Submitted:
06 June 2024
Posted:
07 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Cytokine Release
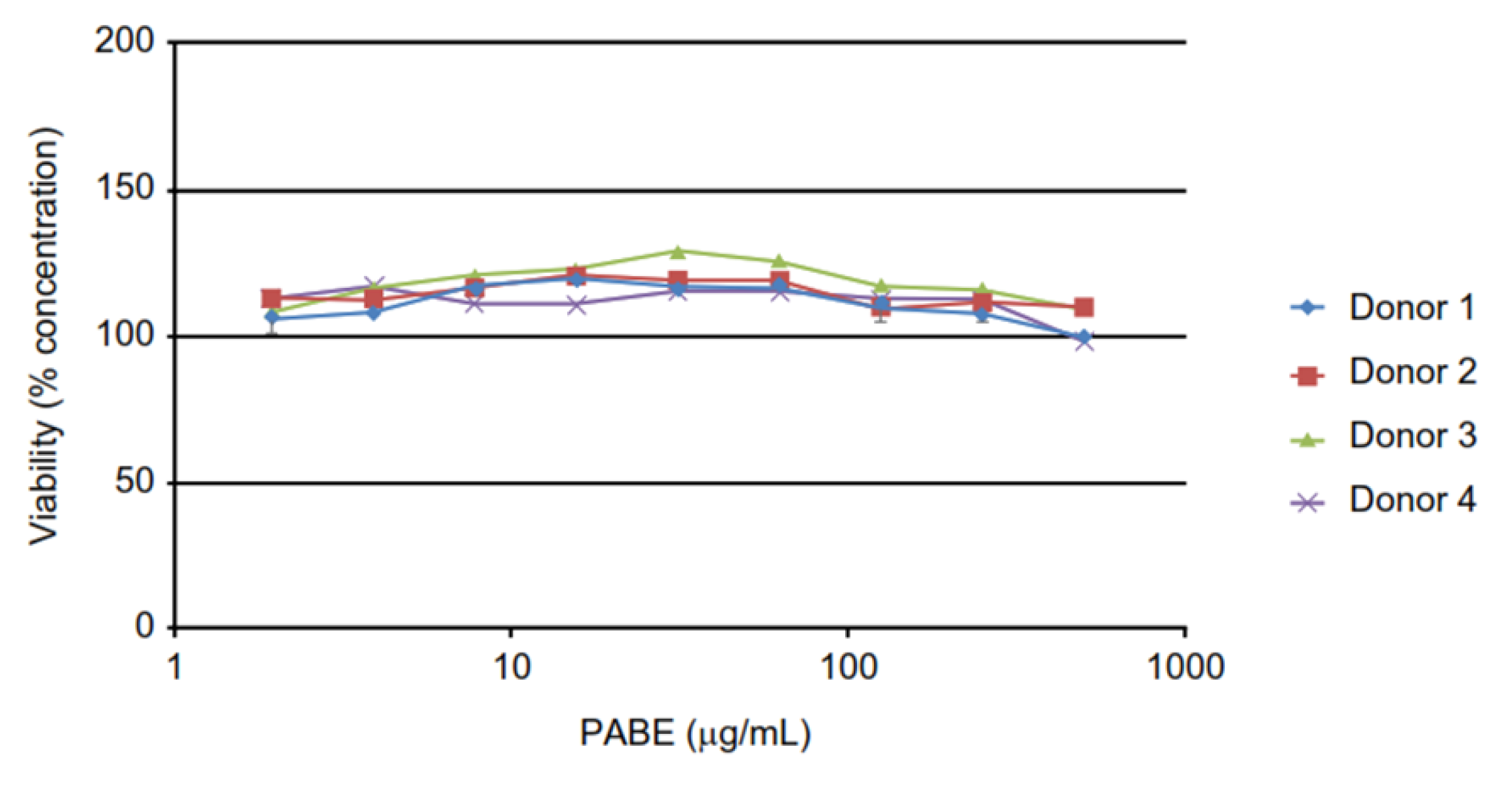
2.2. Viability Assays
3. Discussion
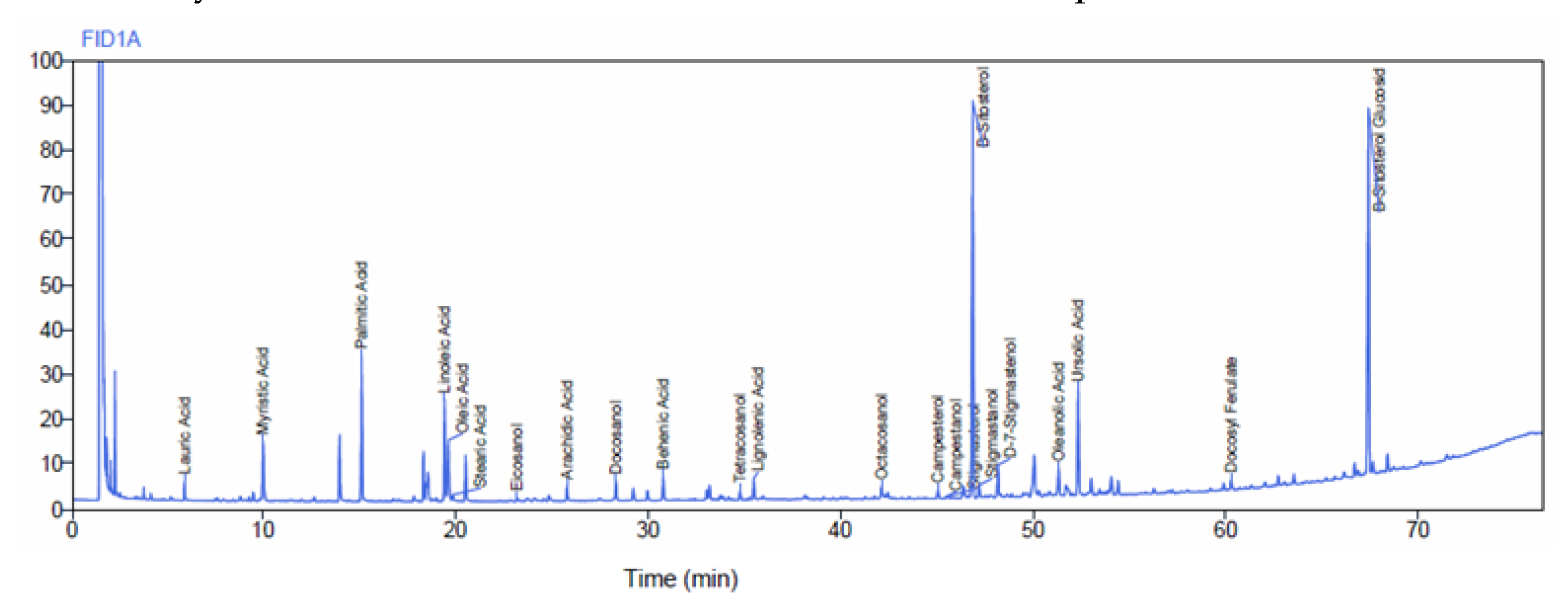
4. Materials and Methods
4.1. Human Peripheral Blood Mononuclear Cells
4.2. Cell Viability Assay
4.3. Cytokine Release Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Untergasser, G.; Madersbacher, S.; Berger, P. Benign prostatic hyperplasia: age-related tissue-remodeling. Exp Gerontol 2005, 40, 121–128. [Google Scholar] [CrossRef]
- Kramer, G.; Steiner, G.E.; Handisurya, A.; Stix, U.; Haitel, A.; Knerer, B.; Gessl, A.; Lee, C.; Marberger, M. Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate 2002, 52, 43–58. [Google Scholar] [CrossRef]
- Steiner, G.; Gessl, A.; Kramer, G.; Schöllhammer, A.; Förster, O.; Marberger, M. Phenotype and function of peripheral and prostatic lymphocytes in patients with benign prostatic hyperplasia. J Urol 1994, 151, 480–484. [Google Scholar] [CrossRef]
- Norström, M.M.; Rådestad, E.; Sundberg, B.; Mattsson, J.; Henningsohn, L.; Levitsky, V.; Uhlin, M. Progression of benign prostatic hyperplasia is associated with pro-inflammatory mediators and chronic activation of prostate-infiltrating lymphocytes. Oncotarget 2016, 7, 23581–23593. [Google Scholar] [CrossRef]
