1. Introduction
Early glottic cancer (T1-T2, N0/Stage I-II) shows favorable outcome after radiotherapy, open surgery or transoral resection [
1,
2]. Therefore, factors influencing the choice of treatment are preferences of doctors and patients, availability of resources, adverse effects and the functional outcome. Since implementation of laser-assistance in microsurgery of the vocal folds, the percentage of open approach in early glottic cancer decreased significantly [
3]. Hence, risks and results of open partial laryngectomy including open cordectomy are difficult to determine in view of declining case-numbers per center. Indications for open approach in early glottic cancer are problems to reach the anterior border of the tumor or the anterior commissure by transoral approach. A unique advantage of open cordectomy is the possibility for direct reconstruction of the vocal fold to restore the voice, e.g. by false cord flaps [
4]. As the switch from open to endoscopic approach proceeded in a short time period long ago, up-to-date comparative results of both approaches are missing [
5]. Here, we describe current results of survival outcome, voice parameters and perioperative complications in patients who underwent microscopic laser-assisted resection or open cordectomy in early glottic cancer.
2. Materials and Methods
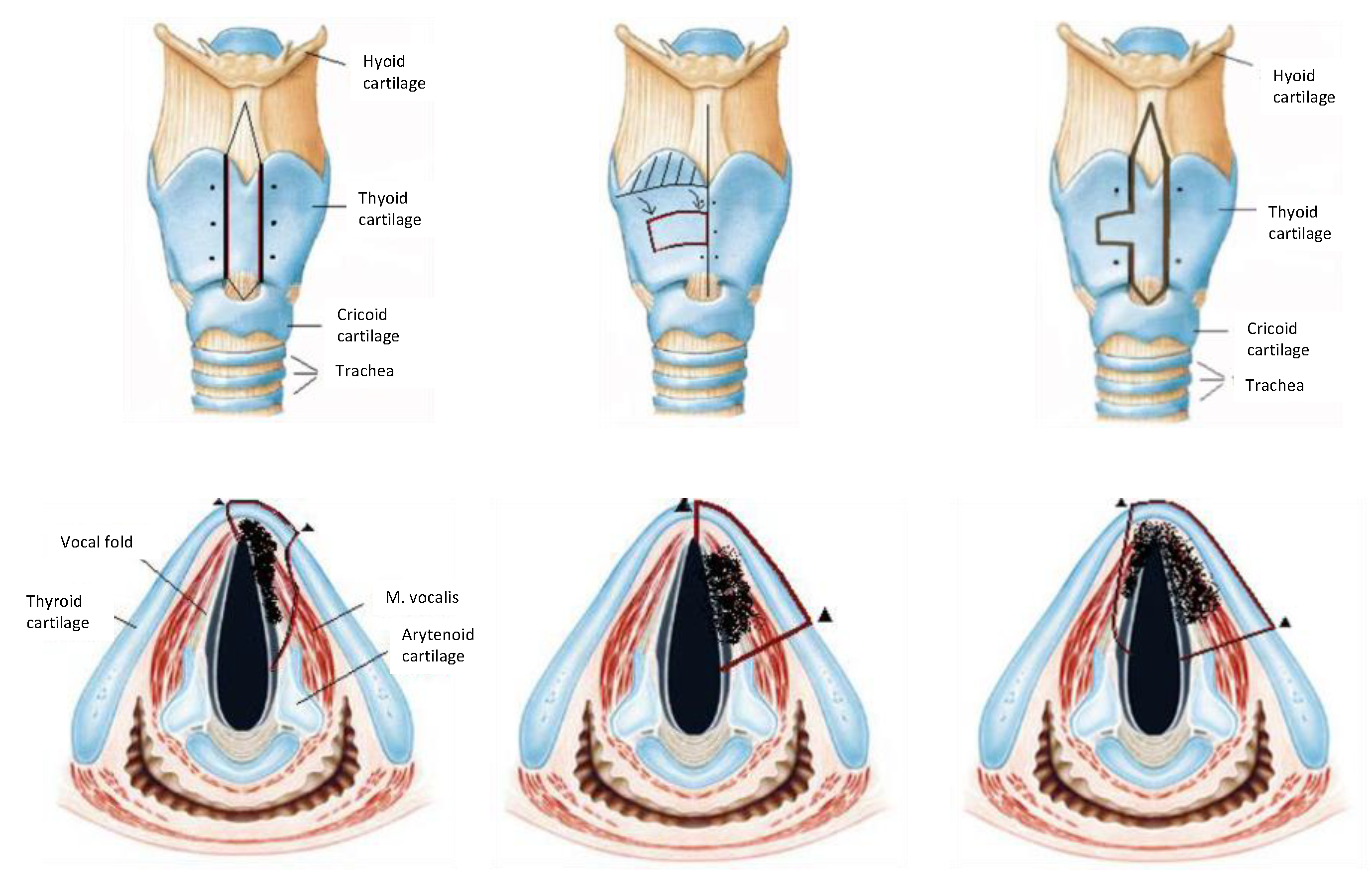
We treated 238 patients with early glottic cancer in the Department of Otorhinolaryngology and Head and Neck surgery in a tertiary care university medical center from 2003 to 2013. 