1. Introduction
Cardiovascular diseases represent the world’s leading cause of death[
1]. Older age is a significant risk factor for cardiovascular disease, which prolongs exposure to hypertension, diabetes, hypercholesterolemia and smoking [
2]. In addition, intrinsic ageing of the heart makes it more prone to stress, with an increase in cardiovascular mortality and morbidity in older adults. Intrinsic cardiac ageing is defined as the slowly progressive structural changes and functional declines with age, without significant cardiovascular risks [
3]. At the same time, the progressive deterioration of immune functions with age (immunosenescence) increases older adults’ susceptibility to infection and their risk of severe outcomes in case of disease. Moreover, ageing may be the cause of infection, but infection can also be the cause of ageing [
4]. Mechanisms may include enhanced inflammation, pathogen-dependent tissue destruction or accelerated cellular ageing through increased turnover. However, the connection between cardiovascular ageing and infections and how the two phenomena can influence each other is still unknown. Our attention was focused on the possible interactions between HIV and the development of cardiovascular diseases with advancing age, considering the increase in life expectancy of these patients thanks to modern therapies.
The human immunodeficiency virus (HIV) is an enveloped retrovirus that contains two copies of a single-stranded RNA genome[
5]. HIV attaches to the CD4 molecule and CCR5 (a chemokine co-receptor), gaining the T-helper lymphocyte. After integration in the host genome, the HIV provirus forms and follows transcription and viral mRNA production, assembling structural proteins in the host cell. Viral budding from host cells can release millions of HIV particles that can infect other cells [
6].
From two to four weeks, HIV enters the body, and the patient may develop symptoms of primary infection. Following this period, a prolonged chronic HIV infection occurs, which can last for decades. The last stage of HIV disease is acquired immunodeficiency syndrome (AIDS), characterised by opportunistic infections and tumours, which are usually fatal without treatment [
7]. Prolonged life expectancy heralded a higher prevalence of diseases of ageing, including CVD-associated morbidity and mortality [
8].
The risk of CVD death is significantly higher among patients with HIV in respect of the general population for every 10-year age group from 25 years to 64 years. In contrast, no significant difference was observed in the 65 to 74-year age group [
9]. It was also found that the risk of CVD death was significantly lower among PWH who had viral suppression than among those without full suppression. Different pathophysiological mechanisms correlate the development of cardiovascular diseases to a gene dysregulation exacerbated by the infection and by the inflammatory substrate connected to it [
10]. Rho-associated kinases ROCK1 and ROCK2, IL-6, MMP9, CCL23, NCF4, and some T-cell components (TLR4, CXCR4) regulate pathological remodelling processes that involve inflammation and fibrosis in cardiovascular disease. Their up/down deregulation is frequent in people living with HIV (PLWH). Their expression varies according to the type of tissue considered and is strongly influenced by sex, race, and age. In this context, we aim to provide a comprehensive quantitative synthesis of genetic pathways involved in HIV and their correlation with CVD, considering age and sex-related differences too [
11].
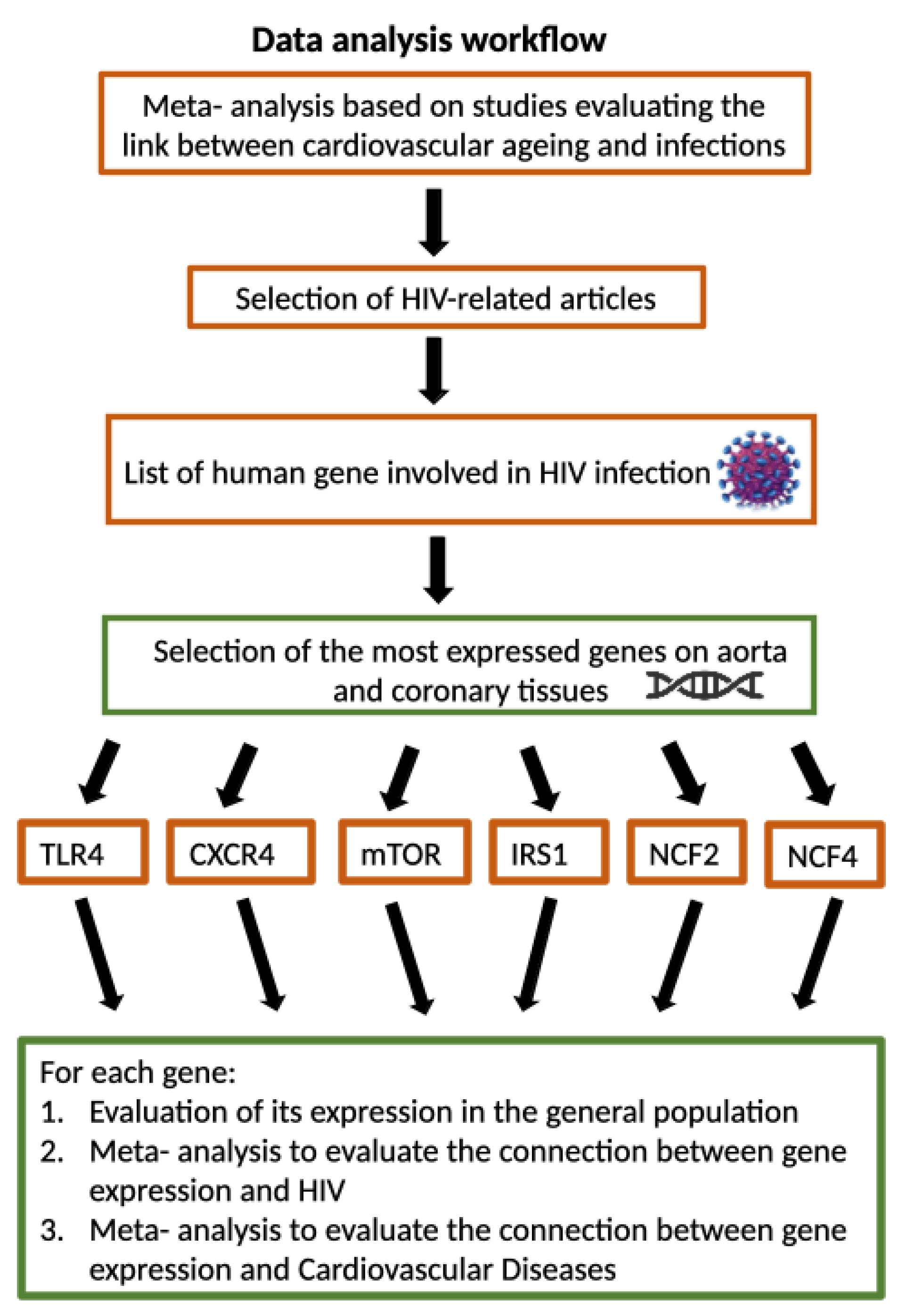
Figure 1 depicts the workflow of analysis.
2. Related Work
The introduction of Highly Active Antiretroviral Therapy (HAART) has reduced AIDS-related mortality, as demonstrated by Hammer et al. [
12]. However, non-HIV-related mortality, such as that attributable to CVD, has become increasingly crucial for the estimated 33.3 million people living with HIV (PLHIV) [
13]. Data from the New York City HIV Surveillance Registry for 2001 to 2012 showed that the proportion of CVD deaths among all deaths increased in the HIV population from 6 per cent to 15 per cent and decreased in the general population [
14].
Despite evidence of the earlier onset of CVD in the PWH population, it is not well known how HIV infection increases the risk of CVD. Smoking remains one of the most significant contributors to the development of CVD among PLWH [
15]. At the same time, HIV infection has been recognized as a prothrombotic condition in which a hypercoagulable state places patients at increased risk for deep vein thrombosis and other ischemic CVD events. Activated platelets favour proinflammatory and thrombogenic effects. However, no genes have been identified as the cause of the increased risk of developing CVD in PLWH.
3. Methods
3.1. Study Selection
We searched Scopus electronic databases using the following keywords and corresponding MeSH (Medical Subject Headings) terms: ( cardiovascular AND ageing, AND infection ) AND ( omics OR sequencing OR genomics OR proteomics OR metabolomics ). We also checked the reference lists of eligible studies and screened scientific abstracts and relevant Web sites. Two investigators (S.D.R., F.B.) independently screened search records to identify eligible trials. No disagreements occurred. Inclusion criteria were randomised, and observational controlled trials were conducted based on the connection between cardiovascular ageing and infections. Exclusion criteria were studies unrelated to the research question, with no control arm; clinical outcome not reported; absence of original article and editorial comment.
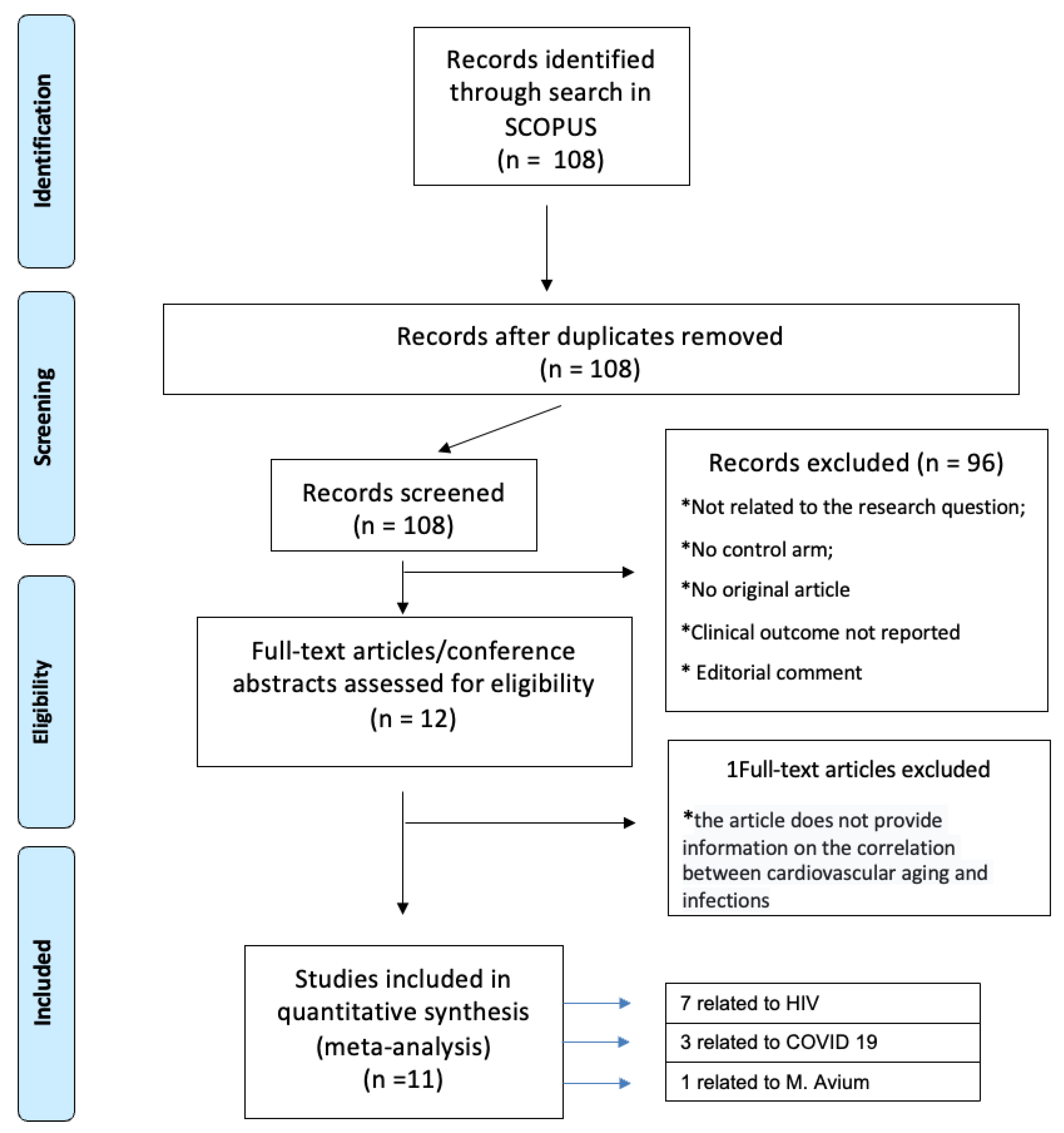
Figure 2 depicts the PRISMA workflow for meta-analysis.
3.2. Data Synthesis and Analysis
We decided to focus our primary data summary on studies evaluating only the link between cardiovascular ageing and HIV using data extracted from the original primary publications. We based our analyses on the genes dis-regulated pathways in patients with HIV, drown up a list of all the genes involved and selected only the genes most expressed in aortic and coronary tissues: TLR4, CXCR4, mTOR, NCF2, NCF4 and IRS1. We used VoyAGEr application [
16] to search expression gene levels in the general population of TLR4, CXCR4, mTOR, NCF2, NCF4 and IRS1, in particular on the aorta and coronary tissues, with related age and sex differences as depicted in
Figure 3.
At the same time, we used these genes for a new integrated Scopus and Pubmed meta-analysis to identify their role in HIV and CVD using the following keywords and corresponding MeSH (Medical Subject Headings) terms: ((TLR4) AND (HIV);((CXCR4) AND (HIV);((mTOR) AND (HIV);((NCF4) AND (HIV);((NCF2) AND (HIV);((IRS1) AND (HIV) for the first meta-analysis and ((TLR4) AND (cardiovascular)) AND (disease);((CXCR4) AND (cardiovascular)) AND (disease);((mTOR) AND (cardiovascular)) AND (disease);((NCF4) AND (cardiovascular)) AND (disease);((NCF2) AND (cardiovascular)) AND (disease);((IRS1) AND (cardiovascular)) AND (disease) for the second meta-analysis. Finally, we compared the variability of these genes in the general population, in patients with HIV, and in patients with cardiovascular diseases to understand how the development of cardiac pathologies in PLWH correlates with the expression of these genes.
3.3. Bioinformatic Data Analysis
The GTEx data portal [
17] is widely recognized as a crucial resource for accessing whole-genome sequencing and RNA-seq data from various individuals. For each specimen, GTEx includes pertinent details about the patient, including the origin tissue, sex, and age, which are categorized into six distinct groups.
As of September 25th, the latest iteration of the GTEx database (version 8) comprises 17,382 samples from 54 different tissues across 948 donors (accessible at
https://gtexportal.org/home/tissueSummaryPage). This version is hosted online and features a user-friendly query interface and data visualization tools. These resources are extensively utilized in numerous studies related to ageing, as referenced in several publications [
18].
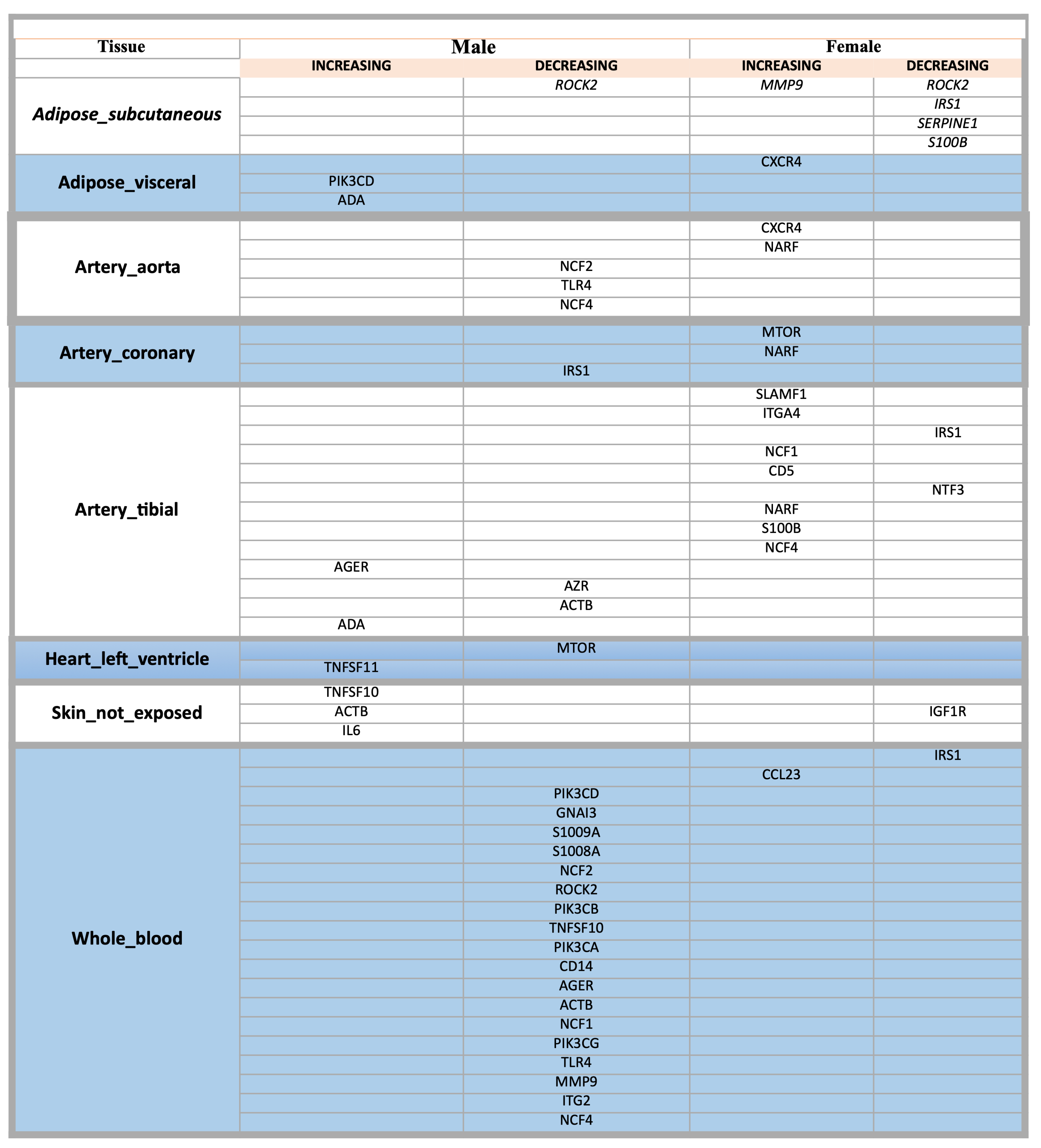
GTEx provides information about the age and sex of the patient, categorized into six different classes: 20-29, 30-39, 40-49, 50-59, 60-69, 70-79. The data also includes details about the tissue of the sample. Samples were grouped by tissue and sex, and genes were identified based on their average expression levels increasing or decreasing with age for each sex. The average expression level for each age group was calculated, and genes showing increased or decreased levels with age were selected. The statistical significance of the increase was tested using an ANOVA test with a corrected p-value of less than 0.05.
