Introduction
Ineffective medication is one of the most important healthcare problems. Many patients with complex diseases do not respond to treatment, or experience serious side-effects. This problem causes enormous suffering and costs for health care, drug development, and production loss. An important reason for ineffective medication is the daunting complexity of diseases. Multi-omics analyses down to the single cell level show that each disease can involve altered interactions among thousands of genes across billions of cells in multiple organs [
1].
Most diseases, including inflammatory, cardiovascular, malignant, and metabolic, can evolve for many years, or even decades, before symptoms manifest themselves and a diagnosis is given. Ineffective treatment increases the risk of comorbidities, and, thereby, a vicious circle of increasing treatment inefficiency ensues.
Moreover, disease progression can differ between different patients with the same diagnosis or within a given patient at different time points. Indeed, health and disease can be seen as variable entities on continuous scales. Such variations depend on genetic or environmental factors, such as pollution, lifestyle, and inequitable health care. The 2030 agenda for sustainable development identified effective and equitable health as priorities. To address this priority adequately, would require identification of factors that predispose to, or protect against, a complex disease in the life of a given patient.
Digital twins (DTs) can contribute to these goals. The DT concept is derived from engineering with the aims of modeling computationally and developing complex systems more effectively and inexpensively in silico than in real life.
Early examples of DTs have already been tested in the clinic, such as the artificial lung and the artificial pancreas [
2,
3,
4]. Ideally, analyses and computational treatment of DTs will radically improve health care by paving the way for predictive, preventive, and personalized treatments [
5].
The medical potential of DTs has been recognized by scientific organizations in the US, Europe, and Asia, and led to international collaborative efforts to implement this computational strategy in health care and clinical trials [
2,
6,
7,
8,
9,
10,
11,
12,
13].
