Submitted:
06 June 2024
Posted:
07 June 2024
You are already at the latest version
Abstract
Keywords:
Introduction:
Materials and Methods:
Results:
Discussion:
Conclusion:
References
- Robinson, S.C.; Brucer, M. Range of normal blood pressure: a statistical and clinical study of 11,383 persons. Arch Intern Med 1939; 64:409-44. [CrossRef]
- Vasan, R.S.; Larson, M.G.; Leip, E.P. , et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001: 345(18):1291-7. [CrossRef]
- Lewington, S.; Clarke, R.; Qizilbash, N. , et al. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet.2002;360(9349):1903-13. [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R. , et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560-72. [CrossRef]
- Mancia, G.; De Backer, G.; Dominiczak, A. , et al. ESH-ESC Task Force on the Management of Arterial Hypertension. 2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens. 2007;25(9):1751-62. [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K. , et al. Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281-357. [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W. , et al. ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018; 39(33):3021-3104. [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M. , et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens. 2023;41(12):1874-2071. [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S. , et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138(17):e426-e483. [CrossRef]
- Gupta, A.K.; Brashear, M.M.; Johnson, W.D. : Coexisting prehypertension and prediabetes in healthy adults: a pathway for accelerated cardiovascular events. Hypertens Res. 2011; 34 (4): 456-461. [CrossRef]
- Gupta, A.K.; McGlone, M.; Greenway, F.L. , et al. Prehypertension in disease-free adults: a marker for an adverse cardiometabolic risk profile. Hypertens Res. 2010; 33 (9): 905-910. [CrossRef]
- Chiang, P.P.; Lamoureux, E.L.; Shankar, A. et al. Cardio-metabolic risk factors and prehypertension in persons without diabetes, hypertension, and cardiovascular disease. BMC Public Health 2013; 13; 730. [CrossRef]
- Chobanian, A.V. Prehypertension revisited. Hypertension. 2006;48:812–814. [CrossRef]
- Levey, A.S.; Stevens, L.A. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 2010; 55:622–7. [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004;27(6):1487—95. [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B. , et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's guidelines and standards committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography 2005; 18: 1440-63. [CrossRef]
- D’Agostino RB, Vasan SR; Pencina MJ, et al. General Cardiovascular Risk Profile for Use in Primary Care: The Framingham Heart Study. Circulation. 2008;117: 743-53. [CrossRef]
