1. Introduction
African swine fever (ASF) is a highly contagious viral disease with a mortality rate approaching 100 %, leading to significant economic losses to swine industry worldwide [
1,
2]. ASF was first discovered in Kenya in 1921, identified in the ancient sylvatic cycle, followed by its spread outside Africa mainly in European countries between 1957 and 1995 [
3]. The ASF was introduced into Georgia in 2007, and spread to Trans-Caucasus, Russian Federation, Eastern Europe and the Baltic countries, into China in 2018 and spread to other Asia countries [
4,
5,
6]. Because there is no effective and safe vaccine or antiviral drugs, the ASF has drawn much public attention and scientific investigation [
7,
8,
9].
ASFV is a member of the
Asfarviridae family, belonging to the nucleoplasmic large DNA virus (NCLDV) group, and it is the only known DNA arbovirus [
10]. The ASFV genome contains a linear double-stranded DNA (170–190 kb) that encodes approximately 150 proteins [
11,
12]. ASFV gene S273R encodes a 31 kD protein, containing a “core domain” with conserved catalytic residues and exhibiting the characteristics of SUMO-1 specific cysteine proteases [
13]. Two ASFV polyprotein precursors, pp220 and pp62, are cleaved by the intrinsic pS273R protease to produce p5, p34, p14, p37, and p150 (derived from pp220) and p8, p15 and p35 (derived from pp62) [
14]. Thus, pS273R is supposed to be involved in the viral late maturational step, which is essential for proper core assembly and infectivity of ASFV [
15].
The pS273R is not only essential for ASFV virion maturation, but also important in host immune evasion [
16,
17,
18,
19,
20,
21]. First, pS273R cleaves gasdermin D in a non-canonical way to inhibit inflammatory pyroptosis [
21], and cleaves G3BP1 to antagonize the antiviral stress granule (SG) formation [
17]. Second, pS273R evades host DNA sensing cGAS-STING pathway mediated interferon (IFN) induction by disturbing the interactions between STING and IKKε[
19], between TBK1 and IRF3 [
16]. Additionally, pS273R was reported to mediate STAT2 degradation to inhibit IFN signaling [
18], and mediate degradation of FoxJ1, a host antiviral factor [
20].
However, in spite of above pS273R biological functions and its crystal structure recently resolved [
22], little is known about the antigenic characteristic of the pS273R protein. Further, the antigenic epitope of pS273R has not been described, which is necessary for development of the detection method as well as candidate vaccine. In this study, we expressed and purified the prokaryotic recombinant pS273R, and used it to immunize mice. Two monoclonal antibodies were generated and both recognized a conserved linear epitope at the N terminal 1-25 amino acids of pS273R. In addition, the epitope based indirect ELISA is capable of specific detection of ASFV antibody in clinical ASFV infection. These results not only provide the biological tools for ASFV research, but also deepen our understanding of the antigenicity of ASFV protease.
2. Materials and Methods
2.1. Mice, Cells, Virus, and Sera
The 6- to 8-week-old, female, BALB/c mice were purchased from the Laboratory Animal Center of Yangzhou University. HEK-293T cells and myeloma cell line SP2/0 were cultured in Dulbecco modified Eagle medium (DMEM, Hyclone Laboratories, USA) supplemented with 100 IU/mL of penicillin plus 100 μg/mL streptomycin and 10% fetal bovine serum (FBS). Primary porcine alveolar macrophages (PAMs) were cultured in RPMI 1640 medium (Hyclone Laboratories) which contains 100 IU/mL of penicillin plus 100 μg/mL streptomycin and 10% FBS. Cells were grown at 37°C in a 5% CO2 humidified incubator. The ASFV strain (genotype II, GenBank accession ON456300) was preserved in the animal biosafety level 3 (ABSL-3) of Yangzhou University approved by the Ministry of Agriculture and Rural Affairs (07140020201109-1), whereas the porcine serum samples were stored in our lab. The animal experiment was in strict accordance with the Guidance for the Care and Use of Laboratory Animals of Yangzhou University (SYXK(JS)-2021-0026).
2.2. Expression and Purification of pS273R Protein
The ASFV pS273R gene coding sequence was PCR amplified from pCAGGS-S273R-HA constructed in our laboratory and cloned into NdeI and XhoI sites of pET-28a vector by Seemless/In-Fusion Cloning (2×MultiF Seamless Assembly Mix, Abclonal, Wuhan, China). The recombinant plasmid was transformed into E. coli BL21/DE3 competent cells and treated with 1 mM isopropyl-β-d-1-thiogalactoside (IPTG) at 16 °C for 14 h to induce pS273R expression. Bacteria pellet was collected by centrifugation, resuspended in PBS and sonicated on ice. After centrifugation, the supernatant was removed, and the pelleted inclusion bodies were resuspended in 8 mM urea buffer with gently shake at 4°C overnight, followed by gradient dialysis and subsequent refolding. The purified protein was confirmed by Western blotting with His mAb (TransGen Biotech, Beijing, China).
2.3. Generation of Anti-pS273R Monoclonal Antibodies (mAbs)
BALB/c mice were first immunized with 100μg purified pS273R protein emulsified in complete Freund’s adjuvant at a 1:1 ratio, and then with purified pS273R emulsified in incomplete Freund’s adjuvant at 2-week intervals. Three days after the final immunization, tail vein blood was taken and serum pS273R antibody was determined by indirect ELISA. Mouse presenting the highest antibody titer was chosen for the final boost without adjuvant and subsequently sacrificed three days later for cell fusion with SP2/0 cells. The hybridomas that secreted pS273R-specific antibodies were screened by indirect ELISA and confirmed by Western blotting. Positive hybridomas were subcloned three times by limiting dilution and tested again for antibody production.
2.4. Mapping of the Linear B Cell Epitope of pS273R Protein
First, the pS273R sequence was separated into two fragments to test the reactivity with pS273R mAbs. Next, based on the reacted fragment, progressive truncations were performed at both N and C terminal ends. The reactivity of different truncated fragments with pS273R mAbs was tested, and the critical amino acids at both N and C ends for reactivity with pS273R mAbs were determined, from which the minimal epitope was deduced. Totally, 12 pS273R fragments (P1-P12) were designed, with all cloning PCR primers listed in
Table S1. All the pS273R fragments were PCR amplified and cloned into the vector pEGFP-N1 by Seemless/In-Fusion Cloning with 2×MultiF Seamless Assembly Mix, and the recombinant plasmids were transfected into 293T cells for protein expressions. The reactivity of different truncated fragments in transfected 293 T cells with pS273R mAbs was tested by using Western blotting.
2.5. Western Blotting
The reactivity of anti-His mAb with the recombinant pS273R protein and anti-pS273R mAb with pS273R in transfected 293T cells and ASFV infected PAMs was evaluated using Western blotting. The cell protein samples were separated by 6-10 % SDS-polyacrylamide gels, and transferred to PVDF membranes. Membranes were blocked using 5% non-fat dry milk Tris-buffered saline, with 0.1% Tween-20 (TBST). Then, the membrane was incubated with the primary mAbs (1 : 1000 anti-HA, and pS273R hybridoma ascites) at 4 ℃ overnight. Next, the membrane was incubated with HRP-conjugated Goat Anti-Mouse IgG (1 : 10000, BBI, Shanghai, China) for 1 h. Protein signals was visualized and captured by Western blot imaging system.
2.6. Immunofluorescence Assay
293T cells were transfected with pCAGGS-S273R-HA for 24 h, whereas primary PAMs were infected with ASFV at a multiplicity of infection (MOI) of 0.1 for 72 h. Subsequently, the cells were fixed in 4 % paraformaldehyde for 30 min, permeabilized by 0.5% Triton X-100, and then blocked with 5% BSA. The treated cells were incubated with pS273R mAbs (1 : 200 ascites) overnight, and then Goat anti-mouse IgG H&L Alexa Fluor 594 (1 : 500, Abcam, Shanghai, China) for 1 h, followed by DAPI staining (Beyotime, Shanghai, China) for 15 min. The stained cells were visualized under fluorescence microscope.