- Manzarbeitia, F.; Vela Navarrete, R.; Fernández-Aceñero, M.J. Early histopathological aspects of benign prostatic hyperplasia: myxoid-inflammatory nodules. Actas Urol Esp 2010, 34, 549–554. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.R.; Yang, L.Y.; Liu, Z.T. Chronic inflammation in benign prostatic hyperplasia: implications for therapy. Med Hypotheses 2008, 70, 1021–1023. [Google Scholar] [CrossRef]
- Naiyila, X.; Li, J.; Huang, Y.; Chen, B.; Zhu, M.; Li, J.; Chen, Z.; Yang, L.; Ai, J.; Wei, Q.; et al. A novel insight into the immune-related interaction of inflammatory cytokines in benign prostatic hyperplasia. J Clin Med 2023, 12, 1821. [Google Scholar] [CrossRef]
- Taille de la, A. Therapeutic approach: The importance of controlling prostatic inflammation. Eur J Urol Suppl 2013, 12, 116–122. [Google Scholar] [CrossRef]
- Anonymous. Pygeum africanum (Prunus africana) (African plum tree) Monograph. Altern Med Rev 2002, 7, 71–74. [Google Scholar]
- Andro, M.C.; Riffaud, J.P. Pigeum africanum extract for the treatment of patients with benign prostatic hyperplasia: review of 25 years of published experience. Curr Ther Res 1995, 56, 796–817. [Google Scholar] [CrossRef]
- Yablonsky, F.; Nicolas, V.; Riffaud, J.P.; Bellamy, F. Antiproliferative effect of Pygeum africanum extract on rat prostatic fibroblasts. J Urol 1997, 157, 2381–2387. [Google Scholar] [CrossRef]
