Submitted:
07 June 2024
Posted:
07 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Graph Machine Model Selection
2.2. Performance of the GM26 Model on the Compounds of the Test Set
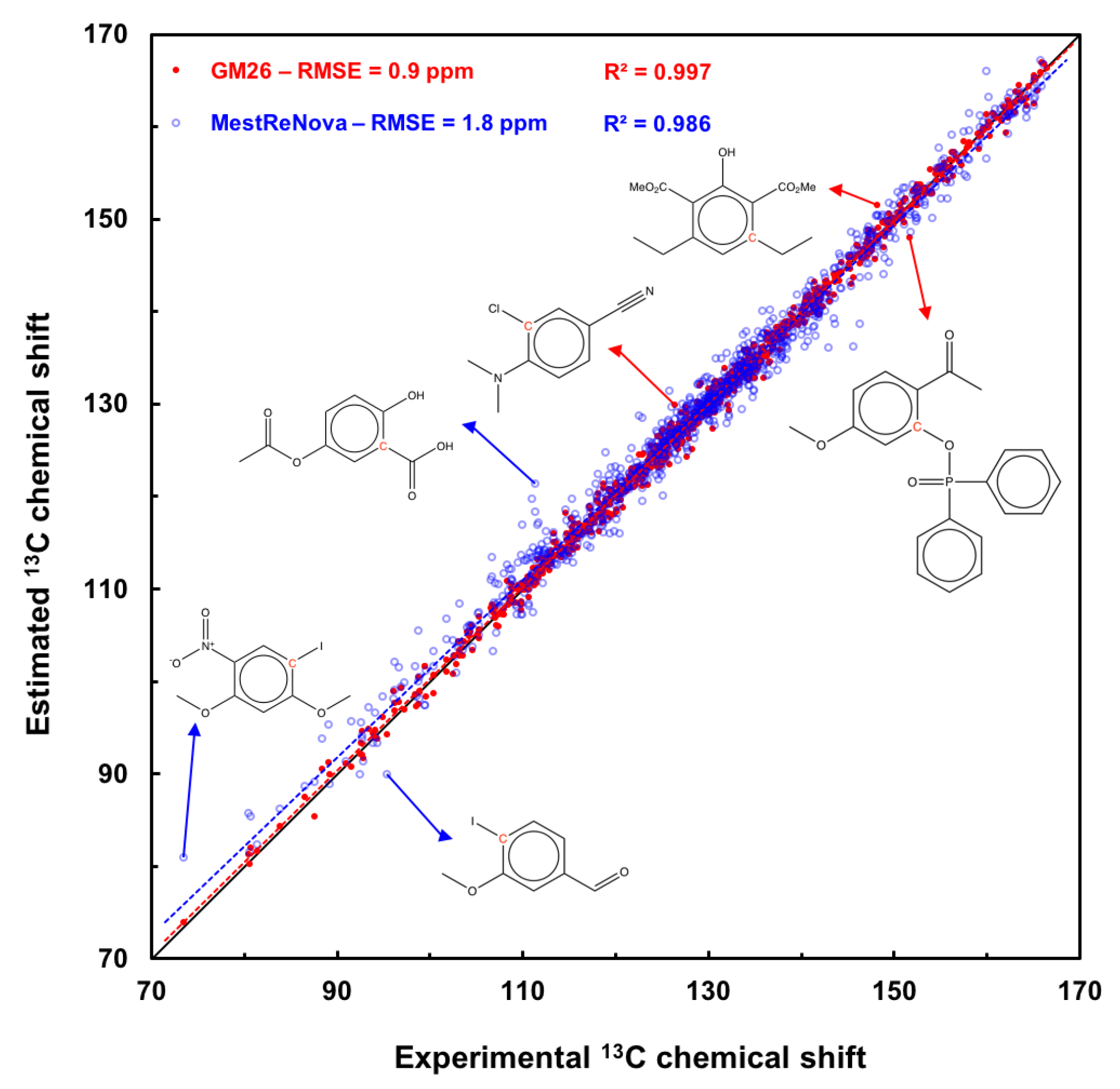
2.3. Scatter plot of the GM26 Model Estimations on Both Sets
3. Discussion
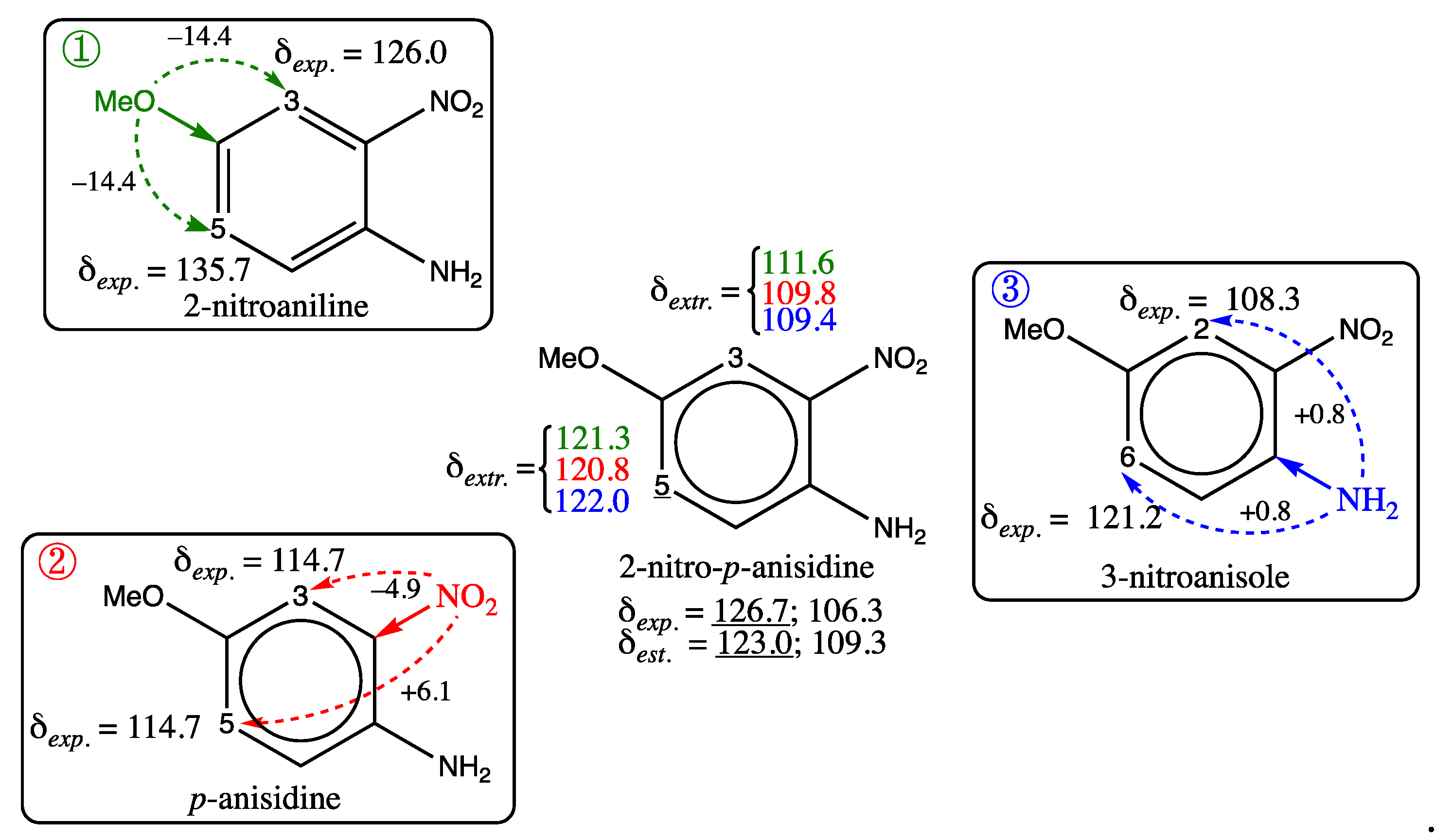
3.1. Analysis of Chemical Shift Estimates by the GM26 model on training and test sets