219 patients were treated by surgery, 19 patients (8.0%) had radiotherapy and were excluded from this study. Biopsy was taken during rigid triple endoscopy for definitive diagnosis, accessibility for transoral approach and exclusion of second primary tumor. Nodal involvement as well as exclusion of paraglottic or thyroid cartilage infiltration was excluded by computed tomography. The approach selection before surgery was mainly upon surgeons’ preference if clear margins could be achieved by transoral approach. Hereby a clear preference for laser-assisted microscopic surgery was made, conversely—open cordectomy was the treatment of second choice (thus both groups are not balanced). Patients above 75 years were not offered open cordectomy in knowledge of more intra- and postoperative risks. Open cordectomy was performed via laryngofissure and optional small volume resection of the thyroid cartilage as a fronto-anterior or frontolateral techniques (
Figure 1) [
6]. Tracheostomy was performed in all cases. Intraoperative reconstruction of the glottic area was performed by mucosal or combined muscular-mucosal flaps, mostly rotation flaps from the false chord [
7].
We collected the following data from patient files: biometry and epidemiology (age, gender, survival) treatment and side-effects (approach, immediate complications, duration of hospital stay, need for revision surgery within 6 months, need for tracheotomy and/or feeding tube) and voice parameters (perception, aerodynamics, acoustic parameters, and self-evaluation before and after therapy). Survival data were analyzed by the Kaplan Meyer method and two-sided log rank-testing. Biometric data, side-effects and voice parameters were compared by non-parametric testing using SPSS (version 28.0.0.0), significance was considered p≤ .05 for all tests. The patients have been informed about participation in the study and have given their written informed consent. The study was approved by local ethics committee of the University of Giessen (protocol code 95/15).
3. Results