- Paubert-Braquet, M.; Cave, A.; Hocquemiller, R.; Delacroix, D.; Dupont, C.; Hedef, N.; Borgeat, P. Effect of Pygeum africanum extract on A23187-stimulated production of lipoxygenase metabolites from human polymorphonuclear cells. J Lipid Mediat Cell Signal 1994, 9, 285–290. [Google Scholar]
- Lucchetta, G.; Weill, A.; Becker, N.; Clavert, A.; Bollack, C. Reactivation of the secretion from the prostatic gland in cases of reduced fertility. Biological study of seminal fluid modifications. Urol Int 1984, 39, 222–224. [Google Scholar] [CrossRef]
- Boulbès, D.; Soustelle, L.; Costa, P.; Haddoum, M.; Bali, J.P.; Hollande, F.; Magous, R. Pygeum africanum extract inhibits proliferation of human cultured prostatic fibroblasts and myofibroblasts. BJU Int 2006, 98, 1106–1113. [Google Scholar] [CrossRef]
- Antoniou, V.; Gauhar, V.; Modi, S.; Somani, B.K. Role of phytotherapy in the management of BPH: A summary of the literature. J Clin Med 2023, 12, 1899. [Google Scholar] [CrossRef]
- Jena, A.K.; Vasisht, K.; Sharma, N.; Kaur, R.; Dhingra, M.S.; Karan, M. Amelioration of testosterone induced benign prostatic hyperplasia by Prunus species. J Ethnopharmacol 2016, 22, 33–45. [Google Scholar] [CrossRef]
- ESCOP Monographs. The Scientific Foundation for Herbal Medicinal Products. Pruni africanae cortex. Pygeum africanum bark. 2020. Available online: https://www.sefit.es/corteza-pigeum-pruni-africanae-cortex-pygeum-africanum-bark-monografia-online-escop/ (accessed on 31 March 2023).
