1. Introduction
Copper is a vital dietary element for humans, and therefore, insufficient or excessive consumption of this trace mineral can have negative effects on well–being. With high copper intake, the body’s ability to absorb copper decreases, while the elimination of copper increases. On the other hand, when copper intake is low, the body reduces the excretion of copper through bile and retains more copper [
1]. Furthermore, considering the natural tendency of copper to enhance the generation of detrimental oxygen free radicals, excessive amounts of copper have the potential to cause tissue damage and subsequent pathological consequences [
2]. The inequitable production and elimination of reactive oxygen species (ROS) may result in the buildup of intermediate ROS products, which are considered harmful and have the potential to trigger oxidative stress [
3]. Copper also has crucial functions in a multitude of physiological processes. These processes include respiration, the removal of harmful free radicals, the regulation of iron and oxygen metabolism, the formation of connective tissues, maturation of the extracellular matrix, the production of energy, the synthesis of neuropeptides, and the facilitation of neuroendocrine signaling [
4]. The potential for oxidation exhibited by copper may contribute to its toxicity in instances of excessive ingestion. At elevated concentrations, copper has been observed to induce oxidative harm to biological systems through the peroxidation of lipids and other macromolecules [
5].
Copper exerts a regulatory influence on the activity of nitric oxide synthase (NOS) and guanylyl cyclase (GC) in vascular structures [
6]. The modified process of synthesizing and releasing vasoactive factors can potentially play a role in the impairment of blood vessel function, ultimately resulting in the emergence of cardiovascular disorders (CVDs), such as atherosclerosis, hypertension, and ischemia. There are several ways in which high serum levels of copper can potentially increase the risk of atherosclerotic CVD. One possible mechanism is through the oxidative modification of low–density lipoprotein cholesterol and the formation of free radicals, which can contribute to the development of atherosclerosis [
7]. Another pathway involves inflammation, as copper is closely associated with ceruloplasmin, an acute phase reactant [
7]. Additionally, copper has been linked to insulin resistance and the pathogenesis of diabetes, which are major risk factors for coronary heart disease (CHD) [
8]. Finally, high levels of copper can lead to luminal narrowing of the arteries, as it promotes the expansion of the arterial neointima, which is primarily composed of copper–containing extracellular matrix molecules [
9].
Understanding the impact of copper supplementation on different aspects of the cardiovascular system remains a topic of ongoing research due to conflicting findings from previous studies.
2. Results
2.1. Rat Body Weight and Body Composition
Body weight gain was not affected. The weights of the internal organs (heart, liver, kidneys, spleen, and brain) did not change. Body fat and lean body parts were not modified.
2.2. Copper, Zinc, Iron, and Selenium in the Rat Serum, Liver and Kidneys
The copper concentration increased in the rat liver (mg/kg wet weight) 3.11 ± 0.18 vs 3.58 ± 0.52 (p = 0.032), in the kidneys 6.25 ± 0.91 vs 7.71 ± 1.68 (p = 0.05), and in the blood serum (mg/L) 1.01 ± 0.31 vs 1.22 ± 0.24 (p = 0.04).
The calculated copper–to–zinc ratios increased in the rat liver (0.133 ± 0.006 vs 0.145 ± 0.017, p = 0.014), kidney (0.271 ± 0.028 vs 0.315 ± 0.063, p = 0.05) and blood serum (1.02 ± 0.23 vs 1.42 ± 0.356, p = 0.04).
Zinc, iron, and selenium were not modified.
2.3. ELISA of COX−1, COX−2, GAPDH, ICAM−1, HO−1, and eNOS in the Rat Serum
These were not modified.
2.4. TAS, MDA, SOD, and CAT in the Rat Serum, Heart, and Aortic Rings
These were not modified.
2.5. NO, O2•−, and H2O2 in Rat Aortic Rings
The following molecules increased in the rat aortic rings: NO (4265 ± 874 vs 6342 ± 987, p = 0.04), O2•− (3254 ± 253 vs 5324 ± 1034, p = 0.023), and H2O2 (5765 ± 753 vs 7789 ± 1075, p = 0.015).
