1. Introduction
In vitro maturation (IVM) of oocyte and the subsequent early embryo development are two key steps in assisted reproductive technology, no matter for the treatment of human infertility; or for high-yield, top-quality and disease-resistant livestock breeding. In order to increase oocyte IVM rate and embryo development rate, great efforts have been made, and multiple parameters have been found involving in these processes [
1,
2]. However, the development rates obtained with these techniques are still limited, particularly in large domestic animal like cattle.
Vitamin C (V
C, also known as Ascorbic Acid/L-Ascorbic Acid) is an essential nutrient that must be obtained through the diet in adequate amounts to prevent hypovitaminosis C, deficiency and its consequences-including the potentially fatal deficiency disease scurvy. As a water-soluble molecule, V
C is well known for its multiple functions such as promotion of wound healing, detoxification, alleviation of diabetes and neurogenic/synaptogenic disease, epigenetic regulation, serving as a cofactor for various enzymes, cytotoxicity against tumor/cancer cells, and antioxidant activity [
3,
4]. Among these functions, V
C fulfils its roles in collagen synthesis, vasculogenesis, aging, cell proliferation, and differentiation by donating electrons in various enzymatic and non-enzymatic reactions [
5]. Recently, it’s demonstrated that treatment with V
C improved the development of mouse IVF embryo derived [
6], and the supplements of V
C as well other molecules seems to be also beneficial to the development of bovine embryos [
7]. Later studies have shown that V
C-treated bovine donor cells improved the development of cloned embryos by influencing embryonic transcription [
8]. Based on its versatile function, V
C improves the developmental competence of porcine oocytes [
9] and enhances the survival of primary ovarian follicles cultured in vitro [
10]. However, the appropriate concentration of V
C still needs to be tuned for bovine oocyte IVM and the subsequent embryo development. Additionally, the effects of combined application of V
