Submitted:
07 June 2024
Posted:
07 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
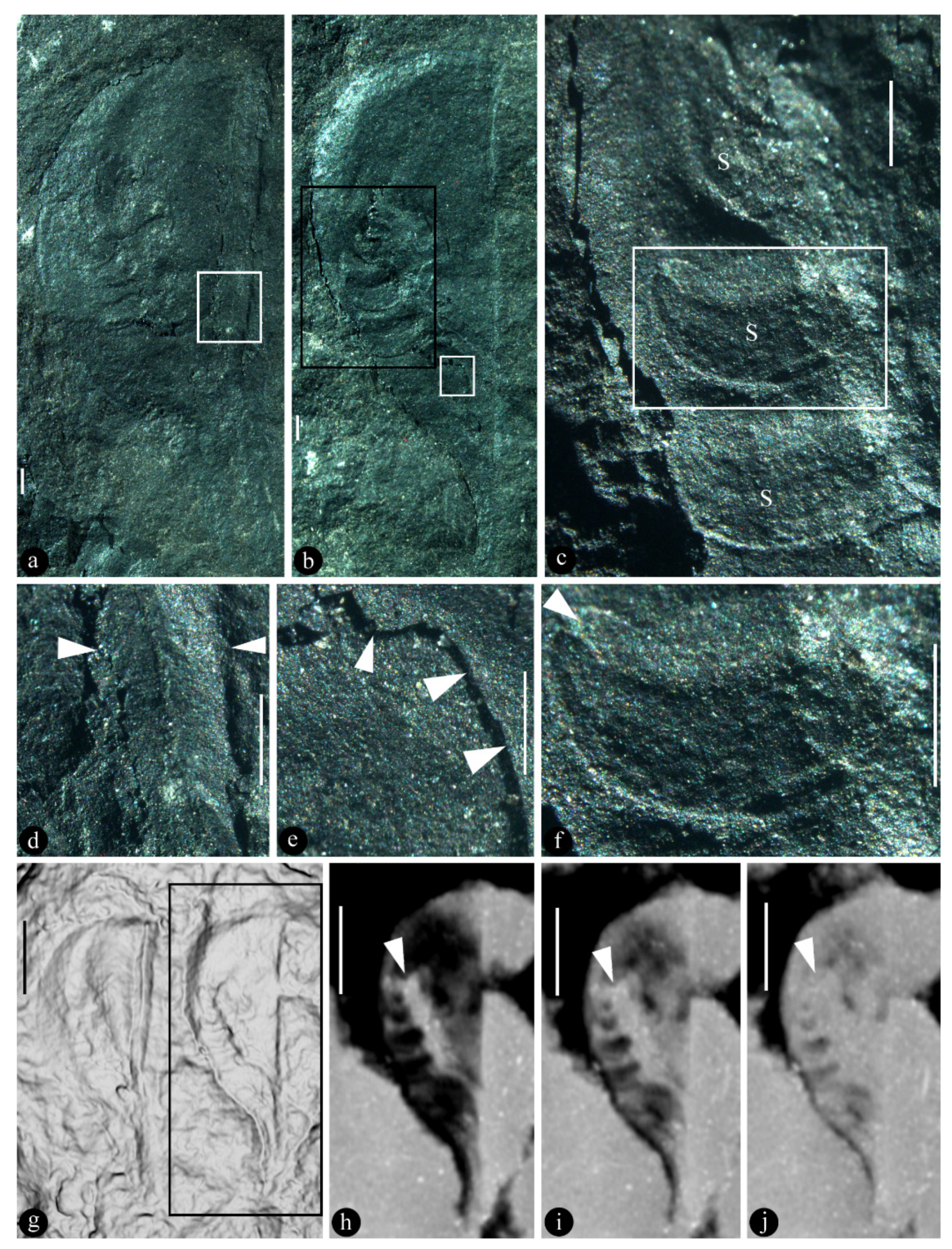
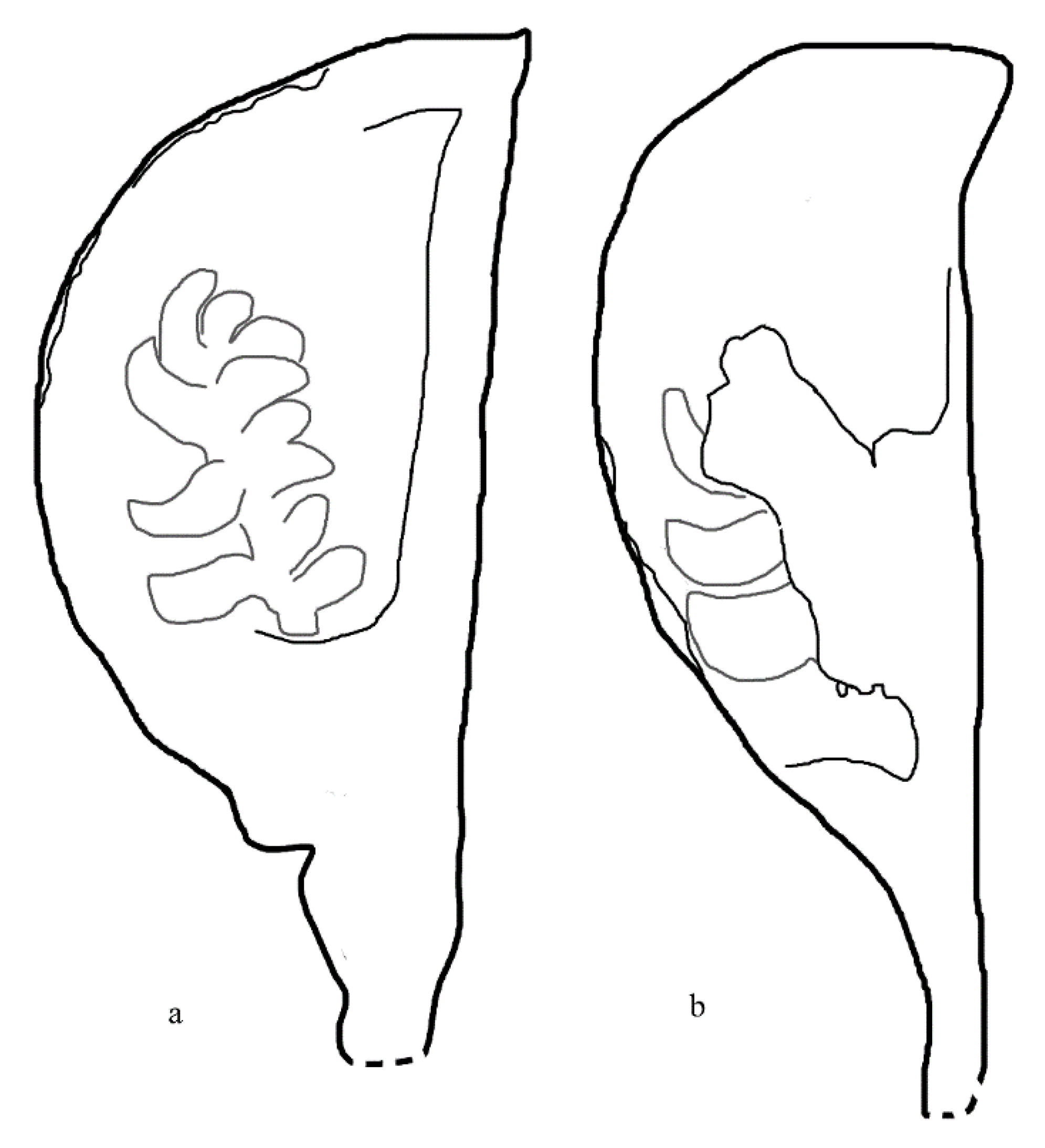
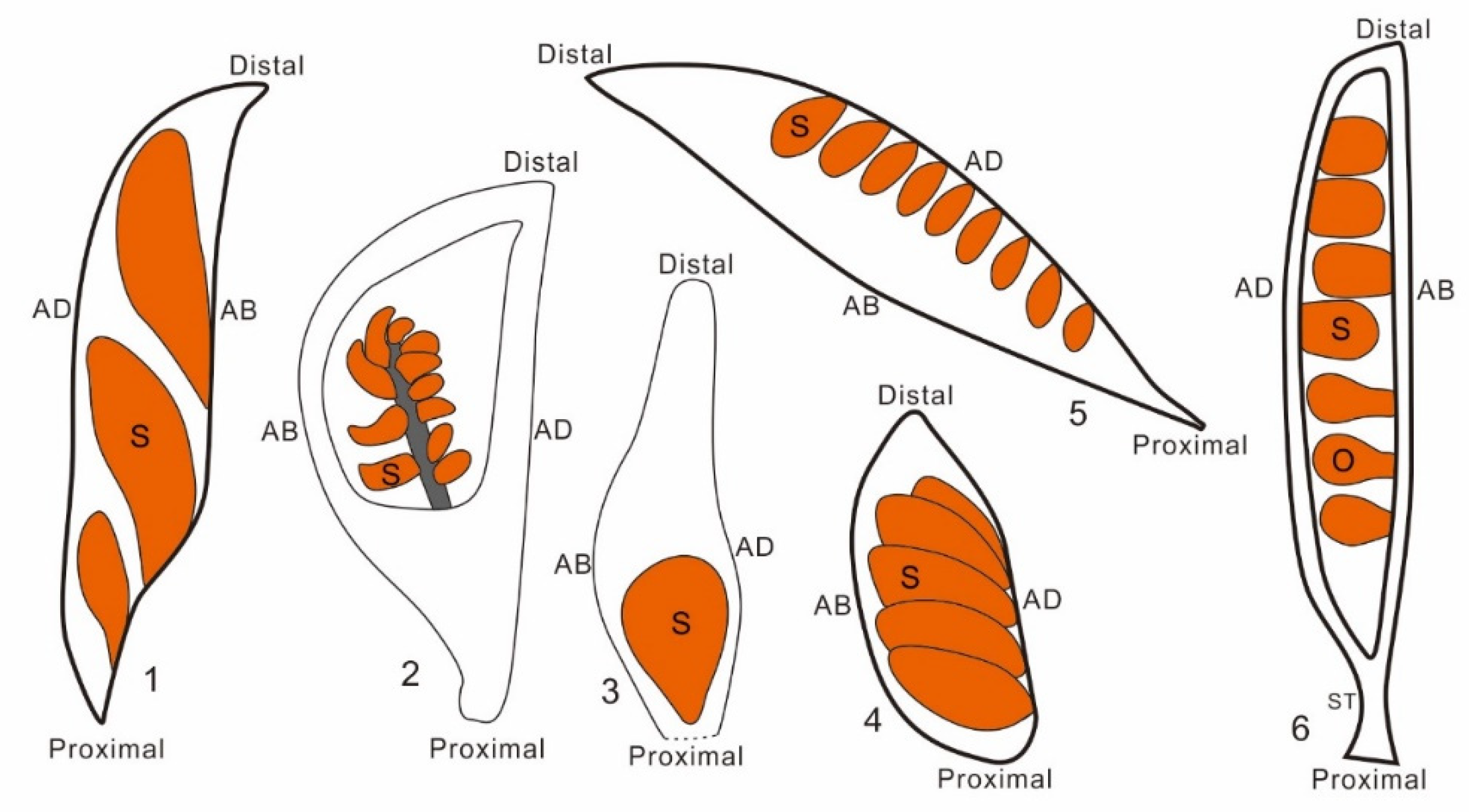
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cronquist, A. The evolution and classification of flowering plants., 2nd ed.; New York Botanical Garden: Bronx, 1988; p. 555. [Google Scholar]
- Wang, X.; Liu, Z.-J.; Liu, W.; Liao, W.; Zhang, X.; Liu, Z.; Hu, G.; Guo, X.; Wang, Y. Stepping out of the shadow of Goethe: for a more scientific plant systematics. Chinese Bulletin of Botany 2020, 55(4), 505–512. [Google Scholar]
- Wang, X. , The Dawn Angiosperms., 2nd ed.; Springer: Cham, Switzerland, 2018; p. 407. [Google Scholar]
- Zhang, X. , Floral ontogeny of Illicium lanceolatum (Schisandraceae) and its implications on carpel homology. Phytotaxa 2019, 416(3), 200–210. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Wang, X. , How the ovules get enclosed in magnoliaceous carpels. PLOS ONE 2017, 12(4), e0174955. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Leslie, A. B.; Herendeen, P. S.; Herrera, F.; Ichinnorov, N.; Takahashi, M.; Knopf, P.; Crane, P. R. , Early Cretaceous Umkomasia from Mongolia: implications for homology of corystosperm cupules. New Phytologist 2016, 210(4), 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Crane, P. R.; Herendeen, P. S.; Herrera, F.; Shi, G., Diversity and homologies of corystosperm seed-bearing structures from the Early Cretaceous of Mongolia and China. In 10th European Palaeobotany and Palynology Conference, McElwain, J., Ed. Trinity College Dublin: Dublin, 2018; p 88.
- Endress, P. K. , The morphological relationship between carpels and ovules in angiosperms: pitfalls of morphological interpretation. Botanical Journal of the Linnean Society 2019, 189(3), 201–227. [Google Scholar] [CrossRef]
- Sun, G.; Dilcher, D. L.; Zheng, S.; Zhou, Z. , In search of the first flower: a Jurassic angiosperm, Archaefructus, from Northeast China. Science 1998, 282, 1692–1695. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Ji, Q.; Dilcher, D. L.; Zheng, S.; Nixon, K. C.; Wang, X. , Archaefructaceae, a new basal angiosperm family. Science 2002, 296, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Li, H.; Bowe, M.; Liu, Y.; Taylor, D. W., Early Cretaceous Archaefructus eoflora sp. nov. with bisexual flowers from Beipiao, Western Liaoning, China. Acta Geologica Sinica (English edition) 2004, 78 (4), 883-896.
- Wang, X.; Zheng, X.-T., Reconsiderations on two characters of early angiosperm Archaefructus. Palaeoworld 2012, 21 (3–4), 193-201.
- Wang, X. , New observation on seed/ovule position in the fruit of Archaeanthus and its systematic implications. China Geology 2021, 4, 752–755. [Google Scholar] [CrossRef]
- Dilcher, D. L.; Crane, P. R. , Archaeanthus: An early angiosperm from the Cenomanian of the Western Interior of North America. Annals of the Missouri Botanical Garden 1984, 71(2), 351–383. [Google Scholar] [CrossRef]
- Zheng, S.-L.; Zhang, W. , Early and Middle Jurassic fossil flora from Tianshifu Liaoning. Liaoning Geology 1990, 3, 212–237. [Google Scholar]
- Zheng, S.-L.; Zhang, W. , Basic characteristics of Tianshifu Flora. Liaoning Geology 1990, 4, 322–334. [Google Scholar]
- Harris, T. M., III. On Williamsoniella, a new type of bennettitalean flower. Philosophical Transactions of the Royal Society of London. Series B 1916, 207, 335–347. [Google Scholar]
- Harris, T. M. , Williamsoniella lignieri: Its pollen and the compression of spherical pollen grains. Palaeontology 1974, 17(1), 125–148. [Google Scholar]
- Han, L.; Zhao, Y.; Zhao, M.; Sun, J.; Sun, B.; Wang, X. , New fossil evidence suggests that angiosperms flourished in the Middle Jurassic. Life 2023, 13(3), 819. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Zhang, M.; Sun, B.; Li, A.; Zhang, J.; Yan, D.; Xie, S.; Wu, J. , An exceptionally well-preserved herbaceous eudicot from the Early Cretaceous (late Aptian-early Albian) of Northwest China. National Science Review 2021, 8(12), nwab084. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Friis, E. M., Sinocarpus decussatus gen. et sp. nov., a new angiosperm with basally syncarpous fruits from the Yixian Formation of Northeast China. Plant Systematics and Evolution 2003, 241, 77-88.