However, clinical implementation of DTs involves multiple challenges, including: 1) dynamic characterization of health and disease-associated molecular changes on population-, organome-, cellulome- and genome-wide scales, as well as environmental factors; 2) computational methods that integrate and organize all changes into DT; 3) prioritization of mechanisms, from which 4) diagnostic biomarkers, and preventive measures or therapeutic targets can be inferred; 5) solutions to connect 1-4 so that DTs can learn from each other; 6) user-friendly interfaces adapted to individuals and care givers; 7) solutions to disseminate DTs on a global scale for equitable and effective health; and 8) solutions to address social, psychological, organizational, ethical, regulatory, and financial challenges and opportunities. As recently highlighted by manifestos about DTs from the European Commission and US National Academy of Sciences, Engineering and Medicine there is a lack of concrete clinical implementations that address these challenges [
12,
13].
Here, we will discuss these challenges and potential solutions and give concrete examples of such solutions.
1. Dynamic and Multi-Scale Characterization of Health and Risk Factors
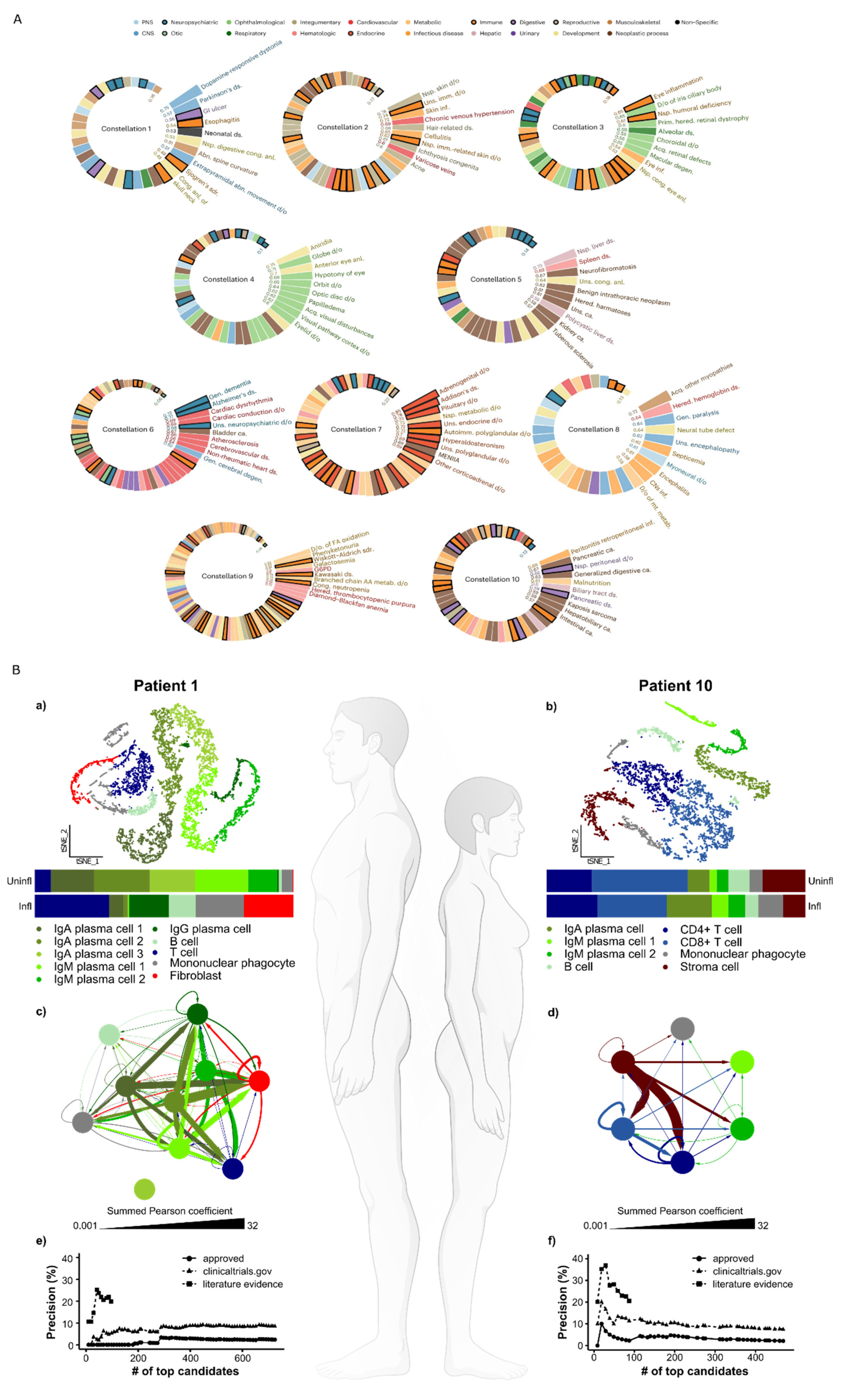
Predictive, preventive, and personalized medicine will require analyses of potential disease causes on multiple scales ranging from populations to individuals, to their tissues, cells, and molecular species. Since multimorbidity is common, population-wide analyses are important to characterize disease constellations. This goal is increasingly feasible because of the availability of longitudinal electronic medical records of populations and large biobanks. As an example, see our analyses of a temporal disease trajectories of over 200 million Americans revealing ten constellations of comorbid diseases (
Figure 1A).
Such analyses can be correlated with genetic variants and environmental factors so that mechanisms for health and diseases can be inferred and ideally used for predictive and preventive medicine on population-wide scales [
14].
On the scale of individuals, detailed characterization of health and disease mechanisms can be achieved using different types of genome-wide analyses (“multi-omics”) down to the level of single cells. The latter is important because analyses of the transcriptomes of thousands of cells give sufficient statistical power to characterize disease-associated changes in an individual patient by comparing sick and healthy tissues. Such changes can vary greatly between two patients with the same diagnosis who require completely different treatments (
Figure 1B) [
15]. Treatment of disease-associated changes is further complicated by involvement of multiple organs with variable mechanisms in the same patients [
1,
16]. A recent scRNA-seq study of a mouse model of arthritis showed involvement of multiple interconnected organs, although only joints showed signs of disease (
Figure 2).