- Grotto, I.; Grossman, E.; Huerta, M. , et al. Prevalence of prehypertension and associated cardiovascular risk profiles among young Israeli adults. Hypertension. 2006;48(2):254-9. [CrossRef]
- Ahn, H.S.; Kim, S.J.; Kim, M.K. , et al. The Difference of Left Ventricular Hypertrophy and the Diastolic Function between Prehypertensives and Normotensives. Korean Circ J 2006; 36:437. [CrossRef]
- Di Bello, V.; Talini, E.; Dell’Omo, G. , et al. Early left ventricular mechanics abnormalities in prehypertension: A two-dimensional strain echocardiography study. Am J Hypertens 2010; 23:405–12. [CrossRef]
- Jang, S.Y.; Kim, S.; Lee, C.K.; Cho, E.J.; Cho, S.J.; Lee, S. Prehypertension and Left Ventricular Diastolic Dysfunction in Middle-Aged Koreans. Korean Circ J. 2016; 46: 536–41. [CrossRef]
- Bajpai, J.K.; Sahay, A.P.; Agarwal, A.K.; De, A.K.; Garg, B.; Goel, A. Impact of prehypertension on left ventricular structure, function and geometry. J Clin Diagnostic Res 2014; 8:7–10. [CrossRef]
- Aktürk, E.; Ermis, N.; Yaǧmur, J. , et al. Early left atrial mechanics and volume abnormalities in subjects with prehypertension: A real time three- dimensional echocardiography study. Echocardiography 2012; 29:1211–7. [CrossRef]
- De Marco, M.; De Simone, G.; Roman, M.J. , et al. Cardiovascular and metabolic predictors of progression of prehypertension into hypertension: The strong heart study. Hypertension 2009; 54:974–80. [CrossRef]
- Kim, B.J.; Lee, H.J.; Sung, K.C. , et al. Comparison of Microalbuminuria in 2 Blood Pressure Categories of Prehypertensive Subjects. Circ J 2007; 71:1283–7. [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I. , et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–72. [CrossRef]
- Ginsberg, H.N.; Packard, C.J.; Chapman, M.J. , et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies – a consensus statement from the European Atherosclerosis Society. Eur Heart J. 2021;42(47):4791–806. [CrossRef]
- Berglund, L.; Brunzell, J.D.; Goldberg, A.C. , et al. Endocrine Society. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;2969–2989. [CrossRef]
- Sarwar, N.; Danesh, J.; Eiriksdottir, G. , et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115(4):450-8. [CrossRef]
- Toth, P.P.; Granowitz, C.; Hull, M. , et al. High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: a real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J Am Heart Assoc. 2018;7(15): e008740. [CrossRef]
- Farniera, M.; Zellera, M.; Massonc, D. , et al. Triglycerides and risk of atherosclerotic cardiovascular disease: An update. Archives of Cardiovascular Disease. 2021; 114: 132—139. [CrossRef]
- Jørgensen, A.B.; Frikke-Schmidt, R.; West, A.S. , et al. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;1826–1833. [CrossRef]
- Sarwar, N.; Sandhu, M.S.; Ricketts, S.L. , et al. Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration, Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;1634–1639. [CrossRef]