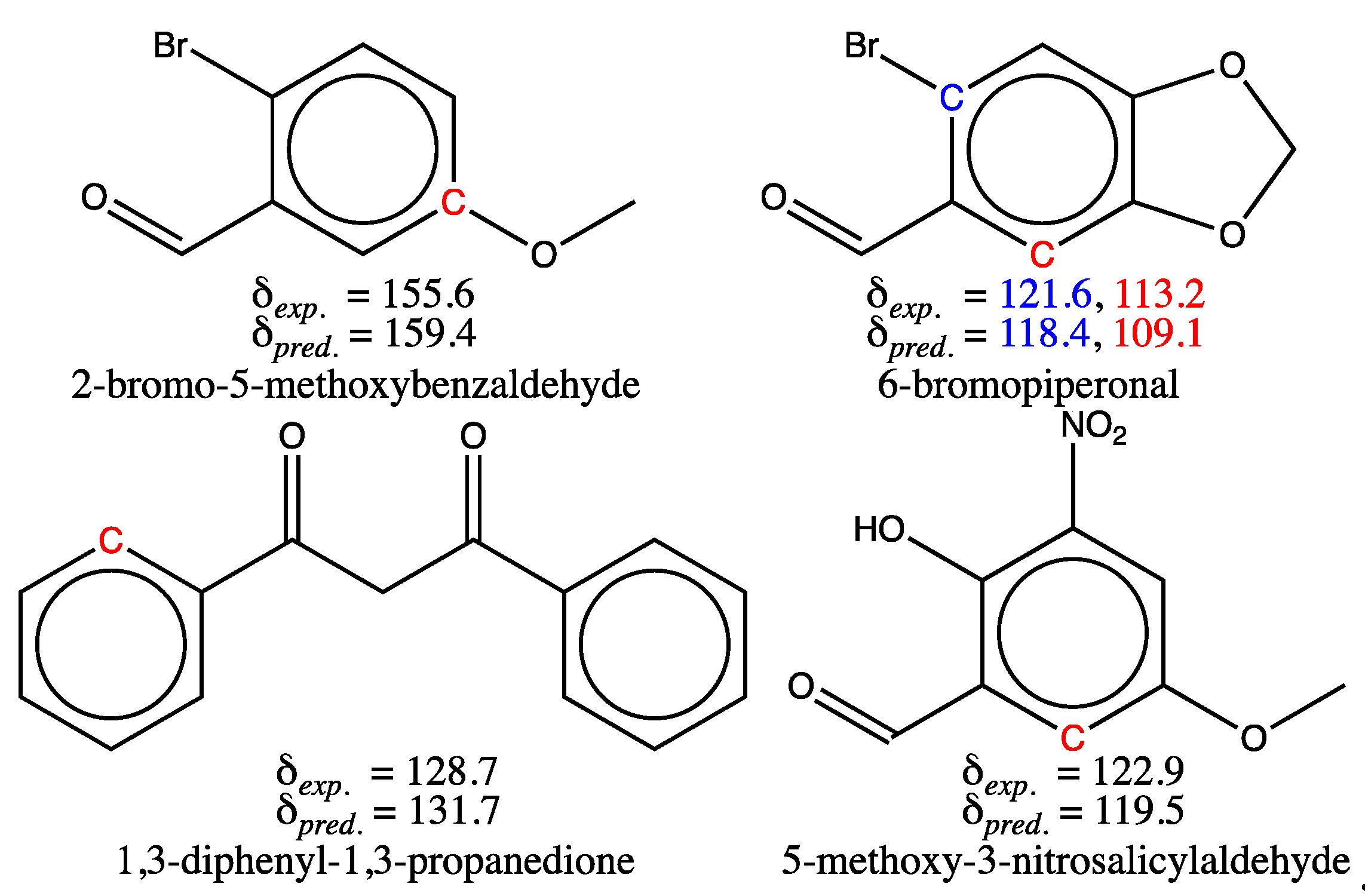
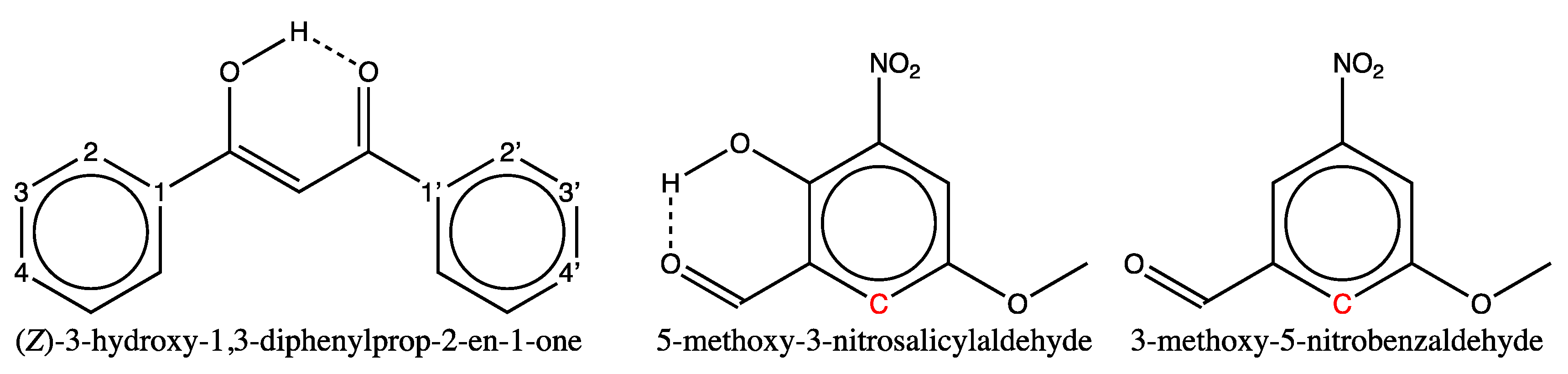
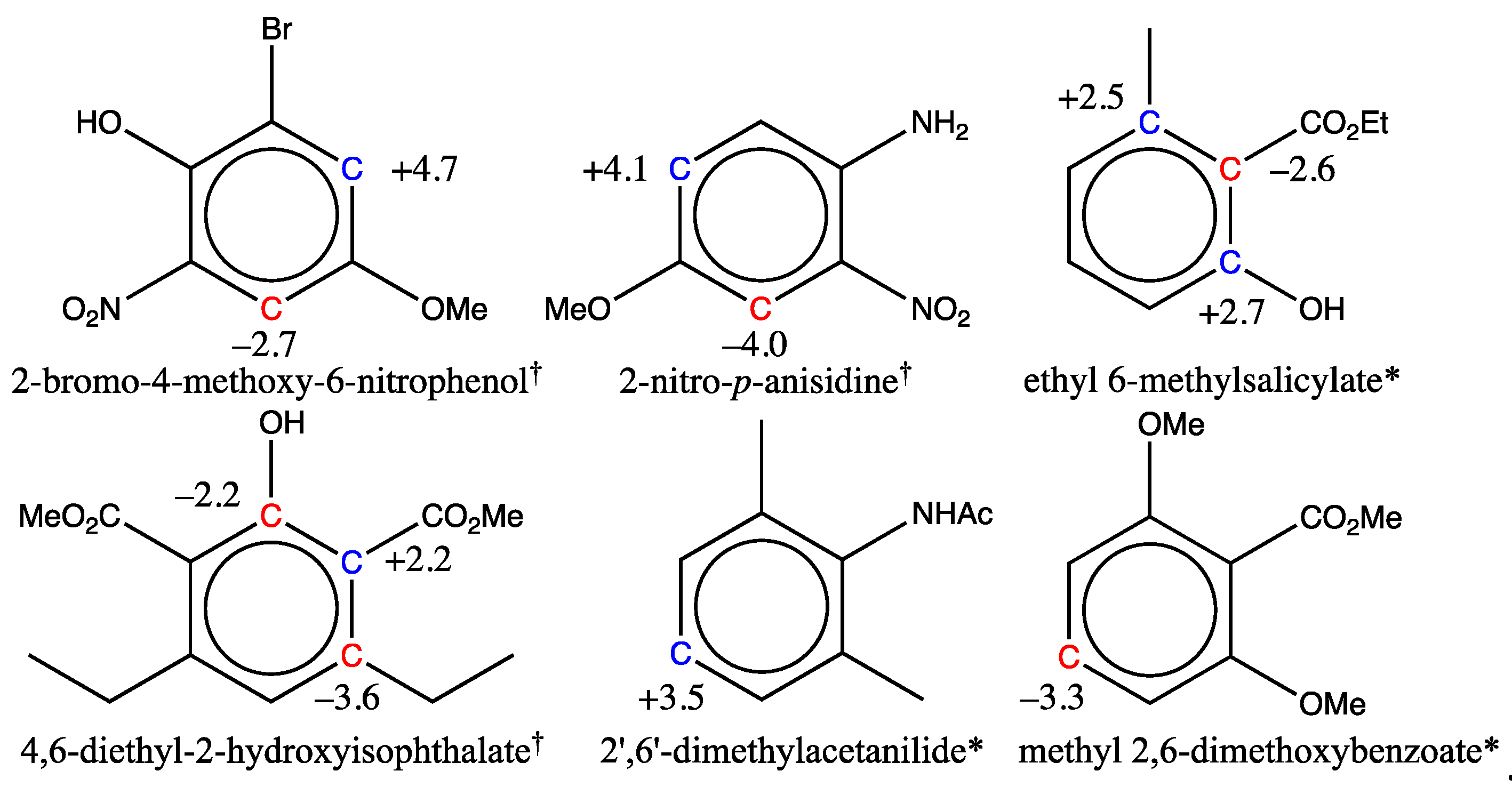
3.1.2. Analysis of Test Set Predictions with absolute errors above 3 ppm
3.2. Design of an extended graph machine-based model
3.3. Comparison of Known Models with the Graph-Machine-Based Model
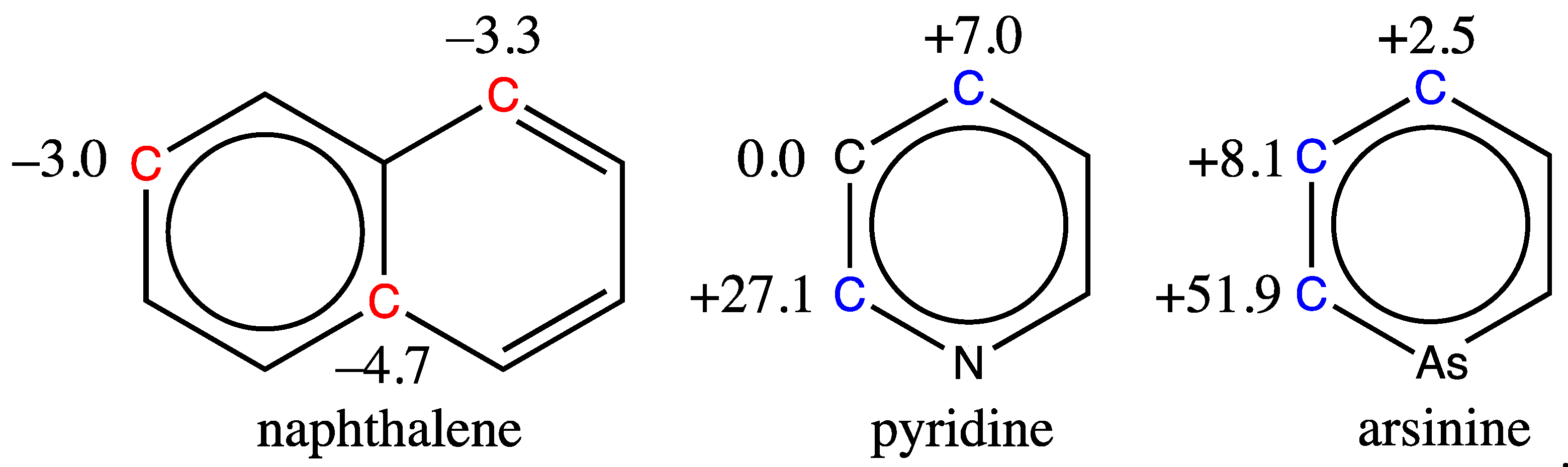