2. Systems-Level Principles That Organize Health and Disease Mechanisms into an Overarching DT Structure for Populations and Individuals
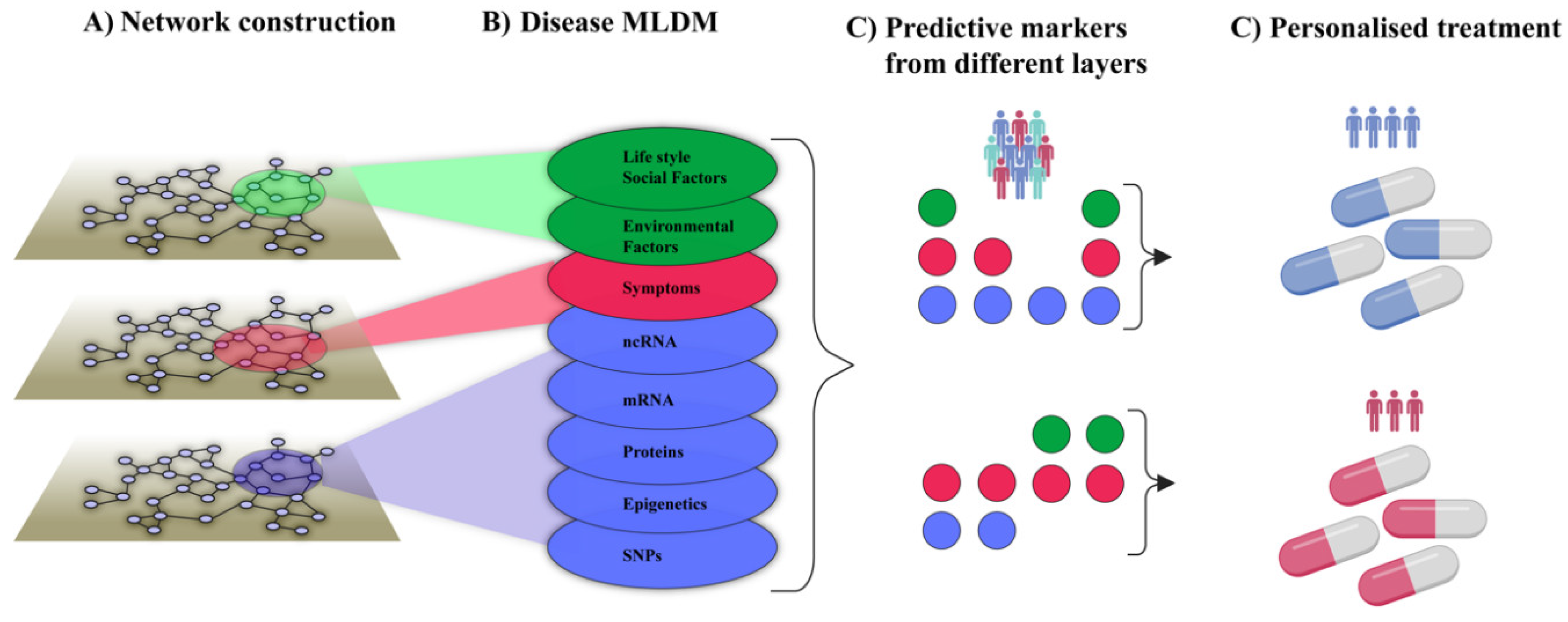
The complexity and heterogeneity of diseases calls for systems levels to organize disease-associated changes into DTs on scales ranging from populations to individuals (henceforth referred to as pop-DTs and indi-DTs, respectively). Pop-DTs should ideally describe combinations of environmental and genetic causes of health or disease. The underlying data are increasingly available in longitudinal electronic medical records, quality registries, and genome-wide databases. Pop-DTs should be continuously updated based on information from the literature and the evolution of different databases. Indi-DTs translate the same principles to individual patients, but at a greater resolution. For example, as shown in
Figure 1B and
Figure 2, disease-associated changes can be described on multi-organ, -cellulome, and genome-wide scales in individual patients. The figures also illustrate how different types of variables can be organized into networks on different scales. For example, in
Figure 1B disease-associated cell types from individual patients were connected into networks using predicted molecular interactions between those cell types. Importantly, networks may provide a systems-level solution that organize multiple types of variables in a complex system, and show how they interact within that system as well as with variables in other complex systems. For example, symptoms and signs of human diseases can be connected into a network. In such a network symptoms and signs of the same disease are interconnected into modules (like pain in the chest and left arm in myocardial infarction). Such modules can, in turn, be connected to underlying cellular and molecular networks. Similarly, networks of environmental factors can be constructed and connected into multi-layer networks that describe diseases in scales ranging from populations to individuals, as well as how they change over time (
Figure 3A and B).