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
For pS273R protein mediated indirect ELISA, the ELISA plate wells were coated with pS273R purified protein diluted in PBS at a final concentration of 0.625 μg/mL at 4°C overnight, followed by washing and blocking with 5% skim milk at 37°C for 2 h. Hybridoma supernatants were added to each wells and incubated for 2 h at 37 °C. After washing with PBST, secondary antibody Goat anti-Mouse IgG-HRP (1:10000 dilution, TransGen Biotech, Beijing, China) was added and incubated at 37°C for 1 h. Next, TMB (50 μL/well) was added and incubated for 15 min at 37 °C in the dark. The reaction was stopped with 0.5 M H2SO4 and the optical density at 450 nm (OD450) was measured. The ratios of hybridoma supernatants to negative supernatant (P/N) were calculated, with P/N ≥ 2.0 as positive.
For epitope mediated indirect ELISA, ELISA plate wells were coated with epitope peptide diluted with PBS at the concentration of 0.3125-10 μg/mL at 4°C overnight, followed by washing and blocking with 5% BSA at 37 °C for 2 hours. Diluted porcine sera (1:5-1:800) were added and incubated at 37 °C for 2 h. After washing with PBST, secondary antibody Goat anti-Swine IgG-HRP (1:10000, Proteintech, Wuhan, China) was added and incubated at 37 °C for 1 h, followed by addition of substrate TMB, stopping with 0.5 M H2SO4 and OD450 measurement. The ratios of positive sera to negative serum (P/N) were calculated.
2.8. Bioinformatics Analysis
To verify the conservation of identified pS273R epitope, the pS273 protein sequences of 171 ASFV strains from GenBank were downloaded. The amino acid sequence alignment and conservation analysis was implemented by Clustal W in MegAlign software, version 7.1.0 (DNAstar). The spatial distribution and the structure of the identified epitope within pS273R (PDB: 6LJB) were visualized by the PyMOL Molecular Graphics System (Version 2.4.0, Schrödinger, LLC).
3. Results
3.1. Production and Characterization of Recombinant pS273R Protein
In this study, the ASFV pS273R coding sequence was cloned into the pET-28a vector and the recombinant plasmid was transformed into
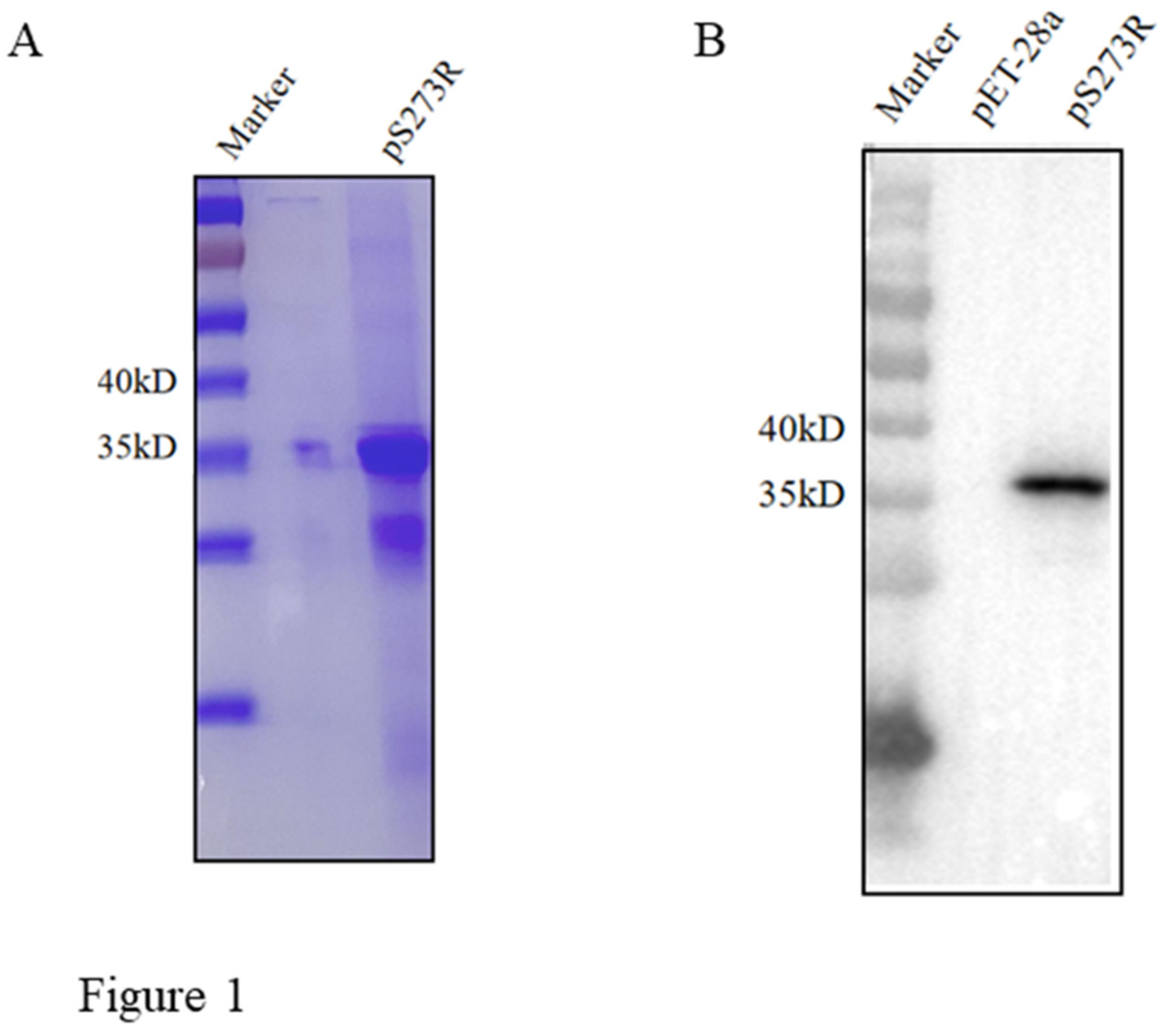
E. coli for protein expression. The recombinant protein was purified from inclusion body and SDS-PAGE analysis showed that the purified pS273R had high purity with a molecular mass of approximately 37 kD (
Figure 1A). Further, Western blotting confirmed that the purified pS273R protein was specifically recognized by anti-His mAb (
Figure 1B).