3.4. Some limitations of the Graph Machine-Based Model
4. Materials and Methods
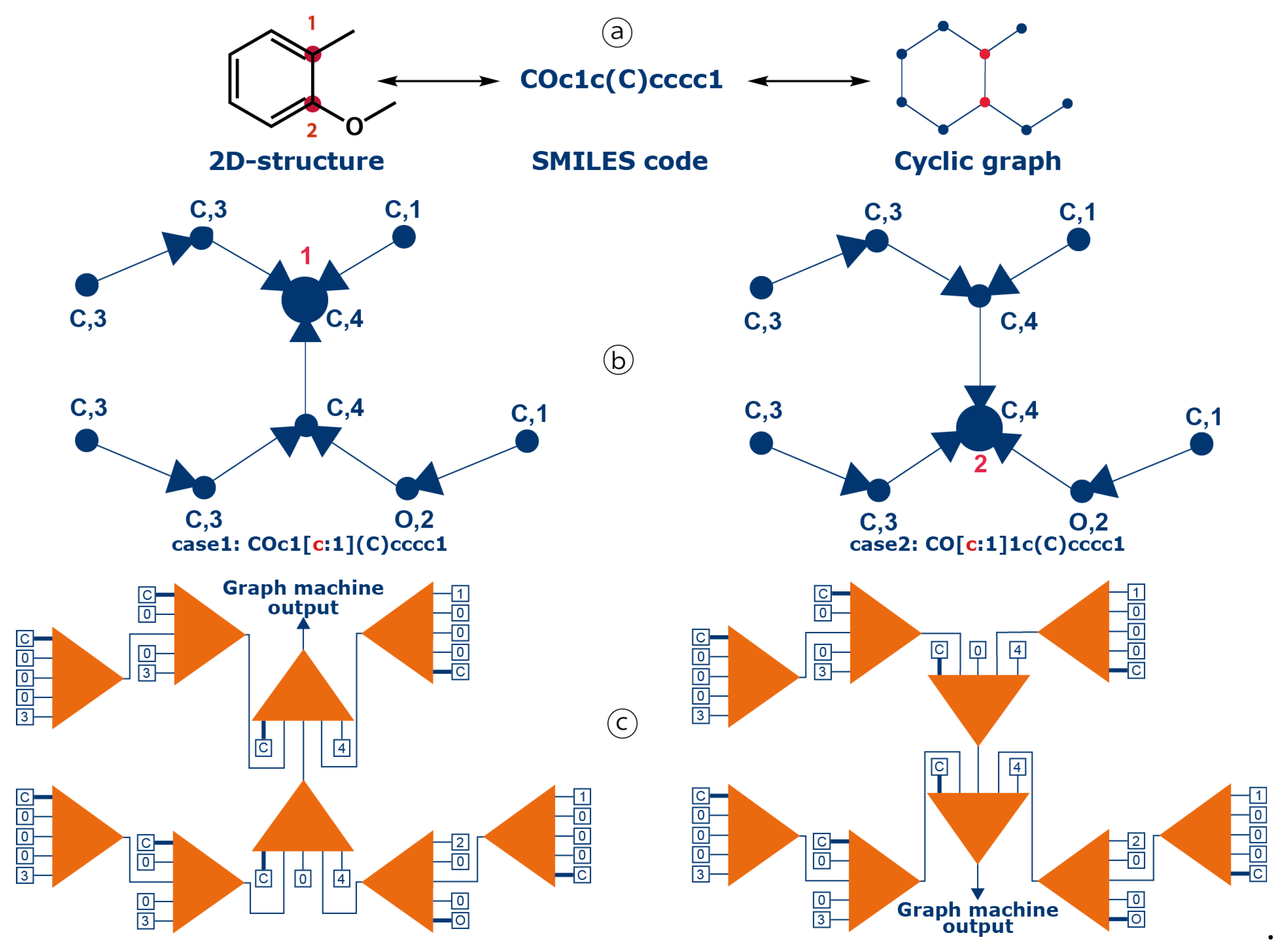
4.1. Graph Machine Modeling
4.2. 13C NMR measurements for the molecules of the test set
4.3. Model Selection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- AIST. Spectral Database for Organic Compounds. Available online: https://sdbs.db.aist.go.jp (accessed on 2024).
