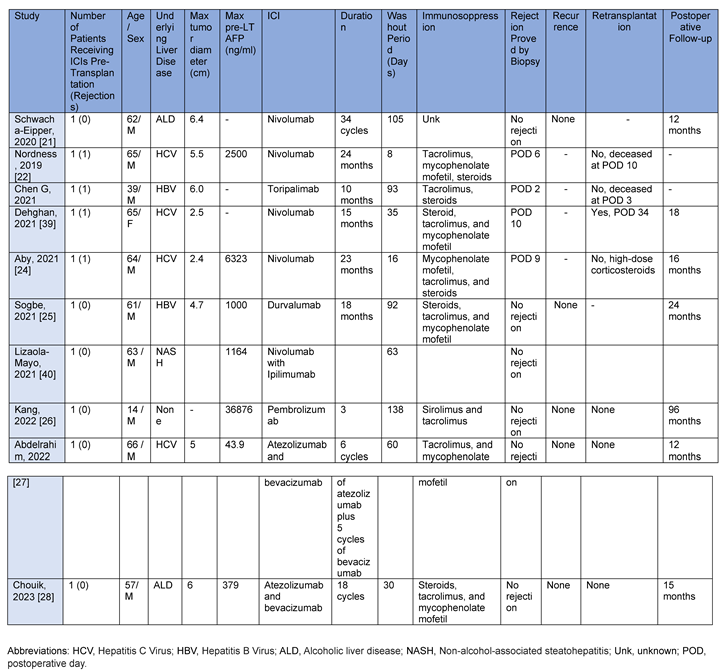
Clinical cases and case reports published, up to March 2024, were collected and described. These studies were described to highlight adverse events or complications deriving from the use of ICIs in the context of liver transplantation. For each clinical case and case report, number of patients, age and sex, underlying liver disease, maximum tumor diameter (cm), maximum pre-LT Alpha-fetoprotein (ng/ml), ICI type, duration of washout period (days), biopsy-proven rejection, HCC recurrence, re-transplantation and postoperative follow-up.
On the basis of these data, ongoing case-control studies were then evaluated. ClinicalTrials.gov was last queried on March 31, 2024 for the terms “checkpoint inhibitors,” “liver transplant,” “hepatocellular carcinoma,” and “immunotherapy.” Only studies involving liver transplant recipients with HCC undergoing ICI were included. We analyzed 9 patient studies and for each we assessed the main outcomes, recruitment status and etiology of liver disease.
3. Results
3.1. Case Reports
Between 2017 and 2020 at Mount Sinai Medical Center (New York), nine patients with HCC were treated with nivolumab and then transplanted. The mean age was 57 years and the male gender was more frequent (67%). HBV infection was the most common underlying liver disease (56%). Five patients (56%) had undergone previous surgery; one transplant (11%) was from a living donor. Nivolumab was administered every 2 weeks at a dosage of 240 mg and eight (89%) patients received last dose within 1 month after LT. No severe allograft rejection/loss, tumour recurrence or death occurred during the median follow-up of 16 months (range, 8-23 months) after LT. A mild acute rejection was reported secondary to low tacrolimus (<6 ng/ml) that responded rapidly to dosage increase. Histological examination of the explanted liver revealed almost complete tumour necrosis (>90%) in one third of the cases [
20].
In 2019, a 62-year-old patient was transplanted in Bern, Switzerland, due to alcohol-related cirrhosis complicated by HCC. The patient first underwent liver resection. Due to multifocal recurrence, he started systemic therapy with sorafenib then regorafenib, discontinued due to skin toxicity. In June 2017, he started therapy with nivolumab for a total of 24 cycles. The nivolumab was discontinued 6 weeks prior to inclusion on the transplant list. One year after LT the patient had neither tumor recurrence nor rejection [
21].
Different is the case of a 65-year-old man with a hepatitis C virus (HCV) infection who underwent a LT in Nashville (Tennessee). Due to the appearance of HCC, the patient underwent laparoscopic resection followed by sorafenib and Y90 transarterial radioembolization. Due to extensive tumour burden nivolumab was started at a dose of 240 mg every 2 weeks. Following the drastic reduction of the tumor disease burden, he was placed on the liver transplant list. The patient had received almost 2 years of nivolumab therapy, with the last dose administered 8 days before the transplant. Due to a progressive increase of liver enzymes, prothrombin time and creatinine levels, he underwent a transjugular liver biopsy which demonstrated acute hepatic necrosis with a profound lymphocyte infiltrative reaction in the portal tracts with subsequent death [
22].
Only a few considerations can be deduced from these case reports: the patient who died after immunotherapy had received therapy with nivolumab for almost 2 years and underwent LT 8 days after the end. However, other patients who received the last dose close to the transplant did not experience fatal events. It should be remembered that nivolumab has a half-life of approximately 4 weeks and therefore a short interval between the last dose and the LT should be avoided [
20,
21,
22].
