1. Introduction
Community-acquired pneumonia (CAP) is the leading cause of hospitalization and mortality worldwide[
1], with community respiratory viruses (CRV) accounting for 15–30% of cases[
2], many of which are associated with severe acute respiratory infection (SARI), an acute respiratory infection requiring hospitalization that may be lethal[
3].
The main CRVs identified in SARI are influenza A and B (IF), human adenovirus (hAdV), respiratory syncytial virus (RSV), human rhinovirus (hRV), human metapneumovirus (hMPV), parainfluenza virus group (PIV 1,2,3,4), human enterovirus (hEV), human bocavirus (hBoV), human coronavirus-hCoV (229E, OC43, HKU1, NL-63)[
4]. CRV infections in pregnant women can result in mild to severe acute respiratory illness[
5].
Physiological changes during pregnancy, including decreased lung capacity, cell-mediated immunity, and increased oxygen consumption, predispose them to poorer outcomes than the general population[
6].
For pregnant women, vaccination is a safe and effective strategy to protect against infectious diseases. Vaccination is an essential element of pre-pregnancy, antenatal, and postpartum care, as it should protect against the risk of complications and progression to SARI during the antenatal or postpartum period[
7].
After the H1N1 pandemic in 2009, the World Health Organization (WHO) designated pregnant women as a risk group for influenza-derived SARI[
8]. Although influenza is the only respiratory virus for which there is immunization, treatment, and a worldwide surveillance network, studies in pregnant women remain limited[
9]. In addition to influenza, only research on RSV in pregnant women has recently become prominent; however, the focus is on the development of future specific vaccines designed to immunize newborns[
10].
Infection with respiratory viruses, other than influenza, during pregnancy remains unclear. This study examined the burden of viral respiratory infections in pregnant women hospitalized with SARI.