3.2. Generation of Monoclonal Antibodies (mAbs) against pS273R Protein
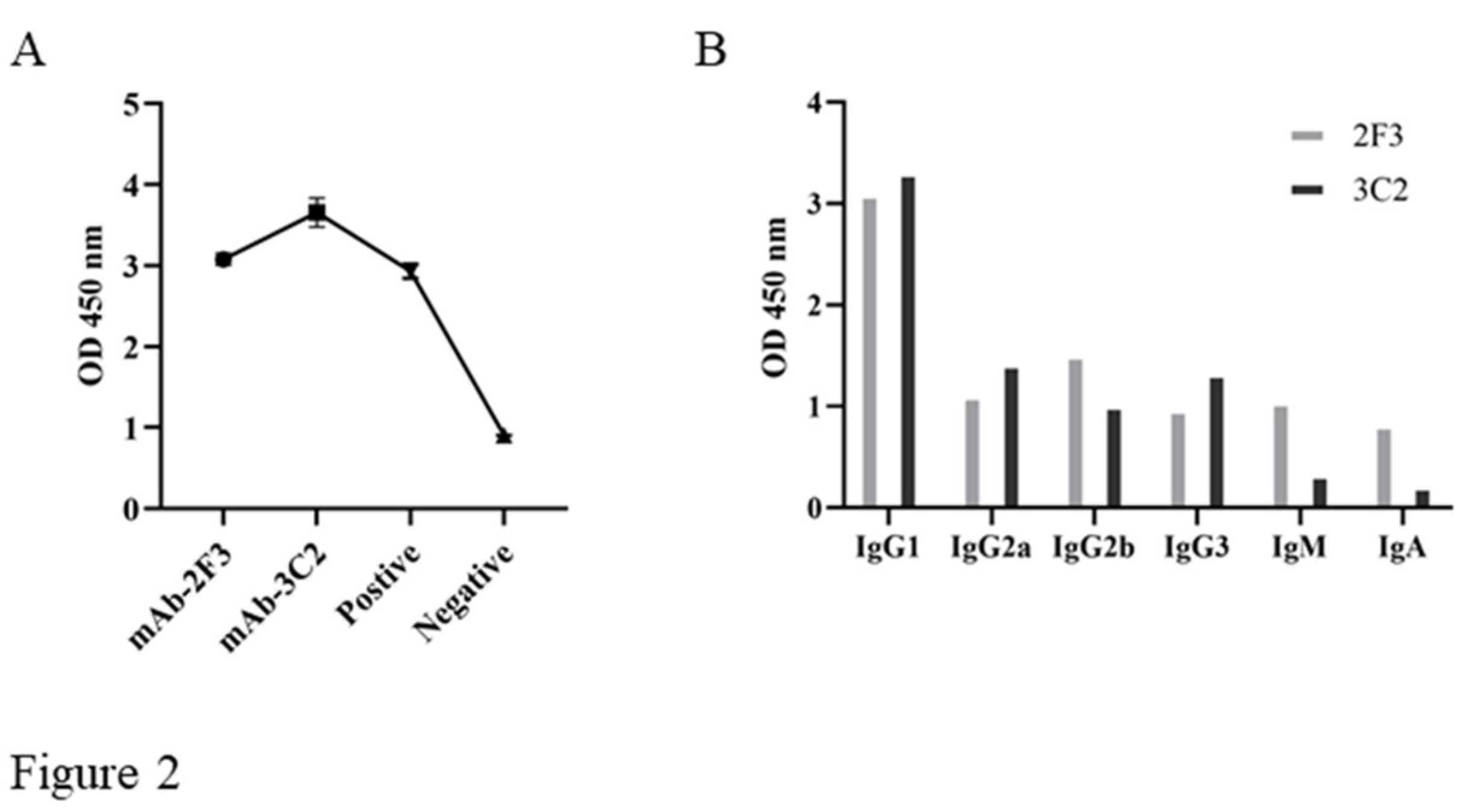
The hybridomas were obtained by regular cell fusion and positive cell clones were selected by pS273R protein based indirect ELISA. After screening and three time subcloning, two hybridoma cell clones named 2F3 and 3C2 were generated (
Figure 2A). The mAbs produced by both 3F3 and 3C2 clones belong to the IgG1 subclass (
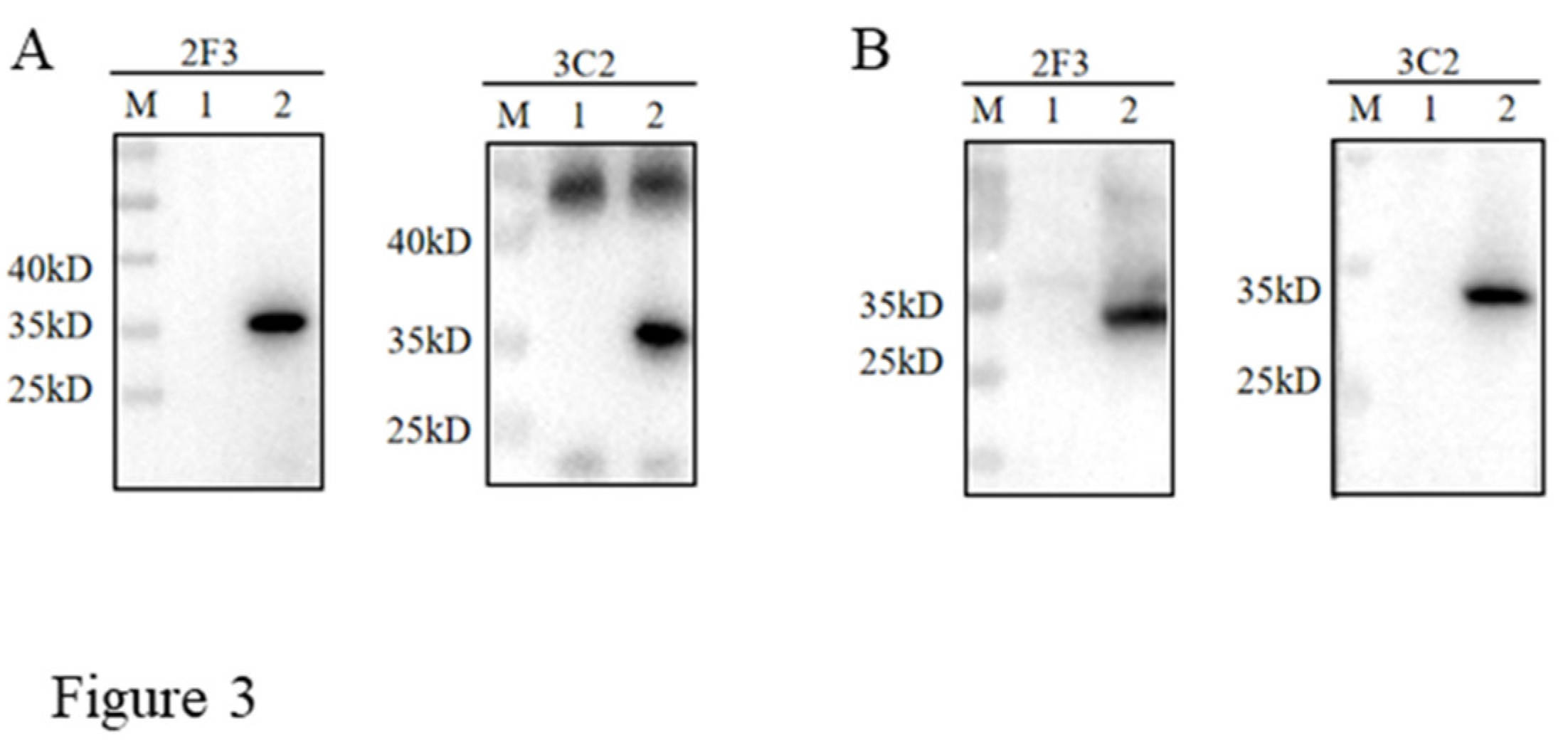
Figure 2B). We further analyzed the reactivity of these two mAbs with the expressed proteins from 293T cells transfected with pCAGGS-S273R-HA (
Figure 3A) and primary PAMs infected with ASFV (
Figure 3B) by using Western blotting. The results showed that both 3F3 and 3C2 mAbs can specifically recognize exogenous pS273R (
Figure 3A) and endogenous pS273R (
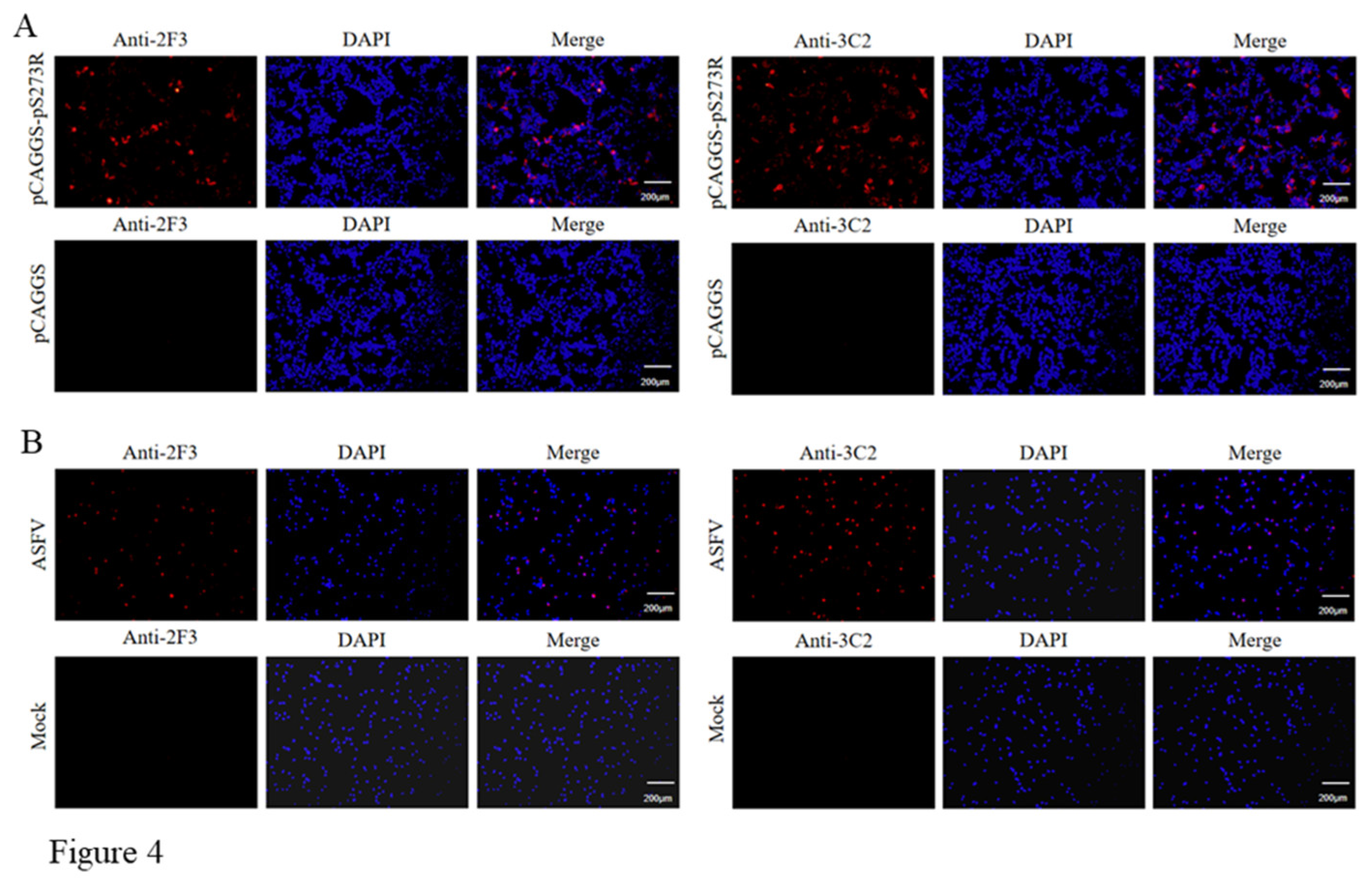
Figure 3B), with the exogenous pS273R a little higher molecular weight than endogenous pS273R. The results also suggested both mAbs recognize linear epitopes of pS273R. Similarly, immunofluorescent assay showed that both mAbs are able to react with the expressed pS273R in transfected 293T cells (
Figure 4A) as well as in ASFV infected PAMs (
Figure 4B), with the pS273R mainly localized in the cytoplasm. Together, the results signified that produced mAbs are specific to ASFV pS273R.