2.6. The Isolated Perfused Heart
The cardiac contractile strength and heart rate obtained from the Langendorff heart assay were not modified.
2.7. Vascular Contraction
We observed no significant difference in the NA–induced contraction between the studied groups under the control conditions (CC,
Figure 1a) or when they were preincubated with either selective COX–1 inhibitor (
Figure 1c) or COX–1 plus COX–2 inhibitors simultaneously (
Figure 1d). Preincubation with the selective iNOS inhibitor potentiated vasoconstriction compared to that in the control group (
Figure 1b), and this effect was also significantly greater than that in the CC in experimental group B but not in control group A (
Figure 1e,f). A selective COX–2 inhibitor (NS–398) decreased vascular contraction in both groups (
Figure 1e,f), and this effect was also observed when both COX–1 and COX–2 were blocked (
Figure 1e,f).
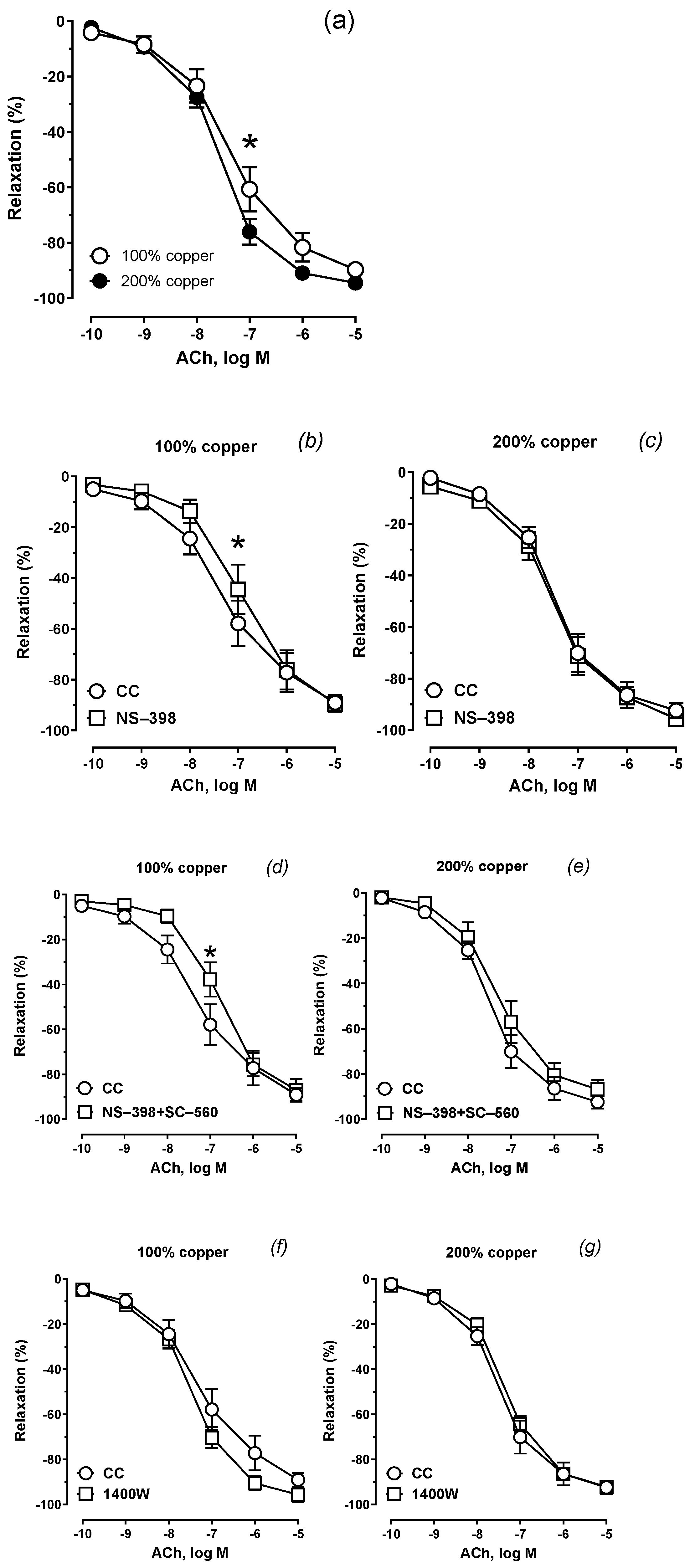
2.8. Vascular Relaxation
Supplementation with 200% copper potentiated the sensitivity to ACh compared to 100% copper, as shown in
Figure 2a. Preincubation with a COX–1 inhibitor (
Figure 2b,c) or a COX–1+COX–2 inhibitors (
Figure 2d,e) attenuated the vasodilator response to acetylcholine. This effect was not observed with the iNOS inhibitor (
Figure 2f,g).