4. Results
One hundred eight articles were indentified through search in SCOPUS. Using a PRISMA-based screening strategy depicted in
Figure 2, we identified 11 studies, seven related to HIV [
19,
20,
21,
22,
23,
24], 3 related to COVID-19 [
25,
26,
27] and one related to Micobactherium Avium [
28]. We focused our analisys only on HIV related studies, drown up a list of all the genes involved and selecting only the genes most expressed in aortic and coronary tissues: TLR4, CXCR4, mTOR, NCF2, NCF4 and IRS1. Our aim was to clarify the variability of these genes in general population, in PLWH and in people with cardiovascular diseases in order to provide a comprehensive quantitative synthesis of genetic pathways involved in HIV and their correlation with CVD, considering age related differences too as depicted in
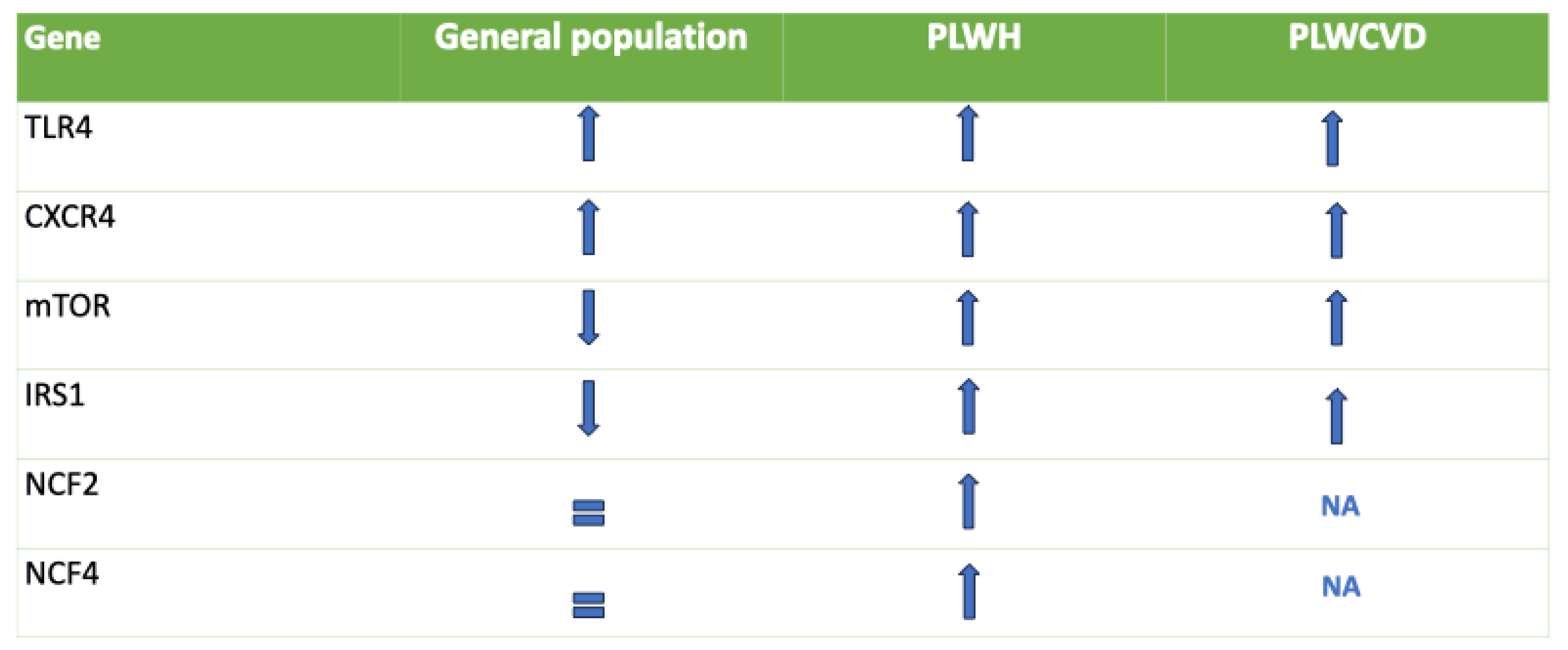
Figure 4.
4.1. Increasing Decreasing Genes in General Population
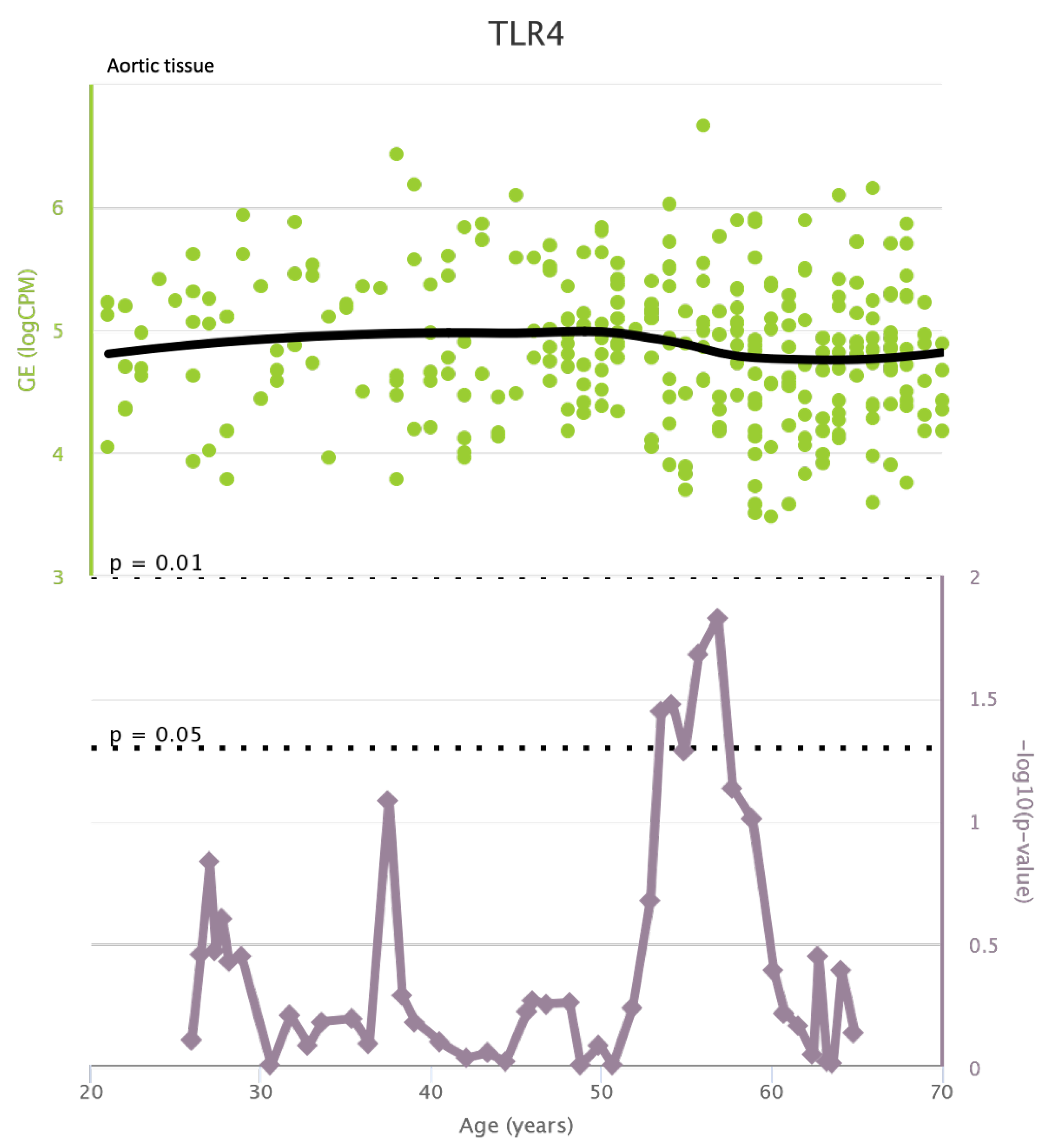
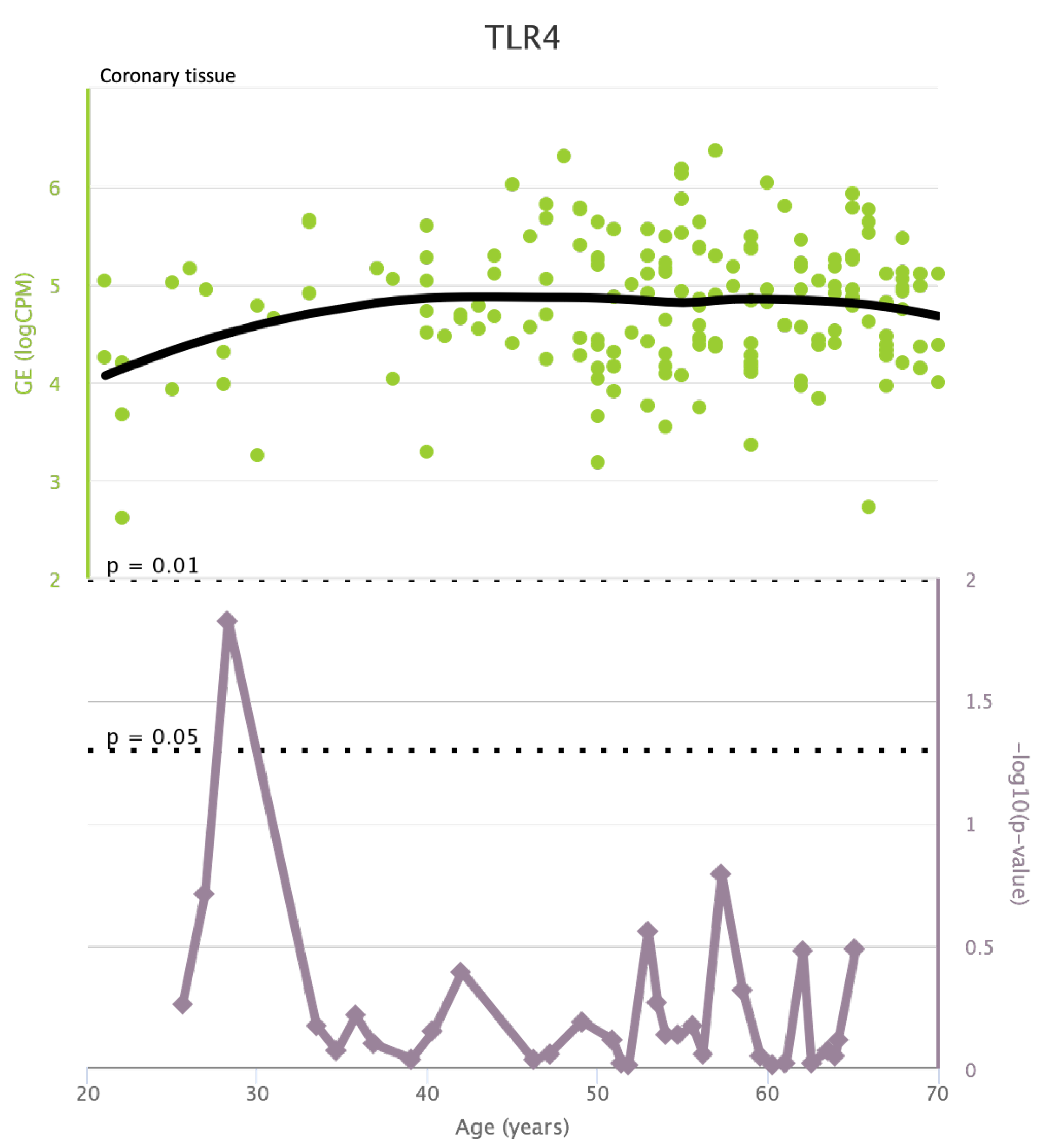
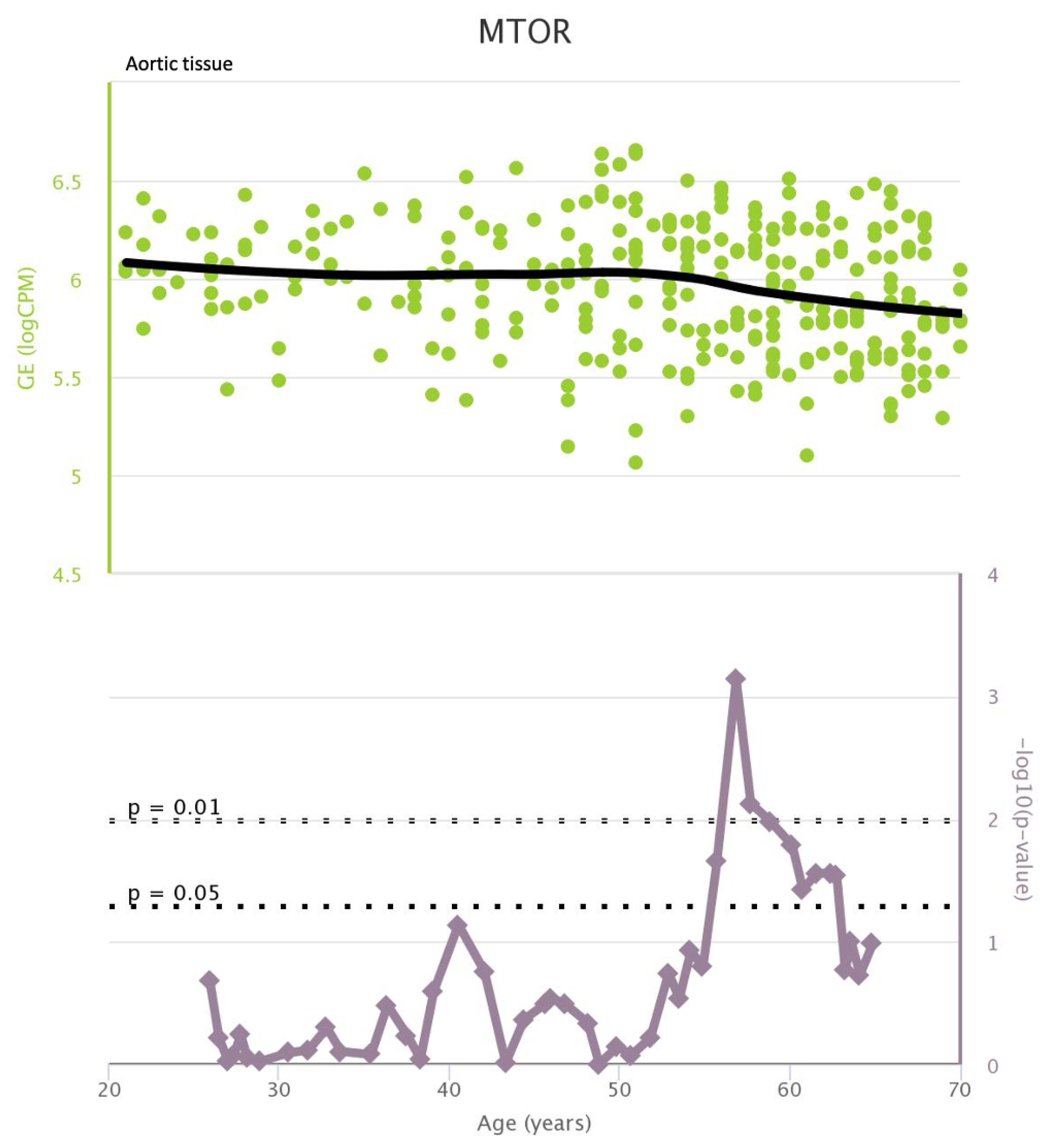
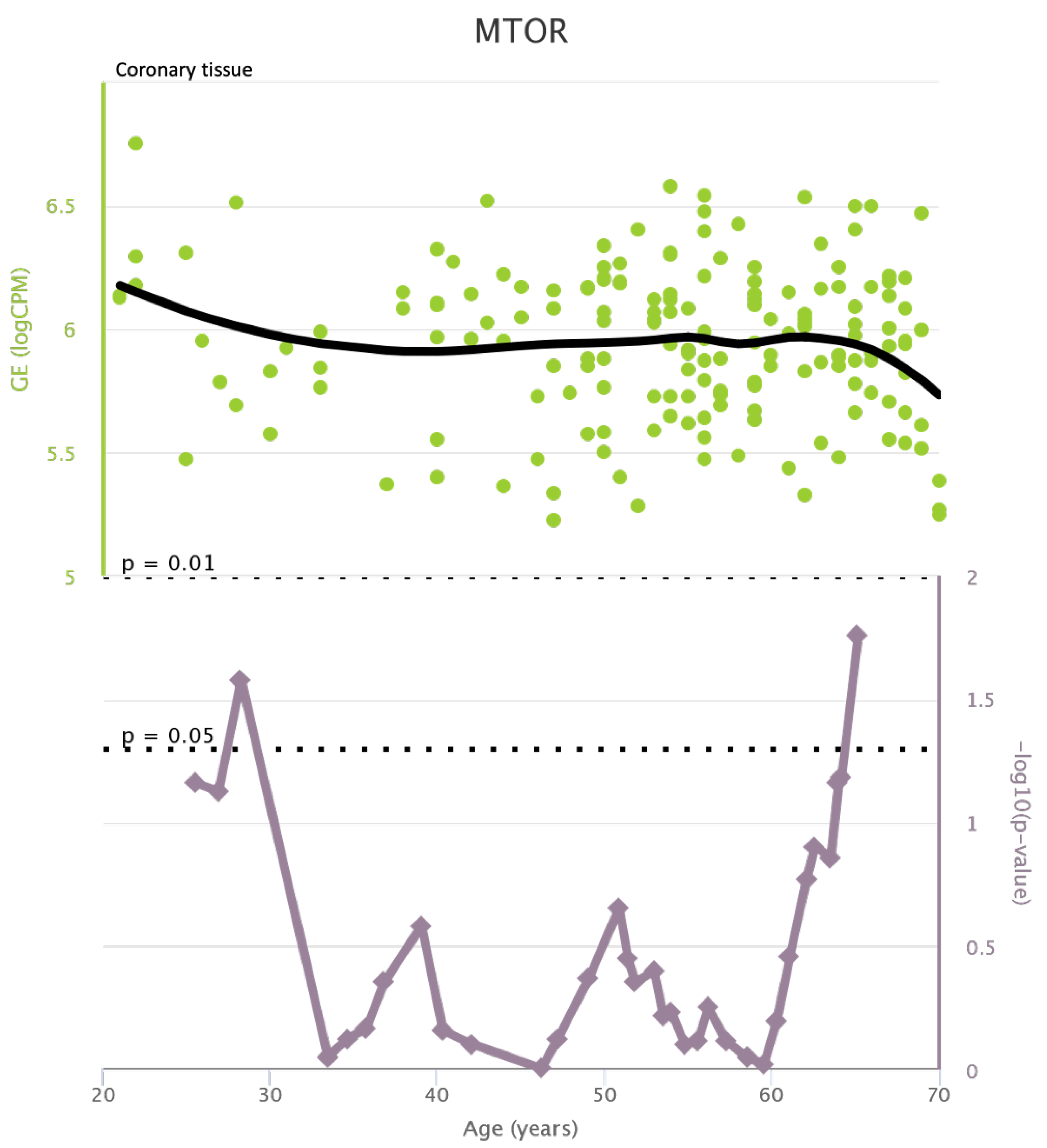