C both in oocyte IVM and embryo culture of cattle are unclear.
To address the questions mentioned above, here we investigated the effects of VC and its combinations in IVM medium and IVC medium on bovine oocyte IVM and early embryonic development, aiming to improve the efficiency of bovine embryo IVC and procure high-quality embryos.
2. Materials and Methods
2.1. Bovine Ovaries and Chemicals
Bovine ovaries were collected from a local abattoir (Changchun Jixing meat industry Co., LTD, Changchun, China) and transported to the laboratory in 0.9% NaCl supplemented with 200 IU/mL penicillin at 36–37.5°C within 2 h. All experimental materials and procedures used here received endorsement from the Animal Welfare and Research Ethics Committee at Jilin University (SY201903002). Unless stated otherwise, chemicals and reagents were all procured from Sigma-Aldrich and the basic medium was acquired from Gibco (CA, USA).
2.2. In Vitro Maturation (IVM)
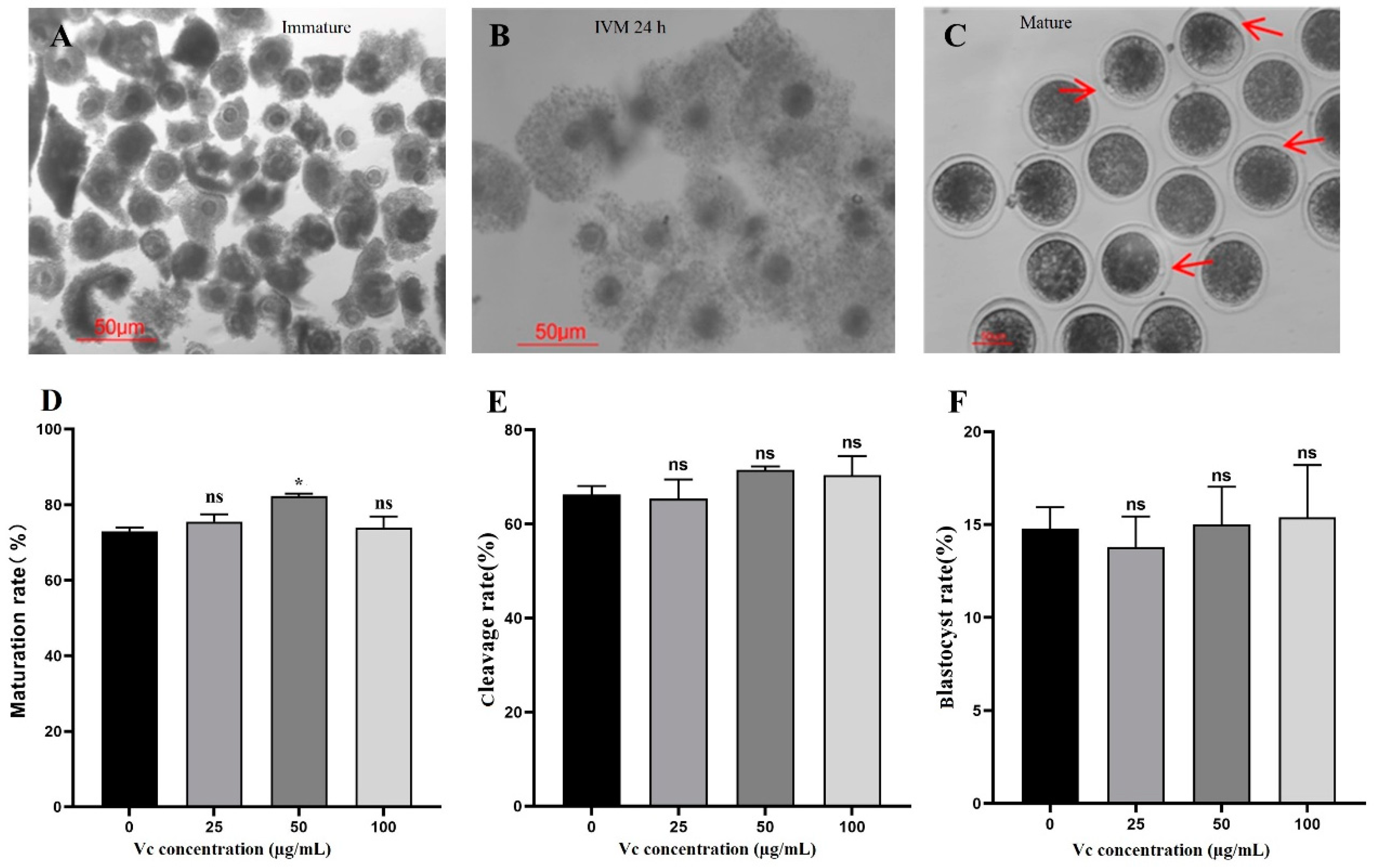
The follicles with a diameter of 2–8 mm were selected, and the oocytes were aspired using a 10 mL syringe with a 12-gauge needle. The cumulus-oocyte complexes (COCs) containing intact and compact cumulus cells were selected under a stereomicroscope. The COCs were washed three times with maturation solution [TCM-199 enriched with 25 mmoL/L NaHCO3, 0.38 mmoL/L sodium pyruvate, 10 μg follicle stimulating hormone (FSH)/mL, 10 μg luteinizing hormone (LH)/mL, 1 μg 17β-estradiol (E2)/mL and 10% (v/v) fetal bovine serum (FBS)]. Next, the COCs were transferred into 100 µL maturing solution microdroplets (10-15 pieces/droplet), and the IVM was conducted at 38.5℃, 5% CO2 and saturated humidity for 20–22 h. VC at concentrations of 0, 25, 50, 100 µg/mL was added into the maturing solution based on our preliminary experiments. The maturation rate was calculated and statistically analyzed after the oocytes being cultured for 23–24 h. After IVM, the oocyte with the first polar body, an homogeneous cytoplasm and at least three layers of cumulus cells, was considered to be a mature oocyte with high morphological quality.