Colleagues at the University of California at San Diego, instead, described a re-transplant after demonstrating severe acute rejection with massive hepatic necrosis and loss of the first allograft, which they attributed to immune-mediated damage resulting from the use of nivolumab before the initial transplant [
23]. A 60-year-old woman suffering from HCV cirrhosis and complicated by HCC underwent transarterial chemoembolization and microwave ablation. Due to persistence of tumor disease, she began treatment with sorafenib and nivolumab with repeated microwave ablation. Sorafenib was stopped after 3 months due to intolerance, but nivolumab was continued (240 mg every 2 weeks in the first month, then 480 mg every 4 weeks for 15 months) and discontinued at 5 weeks before transplantation [
23]. The successful postoperative course of the second LT was attributed to the use of therapeutic plasmapheresis, IV immunoglobulin, and preoperative antithymocyte globulin to reduce DSA and significantly lower the immune response. After bolus of 1000 mg methylprednisolone for 3 days with gradual reduction for acute cellular rejection (RAI 5/9) and placement of plastic stent for biliary anastomotic stricture she was discharged on POD 33 and continues to do well 18 months after transplant [
23].
A case described by the University of Minnesota, the patient developed, within the first two weeks of liver transplantation, acute cellular rejection (ACR) and was promptly treated with high-dose glucocorticoids followed by thymoglobulin with resolution of rejection on biopsy liver follow-up [
24]. The patient was a 64-year-old male with compensated HCV-cirrhosis complicated by HCC and malignant portal vein thrombus. He was treated with locoregional treatments (radioembolization, chemoembolization, microwave ablation) and systemic therapy with sorafenib. Due to disease progression and intolerance to sorafenib, treatment with nivolumab was initiated. He received a total of 23 cycles of nivolumab, 480 mg administered every 4 weeks. Last nivolumab infusion was 16 days before LT [
24]. The liver explant showed no viable HCC, no evidence of vascular invasion, and no transcapsular extrahepatic tumor extension. For histological confirmation on day 9 of acute cellular rejection (ACR) he was treated with high-dose solumedrol and thymoglobulin 100 mg i.v. The patient was discharged with maintenance therapy with mycophenolate mofetil, tacrolimus, and prednisone. Liver chemistry parameters at 16 months are normal [
24].
A 61-year-old male with chronic hepatitis B virus infection complicated by HCC showed recurrence at four months after resection. After progression on Sorafenib, he began monotherapy treatment with durvalumab. To minimize the risk of rejection induced by PDL1 inhibition, waiting list entry was delayed for 3 months after discontinuation of durvalumab. No viable tumor tissue was observed after explantation and no rejection events were observed. The patient remained disease-free 89 months after diagnosis and 24 months after LT [
25].
A fourteen-year-old male, after HCC, underwent three cycles of chemotherapy with cisplatin/doxorubicin/dexrazoxane (PLADO) and transarterial chemoembolization with drug-eluting spheres (DEB-TACE) with doxorubicin. After extended left tri-segmentectomy, for recurrence the patient received three cycles of 2 mg/kg every 3 weeks of pembrolizumab (anti-PD1) then another cycle of DEB-TACE. At 138 days after the last dose of pembrolizumab, the patient underwent LT. Unfortunately, due to hepatic artery dissection, a second LT was required 6 days later. Now, 4 years after the transplant, the patient is doing well with sirolimus and tacrolimus without episodes of transplant rejection or disease recurrence [
26].
A case report published in 2022 described an excellent response of a 66-year-old cirrhosis patient with treated HCV infection and 5-cm undifferentiated hepatocellular carcinoma in the right hepatic lobe [
27]. The patient was treated with the combination atezolizumab and bevacizumab before LT [
27]. After treatment, imaging showed a significant response and liver transplantation was performed 8 weeks after the last dose of immunotherapy. After completion of LT, the patient started treatment with tacrolimus 1 mg capsules twice daily and mycophenolate 500 mg tablets twice daily for immunosuppression [
27].
In 2023, the case of a 57-year-old man was published, with initial unresectable multinodular HCC contraindicated to LT and locoregional therapies, who achieved a complete tumor response after Atezolizumab/Bevacizumab and subsequently underwent LT for liver failure [
28]. Analysis of the explanted liver revealed a complete pathological response with no tumor residues [
28]. The patient suffered from several postoperative complications, but there was no recurrence of HCC or biopsy-confirmed acute rejection 10 months after LT [
28].
A brief description of all reported cases is summarized in
Table 1.