3.3. Identification of the Antigenic Epitope Recognized by Two pS273R mAbs
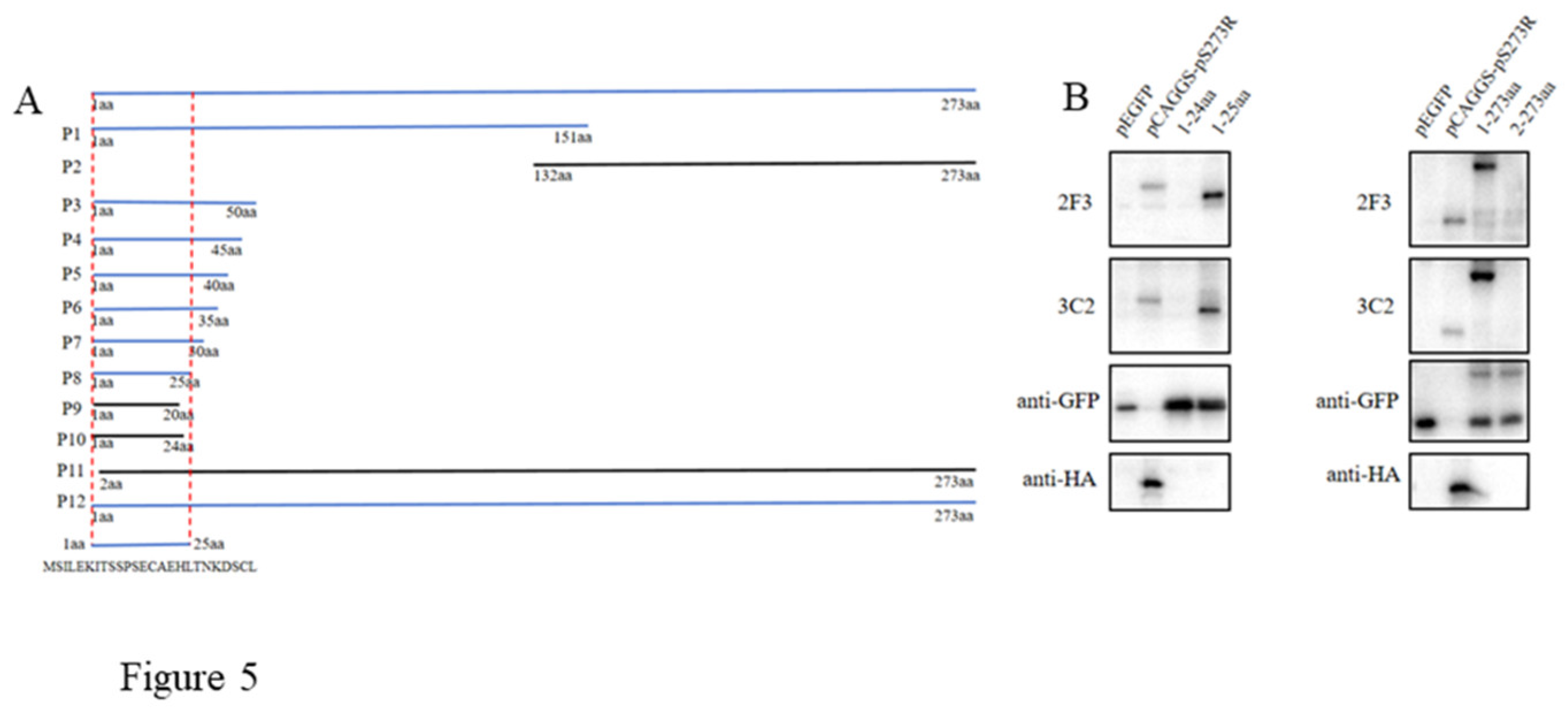
As stated in Methods and shown in the schematic (
Figure 5A), the initial testing with Western botting demonstrated that both mAbs react with N fragment P1, not C fragment P2. Based on the results from P1, the progressive truncations of both N and C ends were performed and the truncated fragments (P3-P12) were all tested for reactivity with pS273R mAbs by Western blotting. It turned out that N terminal amino acid 1 and C terminal amino acid 25 are critical for reactivity with both pS273R mAbs (
Figure 5B). As such, the results clearly showed that both 2F3 and 3C2 mAbs recognize the linear epitope
1MSILEKITSSPSECAEHLTNKDSCL
25.
