1. Introduction
In the last decades we assisted to a significant improvent Metastatic Breast Cancer (MBC) outcomes. Thanks to advances in diagnostic imaging, in new technologies for local treatments and new target therapies, survival of patients with MBC is slowly but steadily improving [
1] and the risk of death is decreasing by 1%–2% each year [
2]
. Even the guidelines are currently being characterized to define the metastatic patient based on the disease burden and the expected prognosis [
3,
4]
. A recent classification of oligometastatic patients has been published in 2020 by Guckemberger et al., classifing different presentation of oligometastatic disease, based on a dynamic model that can present different therapeutic goals according to tumor active burden, time of revaluation and response to therapies. Alongside this need to classify the oligometastatic patient based on the expected prognosis, an increasingly important role of local therapies is emerging to eradicate the macroscopically visible disease [
5]. For breast cancer, over the years, there has been growing interest in the use of metastasis-directed therapy [
6]. This approach is particularly considered in patients with oligometastatic disease, more indolent immunophenotypes (luminal-like) or in case of a driven-guided therapy (BRCA mutation or HER2 amplification [
7]. An unmet need is also how to manage local therapies in patients who are oligopersistent or oligoprogressive from a previous plurimetastatic disease presentation, that could present intermediate prognosis.
Stereotactic-body radiotherapy (SBRT) has been known as an effective and non-invasive local therapy for ablation of macro-metastatic disease. In a study of 2018 by Possanzini et al. [
6] a review of SBRT administration on MBC cohorts showed that SBRT may play an important role in the management of these patients, potentially improving clinical outcomes with minimal toxicity. Furthermore, from the SABR-comet study, we know that patients with oligometastatic disease can benefit from stereotactic treatments, in terms of overall survival (OS), as well as possible advantages of synergisms with systemics therapies [
8]. Nevertheless, this encouraging data, the choice of dose and volumes in this setting is still not unequivocal. For this reason, we focused on treatments of MBC bone lesions with application of a particular SBRT techniques, with administration of a simultaneous integrated boost (SIB) on bone lesion, concomitant to iraddiation of entire bone compartment. In literature there are few data on safety and efficacy of this technique.
Aim of this study is to investigate on outcomes and toxicity application of SIB-IMRT in MBC. In this study we retrospectally analyzed freedom from local failure and survival outcomes in MBC who underwent SIB-intensity modulated (IM)RT on bone metastases.
2. Material and Methods
2.1. Patients Selection
All the patients addressed to our Department for radiotherapy evaluation, underwent evaluation for SIB-IMRT on bone lesions. Inclusion criteria were MBC hystologically proven (all the patients underwent a biopsy if de novo metastatic, and in case of metastatic relapse they underwent a re-biopsy.), ECOG 0-2, an instrumental re-evaluation with computed tomography (CT) scan or a positron emission tomography (PET)/CT scan +/- magnetic resonance (MRI) in the last month, not more than 5 bone lesions who required RT for symptoms or local control, not previous irradiation on selected site, a minimum follow up of six months.
Exclusion criteria were plurimetastatic patients in progression on multiple sites (>5 sites at last revaluation), more than two contiguous bone compartments to be irradiated, patients candidated to surgery.
2.2. Data Collection
From time to enrollment, patient’s data were collected prospectively. In particular, data on patients’ characteristics (age at diagnosis, ECOG, pain symptoms before and after SIB-IMRT, antalgic therapy before and after SIB-IMRT), breast tumor characteristics (data of diagnosis, immunophenotype, stage at diagnosis, data of first metastasis, tumor bone burden at diagnosis, visceral metastases at diagnosis), data on systemic therapies ongoing (type of systemic therapy ongoing, denosumab administration, number of systemic therapies administered at the moment of SIB-IMRT prescription), data on SIB-IMRT administered (bone district treated, volumes prescribed, dose prescribed, data of SIB-IMRT ending), data on instrumental evaluation (imaging pre- SIB-IMRT, imaging post- SIB-IMRT), data on follow up and outcomes (local response to SIB-IMRT, data of local progression, data of systemic progression, data of last follow up, data of death). Toxicities from SIB-IMRT were reported according Common Terminology Criteria for Adverse Events (CTCAE) v5.0 scale.