3. Discussion
We have shown that the vasoconstrictor response to noradrenaline was similar across the two studied groups and that neither selective COX–2 inhibition nor COX inhibition (simultaneous COX–1 and COX–2 inhibition) modified the contractile response. In contrast to our research (with 1.2 mg of copper/L of rat serum detected), other in vitro studies demonstrated that copper concentrations of 10 and 16 µM (0.635 and 1.016 mg/L, respectively) exhibit a dose–dependent inhibitory effect on phenylephrine–induced contraction in isolated rings of the rat thoracic aorta [
10]. A subsequent in vitro study demonstrated that copper pretreatment blocked the vasoconstriction induced by noradrenaline, both dependently and independently of the bioavailability of nitric oxide (NO). This suggests that the effect of copper pretreatment is attributed to a mechanism other than NO [
6]. Through our previous experimental findings, we discovered that increased copper intake in middle–aged rats led to reduced vasoconstriction when exposed to noradrenaline. Additionally, preincubation with a PGF2α analog (which is a selective FP receptor antagonist) rendered the vasoconstrictor response insignificant across the groups, suggesting that FP receptors are involved in reduced contraction in copper–supplemented middle–aged rats [
11]. In the present study, this was not the case, and surprisingly, a selective iNOS inhibitor potentiated vascular contraction in response to noradrenaline, indicating that increased production of NO derived from iNOS constitutes a compensatory mechanism in copper–supplemented rats.
Compared with that in the control group, the vasodilatory response to acetylcholine in the experimental group tended to potentiate vasodilation. It is worth mentioning to the increase in vasodilation in aged rats that was previously observed [
11]. Both selective COX–2 inhibition and nonselective COX inhibition (simultaneous COX–1 and COX–2 inhibition) attenuated the vasodilator net effect of prostanoids on acetylcholine–induced vascular relaxation, indicating the decreased participation of vasodilator prostanoids derived from COX–2 in vascular tone regulation. To some extent, this finding adds to the abovementioned results of our study, which indicate a reduced share of some substances with a vasodilatory effect, which may be compensated for by an increase in the production of NO from iNOS. These substances are vasodilators derived from COX–2. Surprisingly, iNOS did not modify vascular relaxation as it did with the contractile response to noradrenaline, indicating that the primary molecules responsible for vasodilation are prostanoids and not NO from iNOS.
Our research has shown that a higher dietary copper intake (200%) did not have any impact on the levels of COX−1, COX−2, GAPDH, iCAM−1, HO−1, or eNOS in the blood serum of rats. COX–2 is widely recognized as the primary regulator of endothelial prostacyclin synthesis, which helps to maintain the balance of thromboxane synthesis mediated by COX–1 in platelets. Therefore, the specific inhibition of COX–2 is believed to lead to vasoconstriction and platelet aggregation and subsequently increase the occurrence of cardiovascular events [
12]. It has been widely observed that glyceraldehyde–3–phosphate dehydrogenase (GAPDH) is not just an average glycolytic enzyme. In fact, there is a growing body of evidence indicating that GAPDH has multiple functions. Its role as a mediator of cell death has been emphasized. Over the past few years, numerous studies have indicated that a group of GAPDH molecules move to the nucleus in response to various stressors, many of which are linked to oxidative stress [
13]. Unlike young rats, our previous research showed that middle–aged rats experienced a decrease in the levels of COX–1, COX–2, and GAPDH in their blood serum when they were given a greater amount of dietary copper (200%) [
11].
