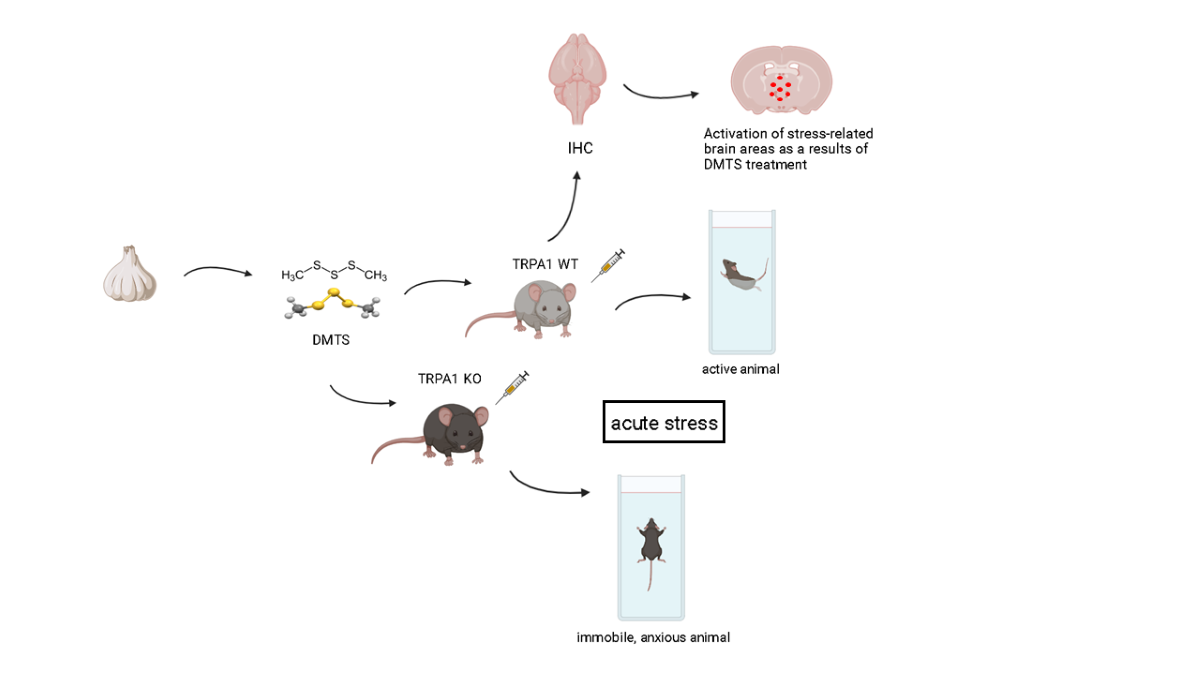
1. Introduction
Stress is the universal response of the organism to any real or perceived external or internal source of threat that compromises the integrity and balance of the organism and forces the individual to adapt in order to survive [
1]. Among stress adaptation disorders depression and anxiety are the most common psychiatric diseases. There is a growing number of drugs available for the treatment of anxiety and depression, but in a significant proportion of patients, these do not lead to improvement, even after weeks of continuous treatment [
2], which raises the importance of research on this topic. Polysulfides are endogenously produced in mammals and generally associated with protective functions: e.g. their antioxidant, neuroprotective, analgesic and anti-inflammatory effects have been described [
3,
4,
5]. Although the role of sulfide in the central nervous system is being actively researched, not much is known about its effects on stress-related behaviour. The biological effects of sulfide are mediated either by reaction with heme-bound iron or by modification of the sulfhydryl groups of cysteine amino acids in proteins [
6]. The latter process actually involves the formation of polysulfides [
7]. Polysulfides have been detected in the central nervous system of mice and humans [
3,
8]. The beneficial effects of sulfides have been reported previously during acute stress. Sodium hydrogen sulfide treatment attenuated the effect of acute stress in the forced swim test (FST) and the tail suspension test (TST) [
9,
10].
Inorganic polysulfide (POLY) is not an ideal candidate for drug development due to its reactivity and short half-life. Dimethyl trisulfide (DMTS), found in garlic, is more stable and has favourable pharmacokinetics [
11]. The effects of DMTS on acute stress have not yet been investigated. Several effects of DMTS (e.g. analgesic and anti-inflammatory effects) are mediated by transient receptor potential ankyrin 1 (TRPA1) ion channel activation [
4,
12].
The TRPA1 ion channel is found in astrocytes, where it is activated by inorganic polysulfide [
13]. Our previous results prove that the human TRPA1 channel expressed by the Chinese hamster ovary (CHO) cell line is activated by both POLY and DMTS [
4,
12,
14,
15]. TRPA1 expression is elevated in the dorsal root ganglion of animals exposed to acute stress [
16]. In our previous experiments, DMTS was shown to greatly reduce motor activity and respiration in mice, depressing the central nervous system, mediated by the TRPA1 channel [
4]. Given that central nervous system inhibitors/depressants (e.g. benzodiazepines and barbiturates) tend to reduce levels of anxiety, we hypothesized that an appropriately chosen dose of DMTS may have an anxiolytic effect on acute stress-related behaviour.
In addition to sensory neurons, substance P (SP) is also localized in the central nervous system [
17]. SP and its neurokinin1 (NK1) receptors are well-known stress mediators. Neurons in some limbic structures express SP and NK1 receptors. Stress can alter SP release or receptor internalization in these brain areas. The tachykinin system has also been implicated in the pathophysiology of mood disorders. Increased SP levels can be detected in the lateral septal nucleus (LS) in response to acute stress. Mice knocked out of the
Tac1 gene encoding SP are less responsive in models of acute stress [
18]. Given that intraperitoneal administration of the H
2S donor NaHS to mice leads to a significant elevation of circulating SP levels, sulfide contributes to the release of SP in the peripheral nervous system [
19]. A similar SP release might occur in the central nervous system, too.
The main components of the endocannabinoid system (ECS) are cannabinoid receptor proteins (CB1 and CB2), ligands (endocannabinoids), and proteins involved in the regulation and metabolism of endocannabinoids. Endocannabinoids mediate retrograde signalling. They are synthesized on demand and are not stored in vesicles [
20]. They are produced postsynaptic and bind to presynaptic CB receptors. The primary enzymes responsible for the hydrolysis of endocannabinoids, namely anandamide and 2-AG (2-arachidonoylglycerol) are fatty acid amide hydrolase (FAAH) and monoacylglyceride lipase (MGL). These two enzymes are important pharmacological therapeutic targets [
21]. FAAH and MGL contain functional disulfide bonds that might be destroyed by DMTS, potentially inhibiting enzyme activity [
22].
Animal studies have provided more direct evidence for the involvement of the ECS in anxiety and depression [
23,
24,
25]. The ECS has been shown to function in several brain regions such as the prefrontal cortex, hippocampus, amygdala, and midbrain periaqueductal grey matter that are involved in various psychiatric disorders [
26]. To date, few studies have measured endocannabinoid levels in psychiatric disorders. Basal serum concentrations of anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are significantly reduced in depression, suggesting a role for this system in the disorder [
27,
28]. Genetic deficiency or chronic inhibition of FAAH has an anti-anxiety and anti-depressant effect [
29] The effect of polysulfides on FAAH is likely because the activity of the enzyme can be inhibited by cysteine modification [
30]. As mentioned above, MGL is also found in the central nervous system and is an important component of the ECS. Its inhibition attenuates the effects of acute stress and depression [
31].
In summary, scientific data suggest that DMTS can 1) activate TRPA1 ion channels [
4,
5], 2) release SP from neurons [
19], which activates its receptor NK1, and 3) inhibit endocannabinoid-degrading enzymes [
22], namely FAAH and MGL.
Our aim was to investigate the effects of DMTS in an acute stress mouse model and the potential mechanisms underlying its effects on acute stress-induced behaviour. We set out to clarify the role of the endocannabinoid system in the process using the CB1 antagonist AM251. This pathway has not yet been explored in detail, so the data obtained may help to understand the possible interactions between the two conserved systems (sulfide signalling and ECS). To test our hypotheses, we used Trpa1 WT, KO, C57BL/6J, NK1 KO, and Tac1 KO strains and performed open field test (OFT), FST, TST, and c-FOS immunohistochemistry.
2. Results
2.1. Finding the suitable dose of DMTS via open field test
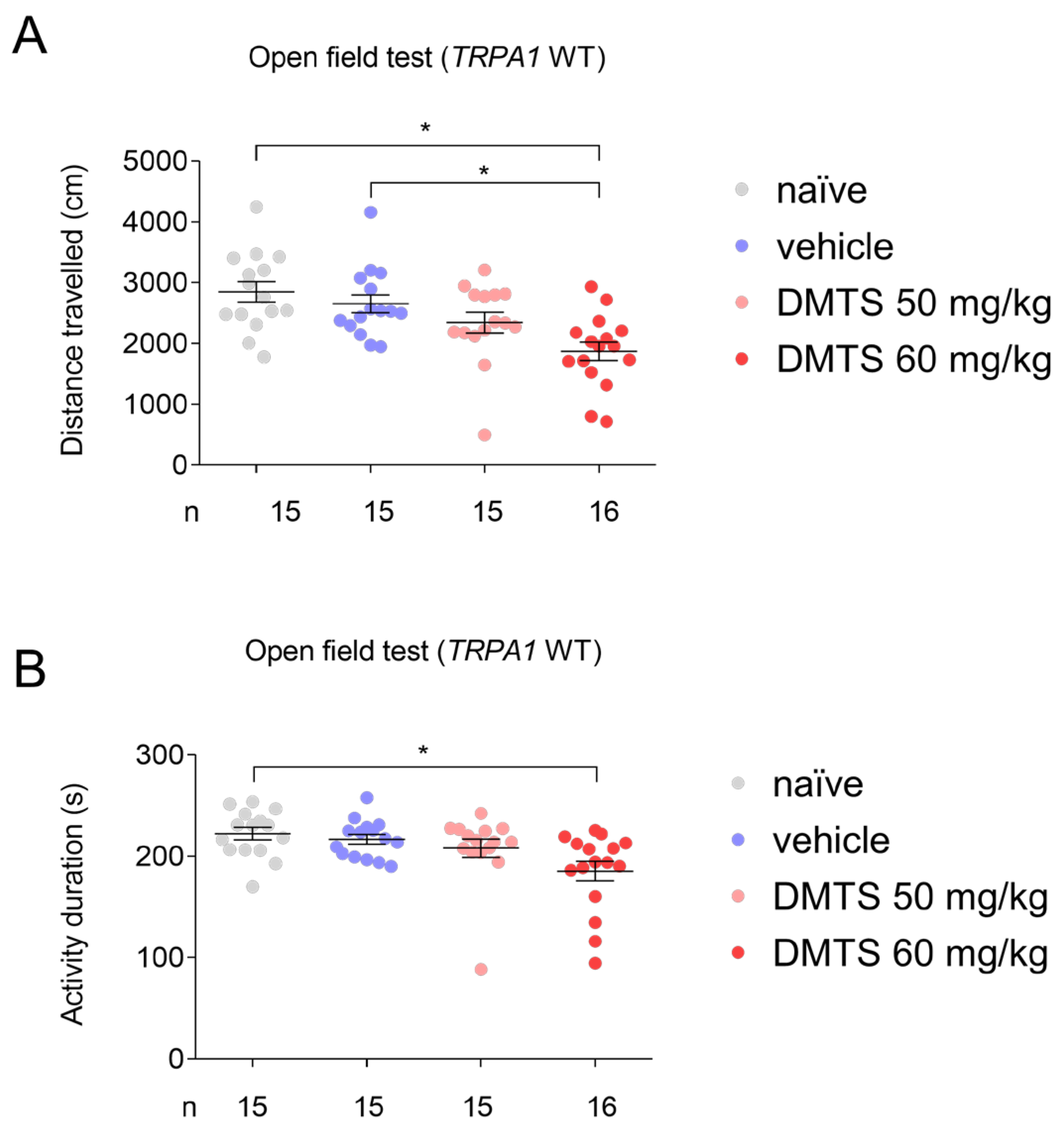
The time spent moving was measured in seconds and the distance covered in centimetres. DMTS at a dose of 60 mg/kg significantly reduced (p<0.05) both the time spent moving and the distance travelled in the observed time frame compared to the untreated group in the TRPA1 WT strain. This negative effect was not observed at a dose of 50 mg/kg DMTS, and this dose was used in the FST and the TST. The vehicle of 50 mg/kg DMTS did not inhibit spontaneous movement of the animals (
Figure 1).