2.3. SIB-IMRT Technique Description
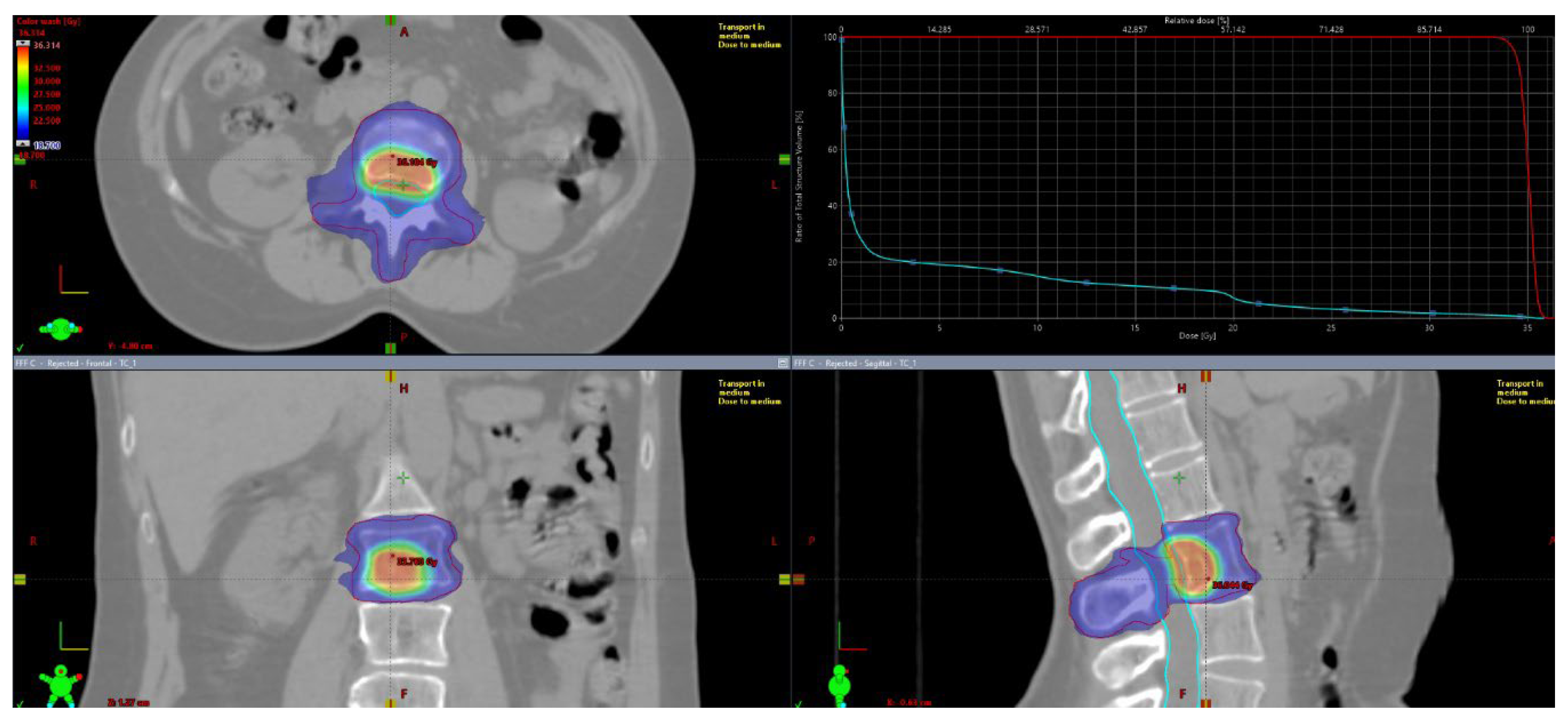
Patients underwent CT simulation with custom immobilization support (Aquaplast® head mask and/or vacuum mattresses). For target delineation, co-registriation with instrumental diagnostic exams (CT with iodine contrast, PET/CT and MRI) was mandatory. Gross tumor volume (GTV) was defined as the visible lesion at diagnostic exams. There was not a dimensional criteria of macroscopic disease visible at instrumental exams used for contouring. “Macroscopic” disease was identified as visible lythic or sclerotic or mixed lesion at II level (CT scan, PET-TC, MRI) used for delineation of planning target volume (PTV)1. Contouring defined two volumes: PTV1, including GTV plus 2-millimeters (mm) margin and PTV2, including entire bone compartment (for long and flat bones in the shoulder or pelvic girdle: the entire bone; for vertebrae: the entire vertebra) plus a 2-mm isotropic margin. For vertebral lesions both spinal canal and spinal cord were contoured. Organ at risks were contoured based on lesion’s site and dose constraints were mantained according to AAPM reports [
8]. The planned doses ranged from 20 to 30 Gy for PTV2 with several fractions from 3 to 10 in according to clinical practice. Three different SIB doses were delivered to PTV1 (35, 40 and 50 Gy). PTV1 coverage was set at 95% of the prescribed dose at 95% of the defined volume. Major deviation for PTV2 was < 77% of the dose prescribed at 95% of the volume, while minor deviation was < 84% of the prescribed dose at 95% of the volume. For PTV1, major deviation was defined as < 79% of the prescribed dose at 95% of the volume, while minor deviation will be < 84% of the prescribed dose at 95% of the volume. Normalization of PTV1 was at 80% of prescribed dose, so inside lesion it was provided also a gradient of heterogeneity since to 120-125% of prescribed dose. Reporting of dose prescription will be done according to International Commission on Radiological Units (ICRU) 83. A representative figure of treatment plan is reported in
Figure 1. Treatment plan reporting was according ICRU 83.