- Thomsen, M.; Varbo, A.; Tybjærg-Hansen, A. , et al. Low nonfasting triglycerides and reduced all-cause mortality: a Mendelian randomization study. Clin Chem. 2014;737–746. [CrossRef]
- Arca, M.; Veronesi, C.; D'Erasmo, L. , et al. Association of hypertriglyceridemia with all-cause mortality and atherosclerotic cardiovascular events in a low-risk Italian population: the TG-REAL retrospective cohort analysis. J Am Heart Assoc. 2020;9(19): e015801. [CrossRef]
- Miller, M.; Stone, N.J.; Ballantyne, C. , et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011; 123:2292–2333. [CrossRef]
| NT (n=103) | PHT (n=140) | HT (n=80) | P• | |
| *SBP (mmHg) | 108.3 (7.1) | 126.9 (80.8) | 143.9 (94.2) | <0.001‡ |
| *DBP (mmHg) | 70.9 (6.2) | 80.8 (6.5) | 94.2 (8.2) | <0.001‡ |
| Sex, m, [n(%)] | 74 (71.8) | 61 (42.7) | 30 (37.5) | <0.001# |
| Age, (g) [median (25%-75%)] | 36 (30 - 42) | 37 (29 - 44) | 39 (33 - 45) | 0.07 |
| **BMI (kg/m2) | 25.6 (22.5 – 28.7) | 26.6 (23.8 - 30) | 28.56 (24.7-32.6) | 0.009 |
| **WC (cm) | 90 (79– 100) | 92 (84 – 102) | 99.5 (89– 110) | 0.001 |
| *Acid uric (µmol/l) | 252 (211 - 307) | 297 (244.5 - 370) | 328 (274 – 412.8) | <0.001 |
| **Glucose (mmol/L) | 4.9 (4.5 – 5.2) | 5 (4.6 – 5.3) | 5.3 (4,8 – 5.6) | <0.001 |
| ±HbA1c (%) | 5.1 (4.9 – 5.4) | 5.1 (4.9 – 5.1) | 5.2 (5 – 5.4) | 0.01 |
| Insulin (mIU/L) | 9.3 (6.6 – 14.6) | 13.4 (8.6 – 17.7) | 14.9 (10 – 25.9) | 0.002 |
| §HOMA-IR (%) | 1.9 (1.4 – 2.7) | 2.9 (1.7 – 4.1) | 3.5 (2.1 – 5.6) | 0.001 |
| *Total cholesterol (mmol/L) | 4.9 (4.3 – 5.6) | 5.2 (4.55 – 5.7) | 5.2 (4.6 – 5.9) | 0.04 |
| HDL-cholesterol (mmol/L) | 1.3 (1.1 – 1.6) | 1.3 (1.1 – 1.7) | 1.3 (1 – 1.6) | 0.35 |
| LDL-cholesterol (mmol/L) | 3.0 (2.4 – 3.6) | 3.1 (2.6 – 3.7) | 3.1 (2.6 – 3.9) | 0.32 |
| *Triglycerides (mmol/L) | 0.9 (0.7 – 1.4) | 1.0 (0.7 – 1.5) | 1.4 (0.9 - 2) | <0.001 |
| Serum potassium (mmol/l) | 4.3 (4.2 – 4.63) | 4.4 (4.1 – 4.7) | 4.3 (4.1 – 4.5) | 0.09 |
| Serum sodium (mmol/l) | 140 (139 - 141) | 140 (139 - 142) | 140 (139 - 141) | 0.48 |
| §Serum creatinine µmol/l) | 68 (63 - 76) | 75 (65 - 86) | 74 (65 – 86.3) | 0.001 |
| CKD-EPI eGFR (ml/min/1,73m2) | 107 (96.3 - 115) | 105 (91 - 113) | 104.5 (94 - 110) | 0.32 |
| ACR urine (mg/g creatinine) | 4.14 (2.64 – 6.05) | 4.1 (2.3 – 6.6) | 4,7 (2,8 - 8,3) | 0.54 |
| A1mCR urine (mg/g creatinine) | 5.6 (3.3 – 7.7) | 4.3 (3.2 - 6,7) | 5.1 (3.6 – 8.4) | 0.07 |
| Na/K ratio, urine | 3.3 (2.5 – 4.5) | 3.3 (2.4 – 5.2) | 3.1 (2.4 – 4.1) | 0.71 |
| Uromodulin (mg/g creatinine) | 43 (27 - 66) | 42.9 (25.5 – 65.3) | 40.6 (24.1 – 60.4) | 0.73 |
| C-reactive protein | 1.8 (0.9 – 2.8) | 1.2 (0.6 – 2.4) | 2 (0.9 – 4.1) | 0.05 |