2. Materials and Methods
A retrospective cross-sectional study was conducted between January 2015 and December 2019 at two tertiary hospitals, in Curitiba, Southern Brazil, using secondary data from medical records reviews. Both hospitals approved the study by there respective Ethics Committees under the identification numbers 15599.8.0000.0096 and 155.99119.8.3001.5225, respectively.
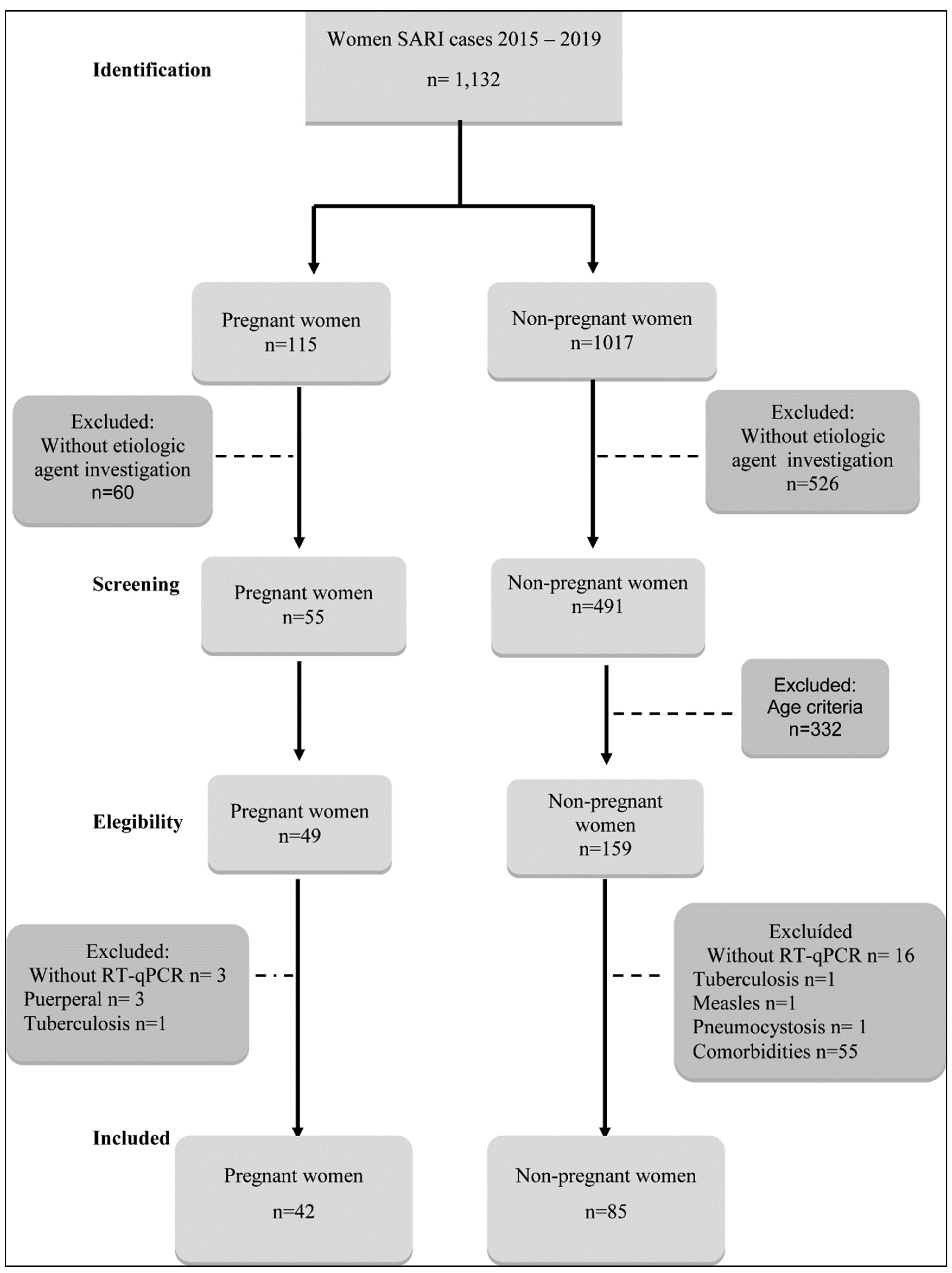
Sampling: Of the 1,132 cases of SARI reported to SIVEP (Epidemiological Surveillance Information Service) between 2015 and 2019 in both tertiary hospitals, forty-nine pregnant women (PW) and 159 non-pregnant women (NPW) were eligible. Of these, 42 (85.7%) PW and 85 (53.4%) NPW were included in the study, according to the inclusion and exclusion criteria, 42/49 PW and 85/159 NPW with SARI were requested for research on respiratory viruses, using reverse transcription – quantitative polymerase chain reaction (RT-qPCR) technique.
Inclusion criteria: PW: aged ≥18 years, at any gestational period, hospitalized with SARI, and investigated for community respiratory viruses (CRV): influenza A and B, hAdV, RSV, hRV, hMPV, hPIV group, hEV, hBoV, hCoV HKU1, 229E, OC43, and NL63 by RT-qPCR. NPW: aged between 18 and 45 years, without underlying diseases, hospitalized with SARI, with the same CRV described above.
Exclusion criteria PW with SARI: Seven excluded: 3 due to lack of RT-qPCR, 3 postpartum, and one with a diagnosis of tuberculosis (
Figure 1).
NPW with SARI: Seventh four excluded (46,5%): 16 due to lack of RT-qPCR, 1 with measles, 1 with pneumonia, one with a diagnosis of tuberculosis, 55 with comorbidities (
Figure 1).
3. Results
From the 1,132 women with SARI cases between 2015 and 2019 in both tertiary hospitals, 49 PW and 159 NPW were eligible to participate. Of these, 42 (86%) PW and 85 (53%) NPW were included, as shown in
Figure 1.