2.4. Endpoints
Primary endpoint of this study was freedom from local progression (FFLP), measured as time from the end of SIB-IMRT RT to first evidence of local progression at radiological (CT scan, PET, MRI) follow up. Patients underwent radiological exams every six months, except in case of clinical suspicious of progression. At radiological exams, local and systemic responses to treatments were classified according to RECIST v1.1 criteria [
9] and/or PERCIST criteria [
10]. Secondary endpoints were rate of disease progression after radiotherapy (DP-AR) and overall survival. DP-AR was measured as time from end of SIB-IMRT RT to first event of disease (local and/or sistemic) progression at instrumental exams. OS was measured as time from data of diagnosis to data of death or data of last follow up. Subgroup analysis (age, immunophenotype, line of therapy) were performed in order to correlate outcomes to possible prognostic factors.
3. Results
From January 2014, among 954 MBC patients who underwent radiotherapy on metastatic lesions, 85 patients underwent SIB-IMRT RT. Patients’ characteristics and breast tumor characteristics are summarized respectively in
Table 1 and
Table 2. Almost one third of patients (26 patients, 30,5%) were metastatic at diagnosis. Sixty-seven patients (78,8%) were on first line systemic therapy, 11 (13%) were on second line and 7 (8,2%) were on subsequent lines. Forty-four patients (52%) were on CDK4/6 inhibitors therapy, 15 (18%) were on anti-HER2 therapy, 11 (13%) were on therapy with citotoxic drugs, 14 (16%) were on endocrine therapy and one (1%) was on PARP inhibitors therapy. Thirty-nine patients (45,8%) had a coadjuvant therapy with denosumab ongoing now of SIB-IMRT administration.
3.1. SIB-IMRT RT Treatments
On 85 patients, a total of 125 lesions underwent SIB-IMRT, according to the following treatment schedules:
A) PTV1 (macroscopic bone lesion(s): 40-30 Gy in 5 fractions (fr); PTV2 (entire bone compartment) 20 Gy in 5 fr.
B) PTV1 (macroscopic bone lesion(s): 30 Gy in 3 fr; PTV2 (entire bone compartment) 21 Gy in 3 fr.
C) PTV1 (macroscopic bone lesion(s): 50 Gy in 10 fr; PTV2 (entire bone compartment) 30 Gy in 10 fr.
Eighty-five lesions were irradiated according to schedule A, 16 lesions according to schedule B and 24 lesions according to schedule C. Among lesions who underwent schedule A, 25 lesions received 40 Gy on PTV1, 47 lesions received 35 Gy on PTV1 and 13 lesions received 30 Gy on PTV1. PTV1 also included multifocal sites of disease visible at instrumental exams in the same bone compartment. Forty-five patients had a basal PET-TC for contouring, 19 had a CT scan with iodine contrast, 15 patients were contoured on MRI imaging, 4 on both PET-TC and MRI and one on both PET-TC and CT with iodine contrast and 1 pt on both MRI imaging and CT with iodine contrast.