- Pagano, E.; Laudato, M.; Griffo, M.; Capasso, R. Phytotherapy of benign prostatic hyperplasia. A minireview. Phytother Res 2014, 28, 949–955. [Google Scholar] [CrossRef]
- Ishani, A.; MacDonald, R.; Nelson, D.; Rutks, I.; Wilt, T.J. Pygeum africanum for the treatment of patients with benign prostatic hyperplasia: a systematic review and quantitative meta-analysis. Am J Med 2000, 109, 654–664. [Google Scholar] [CrossRef]
- Breza, J.; Dzurny, O.; Borowka, A.; Hanus, T.; Petrik, R.; Blane, G.; Chadha-Boreham, H. Efficacy and acceptability of tadenan (Pygeum africanum extract) in the treatment of benign prostatic hyperplasia (BPH): A multicentre trial in central Europe. Curr Med Res Opin 1998, 143, 127–139. [Google Scholar] [CrossRef]
- Cambronero, J.; Osca-García, J.M.; Merino-Salas, S.; Miguel, J.M.; Borralleras, C.; López-Alcina, E. Effectiveness of treatment with Pygeum africanum in patients with lower urinary tract symptoms and benign prostatic hyperplasia: a cross-sectional study in the real-world clinical practice in Spain (The PROFIT Study). Arch Esp Urol 2022, 75, 219–227. [Google Scholar]
- European Medicines Agency. Committee on Herbal Medicinal Products (HMPC). 12 July 2016. EMA/HMPC/680624/2’13. Assessment report on Prunus africana (Hook f.) Kalkm., cortex. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-prunus-africana-hook-f-kalkm-cortex_en.pdf (accessed on 31 March 2023).
- Chughtai, B.; Lee, R.; Te, A.; Kaplan, S. Inflammation and benign prostatic hyperplasia: clinical implications. Curr Urol Rep 2011, 12, 274–277. [Google Scholar] [CrossRef]
- Boulbès, D.; Soustelle, L.; Costa, P.; Haddoum, M.; Bali, J.P.; Hollande, F.; Magous, R. Pygeum africanum extract inhibits proliferation of human cultured prostatic fibroblasts and myofibroblasts. BJU Int 2006, 98, 1106–1113. [Google Scholar] [CrossRef]
- Quiles, M.T.; Arbós, M.A.; Fraga, A.; de Torres, I.M.; Reventós, J.; Morote, J. Antiproliferative and apoptotic effects of the herbal agent Pygeum africanum on cultured prostate stromal cells from patients with benign prostatic hyperplasia (BPH). Prostate 2010, 70, 1044–1053. [Google Scholar] [CrossRef]