3.1. Clinical-epidemiological characteristics
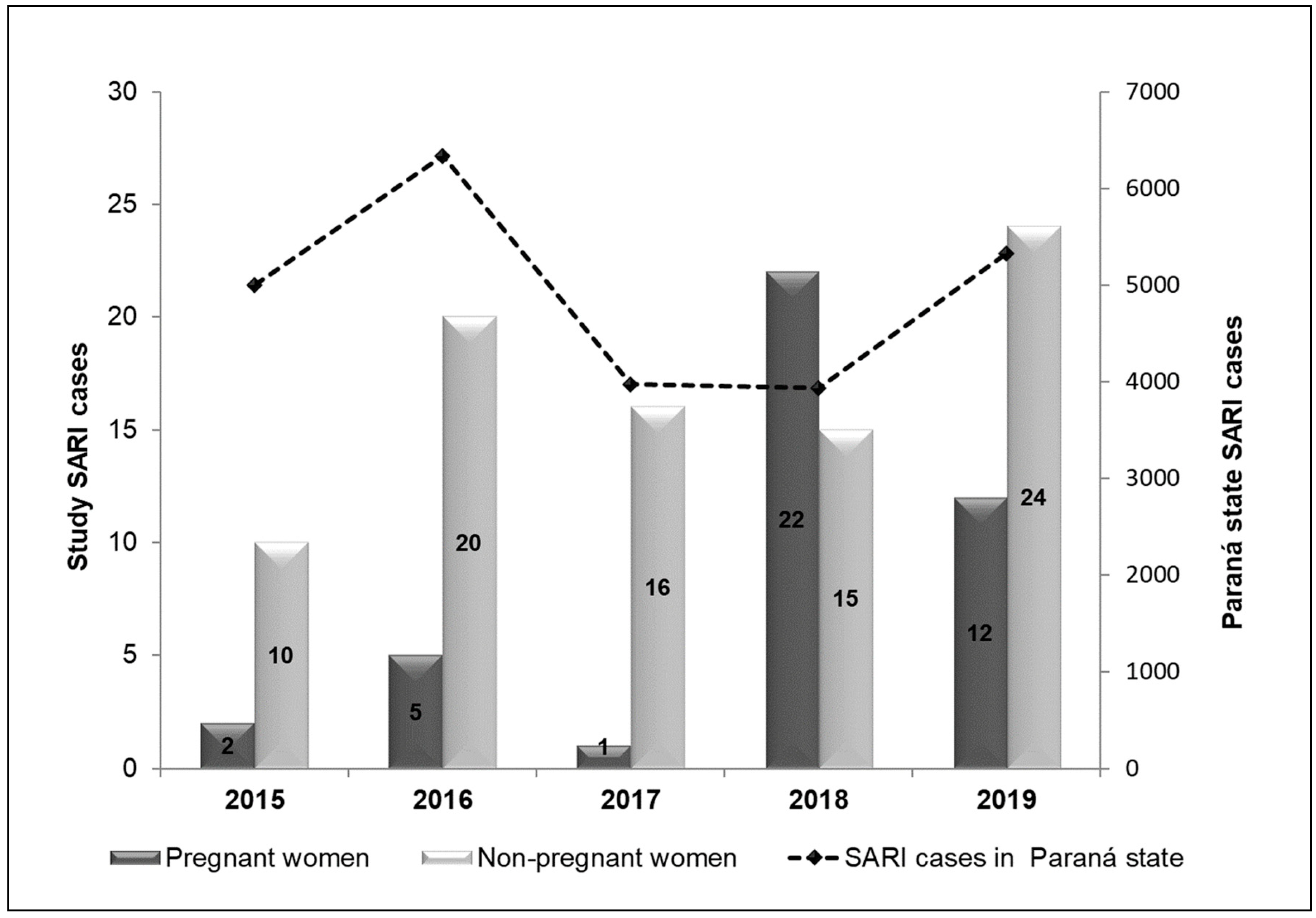
The SARI cases in the study years among PW were concentrated in 2018, while in NPW they were distributed without much discrepancy, with 2015 havind the lowest and 2019 the highest number of cases, as depicted in
Figure 2.