Reactive oxygen species (ROS) play a crucial role as reactive intermediates of molecular oxygen, serving as vital second messengers within cells. However, an imbalance between the production of ROS and the body’s antioxidant defense systems can lead to endothelial dysfunction. This dysfunction is a major contributor to vascular damage in metabolic and atherosclerotic diseases [
14]. There are multiple biomarkers and methods of measurement available to assess antioxidant status. Recent studies have utilized total antioxidant status (TAS) [
15], and we also used this method previously to discover increased TAS in aged rats [
11]. However, in the present study, neither the TAS nor the MDA, SOD or CAT levels in the blood, arteries, or heart of the rats were altered. Surprisingly, the levels of NO, O
2•− and H
2O
2 increased in the aortic rings. The existing scientific literature has not adequately explained the relationship between excess dietary copper and oxidative stress.
Compensatory mechanisms strictly regulate the serum concentrations of copper and zinc, ensuring that they remain stable within specific ranges corresponding to nutritional intake. Nevertheless, there are mechanisms in the blood designed to reduce the amount of zinc and increase the amount of copper during inflammatory conditions. As a result, a common characteristic of various chronic diseases associated with aging is an elevated copper–to–zinc ratio [
16]. In our study, copper and the copper–to–zinc ratio increased in the blood, liver, and kidneys of the rats, contrary to zinc, iron or selenium, which were not modified.
No significant differences were found in body weight gain, fat, or lean body composition. In addition, there were no differences in the weights of the brain, heart, kidney, liver, or spleen. Previous studies support the data presented in this article [
11]. Consistent with our research, Filetti et al. (2023) reported that there were no significant differences in weight between the control group and the groups that were administered different amounts of copper [
17].
4. Materials and Methods
4.1. Substances
The following chemicals were obtained from Sigma–Aldrich (St. Louis, MO, USA): acetylcholine chloride, noradrenaline hydrochloride, 1400 W, NS–398, and SC–560. NS–398 and SC–560 were dissolved in DMSO, and 1400 W was dissolved in methanol. Noradrenaline was dissolved in a solution of NaCl and ascorbic acid at concentrations of 0.9% and 0.01% w/v, respectively. The concentration of the solvent was less than 0.01% (v/v). The solutions were stored at –20°C. On the day of the experiment, the solutions were diluted in Krebs–Henseleit solution (KH in mM: NaCl 115; CaCl
2 2.5; KCl 4.6; KH
2PO
4 1.2; MgSO
4 1.2; NaHCO
3 25; glucose 11.1) [
18]. The copper carbonate sample, which had a purity of at least 99%, was acquired from Poch (Gliwice, Poland).
4.2. Animals and diet
A group of 20 male Wistar Han rats, aged 4 weeks, were provided with a standard rat diet (Diet A) that included 6.45 mg/kg copper, as per the guidelines outlined in references [
11,
18]. For an additional duration of 6 weeks, the rats in Group B (n/group = 10) were administered a diet containing 12.9 mg Cu/kg (200% of the recommended daily dietary quantity of copper), while those in Group A were fed a standard rat diet (Diet A). The rats were housed in accordance with a previously described housing method [
18]. Unrestricted access to food and tap water (a copper concentration of 0.35 mg/L) was provided. Blood samples were obtained from the caudal vena cava of anesthetized animals. Anesthesia was administered through intraperitoneal injections of ketamine and xylazine at dosages of 100 mg/kg and 10 mg/kg of body weight, respectively.
4.3. Body Weight and Body Composition
A Bruker Minispec LF (Ettlingen, Germany) was used to accurately measure fat, fluid, and lean tissue in rats using TD–NMR.