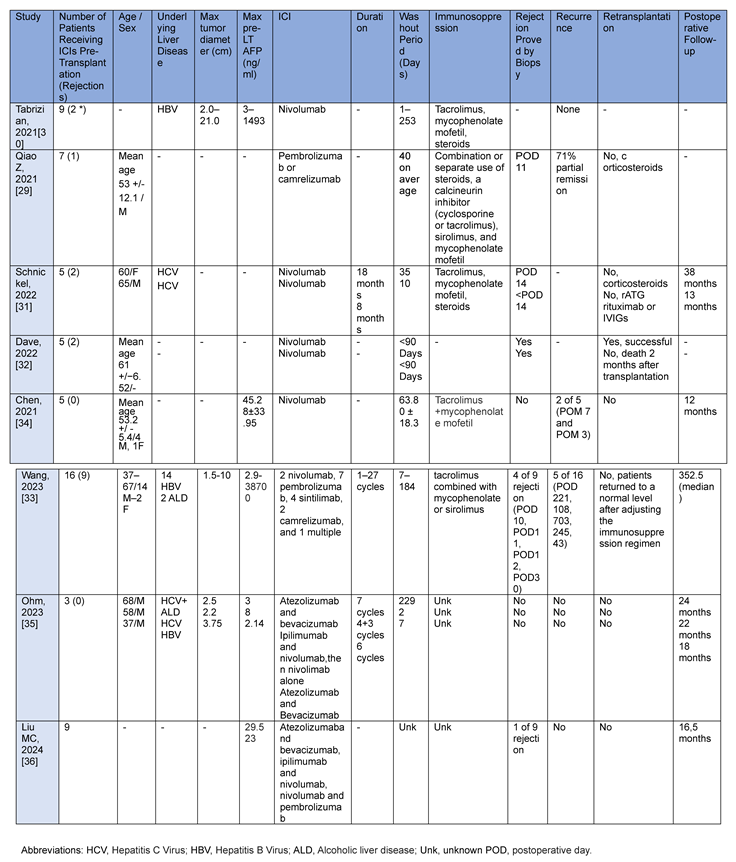
3.2. Case Series
A retrospective cohort study of seven patients evaluated the feasibility and efficacy of pre-transplant immunotherapy. Patients underwent neoadjuvant pembrolizumab (200 mg, 3 weeks per cycle) or camrelizumab (200 mg, 2 weeks per cycle) combined with lenvatinib before LT in 2020 and 2021. The washout period was 42 days and one patient had graft rejection after LT, but it resolved after changing the immunosuppression regimen [
29].
A series of cases were described between 2017 and 2020. These included nine HCC patients who were successfully transplanted after receiving nivolumab pre-liver transplant. Five patients (56%) had previous resection and nivolumab was administered at a dose of 240 mg every 2 weeks. Eight patients (89%) received their last dose within 4 weeks of LT. At a median follow-up of 16 months (range: 8-23 months) after transplantation, there were no serious allograft rejections/losses, tumor recurrences, or deaths [
30].
In another study, five patients who underwent LT for HCC previously treated with immunotherapy were described [
31]. Of these, serious post-transplant complications including liver necrosis and graft loss were observed in the patients with less than 3 months between the last dose of ICI and transplant [
31]. A case-control study included eighty-six patients with HCC subsequently listed for transplant between 2018 and 2020 [
32]. Of these, eight patients received ICI therapy, of which five were transplanted. The median time from last ICI treatment to LT was 105 days (11-354), and all patients were treated with nivolumab. Two patients (40 %) treated with ICI had biopsy-confirmed rejection compared to 3 (6.4%) of non-ICI patients.
There were two graft losses (40 %) in the ICI group. Both patients with biopsy-proven rejection received ICI therapy <90 days before LT. None of the 3 patients who received the last dose of ICI therapy >90 days of LT had rejection [
32].
A Chinese retrospective study enrolled sixteen recipients from November 2018 to November 2021 [
33]. All 16 patients enrolled in this study received a PD1 inhibitor before LT. Two patients received nivolumab at a dose of 3 mg/kg every 2 weeks for 6 and 4 cycles, respectively. Seven patients received pembrolizumab preoperatively at doses of 200 mg every 3 weeks. Four patients received sintilimab at a dose of 200 mg every 3 weeks. Two patients received camrelizumab at a dose of 3 mg/kg every 3 weeks. One patient subsequently received nivolumab, toripalimab, sintilimab, and tislelizumab for a total of 27 cycles. Five patients had tumor recurrence postintervention and the tumor recurrence rate 1 year was 25.0%. The median follow-up time after LT was 352.5 (325.2–758.8) days. Nine patients had impaired liver function postoperatively and four were diagnosed with acute rejection by liver biopsy. In the remaining five, there was improvement after adapting the immunosuppression regimen. After adjusting the immunosuppression regimen, the liver function of the 9 patients with rejection after transplantation returned to the normal level, and no immune-related graft loss and fatal rejection occurred [
33]. This trial is flawed by a variety of different treatments that impedes comparison.
A case series analyzed the data of 5 patients diagnosed with HCC who underwent anti-PD-1 therapy before LT. The mean wash-out interval was 63.80±18.26 days. One patient had a tumor recurrence in the liver, vertebrae and lungs after 7 months, while one patient had a recurrence in the lungs after 3 months and acute rejection occurred in none of the patients [
34].