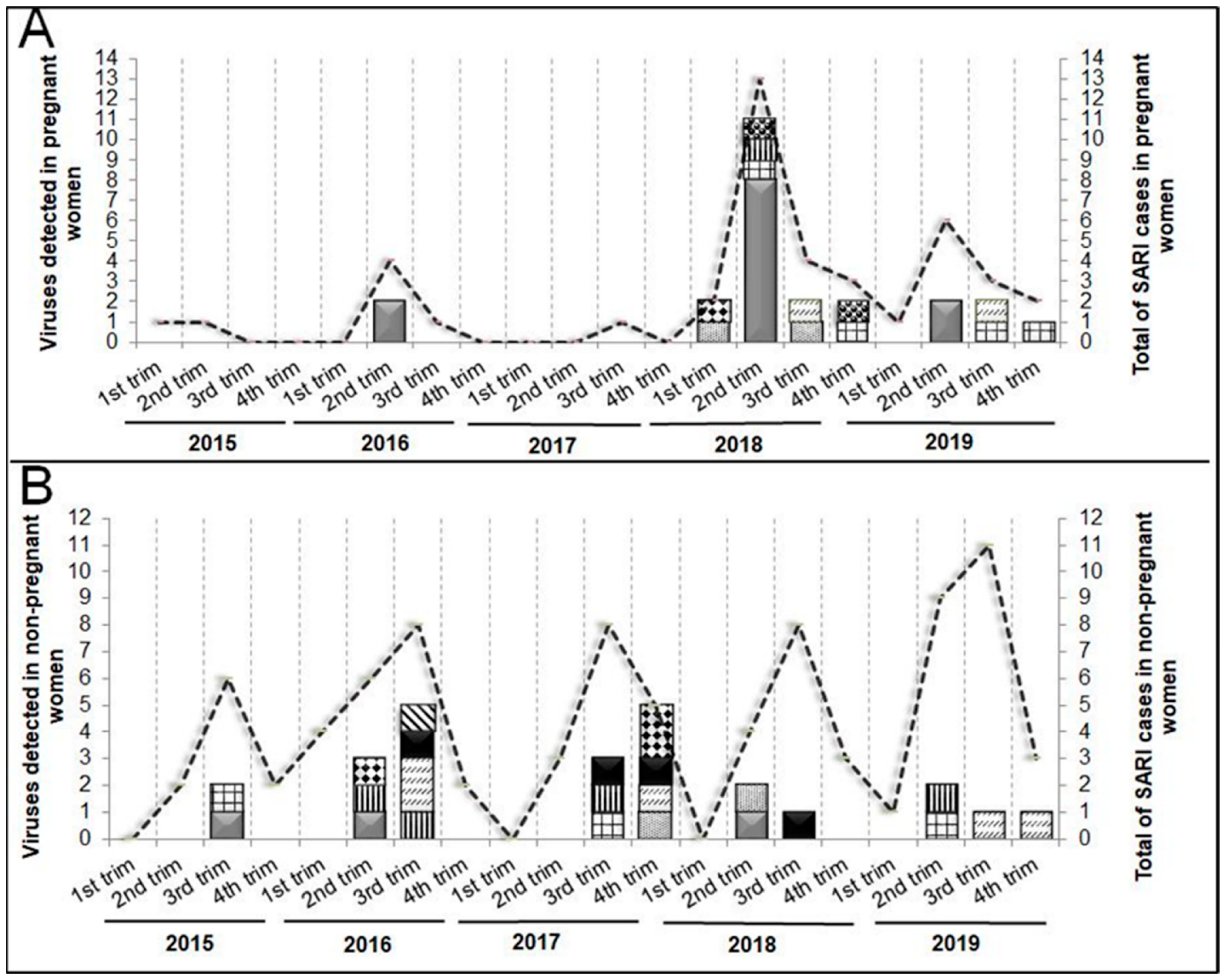
The peak of SARI cases in PW occurred during the coldest months (Apr-Sept), including viral SARI, which was is concentrated in the 2nd trimester of 2018 (Apr-Jun) (
Figure 3A). The NPW showed similar seasonality with a more evenly distributed compared to PW, in cases of SARI, and in viral SARI. However, in 2017, the highest number of viral infections occurred in the 4th trimester (Oct-Dec) (
Figure 3B).
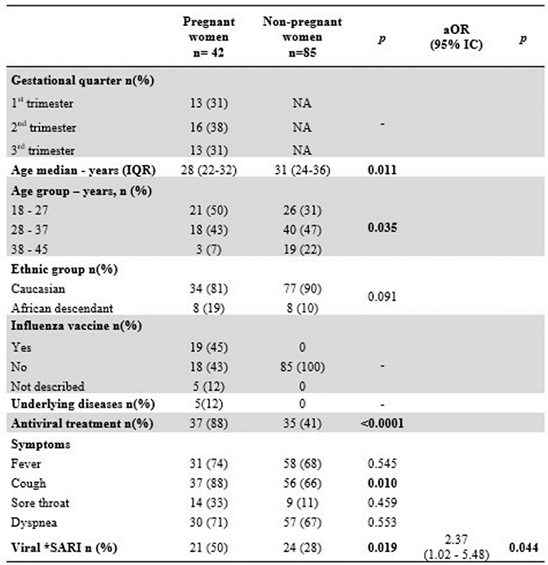
There was no difference in the number of PW hospitalized with SARI by the gestational period. Only 45% of PW were immunized against influenza. Two-thirds of PW with asthma were infected by hRV, and one HIV positive patient was infected with influenza. Of the five PW with comorbidities, only the HIV patients had been vaccinated against influenza. Antiviral treatment was prescribed more frequently in PWs than in NPW (p<0.0001). All PW with viral SARI (21/21) and 10/24 of NPW received antiviral therapy. Multivariate analysis showed that hospitalization rates for viral SARI were higher for PW than for NPW (aOR= 2.37; 95% CI= 1.02 - 5.48; p= 0.044), as shown in
Table 1.