- Haydl, A.M.; Hartwig, J.F. Palladium-Catalyzed Methylation of Aryl, Heteroaryl, and Vinyl Boronate Esters. Organic Letters 2019, 21, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.C.C.; Bernstein, M.; Sýkora, S. An Integrated Approach to Structure Verification Using Automated Procedures. 2015, 445-492. [CrossRef]
- Cobas, C.; Seoane, F.; Vaz, E.; Bernstein, M.A.; Dominguez, S.; Pérez, M.; Sýkora, S. Automatic assignment of 1H-NMR spectra of small molecules. Magnetic Resonance in Chemistry 2013, 51, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational Prediction of 1H and 13C Chemical Shifts: A Useful Tool for Natural Product, Mechanistic, and Synthetic Organic Chemistry. Chemical Reviews 2012, 112, 1839–1862. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.M.; Paul, E.G. Carbon-13 Magnetic Resonance. II. Chemical Shift Data for the Alkanes. Journal of the American Chemical Society 1964, 86, 2984–2990. [Google Scholar] [CrossRef]
- Lindeman, L.P.; Adama, J.Q. Carbon-13 nuclear magnetic resonance spectrometry. Chemical shifts for the paraffins through C9. Analytical Chemistry 1971, 43, 1245–1252. [Google Scholar] [CrossRef]
- Clerc, J.T.; Sommerauer, H. A minicomputer program based on additivity rules for the estimation of 13c-nmr chemical shifts. Analytica Chimica Acta 1977, 95, 33–40. [Google Scholar] [CrossRef]
- Thomas, S.; Brühl, I.; Heilmann, D.; Kleinpeter, E. 13C NMR Chemical Shift Calculations for Some Substituted Pyridines: A Comparative Consideration. Journal of Chemical Information and Computer Sciences 1997, 37, 726–730. [Google Scholar] [CrossRef]
- Thomas, S.; Stroehl, D.; Kleinpeter, E. Computer Application of an Incremental System for Calculating 13C NMR Spectra of Aromatic Compounds. Journal of Chemical Information and Computer Sciences 1994, 34, 725–729. [Google Scholar] [CrossRef]
- Fürst, A.; Pretsch, E. A computer program for the prediction of 13C-NMR chemical shifts of organic compounds. Analytica Chimica Acta 1990, 229, 17–25. [Google Scholar] [CrossRef]
- Zupan, J.; Novič, M.; Bohanec, S.; Razinger, M.; Lah, L.; Tusǎr, M.; Košir, I. Expert system for solving problems in carbon-13 nuclear magnetic resonance spectroscopy. Analytica Chimica Acta 1987, 200, 333–345. [Google Scholar] [CrossRef]
- Ewing, D.F. 13C substituent effects in monosubstituted benzenes. Organic Magnetic Resonance 1979, 12, 499–524. [Google Scholar] [CrossRef]
- Hearmon, R.A.; Liu, H.M.; Laverick, S.; Tayler, P. Microcomputer prediction and assessment of substituted benzene 13C NMR chemical shifts. Magnetic Resonance in Chemistry 1991, 30, 240–248. [Google Scholar] [CrossRef]
- Revvity Signals. ChemDraw v.23.1.1.3 Available online: https://revvitysignals.com/products/research/chemdraw (accessed on 2024).
- Ball, J.W.; Anker, L.S.; Jurs, P.C. Automated model selection for the simulation of carbon-13 nuclear magnetic resonance spectra of cyclopentanones and cycloheptanones. Analytical Chemistry 1991, 63, 2435–2442. [Google Scholar] [CrossRef]
- Small, G.W.; Jurs, P.C. Simulation of carbon-13 nuclear magnetic resonance spectra of cycloalkanols with computer-based structural descriptors. Analytical Chemistry 1989, 55, 1128–1134. [Google Scholar] [CrossRef]
- Sutton, G.P.; Jurs, P.C. Simulation of carbon-13 nuclear magnetic resonance spectra of alkyl-substituted cyclohexanones and decalones. Analytical Chemistry 1989, 61, 863–871. [Google Scholar] [CrossRef]
- Sutton, G.P.; Jurs, P.C. Simulation of carbon-13 nuclear magnetic resonance spectra of alkyl-substituted cyclohexanones and decalones. Analytical Chemistry 1990, 61, 863–871. [Google Scholar] [CrossRef]
- Anker, L.S.; Jurs, P.C. Prediction of carbon-13 nuclear magnetic resonance chemical shifts by artificial neural networks. Analytical Chemistry 1992, 64, 1157–1164. [Google Scholar] [CrossRef]
- Kvasnička, V. An application of neural networks in chemistry. Chemical Papers 1990, 44, 775–792. [Google Scholar]
- Kvasnicka, V.; Sklenak, S.; Pospichal, J. Application of neural networks with feedback connections in chemistry: prediction of carbon-13 NMR chemical shifts in a series of monosubstituted benzenes. THEOCHEM 1992, 96, 87–107. [Google Scholar] [CrossRef]
- Sklenak, S.; Kvasnicka, V.; Pospichal, J. Prediction of 13C NMR chemical shifts by neural networks in a series of monosubstituted benzenes. Chemical Papers 1994, 48, 135–140. [Google Scholar]
- Thomas, S.; Kleinpeter, E. The Assignment of the 13C-NMR Chemical Shifts of Substituted Naphthalenes from Charge Density with an Artificial Neural Network. Journal für Praktische Chemie/Chemiker-Zeitung 1995, 337, 504–507. [Google Scholar] [CrossRef]
- Ivanciuc, O.; Rabine, J.P.; Cabrol-Bass, D.; Panaye, A.; Doucet, J.P. 13C NMR Chemical Shift Prediction of sp2 Carbon Atoms in Acyclic Alkenes Using Neural Networks. Journal of Chemical Information and Computer Sciences 1996, 36, 644–653. [Google Scholar] [CrossRef]
- Meusinger, R.; Himmelreich, U. Neural networks and genetic algorithms applications in nuclear magnetic resonance spectroscopy. 2003, 23, 281–321. [CrossRef]
- Bret, C.L. A General13C NMR Spectrum Predictor Using Data Mining Techniques. SAR and QSAR in Environmental Research 2000, 11, 211–234. [Google Scholar] [CrossRef]
- Blinov, K.A.; Smurnyy, Y.D.; Churanova, T.S.; Elyashberg, M.E.; Williams, A.J. Development of a fast and accurate method of 13C NMR chemical shift prediction. Chemometrics and Intelligent Laboratory Systems 2009, 97, 91–97. [Google Scholar] [CrossRef]
- Meiler, J.; Meusinger, R.; Will, M. Neural Network Prediction of 13C NMR Chemical Shifts of Substituted Benzenes. Monatshefte für Chemie / Chemical Monthly 1999, 130, 1089–1095. [Google Scholar] [CrossRef]
- Bremser, W. Hose — a novel substructure code. Analytica Chimica Acta 1978, 103, 355–365. [Google Scholar] [CrossRef]
- Bremser, W.; Klier, M.; Meyer, E. Mutual assignment of subspectra and substructures—A way to structure elucidation by 13C NMR spectroscopy. Organic Magnetic Resonance 1975, 7, 97–106. [Google Scholar] [CrossRef]
- Schütz, V.; Purtuc, V.; Felsinger, S.; Robien, W. CSEARCH-STEREO: A new generation of NMR database systems allowing three-dimensional spectrum prediction. Fresenius' Journal of Analytical Chemistry 1997, 359, 33–41. [Google Scholar] [CrossRef]
- Steinbeck, C.; Krause, S.; Kuhn, S. NMRShiftDBConstructing a Free Chemical Information System with Open-Source Components. Journal of Chemical Information and Computer Sciences 2003, 43, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- ACD/Labs. ACD/NMR Predictors. Available online: https://www.acdlabs.com/products/spectrus-platform/nmr-predictors/ (accessed on 2024).
- Modgraph Consultants. NMR Predict. Available online: https://mestrelab.com/software/mnova-software/nmr-predict/ (accessed on 2024).
- NMRDB. NMR Predict. Available online: http://www.nmrdb.org/13c/index.shtml?v=v2.138.0 (accessed on 2024).
- Robien, W.; Haider, N. CSEARCH / NMRPREDICT. Available online: https://c13nmr.at/c13robot/robot.php (accessed on 2024).
- Kang, S.; Kwon, Y.; Lee, D.; Choi, Y.-S. Predictive Modeling of NMR Chemical Shifts without Using Atomic-Level Annotations. Journal of Chemical Information and Modeling 2020, 60, 3765–3769. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Lee, D.; Choi, Y.-S.; Kang, M.; Kang, S. Neural Message Passing for NMR Chemical Shift Prediction. Journal of Chemical Information and Modeling 2020, 60, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Lee, D.; Choi, Y.-S.; Kang, S. Molecular search by NMR spectrum based on evaluation of matching between spectrum and molecule. Scientific Reports 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; Li, R.; Pelczer, I.; Rabitz, H. NMR Landscapes for Chemical Shift Prediction. The Journal of Physical Chemistry A 2012, 116, 9142–9157. [Google Scholar] [CrossRef] [PubMed]
- NMRshiftDB. Available online: https://nmrshiftdb.nmr.uni-koeln.de/ (accessed on 2024).