2.3. Parthenogenetic Activation
The cumulus cells of the IVM oocytes were removed by the following steps. Firstly, they were digested for 2–3 min in the operating medium (15 mmol/L Hepes, 5 mmol/L NaHCO3, and 3 mg/mL of bovine serum albumin) containing 0.2% (w/v) hyaluronidase. Post-digestion, the cumulus cells were repeatedly and gently blown away with a 200 µL pipette, and the oocytes were washed three times with the operating medium. After collection of the mature oocytes, parthenogenesis is activated by a combination treatment of ionomycin and 6-dimethylaminopurine (6-DMAP). Briefly, the oocytes were firstly incubated with operating solution containing 5 µmol/L ionomycin for 5 min. Next, the eggs were transferred into the modified synthetic oviductal fluid (mSOF) droplets enriched with 2 mmol/L 6-DMAP, and cultured at 38.5 ℃, 5% CO2 and saturated humidity for 4 h. The mSOF consists of 108 mM NaCl, 7.2 mM KCl, 0.3 mM KH2PO4, 5 mM NaHCO3, 3.3 mM sodium lactate, 0.07 mM kanamycin monosulfate, 0.33 mM pyruvate, 1.7 mM CaCl2·2H2O, 0.3% (w/v) fatty acid-free bovine albumin, 1% (v/v) non-essential amino acid, and 2% (v/v) essential amino acid. The activated oocytes were subsequently transferred into new mSOF droplets covered with mineral oil and continuously cultivated under the conditions mentioned above. The culture medium containing VC (0, 25, 50, 100 µg/mL) was changed every 48 h. The cleavage and blastocyst rates were calculated at 2 d and 7 d respectively.
2.4. In Vitro Fertilization (IVF) and Embryo Culture
The matured oocytes obtained above were used for IVF by procedures as described [
11]. Briefly, frozen bull semen was thawed in a water bath at 39.0℃ for 30–40 s. The semen was washed three times by centrifugation in pre-warmed Dulbecco's phosphate buffered saline (D-PBS). Subsequently, the sperm was washed in 5 mL TALP [Tyrode’s medium base, albumin, lactate and pyruvate, containing 0.67 mg NaCl/mL, 0.024 mg KCl/mL, 0.005 mg NaH
2PO
4/mL, 0.029 mg CaCl
2·2H
2O/mL, 0.01 mg MgCl
2·6H
2O/mL, 0.09 mg glucose/mL, 0.01 mg sodium pyruvate/mL, 0.19 μL sodium lactate (60%, v/v)/mL, 0.21 mg NaHCO
3/mL, 0.3 mg BSA/mL], and incubated at 38.5 ℃, 5% CO
2 and saturated humidity for 30 min. During sperm incubation, the COCs cultured for 23–24 h were washed in TALP for 3 times, transferred into 90 µL TALP droplets with 25–30 COCs per droplet, and continuously incubated at 38.5℃, 5% CO
2 and saturated humidity.
Next, the sperm was washed two times by centrifugation at 1500 r/min for 5 min in TALP, and diluted with TALP to reach a final density of 2–4 ×106/mL. Subsequently, 10 µL sperm was added into 90 µL TALP droplets containing COCs, and the sperm-COCs were coincubated for 24 h.