Such multi-layer networks may be used to analyze the multiple relationships each node within the network has to every other node. For example, environmental effects can be depicted by recognizing the post-translational modifications of proteins in the protein-protein interaction network and their functional consequences. Ideally, tracing such relationships could lead to identification of subnetworks or modules in which the major determinants of every specific disease exist. If so, this could lead to the identification of potential drug targets that can be used to guide therapeutic strategies and drug development, including drug repurposing [
17,
18]. Moreover, multi-layer networks can provide a framework from which highly predictive combinations of variables for different purposes, like personalized treatment, can be inferred with deep learning/Artificial Intelligence (AI) techniques (
Figure 3C and D). These principles will be applied in a recent initiative, The Virtual Child, which aims to construct computational models of individual children’s cancer development to predict, prevent or treat such developments, based on multi-layer networks [
19]. This initiative is based on multidisciplinary team consisting of patient advocates, industry partners, basic and clinical researchers from three continents. Thus, application of network tools to construct multi-layer networks may provide a solution to the challenge of constructing and analyzing pop- and indi-DTs. In the next section, we will discuss how networks can be systematically analyzed to prioritize mechanisms for predictive, preventive, and personalized medicine.
3,4. Prioritization of Mechanisms, from Which Diagnostic Biomarkers, Preventive Measures, or Therapeutic Targets can Be Inferred
Prioritization of disease-relevant environmental, phenotypic, and molecular changes on dynamic population-, organome-, cellulome-, and genome-wide scales are unresolved challenges.
However, recent studies point to potential solutions:
- 1)
On the scale of pop-DTs, analyses of longitudinal data from electronic medical records or biobanks can identify evolution of disease constellations such that the initiating mechanisms of (preclinical) diseases can be identified (
Figure 1A). Combined analyses of molecular data can be used to infer early mechanisms, as well as biomarkers and drug targets for prediction and prevention.
- 2)
On the scale of indi-DTs‚ single-cell-based dynamic multicellular disease models (MCDMs) can be analyzed to find early upstream regulators (URs), which may be both diagnostic and therapeutic targets that predict and prevent disease .
- 3)
Network analyses, such as centrality measures, can be used to prioritize the most central cell types in MCDMs and their modules. Those modules may be computationally matched with thousands of drugs to find the optimal ones for individual patients (
Figure 1B e-f). This approach has been validated by extensive in vitro and in vivo studies [
15], and is ready for clinical trials.
- 4)
Machine and transfer learning can be used to project data about genome-wide drug responses from public databases to individual patients [
20,
21].
5. Solutions to Connect 1-4 so That Medical DTs Can Learn from each Other and Emerging DTs from Other Fields over Time
Pop- and indi-DTs are envisioned to learn and adapt continuously, providing predictive, preventive, and personalized treatment based on diverse data, as described above. The potential of linking medical DTs to emerging DTs in related fields, such as climatology, environmental pollution, and socioeconomics, was recently discussed at a series of seminars organized by the US National Academies of Sciences, Engineering, and Medicine [
13,
22]. Key algorithmic innovations in artificial intelligence and machine learning that may contribute to improving and integrating DTs include self-supervised learning, geometric deep learning, and generative pre-training, followed by fine-tuning [
23].
Self-supervised learning is a form of machine learning in which the system learns to predict part of its input from other parts of its input using a large amount of unlabeled data. In the context of healthcare DTs, the model can learn from vast amounts of medical data without the need for extensive manual labeling. For example, a DT could learn patterns from medical images, electronic health records, or genetic data, identifying relevant features without explicit human annotation. This approach is particularly beneficial in healthcare, where acquiring labeled data can be costly and time-consuming. Geometric deep learning is a recent paradigm in machine learning that generalizes deep neural network models to non-Euclidean domains such as graphs and manifolds [
24]. Since much of the scientific data, especially in biology and healthcare, naturally resides on graph-structured data (like molecular structures, protein-protein interaction networks, or patient similarity networks), geometric deep learning can significantly enhance model accuracy and efficiency. This approach allows the DTs to understand and process the complex relationships and interdependencies in multimodal data more effectively than traditional machine learning models.