- Smurnyy, Y.D.; Blinov, K.A.; Churanova, T.S.; Elyashberg, M.E.; Williams, A.J. Toward More Reliable 13C and 1H Chemical Shift Prediction: A Systematic Comparison of Neural-Network and Least-Squares Regression Based Approaches. Journal of Chemical Information and Modeling 2008, 48, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Meiler, J.; Meusinger, R.; Will, M. Fast Determination of 13C NMR Chemical Shifts Using Artificial Neural Networks. Journal of Chemical Information and Computer Sciences 2000, 40, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Blinov, K.A.; Smurnyy, Y.D.; Elyashberg, M.E.; Churanova, T.S.; Kvasha, M.; Steinbeck, C.; Lefebvre, B.A.; Williams, A.J. Performance Validation of Neural Network Based 13C NMR Prediction Using a Publicly Available Data Source. Journal of Chemical Information and Modeling 2008, 48, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Meiler, J.; Maier, W.; Will, M.; Meusinger, R. Using Neural Networks for 13C NMR Chemical Shift Prediction–Comparison with Traditional Methods. Journal of Magnetic Resonance 2002, 157, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Robien, W. The CSEARCH-NMR data base approach to solve frequent questions concerning substituent effects on 13C NMR chemical shifts. Chemometrics and Intelligent Laboratory Systems 1993, 19, 217–223. [Google Scholar] [CrossRef]
- Robien, W. Computer-assisted peer reviewing of spectral data: the CSEARCH protocol. Monatshefte für Chemie - Chemical Monthly 2019, 150, 927–932. [Google Scholar] [CrossRef]
- Jonas, E.; Kuhn, S. Rapid prediction of NMR spectral properties with quantified uncertainty. Journal of Cheminformatics 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Cobas, C. NMR signal processing, prediction, and structure verification with machine learning techniques. Magnetic Resonance in Chemistry 2020, 58, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Johnson, S.R. Stereo-Aware Extension of HOSE Codes. ACS Omega 2019, 4, 7323–7329. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Kolshorn, H.; Steinbeck, C.; Schlörer, N. Twenty years of nmrshiftdb2: A case study of an open database for analytical chemistry. Magnetic Resonance in Chemistry 2023, 62, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Cobas, C.; Barba, A.; Colreavy-Donnelly, S.; Caraffini, F.; Borges, R.M. Direct deduction of chemical class from NMR spectra. Journal of Magnetic Resonance 2023, 348, 107381. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Tumer, E.; Colreavy-Donnelly, S.; Moreira Borges, R. A pilot study for fragment identification using 2D NMR and deep learning. Magnetic Resonance in Chemistry 2021, 60, 1052–1060. [Google Scholar] [CrossRef]
- Kuhn, S.; Borges, R.M.; Venturini, F.; Sansotera, M. Dataset Size and Machine Learning - Open NMR Databases as a Case Study. 2022, 1632-1636. [CrossRef]