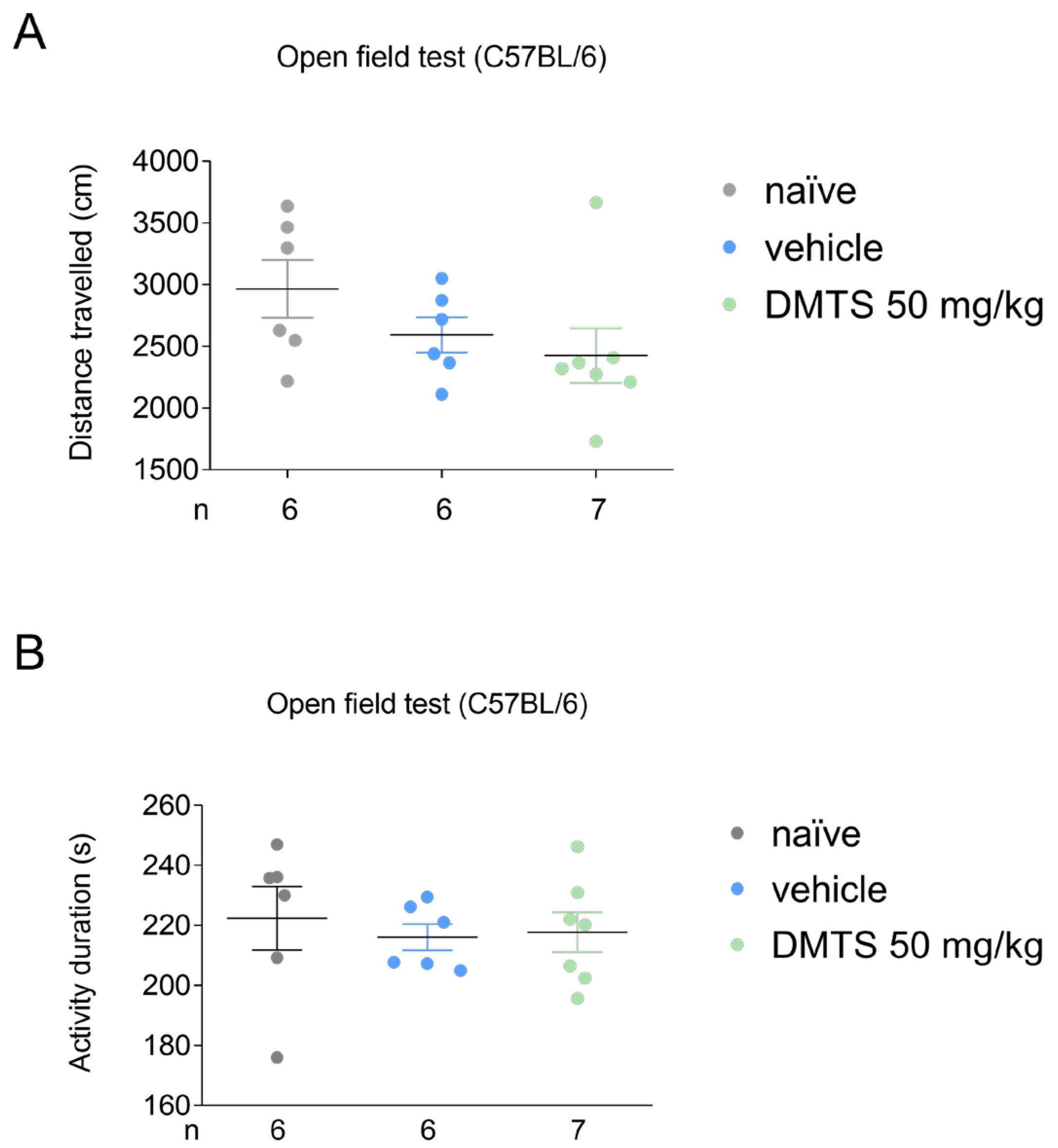
In C57BL/6J mice, DMTS at a dose of 50 mg/kg and vehicle were tested compared to the untreated group. Neither DMTS (50 mg/kg) nor vehicle reduced the time spent moving and the distance travelled during the observed time period (
Figure 2). Based on these results, DMTS at 50 mg/kg and the corresponding vehicle were used for the FST and the TST.
2.2. Behavioural tests
2.2.1. Effect of DMTS on TRPA1 WT and KO animals in the forced swim test
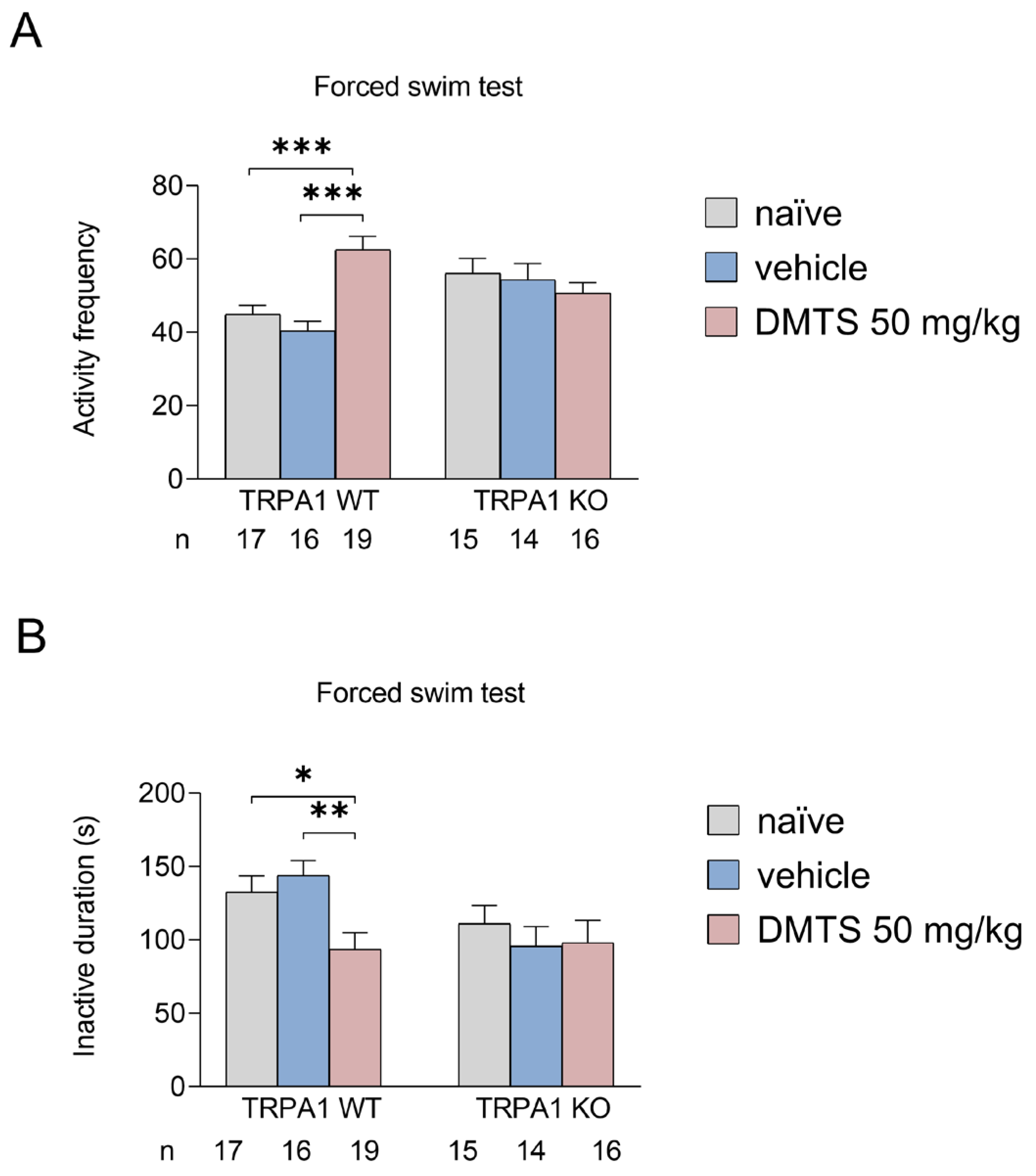
In the behavioural test, we measured the number of times the animal entered a more active state (frequency) and the amount of time it remains inactive (seconds). The more times the animal enters an active phase and the less time it spends inactive, the better the anxiolytic effect, as the animal struggles to escape. In wild type mice, DMTS treatment increased the frequency of active periods compared to both vehicle-treated and untreated groups. Furthermore, vehicle yielded similar results to those obtained in the untreated group. However, this protective effect of DMTS was not observed in animals with genetic TRPA1 ion channel dysfunction. The same trend was also observed for the time spent inactively (
Figure 3). It is important to note that the less time an animal spends in inactivity, the more it struggles, the less stressed it is.