Finally, foundation models [
25,
26], especially large language models, such as GPT-4, are profoundly transforming the paradigm of deep learning. Instead of training many task-specific models, we can now adapt a single, generative, pretrained model to many tasks via few-shot prompting or fine-tuning. Generative pre-training involves training a model on a large dataset to learn a general representation of the data, which can then be fine-tuned for specific tasks. In the realm of virtual cell simulators, this approach can be transformative. This methodology is particularly powerful for hypothesis testing in virtual environments, enabling scientists to explore scenarios and conditions that are difficult to replicate in a physical laboratory.
6. Solutions to Make DTs Explainable to Individuals and Care Givers
The integration of DTs and AI models into clinical settings presents an important challenge in ensuring these technologies are interpretable and transparent to both individuals and caregivers. This is essential for participatory medicine, where joint decision-making between patients and health professionals is based on clear and informed understanding of health and disease management [
6]
. Machine learning models can often seem like black boxes to end-users. Making the models more explainable without compromising the accuracy of information is key. Advances in machine learning research, including model auditing and explainability, produce techniques for making machine learning-based DT models trained on networked datasets more explainable. These techniques include visualization tools that map out how data points are connected and influence each other within the model or algorithms that can break down complex networked predictions into simpler, more understandable components. Attribution maps are one of the techniques used to explain predictions made by machine learning models in DTs. These maps visualize which parts of the graph (which nodes or edges) are most influential in the model’s decision-making process. In a medical DT, this could mean identifying which symptoms, genetic factors, or other clinical parameters are most significant in diagnosing a disease or predicting a treatment outcome. Attribution maps provide a visual representation, making it easier for non-experts to understand the model’s reasoning.
Another important class of explainability techniques is local explainers, which are tools that focus on explaining individual predictions of a model as opposed to providing a global explanation of the model’s overall behavior. This strategy is particularly useful in healthcare, where understanding the specific reasoning behind a diagnosis or treatment recommendation for a particular patient is often more relevant than understanding the model’s general behavior. Another important aspect is to design interfaces of DT that are tailored to patients’ individual preferences, emotions and educational levels. This will likely require interdisciplinary efforts in which patients, clinicians and researchers may need to form new collaborations with for example psychologists, computer game designers and artists.
7. Solutions to Disseminate DTs on a Global Scale for Equitable and Effective Health in Accordance with the 2030 Agenda for Sustainable Development
As evidenced by the Virtual Child Project, which spans three continents, many of the computational solutions underlying DTs are independent of geographical location. This supports that DTs may contribute to improved and equitable health on a global scale based on collaborative efforts between developing and developed countries. There are several successful examples of such collaborations, aiming at global health digitalization, including concrete examples such as an automated pipeline for virtual drug discovery and clinical applications such as digital or AI-supported diagnostic protocols in low-resource settings [
27,
28,
29].
8. Solutions to Address Social, Psychological, Organizational, Ethical, Regulatory and Financial Challenges and Opportunities
Clinical implementation of DTs will involve a wide range of challenges. As recently discussed, many of these challenges are generic for implementation of computational science in different fields [
13]. One important example is gender differences in how digital technologies and health care are perceived, used, and led in different countries [
30,
31]. Such differences can be disadvantageous for women - especially women of racial or ethnic minority backgrounds. Another question can be data ownership: can a patient be asked to donate increasingly detailed information from her DT as a resource for clinicians treating patients with similar characteristics, or for clinical or industrial research, such as drug discovery? Answers to this question will involve intertwined solutions for challenges in ethics, data security, and regulatory issues [
32]. However, despite national differences in evaluation and approval processes, there are already products in the market that use computational modelling for clinical purposes. The FDA has introduced pre-qualifying tools to speed up the regulatory processes of digital tools. Another important need is to protect the privacy and rights of an individual’s DT, especially if it integrates sensitive, multiscale data with evolving computational approaches that protect privacy even in population-based studies (federated data analysis).
Acknowledgements
This paper is supported by US NIH grants R01 HL1551107, R01 HL166137, U01 HG007691, American Heart Association grants AHA957729, AHAMERIT1185447, and EU Horizon Health 2021 grant 101057619 to JL.