3.2. Impact of viral SARI on PW and NPW
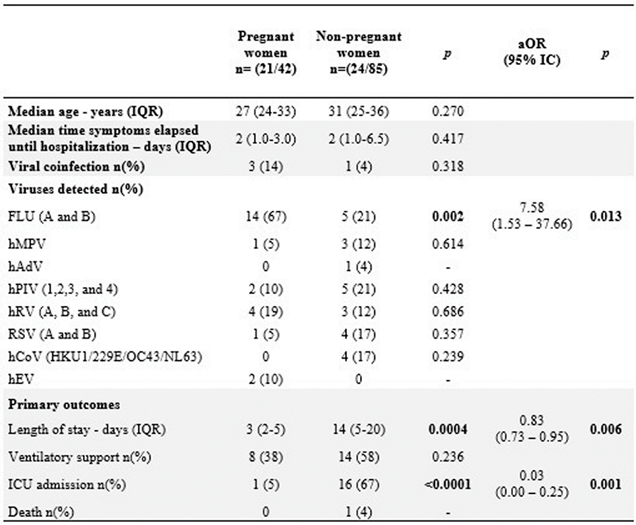
The median age of PW with viral SARI was similar to that of NPW. Of the 127 (42 PW + 85 NPW) SARI cases, 45 (35%) were positive for viruses. Among these, 50% (21/42) were PW, and 28% (24/85) were NPW. Among the 14 viruses investigated by RT-qPCR, hBoV, hCoV, and hAdV was not identified in PW group, and hEV was recognized only in the PW group.
Out of the 21 PW with viral SARI, 14 (67%) were infected with influenza, and 10 (71,4% ) did not receive the influenza vaccine. Of the four that receiver the vaccine: two received the vaccine less than 30 days before the infection, two was infected by influenza B, which was absent in the trivalent vaccine, and only one of the PW diagnosed with influenza SARI had been adequately immunized.
There was no difference in the median time of symptom onset and hospitalization between groups; the medical records of 95% (n=20/21) of the PW and only 71% (n=17/24) of the NPW were available. Three PW were co infected: two with hRV and only one with SARI had influenza and RSV co-infection, admitted on ICU, whithout comorbidities, and was not vaccinated. No cases hCoV infections (HKU, OC43 and NL63) were detected.
In the NPW group, 24 were positive to viral SARI. The distribution of viral etiological agents were more homogeneous. The Influenza was responsible for five (20.8%), the other RVs were detected in 15 patients (
Table 2). From this, one PIV+RSV co-infection was identified, and only the hEV was not identified in NPW group.
The logistic regression showed that the likelihood of viral SARI by influenza was seven times greater in PW than in NPW (aOR=7.58; 95% IC=1.53 - 37.66; p=0.013).
However, the risk of NPW being admitted to the ICU due to viral SARI was 16 times higher than that in PW (aOR=0.028; 95% IC=0.004–0.25; p=0.001). Nonetheless, there was no difference in median age or co-infection rates in the PW group. Linear regression showed a 16-day increase in the length of hospital stay for viral SARI in the NPW group (β=16.38; 95% IC=0.57 - 32.18; p=0.043)
Table 2.
4. Discussion
In this study, viral SARI associate with influenza had a more significant impact on the number of hospitalizations among PW than NPW. The length of hospital and ICU stay was shorter in PW. Influenza vaccine coverage, the rate of neuraminidase inhibitor prescriptions, and early hospitalization likely explain these findings[
11,
12].
The distribution of total SARI cases followed the influenza seasonality in both groups[
13]. The viral SARI rate among PW was higher than NPW, consistent with the findings from the state of Paraná. Influenza was the most frequent among the investigated viruses in PW, followed by hRV, in similar profile as described previously[
5,
14]. In accordance with to Azziz-Baumgartner
et al. (2021)[
15], we did not identify any cases of hAdV among PW.