- Dilcher, D. L.; Cronquist, A.; Stevenson, D. W.; Zimmermann, M. H.; Berry, P. E.; Stevens, P. Angiosperm. https://www.britannica.com/plant/angiosperm (accessed 8 July).
- Wang, X. , Criterion is a touchstone in study of early angiosperms. Open Journal of Plant Science 2021, 6(1), 91–93. [Google Scholar]
- Wang, X. , A novel Early Cretaceous flower and its implications on flower derivation. Biology 2022, 11(7), 1036. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shih, C.; Liu, Z.-J.; Lin, L.; Singh, K. J. , Reconstructing the Callianthus plant–An early aquatic angiosperm from the Lower Cretaceous of China. Cretaceous Research 2021, 128, 104983. [Google Scholar] [CrossRef]
- Fu, Q.; Diez, J. B.; Pole, M.; Garcia-Avila, M.; Liu, Z.-J.; Chu, H.; Hou, Y.; Yin, P.; Zhang, G.-Q.; Du, K.; Wang, X. , An unexpected noncarpellate epigynous flower from the Jurassic of China. eLife 2018, 7, e38827. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Diez, J. B.; Pole, M.; García-Ávila, M.; Wang, X. , Nanjinganthus is an angiosperm, isn’t it? China Geology 2020, 3(3), 359–361. [Google Scholar] [CrossRef]
- Fu, Q.; Hou, Y.; Yin, P.; Diez, J. B.; Pole, M.; García-Ávila, M.; Wang, X. Micro-CT results exhibit ovules enclosed in the ovaries of Nanjinganthus. Scientific Reports 2023, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Fu, X.; Liu, Z.-J.; Wang, X., A new angiosperm genus from the Lower Cretaceous Yixian Formation, Western Liaoning, China. Acta Geologica Sinica (English edition) 2013, 87 (4), 916-925.
- Han, G.; Liu, Z.; Wang, X., A Dichocarpum-like angiosperm from the Early Cretaceous of China. Acta Geologica Sinica (English edition) 2017, 90 (1), 1-8.
- Han, G.; Wang, X., A new infructescence of angiosperms from the Early Cretaceous of China. Acta Geologica Sinica (English edition) 2020, 94 (5), 1711-1713.
- Liu, X.; Ma, L.; Liu, B.; Liu, Z.-J.; Wang, X. , A novel angiosperm including various parts from the Early Cretaceous sheds new light on flower evolution. Historical Biology 2021, 33(11), 2706–2714. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Wang, X. , A perfect flower from the Jurassic of China. Historical Biology 2016, 28(5), 707–719. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-J.; Wang, X. , Yuhania: a unique angiosperm from the Middle Jurassic of Inner Mongolia, China. Historical Biology 2017, 29(4), 431–441. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-J.; Wang, X., A novel angiosperm from the Early Cretaceous and its implications on carpel-deriving. Acta Geologica Sinica (English edition) 2018, 92 (4), 1293-1298.
- Endress, P. K. , Multicarpellate gynoecia in angiosperms: occurrence, development, organization and architectural constraints. BOTANICAL JOURNAL OF THE LINNEAN SOCIETY 2014, 174(1), 1–43. [Google Scholar] [CrossRef]
- Leng, Q.; Friis, E. M., Angiosperm leaves associated with Sinocarpus infructescences from the Yixian Formation (Mid-Early Cretaceous) of NE China. Plant Systematics and Evolution 2006, 262 (3-4), 173-187.