Conflicts of Interest
MB is co-founder of Mavatar Inc, DMA, FM and AR have no financial interests to report. JL is scientific co-founder of Scipher Medicine, Inc.
References
- 1. Lilja S, Li X, Smelik M, Lee EJ, Loscalzo J, Marthanda PB, Hu L, Magnusson M, Sysoev O, Zhang H, et al.: Multi-organ single-cell analysis reveals an on/off switch system with potential for personalized treatment of immunological diseases. Cell Rep Med 2023, 4:100956.
- Zhou C, Chase JG, Knopp J, Sun Q, Tawhai M, Moller K, Heines SJ, Bergmans DC, Shaw GM, Desaive T: Virtual patients for mechanical ventilation in the intensive care unit. Comput Methods Programs Biomed 2021, 199:105912. [CrossRef]
- Iacobucci G: NHS to trial “artificial pancreas” for patients with type 1 diabetes. BMJ 2021, 373:n1538.
- Nagaraj D, Khandelwal P, Steyaert S, Gevaert O: Augmenting digital twins with federated learning in medicine. Lancet Digit Health 2023, 5:e251-e253. [CrossRef]
- Hernandez-Boussard T, Macklin P, Greenspan EJ, Gryshuk AL, Stahlberg E, Syeda-Mahmood T, Shmulevich I: Digital twins for predictive oncology will be a paradigm shift for precision cancer care. Nat Med 2021, 27:2065-2066.
- Bjornsson B, Borrebaeck C, Elander N, Gasslander T, Gawel DR, Gustafsson M, Jornsten R, Lee EJ, Li X, Lilja S, et al.: Digital twins to personalize medicine. Genome Med 2019, 12:4.
- Coorey G, Figtree GA, Fletcher DF, Snelson VJ, Vernon ST, Winlaw D, Grieve SM, McEwan A, Yang JYH, Qian P, et al.: The health digital twin to tackle cardiovascular disease-a review of an emerging interdisciplinary field. NPJ Digit Med 2022, 5:126. [CrossRef]
- Venkatesh KP, Brito G, Kamel Boulos MN: Health Digital Twins in Life Science and Health Care Innovation. Annu Rev Pharmacol Toxicol 2024, 64:159-170.
- Baldwin M, Buckley CD, Guilak F, Hulley P, Cribbs AP, Snelling S: A roadmap for delivering a human musculoskeletal cell atlas. Nat Rev Rheumatol 2023, 19:738-752. [CrossRef]
- Subbiah V: The next generation of evidence-based medicine. Nat Med 2023, 29:49-58.
- Laubenbacher R, Adler F, An G, Castiglione F, Eubank S, Fonseca LL, Glazier J, Helikar T, Jett-Tilton M, Kirschner D, et al.: Forum on immune digital twins: a meeting report. NPJ Syst Biol Appl 2024, 10:19.
-
A Statement of Intent on Development, Evidence, and Adoption in Healthcare Systems. In https://wwwvirtualhumantwinseu/manifesto. https://www.virtualhumantwins.eu/manifesto: European Virtual Human Twins.
-
Opportunities and Challenges for Digital Twins in Biomedical Sciences - A Workshop [https://www.nationalacademies.org/event/01-30-2023/opportunities-and-challenges-for-digital-twins-in-biomedical-sciences-a-workshop].
- Jia G, Li Y, Zhong X, Wang K, Pividori M, Alomairy R, Esposito A, Ltaief H, Terao C, Akiyama M, et al.: The high-dimensional space of human diseases built from diagnosis records and mapped to genetic loci. Nature Computational Science 2023, 3:403-417.
- Schafer S, Smelik M, Sysoev O, Zhao Y, Eklund D, Lilja S, Gustafsson M, Heyn H, Julia A, Kovacs IA, et al.: scDrugPrio: a framework for the analysis of single-cell transcriptomics to address multiple problems in precision medicine in immune-mediated inflammatory diseases. Genome Med 2024, 16:42.