3.4. Bioinformatics Analysis of the Identified Antigenic Epitope
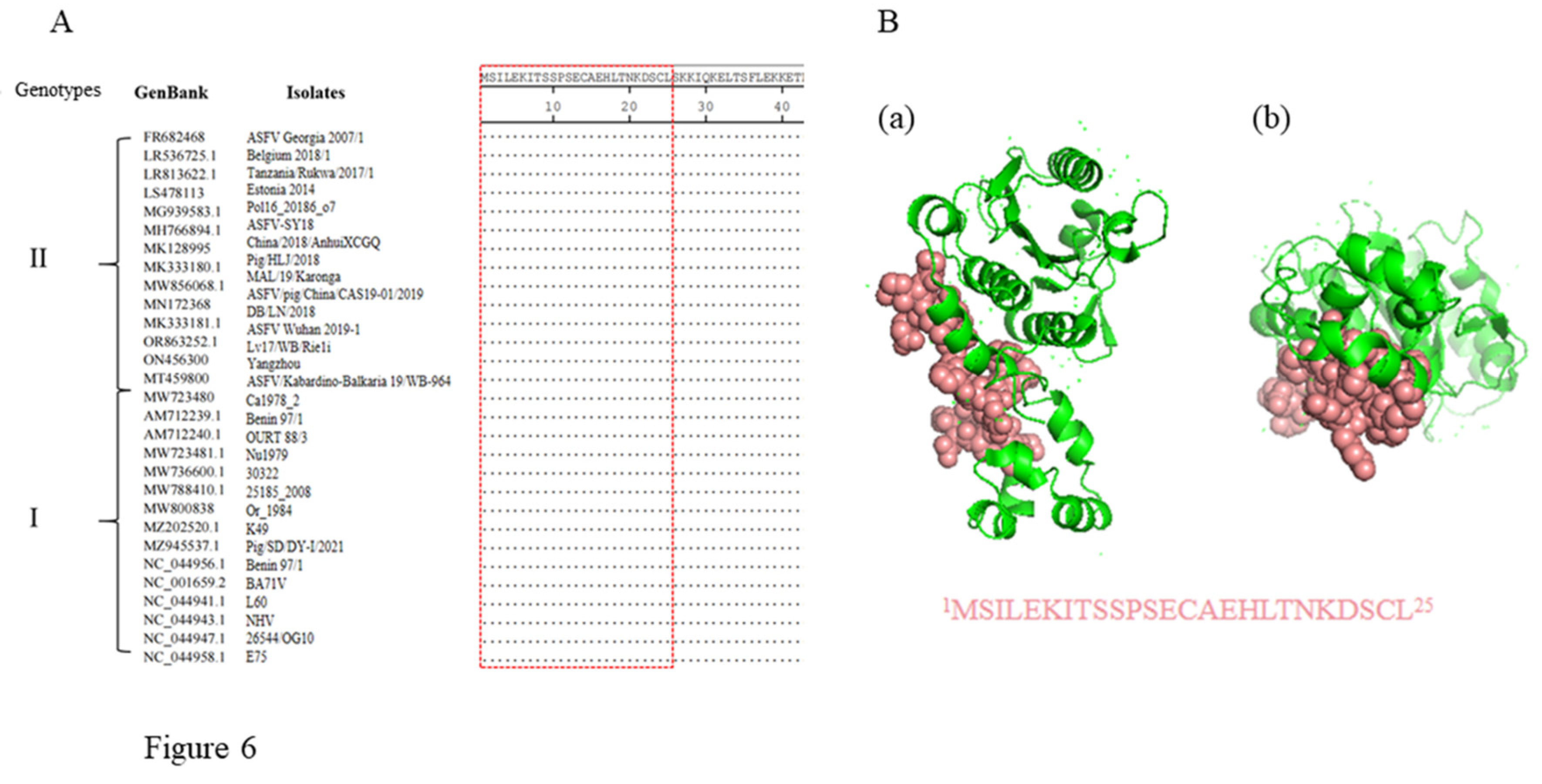
In order to evaluate conservation of the antigenic epitope cross different ASFV strains, the pS273R protein sequences of 171 ASFV strains were downloaded from GenBank and aligned with the epitope sequence we identified. The alignment result showed that the epitope identified here is highly conserved across all ASFV strains except four genotype IX strains (Accession MH025918.1, MH025919.1, MH025920.1 and KM111295.1) (
Table S2). Notably, the epitope sequences are identical in all genotypes I and II ASFV strains, some of which are presented as representatives (
Figure 6A). A 3D structure of ASFV pS273R (ID 6LJB) was obtained from the Protein Data Bank (PDB), and the visualization using PyMOL software revealed that the epitope is exposed on the surface of the pS273R protein in the 3D structure model, confirming its antigenicity (
Figure 6B).
3.5. Establishment of Epitope Based Indirect ELISA Detecting ASFV Antibody
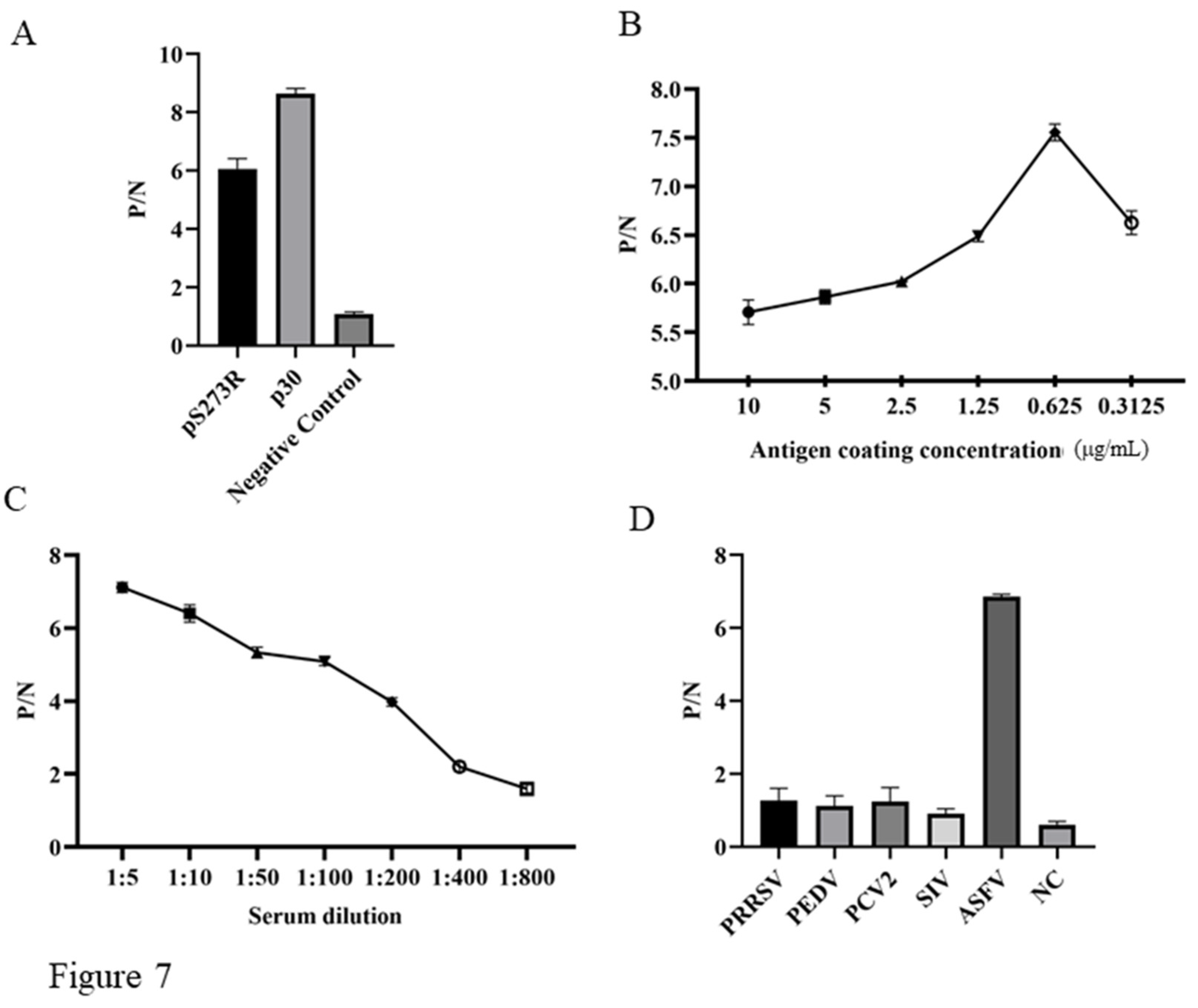
To verify the diagnostic feasibility of pS273R mAb recognized epitope, we developed the epitope peptide based indirect ELISA for detection of ASFV antibody. Preliminary testing was conducted with the coated pS273R epitope, plus a p30 epitope and a non-relevant peptide as the controls, the results showed the pS273R and p30 epitope peptide but not non-relevant peptide were able to detect the antibody from ASFV positive serum (
Figure 7A). Further optimization of the indirect ELISA determined that pS273R epitope peptide coated with the concentration of 0.625 μg/mL gives highest P/N value in detecting ASFV positive serum (
Figure 7B). The dilution of ASFV positive serum was determined to be 1:5, giving the highest P/N value (
Figure 7C). The specificity of the epitope indirect ELISA was tested using different positive sera of PRRSV, PEDV, PCV2, SIV, and ASFV. The results showed that only the ASFV positive serum but not others gives positive P/N value (
Figure 7D). Therefore, our preliminary results suggested that the pS273R mAb recognized epitope can be applied for immunological detection of ASFV infection.
4. Discussion
ASF is a devastating infectious disease in pigs, severely threatening the global pig industry [
1]. World Organization for Animal Health (WOAH) takes ASF as a notifiable disease [
2]. Yet, currently, there is still no commercial vaccine and effective therapeutics, and improving the level of biosafety is still the main means to prevent and control the ASF [
23]. Due to its genetic complexity, many ASFV genes are still poorly studied, which may contribute to the insufficient understanding of its pathogenesis and hinder the progress of ASFV vaccine development and disease control [
24]. According to previous investigations, the ASFV only protease pS273R plays an important role in ASFV infection and host immune evasion, accordingly, mAb against ASFV pS273R may serve as a useful tool for viral research. Here we obtained two specific mAbs against pS273R, which can specifically detect ASFV in various immunological assays including ELISA, Western blotting and Immunofluorescence.