- Roe, J. L.; Nemhauser, J. L.; Zambryski, P. C. , TOUSLED participates in apical tissue formation during gynoecium development in Arabidopsis. Plant Cell 1997, 9(3), 335–353. [Google Scholar] [PubMed]
- Rounsley, S. D.; Ditta, G. S.; Yanofsky, M. F. , Diverse roles for MADS box genes in Arabidopsis development. Plant Cell 1995 7, 1259–1269.
- Skinner, D. J.; Hill, T. A.; Gasser, C. S., Regulation of ovule development. Plant Cell 2004, 16 (suppl_1), S32-S45.
- Mathews, S.; Kramer, E. M. , The evolution of reproductive structures in seed plants: A re-examination based on insights from developmental genetics. New Phytologist 2012, 194(4), 910–923. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Gierl, A.; Saedler, H. , Molecular evidence for pre-Cretaceous angiosperm origins. Nature 1989, 339, 46–48. [Google Scholar] [CrossRef]
- Martin, W.; Gierl, A.; Saedler, H. , Angiosperm origins. Nature 1989, 342, 132. [Google Scholar] [CrossRef]
- Soltis, D. E.; Bell, C. D.; Kim, S.; Soltis, P. S. , Origin and early evolution of angiosperms. Annals of the New York Academy of Sciences 2008, 1133, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Soltis, P. S.; Soltis, D. E. , The origin and diversification of angiosperms. Am. J. Bot. 2004, 91(10), 1614–1626. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-T.; Yi, T.-S.; Gao, L.-M.; Ma, P.-F.; Zhang, T.; Yang, J.-B.; Gitzendanner, M. A.; Fritsch, P. W.; Cai, J.; Luo, Y.; Wang, H.; van der Bank, M.; Zhang, S.-D.; Wang, Q.-F.; Wang, J.; Zhang, Z.-R.; Fu, C.-N.; Yang, J.; Hollingsworth, P. M.; Chase, M. W.; Soltis, D. E.; Soltis, P. S.; Li, D.-Z. , Origin of angiosperms and the puzzle of the Jurassic gap. Nature Plants 2019, 5(5), 461–470. [Google Scholar] [CrossRef] [PubMed]
- Zuntini, A. R.; Carruthers, T.; Maurin, O.; Bailey, P. C.; Leempoel, K.; Brewer, G. E.; Epitawalage, N.; Françoso, E.; Gallego-Paramo, B.; McGinnie, C.; Negrão, R.; Roy, S. R.; Simpson, L.; Toledo Romero, E.; Barber, V. M. A.; Botigué, L.; Clarkson, J. J.; Cowan, R. S.; Dodsworth, S.; Johnson, M. G.; Kim, J. T.; Pokorny, L.; Wickett, N. J.; Antar, G. M.; DeBolt, L.; Gutierrez, K.; Hendriks, K. P.; Hoewener, A.; Hu, A.-Q.; Joyce, E. M.; Kikuchi, I. A. B. S.; Larridon, I.; Larson, D. A.; de Lírio, E. J.; Liu, J.-X.; Malakasi, P.; Przelomska, N. A. S.; Shah, T.; Viruel, J.; Allnutt, T. R.; Ameka, G. K.; Andrew, R. L.; Appelhans, M. S.; Arista, M.; Ariza, M. J.; Arroyo, J.; Arthan, W.; Bachelier, J. B.; Bailey, C. D.; Barnes, H. F.; Barrett, M. D.; Barrett, R. L.; Bayer, R. J.; Bayly, M. J.; Biffin, E.; Biggs, N.; Birch, J. L.; Bogarín, D.; Borosova, R.; Bowles, A. M. C.; Boyce, P. C.; Bramley, G. L. C.; Briggs, M.; Broadhurst, L.; Brown, G. K.; Bruhl, J. J.; Bruneau, A.; Buerki, S.; Burns, E.; Byrne, M.; Cable, S.; Calladine, A.; Callmander, M. W.; Cano, Á.; Cantrill, D. J.; Cardinal-McTeague, W. M.; Carlsen, M. M.; Carruthers, A. J. A.; de Castro Mateo, A.; Chase, M. W.; Chatrou, L. W.; Cheek, M.; Chen, S.; Christenhusz, M. J. M.; Christin, P.