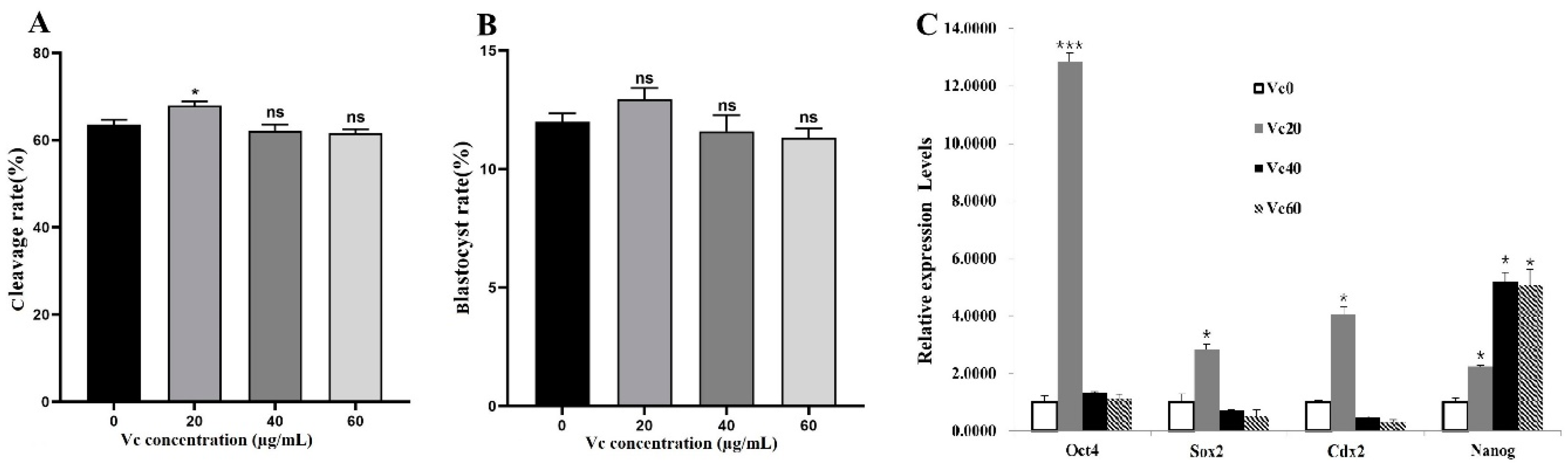
After sperm-COCs coincubation, the putative zygotes were transferred into pre-warmed operating solution (500 µL). The remaining cumulus cells around the oocytes were removed by gentle pipetting repeatedly. The zygotes were washed 3 times with mSOF and transferred into new mSOF containing VC (0, 20, 40 and 60 µg/mL based on preliminary experiments). Generally, 25–30 zygotes were put into 100 µL mSOF with/without VC. The medium was replaced every 48 h. Similarly, the cleavage and blastocyst rates of each group were calculated at cultivation of 2 d and 7 d, respectively.
2.5. Blastocyst Cell Count
Four to five blastocysts from the IVF embryos cultured 7 d were selected for nuclear staining. Briefly, the embryos were washed 3 times with phosphate buffered saline (PBS)/PVA (0.01% polyvinyl alcohol in PBS). The zona pellucida of the blastocysts was removed with the acidic Tyrode’s solution (0.8 mg NaCl/mL, 0.02 mg KCl/mL, 0.024 mg CaCl2·2H2O/mL, 0.0047 mg MgCl2·6H2O/mL, 0.1 mg glucose/mL, and 0.4 mg polyvinylpyrrolidone (PVP)/mL). After washing 3 times with PBS/PVA, the embryos were stabilized using 3.7% paraformaldehyde at room temperature for 3 min, washed 3 times with PBS/PVA, and stained with Hoechst33342 (10 µg/mL) for 10 min in darkness. Finally, the blastocysts were washed 3 times with PBS/PVA, mounted between a cover slip and a glass slide supported by four columns of a mixture of petroleum jelly and paraffin (9:1) and immediately observed under a fluorescence microscope (Nikon, Tokyo, Japan). The number of blastomeres in each blastocyst was counted; the average cell numbers were quantified and statistically compared among groups.
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
The total RNAs of the blastocysts (approximately 25 embryos for each group) was extracted using the DNA/RNA Micro Kit (Qiagen, Germany). The RNA was reverse-transcribed into cDNA using TransScript® II One-Step gDNA Removal and cDNA Synthesis SuperMix AH311-03 Kit (Transgen Biotech, Beijing, China). The reverse transcription was performed in a 20 µL system, using following program: 95 ℃ for 30 s, 95 ℃ for 5 s, and 60 ℃ for 30 s, total 40 cycles, adhering to the protocols provided by the manufacturers. The primer sequences are detailed in
Table 1 and
GAPDH served as the reference gene to normalize the data. The 2
−△△CT method was employed to analyze the relative abundance of mRNA transcripts.