219 patients with early glottic cancer were included. Patients had a mean age of 66.1 years (range 29-90 years,
Table 1). 70.5% of the patients (n=168) received transoral laser-assisted microscopic surgery (TLM), 51 patients (21.4%) underwent transcervical open cordectomy. Patients who received endoscopic surgery were on average older (p=0.003). The mean follow-up time was 40.5 months.
The Chi square test was used to analyze the correlation between tumor stage (T1a vs. T1b and T2) and the applied surgical method. We found a statistically significant association between stage and surgical method (p<0.001).
3.1. Oncological Outcome
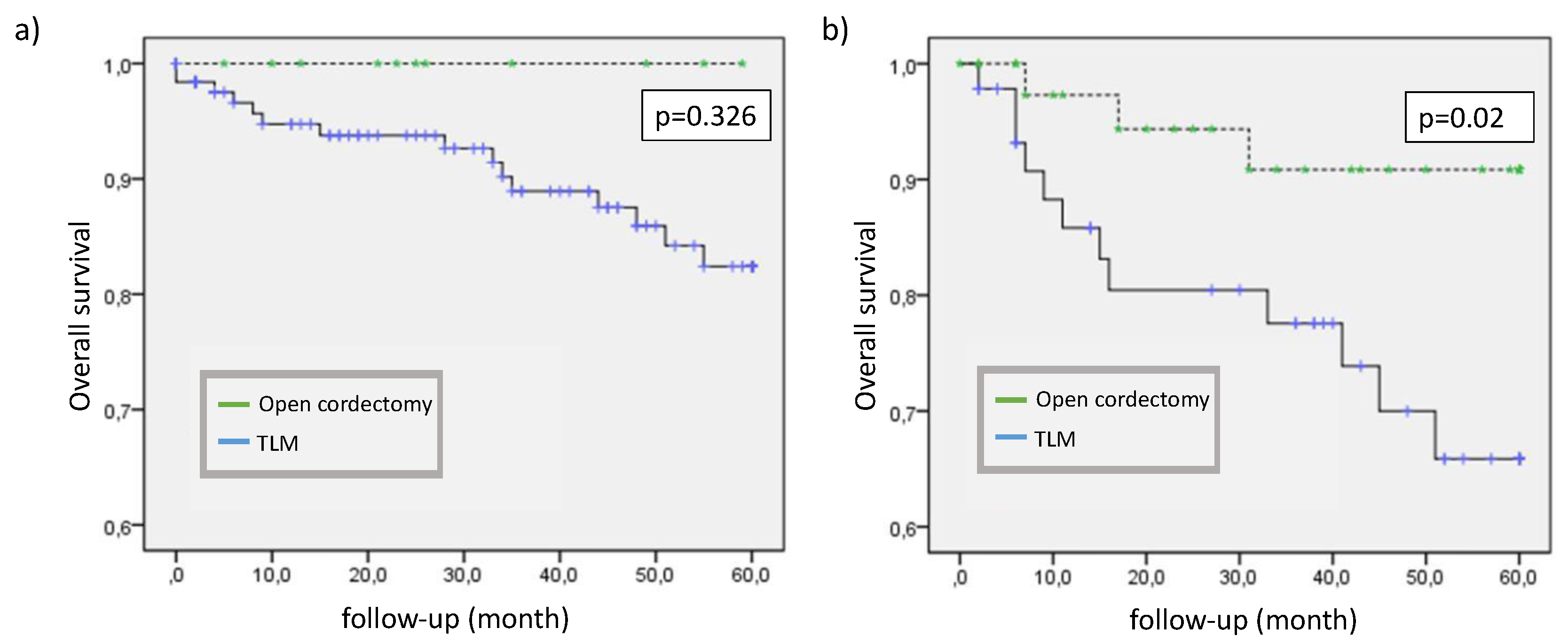
The 2 years overall survival (OS) after endoscopic surgery was 90.5% vs. 95.3% in the transcervically resected group compared with the group treated by open surgery. The 5 years OS was 77.9% vs. 92.0%, respectively. When patients were stratified by TNM category, the 2 years overall survival in T1b and T2 tumors (88.2%) was as expected worse than in T1a tumors (94.2%). Within the T1b/T2 group, patients had a survival benefit from open surgery (p=0.02) while we could not verify a clear difference within T1a patients (p=0.326) (
Figure 2).
Recurrence was found in 21 (9.6%) patients of the total cohort during follow up: 15 (8.9%) patients from the endoscopic surgery group, 6 (11.7%) patients from the external surgery group.
Organ preservation was possible in 98% of the endoscopically treated patient and in 94% of the transcervically resected tumors.
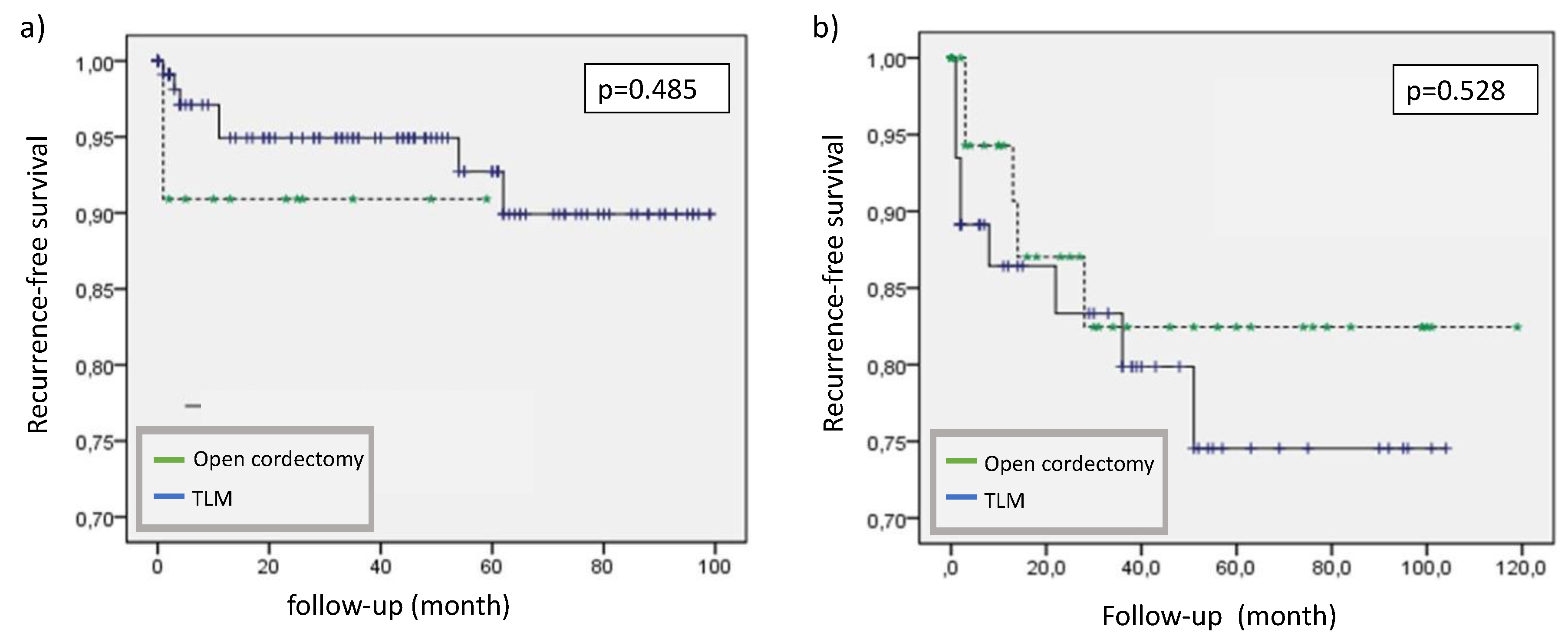
There was a statistically significant difference in recurrence free survival (RFS) within the T1a and T1b/T2 group (p=0.006) but no relevant difference between the two surgical modalities even when the data was stratified by T-categories performing a Cox regression (hazard ratio 1.21, p=0.712).