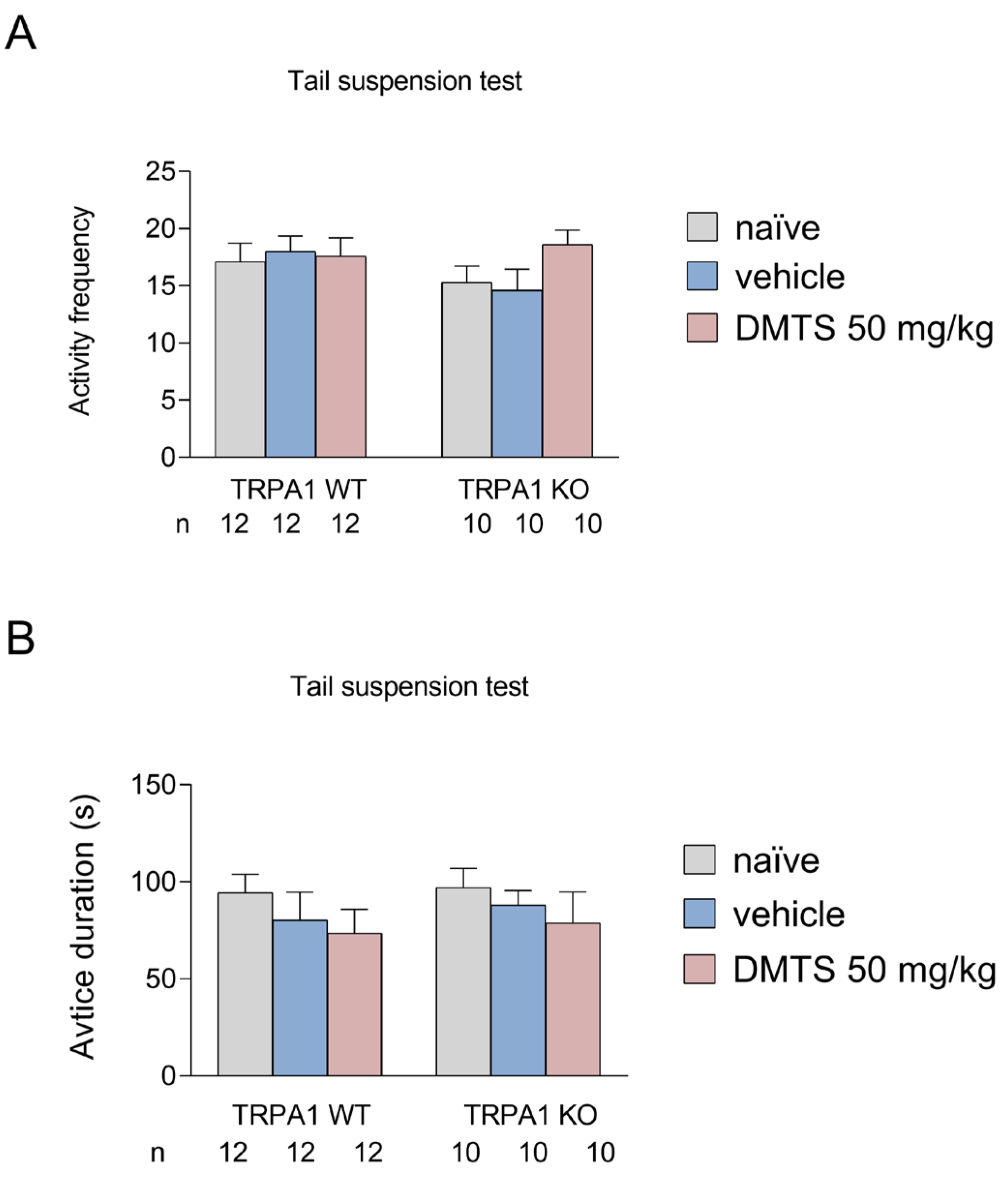
2.2.2. Effect of DMTS on TRPA1 WT and KO animals in the tail suspension test
As with the FST, we also looked at the frequency of active periods and the time spent immobile. The more often the animal engages in active movement or escape and the less time it is immobile, the better the DMTS is in reducing stress-related behaviour. The graphs show the same treatment groups as shown in the previous section: untreated, vehicle-treated, DMTS-treated (50 mg/kg). We found that DMTS did not significantly affect behaviour in any strain. Although a positive trend in inactivity was observed in response to DMTS, there was no significant difference between treatment groups and a similar trend was observed in wild type and KO animals (
Figure 4).
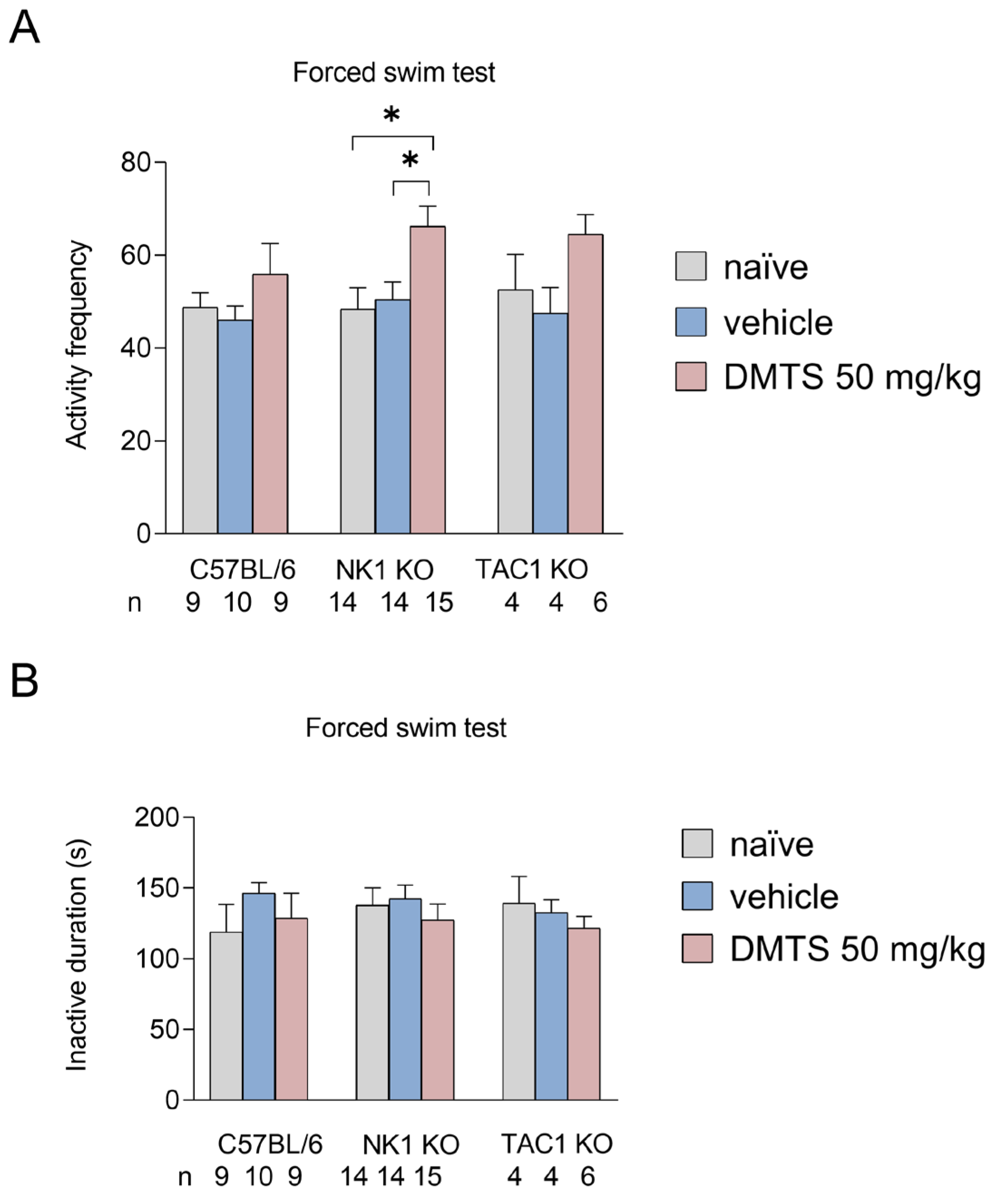
2.2.3. Exploring the involvement of substance P in mediating the effect of DMTS
We examined the time spent immobile in seconds and activity frequency in the FST. No significant differences were observed between treatment groups in the time spent inactive for either NK1 wild type or transgenic animals. In terms of the frequency of active periods in NK1 KO animals, DMTS increased the number of active periods of the animals compared to both the untreated and vehicle-treated groups (p<0.05). A similar trend was observed in C57BL/6J and Tac1 KO animals, but the difference was not statistically significant (
Figure 5).
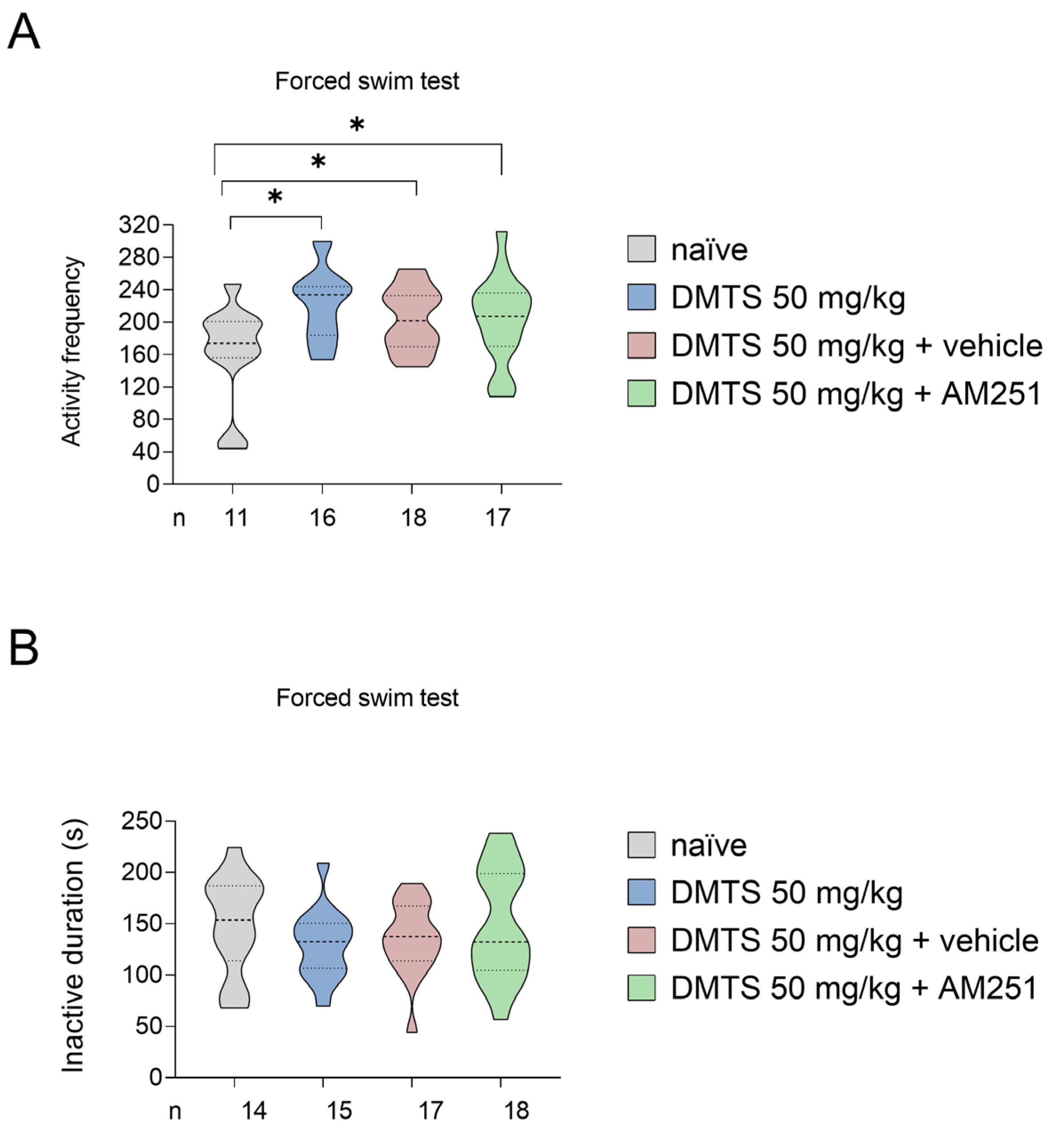
2.2.4. Investigation of the contribution of the endocannabinoid system in mediating the effects of DMTS
We also assessed the time spent immobile and the frequency of active periods in FST in C57BL/6J mice. Both DMTS treatment and DMTS-AM251 co-administration significantly increased the activity frequency (p<0.05). The duration of inactivity was not affected by DMTS treatment or DMTS+AM251 treatment (
Figure 6).