2.7. Statistical Analysis
Data were presented as mean ± SEM and analyzed using IBM SPSS Statistics 19.0 software. P < 0.05 was considered as significant difference (*p < 0.05, **p < 0.01, ***p < 0.001). Replication of each experimental procedure was done three times to ensure reliability.
4. Discussion
Techniques of IVM, IVF, IVC and somatic cell cloned embryos have been successfully applied in research, production, animal breeding and medical fields. Oocyte IVM mainly includes nuclear maturation and cytoplasmic maturation. Many factors can affect oocyte IVM and subsequent embryonic development, such as female age and ovarian function, the temperature and time of ovarian transport, the method for COC aspiration, season, oocyte quality, and culture medium as well supplements, etc. Optimizing these factors is pivotal to procure high-quality oocytes and embryos for animal breeding. Since VC is an essential nutrient having multiple functions, here we evaluated the effects of VC at appropriate concentration as well its combination on bovine oocyte IVM and early embryo development.
Previously, in order to optimize embryo culture conditions, researchers applied varied supplements including V
C to improve oocyte IVM, IVF rate and embryo development. It’s reported that V
C improved the developmental ability of ovine and porcine oocytes and subsequent embryonic development [
12,
13]. However, its effects on bovine oocytes and early embryo development are somewhat controversial. Dalvit et al. reported that V
C has no significant influence on the maturation of bovine oocytes [
14], while Córdova et al. proved that after adding V
C to IVM solution for 12 h in vitro, the subsequent development ability and blastocyst formation rate of bovine oocytes were significantly improved [
15]. It’s also demonstrated that V
C increases the cleavage rate of somatic cloned embryos, but has no positive effects on the blastocyst rate, and even negatively decreased the blastocyst rate and blastocyst quality [
16]. We speculate that, for bovine oocyte IVM and embryo development, V
C concentration needs be tuned appropriately. Based on preliminary experiments, we found that V
C at 50 µg/mL (in gradients of 0, 25, 50, and 100 μg/mL) is significantly beneficial to bovine oocyte IVM, while V
C at 20 µg/mL (in gradients of 0, 20, 40, and 60 μg/mL) is evidently helpful to improve the cleavage rate of bovine IVF embryos. Consistently, supplementing V
C of 20 µg/mL in IVC medium markedly elevated the transcription levels of pluripotent gene
Oct4,
Sox2,
Cdx2, and
Nanog. Interestingly, adding V
C at higher concentration into IVM solution (100 μg/mL) or IVC medium (40 and 60 μg/mL respectively) showed no positive effects on the development of both Parthenogenetic and IVF embryos in cattle. These results verify that our speculation is reasonable and practical. V
C at tuned concentration might also alleviate cytotoxicity during oocyte IVM as reported in yak [
17].