In order to obtain more comprehensive mAbs and the complete epitope map of pS273R, we used the full-length pS273R protein to immunize mice for subsequent cell fusion. Although only two mAbs were obtained, both mAbs recognize one identical linear B cell epitope, indicating the predominance of this antigenic epitope. As far as we know, it is the first reported ASFV pS273R antigenic epitope. Among 150 plus ASFV proteins, there are about 50 viral structural proteins [
11]. The generation of mAbs and identification of antigenic epitopes have been reported for several common viral structure proteins, such as p72 [
25,
26,
27,
28,
29,
30], p54 [
31,
32,
33,
34,
35,
36], p30 [
37,
38,
39,
40,
41,
42] and p17 [
43]. Even though pS273R is a protease, it is existed in the core shell of ASFV virions together with the polyproteins [
13]. Therefore, it is reasonable pS273R exhibits antigenicity during ASFV infection and the epitope recognized by its mAb is able to detect antibody in ASFV positive serum.
ASFV was first discovered in Kenya in 1921 and there have been 24 genotypes in Africa [
1,
44]. Between the 1957 and 1995, genotype I ASFV emerged in Europe, Russia, the Caribbean and South America [
3]. In 2007, genotype II ASFV emerged in the Republic of Georgia and continued to spread through the Caucasus region and subsequently into the Russian Federation and Eastern Europe, where it has continued to circulate and spread [
10]. In August 2018, the first ASF case was reported in China and genotype II ASFV was identified [
5]. Until now, genotype II ASFV strains are mainly epidemic strains in China, whereas genotype I ASFV also emerged in domestic pigs and caused chronic infection in China [
45,
46]. Therefore, the diagnosis of genotype I and/or genotype II ASFV infections are particularly important. The antigenic epitope recognized by pS273R mAbs we generated is highly conservative across different ASFV strains and is identical to all genotype I and genotype II ASFV strains. Thus, the mAbs and antigenic epitope can be used for general detection of genotype I and genotype II ASFV infections.
In this study, we developed two indirect ELISA for detection of ASFV antibody by using pS273R protein and antigenic epitope, respectively. Both ELISA were able to detect ASFV pS273R antibody, and the epitope based ELISA was supposed to be more specific than the pS273R based ELISA. Additionally, the pS273R mAbs we developed can also be used in developing competitive ELISA for detection of ASFV antibody. However, we do not know which ELISA will be better for detection of ASFV antibody in clinical samples. To this end, the three types of ELISA need to be tested side by side with enough number of clinical serum samples, which deserves to be further investigated. Together, our results suggest that ASFV pS273R protein contributes to the development of serological diagnostic kit, either alone or in combination with other immunogenic ASFV proteins.
In summary, we generated two specific mAbs against ASFV pS273R protease, identified a highly conserved linear B-cell epitope of pS273R, and established the indirect ELISA for detection of ASFV antibody. These results contribute to the study on ASFV pS273R function and development of ASFV diagnostic and vaccine candidate.
Supplementary Materials
Table S1. The cloning PCR primers used in this study. Table S2. The identity of pS273R epitope across different ASFV stains.
Author Contributions statement
JZ.Z conceived and designed the experiments; JJ.Z, K.Z, S.S, P.H, D.D, P.Z performed the experiments; W.Z and N.C provided the resources; JJ.Z and JZ.Z wrote the paper. All authors contributed to the article and approved the submitted version.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Acknowledgments
This work was partly supported by the National Key Research and Development Program of China (2021YFD1800105), Jiangsu provincial key R & D plan (BE2020398), Jiangsu agricultural science and technology independent innovation fund project (CX(21)2035), the 111 Project under Grant D18007, and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
- Dixon, L. K.; Sun, H.; Roberts, H., African swine fever. Antiviral Res 2019, 165, 34-41.
- Li, Z.; Chen, W.; Qiu, Z.; Li, Y.; Fan, J.; Wu, K.; Li, X.; Zhao, M.; Ding, H.; Fan, S.; et al. African Swine Fever Virus: A Review. Life 2022, 12, 1255. [CrossRef]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Martínez-López, B. African Swine Fever: An Epidemiological Update. Transbound. Emerg. Dis. 2012, 59, 27–35. [CrossRef]
- Guinat, C.; Gogin, A.; Blome, S.; Keil, G.; Pollin, R.; Pfeiffer, D.U.; Dixon, L. Transmission routes of African swine fever virus to domestic pigs: current knowledge and future research directions. Veter- Rec. 2016, 178, 262–267. [CrossRef]
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African Swine Fever in China, 2018. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [CrossRef]
- Penrith, M.-L.; Van Heerden, J.; Heath, L.; Abworo, E.O.; Bastos, A.D.S. Review of the Pig-Adapted African Swine Fever Viruses in and Outside Africa. Pathogens 2022, 11, 1190. [CrossRef]
- Urbano, A.C.; Ferreira, F. African swine fever control and prevention: an update on vaccine development. Emerg. Microbes Infect. 2022, 11, 2021–2033. [CrossRef]
- Zhang, H.; Zhao, S.; Zhang, H.; Qin, Z.; Shan, H.; Cai, X. Vaccines for African swine fever: an update. Front. Microbiol. 2023, 14. [CrossRef]
- Arabyan, E.; Kotsynyan, A.; Hakobyan, A.; Zakaryan, H. Antiviral agents against African swine fever virus. Virus Res. 2019, 270, 197669. [CrossRef]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African Swine Fever Virus: An Emerging DNA Arbovirus. Front. Vet. Sci. 2020, 7, 215. [CrossRef]
- Alejo, A.; Matamoros, T.; Guerra, M.; Andrés, G. A Proteomic Atlas of the African Swine Fever Virus Particle. J. Virol. 2018, 92. [CrossRef]
- Karger, A.; Pérez-Núñez, D.; Urquiza, J.; Hinojar, P.; Alonso, C.; Freitas, F.B.; Revilla, Y.; Le Potier, M.-F.; Montoya, M. An Update on African Swine Fever Virology. Viruses 2019, 11, 864. [CrossRef]
- Andrés, G.; Alejo, A.; Simón-Mateo, C.; Salas, M.L. African Swine Fever Virus Protease, a New Viral Member of the SUMO-1-specific Protease Family. J. Biol. Chem. 2001, 276, 780–787. [CrossRef]
- Andrés, G.; Alejo, A.; Salas, J.; Salas, M.L. African Swine Fever Virus Polyproteins pp220 and pp62 Assemble into the Core Shell. J. Virol. 2002, 76, 12473–82. [CrossRef]
- Alejo, A.; Andrés, G.; Salas, M.L. African Swine Fever Virus Proteinase Is Essential for Core Maturation and Infectivity. J. Virol. 2003, 77, 5571–5577. [CrossRef]
- Li, H.; Zheng, X.; Li, Y.; Zhu, Y.; Xu, Y.; Yu, Z.; Feng, W.-H. African swine fever virus S273R protein antagonizes type I interferon production by interfering with TBK1 and IRF3 interaction. Virol. Sin. 2023, 38, 911–921. [CrossRef]
- Li, T.; Li, X.; Wang, X.; Chen, X.; Zhao, G.; Liu, C.; Bao, M.; Song, J.; Li, J.; Huang, L.; et al. African swine fever virus pS273R antagonizes stress granule formation by cleaving the nucleating protein G3BP1 to facilitate viral replication. J. Biol. Chem. 2023, 299, 104844. [CrossRef]
- Li, Y.-H.; Peng, J.-L.; Xu, Z.-S.; Xiong, M.-G.; Wu, H.-N.; Wang, S.-Y.; Li, D.; Zhu, G.-Q.; Ran, Y.; Wang, Y.-Y. African Swine Fever Virus Cysteine Protease pS273R Inhibits Type I Interferon Signaling by Mediating STAT2 Degradation. J. Virol. 2023, 97, e0194222. [CrossRef]
- Luo, J.; Zhang, J.; Ni, J.; Jiang, S.; Xia, N.; Guo, Y.; Shao, Q.; Cao, Q.; Zheng, W.; Chen, N.; Zhang, Q.; Chen, H.; Chen, Q.; Zhu, H.; Meurens, F.; Zhu, J., The African swine fever virus protease pS273R inhibits DNA sensing cGAS-STING pathway by targeting IKKε. Virulence 2022, 13, (1), 740-756.