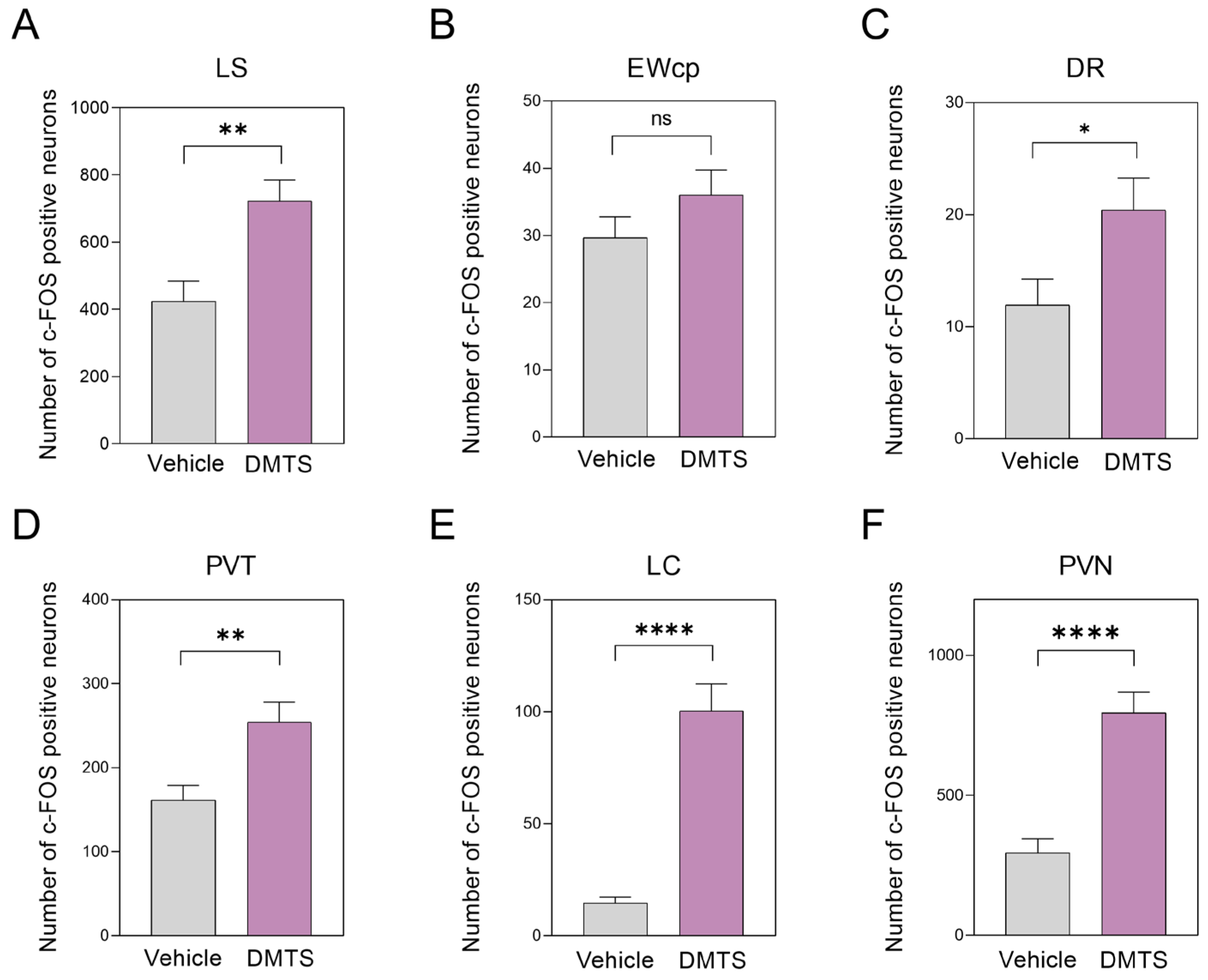
2.3. Immunohistochemistry
c-FOS immunoreactivity was examined in stress-relevant brain areas comparing brain samples from vehicle-treated and DMTS-treated animals to suggest that DMTS treatment has an effect on acute neuronal activity in these brain areas (n=15). DMTS or vehicle were administered to TRPA1 WT animals to identify areas activated by the substance (
Figure 7).
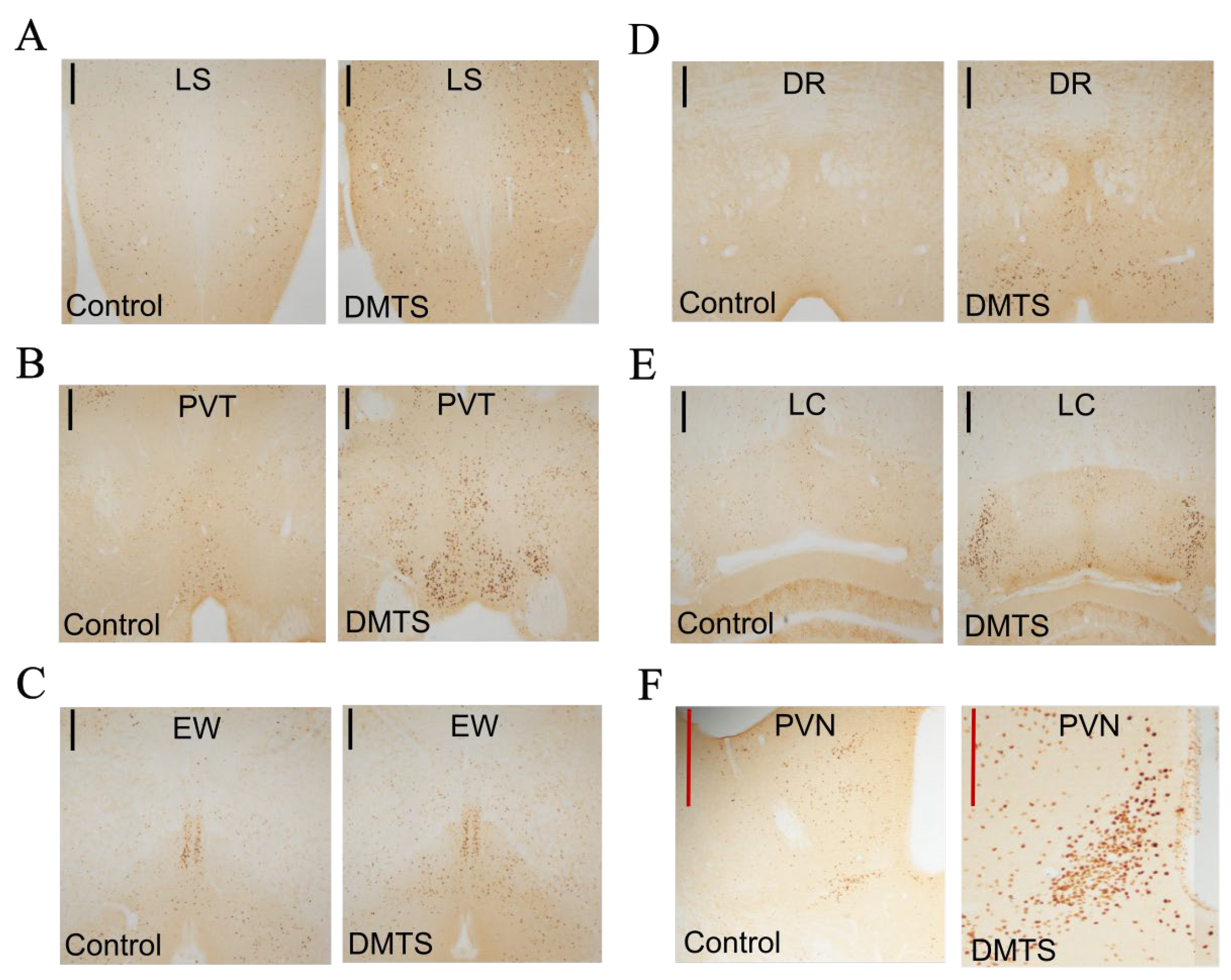
The number of c-FOS positive neurons in the LS brain area increased after treatment, compared to control animals, indicating that significant c-FOS immunoreactivity was induced in this area following DMTS treatment (p<0.01). There was a significant increase in c-FOS immunoreactivity also in the DR area compared to the control group (p<0.05). In the PVT area, DMTS treatment resulted in nearly one and a half times more c-FOS active neurons in comparison to the control group (p <0.01). In the LC, we observed the highest c-FOS immunoreactivity boost in treated animals (p<0.0001) compared to control mice. We also found a nearly triple increase in c-FOS immunoreactivity in the PVN area after DMTS treatment compared to the vehicle-treated group (p<0.0001). In the EWcp, although we observed a mild increase in c-FOS activity after DMTS treatment, the number of c-FOS positive neurons was not statistically significant compared to the control group (
Figure 8).
3. Discussion
Since the effect of DMTS on the general activity of the animals has not been studied before, the first aim of our research was to determine the appropriate dose of it that does not limit spontaneous movement and locomotor activity of the animals. For this purpose, we exposed TRPA1 wild type animals to an OFT. Our results obtained showed that DMTS at a dose of 50 mg/kg is suitable for further experiments and does not negatively affect the locomotor activity of the animals.