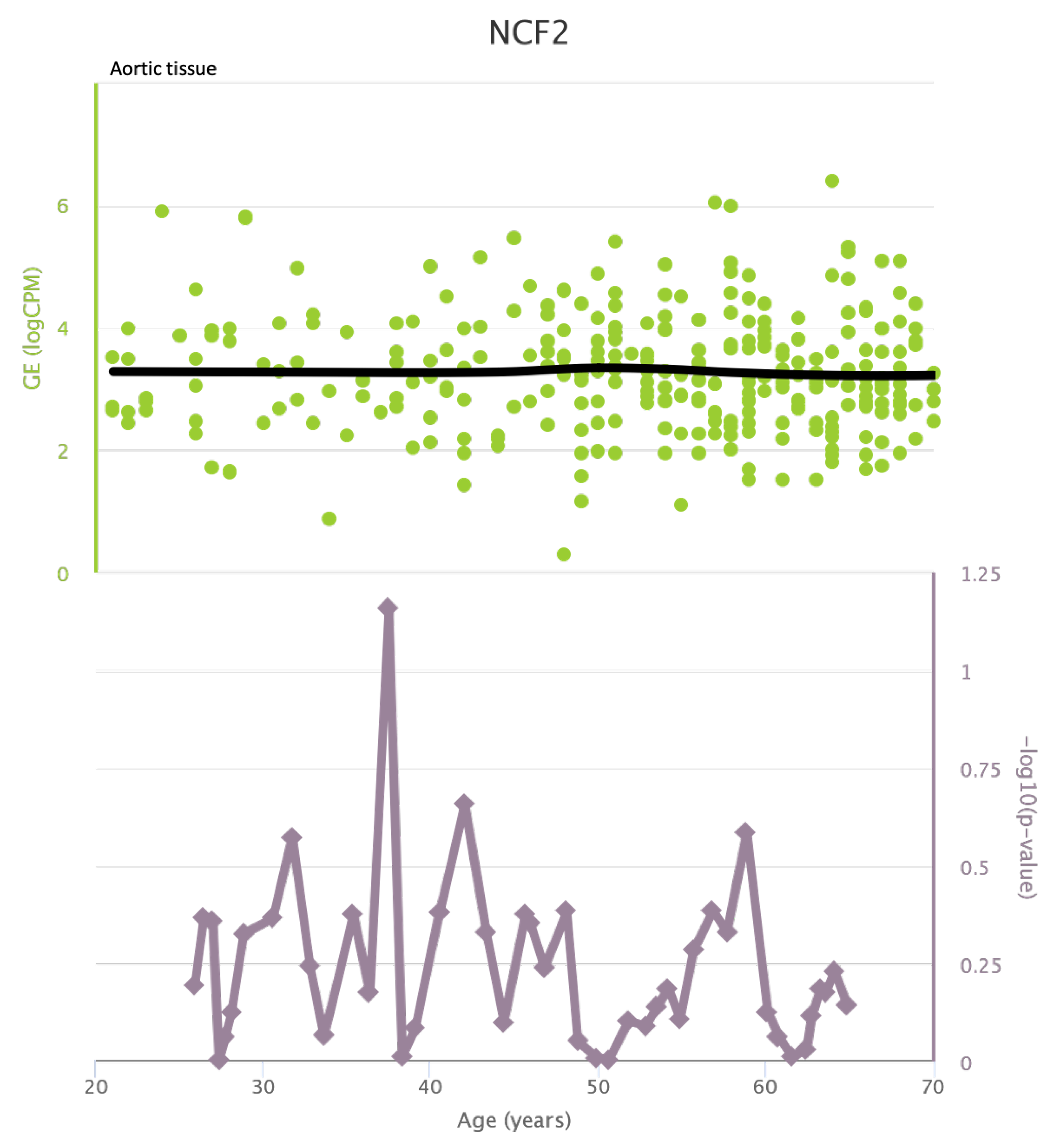
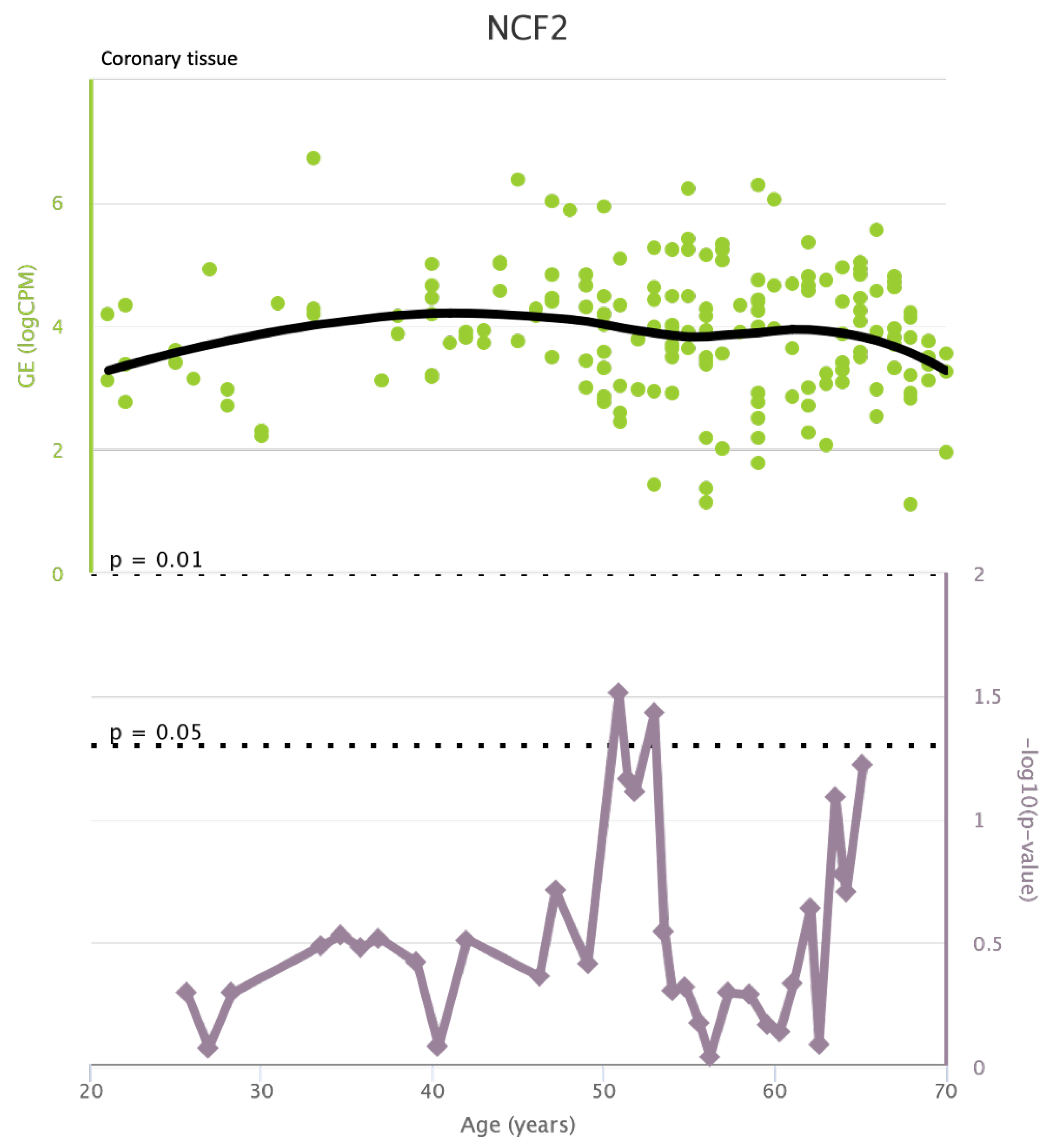
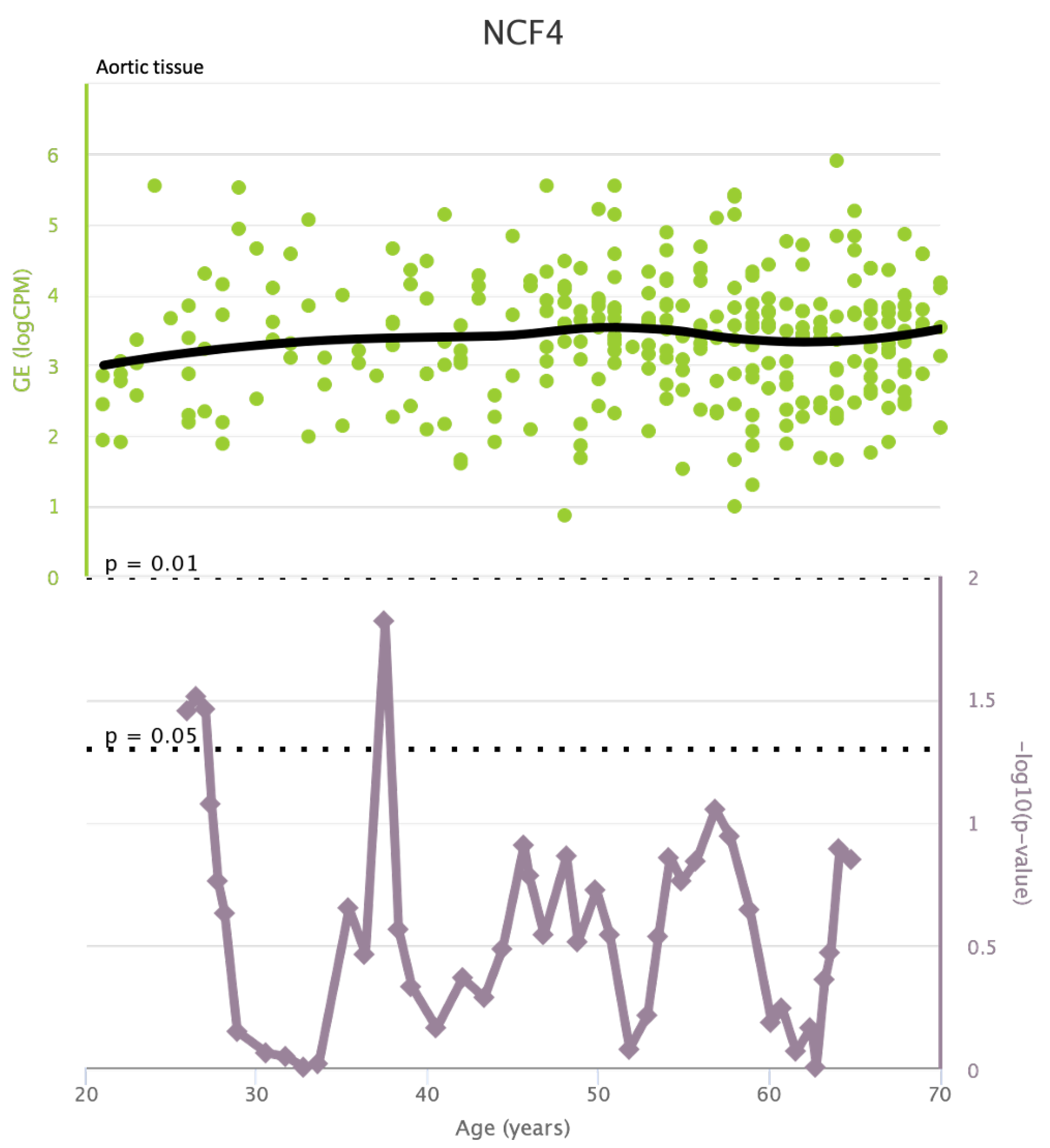
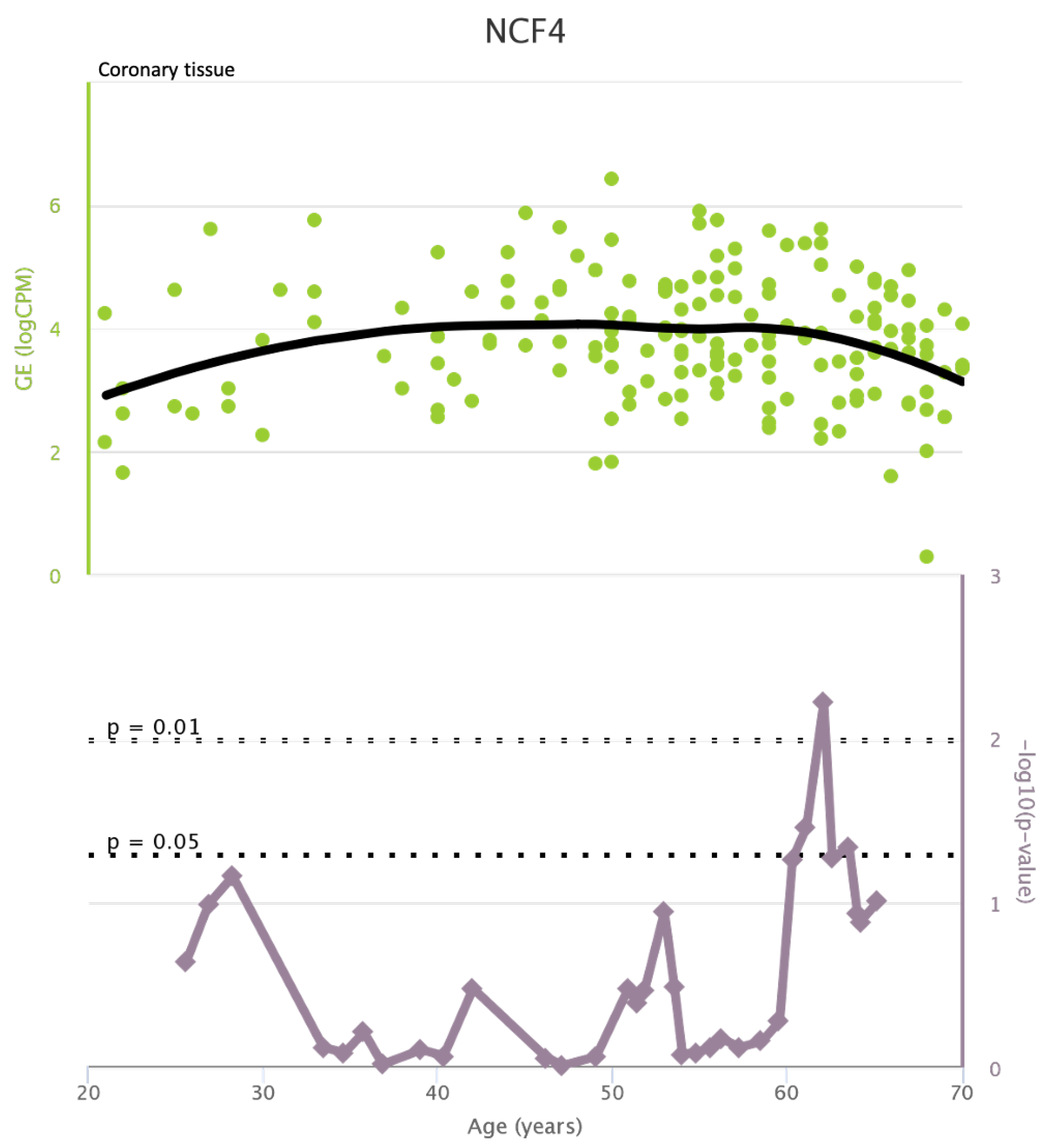
TLR4 levels both in aortic and coronary tissues tend to decrease starting from the age of 50 but the highest rate of genetic alterations occurs at 25 years in coronary tissue and at 55 years in aortic tissue (see
Figure A1 and
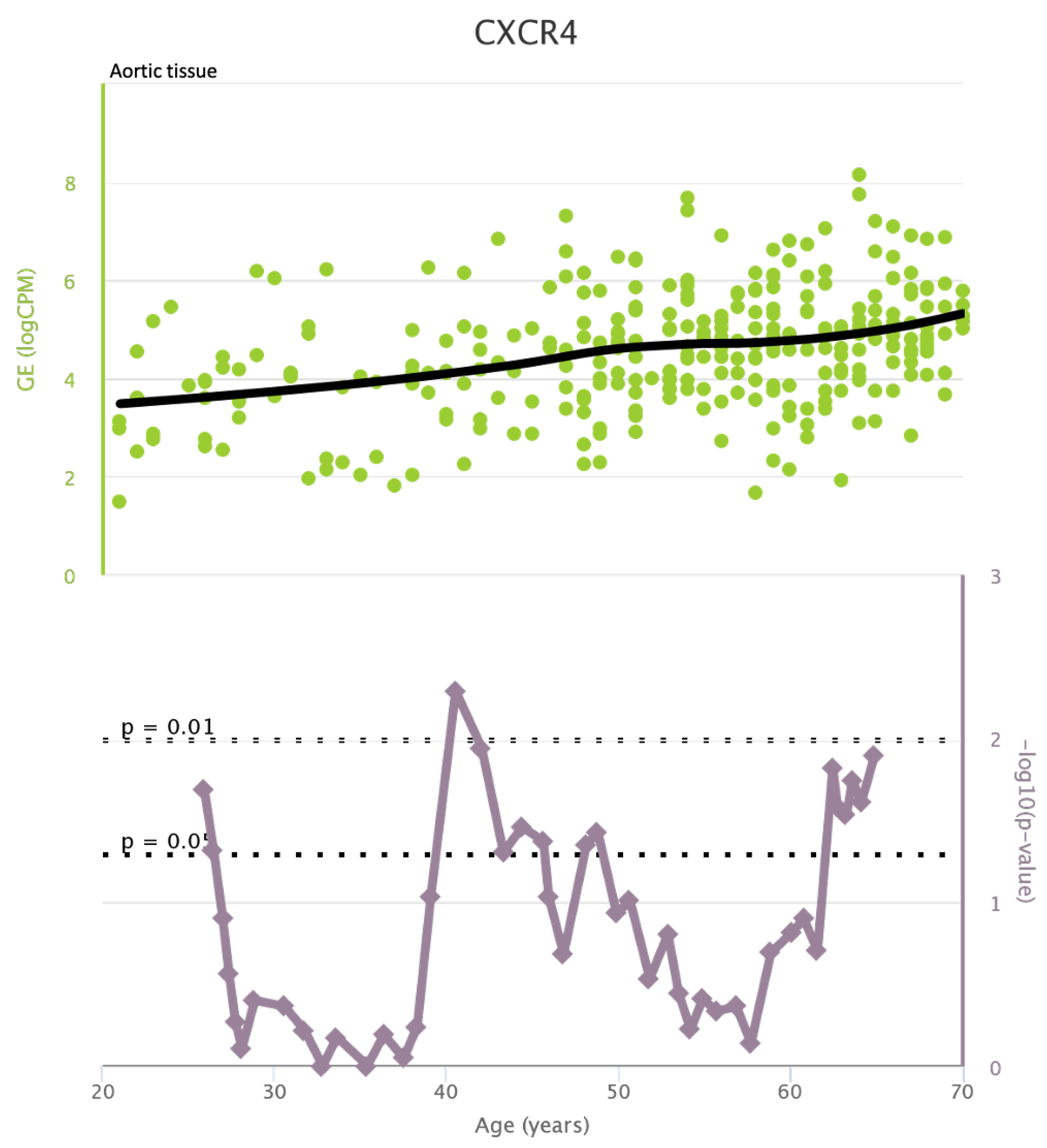
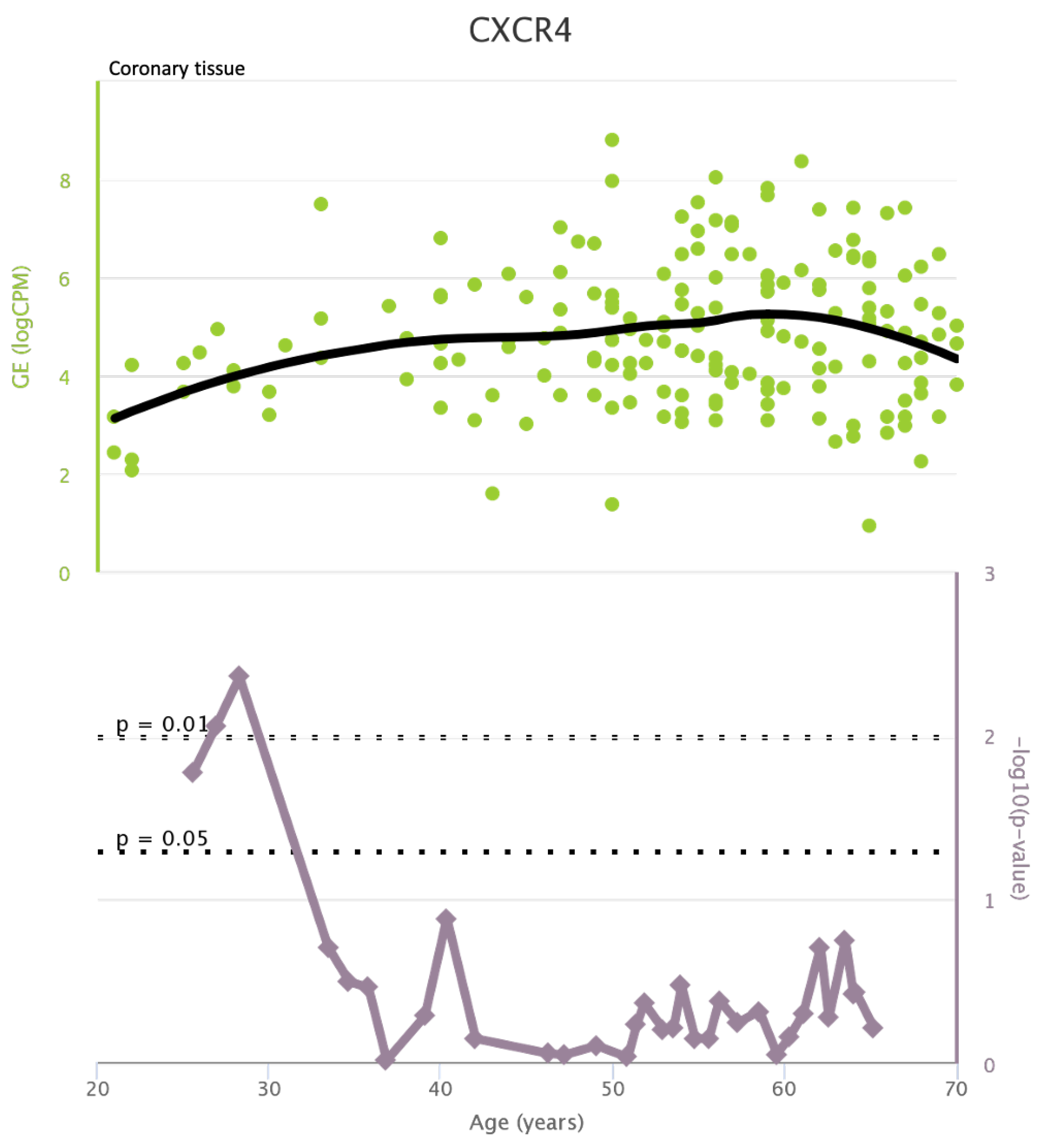
Figure A2). CXCR4 levels in aortic tissue continuously rise throughout life and the highest rate of genetic alterations occurs around 40 years old. Instead in coronary tissue gene’s levels continuously increase up to the age of 60 and then decrease exponentially. In this case the highest rate of genetic alterations occurs during young age (30 years) (see
Figure A3 and
Figure A4).mTOR levels both in aortic and coronary tissues tend to decrease starting from the age of 50 and at the same time the highest rate of genetic alterations occurs in this phase (highest point after 60 years for coronary tissue and at 55 years for aortic tissue) (see