The influenza vaccine coverage in PW was below the target recommended by immunization policies, ranging from 58-76%[
16]. The low the vaccination coverage observed was consistent with current studies that reported vaccination rates between 5-58% among PW[
15,
17], with an average of 59% for the American continent, according to the Pan American Health Organization (PAHO)[
18]. The prevalence of comorbidities among PW was lower than previously described (approximately 30%), with asthma being the most frequent, followed by diabetes and hypertension[
9]. Although antiviral treatment should be prescribed for all SARI patients[
19], prescription was higher for PW than for NPW, especially among viral SARI, for which all PW were treated, unlike the other group, which, because it was not a priority, was not attended to in time to institute treatment[
20].
PW were twice as likely to be hospitalized for viral SARI than NPW, with influenza being the main cause of viral SARI, as previously described[
21,
23] ]and confirmed in the preset study. However, meta-analysis studies have shown that, despite the increase of the need for hospitalization, pregnancy was not associated with more serious flu-associated outcomes such as ICU admission and death[
22,
23]. Consistent with our findings, Mertz
et al. (2019) found a seven-fold increased risk of influenza SARI among PW compared to NPW. PW with viral SARI also had a decreased length of hospital stay, a finding not previously described.
During the (H1N1)pdm09 pandemic, no differences were found in histopathology between severe cases of PW and NPW women[
24]. Although Littauer
et al. (2017) demonstrated in a mice model that pregnancy reduces viral clearance in the lungs, they did not observe any difference between the expression of inflammatory cytokines and chemokines in the lungs of PW and NPW mice[
25,
30] .
Prophylaxis, treatment, and clinical management measures may also explain our findings. We observed that (71,4% ) of PW with influenza did not receive flu vaccine in the present study. A recent meta-analysis study reported that immunization prevented 50%-70% of influenza infections and 45-65% of worse outcomes[
26]. However, adherence to vaccination by PW faces obstacles due to a lack of knowledge regarding the risks of influenza or the benefits of vaccination[
27]. According to the Centers for Disease Control and Prevention (CDC) (2017), 21% of PW did not receive influenza vaccine recommendations from doctors or medical staff[
28].
Antiviral treatment has also been shown to reduce the risk of worse outcomes, including length of hospital stay, ICU admission, and death in PW[
29]. In this study, all PW with viral SARI received antiviral treatment. However, our data on the prescription of antivirals suggest that the clinical management of PW is more precise, targeting interventions in the early stages of the infection, unlike what is observed with NPW, which are clinically managed late, with a risk of worse results[
11]. Although there are national guidelines for the clinical management of SARI[
19], some hospital protocols may differ depending on their participation in the influenza surveillance network or the availability of hospital beds. As NPW are not a risk group for the development of SARI[
11], the majority of these patients tend to be hospitalized in more advanced stages of the disease in a tertiary hospital, in addition to the lack of clinical-epidemiological data due to the retrospective collection of data in medical records. All this date reinforces the findings from the present study, emphasizing the importance of investigating factors associated with the clinical evolution and severity of viral SARI in pregnant women, in order to promote public health decisions in health programs and specific clinical management protocols for this group.
This study had some limitations: the small number of research participants, due to the specificity of the selected group, and the large number of SARI notification forms with incomplete data. Even so, the data presented in this study are extremely relevant and could serve as a basis for expanded studies and for decision-making in public policies to reinforce the indication of immunization of pregnant women to prevent SARI due to influenza.
5. Conclusions
The impact of respiratory viruses on the number of SARI hospitalizations is higher in PW than in NPW. Pregnant women had low vaccination coverage for influenza. However, an inverse association with disease severity was observed, reinforcing the importance of early antiviral treatment. This suggests that broadening the influenza vaccination and providing accurate clinical management can reduce the burden of viral SARI in PW.