We then investigated the effects of a single dose of DMTS in models of acute stress, namely the FST and TST. In the FST, we found that in the TRPA1 WT strain, DMTS treatment increased the frequency of active periods and decreased the time spent immobile. Our findings are in the same line as those of Chen and co-workers, who tested an H
2S donor compound in FST, and found that the treatment reduced inactivity of the animals to the extent that we found in our study [
9]. However, Chen and colleagues used chronic stress to induce depression-like behaviour and also administered the treatment chronically to the animals for 7 days and no organic polysulfide was administered. Given that the effects of sulfide are mediated by polysulfides according to literature [
6,
13,
35], testing DMTS might be more relevant. We did not observe the stress-relieving effect in
Trpa1 KO animals, suggesting that the TRPA1 ion channel is involved in mediating the effects of DMTS on stress-induced behaviour. The role of TRPA1 itself in stress-related processes is well established. A recently published study by Kormos et al. shows that
Trpa1 gene-knockout animals responded differently to the chronic variable mild stress model of depression than wild type ones. In addition, their research was the first to identify the presence of TRPA1 in the EWcp nucleus in mice and in humans, which is known to play an important role in anxiety and mood regulation
via its urocortinergic neurons [
36]. The research team also demonstrated the functional activity of the channel in the EWcp nucleus [
36]. They have also investigated the role of the EWcp nucleus/TRPA1 ion channel in a mouse model of post-traumatic stress disorder (PTSD). Their results show that TRPA1 ion channel mRNA expression is decreased in the PTSD model, with a simultaneous increase of neuronal UCN1 peptide content in EWcp. This proposes the involvement of the cation channel in stress (mal)adaptation contributing to the pathomechanism of depression and PTSD [
38].
In C57BL/6J mice, we expected the same protective effect of DMTS. In C57BL/6J wild type, NK1 KO receptor-deficient and Tac1 KO gene-deficient strains, we observed a similar trend: although DMTS treatment reduced the time spent immobility and increased the frequency of active periods, there was no significant difference between wild type and gene-deficient animals, nor between different treatment groups of each strain. The exception was the NK1 KO strain, where an increase in the frequency of active periods was observed in response to DMTS in the acute stress models.
Taking into account the fact that we used a global knockout strain in this study, we cannot exclude the possibility that the loss of functional TRPA1 channels outside the central nervous system may have contributed to the observed behavioural alterations.
In the TST, no statistically significant difference was found between treatment groups of any mouse strains, although the positive effect of DMTS on the activity of the mice presented as a trend in the results. The fact that we detected no genotype-related difference or effect of DMTS in the TST further supports the concept that effects might be test-specific [
36]. In fact, it has been suggested that although the FST and TST produce similar results in antidepressant-like activity studies, the mechanisms of drug response in the two tests may be different. These results, in agreement with this study, suggest that different stressors (FST/TST) affect locomotor activity differently [
39].
Although we hypothesized the release of SP in response to DMTS and its role in mediating the effect of DMTS [
40], we did not observe any behavioural differences in
NK1 gene knockout animals (receptor for SP). It is worth noting that the time spent immobile in the TST was lower in gene knockout animals, and further decreased in DMTS-treated animals suggesting a stress-relieving effect of SP deficiency (a known stress mediator [
16]) and a contribution of DMTS to this effect. However, the difference was not statistically significant. In the FST, the number of active periods in
NK1 gene knockout animals was increased in DMTS-treated animals, indicating that the absence of the receptor, and thus the inability of SP to act, contributes to the positive effect of DMTS. It would be worthwhile to test the relationship between DMTS and SP in another experimental setting.
Interestingly, both DMTS treatment and the combination of DMTS and AM251 increased the number of active periods in the FST. Considering that DMTS was able to exert its effect even when administered with a CB1 receptor antagonist, it is assumed that the ECS is not involved in our model of acute stress. The role of the ECS might be worth investigating in a chronic stress model.
The
c-Fos gene product (c-FOS protein) is a commonly used and accepted acute neuronal activation marker in neuroscience. Brain samples from animals perfused 1 hour after DMTS treatment were subjected to c-FOS immunohistochemistry. DMTS treatment caused a significant increase of c-FOS expression in almost all brain areas examined. The magnitude of this increase was brain area specific: in the LC, we observed a nearly tenfold increase in c-FOS expression, whereas in the DR and PVT areas it was approximately 2.5 fold. Interestingly, the EWcp nucleus did not respond to the DMTS treatment, despite the fact that this brain area contains the highest expression of
Trpa1 mRNA of all the brain areas studied. This suggests that it is not exclusively the TRPA1 ion channel that mediates the effect of DMTS on stress adaptation. This is confirmed by the elevated activation in the LC brain area after treatment, given that there is no TRPA1 ion channel in this brain area [
41]. Another explanation is that there is secondary activation from an area of the brain in which the TRPA1 ion channel is expressed.
Both serotoninergic neurons in the DR and urocortinergic cells in the EWcp contribute to the development of stress-related mood disorders and anxiety [
42]. The activation of DR neurons results in anxiety-like behaviour, as confirmed by c-FOS. [
43] However, it is important to mention that although the TRPA1 ion channel is detected in certain cells of the DR, these are not the serotonergic cells, implying that there were different ways of mediating the effects of DMTS in this area. Furthermore, EWcp urocortinergic neurons interact with DR serotoninergic neurons, there is a back-and-forth connection between the two nuclei. The EWcp projection is sent to the DR, where there are corticotropin-releasing factor (CRF) receptors, which are affected by UCN1, and affects serotonin release, and therefore mood and anxiety [
44,
45]. It is worth noting that although c-FOS is a widely used and well-validated marker of acute neuronal activation, it does not provide information on neuronal inhibition, although the latter may also play an important role in the observed differences [
46].
Increased basal c-FOS activity in the EWcp, which did not increase further by treatment, may contribute to reduced sensitivity of the HPA axis, suggesting that the positive effect of the treatment on activity during acute stress, in the FST, might be mediated through this area. Interestingly, among the brain areas tested, only the EWcp nucleus shows evidence of TRPA1 ion channel expression in certain neurons [
36,
37], and no effect of DMTS was observed in KO animals in some behavioural tests. The urocortinergic neurons from the EWcp nucleus project primarily to the DR and PVN areas, where they are involved in modulating the stress response [
45,
47,
48], which may further explain why the DR area has the lowest increase in c-FOS activation.
In the future, we plan to test the effect of DMTS in the unpredictable mild stress mouse model of depression to explore other background mechanisms besides the TRPA1 ion channel in mediating the effect of DMTS.
4. Materials and Methods
4.1. Animals and experimental design
Trpa1 WT and KO, C57BL/6J, NK1 KO, and Tac1 KO mouse strains were kept under standard conditions at the Department of Pharmacology and Pharmacotherapy, University of Pécs. TRPA1 WT and KO homozygous lines were bred separately after being backcrossed into C57BL/6J mice for 8 generations. In TRPA1 KO mice, although TRPA1 ion channels are present, they are non-functional. SP receptor is absent in NK1 KO mice, and Tac1 KO animals lack the Tac1 gene, which is responsible for encoding substance P and neurokinin A. The C57BL/6J strain is the wild type of the NK1 KO and Tac1 KO strains. Male, 8-12 weeks old, 25-30 g animals were used in our experiments.
In the first experiment, OFT was used to determine the appropriate intraperitoneal doses of DMTS and vehicle, in which C57BL/6J and TRPA1 WT animals were divided into three treatment groups: untreated (naïve), treated with vehicle, and treated with DMTS. A solution of 3% m/v was prepared by dissolving polysorbate 80 in physiological saline. By dissolving DMTS at a concentration of 10 mg/ml, the DMTS stock solution was obtained. Solutions were diluted further with physiological saline to produce working solutions. Solutions were administered intraperitoneally to the animals. The vehicle used in the experiments contained 1.5% m/v polysorbate 80.
After determining the appropriate dose of DMTS, the animals were subjected to various behavioural tests. We investigated the effect of DMTS in an acute stress model by FST and TST using TRPA1 WT and TRPA1 KO animals, in a similar method to OFT using naive, vehicle, and DMTS-treated groups. The potential impact of substance P in acute stress situations was examined only by FST in C57BL/6, NK1 KO, and TAC1 KO mice, also dividing the animals into the three groups mentioned above.
The role of the endocannabinoid system in the behaviour during acute stress was evaluated in C57BL/6J strain using FST. AM251 were prepared in DMSO (10 mg/ml) and in this case the animals were divided into 4 groups: naïve, DMTS (50 mg/kg) treated, DMTS and vehicle co-treated, and DMTS and AM251 (3 mg/kg) co-treated. DMTS was administered intraperitoneally half an hour before the experiments and AM251 was administered subcutaneously into the interscapular region half an hour before the DMTS treatment to avoid drug interaction.
Finally, stress-related brain areas were analysed in the TRPA1 WT strain by c-FOS immunohistochemical staining using vehicle-treated and DMTS-treated groups. A separeta groups of animals were used that did not participate in behavioural tests.
4.2. Behavioural tests
4.2.1. Open field test
The OFT was used to determine the appropriate dose of DMTS that does not reduce locomotor activity and spontaneous movement of the animals. Although the OFT is a test used to assess locomotor activity, anxiety and exploratory behaviour in rodents, we used this method only to determine whether different doses of DMTS and their respective vehicles caused the animal to move less, the same or more in the box [
32]. The evaluation started 30 seconds after the appearance of the animal (this is how long we allowed them to explore the new environment) and lasted for 5 minutes (
Figure 9). The time spent moving and the total distance covered during the observed interval were recorded using Noldus EthoVision XT 15 software. Doses of 50 mg/kg and 60 mg/kg of DMTS and its associated vehicle were tested. Two strains of mice were treated: male
TRPA1 WT and C57BL/6J animals.
4.2.2. Forced swim test and tail suspension test
After establishing the appropriate dose of DMTS, the experimental animals were studied in acute stress tests - FST and TST. These tests represent acute stress situations in which we can quantify depression-like behaviour in animals. FST was performed with male
TRPA1 WT and KO, C56BL/6J,
NK1 KO and
Tac1 KO strains. TST was carried out on
TRPA1 WT and KO strains. The animals were first exposed to the arrangement during the experiment. Treatment was performed with a single dose of DMTS or the appropriate vehicle 30 min before the behavioural tests (
Figure 10).
Animals were placed in a cylindrical transparent bottle filled with 24 °C tap water to a height that they could not climb out of. Animals were placed in the water-filled bottle for 6 min each and the last 4 min were recorded to determine how much time they spent immobile and how many times they entered a high-activity phase. FST results were processed using Noldus EthoVision XT 15 software. An animal was considered inactive if it floated vertically in the water and only moved the legs to keep its head above the water. Depression-like behaviour is characterised by the animal giving up struggle and floating in the water.
During TST, mice were suspended by their tails for 6 min at a height of 50 cm. During the last 4 min, the time spent motionless and the number of times the animals entered a high-activity phase were recorded.
Experiments were carried out in a separate room so the animals could not see each other swim and the bottles were always washed after each animal. Mice were taken out of the animal facility an hour before treatment for habituation, and were acclimatised for 60-60 minutes on the 3 days before the experiment.
2.3. Perfusion and tissue collection
Immunohistochemistry was performed in a separate group of animals to investigate the effect of DMTS treatment on specific brain areas in the TRPA1 wild type strain. Animals were anaesthetised with urethane (intraperitoneal, 2.4 g/kg), and transcardially perfused with 100 µM phosphate buffered saline (PBS) followed by 4% m/V paraformaldehyde (in 200 µM Millonig buffer). Brain tissue was collected from each animal and postfixed in 4% m/V paraformaldehyde. Coronal sections of 30 µm thickness were made using Leica VT1000S vibratome. The sections were stored in PBS containing 0.01% sodium azide in 4°C. Immunohistochemistry was performed to detect the expression of c-FOS protein in the centrally projecting Edinger-Westphal nucleus (EWcp) (Bregma -2.92 mm to -4.04 mm), dorsal raphe nucleus (DR) (Bregma -4.04 to -4.16 mm), locus coeruleus (LC) (Bregma -5.34 to -5.40 mm), lateral septum (LS) (Bregma 0.26 to -0.10 mm), paraventricular nucleus of the thalamus (PVT) (Bregma -0.22 to -0.70 mm) and paraventricular nucleus of the hypothalamus (PVN) (Bregma 0.26 mm).