Figure A5 and
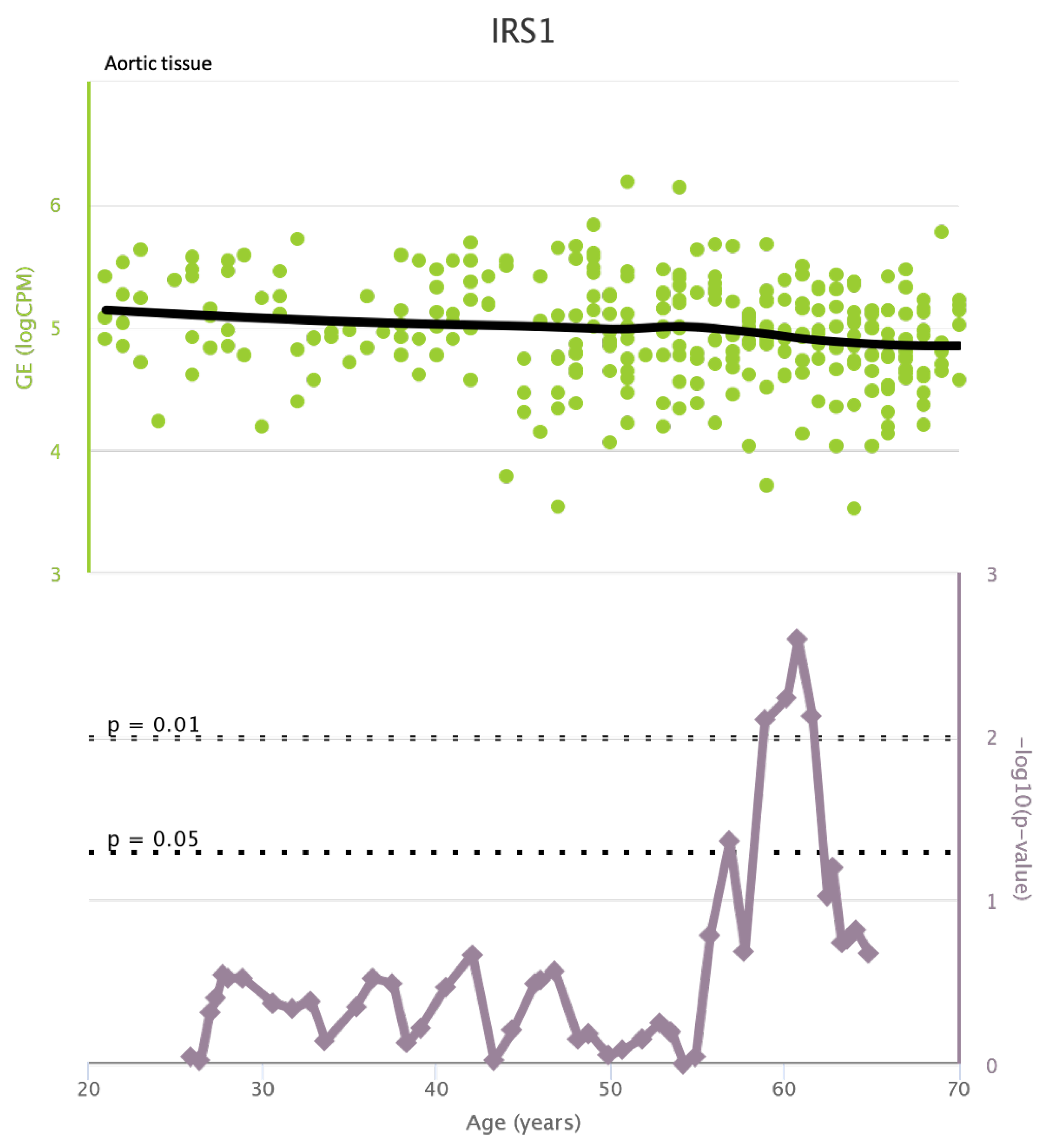
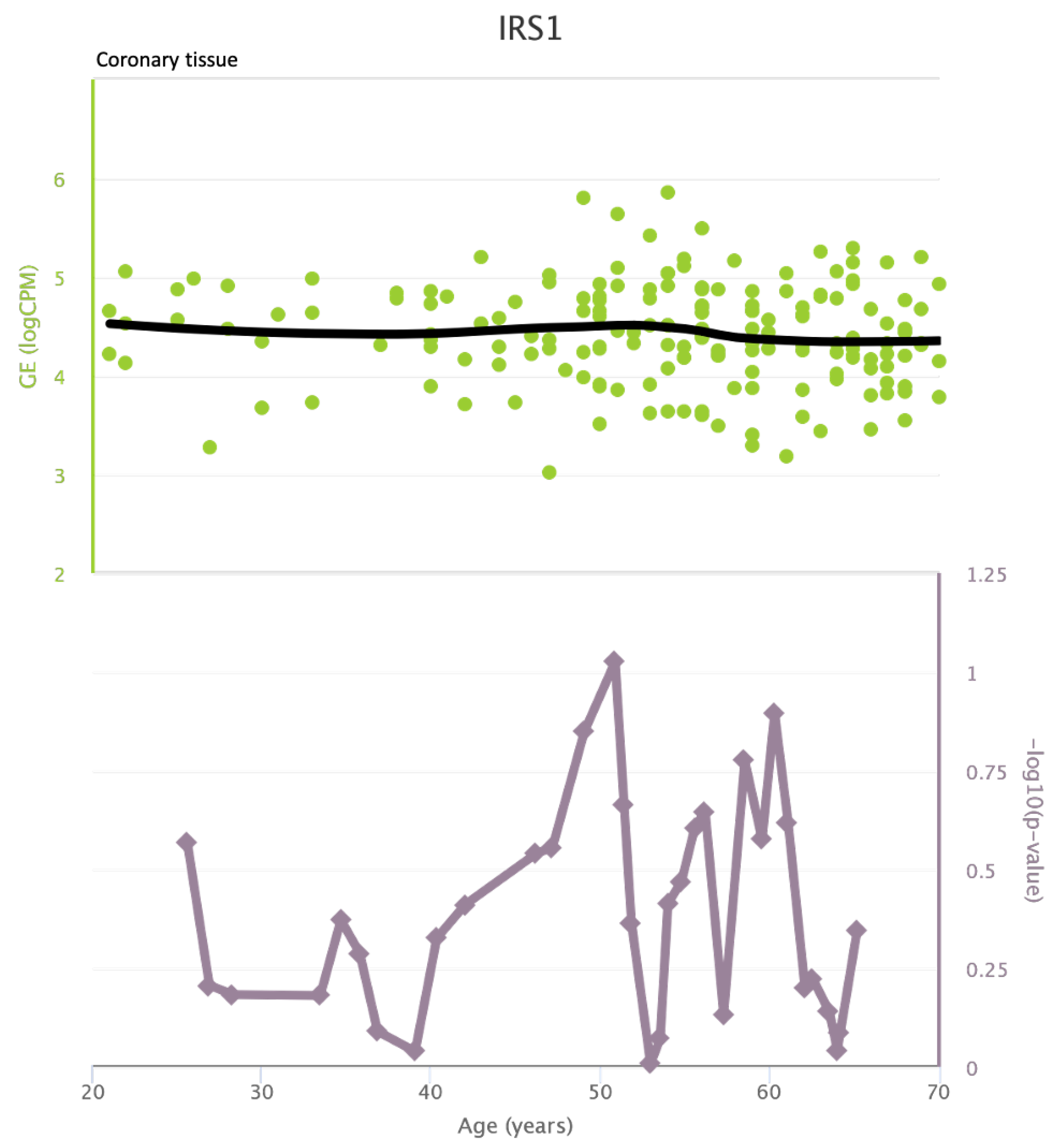
Figure A6).IRS1 levels both in aortic and coronary tissues tend to decrease starting from the age of 50 and at the same time the highest rate of genetic alterations occurs in this phase (highest point at 50 years for coronary tissue and 60 years for aortic tissue) (see
Figure A7 and
Figure A8 of the
Appendix A). NCF2 levels in aortic tissue do not vary throughout life and the highest rate of genetic alterations occurs around 35 years old. Instead in coronary tissue gene’s levels tend to decrease starting from the age of 60 years. In this case the highest rate of genetic alterations occurs at 50 years (see
Figure A9 and
Figure A10 of the
Appendix A). NCF4 levels in aortic tissue do not vary throughout life and the highest rate of genetic alterations occurs around 35 years old. Instead in coronary tissue gene’s levels tend to decrease starting from the age of 60 years. In this case the highest rate of genetic alterations occurs at 60 years (see
Figure A11 and
Figure A12 of the
Appendix A).