- Rull, H.; Fischer, M.; Kuhn, S. NMR shift prediction from small data quantities. Journal of Cheminformatics 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Jonas, E.; Kuhn, S.; Schlörer, N. Prediction of chemical shift in NMR: A review. Magnetic Resonance in Chemistry 2021, 60, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, P.H.; Jansma, M.J.; Hoye, T.R. A guide to small-molecule structure assignment through computation of (1H and 13C) NMR chemical shifts. Nature Protocols 2014, 9, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhang, J.; Peng, Q.; Zhang, J.; Glezakou, V.-A. General Protocol for the Accurate Prediction of Molecular 13C/1H NMR Chemical Shifts via Machine Learning Augmented DFT. Journal of Chemical Information and Modeling 2020, 60, 3746–3754. [Google Scholar] [CrossRef] [PubMed]
- Unzueta, P.A.; Greenwell, C.S.; Beran, G.J.O. Predicting Density Functional Theory-Quality Nuclear Magnetic Resonance Chemical Shifts via Δ-Machine Learning. Journal of Chemical Theory and Computation 2021, 17, 826–840. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, W.; Bratholm, L.A.; Packer, M.J.; Mulholland, A.J.; Glowacki, D.R.; Butts, C.P. IMPRESSION – prediction of NMR parameters for 3-dimensional chemical structures using machine learning with near quantum chemical accuracy. Chemical Science 2020, 11, 508–515. [Google Scholar] [CrossRef] [PubMed]
- SciFinder; Chemical Abstracts Service: Columbus, O. Available online: https://scifinder-n.cas.org/ (accessed on 2024).
- Hyodo, K.; Hasegawa, G.; Oishi, N.; Kuroda, K.; Uchida, K. Direct and Catalytic Amide Synthesis from Ketones via Transoximation and Beckmann Rearrangement under Mild Conditions. The Journal of Organic Chemistry 2018, 83, 13080–13087. [Google Scholar] [CrossRef] [PubMed]
- Morisset, E.; Chardon, A.; Rouden, J.; Blanchet, J. Phenysilane and Silicon Tetraacetate: Versatile Promotors for Amide Synthesis. European Journal of Organic Chemistry 2020, 2020, 388–392. [Google Scholar] [CrossRef]
- Brasche, G.; García-Fortanet, J.; Buchwald, S.L. Twofold C−H Functionalization: Palladium-Catalyzed Ortho Arylation of Anilides. Organic Letters 2008, 10, 2207–2210. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, Z.-L.; Wan, H.-L.; He, Y.-H.; Guan, Z. Visible-Light-Induced Beckmann Rearrangement by Organic Photoredox Catalysis. Organic Letters 2020, 22, 6182–6186. [Google Scholar] [CrossRef] [PubMed]
- Stuart, J.G.; Khora, S.; McKenney, J.D.; Castle, R.N. The synthesis of dimethoxy- and trimethoxy[1]benzothieno[2,3-c]quinolines. Journal of Heterocyclic Chemistry 2009, 24, 1589–1594. [Google Scholar] [CrossRef]
- Cakmak, S.; Kutuk, H.; Odabasoglu, M.; Yakan, H.; Buyukgungor, O. Spectroscopic Properties and Preparation of Some 2,3-Dimethoxybenzamide Derivatives. Letters in Organic Chemistry 2016, 13, 181–194. [Google Scholar] [CrossRef]
- Hayrapetyan, D.; Rit, R.K.; Kratz, M.; Tschulik, K.; Gooßen, L.J. Electrochemical C−H Cyanation of Electron-Rich (Hetero)Arenes. Chemistry – A European Journal 2018, 24, 11288–11291. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Ji, L.; Ge, Z.-m.; Wang, X.; Li, R.-t. A continuous-flow synthesis of primary amides from hydrolysis of nitriles using hydrogen peroxide as oxidant. Tetrahedron 2018, 74, 1527–1532. [Google Scholar] [CrossRef]
- Filleux-Blanchard, M.L.; Fieus, J.; Hallé, J.C. Processus de rotation empéchée autour de la liaison C–N dans les anilines. Organic Magnetic Resonance 1973, 5, 221–225. [Google Scholar] [CrossRef]