Author Contributions
“Conceptualization, M.B.N.; S.M.L. and M.C.D.; methodology, S.M.L. and M.P.; software, B.A.L.; M.E.G.; S.R.; validation, B.A.L.; S.M.L.; formal analysis, M.B.N.; S.M.R.; J.C.O. and N.S.C.; investigation, S.M.L.; L.A.P; M.P; S.R. and M.E.G.; resources, M.B.N.; M.C.D.; SR; and M.E.G.; writing—original draft preparation, B.A.L. and S.M.L.; writing—review and editing, M.B.N.; S.M.R.; J.C.O. and N.S.C; visualization, S.M.L.; B.A.L.; M.E.G.; S.R.; M.C.D.; M.P.; L.A.P.; S.M.R.; N.S.C.; J.C.O. and M.B.N.; supervision, M.B.N.; project administration, M.B.N.; funding acquisition, M.B.N.; M.C.D; S.R.;. and M.E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank the employees of the Virology Laboratory of the Clinical Hospital of the Federal University of Paraná, Hospital do Trabalhador, and Central Laboratory of the State of Paraná (LACEN), for data collection of clinical samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alimi Y, Lim WS, Lansbury L, Leonardi-Bee J, Nguyen-Van-Tam JS. Systematic review of respiratory viral pathogens identified in adults with community-acquired pneumonia in Europe. J Clin Virol. 2017;95:26-35. [CrossRef]
- Johnstone J, Majumdar SR, Fox JD, Marrie TJ. Viral infection in adults hospitalized with community-acquired pneumonia: Prevalence, pathogens, and presentation. Chest. 2008;134(6):1141-1148. [CrossRef]
- Fitzner J, Qasmieh S, Mounts AW, Alexander B, Besselaar T, Briand S; et al. Revision of clinical case definitions: Influenza-like illness and severe acute respiratory infection. Bull World Health Organ. 2018;96(2):122-128. [CrossRef]
- Pavia AT. What is the role of respiratory viruses in community-acquired pneumonia?: What is the best therapy for influenza and other viral causes of community-acquired pneumonia?. Infect Dis Clin North Am. 2013;27(1):157-175. [CrossRef]
- Hause AM, Avadhanula V, Maccato ML, Pinell PM, Bond N, Santarcangelo P; et al. Clinical characteristics and outcomes of respiratory syncytial virus infection in pregnant women. Vaccine. 2019;37(26):3464-3471. [CrossRef]
- Leeper C, Lutzkanin A 3rd. Infections During Pregnancy. Prim Care. 2018;45(3):567-586. [CrossRef]
- Silverman, N. S.; Beigi, R.; Influenza Vaccination During Pregnancy, Obstetrics, and Gynecology, 4rd ed.; APR 2018, Volume: 131 Páginas: E109-E114.
- Ruuskanen O, Järvinen A. What is the real role of respiratory viruses in severe community-acquired pneumonia?. Clin Infect Dis. 2014;59(1):71-73. [CrossRef]
- Wang R, Yan W, Du M, Tao L, Liu J. The effect of influenza virus infection on pregnancy outcomes: A systematic review and meta-analysis of cohort studies. Int J Infect Dis. 2021;105:567-578. [CrossRef]
- Sebghati M, Khalil A. Uptake of vaccination in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2021;76:53-65. [CrossRef]
- WHO – World Health Organization. . Global influenza strategy 2019-2030. Geneva (2019). Available at http://apps.who.int/iris; Accessed in 17/05/2024.
- Jarvis J. R. Dorey R. B, Warricker F. D. M, Alwan N. A, Jones C. E. The effectiveness of influenza vaccination in pregnancy in relation to child health outcomes: Systematic review and meta-analysis. Vaccine, v. 38, n. 7, p. 1601–1613, 2020.
- Li Y, Reeves RM, Wang X, Bassat Q, Brooks WA, Cohen C, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: A systematic analysis. Lancet Glob Health. 2019;7(8):e1031-e1045. [CrossRef]
- Paño-Pardo JR, Martínez-Sánchez N, Martín-Quirós A, Romero-Gómez MP, Muñoz-Muñiz M, Sánchez-Pastor M; et al. Influenza-like illness in pregnant women during summertime: Clinical, epidemiological and microbiological features. Eur J Clin Microbiol Infect Dis. 2011;30(12):1497-1502. [CrossRef]
- Azziz-Baumgartner E, Veguilla V, Calvo A, Franco D, Dominguez R, Rauda R; et al. Incidence of influenza and other respiratory viruses among pregnant women: A multi-country, multiyear cohort [published online ahead of print, 2021 Nov 12]. Int J Gynaecol Obstet. 2021;10.1002/ijgo.14018. [CrossRef]
- MS – Ministério da Saúde. Informe técnico – 23º campanha nacional de vacinação contra influenza. Secretaria de vigilância da saúde. Departamento de imunização e vigilância de doenças transmissíveis. Coordenação geral do programa nacional de imunizações. Brasília (2021). Available at < https://www.gov.br/saude/pt-br/media/pdf/2021/marco/16/informe-tecnico-influenza-2021.pdf>; Accessed in 11/02/2021.