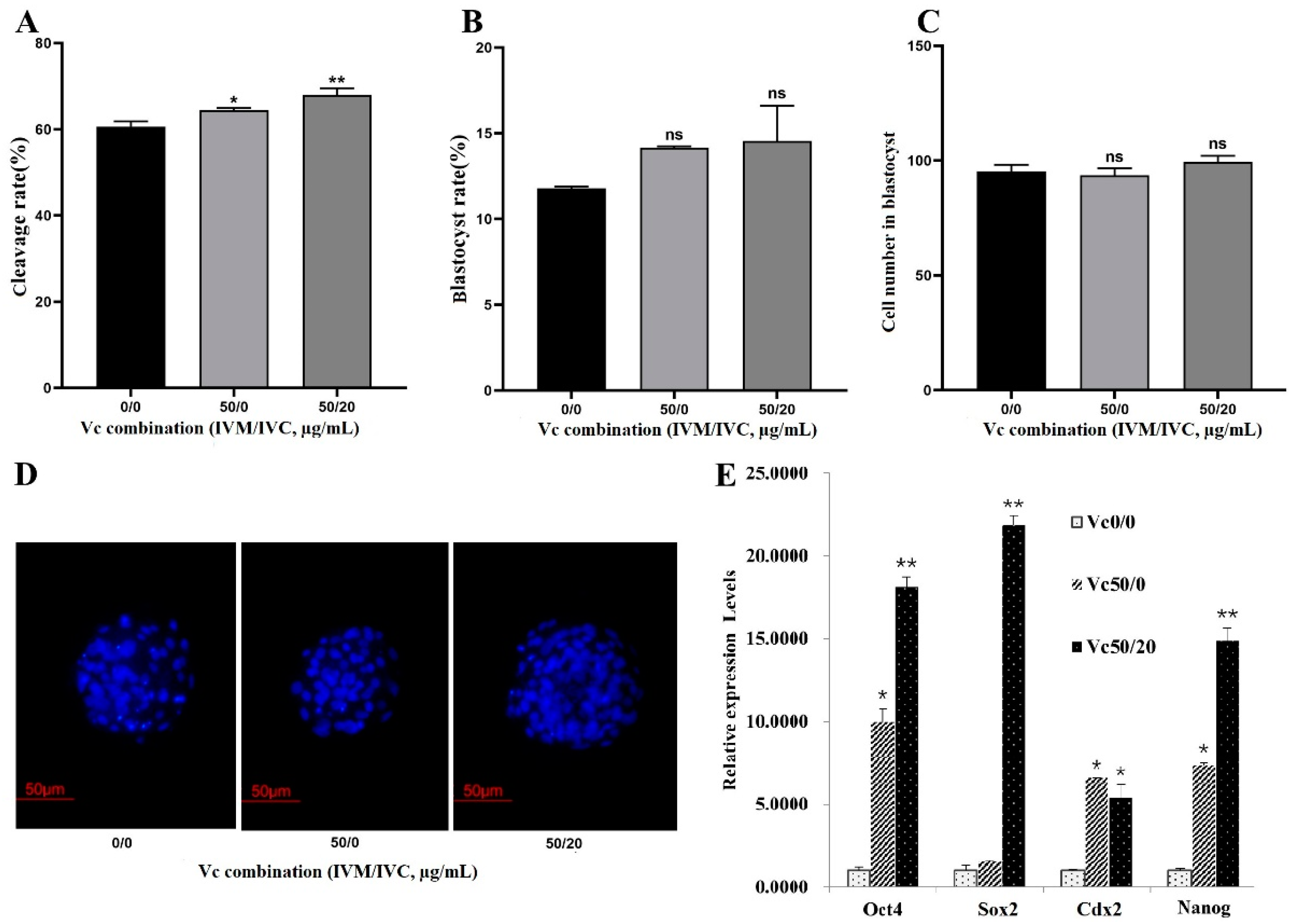
In order to achieve a better effect, IVM solution containing 50 μg/mL V
C + IVC medium containing 20 μg/mL V
C (V
C 50/20 combination) was employed. Consistently, both V
C 50/0 and V
C 50/20 showed statistical positive role on the cleavage rate and slight increasing effect of the blastocyst rate. These combination effects were further confirmed by the up-regulated expressions of
Oct4,
Sox2,
Cdx2, and
Nanog in the IVF blastocysts. It’s well documented that these pluripotency-related genes are important factors in maintaining the proliferation and differentiation of bovine embryonic stem cells and induced pluripotent stem cells [
18,
19,
20,
21]. Thus, appropriate combination of V
C might enhance the developmental capacity by regulating endogenous pluripotency factors.