Figure 3.
Recurrence-free survival stratified by tumor stage and surgical modality (a) T1a, b) T1b/T2), (TLM=transoral laser-assisted microscopic surgery).
Figure 3.
Recurrence-free survival stratified by tumor stage and surgical modality (a) T1a, b) T1b/T2), (TLM=transoral laser-assisted microscopic surgery).
3.2. Functional Outcome
As male and female voices are not comparable in variant parameters and due to the small group, we decided to exclude female patients from voice functional outcome analysis. Voice examination was performed in 65 patients, 43 from the TES-group and 22 from ETC group. In general, the pre- and postoperative comparison of ELS protocol functional data indicates a change in vocal performance with mostly deterioration after operation, but differences within the individual data.
The most simple but meaningful aerodynamic parameter is the maximum phonation time in seconds. Analyzing the pre- and postoperative findings in the two surgical groups, there is a decrease from meanly 14 to 11 seconds. This parameter has to be corrected by including the vital capacity and using the phonation quotient (PQ). This examination also shows a relevant difference between pre- and postoperative values (226.1ml/s vs. 265.9ml/s, p=0.001). The acoustic parameters jitter, shimmer, dynamic, frequency range and voice intensity also exhibit a deterioration from pre- to postoperative measurement. In contrast patient`s self-evaluation of voice quality with means of Visual Analogue Scale from 1 to 5 (1=very good, 5= very bad) improved from in the mean 3 to 4 points.
Comparing voice parameters of T1a-tumor patients with the T1b/T2 group, the pre-/postoperative differences in the T1a group are obviously smaller. The maximum phonation time changed from 12 to 11 sec. (mean) in the T1 group (p= 0.021) vs. 20 to 10 sec. (mean) in the T1b/T2 group (p>0.001), the phonation quotient from 233.3 ml/s to 266.25 ml/s in the T1 group (p= 0.114) vs. 184.38 ml/s to 269.23 ml/s in the T1b/T2 group (p=0.002). This data is supported by respective changes in the acoustic parameters, e.g. jitter and shimmer. Comparing the two surgical groups according to their postoperative voice functional outcome, there is no difference in auditory perceptual assessment (hoarseness, breathiness) and only marginal difference in acoustic parameters with a slight but not statistically significant discrepancy in favor of the endoscopically resected group. Patient’s self-evaluation of postoperative voice quality (ranked as above) was 3.0 in the endoscopic group versus 3.25 in the transcervically resected group also without statistical significance (
Table 2).
3.3. Perioperative Course and Complications
Tracheostomy was necessary in 2 patients (1.2%) of the endoscopically resected group while in the open surgery group tracheostomy was performed in 50 patients (98%, p<0.001). Within this group the mean duration of tracheostomy was 9.5 days. Hospitalization was significantly longer in the open surgery group (3 vs. 12.5 days in the mean, p<0.001). A feeding tube was necessary in 10 patients with open resection (19.6%) while none of the endoscopically resected patients needed additional nutrition. Comparing the two groups with regard to peri- and postoperative complications there was no relevant difference concerning infections, especially pneumonia and dysphagia. 7 cases of intra- and postoperative bleeding occurred in the endoscopical group, none in the openly resected patients which could be interpreted as a trend but without statistical significance in Fisher’s exact test (
Table 3).
4. Discussion
Survival after different therapies in early glottis cancer has been examined in multiple studies, resulting in comparable disease specific survival and control rates [
8,
9,
10,
11,
12]. Regarding the surgical options treating small (T1/T2) laryngeal cancer in the last decades the management changed from open partial laryngectomy to endoscopic microsurgical resection, mostly performed with CO2-laser. Nevertheless, this therapeutical way is limited by several facts: at first the laryngeal exposure, but also tumor invasion of the anterior commissure, the paraglottic or pre-epiglottic space or laryngeal framework involvement [
13,
14]. Comparing the two surgical options within the cohort of 219 patients we did not see significant advantages in OS, recurrence or larynx preservation comparing T1a-tumors. As expected, patients with T1b/T2-tumors in general had a significantly worse recurrence-free survival. In this group we found a significantly better OS after open cordectomy. This might be an effect of better intraoperative overview in advanced tumor size.