- Vicari M, Mirzazadeh R, Nilsson A, Shariatgorji R, Bjarterot P, Larsson L, Lee H, Nilsson M, Foyer J, Ekvall M, et al.: Spatial multimodal analysis of transcriptomes and metabolomes in tissues. Nat Biotechnol 2023.
- Cheng F, Desai RJ, Handy DE, Wang R, Schneeweiss S, Barabasi AL, Loscalzo J: Network-based approach to prediction and population-based validation of in silico drug repurposing. Nat Commun 2018, 9:2691.
- Maron BA, Wang RS, Shevtsov S, Drakos SG, Arons E, Wever-Pinzon O, Huggins GS, Samokhin AO, Oldham WM, Aguib Y, et al.: Individualized interactomes for network-based precision medicine in hypertrophic cardiomyopathy with implications for other clinical pathophenotypes. Nat Commun 2021, 12:873.
- Gilbertson RJ, Behjati S, Bottcher AL, Bronner ME, Burridge M, Clausing H, Clifford H, Danaher T, Donovan LK, Drost J, et al.: The Virtual Child. Cancer Discov 2024, 14:663-668.
- Lotfollahi M, Klimovskaia Susmelj A, De Donno C, Hetzel L, Ji Y, Ibarra IL, Srivatsan SR, Naghipourfar M, Daza RM, Martin B, et al.: Predicting cellular responses to complex perturbations in high-throughput screens. Mol Syst Biol 2023, 19:e11517.
- Tang C, Fu S, Jin X, Li W, Xing F, Duan B, Cheng X, Chen X, Wang S, Zhu C, et al.: Personalized tumor combination therapy optimization using the single-cell transcriptome. Genome Med 2023, 15:105.
- Ektefaie Y, Dasoulas G, Noori A, Farhat M, Zitnik M: Multimodal learning with graphs. Nature Machine Intelligence 2023, 5:340-350.
- Wang H, Fu T, Du Y, Gao W, Huang K, Liu Z, Chandak P, Liu S, Van Katwyk P, Deac A, et al.: Scientific discovery in the age of artificial intelligence. Nature 2023, 620:47-60.
- Li MM, Huang K, Zitnik M: Graph representation learning in biomedicine and healthcare. Nature Biomedical Engineering 2022, 6:1353-1369.
- Singhal K, Azizi S, Tu T, Mahdavi SS, Wei J, Chung HW, Scales N, Tanwani A, Cole-Lewis H, Pfohl S, et al.: Large language models encode clinical knowledge. Nature 2023, 620:172-180.
- Jiang LY, Liu XC, Nejatian NP, Nasir-Moin M, Wang D, Abidin A, Eaton K, Riina HA, Laufer I, Punjabi P, et al.: Health system-scale language models are all-purpose prediction engines. Nature 2023, 619:357-362.
- Naher AF, Vorisek CN, Klopfenstein SAI, Lehne M, Thun S, Alsalamah S, Pujari S, Heider D, Ahrens W, Pigeot I, et al.: Secondary data for global health digitalisation. Lancet Digit Health 2023, 5:e93-e101.
- Borges do Nascimento IJ, Abdulazeem HM, Vasanthan LT, Martinez EZ, Zucoloto ML, Ostengaard L, Azzopardi-Muscat N, Zapata T, Novillo-Ortiz D: The global effect of digital health technologies on health workers’ competencies and health workplace: an umbrella review of systematic reviews and lexical-based and sentence-based meta-analysis. Lancet Digit Health 2023, 5:e534-e544.
- Holst C, Sukums F, Radovanovic D, Ngowi B, Noll J, Winkler AS: Sub-Saharan Africa-the new breeding ground for global digital health. Lancet Digit Health 2020, 2:e160-e162.
- Jack BW, Bickmore T, Yinusa-Nyahkoon L, Reichert M, Julce C, Sidduri N, Martin-Howard J, Zhang Z, Woodhams E, Fernandez J, et al.: Improving the health of young African American women in the preconception period using health information technology: a randomised controlled trial. Lancet Digit Health 2020, 2:e475-e485.
- The Lancet Digital H: Empowering women in health technology. Lancet Digit Health 2022, 4:e149.
- Reddy S, Allan S, Coghlan S, Cooper P: A governance model for the application of AI in health care. J Am Med Inform Assoc 2020, 27:491-497.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).