- Irving SA, Ball SW, Booth SM, Regan AK, Naleway AL, Buchan SA; et al. A multi-country investigation of influenza vaccine coverage in pregnant individuals, 2010-2016. Vaccine. 2021;39(52):7598-7605. [CrossRef]
- PAHO - Pan American Health Organization. Evaluación multicéntrica de la efectividad de la vacuna de influenza estacional en América Latina y el Caribe. Rede REVELAC-i (2018). Available at < https://www.paho.org/es/documentos/evaluacion-multicentrica-efectividad-vacuna-influenza-estacional-america-latina-caribe>; Accessed 11/02/2021.
- MS - Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Protocolo de tratamento de Influenza: 2017 Brasília (2018); Available at <http://bvsms.saude.gov.br/publicacoes/protocolo_tratamento_influenza_2017>; Accessed in 11/02/2021.
- Kumari R, Shara S. D, Kumar A, Ende Z, Mishina M, Wang Y; et al. Antiviral Approaches against In fl uenza Virus. n. January, p. 1–43, 2023.
- Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS; et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374(9688):451-458. [CrossRef]
- Mertz D, Kim TH, Johnstone J, Lam PP, Science M, Kuster SP; et al. Populations at risk for severe or complicated influenza illness: Systematic review and meta-analysis. BMJ. 2013;347:f5061. [CrossRef]
- Mertz D, Lo CK, Lytvyn L, Ortiz JR, Loeb M; FLURISK-INVESTIGATORS. Pregnancy as a risk factor for severe influenza infection: An individual participant data meta-analysis. BMC Infect Dis. 2019;19(1):683. Published 2019 Aug 2. [CrossRef]
- Van Riel D, Mittrücker HW, Engels G, Klingel K, Markert UR, Gabriel G. Influenza pathogenicity during pregnancy in women and animal models. Semin Immunopathol. 2016;38(6):719-726. [CrossRef]
- Littauer EQ, Esser ES, Antao OQ, Vassilieva EV, Compans RW, Skountzou I. H1N1 influenza virus infection results in adverse pregnancy outcomes by disrupting tissue-specific hormonal regulation. PLoS Pathog. 2017;13(11):e1006757. Published 2017 Nov 27. [CrossRef]
- Bansal A, Trieu MC, Mohn KGI, Cox RJ. Safety, Immunogenicity, Efficacy and Effectiveness of Inactivated Influenza Vaccines in Healthy Pregnant Women and Children Under 5 Years: An Evidence-Based Clinical Review. Front Immunol. 2021;12:744774. Published 2021 Oct 6. [CrossRef]
- Ortiz JR, Perut M, Dumolard L, Wijesinghe PR, Jorgensen P, Ropero AM; et al. A global review of national influenza immunization policies: Analysis of the 2014 WHO/UNICEF Joint Reporting Form on immunization. Vaccine. 2016;34(45):5400-5405. [CrossRef]
- Quach THT, Mallis NA, Cordero JF. Influenza Vaccine Efficacy and Effectiveness in Pregnant Women: Systematic Review and Meta-analysis. Matern Child Health J. 2020;24(2):229-240. [CrossRef]
- Chow EJ, Beigi RH, Riley LE, Uyeki TM. Clinical Effectiveness and Safety of Antivirals for Influenza in Pregnancy. Open Forum Infect Dis. 2021;8(6):ofab138. Published 2021 Mar 20. [CrossRef]
- Dawwod F. S, Garg S, Fink R. V, Russell M. L, Regan A. K, Katz M. A; et al. Epidemiology and Clinical Outcomes of Hospitalizations for Acute Respiratory or Febrile Illness and Laboratory-Confirmed Influenza among Pregnant Women during Six Influenza Seasons, 2010-2016. Journal of Infectious Diseases, v. 221, n. 10, p. 1703–1712, 2020.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).