4.4. Vascular Reactivity Studies
As previously described, the aortic rings were incubated in 5 mL chambers (Graz, Barcelona, Spain) under a preload tension of 1 g (FT20, TAM–A, Hugo Sachs Elektronik, March, Germany) [
18]. The functional integrity of the aortic rings was assessed using high concentrations of the vasoconstrictor KCl (75 mM) and the vasodilator acetylcholine (10 µM). In addition, the aortic rings were subjected to a 30–minute preincubation with various substances, including a selective cyclooxygenase–1 (COX–1) inhibitor (SC–560, 10 µM), a selective cyclooxygenase–2 (COX–2) inhibitor (NS–398, 10 µM), and an inducible nitric oxide synthase (iNOS) inhibitor (1400 W, 1 µM). The vasodilatory response was analyzed by adding cumulative concentrations (CCs) of acetylcholine (ranging from 0.1 nM to 10 µM) to the incubation chambers [
18]. The response to noradrenaline (0.1 µM) was also studied.
4.5. The Langendorff Heart Studies
The isolated heart studies were conducted using the Langendorff system equipped with the ISOHEART software 73–0161 (Hugo Sachs Elektronik, March, Germany), following the methodology outlined in a previous study [
18].
4.6. Analysis of Copper, Zinc, Iron, and Selenium in Rat Blood, Liver and Kidneys
4.6.1. Sample Preparation
Each analyzed liver and kidney sample was cut into small pieces with a ceramic knife and mixed to homogenize the sample. Three independent samples were prepared from each organ (n=3). Approximately 0.2 g of the wet samples, 3 mL of 65% nitric acid and 0.5 mL of 30% hydrogen peroxide were used for digestion. The digestion proceeded in two steps: permineralization for 3 h and mineralization in a closed system. The samples were mineralized with the use of a microwave mineralizer (EthoS One, Milestone, Sorisole, Italy). The heating program during mineralization in a closed system was as follows: 20 min ramp time to 220°C, 30 min at 220°C and 20 min of cooling. The power of the total process was 1500 W. The digested samples were diluted with distilled water (Direct–Q–3 UV, Merck, Darmstadt, Germany) to a volume of 50 mL. The certified reference material ERM, BB184 Bovine Muscle (IRMM, Geel, Belgium), was used to evaluate the accuracy of the measurements and was prepared in the same way as the analyzed samples.
4.6.2. Elements Measurements
Inductively coupled plasma–mass spectrometry (ICP–MS 7100x Agilent, Santa Clara, CA, USA) was used for analysis. The instrumental parameters were automatically optimized using Tuning Solution (Agilent). For the reduction of interferences, helium mode was used. The interferences were reduced by using helium mode and an internal standard solution containing 10 µg L–1 Rh and Tb. Calibration solutions were prepared by appropriate dilution of 10 mg L–1 multielement standard solution (ICP Standard, Perkin Elmer, Darmstadt, Germany). Calibration curves were constructed in the range of 0.01–100 µg/L. High purity argon (99.999%) was used as the nebulizer, auxiliary, and plasma gas for the ICP–MS (Messer, Chorzów, Poland).
4.6.3. Quality Assessment
The validity of the analytical method was assessed by analyzing the certified reference material ERM BB184 Bovine Muscle (IRMM, Geel, Belgium). Validation parameters such as linearity, precision, the limit of detection (LOD), and trueness were evaluated. The linearity of the calibration curves, calculated as the correlation coefficient R, was greater than 0.9996 for all analytes. The LOD was defined as 3.3 s/b, where s is the standard deviation corresponding to 10 blank injections and b is the slope of the calibration graph. The LODs were as follows: 0.008 µg/g for Cu, 0.012 µg/g for Zn, 0.075 µg/g for Se and 0.214 µg/g for Fe. The precision values were calculated as the coefficient of variation (CV) (%) and ranged from 1.2% to 3.4% for all the elements. Trueness was evaluated by applying the certified reference material and expressed as recovery values (%) ranging from 95% to 103%.