2.3.1. c-FOS immunohistochemistry
Sections were washed in 0.1M PBS (pH 7.6) for 3x10 min. Inhibition of endogenous peroxidase activity was performed in 1% H2O2 solution in PBS for 30 min. After washing steps, sections were treated with 0.5% Triton X-100 and aspecific binding sites were blocked by 2% normal goat serum in PBS for 30 min. Sections were incubated overnight at room temperature with rabbit polyclonal anti-c-FOS antibody in a 1:500 diluted solution (Santa Cruz Biotechnology Inc., sc-52, Santa Cruz, CA, USA) which was dissolved in PBS containing 2% normal goat serum. After washing steps, sections were incubated with biotinylated secondary antibody (biotinylated anti-rabbit gamma globulin; Vectastain Elite ABC-HRP Kit). This was followed by incubation for one hour in avidin-biotin complex (Vectastain Elite ABC Kit) at 20 °C. After washing in PBS, Tris-buffer containing 0.02% DAB and 0.00003% H2O2 was added to the sections. The reaction was monitored under a microscope, measured with a stopwatch, and stopped with PBS when the optimal contrast background was reached. The sections were mounted on gelatine-coated slides and dried overnight at room temperature. Slides were then dehydrated in alcohol (70%, 96%, absolute alcohol 10-10 min), followed by xylene treatment (2x20-60 min). Slides were coverslipped with DePex.
2.4. Evaluation methods
2.4.1. Noldus EthoVision XT 15
The Noldus EthoVision XT system was developed to monitor animal movements in a laboratory environment. Using an overhead camera, the software can track animals based on black and white contrast. This type of evaluation is reliable compared to subjective methods, provides a wide range of data and can be consistently applied in different laboratories [
33].
In the OFT animals were separated from the background by the software based on the black and white contrast. The evaluation starts 30 seconds after the animal appears and lasts for 5 minutes. The time spent moving and the distance travelled during this time are assessed.
In the case of the FST, once the animal was in the water, the 6-minute recording started. The last 4 minutes were evaluated. We measured how long the animal was inactive. We also looked at how many times the animal entered a higher activity state. Activity detection is based on the changes of the outline of the animals on the frames of the recordings.
2.4.2. ImageJ
After photographing the histological sections, we used ImageJ and the IHC Toolbox. By outlining the brain area to be examined, setting the appropriate thresholds, the program separates the cells from the background based on density, counts the number of cells within the area, and calculates the size of the outlined area. The IHC Toolbox extension also includes a model optimised specifically for DAB staining. The program allows manual or automatic cell counting. In our case, manual counting was not practical given the large number of cells, so we used automatic counting. We converted the RGB image to greyscale (8-bit). Calculation was based on the area of the image. In the next step, we highlighted all the structures to be counted using the Threshold command. Finally, by clicking on the Analyse command, the program calculated the number of structures highlighted relative to the area selected.
2.5. Statistics
The data presented show the mean and standard error of the mean of the experimental groups. Unpaired t-test was used to evaluate the immunohistochemical data. The normality of the data distribution was checked by the Shapiro-Wilk test and the homogenity of variance by Bartlett's Chi-squared test [
34]. The data obtained in the Noldus system evaluated by one-way analysis of variance (ANOVA) in the case of OFT. In the FST to assess the effect of AM251, we used also a one-way ANOVA. In all other cases, we evaluated data by 2-way ANOVA. All
post hoc analyses were performed by the Fisher test based on first- or second-order effects in the ANOVA. Statistical evaluation was performed using GraphPad Prism 8.
5. Conclusions
We provide, to our knowledge, the first evidence for the effect of DMTS in the central nervous system, which may be mediated in part through the TRPA1 ion channel.
DMTS may have a specific regulatory function of acute stress-induced behaviour in part through regulation of the TRPA1 ion channel. Since similar results were obtained in C57BL/6 wild type, NK1 KO receptor-deficient, and Tac1 KO gene-deficient animals, we excluded a role of SP in this process. In our ongoing research, we are investigating the effects of DMTS in a chronic unpredicted mild stress model of depression by examining the involvement of the TRPA1 ion channel and the endocannabinoid system.
DMTS may be an ideal candidate for further study as a potential substance in the regulation of stress adaptation.
6. Limitations
The behavioural phenotype of our global knockout mouse strain may be due to developmental compensation. We cannot exclude the possibility that other peripheral or central mechanisms, which we did not study here, may contribute to the behavioural phenotype of the knockout mice, as the functional receptor was deleted both peripherally and centrally. In this study, female mice and tissue samples were not examined. Responses in female mice may be influenced by the phase of the oestrous cycle, as some of the examined brain areas express the oestrogen receptor ß [
49,
50,
51,
52,
53].
Another limitation is related to the substance used in our research study, as DMTS has not been tested in behavioural tests before, so there is not enough data on the possible side effects of DMTS on the central nervous system and animal behaviour due to the lack of previous research.
Author Contributions
Conceptualization, G.P.; methodology, G.P., K.G., V.K. and A.D.; validation G.P.; formal analysis, G.P.; writing—original draft preparation, K.G.; writing-review and editing, G.P.; visualization, G.P., K.G. and V.K.; supervision, G.P.; funding acquisition, G.P. and E.P. E.P. provided the C57BL6J, Trpa1 KO and WT mice. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by OTKA FK 132454 from the National Research, Development and Innovation Office, Hungary. VK was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (BO/00750/22/5), by the New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund (ÚNKP-23-5-PTE-1991) and the Research grant of Medical School, University of Pécs (KA-2022-29).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, the 1998/XXVIII Act of the Hungarian Parliament on Animal Protection and Consideration Decree of Scientific Procedures of Animal Experiments (243/1998), the European Communities Council Directive of 2010/63/EU, the requirements of the International Association for the Study of Pain (IASP) and was approved by the Ethics Committee on Animal Research of University of Pécs (license number BA02/2000-2/2020, approved on 26 February 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We wish to thank Teréz Bagoly and Csenge Sánta for their expert technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
7. Abbreviations
TRPA1, transient receptor potential ankyrin 1; DMTS, dimethyl trisulfide; DMSO, dimethyl sulfoxide; WT, wild type; KO, knockout; CHO, Chinese hamster ovary; CB1, cannabinoid receptor 1; AEA, anandamide; 2-AG, 2-arachidonoylglycerol; MGLL, monoacylglycerol lipase; FAAH, fatty-acid amide hydrolase; LC, locus coeruleus; DR, dorsal raphe nucleus; LS, lateral septum; PVN, paraventricular nucleus of the hypothalamus; PVT, paraventricular nucleus of the thalamus; EWcp, centrally projecting Edinger-Westphal nucleus; SP, substance P; NK1, neurokinin 1 receptor; FTS, forced swim test; TST, tail suspension test; OFT, open field test; MBT, marble burying test; SPT, sucrose preference test; POLY, inorganic polysulfide; Tac1, tachykinin precursor 1; PBS, phosphate buffered saline; IHC, immunohistochemistry; DAB, 3,3′-diaminobenzidine; ANOVA, analysis of variance; PTSD, post-traumatic stress disorder; UCN1, urocortin 1; H2S, hydrogen sulfide; NaHS, sodium hydrosulfide; ECS, endocannabinoid system; CRF, corticotropin-releasing factor
References
- McEwen, B.S. The Neurobiology of Stress: From Serendipity to Clinical Relevance. Brain Research 2000.