- Ma, C.; Li, S.; Yang, F.; Cao, W.; Liu, H.; Feng, T.; Zhang, K.; Zhu, Z.; Liu, X.; Hu, Y.; et al. FoxJ1 inhibits African swine fever virus replication and viral S273R protein decreases the expression of FoxJ1 to impair its antiviral effect. Virol. Sin. 2022, 37, 445–454. [CrossRef]
- Zhao, G.; Li, T.; Liu, X.; Zhang, T.; Zhang, Z.; Kang, L.; Song, J.; Zhou, S.; Chen, X.; Wang, X.; et al. African swine fever virus cysteine protease pS273R inhibits pyroptosis by noncanonically cleaving gasdermin D. J. Biol. Chem. 2021, 298, 101480. [CrossRef]
- Li, G.; Liu, X.; Yang, M.; Zhang, G.; Wang, Z.; Guo, K.; Gao, Y.; Jiao, P.; Sun, J.; Chen, C.; et al. Crystal Structure of African Swine Fever Virus pS273R Protease and Implications for Inhibitor Design. J. Virol. 2020, 94. [CrossRef]
- Danzetta, M.L.; Marenzoni, M.L.; Iannetti, S.; Tizzani, P.; Calistri, P.; Feliziani, F. African Swine Fever: Lessons to Learn From Past Eradication Experiences. A Systematic Review. Front. Veter- Sci. 2020, 7, 296. [CrossRef]
- Yang, S.; Miao, C.; Liu, W.; Zhang, G.; Shao, J.; Chang, H. Structure and function of African swine fever virus proteins: Current understanding. Front. Microbiol. 2023, 14, 1043129. [CrossRef]
- Yin, D.; Geng, R.; Shao, H.; Ye, J.; Qian, K.; Chen, H.; Qin, A. Identification of novel linear epitopes in P72 protein of African swine fever virus recognized by monoclonal antibodies. Front. Microbiol. 2022, 13, 1055820. [CrossRef]
- Hu, Y.; Wang, A.; Yan, W.; Li, J.; Meng, X.; Chen, L.; Li, S.; Tong, W.; Kong, N.; Yu, L.; et al. Identification of Linear Epitopes in the C-Terminal Region of ASFV p72 Protein. Microorganisms 2023, 11, 2846. [CrossRef]
- Chang, Z.; Du, Y.; Li, R.; Sun, X.; Chen, Y.; Li, M.; Fan, L.; Liu, S.; Wang, S.; Ding, P.; et al. Development and characterization of monoclonal antibody against the critical loop structure of african swine fever virus P72 protein. Veter- Microbiol. 2023, 283, 109776. [CrossRef]
- Duan, X.; Liu, Y.; Chen, Z.; Xie, Z.; Tian, C.; Li, Y.; Lv, L.; Wang, R.; Liu, J.; Chen, H. Identification of monoclonal antibody targeting epitope on p72 trimeric spike of African swine fever virus. Virus Genes 2023, 59, 582–590. [CrossRef]
- Miao, C.; Yang, S.; Shao, J.; Zhou, G.; Ma, Y.; Wen, S.; Hou, Z.; Peng, D.; Guo, H.; Liu, W.; et al. Identification of p72 epitopes of African swine fever virus and preliminary application. Front. Microbiol. 2023, 14, 1126794. [CrossRef]
- Heimerman, M.E.; Murgia, M.V.; Wu, P.; Lowe, A.D.; Jia, W.; Rowland, R.R. Linear epitopes in African swine fever virus p72 recognized by monoclonal antibodies prepared against baculovirus-expressed antigen. J. Veter- Diagn. Investig. 2018, 30, 406–412. [CrossRef]
- Cao, Y.; Han, D.; Zhang, Y.; Zhang, K.; Du, N.; Tong, W.; Li, G.; Zheng, H.; Liu, C.; Gao, F.; et al. Identification of one novel epitope targeting p54 protein of African swine fever virus using monoclonal antibody and development of a capable ELISA. Res. Veter- Sci. 2021, 141, 19–25. [CrossRef]
- Zhao, H.; Wang, G.; Dong, H.; Wu, S.; Du, Y.; Wan, B.; Ji, P.; Wu, Y.; Jiang, D.; Zhuang, G.; et al. Identification of a Linear B Cell Epitope on p54 of African Swine Fever Virus Using Nanobodies as a Novel Tool. Microbiol. Spectr. 2023, 11, e0336222. [CrossRef]
- Zheng, N.; Li, C.; Hou, H.; Chen, Y.; Zhang, A.; Han, S.; Wan, B.; Wu, Y.; He, H.; Wang, N.; et al. A Novel Linear B-Cell Epitope on the P54 Protein of African Swine Fever Virus Identified Using Monoclonal Antibodies. Viruses 2023, 15, 867. [CrossRef]
- Gao, Y.; Xia, T.; Bai, J.; Zhang, L.; Zheng, H.; Jiang, P. Preparation of Monoclonal Antibodies against the Viral p54 Protein and a Blocking ELISA for Detection of the Antibody against African Swine Fever Virus. Viruses 2022, 14, 2335. [CrossRef]
- Wang, A.; Jiang, M.; Liu, H.; Liu, Y.; Zhou, J.; Chen, Y.; Ding, P.; Wang, Y.; Pang, W.; Qi, Y.; et al. Development and characterization of monoclonal antibodies against the N-terminal domain of African swine fever virus structural protein, p54. Int. J. Biol. Macromol. 2021, 180, 203–211. [CrossRef]
- Petrovan, V.; Murgia, M.V.; Wu, P.; Lowe, A.D.; Jia, W.; Rowland, R.R. Epitope mapping of African swine fever virus (ASFV) structural protein, p54. Virus Res. 2020, 279, 197871. [CrossRef]
- Tian, P.; Sun, Z.; Wang, M.; Song, J.; Sun, J.; Zhou, L.; Jiang, D.; Zhang, A.; Wu, Y.; Zhang, G. Identification of a novel linear B-cell epitope on the p30 protein of African swine fever virus using monoclonal antibodies. Virus Res. 2024, 341, 199328. [CrossRef]
- Zhou, J.; Ni, Y.; Wang, D.; Fan, B.; Zhu, X.; Zhou, J.; Hu, Y.; Li, L.; Li, B. Development of a Competitive Enzyme-Linked Immunosorbent Assay Targeting the-p30 Protein for Detection of Antibodies against African Swine Fever Virus. Viruses 2023, 15, 154. [CrossRef]
- Zhou, G.; Shi, Z.; Luo, J.; Cao, L.; Yang, B.; Wan, Y.; Wang, L.; Song, R.; Ma, Y.; Tian, H.; et al. Preparation and epitope mapping of monoclonal antibodies against African swine fever virus P30 protein. Appl. Microbiol. Biotechnol. 2022, 106, 1199–1210. [CrossRef]
- Zhang, X.; Liu, X.; Wu, X.; Ren, W.; Zou, Y.; Xia, X.; Sun, H. A colloidal gold test strip assay for the detection of African swine fever virus based on two monoclonal antibodies against P30. Arch. Virol. 2021, 166, 871–879. [CrossRef]
- Wu, P.; Lowe, A.D.; Rodríguez, Y.Y.; Murgia, M.V.; Dodd, K.A.; Rowland, R.R.; Jia, W. Antigenic regions of African swine fever virus phosphoprotein P30. Transbound. Emerg. Dis. 2020, 67, 1942–1953. [CrossRef]
- Petrovan, V.; Yuan, F.; Li, Y.; Shang, P.; Murgia, M.V.; Misra, S.; Rowland, R.R.; Fang, Y. Development and characterization of monoclonal antibodies against p30 protein of African swine fever virus. Virus Res. 2019, 269, 197632. [CrossRef]
- Li, L.; Qiao, S.; Wang, S.; Liu, J.; Zhao, K.; Zhou, Y.; Li, G.; Jiang, Y.; Liu, C.; Tong, G.; et al. Expression of ASFV p17 in CHO cells and identification of one novel epitope using a monoclonal antibody. Virus Res. 2023, 336, 199194. [CrossRef]
- Qu, H.; Ge, S.; Zhang, Y.; Wu, X.; Wang, Z. A systematic review of genotypes and serogroups of African swine fever virus. Virus Genes 2022, 58, 77–87. [CrossRef]
- Sun, E.; Huang, L.; Zhang, X.; Zhang, J.; Shen, D.; Zhang, Z.; Wang, Z.; Huo, H.; Wang, W.; Huangfu, H.; et al. Genotype I African swine fever viruses emerged in domestic pigs in China and caused chronic infection. Emerg. Microbes Infect. 2021, 10, 2183–2193. [CrossRef]
- Sun, E.; Zhang, Z.; Wang, Z.; He, X.; Zhang, X.; Wang, L.; Wang, W.; Huang, L.; Xi, F.; Huangfu, H.; et al. Emergence and prevalence of naturally occurring lower virulent African swine fever viruses in domestic pigs in China in 2020. Sci. China Life Sci. 2021, 64, 752–765. [CrossRef]
Figure 1.