4.2. Increasing and Decreasing Genes in Patients with HIV
From an integrated Scopus and Pubmed search, we found clinical data only for TLR4, mTOR, CXCR4, IRS1, and NCF4.
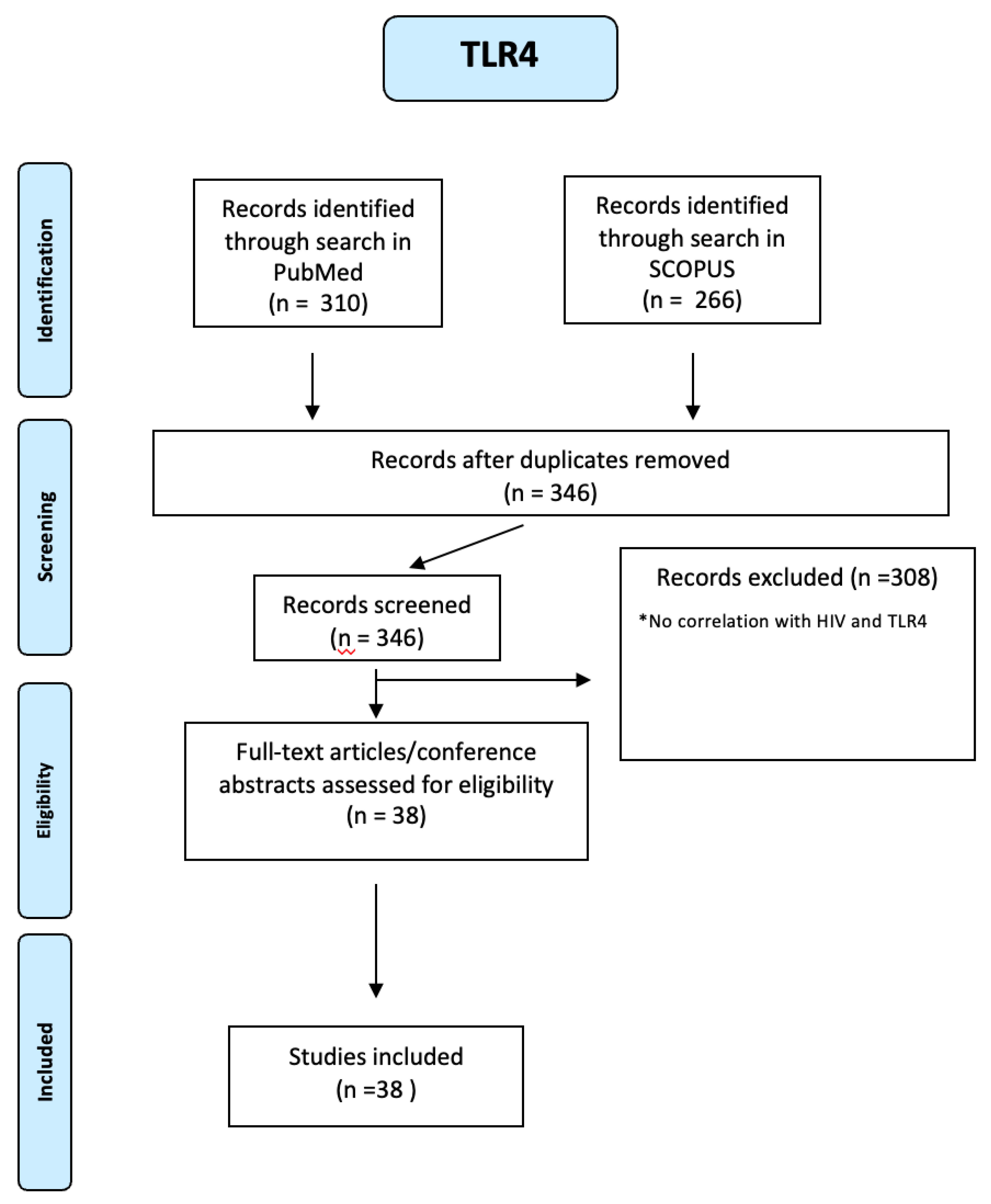
266 TLR4 articles were identified through a search in SCOPUS and 310 through a search in PUBMED. Using a PRIMSA-based screening strategy (see
Figure A13 of the
Appendix A), we identified 38 studies. Many studies using several cell types from HIV-infected patients indicate that TLR4 plays a crucial role in regulating the expression of proinflammatory cytokines and viral pathogenesis [
29]. An increase of TLR4 expression and production of proinflammatory cytokines were observed in HIV patients and, remarkably, some studies found the expression was higher in cells from patients who do not use HAART [
30,
31].
Ten studies have highlighted the correlation between HIV and specific TLR4 polymorphisms: the presence of the TLR4 gene Gly polymorphic allele in the genome increases the risk of HIV/HCV-coinfection development[
32]. The main TLR4 SNPs studied include A+896G (rs4986790) and C+1196T (rs4986791). Compared to non-carriers, carriers of A+896G/C+1196T showed blunted inflammatory responses to inhaled LPS [
33] while single nucleotide polymorphisms (SNPs) TLRs such as TLR4 1063A/G and 1363C/T are associated with changes in CD4 count, viral load (VL), and disease progression during HIV infection [
34]. The TLR4 Asp299Gly heterozygous genotype and the mutant allele G were higher in HIV-1 infection than healthy controls, and in stage, I compared to different clinical stages of infection [
35].
Finally, TLR4 Asp299Gly polymorphism is independently associated with the occurrence of CVDs in HIV-infected patients. The pro-inflammatory profile related to this variant could be involved in the development of atherosclerotic pathologies[
36]. At the same time, HIV entry in the host cell requires interaction with the CD4 membrane receptor and depends on the activation of coreceptor CXCR4. [
37]. Therefore, a tendency for greater activation of CXCR4+CD4+ T cells in patients with advanced disease was observed. [
38]. In particular, 3825 CXCR4 articles were identified through a search in SCOPUS and 3970 through a search in PUBMED. Using a PRIMSA-based screening strategy (see
Figure A14 of the
Appendix A), we identified 62 studies. The natural ligands for CXCR4 can inhibit viral entry. In particular, several peptidic compounds, T22 (an 18-mer), T134 (a 14-mer), ALX40-4C (a 9-mer) and CGP 64222 (also a 9-mer), have been identified as CXCR4 antagonists and show anti-HIV activity [
39,
40,
41]. Another new technology consists of a small-molecule inhibitor, ALX40-4C, that inhibits HIV-1 envelope (Env)-mediated membrane fusion and viral entry directly at the level of coreceptor use. [
42] In the future, HIV entry/fusion inhibitors will become important new antiviral agents to combat AIDS. Mutations in the CXCR4 gene are generally rare and have not been implicated in HIV-1/AIDS pathogenesis. Comprehensive mutation analysis of the CXCR4 gene confirmed a high degree of genetic conservation within the coding region of this ancient population. [
43].
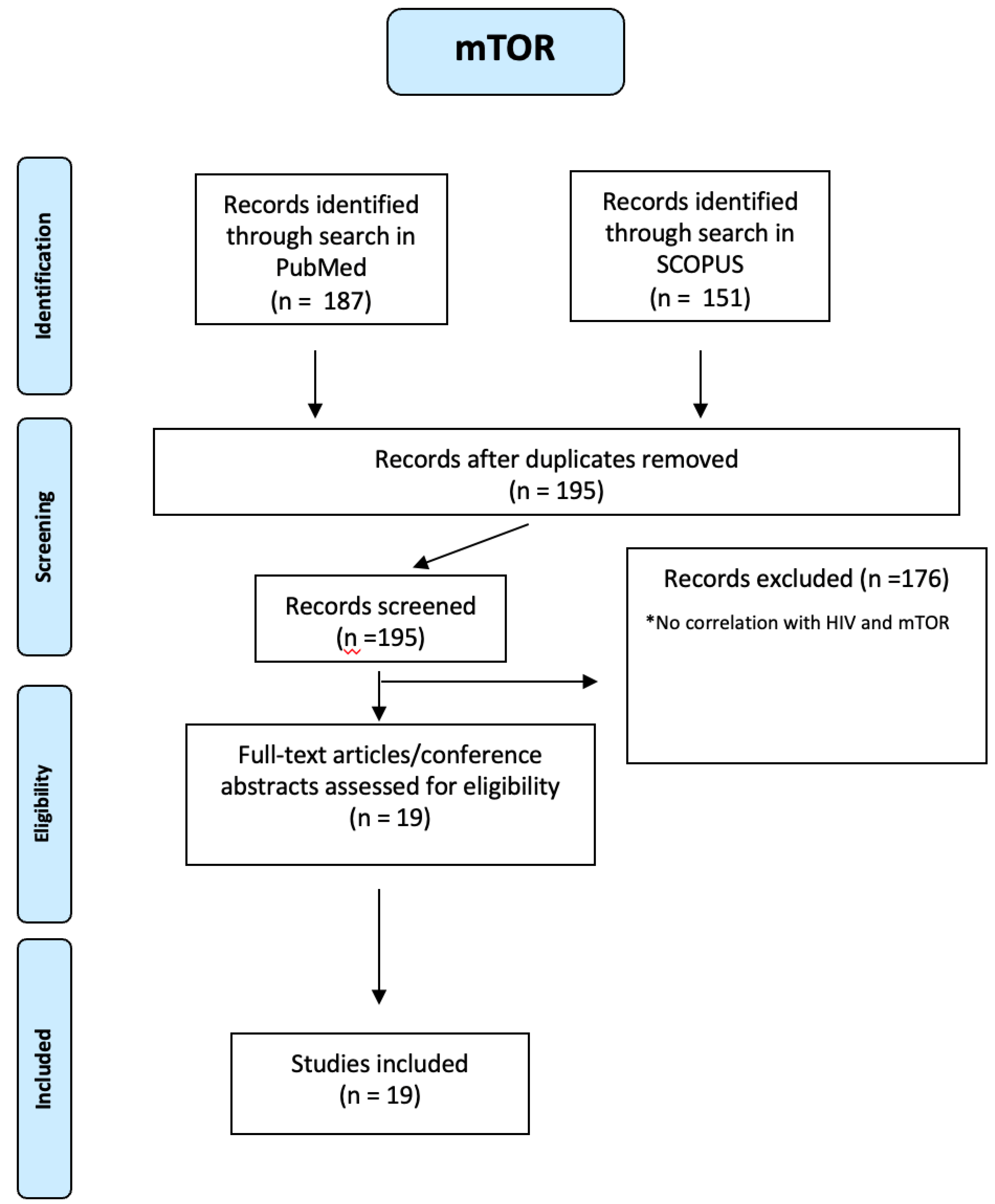
mTOR plays a crucial role, too. One hundred fifty-one mTOR articles were identified through a search in SCOPUS and 187 through a search in PUBMED. Using a PRIMSA-based screening strategy (see
Figure A15 of the
Appendix A), we identified 19 studies. CD4+ and CD8+ T cells from PLWH play a role in cell adhesion, apoptosis and migration processes involved in atherosclerosis, and the upregulated mTOR pathway mediates these atherogenic processes [
20,
44]. The HIV-1 viral life cycle depends on mTOR because it drives signalling and metabolic pathways required for viral entry, replication, and latency. In HIV-1 pathogenesis, mTOR alters host cell metabolism to create an optimal environment for viral replication [
44]. Preclinical evidence indicates that selective inhibitors of mTOR, such as rapamycin, could represent a novel therapeutic approach for the treatment of these pathologies [
45].
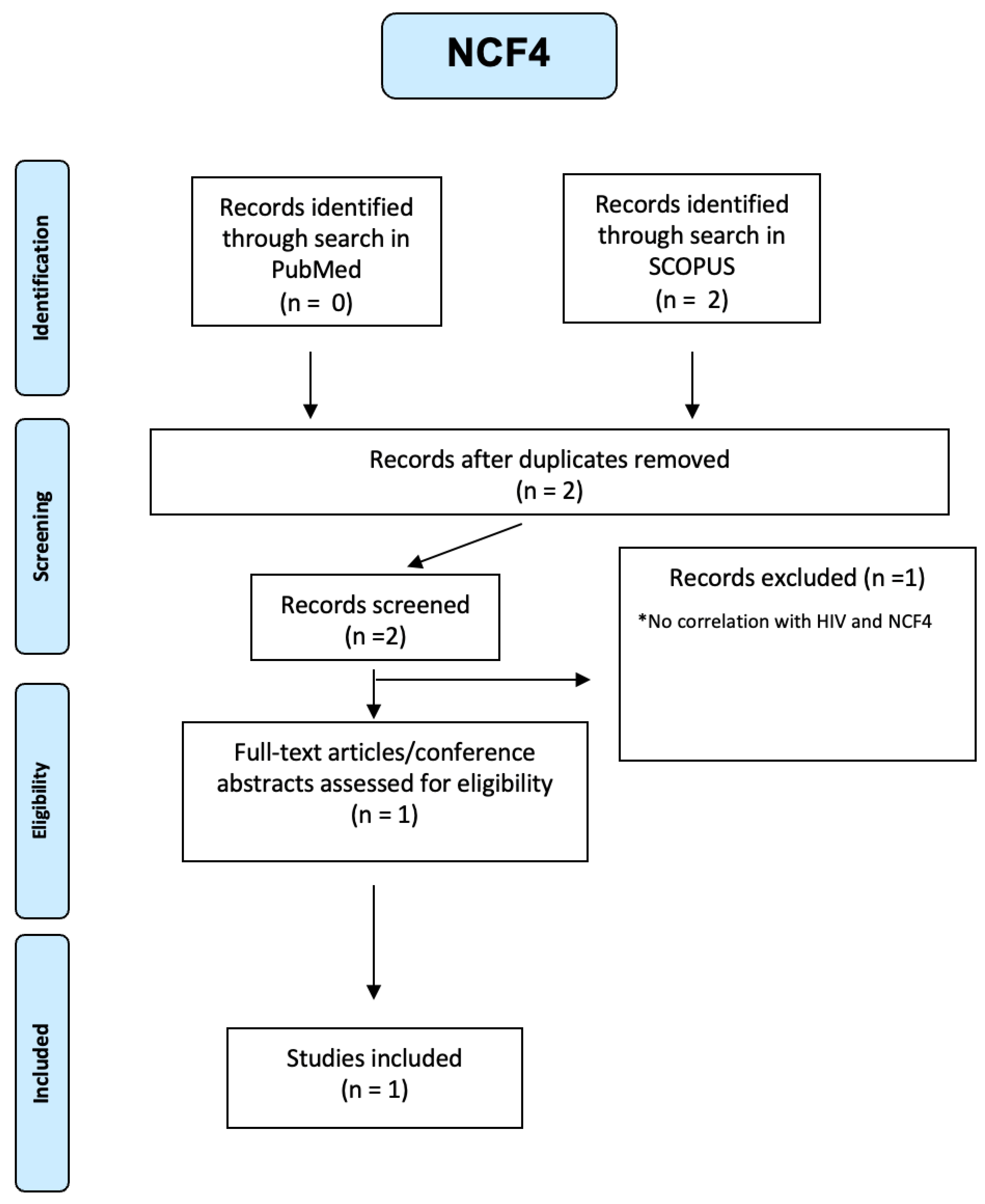
In PLWH monocytes, NCF genes are involved in superoxide production and are a positive regulator of P13K signalling. [
20] From our integrated search on Pubmed and Scopus, only one article was selected (see
Figure A16 of the
Appendix A), where microarray data analysis of datasets that involved HIV cases was done to scrutinize the differentially expressed genes. NCF4 might have the potential to be exploited as a possible drug target and biomarker in the diagnosis, prognosis as well as treatment of HIV and its comorbidities[
46].