- Wong, M.-L.; Licinio, J. Research and Treatment Approaches to Depression. Nat Rev Neurosci 2001, 2, 343–351. [Google Scholar] [CrossRef]
- Sun, H.-J.; Wu, Z.-Y.; Nie, X.-W.; Bian, J.-S. Role of Hydrogen Sulfide and Polysulfides in Neurological Diseases: Focus on Protein S-Persulfidation. CN 2021, 19, 868–884. [Google Scholar] [CrossRef] [PubMed]
- Pozsgai, G.; Payrits, M.; Sághy, É.; Sebestyén-Bátai, R.; Steen, E.; Szőke, É.; Sándor, Z.; Solymár, M.; Garami, A.; Orvos, P.; et al. Analgesic Effect of Dimethyl Trisulfide in Mice Is Mediated by TRPA1 and Sst 4 Receptors. Nitric Oxide 2017, 65, 10–21. [Google Scholar] [CrossRef]
- Dombi, Á.; Sánta, C.; Bátai, I.Z.; Kormos, V.; Kecskés, A.; Tékus, V.; Pohóczky, K.; Bölcskei, K.; Pintér, E.; Pozsgai, G. Dimethyl Trisulfide Diminishes Traumatic Neuropathic Pain Acting on TRPA1 Receptors in Mice. IJMS 2021, 22, 3363. [Google Scholar] [CrossRef]
- Kimura, H. Signalling by Hydrogen Sulfide and Polysulfides via Protein S-sulfuration. British Pharmacological Society, 2020; 177, 720–733. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Kuhnle, G.G.C.; Dyson, A.; Fernandez, B.O.; Grman, M.; DuMond, J.F.; Barrow, M.P.; McLeod, G.; Nakagawa, H.; Ondrias, K.; et al. Key Bioactive Reaction Products of the NO/H 2 S Interaction Are S/N-Hybrid Species, Polysulfides, and Nitroxyl. Proc. Natl. Acad. Sci. U.S.A. 2015, 112. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Kawamura, K.; Kimura, Y.; Shibuya, N.; Kimura, H.; Ogasawara, Y. Analysis of Endogenous H2S and H2Sn in Mouse Brain by High-Performance Liquid Chromatography with Fluorescence and Tandem Mass Spectrometric Detection. Free Radical Biology and Medicine 2017, 113, 355–362. [Google Scholar] [CrossRef]
- Chen, W.-L.; Xie, B.; Zhang, C.; Xu, K.-L.; Niu, Y.-Y.; Tang, X.-Q.; Zhang, P.; Zou, W.; Hu, B.; Tian, Y. Antidepressant-like and Anxiolytic-like Effects of Hydrogen Sulfide in Behavioral Models of Depression and Anxiety. Behavioural Pharmacology 2013, 24, 590–597. [Google Scholar] [CrossRef]
- Nagy, P.; Pálinkás, Z.; Nagy, A.; Budai, B.; Tóth, I.; Vasas, A. Chemical Aspects of Hydrogen Sulfide Measurements in Physiological Samples. Biochimica et Biophysica Acta (BBA) - General Subjects 2014, 1840, 876–891. [Google Scholar] [CrossRef]
- De Silva, D.; Lee, S.; Duke, A.; Angalakurthi, S.; Chou, C.-E.; Ebrahimpour, A.; Thompson, D.E.; Petrikovics, I. Intravascular Residence Time Determination for the Cyanide Antidote Dimethyl Trisulfide in Rat by Using Liquid-Liquid Extraction Coupled with High Performance Liquid Chromatography. Journal of Analytical Methods in Chemistry 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Bátai, I.Z.; Sár, C.P.; Horváth, Á.; Borbély, É.; Bölcskei, K.; Kemény, Á.; Sándor, Z.; Nemes, B.; Helyes, Z.; Perkecz, A.; et al. TRPA1 Ion Channel Determines Beneficial and Detrimental Effects of GYY4137 in Murine Serum-Transfer Arthritis. Front. Pharmacol. 2019, 10, 964. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Mikami, Y.; Osumi, K.; Tsugane, M.; Oka, J.; Kimura, H. Polysulfides Are Possible H 2 S-derived Signaling Molecules in Rat Brain. FASEB j. 2013, 27, 2451–2457. [Google Scholar] [CrossRef] [PubMed]
- Kiss, L.; Holmes, S.; Chou, C.-E.; Dong, X.; Ross, J.; Brown, D.; Mendenhall, B.; Coronado, V.; De Silva, D.; Rockwood, G.A.; et al. Method Development for Detecting the Novel Cyanide Antidote Dimethyl Trisulfide from Blood and Brain, and Its Interaction with Blood. Journal of Chromatography B, 2017; 1044–1045. [Google Scholar] [CrossRef]
- Kiss, L.; Bocsik, A.; Walter, F.R.; Ross, J.; Brown, D.; Mendenhall, B.A.; Crews, S.R.; Lowry, J.; Coronado, V.; Thompson, D.E.; et al. From the Cover: In Vitro and In Vivo Blood-Brain Barrier Penetration Studies with the Novel Cyanide Antidote Candidate Dimethyl Trisulfide in Mice. Toxicological Sciences 2017, 160, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y. ; The 173rd Clinical Department of PLA 421rd Hospital, Guangdong, China; Huang, C.; The 173rd Clinical Department of PLA 421rd Hospital, Guangdong, China; Deng, H.; Department of Anesthesiology, Guangzhou General Hospital of Guangzhou Military Command, Guangdong, China; Jia, J.; Department of Anesthesiology, Guangzhou General Hospital of Guangzhou Military Command, Guangdong, China; Wu, Y.; Department of Anesthesiology, Guangzhou General Hospital of Guangzhou Military Command, Guangdong, China; et al. TRPA1 and Substance P Mediate Stress Induced Duodenal Lesions in Water Immersion Restraint Stress Rat Model. Turk J Gastroenterol, 2018; 692–700. [Google Scholar] [CrossRef]
- Hökfelt, T.; Kellerth, J.O.; Nilsson, G.; Pernow, B. Substance P: Localization in the Central Nervous System and in Some Primary Sensory Neurons. Science 1975, 190, 889–890. [Google Scholar] [CrossRef] [PubMed]
- Bilkei-Gorzo, A.; Racz, I.; Michel, K.; Zimmer, A. Diminished Anxiety- and Depression-Related Behaviors in Mice with Selective Deletion of the Tac1 Gene. J. Neurosci. 2002, 22, 10046–10052. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M. H2S and Substance P in Inflammation. In Methods in Enzymology; Elsevier, 2015; Vol. 555, pp. 195–205 ISBN 978-0-12-801511-7.
- Egertova, M.; Giang, D.K.; Cravatt, B.F.; Elphick, M.R. A New Perspective on Cannabinoid Signalling: Complementary Localization of Fatty Acid Amide Hydrolase and the CB1 Receptor in Rat Brain. 1998.
- Huang, W.-J.; Chen, W.-W.; Zhang, X. Endocannabinoid System: Role in Depression, Reward and Pain Control (Review). Molecular Medicine Reports 2016, 14, 2899–2903. [Google Scholar] [CrossRef] [PubMed]
- Kapanda, C.N.; Muccioli, G.G.; Labar, G.; Poupaert, J.H.; Lambert, D.M. Bis(Dialkylaminethiocarbonyl)Disulfides as Potent and Selective Monoglyceride Lipase Inhibitors. J. Med. Chem. 2009, 52, 7310–7314. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Hillard, C.J. Role of Endocannabinoid Signaling in Anxiety and Depression. In Behavioral Neurobiology of the Endocannabinoid System; Kendall, D., Alexander, S., Eds.; Current Topics in Behavioral Neurosciences; Springer Berlin Heidelberg: Berlin, Heidelberg, 2009; Volume 1, pp. 347–347. ISBN 978-3-540-88954-0. [Google Scholar]
- Saito, V.M.; Wotjak, C.T.; Moreira, F.A. Exploração Farmacológica Do Sistema Endocanabinoide: Novas Perspectivas Para o Tratamento de Transtornos de Ansiedade e Depressão? Rev. Bras. Psiquiatr. 2010, 32, 57–514. [Google Scholar] [CrossRef]
- Viveros, M.; Marco, E.; File, S. Endocannabinoid System and Stress and Anxiety Responses. Pharmacology Biochemistry and Behavior 2005, 81, 331–342. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.B.; Little, M.D.; Johnson, M.R.; Melvin, L.S.; De Costa, B.R.; Rice, K.C. Cannabinoid Receptor Localization in Brain. Proc. Natl. Acad. Sci. U.S.A. 1990, 87, 1932–1936. [Google Scholar] [CrossRef]
- Hill, M.N.; Hillard, C.J.; Bambico, F.R.; Patel, S.; Gorzalka, B.B.; Gobbi, G. The Therapeutic Potential of the Endocannabinoid System for the Development of a Novel Class of Antidepressants. Trends in Pharmacological Sciences 2009, 30, 484–493. [Google Scholar] [CrossRef]
- Tejeda-Martínez, A.R.; Viveros-Paredes, J.M.; Hidalgo-Franco, G.V.; Pardo-González, E.; Chaparro-Huerta, V.; González-Castañeda, R.E.; Flores-Soto, M.E. Chronic Inhibition of FAAH Reduces Depressive-Like Behavior and Improves Dentate Gyrus Proliferation after Chronic Unpredictable Stress Exposure. Behavioural Neurology 2021, 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Ware, T.B.; Lee, H.-C.; Hsu, K.-L. Lipid-Metabolizing Serine Hydrolases in the Mammalian Central Nervous System: Endocannabinoids and Beyond. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2019, 1864, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Gomes-de-Souza, L.; A Oliveira, L.; Benini, R.; Rodella, P.; Costa-Ferreira, W.; Crestani, C.C. Involvement of Endocannabinoid Neurotransmission in the Bed Nucleus of Stria Terminalis in Cardiovascular Responses to Acute Restraint Stress in Rats. British Journal of Pharmacology 2016, 2016, 173. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. JoVE 2015, 52434. [Google Scholar] [CrossRef]
- Cohen, I.L.; Gardner, J.M.; Karmel, B.Z.; Kim, S.-Y. Rating Scale Measures Are Associated with Noldus EthoVision-XT Video Tracking of Behaviors of Children on the Autism Spectrum. Mol Autism 2014, 5, 15. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). 2024.
- Kimura, H. Hydrogen Sulfide (H2S) and Polysulfide (H2Sn) Signaling: The First 25 Years. Biomolecules 2021, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Kormos, V.; Kecskés, A.; Farkas, J.; Gaszner, T.; Csernus, V.; Alomari, A.; Hegedüs, D.; Renner, É.; Palkovits, M.; Zelena, D.; et al. Peptidergic Neurons of the Edinger–Westphal Nucleus Express TRPA1 Ion Channel That Is Downregulated Both upon Chronic Variable Mild Stress in Male Mice and in Humans Who Died by Suicide. jpn 2022, 47, E162–E175. [Google Scholar] [CrossRef]
- Al-Omari, A.; Kecskés, M.; Gaszner, B.; Biró-Sütő, T.; Fazekas, B.; Berta, G.; Kuzma, M.; Pintér, E.; Kormos, V. Functionally Active TRPA1 Ion Channel Is Downregulated in Peptidergic Neurons of the Edinger-Westphal Nucleus upon Acute Alcohol Exposure. Front. Cell Dev. Biol. 2023, 10, 1046559. [Google Scholar] [CrossRef]
- Konkoly, J.; Kormos, V.; Gaszner, B.; Correia, P.; Berta, G.; Biró-Sütő, T.; Zelena, D.; Pintér, E. Transient Receptor Potential Ankyrin 1 Ion Channel Expressed by the Edinger-Westphal Nucleus Contributes to Stress Adaptation in Murine Model of Posttraumatic Stress Disorder. Front. Cell Dev. Biol. 2022, 10, 1059073. [Google Scholar] [CrossRef]
- Renard, C.E.; Dailly, E.; David, D.J.P.; Hascoet, M.; Bourin, M. Monoamine Metabolism Changes Following the Mouse Forced Swimming Test but Not the Tail Suspension Test. Fundamemntal Clinical Pharma 2003, 17, 449–455. [Google Scholar] [CrossRef]
- Bhatia, M. H2S and Substance P in Inflammation. In Methods in Enzymology; Elsevier, 2015; Vol. 555, pp. 195–205 ISBN 978-0-12-801511-7.