Regarding 5 years OS rates, no significant difference between patients after open surgical and endoscopic resection could be demonstrated. Nevertheless, the difference between the 5-year overall survival of 77% in the endoscopically resected group and 93% in the openly resected patients shows a trend that could also be seen in other studies [
15,
16,
17,
18]. What could be the reason of such a result or trend? At first age may lead to a bias between the groups. In our cohort, patients in the transorally resected group were older, and this trend has to be expected in general, because an external partial laryngectomy requires a performance status allowing longer operation times than transorally performed cordectomy. This information should also be considered when comparing survival rates, because early glottic cancer is rarely a cause of death. Rather, secondary cancer or general health are factors affecting survival in these patients.
Furthermore, it has to be discussed if transoral resections lead to closer surgical resection margins and therefore less oncological safety than external resections. The prognostic significance of surgical margins in early glottic cancer is not clear yet [
19,
20]. Positive margins are reported in up to 50% of transorally laser resected tumors due to narrow free tissue resulting from anatomical and functional reasons [
21,
22]. An Italian study examined the impact of resection margin status on oncological outcome after laser resection and showed that a positive margin status had a statistically significant prognostic impact on local control and organ preservation, but not on overall survival [
23]. A higher rate of close or positive margins in transoral laser resection compared to open partial laryngectomy may be assumed, but there are no controlled studies covering this fact.
Comparing the T1b/T2 groups there is a significant survival advantage in this study for the patients treated by open surgery. Further studies also showed a higher risk of cartilage infiltration and therefore insufficient transoral resection in T1b tumors [
24]. Infiltration of the anterior commissure seems to be an independent risk factor for tumor recurrence and poorer survival as supported by our data so far [
25,
26,
27].
Laryngeal preservation is also an important factor in assessing treatment options. This study showed organ preservation in 98% of the endoscopically treated patient and in 94% of the transcervically resected tumors without statistical significance most probably due to the low absolute number of laryngectomies. However, clear data concerning this point is lacking in the literature.
According to the peri- and postoperative course and complications the study showed a clear benefit for patients with transorally resected tumors. As expected, the hospitalization time was significantly shorter (mean 3.0 vs. 12.5 days) than in patients with external partial laryngectomy. As country-specific differences due to different health systems play a major role in this point, the absolute count of days is not comparable but the trend is supported by further data in different studies [
5,
15]. As tracheostomy is a common procedure performing a transcervical laryngeal resection it is obvious that the number of tracheostomies is significantly higher in the transcervically resected group (98% vs. 1.2%). As was also expected, there was a higher number of patients needing a postoperative feeding tube. This may be caused by the fact that a feeding tube is sometimes used preventively with regard to wound infections. In general, peri- and postoperative complications did not differ between the two groups regarding wound infections, dysphagia or pneumonia. We saw a trend of more bleeding complications in the endoscopically resected group but without statistical significance.
Concerning the voice functional outcome after therapy of laryngeal carcinoma it seems to be difficult to foresee voice results e.g. by simply comparing tissue defects. In general, there is a dependency on the type and extent of the surgical procedure, but this relationship is not linear and depends not only on the type of cordectomy [
28,
29] but also, for example, on the region affected. Patients treated by radiotherapy don’t have a voice functional advantage even though the tissue defect is hardly as big as in resected tumors [
30,
31]. Many different parameters affect the voice functional result and one important parameter is the infiltration of anterior commissure.
The measurement of voice functional outcome in this examination differs in subjective and objective parameters. As it was seen before in laryngological studies objective and subjective measurements are not always congruent [
32,
33,
34,
35,
36]. The influence of the resection itself on the voice results therefore showed this discrepancy in both treatment variations: while the majority of the objectively measured parameters was worsened by the operation, a rather positive trend occurred in the self-assessment of the patients (VAS median preoperative 4 to median postoperative 3).
As mentioned before, the resection of bilateral laryngeal tumors (T1b, T2) tends to deteriorate voice function more than unilateral resections. This is mainly caused by affection of the integrity of the vocal tract by destroying the anterior commissure, which has a key role in the voice functional result [
37,
38]. Therefore -as expected- comparing the voice functional outcome within the tumor stages, the study showed a clear difference concerning especially the acoustic measurements, but also aerodynamic parameters as the maximum phonation time. In all measurements the pre-/postoperative differences in the T1a group were obviously smaller than in the T1b/T2 group. This fact is supported by other studies on similar issues, which showed the dependency of voice functional outcome on type of cordectomy [
28,
29,
39].