Six patients receive treatment on shoulder girdle lesions, 46 on vertebrae, 23 on pelvic girdle, 8 on both vertebrae and pelvic girdle, one on both shoulder girdle and vertebrae and one on both shoulder and pelvic girdle.
3.2. Survival Outcomes
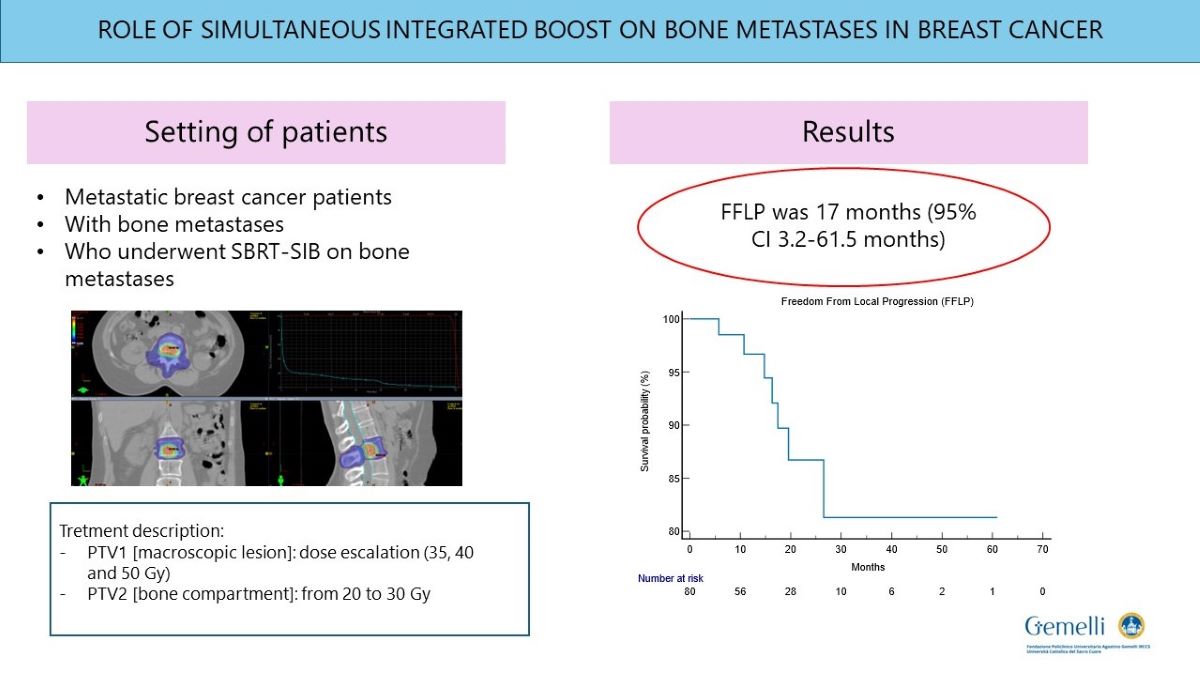
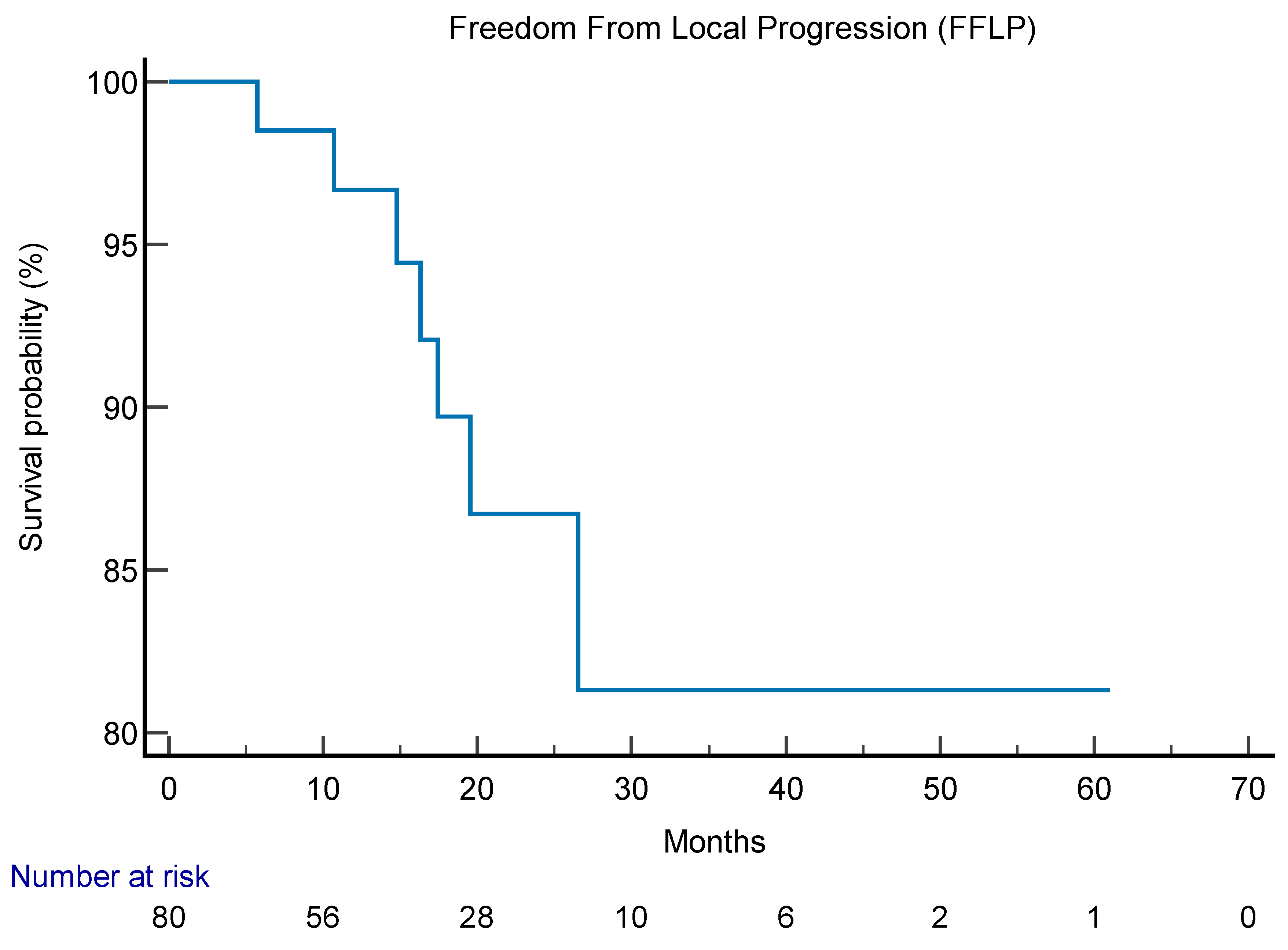
Mean follow up was 41 months (6-61.5 months). At 6 months after SIB-IMRT, complete response was reported in 31 patients, partial response in 19, stable disease in 34 and progression disease in one. Among patients, 53 underwent PET-TC re-evaluation after SIB-IMRT reporting a complete metabolic response in 33 patients, a partial metabolic response in 13, a stable metabolic response in 6 and one progressive metabolic response. FFLP was 17 months (95% CI 3.2-61.5 months) (
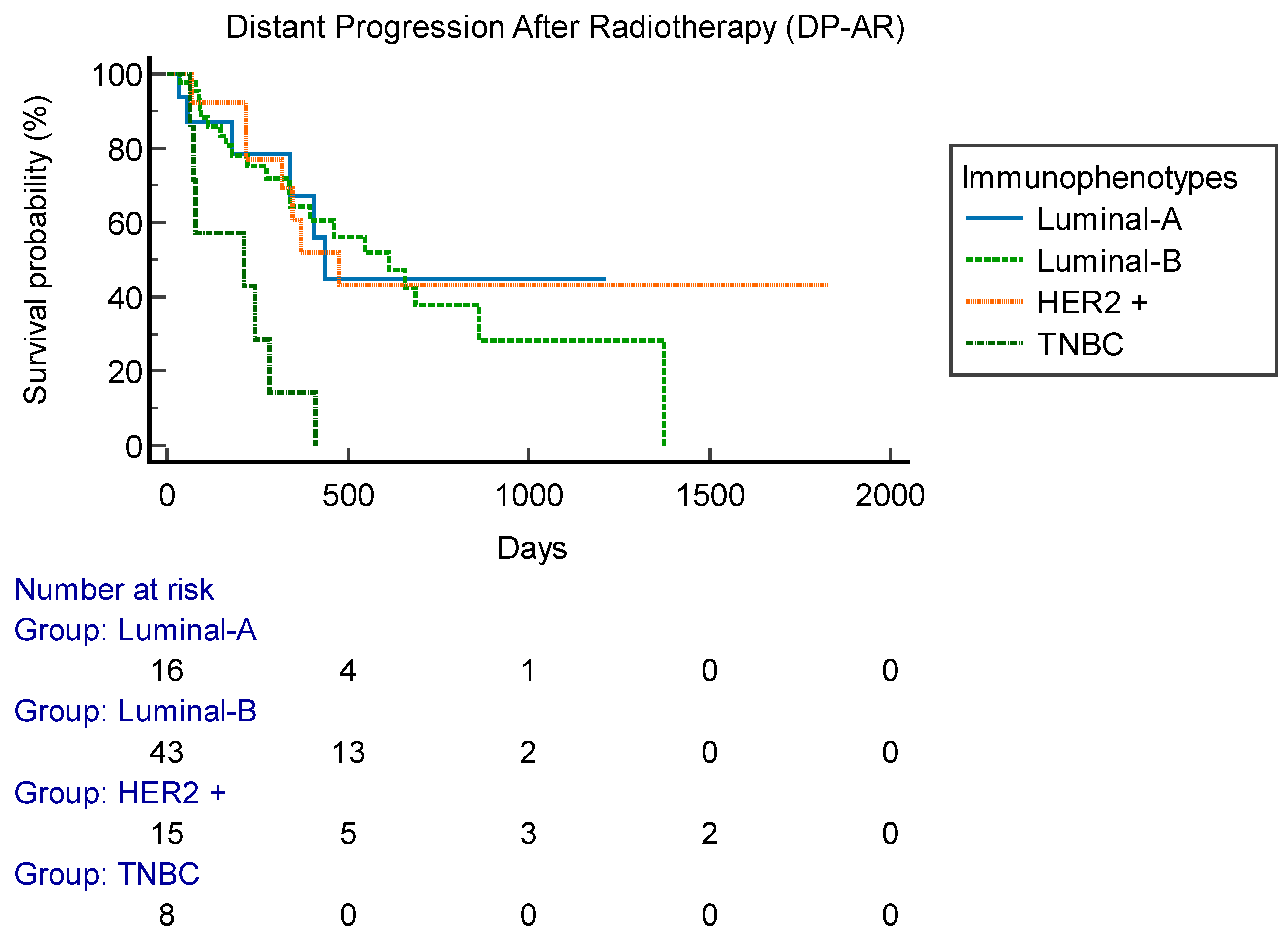