- Milicic, M.; Gaszner, B.; Berta, G.; Pintér, E.; Kormos, V. The Lack of TRPA1 Ion Channel Does Not Affect the Chronic Stress-Induced Activation of the Locus Ceruleus. IJMS 2024, 25, 1765. [Google Scholar] [CrossRef] [PubMed]
- Kozicz, T.; Tilburg-Ouwens, D.; Faludi, G.; Palkovits, M.; Roubos, E. Gender-Related Urocortin 1 and Brain-Derived Neurotrophic Factor Expression in the Adult Human Midbrain of Suicide Victims with Major Depression. Neuroscience 2008, 152, 1015–1023. [Google Scholar] [CrossRef]
- Bouwknecht, J.A.; Spiga, F.; Staub, D.R.; Hale, M.W.; Shekhar, A.; Lowry, C.A. Differential Effects of Exposure to Low-Light or High-Light Open-Field on Anxiety-Related Behaviors: Relationship to c-Fos Expression in Serotonergic and Non-Serotonergic Neurons in the Dorsal Raphe Nucleus. Brain Research Bulletin 2007, 72, 32–43. [Google Scholar] [CrossRef]
- Neufeld-Cohen, A.; Tsoory, M.M.; Evans, A.K.; Getselter, D.; Gil, S.; Lowry, C.A.; Vale, W.W.; Chen, A. A Triple Urocortin Knockout Mouse Model Reveals an Essential Role for Urocortins in Stress Recovery. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 19020–19025. [Google Scholar] [CrossRef]
- Kozicz, T. The Missing Link; the Significance of Urocortin 1/Urocortin 2 in the Modulation of the Dorsal Raphe Serotoninergic System. Mol Psychiatry 2010, 15, 340–341. [Google Scholar] [CrossRef]
- Kovács, K.J. Measurement of Immediate-Early Gene Activation- C-fos and Beyond. J Neuroendocrinology 2008, 20, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, J.C.; Vaughan, J.; Arias, C.; Rissman, R.A.; Vale, W.W.; Sawchenko, P.E. Urocortin Expression in Rat Brain: Evidence Against a Pervasive Relationship of Urocortin-Containing Projections With Targets Bearing Type 2 CRF Receptors. J. Comp. Neurol. 1999, 415, 285–312. [Google Scholar] [CrossRef]
- Kozicz, T. On the Role of Urocortin 1 in the Non-Preganglionic Edinger–Westphal Nucleus in Stress Adaptation. General and Comparative Endocrinology 2007, 153, 235–240. [Google Scholar] [CrossRef]
- Derks, N.M.; Roubos, E.W.; Kozicz, T. Presence of Estrogen Receptor β in Urocortin 1-Neurons in the Mouse Non-Preganglionic Edinger–Westphal Nucleus. General and Comparative Endocrinology 2007, 153, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Isgor, C.; Cecchi, M.; Kabbaj, M.; Akil, H.; Watson, S.J. Estrogen Receptor β in the Paraventricular Nucleus of Hypothalamus Regulates the Neuroendocrine Response to Stress and Is Regulated by Corticosterone. Neuroscience 2003, 121, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Silva, I.A.; Helena, C.V.V.; Franci, C.R.; Lucion, A.B.; Anselmo-Franci, J.A. Modulatory Role of Locus Coeruleus and Estradiol on the Stress Response of Female Rats. Endocr 2009, 35, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Kawano, J.; Yanai, A.; Fujinaga, R.; Tanaka, M.; Watanabe, Y.; Shinoda, K. Expression of Estrogen Receptors (α, β) and Androgen Receptor in Serotonin Neurons of the Rat and Mouse Dorsal Raphe Nuclei; Sex and Species Differences. Neuroscience Research 2004, 49, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Hasunuma, K.; Murakawa, T.; Takenawa, S.; Mitsui, K.; Hatsukano, T.; Sano, K.; Nakata, M.; Ogawa, S. Estrogen Receptor β in the Lateral Septum Mediates Estrogen Regulation of Social Anxiety-like Behavior in Male Mice. Neuroscience 2024, 537, 126–140. [Google Scholar] [CrossRef]
Figure 1.
Distance travelled (A) and activity duration (time spent moving; B) in different treatment groups of TRPA1 WT mice in the open field test. Dimethyl trisulfide (DMTS) at 60 mg/kg dose reduced, but at 50 mg/kg dose did not affect either distance travelled or activity duration compared to untreated and vehicle-treated groups. One-way ANOVA and Fisher post hoc test, *p<0.05 vs. indicated group.
Figure 1.
Distance travelled (A) and activity duration (time spent moving; B) in different treatment groups of TRPA1 WT mice in the open field test. Dimethyl trisulfide (DMTS) at 60 mg/kg dose reduced, but at 50 mg/kg dose did not affect either distance travelled or activity duration compared to untreated and vehicle-treated groups. One-way ANOVA and Fisher post hoc test, *p<0.05 vs. indicated group.
Figure 2.
Distance travelled (A) and activity duration (time spent moving; B) in different treatment groups of C57BL/6J strain in the open field test. Dimethyl trisulfide (DMTS) at 50 mg/kg dose did not diminish either distance travelled or activity duration in C57BL/6 mice. n=6-7, one-way ANOVA and Fisher post hoc test.
Figure 2.
Distance travelled (A) and activity duration (time spent moving; B) in different treatment groups of C57BL/6J strain in the open field test. Dimethyl trisulfide (DMTS) at 50 mg/kg dose did not diminish either distance travelled or activity duration in C57BL/6 mice. n=6-7, one-way ANOVA and Fisher post hoc test.
Figure 3.
Activity frequency (A) and immobility time (B) in the forced swim test. Dimethyl trisulfide (DMTS; 50 mg/kg) increased activity frequency and decreased the time spent immobile compared to untreated or vehicle-treated groups in the TRPA1 WT strain. No treatment effect was observed in TRPA1 KO animals. Shapiro–Wilk test and Bartlett’s Chi square test followed by two-way ANOVA and Fisher post hoc test, n=15-19, *p<0.05, **p<0.01, ***p<0.001 vs. indicated group.
Figure 3.
Activity frequency (A) and immobility time (B) in the forced swim test. Dimethyl trisulfide (DMTS; 50 mg/kg) increased activity frequency and decreased the time spent immobile compared to untreated or vehicle-treated groups in the TRPA1 WT strain. No treatment effect was observed in TRPA1 KO animals. Shapiro–Wilk test and Bartlett’s Chi square test followed by two-way ANOVA and Fisher post hoc test, n=15-19, *p<0.05, **p<0.01, ***p<0.001 vs. indicated group.
Figure 4.
Activity frequency (A) and time spent immobile (B) in different treatment groups of TRPA1 WT and KO mice in the tail suspension test. There was no significant effect of treatment on neither active frequency nor active duration in either group. Shapiro–Wilk test and Bartlett’s Chi square test followed by two-way ANOVA and Fisher post hoc test. n=1-12. DMTS: dimethyl trisulfide.
Figure 4.
Activity frequency (A) and time spent immobile (B) in different treatment groups of TRPA1 WT and KO mice in the tail suspension test. There was no significant effect of treatment on neither active frequency nor active duration in either group. Shapiro–Wilk test and Bartlett’s Chi square test followed by two-way ANOVA and Fisher post hoc test. n=1-12. DMTS: dimethyl trisulfide.
Figure 5.
Number of active periods (A) and immobilization time (B) in different treatment groups of C57BL/6, NK1 KO and Tac1 KO strains in the forced swim test. The group treated with dimethyl trisulfide (DMTS) had a higher number of active periods compared to the other groups. Inactive duration was not affected by treatment in any group. Shapiro–Wilk test and Bartlett’s Chi square test followed by two-way ANOVA and Fisher post hoc test, n=4-15, *p<0.05 vs. indicated group.
Figure 5.
Number of active periods (A) and immobilization time (B) in different treatment groups of C57BL/6, NK1 KO and Tac1 KO strains in the forced swim test. The group treated with dimethyl trisulfide (DMTS) had a higher number of active periods compared to the other groups. Inactive duration was not affected by treatment in any group. Shapiro–Wilk test and Bartlett’s Chi square test followed by two-way ANOVA and Fisher post hoc test, n=4-15, *p<0.05 vs. indicated group.
Figure 6.
Activity frequency (A) and time spent immobile (B) in different treatment groups of C57BL/6J strain in the forced swim test. Compared to the untreated group, all three treatments increased active frequency but had no effect on inactive duration. Shapiro–Wilk test and Bartlett’s Chi square test followed by one-way ANOVA and Fisher post hoc test, n=11-18, *p<0.05 vs. indicated group. DMTS: dimethyl trisulfide
Figure 6.
Activity frequency (A) and time spent immobile (B) in different treatment groups of C57BL/6J strain in the forced swim test. Compared to the untreated group, all three treatments increased active frequency but had no effect on inactive duration. Shapiro–Wilk test and Bartlett’s Chi square test followed by one-way ANOVA and Fisher post hoc test, n=11-18, *p<0.05 vs. indicated group. DMTS: dimethyl trisulfide
Figure 7.
c-FOS immunoreactivity in different brain areas in vehicle-treated and dimethyl trisulfide (DMTS)-treated animals in the TRPA1 WT mouse strain. Compared to the untreated groups, DMTS (50 mg/kg) treatment significantly increased c-FOS immune activity in all brain areas tested, except the centrally projecting Edinger-Whestphal nucleus (EWcp). PVN: paraventricular nucleus of the hypothalamus; LC: locus coeruleus; PVT: paraventricular nucleus of the thalamus; DR: dorsal raphe nucleus; LS: lateral septum. Unpaired T test, n=15, *p<0.05, ** p<0.01, ****p<0.0001 vs. indicated group.
Figure 7.
c-FOS immunoreactivity in different brain areas in vehicle-treated and dimethyl trisulfide (DMTS)-treated animals in the TRPA1 WT mouse strain. Compared to the untreated groups, DMTS (50 mg/kg) treatment significantly increased c-FOS immune activity in all brain areas tested, except the centrally projecting Edinger-Whestphal nucleus (EWcp). PVN: paraventricular nucleus of the hypothalamus; LC: locus coeruleus; PVT: paraventricular nucleus of the thalamus; DR: dorsal raphe nucleus; LS: lateral septum. Unpaired T test, n=15, *p<0.05, ** p<0.01, ****p<0.0001 vs. indicated group.
Figure 8.
Representative images used for the calculation of the density of c-FOS activation in brain areas of TRPA1 WT animals. Immunohistochemistry was visualized with 3,3’-diaminobenzidine. Images were taken with a 10× objective. Apart from the centrally projecting Edinger-Westphal nucleus (EWcp) nucleus, significantly stronger c-FOS activity was found in all brain areas examined after dimethyl trisulfide (DMTS) treatment. A. LS (lateral septum) B. PVT (paraventricular nucleus of the thalamus) C. EWcp D. DR (dorsal raphe nucleus) E. LC (locus coeruleus) F. PVN (paraventricular nucleus of the hypothalamus). Black line: scale bar 200 m; red line: scale bar 500 μm.
Figure 8.
Representative images used for the calculation of the density of c-FOS activation in brain areas of TRPA1 WT animals. Immunohistochemistry was visualized with 3,3’-diaminobenzidine. Images were taken with a 10× objective. Apart from the centrally projecting Edinger-Westphal nucleus (EWcp) nucleus, significantly stronger c-FOS activity was found in all brain areas examined after dimethyl trisulfide (DMTS) treatment. A. LS (lateral septum) B. PVT (paraventricular nucleus of the thalamus) C. EWcp D. DR (dorsal raphe nucleus) E. LC (locus coeruleus) F. PVN (paraventricular nucleus of the hypothalamus). Black line: scale bar 200 m; red line: scale bar 500 μm.
Figure 9.
The open field test (OFT) was used to determine the appropriate dose of dimethyl trisulfide. Movement time (s) and total distance travelled (cm) were investigated. Prior to test we took the animals to the laboratory to habituate to the environment. Video recording lasted 10 minutes from which 5 minutes were evaluated.
Figure 9.
The open field test (OFT) was used to determine the appropriate dose of dimethyl trisulfide. Movement time (s) and total distance travelled (cm) were investigated. Prior to test we took the animals to the laboratory to habituate to the environment. Video recording lasted 10 minutes from which 5 minutes were evaluated.
Figure 10.
To investigate the depression-like behaviour we used forced swim test (FST) and tail suspension test (TST). Highly active duration (s) and activity frequency parameters were utilised. Duration of immobility (s) was calculated by subtracting highly active duration from the total duration of observation.
Figure 10.
To investigate the depression-like behaviour we used forced swim test (FST) and tail suspension test (TST). Highly active duration (s) and activity frequency parameters were utilised. Duration of immobility (s) was calculated by subtracting highly active duration from the total duration of observation.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).