Another case series confirmed the safety of immunotherapy up to the follow-up period before transplantation [
35]. Three cases of patients who received immunotherapy with atezolizumab/bevacizumab or ipilimumab/nivolumab prior to liver transplantation were described in these case series. One case demonstrated a 45% reduction in HCC tumor dimension, while another showed disease stability. No organ rejection or non-healing of the wound after transplantation was observed [
35].
A latest case report published in 2024 described nine cases of HCC outside the MC undergoing atezolizumab/bevacizumab, ipilimumab/nivolumab, nivolumab or pembrolizumab [
36]. Of these, four received a transplant and one was cancelled due to its exceptional therapeutic response. The median duration of treatment was three months. Three patients received methylprednisolone for induction of immunosuppression and one received thymoglobulin. Acute transplant rejection was observed in one patient at 5 weeks, 9 weeks and 5 months. This rejection was successfully treated and considered not secondary to therapy as they observed a late onset of rejection. During a mean follow-up of 16.5 months, no tumor recurrence occurred [
36].
A brief description of all reported cases series is summarized in
Table 2.
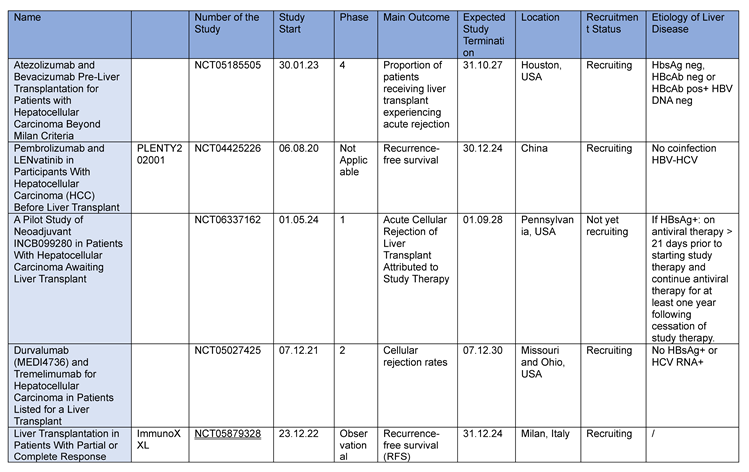
3.3. Clinical Trials
The XXL study [
37] is the first prospective study to extend the Milan criteria, validating that effective and prolonged downstage therapy improves prognosis after transplantation [
37].
The results of this study have revolutionized the HCC treatment paradigm, indicating a new standard of care for intermediate-stage HCC unresponsive to local therapies and advanced stage. Currently, there is little data to support systemic treatments with immunotherapy as a bridging or downstaging strategy to liver transplantation.
In the trial entitled “Atezolizumab and Bevacizumab Pre-Liver Transplantation for Patients with Hepatocellular Carcinoma Beyond Milan Criteria” (NCT05185505), patients with HCC beyond MC treated with neoadjuvant/downstaging atezolizumab for 6 months plus bevacizumab and transarterial chemoembolization (TACE) before liver transplantation. This multi-site study will involve a site in the US and a site in Canada. Combined enrollment from these sites envisages the recruitment of up to 30 patients
The authors hypothesize that atezolizumab and bevacizumab can be used in the pre-transplant setting without increasing the risk of rejection at 1 year post-transplant.
The Dulect2020-1 study (Durvalumab and Lenvatinib in participants with locally advanced and metastatic HCC - NCT04443322), designed as a prospective, open-label, 20-patient study, will evaluate the safety and efficacy of durvalumab in combination with lenvatinib in patients with advanced HCC prior to liver transplantation.
Most of these studies take place in North America and China their results could also have an impact on the number of patients not eligible for transplantation due to tumor burden. Ongoing studies are listed in
Table 3 (
www.clinicaltrials.gov).
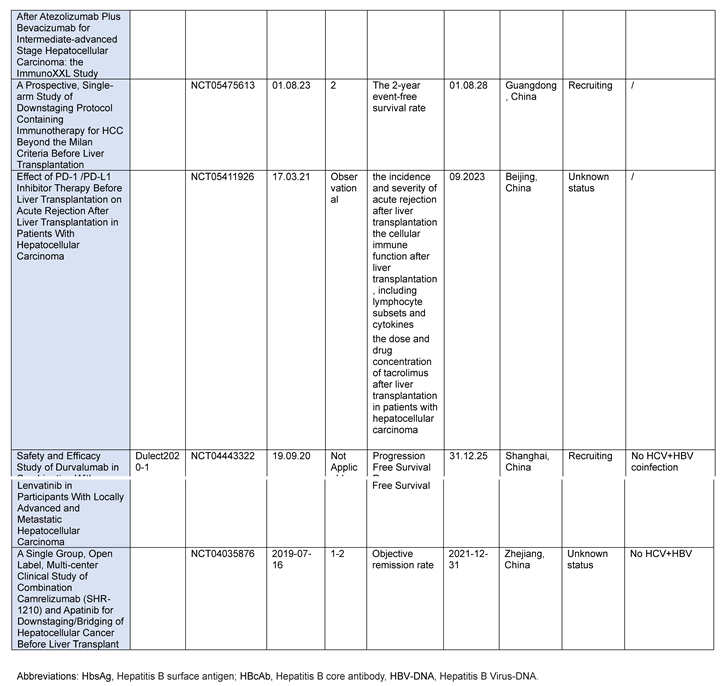
ESR-20-21010 is a Phase II (NCT05027425), open-label, single-arm, multicenter clinical trial designed to evaluate the safety (in terms of 30-day post-transplant rejection) and efficacy of durvalumab and tremelimumab for the treatment of patients with HCC before liver transplantation. Thirty patients will be enrolled and an interim analysis will be performed after ten patients to ensure safety. Patients will be treated with the immunotherapy combination for up to 4 months. After a minimum washout period of 28 days, they will undergo locoregional therapy according to the guidelines and after a minimum washout period of 72 days, they will undergo liver transplantation.