Analyzing the postoperative voice functional results of the two different surgical groups, the study did not show significant differences between transcervically resected and endoscopically operated patients. There was a non-statistically proven trend to poorer median acoustic parameters in the transcervically resected group. Due to the strongly overlapping distribution of values, no statistical significance can be determined. Literature research shows similar results of related research projects [
22,
23].
5. Conclusions
In the last decades, transthyreoidal resection of laryngeal tumors has been almost completely replaced by endoscopic laser resections, not at least because the operative technique is easier to perform and patient’s morbidity seems to be lower. As there are still indications for the open resection, there is a need to indicate what selection criteria have to lead to an open resection and if this operation technique can be performed with regard to oncological safety and voice functional outcome.
Our study is limited by its observational nature and the single center experience. Nevertheless, the present data shows that the oncological outcome is better in T1b/T2 tumors openly resected. The oncological outcome regarding overall survival, recurrence, or larynx preservation in T1a tumors did not differ between the surgical options.
With regard to voice functional outcome we did not find any significant differences but a trend towards poorer acoustic parameters in the openly resected group. Future confirmative analyses of single voice functional parameters could help to confirm this trend.
In conclusion, the present data show that open surgery in laryngeal cancer offer comparable oncological and voice functional results to endoscopic surgical procedures at the cost of a higher acute morbidity. We therefore conclude that open laryngeal resection is a valid treatment option even when transoral endoscopic resection is not possible.
Author Contributions
Conceptualization: Langer, C, Glatt, F and Wittekindt C; methodology: Langer, C, Glatt, F. and Wittekindt C.; validation:Wagner, S., Sharma, SJ.,.; formal analysis: Wagner, S.; Glatt, F writing—original draft preparation: Langer, C, Wittekindt C.; writing—review and editing: Wagner, S. Sharma SJ and Knitschke M; visualization: Glatt, F.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Justus-Liebig-University of Giessen (protocol code 95/15, 19.10.2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are not available due to ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Warner L, Chudasama J, Kelly CG, et al. Radiotherapy versus open surgery versus endolaryngeal surgery (with or without laser) for early laryngeal squamous cell cancer. Cochrane Database Syst Rev. 2014, 2014, CD002027. Published 2014 Dec 12.
- Arboleda LPA, Neves AB, Kohler HF, Vartanian JG, Candelária LM, Borges MF, Fernandes GA, de Carvalho GB, Kowalski LP, Brennan P, Santos Silva AR, Curado MP. Overview of glottic laryngeal cancer treatment recommendation changes in the NCCN guidelines from 2011 to 2022. Cancer Rep (Hoboken). 2023, 6, e1837, Epub 2023 Jun 7. [CrossRef] [PubMed] [PubMed Central]
- Thomas L, Drinnan M, Natesh B, Mehanna H, Jones T, Paleri V. Open conservation partial laryngectomy for laryngeal cancer: a systematic review of English language literature. Cancer Treat Rev. 2012 May;38, 203-11 Epub 2011 Jul 20. [CrossRef] [PubMed]
- Luna-Ortiz K, Campos-Ramos E, Villavicencio-Valencia V, Contreras-Buendía M, Pasche P, Gómez AH. Vertical partial hemilaryngectomy with reconstruction by false cord imbrication. ANZ J Surg. 2010, 80, 358–363. [Google Scholar] [CrossRef] [PubMed]
- de Campora E, Radici M, de Campora L. External versus endoscopic approach in the surgical treatment of glottic cancer. Eur Arch Otorhinolaryngol. 2001, 258, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Glatt, F. Onkologisches und funktionelles Outcome nach Therapie des frühen Stimmlippenkarzinoms. Inauguraldissertation des Fachbereichs Medizin der Justus Liebig Universität Giessen. Giessen, 2018.