| Kruskal–Wallis test [median (25%-75%)] •; χ2 test #; ANOVA‡; post hoc Conover: * significant differences btw. NT vs. PHT, NT vs. HT, and PHT vs. HT; ** significant differences btw. NT vs. HT and PHT vs. HT; § significant differences btw. NT vs. PHT and NT vs.HT; §§ significant difference btw. NT vs.HT; ±significant difference btw. PHT vs. HT; NT, normotensive; PHT, prehypertensive; HT, hypertensive; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; WC, waist circumference; HbA1c, glycated hemoglobin A1c; HOMA-IR, Homeostasis Model Assessment for Insulin Resistance; HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration Estimated Glomerular Filtration Rate; ACR, albumin/creatinine ratio in spot urine; A1mCR, alpha-1-microglobulin creatinine ratio in spot urine; spot urine Na/K ratio, sodium/potassium ratio in spot urine. | ||||
| NT (n=41) | PHT (n=52) | HT (n=50) | P* | |
| LA (mm) §§§ | 32.5 (30.3 - 36) | 33 (30 - 36) | 36.5 (32.8 - 39) | 0.007 |
| LAVI (ml/m2) | 20.8 (16.8 – 23.6) | 19.1 (16.5 – 22.7) | 21.2 (17.4 - 26) | 0.25 |
| LV (mm) | 47 (44 – 50.5) | 49 (45 - 51) | 46.5 (43 - 52) | 0.43 |
| IVS (mm) §§ | 10 (8 - 10) | 10 (9 - 11) | 10 (9 - 12) | 0.02 |
| LVPW (mm) § | 9 (8 – 10.5) | 10 (9 - 11) | 11 (10 - 12) | <0.001 |
| LVM (g) § | 142.1 (122.3 – 180.9) | 163.72 (146.4 -193.8) | 190.4 (145.9 – 228.3) | 0.001 |
| LVMI (g/m2) §§ | 77.5 (67.6-88.3) | 84.3 (73.3-95.9) | 87.7 (74.3-107.4) | 0.01 |
| RWT (cm) § | 0.4 (0.4 – 0.4) | 0.4 (0.4-0.5) | 0.5 (0.4 – 0.6) | <0.001 |
| E/A | 1.3 (1 – 1.5) | 1.3 (1 – 1.5) | 1.3 (1 – 1.5) | 0.89 |
| E/e' §§ | 7 (5.6 – 8.7) | 7.4 (6.3 - 9) | 8.2 (6.8 – 9.4) | 0.03 |
| SV (ml) | 67.5 (58.5 – 82.8) | 74 (63.5 - 86) | 74 (61 – 95.5) | 0.24 |
| EFLV (%) | 66 (62 - 70) | 64 (60 - 69) | 67 (62 – 68.3) | 0.34 |
| * Kruskal–Wallis test (median and interquartile range); NT, normotensive; PHT, prehypertensive; HT, hypertensive; LA, left atrium; LAVI, indexed left atrium volume; LV, left ventricle; IVS, interventricular septum; LVPW, left ventricular posterior wall; LVM, left ventricular mass; LVMI, left ventricular mass indexed; RWT, relative wall thickness; E/A, the ratio of peak velocity blood flow from left ventricular relaxation in early diastole (the E wave) to peak velocity flow in late diastole caused by atrial contraction (the A wave); E/e', the ratio of early filling velocity on transmittal Doppler (E) with the early relaxation velocity on tissue Doppler (E′); SV, stroke volume; EFLV, left ventricular ejection fraction; post hoc Conover : § NT vs. PHT, NT vs.HT, and PHT vs.HT; §§ NT vs. HT; §§§ NT vs.HT and PHT vs.HT. | ||||
| Parameter | ß | Standard error | Wald | p | Odds ratio (Exp ß) | 95% CI za Exp ß |
| Triglycerides (mmol/L) | 0.63 | 0.28 | 4.96 | 0.03 | 1.88 | 1.08 do 3.28 |
| Constant | -2.8 | 0.73 | 15.0 | <0.001 |
| Parameters | AUC | 95% CI | Sensitivity | Specificity | Cut-off | Youden index |
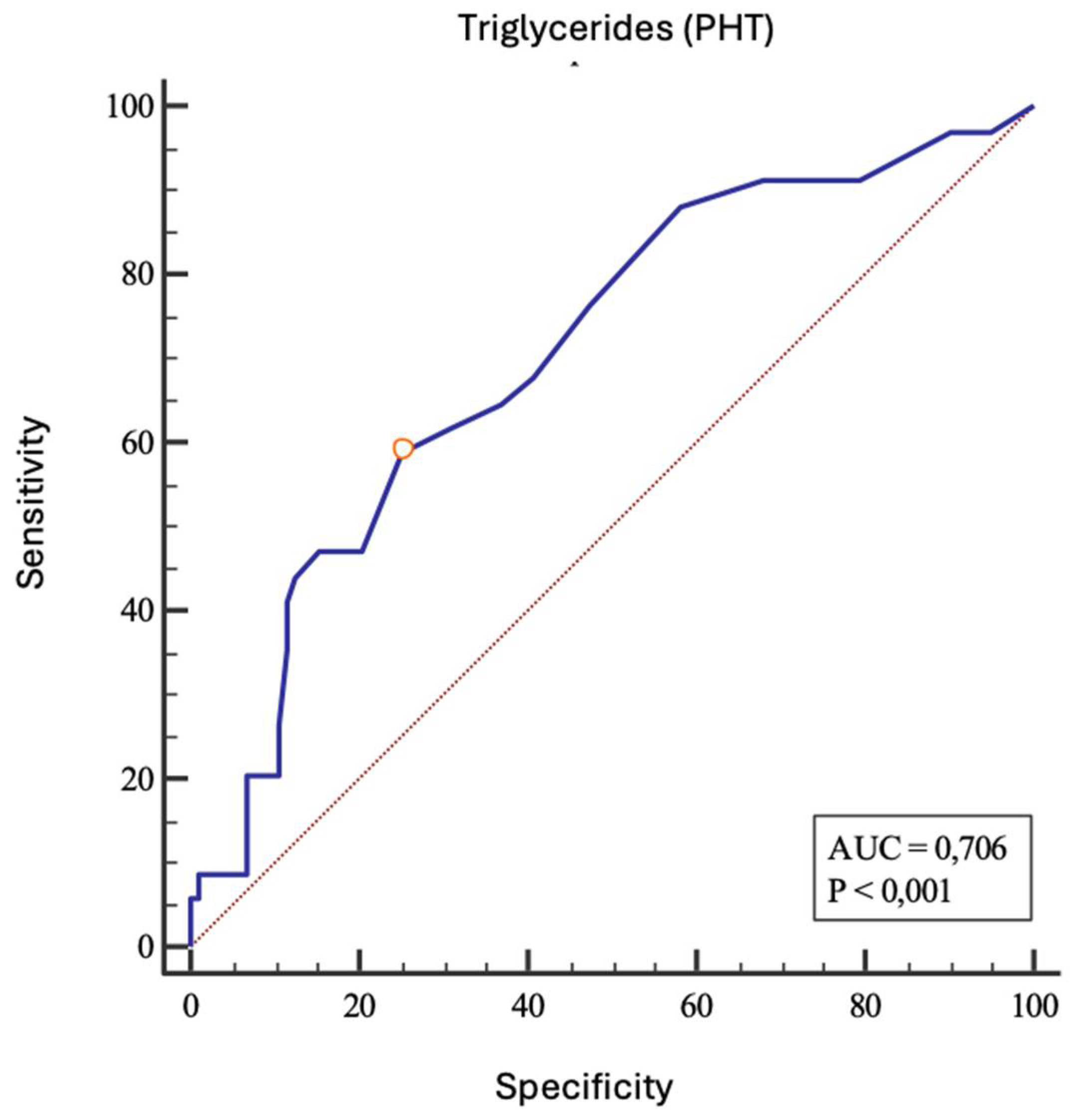
P |
| Triglycerides (mmol/L) | 0.706 | 0.622-0.781 | 58.8 | 74.8 | > 1.3 | 0.34 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