A single-center, prospective, non-interventional, real-world data-based cohort study (NCT05411926) will enroll 60 patients prior to liver transplantation for HCC (30 with a history of PD-1/PD-L1 monotherapy and 30 patients with no history of PD-1/PD-L1 monotherapy PD-1/PD-L1).
The incidence of acute rejection after liver transplantation, the time of acute rejection, Banff classification and acute rejection-related mortality, cellular immune function after liver transplantation, the dose and drug concentration of tacrolimus after liver transplantation, overall survival (OS) and recurrence-free survival (RFS) will be the endpoints.
The study (SHR-1210 - NCT04035876) will evaluate the primary effects and safety of camrelizumab in combination with apatinib in the same patient setting. Each patient will be administered a regimen of camrelizumab 200 mg every 2 weeks iv and apatinib 250 mg once daily PO every 4 weeks as a course. Each patient will receive camrelizumab treatment for at least 2 cycles and will discontinue camrelizumab 5 weeks before liver transplantation. Apatinib will be stopped 1 week before liver transplant. In this study, objective remission rate (ORR), relapse-free survival (RFS), overall survival (OS), time to progression (TTP) will be evaluated.
The PLENTY202001 study (NCT04425226) will evaluate the safety and efficacy of pembrolizumab in combination with lenvatinib in patients with HCC exceeding Milan criteria prior to liver transplantation.
A trial proposed by the Sun Yat-sen Memorial Hospital Organ Transplant Center of Sun Yat-sen University (NCT05913583) will analyze the correlation between the use of ICIs and the incidence of graft rejection and rejection-related death or graft loss after LT. The primary aim of this study will be to analyze the correlation between the use of ICI and the incidence of graft rejection, rejection-related death, or graft loss within 1 year of liver transplantation. The secondary objective will be to analyze risk factors for transplant rejection and evaluate the correlation between ICI exposure and post-transplant complications (incidence of early allograft dysfunction (EAD), bleeding, infection, biliary and vascular complications).
4. Discussion
4.1. Prevention of Graft Rejection after Conversion with ICI
Immunosuppressive therapy after liver transplantation in the above-mentioned studies is relatively standard. Induction is performed with methylprednisolone followed by mycophenolate mofetil (MMF), a calcineurin inhibitor, or an mTOR inhibitor. Some groups have also used basiliximab or antithymocyte globulin (ATG), although this did not completely prevent rejection.
After LT, acute rejection was treated with methylprednisolone, with a 90% success rate in reversing rejection. It is believed that the use of plasmapheresis increases the likelihood of overcoming acute rejection.
However, although the use of immunosuppressants saved the organ in the majority of cases some patients needed a new transplant.
In addition to the half-life of the drug, the wash-out of the drug could also be favored by the liver explantation technique which still involves significant blood loss favouring the elimination of circulating ICIs.
4.2. Expert Guidelines
In consideration of the very interesting but very complex topic from a clinical and outcome point of view, the European Society of Organ Transplantation (ESOT) has convened a working group to address the current state of downstaging, bridging and immunotherapy in liver transplantation for HCC [
25]. To date, due to insufficient evidence, no conclusive recommendation can be made about the use of immunotherapy before liver transplantation [
25]. Notably, further studies are needed to accurately analyze both safety and oncologic outcomes of that approach. Many open issues remain.
5. Future Directions
Several key areas require focused research to enhance the success of liver transplantation following immune checkpoint inhibitor therapy in advanced hepatocellular carcinoma patients. One critical avenue is the development of predictive biomarkers to better identify patients who will respond to ICIs and subsequently benefit from transplantation. Advances in immunogenetics and personalized medicine could enable tailored immunosuppressive regimens that minimize the risk of graft rejection while maintaining anti-tumor immunity. Integrating novel imaging techniques and liquid biopsy technologies could provide real-time monitoring of tumor dynamics and immune status, facilitating more precise decision-making. Additionally, exploring combination therapies that include ICIs, targeted therapies, and local treatments may offer synergistic benefits and improve survival rates and quality of life for patients with advanced HCC.