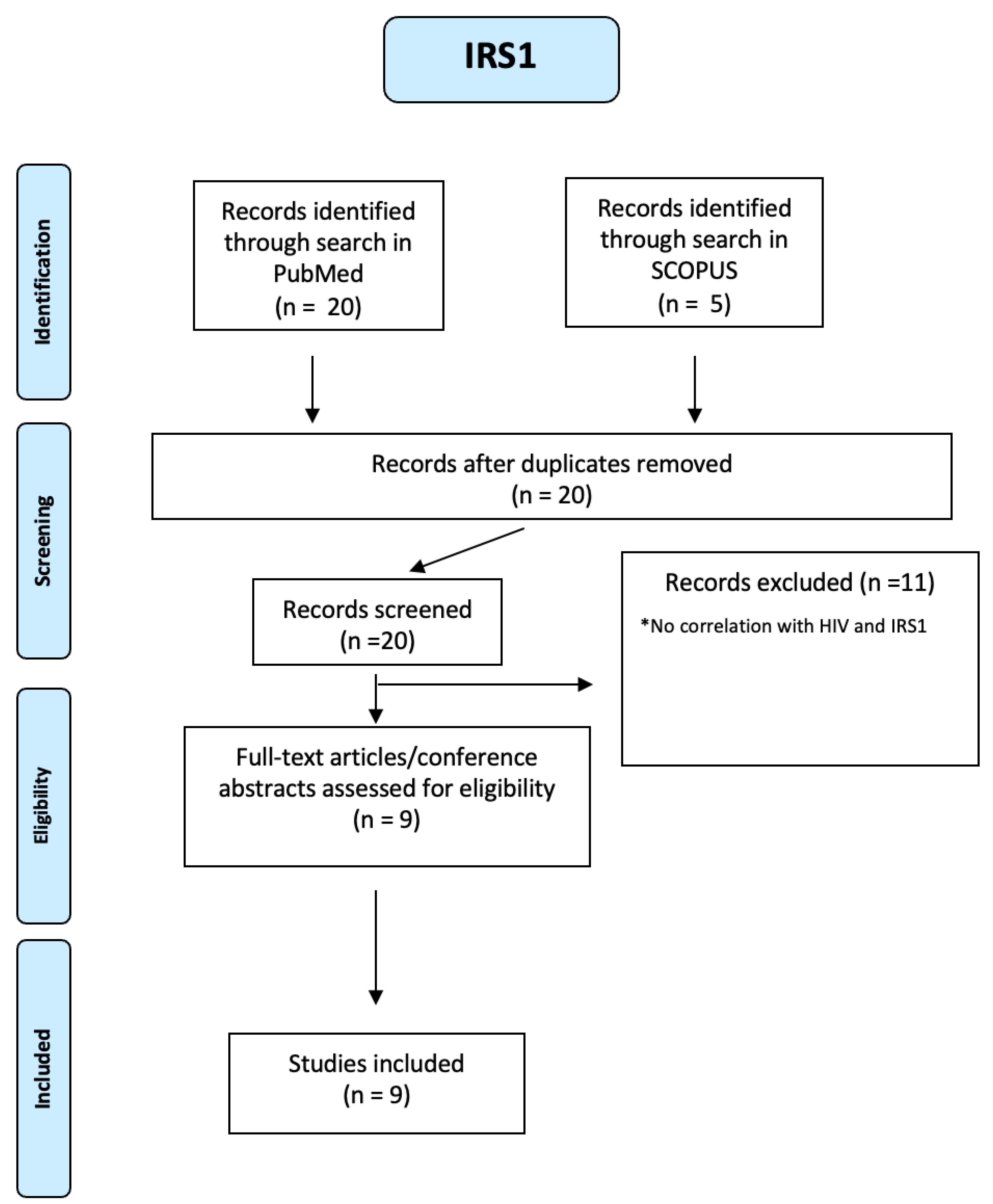
Finally, blood sugar metabolism abnormalities have been identified in HIV-infected individuals and associated with HIV-associated neurocognitive disorders (HAND). [
47] In HIV untreated patients, there is severe insulin resistance with increased LPS and cytokines that involve the liver, hypothalamus, muscle, vessels and adipose tissue. Five IRS1 articles were identified through a search in SCOPUS and 20 through a search in PUBMED. We identified nine studies using a PRIMSA-based screening strategy (see
Figure A17 of the
Appendix A).
The increase in LPS circulating levels in HIV patients will induce an increase in circulating inflammatory cytokines. These increases will activate TLR4, IL-6, and TNFalpha receptors, which will induce mitochondrial dysfunction, activation of the inflammasome and an increase in intracellular lipid accumulation [
48]. Then, PKR, JNK, and IKKbeta/NF-KB pathways will be activated in the liver, muscle, adipose tissue, macrophages and other tissues. Activating these serine kinases (PKR, JNK, and IKKbeta) will induce serine phosphorylation of the IRS1/2 and, consequently, a downregulation in insulin signalling [
48]. Finally, the same antiretroviral therapy with indinavir, lopinavir and nelfinavir causes insulin resistance [
49,
50,
51].
4.3. Increasing and Decreasing Genes in Patients with CVD
The integrated Scopus and Pubmed search found clinical data for TLR4, mTOR, CXCR4, and IRS1. It was of great interest to require information about baseline characteristics of patients with cardiovascular disease and the de-regulation of a specific gene expression.
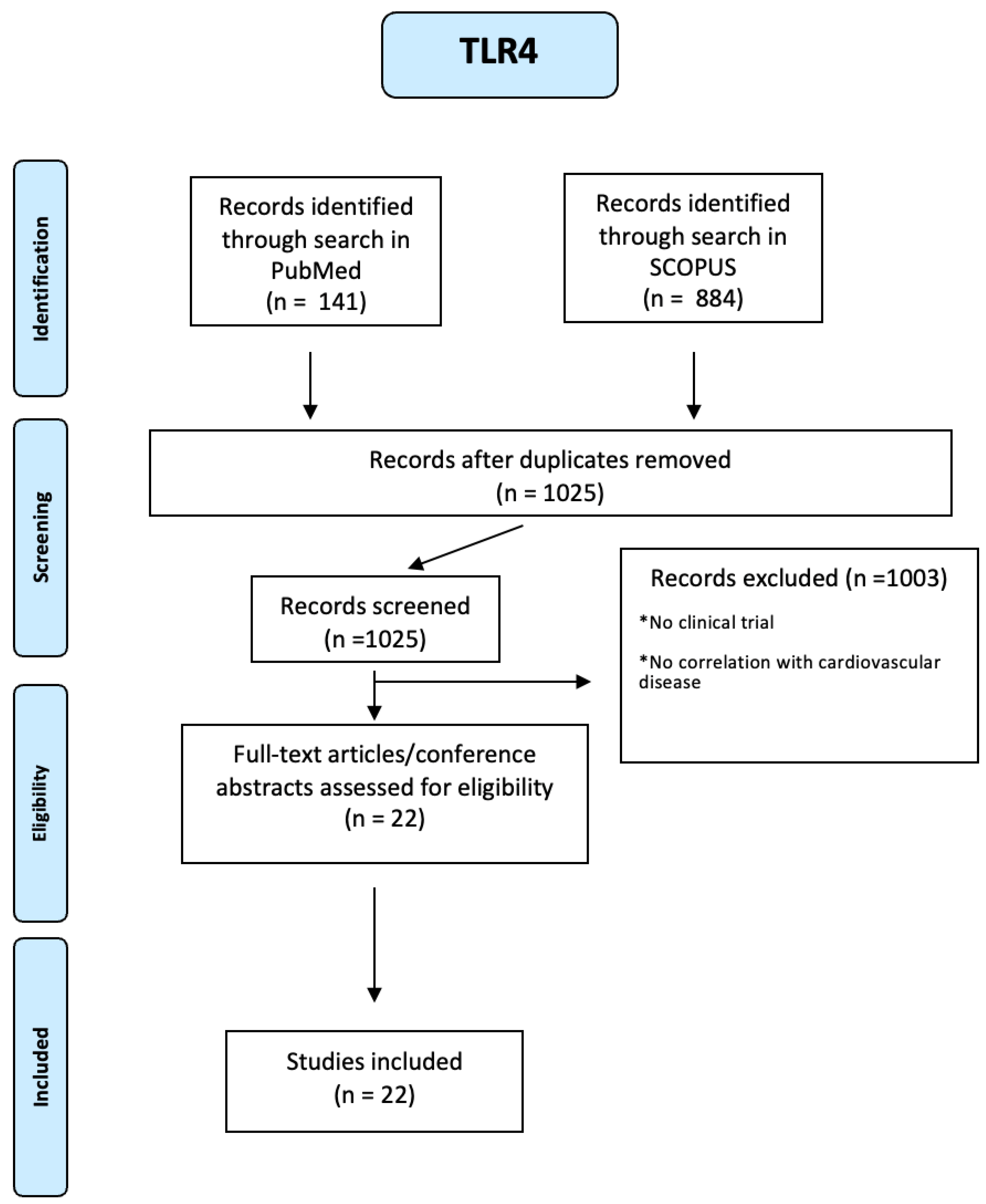
884 TLR4 articles were identified through a search in SCOPUS and 141 through a search in PUBMED. Using a PRISMA-based screening strategy, we identified 22 studies. (see
Figure A18 of the
Appendix A) People included were both male and female, of any age from eighteen years and with many diseases such as diabetes mellitus, hypertension, dyslipidemia, obesity, bicuspid aortic valve, abdominal aortic aneurysm, peripheral artery disease, the story of acute myocardial infarction or unstable angina. Six studies have highlighted the correlation between cardiovascular diseases and specific TLR4 polymorphisms [
52,
53,
54,
55,
56,
57]: rs1927914 TC, TC/CC genotypes and TLR4 rs1927914 TC genotype were associated with aortic aneurysm; rs10759932 polymorphism was associated with a reduced risk of AAD. The C/T genotype of the rs4986791 polymorphism was significantly associated with severe non-coronary atherosclerosis. The frequency of SNP896A ⁄G in the TLR4 gene was not significantly different between AMI patients and controls. Finally, 299Gly carriers (with a story of CCS) had a lower risk of cardiovascular events during follow-up with pravastatin. Instead sixteen studies demonstrated that the specific cardiovascular disease (aortic aneurysm, acute or chronic coronary syndrome etc.) was associated with higher TLR4 blood levels [
58,
59,
60,
61,
62,
63,
64,
65,
66,
67,
68].
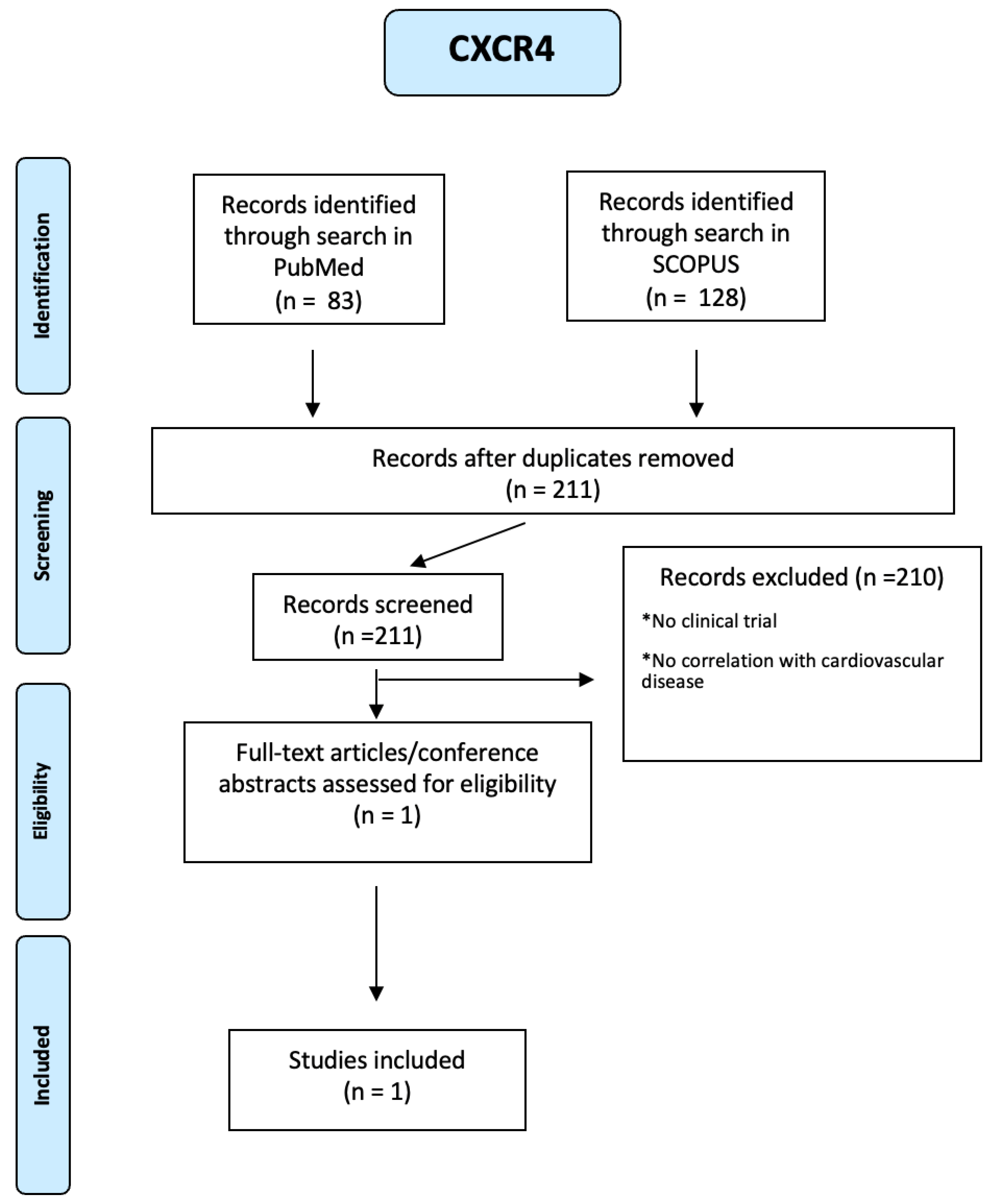
About CXCR4, 128 articles were identified through a search in SCOPUS and 83 through a search in PUBMED. Using a PRISMA-based screening strategy as reported in
Figure A19 of the
Appendix A), we identified 1 study. People included were both male and female, of any age from eighteen years and with many diseases such as diabetes mellitus, hypertension, dyslipidemia, atrial fibrillation, the story of acute myocardial infarction or unstable angina. In this study, platelet surface expression of CXCR4 was measured in 284 patients with symptomatic CAD at the time of percutaneous coronary intervention (PCI). The primary combined endpoint was defined as all-cause death and myocardial infarction (MI) during a 12-month follow-up. There were differences in CXCR4 values in patients who developed a combined end point compared with event-free patients and in patients who subsequently died. In fact, lower platelet CXCR4 levels were independently and significantly associated with all-cause mortality (hazard ratio 0.24, 95 per cent CI 0.07-0.87) and the primary combined end point of all-cause death and MI (hazard ratio 0.30, 95 per cent CI 0.13-0.72) [
69].
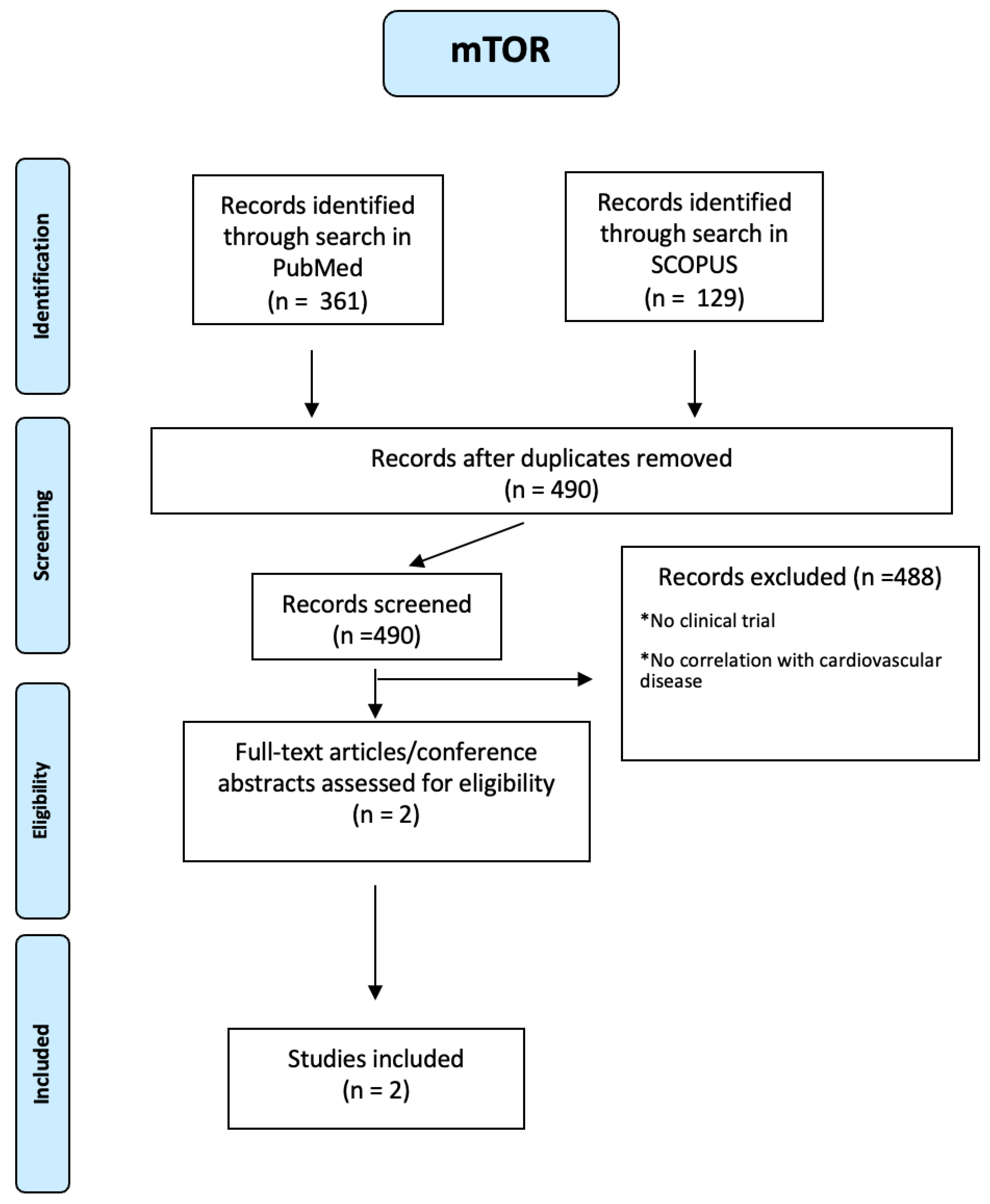
One hundred twenty-nine articles on mTOR were identified through a search in SCOPUS and 361 through a search in PUBMED. Using a PRIMSA-based screening strategy (see
Figure A20 of the
Appendix A), we identified two studies. People included were both male and female, of any age from eighteen years and with many diseases such as diabetes mellitus, hyper- or hypothyroidism, severe infection, malignancy, CVD, stroke/TIA, Rheumatoid Arthritis, and acute myocardial infarction. One study demonstrated that mTOR inhibition using everolimus at the time of an acute STEMI reduces LV infarct size following successful PCI[
70]. In the other study, patients with Rheumatoid Arthritis showed hypoexpression of Raptor, a positive regulator of mTOR activity, resulting in decreased LDL levels[
71].