Production and identification of the pS273R recombinant protein. (A) The purified pS273R was verified by SDS-PAGE and Coomassie blue staining, with the major band about 37 kD. (B) The purified pS273R protein was verified by Western blotting using the anti-His mAb. The pET28a vector transformed bacterial protein was used as a control.
Figure 1.
Production and identification of the pS273R recombinant protein. (A) The purified pS273R was verified by SDS-PAGE and Coomassie blue staining, with the major band about 37 kD. (B) The purified pS273R protein was verified by Western blotting using the anti-His mAb. The pET28a vector transformed bacterial protein was used as a control.
Figure 2.
Preparation and characterization of anti-pS273R monoclonal antibodies. (A) The reactivity of mAbs was tested in pS273R protein based indirect ELISA. The cell supernatants of hybridoma clones 2F3 and 3C2 were used as the primary antibodies, the SP2/0 cell supernatant was used as the negative control, and the serum of immunized mice was used as positive control. (B) Subclass of mAbs 2F3 and 3C2 was determined by the monoclonal antibody subclass identification kit (C060101) from CELLWAY-LAB (Luoyang, China).
Figure 2.
Preparation and characterization of anti-pS273R monoclonal antibodies. (A) The reactivity of mAbs was tested in pS273R protein based indirect ELISA. The cell supernatants of hybridoma clones 2F3 and 3C2 were used as the primary antibodies, the SP2/0 cell supernatant was used as the negative control, and the serum of immunized mice was used as positive control. (B) Subclass of mAbs 2F3 and 3C2 was determined by the monoclonal antibody subclass identification kit (C060101) from CELLWAY-LAB (Luoyang, China).
Figure 3.
The specific reactivity of pS273R mAbs was analyzed by Western blotting. 293T cells were transfected with pCAGGS-pS273R-2HA (lane 2) and pCAGGS vector control (lane 1). Cell were harvested and cell lysates were detected for exogenous pS273R by Western blotting with 2F3 and 3C2 mAbs as primary antibodies. (B) Primary PAMs were infected with ASFV (MOI 0.1) (lane 2) and mock infected (lane 1) for 96 h, and cell lysates were detected for endogenous pS273R by Western blotting with the mAbs 2F3 and 3C2 as primary antibodies.
Figure 3.
The specific reactivity of pS273R mAbs was analyzed by Western blotting. 293T cells were transfected with pCAGGS-pS273R-2HA (lane 2) and pCAGGS vector control (lane 1). Cell were harvested and cell lysates were detected for exogenous pS273R by Western blotting with 2F3 and 3C2 mAbs as primary antibodies. (B) Primary PAMs were infected with ASFV (MOI 0.1) (lane 2) and mock infected (lane 1) for 96 h, and cell lysates were detected for endogenous pS273R by Western blotting with the mAbs 2F3 and 3C2 as primary antibodies.
Figure 4.
The specific reactivity of pS273R mAbs was analyzed with Immunofluorescence. 293T cells were transfected pCAGGS-pS273R and pCAGGS vector, respectively. Cells were fixed at 24 h post-transfection and stained with 2F3 or 3C2 mAbs, together with Goat anti-mouse IgG H&L Alexa Fluor 594. Cellular nuclei were counterstained with 4′, 6′-diamidino-2-phenylindole (DAPI). (B) Primary PAMs were infected with ASFV (MOI 0.1). Cells were fixed at 72 h post-infection and stained with 2F3 or 3C2, together with secondary antibody and DAPI.
Figure 4.
The specific reactivity of pS273R mAbs was analyzed with Immunofluorescence. 293T cells were transfected pCAGGS-pS273R and pCAGGS vector, respectively. Cells were fixed at 24 h post-transfection and stained with 2F3 or 3C2 mAbs, together with Goat anti-mouse IgG H&L Alexa Fluor 594. Cellular nuclei were counterstained with 4′, 6′-diamidino-2-phenylindole (DAPI). (B) Primary PAMs were infected with ASFV (MOI 0.1). Cells were fixed at 72 h post-infection and stained with 2F3 or 3C2, together with secondary antibody and DAPI.
Figure 5.
Identification of the antigenic epitopes recognized by pS273R monoclonal antibodies. Schematic of the strategy for mapping the epitope. The fragments with reactivity with pS273R mAbs are blue marked, whereas those without reactivity with mAbs are black marked. (B) Western blotting analysis of the critical C terminal amino acid (left) and N terminal amino acid (right) for reactivity of pS273R fragments with the two mAbs 2F3 and 3C2. The aa is an abbreviation for amino acid.
Figure 5.
Identification of the antigenic epitopes recognized by pS273R monoclonal antibodies. Schematic of the strategy for mapping the epitope. The fragments with reactivity with pS273R mAbs are blue marked, whereas those without reactivity with mAbs are black marked. (B) Western blotting analysis of the critical C terminal amino acid (left) and N terminal amino acid (right) for reactivity of pS273R fragments with the two mAbs 2F3 and 3C2. The aa is an abbreviation for amino acid.
Figure 6.
Conservation analysis of identified novel linear epitopes of the pS273R protein. Alignment of the epitope (1MSILEKITSSPSECAEHLTNKDSCL25) in 30 representative genotypes I and II ASFV strains. The red box indicated the identified conserved epitope. (B) Prediction of the pS273R structure by using PyMOL. The epitope recognized by two mAbs is displayed in pink color, and shown in the front view (a) and bottom view (b), respectively.
Figure 6.
Conservation analysis of identified novel linear epitopes of the pS273R protein. Alignment of the epitope (1MSILEKITSSPSECAEHLTNKDSCL25) in 30 representative genotypes I and II ASFV strains. The red box indicated the identified conserved epitope. (B) Prediction of the pS273R structure by using PyMOL. The epitope recognized by two mAbs is displayed in pink color, and shown in the front view (a) and bottom view (b), respectively.
Figure 7.
Establishment of indirect ELISA for detecting of ASFV antibody. The pS273R epitope peptide, the positive p30 peptide (ETNECTSSFET), and a non-relevant control peptide (RSVPFEYYRIRKVKV) were used for coating at a concentration of 1μg/mL (peptides were synthesized by GeneCreate Wuhan China). The epitope based ELISA were tested for detection of ASFV positive serum, with a serum dilution of 1:10. (B) The optimization of coating epitope peptide in indirect ELISA for detection of ASFV positive serum (1:10 dilution). (C) The optimization of serum dilution in in indirect ELISA with peptide coating concentration at 0.625 μg/mL. (D) Detection specificity of the established epitope based indirect ELISA. PRRSV, PEDV, PCV2, SIV and ASFV positive porcine sera and negative porcine serum were used.
Figure 7.
Establishment of indirect ELISA for detecting of ASFV antibody. The pS273R epitope peptide, the positive p30 peptide (ETNECTSSFET), and a non-relevant control peptide (RSVPFEYYRIRKVKV) were used for coating at a concentration of 1μg/mL (peptides were synthesized by GeneCreate Wuhan China). The epitope based ELISA were tested for detection of ASFV positive serum, with a serum dilution of 1:10. (B) The optimization of coating epitope peptide in indirect ELISA for detection of ASFV positive serum (1:10 dilution). (C) The optimization of serum dilution in in indirect ELISA with peptide coating concentration at 0.625 μg/mL. (D) Detection specificity of the established epitope based indirect ELISA. PRRSV, PEDV, PCV2, SIV and ASFV positive porcine sera and negative porcine serum were used.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).