6. Conclusions
The integration of ICI in the treatment of advanced hepatocellular carcinoma offers significant opportunities and complex challenges. Although evidence indicates that liver transplantation after ICI therapy may be feasible and potentially beneficial, there are several aspects that need to be addressed for graft safety.
The effects of ICIs require a deeper understanding of how these drugs alter immune responses and affect transplantation.
This includes assessing the risk of rejection of the transplanted organ in the pre-transplant and developing strategies to mitigate these risks through standardised immunosuppressive protocols tailored to the patient.
The timing of liver transplantation after ICI treatment is a crucial issue for both reducing the risk of tumour progression and preventing graft rejection. The former problem could be mitigated by the application of other treatment strategies, the latter by a washout period determined by the half-life of the drug. In addition, the selection criteria for liver transplant candidates need to be refined to identify which are most likely to benefit from transplantation after ICI therapy. These include liver function, tumour characteristics, response to ICIs, and the presence of comorbidities. There is an urgent need for standardised guidelines and protocols, which can be produced from data derived from ongoing trials. The multidisciplinary team of oncologists, hepatologists, transplant surgeons and immunologists will be essential to develop these guidelines and to ensure their application in clinical practice.
In conclusion, the use of ICI therapy aimed at liver transplantation in patients with advanced HCC is promising, but still requires standardisation.
By addressing the immunological, temporal and clinical challenges associated with this conversion, we can improve preparedness to address this new frontier in the treatment of HCC and ultimately improve patient outcomes.
Author Contributions
Conceptualization, L.M. and G.S.; methodology, L.M. and G.S.; resources, L.M., G.S. and C.T; writing—original draft preparation, L.M.; writing—review and editing, G.S and S.G; supervision, S.G and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011 Mar-Apr;61(2):69-90. Epub 2011 Feb 4. Erratum in: CA Cancer J Clin. 2011 Mar-Apr;61(2):134. [CrossRef]
- Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016 Apr;150(4):835-53. Epub 2016 Jan 12. [CrossRef]
- Vitale A, Cabibbo G, Iavarone M, Viganò L, Pinato DJ, Ponziani FR, Lai Q, Casadei-Gardini A, Celsa C, Galati G, Gambato M, Crocetti L, Renzulli M, Giannini EG, Farinati F, Trevisani F, Cillo U; HCC Special Interest Group of the Italian Association for the Study of the Liver. Personalised management of patients with hepatocellular carcinoma: a multiparametric therapeutic hierarchy concept. Lancet Oncol. 2023 Jul;24(7):e312-e322. [CrossRef]
- Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017 Apr;14(4):203-217. Epub 2017 Jan 5. [CrossRef]
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996 Mar 14;334(11):693-9. [CrossRef]
- O’Rourke JM, Shetty S, Shah T, Perera MTPR. Liver transplantation for hepatocellular carcinoma: pushing the boundaries. Transl Gastroenterol Hepatol. 2019 Jan 2;4:1. eCollection 2019. No abstract available. [CrossRef]
- Sapisochin G, Goldaracena N, Laurence JM, Dib M, Barbas A, Ghanekar A, Cleary SP, Lilly L, Cattral MS, Marquez M, Selzner M, Renner E, Selzner N, McGilvray ID, Greig PD, Grant DR. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: A prospective validation study. Hepatology. 2016 Dec;64(6):2077-2088. Epub 2016 Jun 30. [CrossRef]
- Gorgen A, Rosales R, Sadler E, Beecroft R, Knox J, Dawson LA, Ghanekar A, Grant D, Greig PD, Sapisochin G. Patterns and Predictors of Mortality After Waitlist Dropout of Patients With Hepatocellular Carcinoma Awaiting Liver Transplantation. Transplantation. 2019 Oct;103(10):2136-2143. [CrossRef]
- Lingiah VA, Niazi M, Olivo R, Paterno F, Guarrera JV, Pyrsopoulos NT. Liver Transplantation Beyond Milan Criteria. J Clin Transl Hepatol. 2020 Mar 28;8(1):69-75. Epub 2020 Mar 30. [CrossRef]
- Hermann RE, Lonsdale D. Chemotherapy, radiotherapy, and hepatic lobectomy for hepatoblastoma in an infant: report of a survival. Surgery. 1970 Aug;68(2):383-8. PMID: 4317928.
- Finn RS, Qin S, Ikeda M, et al. Imbrave150: Updated Overall Survival (Os) Data from a Global, Randomized, Open-Label Phase Iii Study of Atezolizumab (Atezo) + Bevacizumab (Bev) Versus Sorafenib (Sor) in Patients (Pts) with Unresectable Hepatocellular Carcinoma (Hcc). Journal of Clinical Oncology. 2021;39(3_suppl):267-267.
- Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Van Dao T, De Toni EN, Rimassa L, Breder V, Vasilyev A, Heurgué A, Tam VC, Mody K, Thungappa SC, Ostapenko Y, Yau T, Azevedo S, Varela M, Cheng AL, Qin S, Galle PR, Ali S, Marcovitz M, Makowsky M, He P, Kurland JF, Negro A, Sangro B. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022 Aug;1(8):EVIDoa2100070. Epub 2022 Jun 6. [CrossRef]
- Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016 Feb;39(1):98-106. [CrossRef]
- Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018 Jun 10;36(17):1714-1768. Epub 2018 Feb 14. [CrossRef]
- Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, Lyon AR, Wick W, Kostine M, Peters S, Jordan K, Larkin J; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022 Dec;33(12):1217-1238. Epub 2022 Oct 18. [CrossRef]
- Liu JC, Yu HJ. A Review of the Pharmacokinetic Characteristics of Immune Checkpoint Inhibitors and Their Clinical Impact Factors. Pharmgenomics Pers Med. 2023 Jan 20;16:29-36. [CrossRef]
- Fisher J, Zeitouni N, Fan W, Samie FH. Immune checkpoint inhibitor therapy in solid organ transplant recipients: A patient-centered systematic review. J Am Acad Dermatol. 2020 Jun;82(6):1490-1500. [CrossRef]
- Shi XL, Mancham S, Hansen BE, de Knegt RJ, de Jonge J, van der Laan LJ, Rivadeneira F, Metselaar HJ, Kwekkeboom J. Counter-regulation of rejection activity against human liver grafts by donor PD-L1 and recipient PD-1 interaction. J Hepatol. 2016 Jun;64(6):1274-82. Epub 2016 Mar 3. [CrossRef]
- Wassmer CH, El Hajji S, Papazarkadas X, Compagnon P, Tabrizian P, Lacotte S, Toso C. Immunotherapy and Liver Transplantation: A Narrative Review of Basic and Clinical Data. Cancers (Basel). 2023 Sep 15;15(18):4574. [CrossRef]
- Tabrizian P, Florman SS, Schwartz ME. PD-1 inhibitor as bridge therapy to liver transplantation? Am J Transplant. 2021 May;21(5):1979-1980. Epub 2021 Jan 2. [CrossRef]
- Schwacha-Eipper B, Minciuna I, Banz V, Dufour JF. Immunotherapy as a Downstaging Therapy for Liver Transplantation. Hepatology. 2020 Oct;72(4):1488-1490. [CrossRef]
- Nordness MF, Hamel S, Godfrey CM, Shi C, Johnson DB, Goff LW, O’Dell H, Perri RE, Alexopoulos SP. Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for HCC: Are checkpoint inhibitors safe for the pretransplant patient? Am J Transplant. 2020 Mar;20(3):879-883. Epub 2019 Oct 28. [CrossRef]
- Dehghan Y, Schnickel GT, Hosseini M, Burgoyne AM, Ajmera VH, Morris GP, Mendler MH, Parekh JR, Abushamat F, Vodkin I, Kono Y. Rescue liver re-transplantation after graft loss due to severe rejection in the setting of pre-transplant nivolumab therapy. Clin J Gastroenterol. 2021 Dec;14(6):1718-1724. Epub 2021 Oct 13. [CrossRef]
- Aby ES, Lake JR. Immune Checkpoint Inhibitor Therapy Before Liver Transplantation-Case and Literature Review. Transplant Direct. 2022 Mar 10;8(4):e1304. [CrossRef]
- Sogbe M, López-Guerra D, Blanco-Fernández G, Sangro B, Narváez-Rodriguez I. Durvalumab as a Successful Downstaging Therapy for Liver Transplantation in Hepatocellular Carcinoma: The Importance of a Washout Period. Transplantation. 2021 Dec 1;105(12):e398-e400. [CrossRef]
- Kang E, Martinez M, Moisander-Joyce H, Saenger YM, Griesemer AD, Kato T, Yamashiro DJ, Remotti H, Gartrell RD. Stable liver graft post anti-PD1 therapy as a bridge to transplantation in an adolescent with hepatocellular carcinoma. Pediatr Transplant. 2022 May;26(3):e14209. Epub 2021 Dec 15. [CrossRef]
- Abdelrahim M, Esmail A, Umoru G, Westhart K, Abudayyeh A, Saharia A, Ghobrial RM. Immunotherapy as a Neoadjuvant Therapy for a Patient with Hepatocellular Carcinoma in the Pretransplant Setting: A Case Report. Curr Oncol. 2022 Jun 15;29(6):4267-4273. [CrossRef]
- Chouik Y, Erard D, Demian H, Schulz T, Mazard T, Hartig-Lavie K, Antonini T, Mabrut JY, Mohkam K, Rode A, Merle P. Case Report: Successful liver transplantation after achieving complete clinical remission of advanced HCC with Atezolizumab plus Bevacizumab combination therapy. Front Immunol. 2023 Jun 12;14:1205997. [CrossRef]