In the process of embryo IVC, cell apoptosis might occur in its early development due to changes in the culture environment [
22]. It’s reported that cell apoptosis mainly occurs at the stage of 9-16 cells and compacted morula [
23]. Therefore, we next asked whether the effects of V
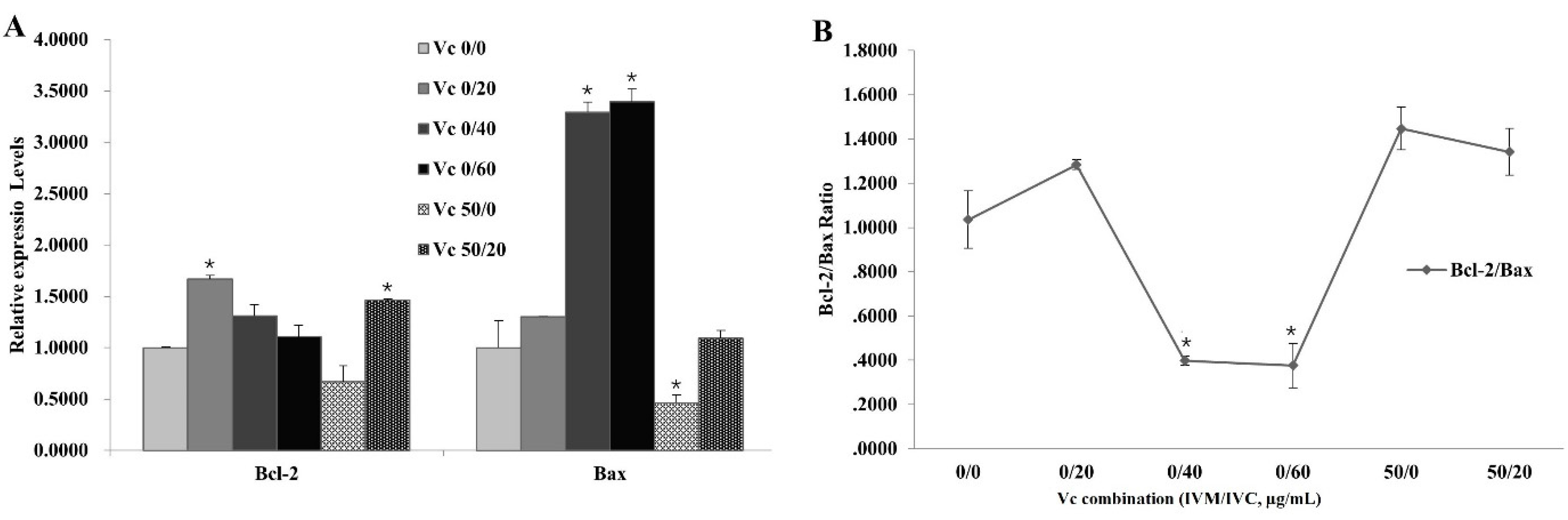
C combinations are related to their anti-apoptosis role in bovine embryos. Our data indicate that V
C 0/20 and V
C 50/20 indeed boost anti-apoptotic gene
Bcl-2 but inhibit pro-apoptotic gene
Bax. The factors leading to embryo damage are variable, sometimes even including electromagnetic waves. Recently, it’s demonstrated that an in ovo V
C injection reduces the harmful effects of electromagnetic waves emitted from mobile phones on chicken embryos [
24]. Commonly, exposure to radiation and other detrimental factors increases the concentration of free radicals in embryos and causes oxidative stress damage. Our results and the above report suggest that V
C and its combination prevent embryo damage and death possibly by anti-apoptosis and anti-oxidation activities.
Figure 1.
Effects of VC on bovine oocyte IVM and parthenogenetic embryo development. (A-C): Oocyte morphology at different stages (Immature, IVM 24 h, and Mature, respectively) was shown with arrows showing the first polar bodies; (D): IVM rates (Oocyte number = 89, 78, 94 and 81 for different groups, respectively); (E, F): Cleavage and blastocyst rates of the parthenogenetic embryos (Oocyte number = 102, 96, 111, and 85, respectively). *P < 0.05; Scale bar = 50 μm.
Figure 1.