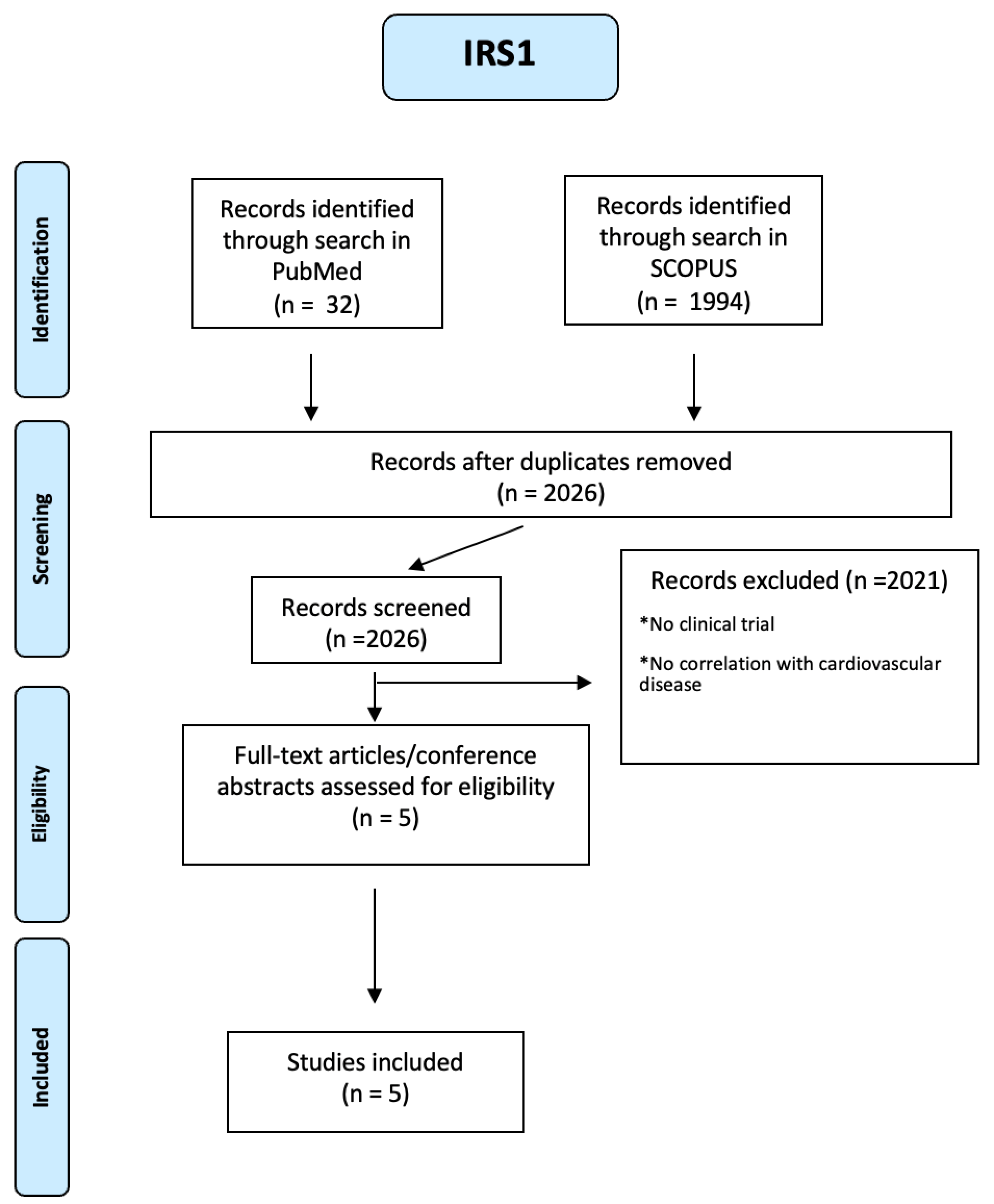
One thousand nine hundred ninety-four articles were identified about IRS1 through a search in SCOPUS and 32 through a search in PUBMED (see
Figure A21 of the
Appendix A). Using a PRISMA-based screening strategy , we identified following studies [
71,
72,
73,
74]. People included were both male and female, of any age from eighteen years and with many diseases such as diabetes mellitus, obesity, hypertension, dyslipidemia, previous acute myocardial infarction, multivessel CAD, prior stroke/TIA, chronic renal insufficiency. Five studies have highlighted the correlation between cardiovascular diseases and specific IRS1 polymorphisms: the C allele of the IRS1 gene (rs2943640) in both homozygous and heterozygous states may indicate an increased risk of dyslipidemia in type 2 diabetic patients with comorbidities. Arg/Arg and Gly/Arg polymorphism of the IRS-1 gene is associated with such components of the metabolic syndrome as hypertriglyceridemia and fasting hyperglycemia. In another study, type 2 DM patients who are carriers of the C allele of the rs956115 marker of the IRS-1 gene have a hyperreactive platelet phenotype and increased risk of MACE, while hypertensive patients with the GA genotype Gly972Arg polymorphism of the IRS-1 gene are predisposed to insulin resistance and disorders of lipid metabolism.
Finally, the Arg972 variant in insulin receptor substrate-1 is associated with an atherogenic profile in type 2 diabetic patients.
5. Discussion
Antiretroviral therapy (ART) has improved quality of life and increased life expectancy among human immunodeficiency virus (HIV)–infected individuals. Consequently, patterns of mortality and morbidity are changing among the human immunodeficiency virus (HIV)–-infected population [
75].
Several studies suggest that CVD events in HIV-positive patients occur at higher rates compared with HIV-negative or general populations of similar age [
76]
It is well known that the risk of CVD increases with age, but it remains unclear whether this age-related increase is more rapid in HIV-positive people than in the general HIV-negative population.
We based our analyses on the genes dis-regulated pathways in patients with HIV, selecting genes simultaneously most expressed in aortic and coronary tissue. We compared the gene expression of TLR4, CXCR4, mTOR, NCF2, NCF4, and IRS1 in the general population, in patients with HIV, and in patients with cardiovascular disease, obtaining interesting results.
The findings presented in the analysis highlight the intricate relationship between genetic pathways involved in HIV infection and cardiovascular diseases. The dysregulation of genes such as TLR4, CXCR4, mTOR, NCF2, NCF4, and IRS1 in individuals living with HIV not only impacts viral pathogenesis but also contributes to the development of cardiovascular complications.
The upregulation of TLR4, a key regulator of proinflammatory cytokines, in HIV patients, particularly in those not on antiretroviral therapy, underscores the role of inflammation in HIV pathogenesis and its potential link to cardiovascular diseases. Similarly, the increased activation of CXCR4 in advanced HIV disease indicates its involvement in viral entry and immune dysregulation, which can also impact cardiovascular health.
Furthermore, the dysregulation of mTOR, NCF2, NCF4, and IRS1 in HIV-infected individuals sheds light on the metabolic disturbances and insulin resistance observed in these patients, which are known risk factors for cardiovascular diseases. The interplay between these genetic pathways not only affects the progression of HIV-related complications but also influences the development of cardiovascular events in this population.
The identification of specific polymorphisms and gene expressions associated with cardiovascular diseases in individuals living with HIV provides valuable insights into potential genetic markers for risk stratification and targeted interventions. Understanding these genetic pathways’ molecular mechanisms can pave the way for personalized approaches to managing cardiovascular risks in PLWH.
Overall, the integration of genetic studies in the context of HIV infection and cardiovascular diseases offers a comprehensive understanding of the intricate interplay between viral pathogenesis, immune responses, metabolic dysregulation, and cardiovascular complications.
6. Conclusions
In conclusion, with our research we have identified possible correlations between the genetic pathways involved in HIV and the development of cardiovascular diseases. Further research in this area is essential to elucidate the causal relationships between these genetic pathways and disease outcomes, ultimately guiding the development of novel therapeutic strategies for improving the health outcomes of individuals living with HIV.
Abbreviations
| AIDS |
Acquired Immunodeficiency Syndrome |
| HIV |
Human Immunodeficiency Virus |
| PLWH |
People Living With HIV |
| CVD |
Cardiovascular Diseases |
| PWH |
People With HIV |
| HAART |
Highly Active Antiretroviral Therapy |
| CCS |
Chronic Coronary Syndrome |
| AMI |
Acute Myocardial Infarction |
| CAD |
Coronary Artery Disease |
| TIA |
Transient Ischemic Attack |
| DM |
Diabetes Mellitus |
| MACE |
Major Adverse Cardiovascular Events |
| ART |
Anti Retroviral Theraphy |
Appendix A
This section contains a set of supplementary Figures related to the analysis of the paper.
Figure A1.
TLR4 levels in aortic tissue and rate of genetic alterations
Figure A1.
TLR4 levels in aortic tissue and rate of genetic alterations
Figure A2.
TLR4 levels in coronary tissue and rate of genetic alterations
Figure A2.
TLR4 levels in coronary tissue and rate of genetic alterations
Figure A3.
CXCR4 levels in aortic tissue and rate of genetic alterations
Figure A3.
CXCR4 levels in aortic tissue and rate of genetic alterations
Figure A4.
CXCR4 levels in coronary tissue and rate of genetic alterations
Figure A4.
CXCR4 levels in coronary tissue and rate of genetic alterations
Figure A5.
mTOR levels in aortic tissue and rate of genetic alterations
Figure A5.
mTOR levels in aortic tissue and rate of genetic alterations
Figure A6.
mTOR levels in coronary tissue and rate of genetic alterations
Figure A6.
mTOR levels in coronary tissue and rate of genetic alterations
Figure A7.
IRS1 levels in aortic tissue and rate of genetic alterations
Figure A7.
IRS1 levels in aortic tissue and rate of genetic alterations
Figure A8.
IRS1 levels in coronary tissue and rate of genetic alterations
Figure A8.
IRS1 levels in coronary tissue and rate of genetic alterations
Figure A9.
NCF2 levels in aortic tissue and rate of genetic alterations
Figure A9.
NCF2 levels in aortic tissue and rate of genetic alterations
Figure A10.
NCF2 levels in coronary tissue and rate of genetic alterations
Figure A10.
NCF2 levels in coronary tissue and rate of genetic alterations
Figure A11.
NCF4 levels in aortic tissue and rate of genetic alterations
Figure A11.
NCF4 levels in aortic tissue and rate of genetic alterations
Figure A12.
NCF4 levels in coronary tissue and rate of genetic alterations
Figure A12.
NCF4 levels in coronary tissue and rate of genetic alterations
Figure A13.
PRISMA-based screening strategy for TLR4
Figure A13.
PRISMA-based screening strategy for TLR4
Figure A14.
PRISMA-based screening strategy for CXCR4
Figure A14.
PRISMA-based screening strategy for CXCR4
Figure A15.
PRISMA-based screening strategy for mTOR
Figure A15.
PRISMA-based screening strategy for mTOR
Figure A16.
PRISMA-based screening strategy for NCF4
Figure A16.
PRISMA-based screening strategy for NCF4
Figure A17.
PRISMA-based screening strategy for IRS1
Figure A17.
PRISMA-based screening strategy for IRS1
Figure A18.
PRISMA-based screening strategy for TLR4
Figure A18.
PRISMA-based screening strategy for TLR4
Figure A19.
PRISMA-based screening strategy for CXCR4
Figure A19.
PRISMA-based screening strategy for CXCR4
Figure A20.
PRISMA-based screening strategy for mTOR
Figure A20.
PRISMA-based screening strategy for mTOR
Figure A21.
PRISMA-based screening strategy for IRS1
Figure A21.
PRISMA-based screening strategy for IRS1
References
- Bykowska-Derda, A.; Spychala, M.; Czlapka-Matyasik, M.; Sojka, M.; Bykowski, J.; Ptak, M. The Relationship between Mortality from Cardiovascular Diseases and Total Drinking Water Hardness: Systematic Review with Meta-Analysis. Foods 2023, 12, 3255. [Google Scholar] [CrossRef] [PubMed]
- Sniderman, A.D.; Furberg, C.D. Age as a modifiable risk factor for cardiovascular disease. The Lancet 2008, 371, 1547–1549. [Google Scholar] [CrossRef]
- Obas, V.; Vasan, R.S. The aging heart. Clinical Science 2018, 132, 1367–1382. [Google Scholar] [CrossRef]
- High, K.P. Infection as a cause of age-related morbidity and mortality. Ageing Research Reviews 2004, 3, 1–14. [Google Scholar] [CrossRef]
- Hardy, W.D. The human immunodeficiency virus. Medical Clinics 1996, 80, 1239–1261. [Google Scholar] [CrossRef]
- Hanson, H.M.; Willkomm, N.A.; Yang, H.; Mansky, L.M. Human retrovirus genomic RNA packaging. Viruses 2022, 14, 1094. [Google Scholar] [CrossRef]
- Schuman, J.S.; Orellana, J.; Friedman, A.H.; Teich, S.A. Acquired immunodeficiency syndrome (AIDS). Survey of ophthalmology 1987, 31, 384–410. [Google Scholar] [CrossRef]
- Stott, D.J.; Lowe, G.D. Cardiovascular Disease and Health in the Older Patient: Expanded from’Pathy’s Principles and Practice of Geriatric Medicine; John Wiley & Sons, 2012.
- Petoumenos, K.; Reiss, P.; Ryom, L.; Rickenbach, M.; Sabin, C.; El-Sadr, W.; d’Arminio Monforte, A.; Phillips, A.N.; De Wit, S.; Kirk, O.; others. Increased risk of cardiovascular disease (CVD) with age in HIV-positive men: a comparison of the D: A: D CVD risk equation and general population CVD risk equations. HIV medicine 2014, 15, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, G.; Malagoli, A.; Calcagno, A.; Mussi, C.; Celesia, B.; Carli, F.; Piconi, S.; De Socio, G.; Cattelan, A.; Orofino, G.; others. The increasing burden and complexity of multi-morbidity and polypharmacy in geriatric HIV patients: a cross sectional study of people aged 65–74 years and more than 75 years. BMC geriatrics 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aberg, J.A. Aging and HIV infection: focus on cardiovascular disease risk. Topics in Antiviral Medicine 2020, 27, 102. [Google Scholar]
- Hammer, S.M.; Saag, M.S.; Schechter, M.; Montaner, J.S.; Schooley, R.T.; Jacobsen, D.M.; Thompson, M.A.; Carpenter, C.C.; Fischl, M.A.; Gazzard, B.G.; others. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society–USA panel. Jama 2006, 296, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Cockerham, L.; Scherzer, R.; Zolopa, A.; Rimland, D.; Lewis, C.E.; Bacchetti, P.; Grunfeld, C.; Shlipak, M.; Tien, P.C. Association of HIV infection, demographic and cardiovascular risk factors with all-cause mortality in the recent HAART era. JAIDS Journal of Acquired Immune Deficiency Syndromes 2010, 53, 102–106. [Google Scholar] [CrossRef]
- Hanna, D.B.; Ramaswamy, C.; Kaplan, R.C.; Kizer, J.R.; Anastos, K.; Daskalakis, D.; Zimmerman, R.; Braunstein, S.L. Trends in cardiovascular disease mortality among persons with HIV in New York City, 2001–2012. Clinical Infectious Diseases 2016, 63, 1122–1129. [Google Scholar] [CrossRef]
- Petoumenos, K.; Worm, S.; Reiss, P.; De Wit, S.; d’Arminio Monforte, A.; Sabin, C.; Friis-Møller, N.; Weber, R.; Mercie, P.; Pradier, C.; others. Rates of cardiovascular disease following smoking cessation in patients with HIV infection: results from the D: A: D study. HIV medicine 2011, 12, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.L.; Martins-Silva, R.; Kaizeler, A.; Saraiva-Agostinho, N.; Barbosa-Morais, N.L. voyAGEr: free web interface for the analysis of age-related gene expression alterations in human tissues 2022. [CrossRef]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; others. The genotype-tissue expression (GTEx) project. Nature genetics 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Guzz, P.H.; Lomoio, U.; Veltri, P. GTExVisualizer: a web platform for supporting ageing studies. Bioinformatics 2023, 39, btad303. [Google Scholar] [CrossRef]
- Vos, W.A.; Groenendijk, A.L.; Blaauw, M.J.; Van Eekeren, L.E.; Navas, A.; Cleophas, M.C.; Vadaq, N.; Matzaraki, V.; Dos Santos, J.C.; Meeder, E.M.; others. The 2000HIV study: Design, multi-omics methods and participant characteristics. Frontiers in immunology 2022, 13, 982746. [Google Scholar] [CrossRef]
- Palshikar, M.G.; Palli, R.; Tyrell, A.; Maggirwar, S.; Schifitto, G.; Singh, M.V.; Thakar, J. Executable models of immune signaling pathways in HIV-associated atherosclerosis. NPJ systems biology and applications 2022, 8, 35. [Google Scholar] [CrossRef]
- Kundu, S.; Freiberg, M.S.; Tracy, R.P.; So-Armah, K.A.; Koethe, J.R.; Duncan, M.S.; Tindle, H.A.; Beckman, J.A.; Feinstein, M.J.; McDonnell, W.J.; others. Circulating T cells and cardiovascular risk in people with and without HIV infection. Journal of the American College of Cardiology 2022, 80, 1633–1644. [Google Scholar] [CrossRef]
- Tsukamoto, T. HIV accelerates clonal hematopoiesis and cardiovascular aging. AIDS 2022, 36, 1599–1601. [Google Scholar] [CrossRef]
- Villanueva-Millán, M.J.; Pérez-Matute, P.; Recio-Fernández, E.; Lezana Rosales, J.M.; Oteo, J.A. Characterization of gut microbiota composition in HIV-infected patients with metabolic syndrome. Journal of physiology and biochemistry 2019, 75, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Babu, H.; Ambikan, A.T.; Gabriel, E.E.; Svensson Akusjärvi, S.; Palaniappan, A.N.; Cheedarla, N.; Hanna, L.E.; Neogi, U. Systemic inflammation and the increased risk of inflamm-aging and age-associated diseases in people living with HIV on long term suppressive antiretroviral therapy. Frontiers in immunology 2019, 10, 474418. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Chuffa, L.G.; Freire, P.P.; dos Santos Souza, J.; de Mello, M.C.; de Oliveira Neto, M.; Carvalho, R.F. Aging whole blood transcriptome reveals candidate genes for SARS-CoV-2-related vascular and immune alterations. Journal of Molecular Medicine 2022, 100, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.P.; Seet, R.C.S.; Kennedy, B.K. Does eNOS derived nitric oxide protect the young from severe COVID-19 complications? Ageing Research Reviews 2020, 64, 101201. [Google Scholar] [CrossRef] [PubMed]
- Nicin, L.; Abplanalp, W.T.; Mellentin, H.; Kattih, B.; Tombor, L.; John, D.; Schmitto, J.D.; Heineke, J.; Emrich, F.; Arsalan, M.; others. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. European heart journal 2020, 41, 1804–1806. [Google Scholar] [CrossRef]
- Headley, C.A.; Gerberick, A.; Mehta, S.; Wu, Q.; Yu, L.; Fadda, P.; Khan, M.; Ganesan, L.P.; Turner, J.; Rajaram, M.V. Nontuberculous mycobacterium M. avium infection predisposes aged mice to cardiac abnormalities and inflammation. Aging Cell 2019, 18, e12926. [Google Scholar] [CrossRef] [PubMed]
- Ben Haij, N.; Planès, R.; Leghmari, K.; Serrero, M.; Delobel, P.; Izopet, J.; BenMohamed, L.; Bahraoui, E. HIV-1 Tat protein induces production of proinflammatory cytokines by human dendritic cells and monocytes/macrophages through engagement of TLR4-MD2-CD14 complex and activation of NF-κB pathway. PloS one 2015, 10, e0129425. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.C.; Stevenson, M.; Latz, E.; Urcuqui-Inchima, S. HIV type 1 infection up-regulates TLR2 and TLR4 expression and function in vivo and in vitro. AIDS research and human retroviruses 2012, 28, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.C.; Arteaga, J.; Paul, S.; Kumar, A.; Latz, E.; Urcuqui-Inchima, S. Up-regulation of TLR2 and TLR4 in dendritic cells in response to HIV type 1 and coinfection with opportunistic pathogens. AIDS research and human retroviruses 2011, 27, 1099–1109. [Google Scholar] [CrossRef]
- Koval, T.I.; Syzova, L.M.; Dubynska, H.M.; Pryimenko, N.O.; Rudenko, S.S.; Marchenko, O.H.; Shlykova, O. TLR4 and TLR7 genetic polymorphism in patients with HIV/HCV-coinfection: prevalence and gender-related features 2018.