Figure 2). Six patients (7%) had local relapse. FFLP was not related to RT regimen administered (p 0.73). DP-AR was 13,2 months (95% CI 3.1-56.9 months). OS was 82,7 months (95% CI 10.6-343 months). Local-relapse was not associated with age, immunophenotype or sistemic line ongoing. Among secondary outcomes, DP-AR resulted associated to immunophenotype (p 0.002) [
Figure 3]. DP-AR and OS were not significantly associated with local relapse (p 0.148 and p 0.4, respectively). About safety and tolerance, event of pain flare during SIB-IMRT had an incidence of 37% and were managed with steroids administration, while about late toxicity, no events of bone fracture were observed during follow up.
4. Discussion
MBC is a challenging setting of care and thanks to implementation of treatments, we are assisting to a progressive implementation of outcomes in the last years [
2]. In particular, among the spectrum of MBC, oligometastatic breast cancer seems to have better prognosis probably due to its unique molecular signature [
11,
12]and data on SBRT administration in this setting are encouraging in terms of progression-free survival (PFS) [
13,
14,
15,
16,
17]. Limitations of these cohorts are the absence of randomization and usually are mixed cohort with different primary tumors. Less clear data and indications for locoregional therapy are instead available for oligorecurrent or oligoprogressive disease.
In recent decades, clinical trials have been increasingly aimed at responding to an unmet need for ever clearer data on what are the ideal volumes and doses for SBRT treatments on metastatic lesions [
18,
19,
20,
21,
22]. In Marazzi et al. an algorithm for local treatment of bone metastases was proposed to choose dose and volumes for radiotherapy on bone metastases according to prognosis [
7]. According to these literature review, in case of patients with an intermediate-good prognosis, an ablative radiotherapy treatment (Biological Equivalent Dose (BED)>75 Gy) should be proposed to patients.