Effects of VC on bovine oocyte IVM and parthenogenetic embryo development. (A-C): Oocyte morphology at different stages (Immature, IVM 24 h, and Mature, respectively) was shown with arrows showing the first polar bodies; (D): IVM rates (Oocyte number = 89, 78, 94 and 81 for different groups, respectively); (E, F): Cleavage and blastocyst rates of the parthenogenetic embryos (Oocyte number = 102, 96, 111, and 85, respectively). *P < 0.05; Scale bar = 50 μm.
Figure 2.
Effects of VC on the development of bovine IVF embryos. (A, B): The cleavage and blastocyst rates of IVF embryos (Oocyte number = 96, 106, 93 and 100 for different groups, respectively); (C): Relative expressions of pluripotency-related gene Oct4, Sox2, Cdx2, and Nanog in IVF embryos. *p < 0.05, ***p < 0.001.
Figure 2.
Effects of VC on the development of bovine IVF embryos. (A, B): The cleavage and blastocyst rates of IVF embryos (Oocyte number = 96, 106, 93 and 100 for different groups, respectively); (C): Relative expressions of pluripotency-related gene Oct4, Sox2, Cdx2, and Nanog in IVF embryos. *p < 0.05, ***p < 0.001.
Figure 3.
Effects of VC combination on the development of bovine IVF embryo. (A-C): The cleavage and blastocyst rates as well as the blastomere numbers in IVF embryos (Oocyte number = 79, 88, and 95 for different groups, respectively); (D): Representative Hoechst33342 staining images of the IVF blastocysts (200×); (E): mRNA expression of Oct4, Sox2, Cdx2, and Nanog in IVF embryos. *p < 0.05, **p < 0.01, Scale bar = 50 μm.
Figure 3.
Effects of VC combination on the development of bovine IVF embryo. (A-C): The cleavage and blastocyst rates as well as the blastomere numbers in IVF embryos (Oocyte number = 79, 88, and 95 for different groups, respectively); (D): Representative Hoechst33342 staining images of the IVF blastocysts (200×); (E): mRNA expression of Oct4, Sox2, Cdx2, and Nanog in IVF embryos. *p < 0.05, **p < 0.01, Scale bar = 50 μm.
Figure 4.
Effect of Vc combinations on the mRNA expressions of apoptosis-related genes in IVF blastocysts. (A): mRNA levels of Bcl-2 and Bax in groups containing different Vc combinations in IVM solution + IVC medium (VC 0/0, VC 0/20, VC 0/40, VC 0/60, VC 50/0, VC 50/20). (B): mRNA expression ratios of Bcl-2/Bax. *P < 0.05.
Figure 4.
Effect of Vc combinations on the mRNA expressions of apoptosis-related genes in IVF blastocysts. (A): mRNA levels of Bcl-2 and Bax in groups containing different Vc combinations in IVM solution + IVC medium (VC 0/0, VC 0/20, VC 0/40, VC 0/60, VC 50/0, VC 50/20). (B): mRNA expression ratios of Bcl-2/Bax. *P < 0.05.
Table 1.
Table 1. Primers used for qRT-PCR.
Table 1.
Table 1. Primers used for qRT-PCR.
| Gene |
Forward Primer (5'-3') |
Reverse Primer (5'-3') |
| GAPDH |
CGGCACAGTCAAGGCAGAGAAC |
CGGCACAGTCAAGGCAGAGAAC |
| Oct4 |
GATTTGGATGAGTTTTTAAGGGTT |
ACTCCAACTTCTCCTTATCCAACTT |
| Sox2 |
CTATGACCAGCTCGCAGA |
GGAAGAAGAGGTAACCACG |
| Cdx2 |
CTTTCCTCCGGATGGTGATA |
AGCCAAGTGAAAACCAGGAC |
| Nanog |
AAACAACTGGCCGAAGGAATA |
AGGAGTGGTTGVTCCAAGAC |
| Bcl-2 |
GAGTCGGATCGCAACTTGGA |
CTCTCGGCTGCTGCATTGT |
| Bax |
GCGCATCGGAGATGAATTG |
CCACAGCTGCGATCATCCT |