-A.; Clements, M. A.; Coffey, S. C.; Conran, J. G.; Cornejo, X.; Couvreur, T. L. P.; Cowie, I. D.; Csiba, L.; Darbyshire, I.; Davidse, G.; Davies, N. M. J.; Davis, A. P.; van Dijk, K.-j.; Downie, S. R.; Duretto, M. F.; Duvall, M. R.; Edwards, S. L.; Eggli, U.; Erkens, R. H. J.; Escudero, M.; de la Estrella, M.; Fabriani, F.; Fay, M. F.; Ferreira, P. d. L.; Ficinski, S. Z.; Fowler, R. M.; Frisby, S.; Fu, L.; Fulcher, T.; Galbany-Casals, M.; Gardner, E. M.; German, D. A.; Giaretta, A.; Gibernau, M.; Gillespie, L. J.; González, C. C.; Goyder, D. J.; Graham, S. W.; Grall, A.; Green, L.; Gunn, B. F.; Gutiérrez, D. G.; Hackel, J.; Haevermans, T.; Haigh, A.; Hall, J. C.; Hall, T.; Harrison, M. J.; Hatt, S. A.; Hidalgo, O.; Hodkinson, T. R.; Holmes, G. D.; Hopkins, H. C. F.; Jackson, C. J.; James, S. A.; Jobson, R. W.; Kadereit, G.; Kahandawala, I. M.; Kainulainen, K.; Kato, M.; Kellogg, E. A.; King, G. J.; Klejevskaja, B.; Klitgaard, B. B.; Klopper, R. R.; Knapp, S.; Koch, M. A.; Leebens-Mack, J. H.; Lens, F.; Leon, C. J.; Léveillé-Bourret, É.; Lewis, G. P.; Li, D.-Z.; Li, L.; Liede-Schumann, S.; Livshultz, T.; Lorence, D.; Lu, M.; Lu-Irving, P.; Luber, J.; Lucas, E. J.; Luján, M.; Lum, M.; Macfarlane, T. D.; Magdalena, C.; Mansano, V. F.; Masters, L. E.; Mayo, S. J.; McColl, K.; McDonnell, A. J.; McDougall, A. E.; McLay, T. G. B.; McPherson, H.; Meneses, R. I.; Merckx, V. S. F. T.; Michelangeli, F. A.; Mitchell, J. D.; Monro, A. K.; Moore, M. J.; Mueller, T. L.; Mummenhoff, K.; Munzinger, J.; Muriel, P.; Murphy, D. J.; Nargar, K.; Nauheimer, L.; Nge, F. J.; Nyffeler, R.; Orejuela, A.; Ortiz, E. M.; Palazzesi, L.; Peixoto, A. L.; Pell, S. K.; Pellicer, J.; Penneys, D. S.; Perez-Escobar, O. A.; Persson, C.; Pignal, M.; Pillon, Y.; Pirani, J. R.; Plunkett, G. M.; Powell, R. F.; Prance, G. T.; Puglisi, C.; Qin, M.; Rabeler, R. K.; Rees, P. E. J.; Renner, M.; Roalson, E. H.; Rodda, M.; Rogers, Z. S.; Rokni, S.; Rutishauser, R.; de Salas, M. F.; Schaefer, H.; Schley, R. J.; Schmidt-Lebuhn, A.; Shapcott, A.; Al-Shehbaz, I.; Shepherd, K. A.; Simmons, M. P.; Simões, A. O.; Simões, A. R. G.; Siros, M.; Smidt, E. C.; Smith, J. F.; Snow, N.; Soltis, D. E.; Soltis, P. S.; Soreng, R. J.; Sothers, C. A.; Starr, J. R.; Stevens, P. F.; Straub, S. C. K.; Struwe, L.; Taylor, J. M.; Telford, I. R. H.; Thornhill, A. H.; Tooth, I.; Trias-Blasi, A.; Udovicic, F.; Utteridge, T. M. A.; Del Valle, J. C.; Verboom, G. A.; Vonow, H. P.; Vorontsova, M. S.; de Vos, J. M.; Al-Wattar, N.; Waycott, M.; Welker, C. A. D.; White, A. J.; Wieringa, J. J.; Williamson, L. T.; Wilson, T. C.; Wong, S. Y.; Woods, L. A.; Woods, R.; Worboys, S.; Xanthos, M.; Yang, Y.; Zhang, Y.