- Arens, C.; Schwemmle, C.; Voigt-Zimmermann, S. [Surgical reconstruction in laryngeal carcinoma]. HNO 2020, 68, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Elicin, O.; Giger, R. Comparison of Current Surgical and Non-Surgical Treatment Strategies for Early and Locally Advanced Stage Glottic Laryngeal Cancer and Their Outcome. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Higgins, K.M.; Shah, M.D.; Ogaick, M.J.; Enepekides, D. Treatment of early-stage glottic cancer: meta-analysis comparison of laser excision versus radiotherapy. J Otolaryngol Head Neck Surg 2009, 38, 603–612. [Google Scholar] [PubMed]
- Kujath, M.; Kerr, P.; Myers, C.; Bammeke, F.; Lambert, P.; Cooke, A.; Sutherland, D. Functional outcomes and laryngectomy-free survival after transoral CO(2) laser microsurgery for stage 1 and 2 glottic carcinoma. J Otolaryngol Head Neck Surg 2011, 40 Suppl 1, S49–58. [Google Scholar]
- Vaculik, M.F.; MacKay, C.A.; Taylor, S.M.; Trites, J.R.B.; Hart, R.D.; Rigby, M.H. Systematic review and meta-analysis of T1 glottic cancer outcomes comparing CO2 transoral laser microsurgery and radiotherapy. J Otolaryngol Head Neck Surg 2019, 48, 44. [Google Scholar] [CrossRef] [PubMed]
- Campo F, Zocchi J, Ralli M, De Seta D, Russo FY, Angeletti D, Minni A, Greco A, Pellini R, de Vincentiis M. Laser Microsurgery Versus Radiotherapy Versus Open Partial Laryngectomy for T2 Laryngeal Carcinoma: A Systematic Review of Oncological Outcomes. Ear Nose Throat J. 2021 Feb;100(1_suppl):51S-58S. [CrossRef] [PubMed]
- Peretti, G.; Piazza, C.; Mora, F.; Garofolo, S.; Guastini, L. Reasonable limits for transoral laser microsurgery in laryngeal cancer. Curr Opin Otolaryngol Head Neck Surg 2016, 24, 135–139. [Google Scholar] [CrossRef]
- Jacobi C, Freundorfer R, Reiter M. Transoral laser microsurgery in early glottic cancer involving the anterior commissure. Eur Arch Otorhinolaryngol. 2019 Mar;276, 837-845. Epub 2019 Jan 2. [CrossRef] [PubMed]
- Milovanovic, J.; Jotic, A.; Djukic, V.; Pavlovic, B.; Trivic, A.; Krejovic-Trivic, S.; Milovanovic, A.; Milovanovic, A.; Artiko, V.; Banko, B. Oncological and functional outcome after surgical treatment of early glottic carcinoma without anterior commissure involvement. Biomed Res Int 2014, 2014, 464781. [Google Scholar] [CrossRef]
- Pantel, M.; Wittekindt, C.; Altendorf-Hofmann, A.; Boeger, D.; Buentzel, J.; Esser, D.; Mueller, A.; Wendt, T.G.; Guntinas-Lichius, O.; Thuringia, H.; et al. Diversity of treatment of T2N0 glottic cancer of the larynx: lessons to learn from epidemiological cancer registry data. Acta Otolaryngol 2011, 131, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Sachse, F.; Stoll, W.; Rudack, C. Evaluation of treatment results with regard to initial anterior commissure involvement in early glottic carcinoma treated by external partial surgery or transoral laser microresection. Head Neck 2009, 31, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn AH, Kiagiadaki D, Lawson G, Remacle M. CO2 laser cordectomy for glottic squamous cell carcinoma involving the anterior commissure: voice and oncologic outcomes. Eur Arch Otorhinolaryngol. 2015 Feb;272, 413-8. Epub 2014 Oct 29. [CrossRef] [PubMed]
- Hendriksma M, Montagne MW, Langeveld TPM, Veselic M, van Benthem PPG, Sjögren EV. Evaluation of surgical margin status in patients with early glottic cancer (Tis-T2) treated with transoral CO2 laser microsurgery, on local control. Eur Arch Otorhinolaryngol. 2018 Sep;275, 2333-2340. Epub 2018 Jul 19. [CrossRef] [PubMed]
- Fiz I, Koelmel JC, Sittel C. Nature and role of surgical margins in transoral laser microsurgery for early and intermediate glottic cancer. Curr Opin Otolaryngol Head Neck Surg. 2018 Apr;26, 78-83. [CrossRef] [PubMed]
- Peretti, G.; Nicolai, P.; Redaelli De Zinis, L.O.; Berlucchi, M.; Bazzana, T.; Bertoni, F.; Antonelli, A.R. Endoscopic CO2 laser excision for tis, T1, and T2 glottic carcinomas: cure rate and prognostic factors. Otolaryngol Head Neck Surg 2000, 123, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Zapater, E.; Hernandez, R.; Reboll, R.; Perez, A.; Alba, J.R.; Basterra, J. Pathological examination of cordectomy specimens: analysis of negative resection. Auris Nasus Larynx 2009, 36, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Aluffi Valletti, P.; Taranto, F.; Chiesa, A.; Pia, F.; Valente, G. Impact of resection margin status on oncological outcomes after CO2 laser cordectomy. Acta Otorhinolaryngol Ital 2018, 38, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.M.; Ferlito, A.; Brasnu, D.F.; Langendijk, J.A.; Rinaldo, A.; Silver, C.E.; Wolf, G.T. Evidence-based review of treatment options for patients with glottic cancer. Head Neck 2011, 33, 1638–1648. [Google Scholar] [CrossRef]