- Van Damme, J.; van den Berg, O.; Brancart, J.; Van Assche, G.; Du Prez, F. A novel donor-π-acceptor anthracene monomer: Towards faster and milder reversible dimerization. Tetrahedron 2019, 75, 912–920. [Google Scholar] [CrossRef]
- Yong, Q.; Sun, B.; Zhang, F.-L. Palladium-catalyzed ortho-C(sp2) H bromination of benzaldehydes via a monodentate transient directing group strategy. Tetrahedron Letters 2019, 60, 151263. [Google Scholar] [CrossRef]
- Hou, J.; Li, Z.; Jia, X.-D.; Liu, Z.-Q. Bromination of Arenes Using I2O5-KBr in Water. Synthetic Communications 2013, 44, 181–187. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.-Q.; Guo, J.; Chen, L.-L.; Wang, Y.-B.; Zhang, X. An effective preparation of both 1,3-diketones and nitriles from alkynones with oximes as hydroxide sources. Organic & Biomolecular Chemistry 2018, 16, 8336–8344. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Castro, J.L. Total synthesis of (+)-macbecin I. Journal of the Chemical Society, Perkin Transactions 1 1990, 47. [Google Scholar] [CrossRef]
- Brandt, G.E.L.; Blagg, B.S.J. Monoenomycin: A Simplified Trienomycin A Analogue That Manifests Anticancer Activity. ACS Medicinal Chemistry Letters 2011, 2, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, T.; Wildman, T.A.; Salman, S.R. The perpendicular conformation of 2-hydroxythiophenol. Intramolecular hydrogen bonding to a specific lone pair. Journal of the American Chemical Society 1980, 102, 107–110. [Google Scholar] [CrossRef]
- Schaefer, T.; McKinnon, D.M.; Sebastian, R.; Peeling, J.; Penner, G.H.; Veregin, R.P. Concerning lone-pair stereospecificity of intramolecular OH hydrogen bonds to oxygen and sulfur in solution. Canadian Journal of Chemistry 1987, 65, 908–914. [Google Scholar] [CrossRef]
- Schaefer, T.; Penner, G.H. Mechanisms of long-range 13C, 13C spin–spin coupling in thioanisole and its derivatives. Conformational applications. Canadian Journal of Chemistry 1988, 66, 1229–1238. [Google Scholar] [CrossRef]
- Goussard, V.; Duprat, F.; Gerbaud, V.; Ploix, J.-L.; Dreyfus, G.; Nardello-Rataj, V.; Aubry, J.-M. Predicting the Surface Tension of Liquids: Comparison of Four Modeling Approaches and Application to Cosmetic Oils. Journal of Chemical Information and Modeling 2017, 57, 2986–2995. [Google Scholar] [CrossRef] [PubMed]
- Goussard, V.; Duprat, F.; Ploix, J.-L.; Dreyfus, G.; Nardello-Rataj, V.; Aubry, J.-M. A New Machine-Learning Tool for Fast Estimation of Liquid Viscosity. Application to Cosmetic Oils. Journal of Chemical Information and Modeling 2020, 60, 2012–2023. [Google Scholar] [CrossRef] [PubMed]
- Duprat, F.; Ploix, J.-L.; Aubry, J.-M.; Gaudin, T. Fast and Accurate Prediction of Refractive Index of Organic Liquids with Graph Machines. Molecules 2023, 28, 6805. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.R.; Lechner, B.; Mikhova, B. NMR Data for Carbon-13: Aromatic Compounds; Gupta, R.R., Lechner, M.D., Eds.; Springer-Verlag: Berlin Heidelberg, 2005; Volume III. [Google Scholar]