- Paubert-Braquet, M.; Monboisse, J.C.; Servent-Saez, N.; Serikoff, A.; Cavé, A.; Hocquemiller, R.; Dupont, C.; Forneau, C.; Borel, J.P. Inhibition of bFGF and EGF-induced proliferation of 3T3 fibroblasts by extract of Pygeum africanum (Tadenan®). Biomed Pharmacother 1994, 48 (Suppl. 1), 43s–47s. [Google Scholar]
- Penna, G.; Fibbi, B.; Amuchastegui, S.; Cossetti, C.; Aquilano, F.; Laverny, G.; Gacci, M.; Crescioli, C.; Maggi, M.; Adorini, L. Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno-mediated inflammation. J Immunol 2009, 182, 4056–4064. [Google Scholar] [CrossRef]
- Kramer, G.; Marberger, M. Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr Opin Urol 2006, 16, 25–29. [Google Scholar] [CrossRef]
- Szolnoki, E.; Reichart, E.; Marchal, S.; Szegedi, G. The effect of Pygeum africanum on fibroblast growth factor (FGF) and transforming growth factor beta (TGF beta 1/LAP) expression in animal model. Acta Microbiol Immunol Hung 2001, 48, 1–9. [Google Scholar] [CrossRef]
- Sidoti, C.; Hedef, N.; Delacroix, D.; Cavet, A.; Dupontt, C.H.; Borgeat, H. Inhibitory effect of Pygeum africanum extract (Tadenan®) on A23187-stimulated lipoxygenase metabolite production from human polymorphonuclear cells. The Pharmacologist 1993, 35, 196. [Google Scholar]
- United States Pharmacopeia. Dietary Supplement Monographs, Pygeum Extract. USP-NF. Rockville, MD: United States Pharmacopeia, 2023. [CrossRef]
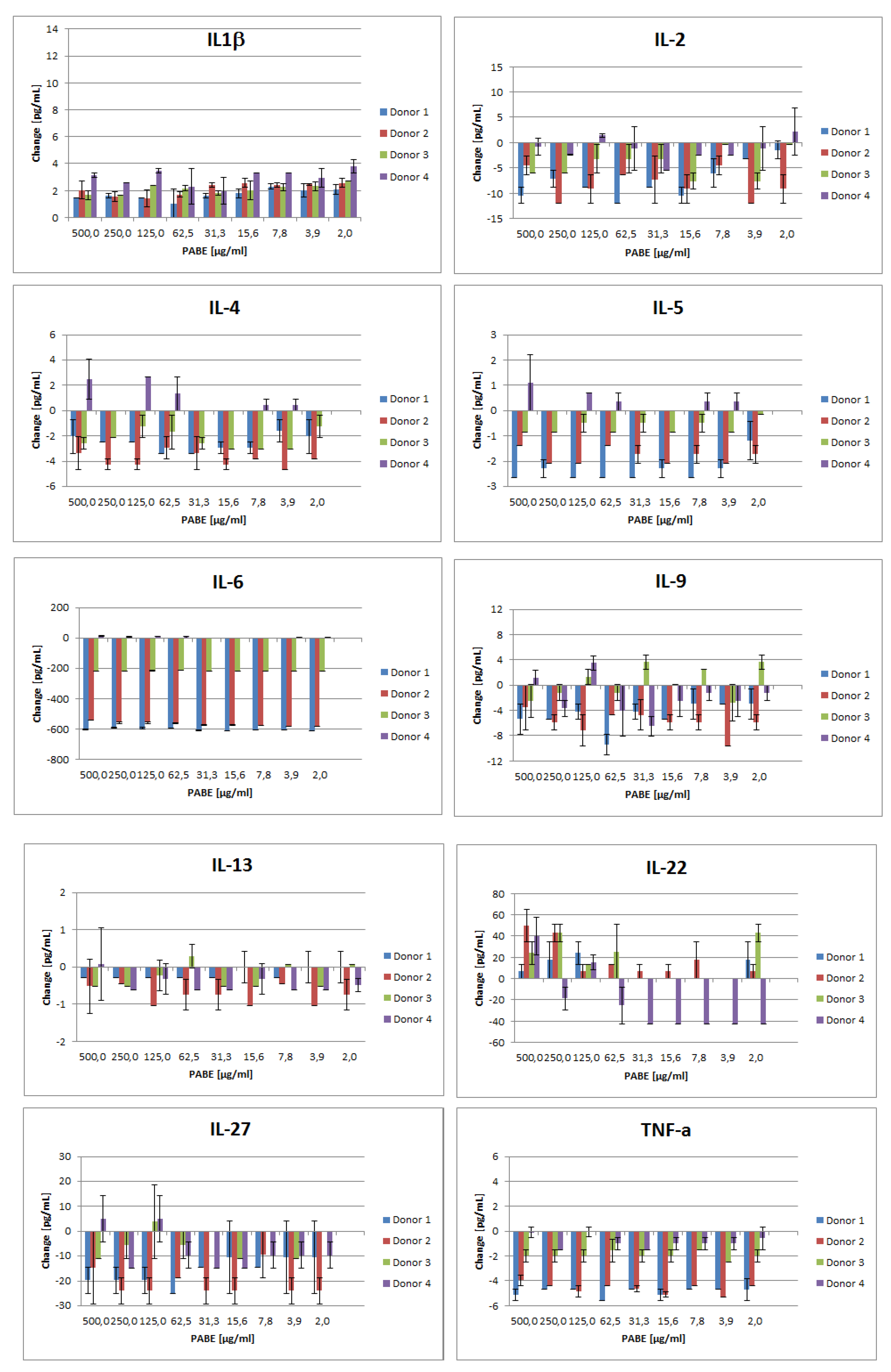
| Cytokines pg/mL |
Donor 1 | Donor 2 | Donor 3 | Donor 4 |
|---|---|---|---|---|
| IL-1β | 15.43 (2.81) | 19.08 (2.90) | 18.95 (1.98) | 26.79 (3.86) |
| IL-2 | -68.67 (9.34) | -74.08 (16.70) | -38.14 (11.54) | -12.53 (15.33) |
| IL-4 | -21.15 (3.13) | -30.86 (4.92) | -19.36 (3.13) | 7.41 (3.83) |
| IL-5 | -21.20 (1.80) | -16.20 (1.05) | -5.90 (1.05) | 2.85 (2.15) |
| IL-6 | -5439.97 (18.48) | -5109.79 (18.09) | -1972.39 (6.58) | 31.83 (13.65) |
| IL-9 | -42.65 (11.22) | -53.08 (13.19) | 3.24 (11.49) | -16.84 (16.54) |