- Chone, C.T.; Yonehara, E.; Martins, J.E.; Altemani, A.; Crespo, A.N. Importance of anterior commissure in recurrence of early glottic cancer after laser endoscopic resection. Arch Otolaryngol Head Neck Surg 2007, 133, 882–887. [Google Scholar] [CrossRef]
- Tulli, M.; Re, M.; Bondi, S.; Ferrante, L.; Dajko, M.; Giordano, L.; Gioacchini, F.M.; Galli, A.; Bussi, M. The prognostic value of anterior commissure involvement in T1 glottic cancer: A systematic review and meta-analysis. Laryngoscope 2020, 130, 1932–1940. [Google Scholar] [CrossRef]
- Carreras A, Martínez-Torre MI, Zabaleta M, Sanchez-Del-Rey A, Santaolalla F, Diaz-de-Cerio P. Prognosis and Outcomes in Early Stage Glottic Carcinoma Involving the Anterior Commissure Treated with Laser CO2 Surgery: A Retrospective Observational Analysis. Indian J Otolaryngol Head Neck Surg. 2022 Dec;74(Suppl 3):6048-6053. Epub 2021 Jun 27. [CrossRef] [PubMed]
- Lechien JR, Crevier-Buchman L, Circiu MP, Lisan Q, Hans S. Evolution of Voice Quality in Type 1-2 Transoral CO2 Laser Cordectomy: A Prospective Comparative Study. Laryngoscope. 2022 Jul;132, 1421-1426. Epub 2021 Oct 27. [CrossRef] [PubMed]
- Colizza A, Ralli M, D’Elia C, Greco A, de Vincentiis M. Voice quality after transoral CO2 laser microsurgery (TOLMS): systematic review of literature. Eur Arch Otorhinolaryngol. 2022 Sep;279, 4247-4255. Epub 2022 May 3. [CrossRef] [PubMed]
- Greulich, M.T.; Parker, N.P.; Lee, P.; Merati, A.L.; Misono, S. Voice outcomes following radiation versus laser microsurgery for T1 glottic carcinoma: systematic review and meta-analysis. Otolaryngol Head Neck Surg 2015, 152, 811–819. [Google Scholar] [CrossRef]
- Ma Y, Green R, Pan S, McCabe D, Goldberg L, Woo P. Long-term Voice Outcome Following Radiation Versus Laser Microsurgery in Early Glottic Cancer. J Voice. 2019 Mar;33, 176-182. Epub 2017 Dec 8. [CrossRef] [PubMed]
- Bindewald, J.; Herrmann, E.; Dietz, A.; Wulke, C.; Meister, E.; Wollbruck, D.; Singer, S. [Quality of life and voice intelligibility in laryngeal cancer patients--relevance of the “satisfaction paradox”]. Laryngorhinootologie 2007, 86, 426–430. [Google Scholar] [CrossRef]
- Friedrich, G.; Dejonckere, P.H. [The voice evaluation protocol of the European Laryngological Society (ELS) -- first results of a multicenter study]. Laryngorhinootologie 2005, 84, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.; Cuccarini, V.; Bottero, A.; Dobrea, C.; Capaccio, P.; Ottaviani, F. Long-term vocal functional results after glottectomy: a multi-dimensional analysis. Eur Arch Otorhinolaryngol 2007, 264, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Bohlender, JE. Patient-Reported Outcome Measures zur Erfassung der gesundheitsbezogenen Lebensqualität bei Patienten mit Stimm- und Schluckstörungen [Patient-reported outcome measures for assessing health-related quality of life in patients with voice and swallowing disorders]. HNO. 2023 Sep;71, 549-555. German. Epub 2023 Aug 7. [CrossRef] [PubMed]
- Narasimhan SV, Rashmi R. Multiparameter Voice Assessment in Dysphonics: Correlation Between Objective and Perceptual Parameters. J Voice. 2022 May;36, 335-343. Epub 2020 Jul 7. [CrossRef] [PubMed]
- Böckler, R.; Bonkowsky, V.; Seidler, T.; Hacki, T. [Comparative voice quality evaluation after laser surgical versus fronto-lateral partial laryngectomy in T1b and T2 vocal cord carcinoma]. Laryngorhinootologie 1999, 78, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Sittel, C.; Eckel, H.E.; Eschenburg, C.; Vossing, M.; Pototschnig, C.; Zorowka, P. [Voice quality after partial laser laryngectomy]. Laryngorhinootologie 1998, 77, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lechien JR, Crevier-Buchman L, Circiu MP, De Mones E, de Pemille GV, Julien-Laferriere A, Saussez S, Baudouin R, Remacle M, Hans S. Voice Quality Outcomes After Transoral CO2 Laser Cordectomy: A Longitudinal Prospective Study. Otolaryngol Head Neck Surg. 2023 Mar;168, 422-428. Epub 2023 Jan 29. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).