- Yong, Y.K.; Shankar, E.M.; Solomon, A.; Spelman, T.; Fairley, C.K.; Elliott, J.H.; Hoy, J.; Cameron, P.U.; Kamarulzaman, A.; Lewin, S.R. Polymorphisms in the CD14 and TLR4 genes independently predict CD4+ T-cell recovery in HIV-infected individuals on antiretroviral therapy. Aids 2016, 30, 2159–2168. [Google Scholar] [CrossRef]
- Said, E.; Al-Yafei, F.; Zadjali, F.; Al-Balushi, M.; Hasson, S.; Al-Mahroqi, S.; Koh, C.; Al-Naamani, K.; Al-Busaidi, J.; Idris, M.; others. Frequency of TLR4 (1063A/G and 1363C/T) polymorphisms in healthy and HIV-infected Omani individuals and their relationship to viral load and T cell count. Genet Mol Res 2016, 15, 10–4238. [Google Scholar] [CrossRef] [PubMed]
- Vidyant, S.; Chatterjee, A.; Dhole, T. A single-nucleotide polymorphism in TLR4 is linked with the risk of HIV-1 infection. British journal of biomedical science 2019, 76, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Tarancon-Diez, L.; De Pablo-Bernal, R.S.; Jiménez, J.L.; Álvarez-Ríos, A.I.; Genebat, M.; Rosado-Sánchez, I.; Muñoz-Fernández, M.Á.; Ruiz-Mateos, E.; Leal, M. Role of toll-like receptor 4 Asp299Gly polymorphism in the development of cardiovascular diseases in HIV-infected patients. AIDS 2018, 32, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Grande, F.; Occhiuzzi, M.A.; Rizzuti, B.; Ioele, G.; De Luca, M.; Tucci, P.; Svicher, V.; Aquaro, S.; Garofalo, A. CCR5/CXCR4 dual antagonism for the improvement of HIV infection therapy. Molecules 2019, 24, 550. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.A.; Justement, S.J.; Catanzaro, A.; Hallahan, C.A.; Ehler, L.A.; Mizell, S.B.; Kumar, P.N.; Mican, J.A.; Chun, T.W.; Fauci, A.S. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. The Journal of Immunology 1998, 161, 3195–3201. [Google Scholar] [CrossRef]
- De Clercq, E.; Schols, D. Inhibition of HIV infection by CXCR4 and CCR5 chemokine receptor antagonists. Antiviral chemistry & chemotherapy 2001, 12, 19–31. [Google Scholar]
- Choi, W.T.; Kumar, S.; Madani, N.; Han, X.; Tian, S.; Dong, C.Z.; Liu, D.; Duggineni, S.; Yuan, J.; Sodroski, J.G.; others. A novel synthetic bivalent ligand to probe chemokine receptor CXCR4 dimerization and inhibit HIV-1 entry. Biochemistry 2012, 51, 7078–7086. [Google Scholar] [CrossRef]
- Xu, Y.; Duggineni, S.; Espitia, S.; Richman, D.D.; An, J.; Huang, Z. A synthetic bivalent ligand of CXCR4 inhibits HIV infection. Biochemical and biophysical research communications 2013, 435, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Doranz, B.J.; Grovit-Ferbas, K.; Sharron, M.P.; Mao, S.H.; Goetz, M.B.; Daar, E.S.; Doms, R.W.; O’Brien, W.A. A small-molecule inhibitor directed against the chemokine receptor CXCR4 prevents its use as an HIV-1 coreceptor. The Journal of experimental medicine 1997, 186, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Petersen, D.C.; Glashoff, R.H.; Shrestha, S.; Bergeron, J.; Laten, A.; Gold, B.; van Rensburg, E.J.; Dean, M.; Hayes, V.M. Risk for HIV-1 infection associated with a common CXCL12 (SDF1) polymorphism and CXCR4 variation in an African population. JAIDS Journal of Acquired Immune Deficiency Syndromes 2005, 40, 521–526. [Google Scholar] [CrossRef]
- Crater, J.M.; Nixon, D.F.; Furler O’Brien, R.L. HIV-1 replication and latency are balanced by mTOR-driven cell metabolism. Frontiers in Cellular and Infection Microbiology 2022, 12, 1068436. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Fagone, P.; Meroni, P.; McCubrey, J.; Bendtzen, K. mTOR as a multifunctional therapeutic target in HIV infection. Drug discovery today 2011, 16, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, K.; Verma, A.; Rathi, B. An Integrative Bioinformatics Analysis for Identifying Hub Genes in Human Immunodeficiency Virus. Current Trends in Biotechnology and Pharmacy 2021, 15, 67–79. [Google Scholar]
- Gerena, Y.; Skolasky, R.L.; Velez, J.M.; Toro-Nieves, D.; Mayo, R.; Nath, A.; Wojna, V. Soluble and cell-associated insulin receptor dysfunction correlates with severity of HAND in HIV-infected women. PLoS One 2012, 7, e37358. [Google Scholar] [CrossRef] [PubMed]
- Pedro, M.N.; Rocha, G.Z.; Guadagnini, D.; Santos, A.; Magro, D.O.; Assalin, H.B.; Oliveira, A.G.; Pedro, R.d.J.; Saad, M.J. Insulin resistance in HIV-patients: causes and consequences. Frontiers in endocrinology 2018, 9, 514. [Google Scholar] [CrossRef]
- Djedaini, M.; Peraldi, P.; Drici, M.D.; Darini, C.; Saint-Marc, P.; Dani, C.; Ladoux, A. Lopinavir co-induces insulin resistance and ER stress in human adipocytes. Biochemical and biophysical research communications 2009, 386, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Ismail, W.I.W.; King, J.A.; Anwar, K.; Pillay, T.S. Indinavir and nelfinavir inhibit proximal insulin receptor signaling and salicylate abrogates inhibition: potential role of the NFkappa B pathway. Journal of Cellular Biochemistry 2013, 114, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Liu, K.; Hamblin, M.; Lasky, J.A.; Agrawal, K.C. Nelfinavir suppresses insulin signaling and nitric oxide production by human aortic endothelial cells: protective effects of thiazolidinediones. Ochsner Journal 2013, 13, 76–90. [Google Scholar]
- Boekholdt, S.M.; Agema, W.R.; Peters, R.J.; Zwinderman, A.H.; van der Wall, E.E.; Reitsma, P.H.; Kastelein, J.J.; Jukema, J.W. Variants of toll-like receptor 4 modify the efficacy of statin therapy and the risk of cardiovascular events. Circulation 2003, 107, 2416–2421. [Google Scholar] [CrossRef]
- Li, T.; Jing, J.; Dong, N.; Liu, X.; Ma, C.; Yang, J. TLR4 rs1927914 polymorphism contributes to serum TLR4 levels in patients with aortic aneurysm. Experimental and Molecular Pathology 2021, 119, 104609. [Google Scholar] [CrossRef]
- Li, T.; Liu, X.; Ning, H.; Li, X.; Yang, J.; Ma, C.; others. Association of Toll-like receptor 4 gene polymorphisms with acute aortic dissection in a Chinese Han population. BioMed Research International 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jing, J.; Sun, L.; Jiang, B.; Xin, S.; Yang, J.; Yuan, Y. TLR4 and MMP2 polymorphisms and their associations with cardiovascular risk factors in susceptibility to aortic aneurysmal diseases. Bioscience Reports 2019, 39, BSR20181591. [Google Scholar] [CrossRef] [PubMed]
- Kutikhin, A.G.; Ponasenko, A.V.; Khutornaya, M.V.; Yuzhalin, A.E.; Zhidkova, I.I.; Salakhov, R.R.; Golovkin, A.S.; Barbarash, O.L.; Barbarash, L.S. Association of TLR and TREM-1 gene polymorphisms with atherosclerosis severity in a Russian population. Meta Gene 2016, 9, 76–89. [Google Scholar] [CrossRef]
- Džumhur, A.; Zibar, L.; Wagner, J.; Šimundić, T.; Dembić, Z.; Barbić, J. Association studies of gene polymorphisms in toll-like receptors 2 and 4 in Croatian patients with acute myocardial infarction. Scandinavian journal of immunology 2012, 75, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tao, T.; Wang, H.; Zhao, H.; Lu, L.; Wu, F. Arterial thrombosis is accompanied by elevated mitogen-activated protein kinase (MAPK) and cyclooxygenase-2 (COX-2) expression via toll-like receptor 4 (TLR-4) activation by S100A8/A9. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 2018, 24, 7673. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Marullo, A.G.; Madonna, M.; Cavarretta, E.; Allegra, A.; Cesarini, V.; Iaccarino, A.; Schiavon, S.; Peruzzi, M.; Greco, E.; others. Deregulation of TLR4 signaling pathway characterizes Bicuspid Aortic valve syndrome. Scientific Reports 2019, 9, 11028. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Aiqun, M.; Jiwu, L.; Ping, Z. TLR3 and TLR4 as potential clinical biomarkers for in-stent restenosis in drug-eluting stents patients. Immunologic research 2016, 64, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Satoh, S.; Yada, R.; Inoue, H.; Omura, S.; Ejima, E.; Mori, T.; Takenaka, K.; Kawamura, N.; Numaguchi, K.; Mori, E.; others. Toll-like receptor-4 is upregulated in plaque debris of patients with acute coronary syndrome more than Toll-like receptor-2. Heart and Vessels 2016, 31, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, Y.; Bellien, J.; Armengol, G.; Brakenhielm, E.; Adriouch, S.; Iacob, M.; Remy-Jouet, I.; Le Cam-Duchez, V.; Monteil, C.; Renet, S.; others. Role of Toll-like receptors 2 and 4 in mediating endothelial dysfunction and arterial remodeling in primary arterial antiphospholipid syndrome. Arthritis & rheumatology 2014, 66, 3210–3220. [Google Scholar]
- Katoh, S.; Honda, S.; Watanabe, T.; Suzuki, S.; Ishino, M.; Kitahara, T.; Funayama, A.; Netsu, S.; Sasaki, T.; Shishido, T.; others. Atrial endothelial impairment through Toll-like receptor 4 signaling causes atrial thrombogenesis. Heart and vessels 2014, 29, 263–272. [Google Scholar] [CrossRef]
- Wyss, C.A.; Neidhart, M.; Altwegg, L.; Spanaus, K.S.; Yonekawa, K.; Wischnewsky, M.B.; Corti, R.; Kucher, N.; Roffi, M.; Eberli, F.R.; others. Cellular actors, Toll-like receptors, and local cytokine profile in acute coronary syndromes. European heart journal 2010, 31, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Cianflone, D.; Vecchio, V.; Banfi, M.; Vermi, A.C.; De Metrio, M.; Grigore, L.; Pellegatta, F.; Pirillo, A.; Garlaschelli, K.; others. Effector memory T cells are associated with atherosclerosis in humans and animal models. Journal of the American Heart Association 2012, 1, e000125. [Google Scholar] [CrossRef]
- Kashiwagi, M.; Imanishi, T.; Ozaki, Y.; Satogami, K.; Masuno, T.; Wada, T.; Nakatani, Y.; Ishibashi, K.; Komukai, K.; Tanimoto, T.; others. Differential expression of Toll-like receptor 4 and human monocyte subsets in acute myocardial infarction. Atherosclerosis 2012, 221, 249–253. [Google Scholar] [CrossRef]
- Sheu, J.J.; Chang, L.T.; Chiang, C.H.; Youssef, A.A.; Wu, C.J.; Lee, F.Y.; Yip, H.K. Prognostic value of activated toll-like receptor-4 in monocytes following acute myocardial infarction. International Heart Journal 2008, 49, 1–11. [Google Scholar] [CrossRef]
- Satoh, M.; Shimoda, Y.; Maesawa, C.; Akatsu, T.; Ishikawa, Y.; Minami, Y.; Hiramori, K.; Nakamura, M. Activated toll-like receptor 4 in monocytes is associated with heart failure after acute myocardial infarction. International journal of cardiology 2006, 109, 226–234. [Google Scholar] [CrossRef]
- Rath, D.; Chatterjee, M.; Borst, O.; Müller, K.; Langer, H.; Mack, A.; Schwab, M.; Winter, S.; Gawaz, M.; Geisler, T. Platelet surface expression of stromal cell–derived factor-1 receptors CXCR4 and CXCR7 is associated with clinical outcomes in patients with coronary artery disease. Journal of Thrombosis and Haemostasis 2015, 13, 719–728. [Google Scholar] [CrossRef]
- Klingenberg, R.; Stähli, B.E.; Heg, D.; Denegri, A.; Manka, R.; Kapos, I.; von Eckardstein, A.; Carballo, D.; Hamm, C.W.; Vietheer, J.; others. Controlled-Level EVERolimus in Acute Coronary Syndrome (CLEVER-ACS)-A phase II, randomized, double-blind, multi-center, placebo-controlled trial. American Heart Journal 2022, 247, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Samimi, Z.; Izadpanah, A.; Feizollahi, P.; Roghani, S.A.; Assar, S.; Zafari, P.; Taghadosi, M. The association between the plasma sugar and lipid profile with the gene expression of the regulatory protein of mTOR (Raptor) in patients with rheumatoid arthritis. Immunological investigations 2021, 50, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Marushchak, M.; Hevko, U.; Krynytska, I. Insulin receptor substrate 1 gene variations and lipid profile characteristics in the type 2 diabetic patients with comorbid obesity and chronic pancreatitis. Endocrine Regulations 2022, 56, 1–9. [Google Scholar] [CrossRef]
- Dziwura, J.; Pełka-Lalik, B.; Widecka, K. The effect of dietary salt load on insulin resistance in hypertensive patients with the Gly972Arg polymorphism of the insulin receptor substrate 1 (IRS-1) gene. Arterial Hypertension 2007, 11, 12–20. [Google Scholar]
- Marini, M.A.; Frontoni, S.; Mineo, D.; Bracaglia, D.; Cardellini, M.; De Nicolais, P.; Baroni, A.; D’Alfonso, R.; Perna, M.; Lauro, D.; others. The Arg972 variant in insulin receptor substrate-1 is associated with an atherogenic profile in offspring of type 2 diabetic patients. The Journal of Clinical Endocrinology & Metabolism 2003, 88, 3368–3371. [Google Scholar]
- Collaboration, H.C.; others. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. Aids 2010, 24, 123–137. [Google Scholar]
- Triant, V.A.; Lee, H.; Hadigan, C.; Grinspoon, S.K. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. The Journal of Clinical Endocrinology & Metabolism 2007, 92, 2506–2512. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).