| IL-13 | -1.76 (1.25) | -6.71 (1.98) | -2.36 (0.73) | -4.15 (1.98) |
| IL-22 | 65.52 (52.03) | 150.29 (67.43) | 148.46 (52.11) | -202.86 (53.73) |
| IL-27 | -144.31 (60.04) | -186.46 (56.07) | -40.19 (25.36) | -73.58 (40.03) |
| TNF-α | -43.92 (1.86) | -41.39 (1.38) | -17.48 (3.77) | -7.70 (3.65) |
| Cytokine | Characteristics |
| IL-1β | Pro-inflammatory cytokine expressed by monocytes, macrophages, and dendritic cells, synthesized in response to inflammatory stimuli (e.g. pathogens). |
| IL-2 | Plays a central role in activation and proliferation of lymphocytes that have been primed by antigens; pivotal for expansion of most T-cells, natural killer [NK] cells, and B-cells during various phases of the immune response- |
| IL-4 | Anti-inflammatory cytokine, secreted by activated T-cells and NK cells. |
| IL-5 | Acts as hematopoietic growth factor, promoting proliferation, activation, and differentiation of certain bone marrow cells. |
| IL-6 | Pleiotropic cytokine produced mainly by monocytes; plays a central role in host defense mechanisms. |
| IL-9 | Pro-inflammatory cytokine having pleiotropic effects on Th2 lymphocytes and B-cells and mast cells; implicated in asthma and other allergies. |
| IL-13 | Pleiotropic cytokine expressed by activated T-helper, T-suppressor and NK cells; suppresses macrophage cytotoxic activity and inflammatory cytokine expression. |
| IL-22 | Regulates production of acute phase proteins of the immunological response; involved in the inflammatory immune response. |
| TNF-α | Pleiotropic cytokine that plays key roles in innate and adaptive immunity; most often associated with regulation of pro-inflammatory properties. |
| Sample identifier |
Gender | Aye, years | Ethnicity/race | ABO group/Rh | Antigen |
| HHU20130318 | Male | 38 | Asian | A/positive | SFC |
| HHU20130715 | Male | 41 | Hispanic | 0/positive | SFC |
| HHU20150826 | Male | 49 | Caucasian | Unknown | SFC |
| HHU20150910 | Female | 23 | African American | Unknown | SFC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