-X.; Zhou, M.-Y.; Zmarzty, S.; Zuloaga, F. O.; Antonelli, A.; Bellot, S.; Crayn, D. M.; Grace, O. M.; Kersey, P. J.; Leitch, I. J.; Sauquet, H.; Smith, S. A.; Eiserhardt, W. L.; Forest, F.; Baker, W. J. , Phylogenomics and the rise of the angiosperms. Nature 2024. [Google Scholar] [CrossRef] [PubMed]
- Mandel, J. R. , A Jurassic leap for flowering plants. Nature Plants 2019, 5, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Herendeen, P. S.; Friis, E. M.; Pedersen, K. R.; Crane, P. R. , Palaeobotanical redux: revisiting the age of the angiosperms. Nature Plants 2017, 3, 17015. [Google Scholar] [CrossRef] [PubMed]
- Hochuli, P. A.; Feist-Burkhardt, S. , A boreal early cradle of angiosperms? angiosperm-like pollen from the Middle Triassic of the Barents Sea (Norway). Journal of Micropalaeontology 2004, 23, 97–104. [Google Scholar] [CrossRef]
- Hochuli, P. A.; Feist-Burkhardt, S. , Angiosperm-like pollen and Afropollis from the Middle Triassic (Anisian) of the Germanic Basin (Northern Switzerland). Frontiers in Plant Science 2013, 4, 344. [Google Scholar] [CrossRef]
- Prasad, V.; Strömberg, C. A. E.; Leaché, A. D.; Samant, B.; Patnaik, R.; Tang, L.; Mohabey, D. M.; Ge, S.; Sahni, A. , Late Cretaceous origin of the rice tribe provides evidence for early diversification in Poaceae. Nature Communication 2011, 2, 480. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; You, H.-L.; Li, X.-Q. , Dinosaur-associated Poaceae epidermis and phytoliths from the Early Cretaceous of China. National Science Review 2018, 5, 721–727. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Lu, A.-M.; Tang, Y.-C.; Chen, Z.-D.; Li, D.-Z. , Synopsis of a new “polyphyletic-polychronic-polytopic” system of the angiosperms. Acta Phytotaxonomica Sinica 2002, 40(4), 289–322. [Google Scholar]
- Han, G.; Liu, Z.-J.; Liu, X.; Mao, L.; Jacques, F. M. B.; Wang, X., A whole plant herbaceous angiosperm from the Middle Jurassic of China. Acta Geologica Sinica (English edition) 2016, 90 (1), 19-29.
- Cui, D.-F.; Hou, Y.; Yin, P.; Wang, X., A Jurassic flower bud from China. Geological Society, London, Special Publications 2022, 521, 81-93.
- Wang, X.; Fu, Q. , Taiyuanostachya: An abominable angiosperm from the Early Permian of China. Journal of Biotechnology and Biomedicine 2023, 6(3), 371–379. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