4.6.4. Blood Analysis Was Performed as Previously Described [11].
4.7. TAS, MDA, SOD and CAT
The blood serum total antioxidant status (TAS) was measured using a Randox kit through a spectrophotometric method, malondialdehyde (MDA) was quantified with a fluorometric assay kit (ab118970), and catalase (CAT) and superoxide dismutase (SOD) activities were determined as previously described following the manufacturer’s instructions [
18].
4.8. The ELISA Protocol
Blood serum was analyzed using commercial ELISA kits to determine the levels of COX–1, COX–2, heme oxygenase–1 (HO–1), endothelial nitric oxide synthase 3 (NOS3), glyceraldehyde 3–phosphate dehydrogenase (GAPDH), intercellular adhesion molecule 1 (ICAM–1), malondialdehyde (MDA), catalase (CAT) and superoxide dismutase (SOD). A Thermo Scientific microplate reader (Varioskan LUX, Bremen, Germany) was used to measure the absorbance in the ELISA test plate at a wavelength of κ = 450 nm.
4.9. The Detection of NO, O2•–, and H2O2
The levels of nitric oxide (NO), superoxide anion (O
2•–), and hydrogen peroxide (H
2O
2) in situ were assessed using diaminofluorescein (DAF), dihydroethidium (DHE), and 2’,7’-dichlorofluorescein diacetate (DCF), following the methodology described in a previous study [
11]. The cells were incubated with the nuclear dye 4’,6–diamidino–2–phenylindole (DAPI, 10 µg/mL) in the following combinations: DAF+DAPI, DHE+DAPI, and DCF+DAPI. The incubation was carried out for 30 minutes in an oven at 37°C. Fluorescence images were acquired using a Leica (TCS ST2DM IRE2) laser scanning confocal microscope at wavelengths of 568 nm and 410–475 nm for the DAPI dye.
4.10. Data Analysis and Statistics
Vascular contraction in response to noradrenaline (0.1 μM) was quantified in milligrams of tension, while vascular relaxation in response to acetylcholine was measured as a percentage of the contractile response to noradrenaline. The log agonist vs. response (nonlinear regression model) was employed to analyze the cumulative concentration–response curves (CCRCs). Comparisons between groups were conducted using either a parametric test (t–test) or a nonparametric test (Mann–Whitney U test or Kruskal–Wallis test) with a sample size of n = 10 rats. The CCRCs were subjected to analysis via two–way ANOVA with Šídák’s multiple comparisons test. The results are typically presented as the means ± SEMs (for CCRCs) and means ± SDs. The significance level was set at *p ≤ 0.05.
5. Conclusions
Our study revealed that supplementation with 200% of the recommended daily dietary quantity of copper did not modify vascular contraction in young rats, unlike in older rats, as previously described. Vascular relaxation tended to increase, similar to that in older animals; however, the participation of vasodilator prostanoids was attenuated. Increased nitric oxide derived from iNOS may constitute a compensatory mechanism for the decreased effect of COX–2-derived vasodilator prostanoids. It is imperative to conduct further investigations concerning the ramifications of elevated dietary copper on living organisms and their growth as a consequence of increasing levels of environmental food and water contamination.