- Qiao ZY, Zhang ZJ, Lv ZC, Tong H, Xi ZF, Wu HX, Chen XS, Xia L, Feng H, Zhang JJ, Xia Q. Neoadjuvant Programmed Cell Death 1 (PD-1) Inhibitor Treatment in Patients With Hepatocellular Carcinoma Before Liver Transplant: A Cohort Study and Literature Review. Front Immunol. 2021 Jul 19;12:653437. [CrossRef]
- Tabrizian P, Florman SS, Schwartz ME. PD-1 inhibitor as bridge therapy to liver transplantation? Am J Transplant. 2021 May;21(5):1979-1980. Epub 2021 Jan 2. [CrossRef]
- Schnickel GT, Fabbri K, Hosseini M, Misel M, Berumen J, Parekh J, Mekeel K, Dehghan Y, Kono Y, Ajmera V. Liver transplantation for hepatocellular carcinoma following checkpoint inhibitor therapy with nivolumab. Am J Transplant. 2022 Jun;22(6):1699-1704. Epub 2022 Feb 8. [CrossRef]
- Dave S, Yang K, Schnickel GT, Kono Y, Delebecque F, Arellano D, Liu A, Zhang X, Tu XM, Ajmera V. The Impact of Treatment of Hepatocellular Carcinoma With Immune Checkpoint Inhibitors on Pre- and Post-liver Transplant Outcomes. Transplantation. 2022 Jun 1;106(6):e308-e309. Epub 2022 May 23. [CrossRef]
- Wang T, Chen Z, Liu Y, Jia Y, Ju W, Chen M, Zhao Q, Wang D, Guo Z, Tang Y, He X. Neoadjuvant programmed cell death 1 inhibitor before liver transplantation for HCC is not associated with increased graft loss. Liver Transpl. 2023 Jun 1;29(6):598-606. Epub 2023 Feb 8. [CrossRef]
- Chen Z, Hong X, Wang T, Guo Y, Huang C, Li M, He X, Ju W, Chen M. Prognosis after liver transplantation in patients treated with anti-PD-1 immunotherapy for advanced hepatocellular carcinoma: case series. Ann Palliat Med. 2021 Sep;10(9):9354-9361. [CrossRef]
- Ohm H, Khwaja R, Karachiwala H. Immunotherapy before liver transplant in unresectable hepatocellular carcinoma: a case report. J Gastrointest Oncol. 2023 Dec 31;14(6):2644-2649. Epub 2023 Dec 12. [CrossRef]
- Liu MC, Lizaola-Mayo B, Jayasekera CR, Mathur AK, Katariya N, Aqel B, Byrne TJ, Chascsa DMH. Downstaging Hepatocellular Carcinoma with Checkpoint Inhibitor Therapy Improves Access to Curative Liver Transplant. J Gastrointest Cancer. 2024 Mar 14. [CrossRef]
- Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, Colledan M, Salizzoni M, Romagnoli R, Antonelli B, Vivarelli M, Tisone G, Rossi M, Gruttadauria S, Di Sandro S, De Carlis R, Lucà MG, De Giorgio M, Mirabella S, Belli L, Fagiuoli S, Martini S, Iavarone M, Svegliati Baroni G, Angelico M, Ginanni Corradini S, Volpes R, Mariani L, Regalia E, Flores M, Droz Dit Busset M, Sposito C. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020 Jul;21(7):947-956. Erratum in: Lancet Oncol. 2020 Aug;21(8):e373. [CrossRef]
- Claasen MPAW, Sneiders D, Rakké YS, Adam R, Bhoori S, Cillo U, Fondevila C, Reig M, Sapisochin G, Tabrizian P, Toso C. European Society of Organ Transplantation (ESOT) Consensus Report on Downstaging, Bridging and Immunotherapy in Liver Transplantation for Hepatocellular Carcinoma. Transpl Int. 2023 Sep 14;36:11648. [CrossRef]
- Chen GH, Wang GB, Huang F, Qin R, Yu XJ, Wu RL, Hou LJ, Ye ZH, Zhang XH, Zhao HC. Pretransplant use of toripalimab for hepatocellular carcinoma resulting in fatal acute hepatic necrosis in the immediate postoperative period. Transpl Immunol. 2021 Jun;66:101386. Epub 2021 Mar 18. [CrossRef]
- Lizaola-Mayo BC, Mathur AK, Borad MJ, Jadlowiec CC, Lam-Himlin DM, Corey RL, Iqbal S, Okubo K, Byrne TJ, Moss AA, Aqel BA, Chascsa DM. Immunotherapy as a Downstaging Tool for Liver Transplantation in Hepatocellular Carcinoma. Am J Gastroenterol. 2021 Dec 1;116(12):2478-2480. [CrossRef]
Table 1.
Case reports published in the literature.
Table 1.
Case reports published in the literature.
Table 2.
Case series published in the literature.
Table 2.
Case series published in the literature.
Table 3.
Current and ongoing studies using ICIs before liver transplantation.
Table 3.
Current and ongoing studies using ICIs before liver transplantation.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).