- Dioury, F.; Duprat, A.; Dreyfus, G.; Ferroud, C.; Cossy, J. QSPR Prediction of the Stability Constants of Gadolinium(III) Complexes for Magnetic Resonance Imaging. Journal of Chemical Information and Modeling 2014, 54, 2718–2731. [Google Scholar] [CrossRef] [PubMed]
- Goulon, A.; Picot, T.; Duprat, A.; Dreyfus, G. Predicting activities without computing descriptors: graph machines for QSAR. SAR and QSAR in Environmental Research 2007, 18, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Daylight Chemical Information Systems. Daylight Theory Manual. Available online: https://www.daylight.com/dayhtml/doc/theory/ (accessed on 2024).
- Dreyfus, G. Neural Networks: Methodology and Applications; Springer: Berlin ; New York, 2005; p. 497.
- Monari, G.; Dreyfus, G. Local Overfitting Control via Leverages. Neural Computation 2002, 14, 1481–1506. [Google Scholar] [CrossRef] [PubMed]
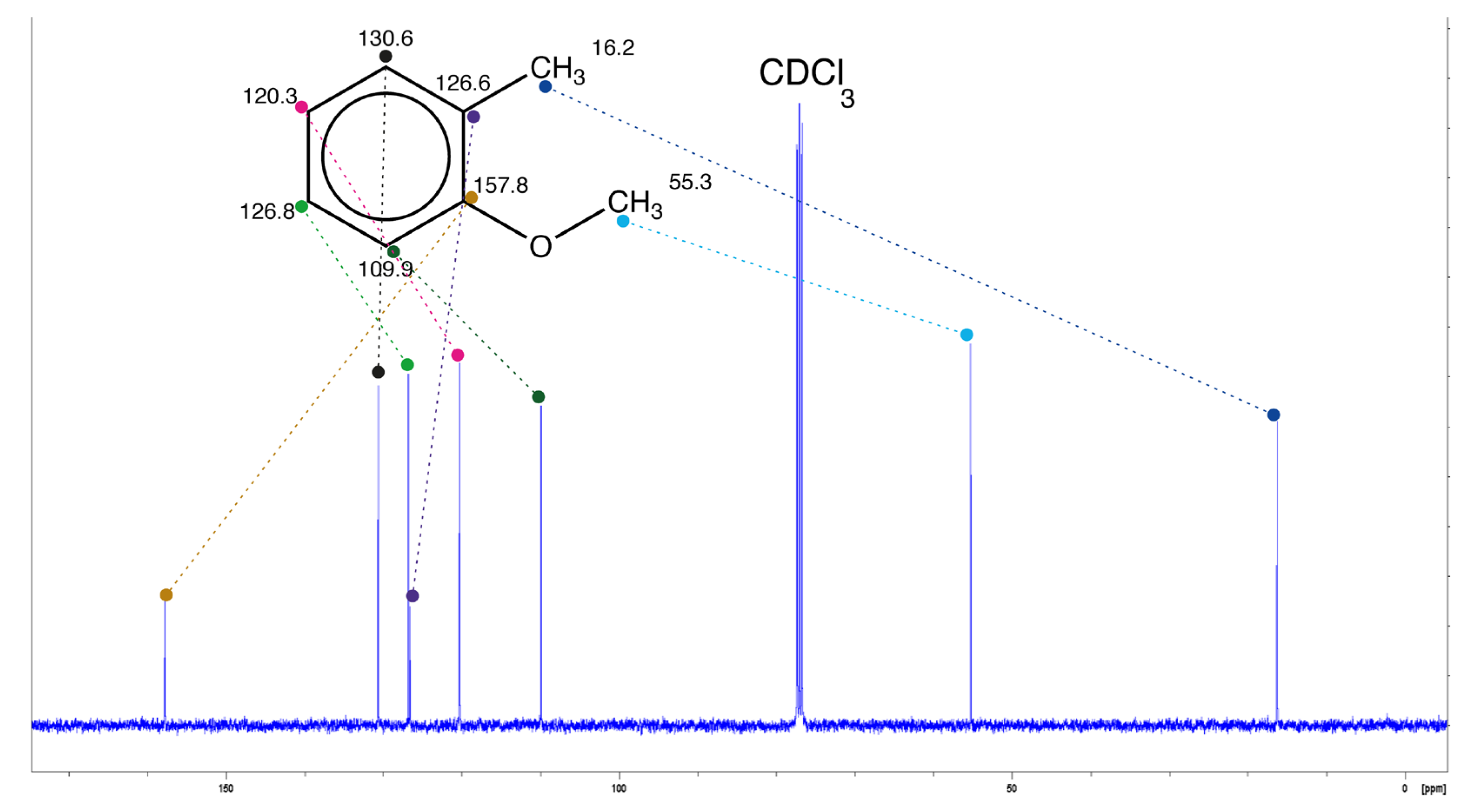
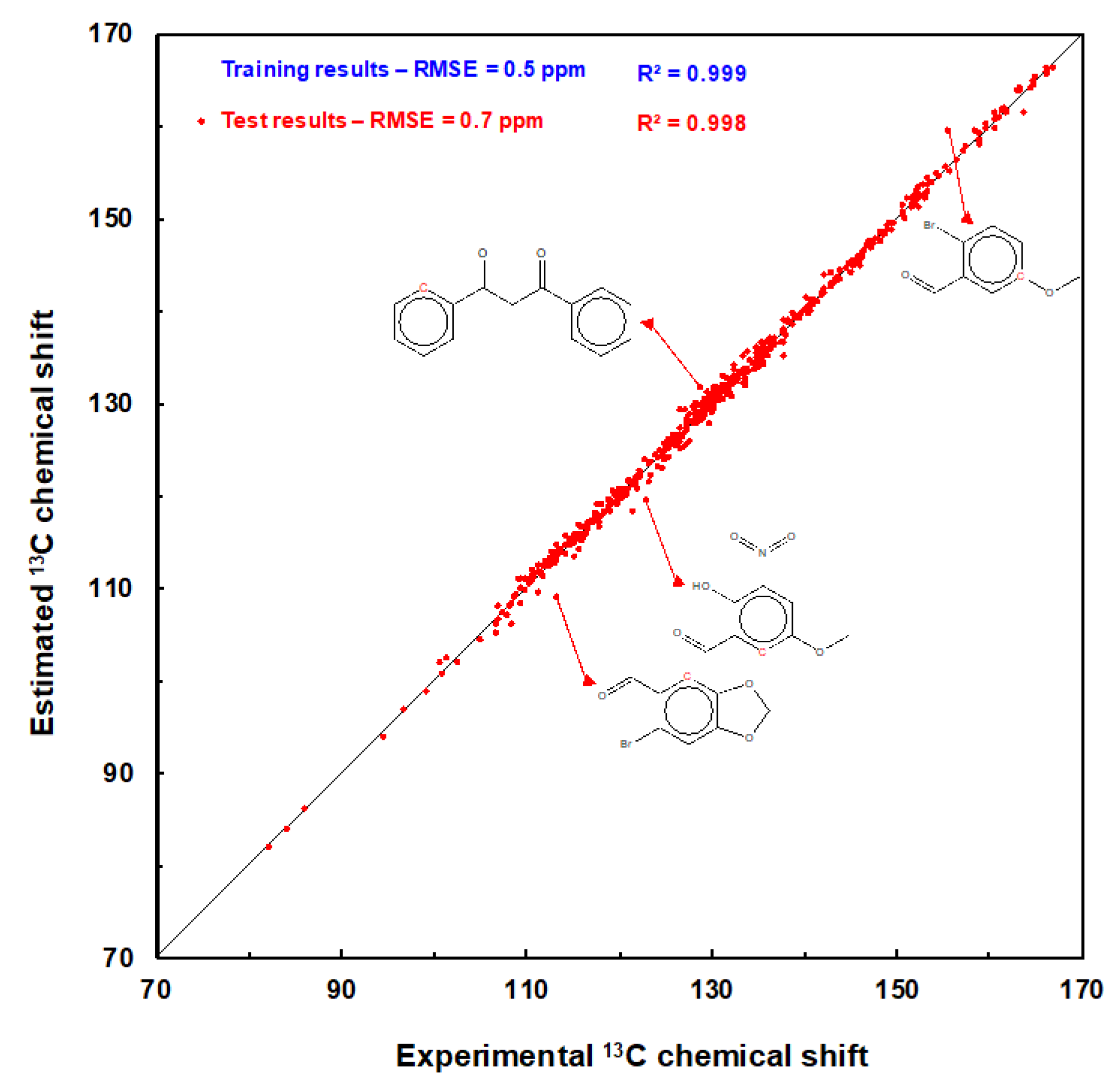
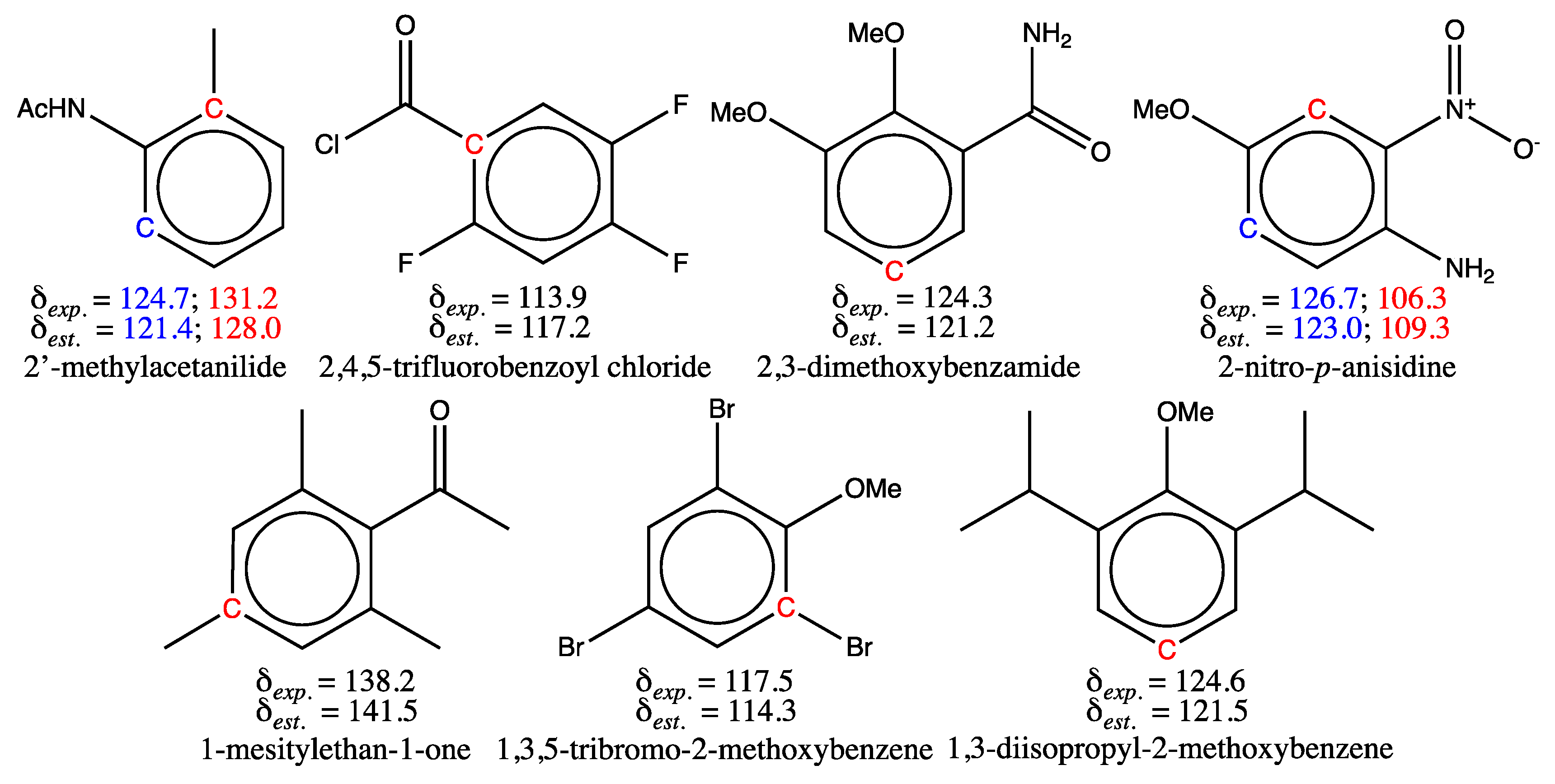
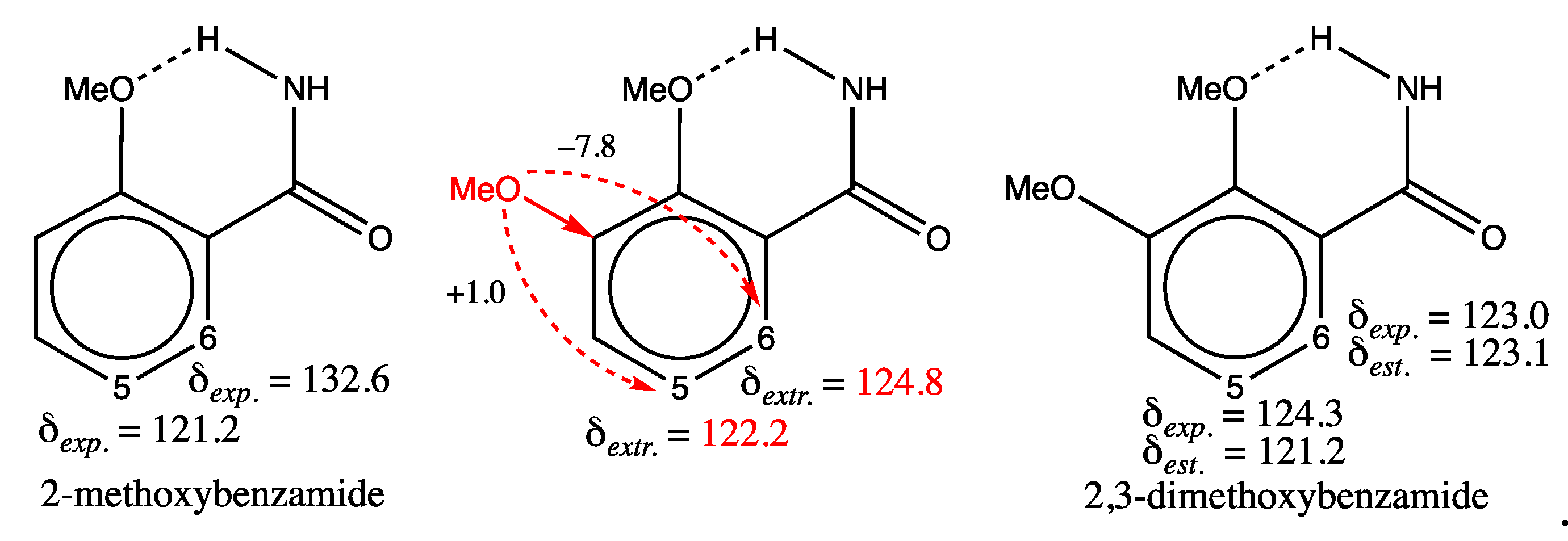
|
Model algorithm |
NMRshiftDB HOSE [NN] |
ChemDraw increments |
MestReNova ensemble |
ACD HOSE+NN |
Gaussian DFT |
| shift (ppm) | 156.1 [157.7] | 158.6 | 157.8 | 157.8 | 156.5 |
| deviation (ppm) | 1.7 [0.1] | 0.8 | 0.0 | 0.0 | 1.3 |
|
Model algorithm |
Nmrdb HOSE |
NMRshiftDB HOSE [NN] 2 |
Nmr predict HOSE+NN |
ChemDraw increments |
MestReNova ensemble |
ACD HOSE+NN |
| RMSE (ppm) 1 | 4.7 | 6.6 [1.1] | 3.8 | 3.3 | 2.2 | 1.9 |
| Number of hidden neurons 1 |
14 | 16 | 18 | 20 | 22 | 24 | 26 | 28 | 30 |
|---|---|---|---|---|---|---|---|---|---|
| RMSTE 2 | 1.08 | 0.97 | 0.86 | 0.79 | 0.73 | 0.67 | 0.63 | 0.58 | 0.55 |
| VLOO score 3 (ppm) | 1.20 (0.003) | 1,08 (0.008) | 0.99 (0.002) | 0.92 (0.001) | 0.87 (0.002) | 0.82 (0.003) | 0.78 (0.004) | 0.75 (0.005) | 0.72 (0.001) |
| Computation time 4 | 0.9 | 1.1 | 1.3 | 1.7 | 2.1 | 2.6 | 3.2 | 4.1 | 5.0 |
| Dataset | 1 | RMSE 2 | MAE 2 | R2 3 | MIN 4 | MAX 5 |
|---|---|---|---|---|---|---|
| Training | 8431 | 0.5 | 0.4 | 0.998 | −3.4 | 3.9 |
| Test | 584 | 0.7 | 0.5 | 0.997 | −3.8 | 4.1 |
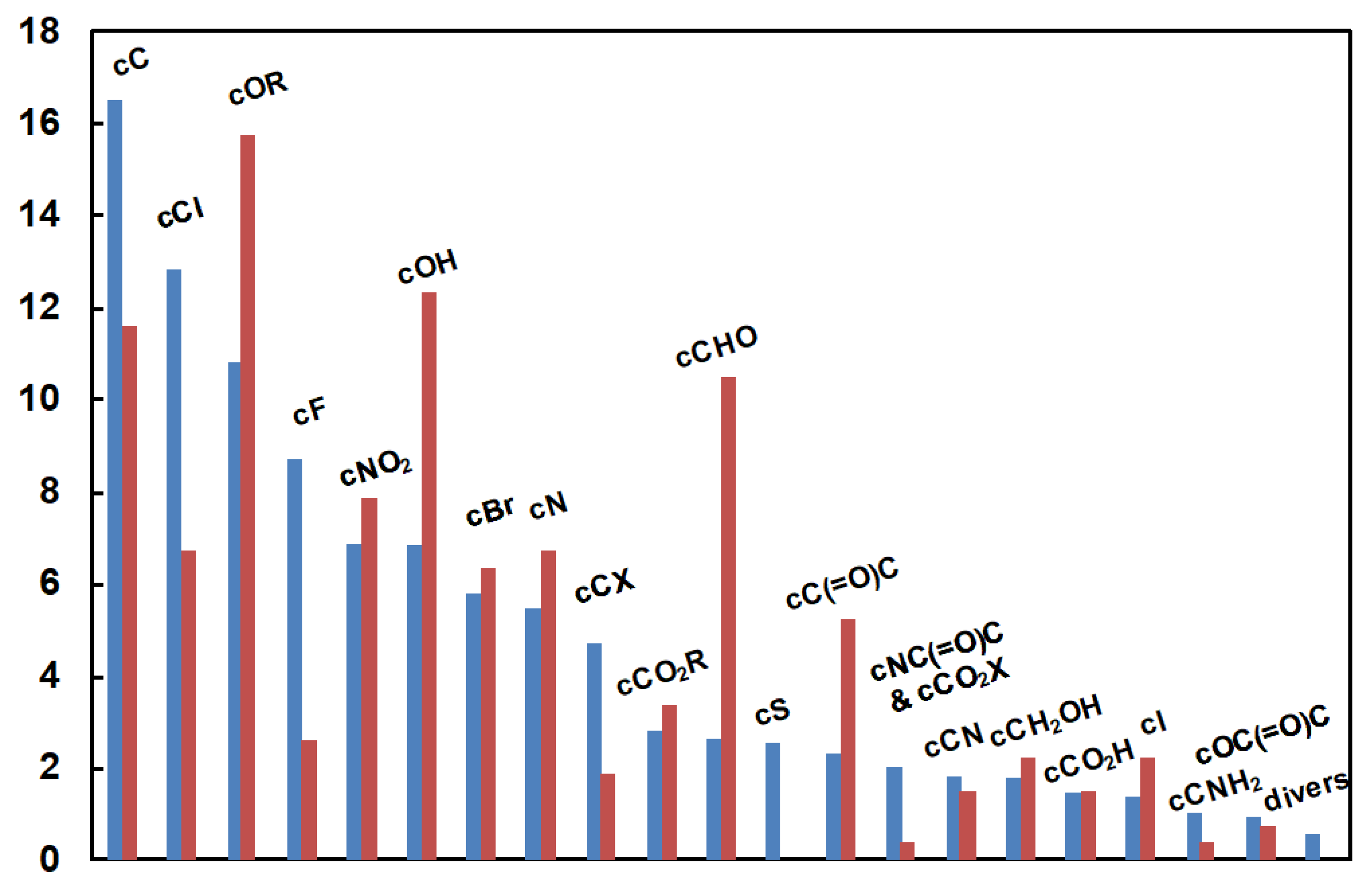
| Functionality or atom in the substituent |
Number of molecules | Example of benzene substituent |
|---|---|---|
| Phenoxy | 30 | p-CH(=O)C6H4O |
| 1,3-diketone | 14 | C6H5C(=O)CH2C(=O) |
| Sulfoxide | 17 | H3CS(=O) |
| Acetic acid | 4 | HO2CCH2 |
| Acetonitrile | 9 | NCCH2 |
| Benzoyl | 7 | C6H5C(=O) |
| Azide | 28 | N3 |
| Crowded carbon | 35 | t-Bu in position 2,4,6 |
| P | 34 | C6H5OPH(=O)O |
| Si | 21 | Me2SiH |
| Dataset | 1 | RMSE 2 | MAE 2 | R2 | MIN 3 | MAX 4 |
|---|---|---|---|---|---|---|
| Training | 10577 | 0.6 | 0.4 | 0.997 | −3.5 | 3.6 |
| Outliers | 125 | 1.9 (3.3) 6 | 1.6 | 0.986 | −2.5 | 2.6 |
| Test | 156 | 1.0 | 0.7 | 0.995 | −3.4 | 5.0 |
| Model | RMSE | MAE | R2 | MIN 2 | MAX 3 | No C4 |
|---|---|---|---|---|---|---|
| GM26 | 0.9 1 | 0.7 1 | 0.997 | −3.6 | 3.6 | 9 |
| ChemDraw | 3.4 | 2.2 | 0.956 | −17.2 | 27.6 | 256 |
| MestReNova | 1.9 | 1.4 | 0.986 | −10.1 | 9.5 | 103 |
| ACD | 1.8 5 | 1.2 | 0.988 | −8.4 | 8.5 | 95 |
| NMRshiftDB (NN) | 1.1 5 | 0.8 | 0.995 | −4.5 | 4.3 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