Author Contributions
All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by UWM Statutory Funding, grant number 61.610.007-110, which was granted to M.M.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Olsztyn, Poland (90/2019 from November 26th, 2019), and conformed to European guidelines, including the ARRIVE guidelines.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, L; Min, J; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct Target Ther. 2022, 7(1), 378. [Google Scholar] [CrossRef] [PubMed]
- ang, T; Xiang, P; Ha, J.H.; et al. Copper supplementation reverses dietary iron overload-induced pathologies in mice. J Nutr Biochem. 2018, 59, 56-63. [CrossRef]
- Hajam YA, Rani R, Ganie SY, et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells. 2022, 11(3), 552. [CrossRef]
- Kitala, K.; Tanski, D.; Godlewski, J.; Krajewska-Włodarczyk, M.; Gromadziński, L.; Majewski, M. Copper and Zinc Particles as Regulators of Cardiovascular System Function—A Review. Nutrients 2023, 15, 3040. [Google Scholar] [CrossRef] [PubMed]
- El-Ta’alu, A; Ahmad, M.M. Age-Dependent Effects of Copper Toxicity on Connective Tissue Structural Stability in Wistar Rats Skin. Niger J Physiol Sci. 2022, 37(1), 93-99. [CrossRef]
- Wang, ; Chao-Wei, Hu; Ming-Yu, Liu; Hong-Chao, Jiang; Rong, Huo; De-Li, Dong. Copper induces vasorelaxation and antagonizes noradrenaline -Induced vasoconstriction in rat mesenteric artery. Cellular Physiology and Biochemistry, 2013 32(5), pp. 1247–1254. [CrossRef]
- Kunutsor, S.K.; Dey, R.S.; Laukkanen, J.A. Circulating Serum Copper Is Associated with Atherosclerotic Cardiovascular Disease, but Not Venous Thromboembolism: A Prospective Cohort Study. Pulse (Basel). 2021, 9(3-4), 109-115. [CrossRef]
- Tanaka, A; Kaneto, H; Miyatsuka, T; et al. Role of copper ion in the pathogenesis of type 2 diabetes. Endocr J. 2009, 56(5), 699–706. [Google Scholar] [CrossRef] [PubMed]
- Ferns, G.A.; Lamb, D.J.; Taylor, A. The possible role of copper ions in atherogenesis: the Blue Janus. Atherosclerosis. 1997, 133(2), 139–152. [Google Scholar] [CrossRef] [PubMed]
- Yan, M; Liu, D.L; Chua, Y.L; Chen, C; Lim, Y.L. Effects of micromolar concentrations of manganese, copper, and zinc on alpha1-adrenoceptor-mediating contraction in rat aorta. Biol Trace Elem Res. 2001, 82(1-3), 159-166. [CrossRef]
- Kitala-Tańska, K.; Socha, K.; Juśkiewicz, J.; Krajewska-Włodarczyk, M.; Majewski, M. The Effect of an Elevated Dietary Copper Level on the Vascular Contractility and Oxidative Stress in Middle-Aged Rats. Nutrients 2024, 16, 1172. [Google Scholar] [CrossRef] [PubMed]
- Luo, W; Liu,, B; Zhou, Y. The endothelial cyclooxygenase pathway: Insights from mouse arteries. European journal of pharmacology 2016, 780, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.R.; Cascio, M.B.; Sawa, A. GAPDH as a sensor of NO stress. Biochim Biophys Acta. 2006, 1762(5), 502–509. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M. A; D’Oria, R; Natalicchio, A; Perrini, S; Laviola, L; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, F; Demir, F; Yılmaz, R; Akıl, E. Total oxidant/antioxidant status, copper and zinc levels in acute ischemic stroke patients after mechanical thrombectomy. Clin Neurol Neurosurg. 2023, 229, 107718. [CrossRef]
- Malavolta, M; Piacenza, F; Basso, A; Giacconi, R; Costarelli, L; Mocchegiani, E. Serum copper to zinc ratio: Relationship with aging and health status. Mech Aging Dev. 2015, 151, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Filetti, F.M; Schereider, I.R.G; Wiggers, G.A; Miguel, M; Vassallo, D.V; Simões, M.R. Cardiovascular Harmful Effects of Recommended Daily Doses (13 µg/kg/day), Tolerable Upper Intake Doses (0.14 mg/kg/day) and Twice the Tolerable Doses (0.28 mg/kg/day) of Copper. Cardiovasc Toxicol. 2023, 23(5-6), 218-229. [CrossRef]
- Majewski, M., Gromadziński, L., Cholewińska, E., Ognik, K., Fotschki, B., & Juśkiewicz, J. The Interaction of Dietary Pectin, Inulin, and Psyllium with Copper Nanoparticle Induced Changes to the Cardiovascular System. Nutrients 2023, 15(16), 3557.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).