SIB found a recent application in radiotherapy treatments, in general it was originally applied in H&N and prostate cancer dose escalation [
23,
24,
25]. Some in silico studies in the last years tested feasibility of SIB-IMRT application to bone metastases. In Lee YK et al. IMRT and Volumetric Modulated Arc Therapy (VMAT) techniques were compared for treatment planning and delivery of SIB-IMRT on vertebrae lesions. PTV coverage was equivalent with both tecniques, but VMAT was superior in terms of spinal cord sparing (p 0.04) and of mean delivery times (3,5 vs. 10.5 min), reducing risk of acute and late toxicities and of target missing [
26]. A literature review of clinical data is reported in
Table 3. As reported, in literature very few data on small cohorts (less than 100 patients) are reported. These cohorts included mixed solid tumors but encouraging data on good local control after 2 years from radiotherapy with low rates of toxicities are reported. Ongoing trials that are enrolling patients to test SIB-IMRT on bone metastases are:
- -
NCT02832765 [
27], a single-center, prospective, randomized, controlled trial, to test four treatment arms planned: IMRT with 30 Gy in ten fractions, IMRT with 30 Gy in ten fractions and SIB to 40 Gy, IMRT with 20 Gy in five fractions, and IMRT with 20 Gy in five fractions and SIB to 30Gy in five fractions will be compared. Primary endpoint is local control.
- -
NCT03597984 [
18], a phase 3, open-label, multicentric trial randomized patients to standard conventional radiotherapy involving 4 Gy × 5 fractions (fx) to the whole involved vertebra or SBRT by intensity modulated radiotherapy with simultaneous integrated boost (IMRT-SIB) involving 7 Gy × 3 fx to the whole involved vertebra + 10 Gy × 3 fx on the macroscopic lesion. Primary endpoint is overall pain reduction.
In this study, results on feasibility, tolerance and clinical outcomes of a SIB-IMRT administered on bone lesions from BC was presented. For our knowledge, it is the first such large cohort of patients with MBC treated with SIB-IMRT presented in the literature. All the outcomes in terms of safety and tolerance are in line with data reported in linteratures. Mean FFLP was 17 months, this result is more than how reported by other SIB-IMRT cohorts in which probably are enrolled different type of solid tumor, particularly with the worse prognosis of breast cancers. This study presents some limitation due to retrospective analysis and absence of a control arm. Although this technique is not extensively used at present and there are still not specific guidelines on its application, it has been shown to improve the therapeutic ratio and may improve local control, in addition to shortening the course of radiotherapy [28]. Surely for breast cancer, at the presente time we had the possibility of different systemic therapies, some of them with very long PFS when administered on first line [
4]. From literature, we know that there are also some possible sinergic effect of combining systemic therapies and radiotherapy. For example, pre-clinical data suggests a potential synergy between radiation therapy (RT) and CDK4/6 inhibitors, the addition of RT to palbociclib, have shown to increase in the DNA damage marker, γH2AX and the apoptotic marker [28]. Another interesting mechanism of possible sinergic effect is represented by association of PARP inhibitors and SBRT. In fact, many pre-clinical models have described that the combination of PARP inhibitors and radiotherapy can enhance intensify DNA damage and promote cancer cell death [29]. For these reasons, the possibility to administer a dose-escalating treatment on macroscopic disease, with few numbers of fractions, to enhance sinergic effect with systemic therapies can be considered expecially for patients with low tumor burden and during first and second lines of treatments.
5. Conclusions
Results of this study showed that pts with breast tumor can be treated with SIB-IMRT on bone metastases with a 93% local control, 17 months after treatment. Further data are needed to individuate patients who can benefit of dose-escalation and sinergic effect with systemic therapies to possibly increase DP-AR.
Author Contributions
Conceptualization, FM, VM, MAG and FC; Methodology, FM, VM and FC; Validation, AF, SM, BC, VL, CM, SL, MDA, FM, ADL, AO, SB, GFC, MM, LB, LT, GF, EB, RM, VV, MAG, and FC; Formal analysis, VM; Investigation, FM; Resources, LT and FC; Data curation, VM; Writing – original draft, VM; Writing – review & editing, FC; Supervision, FC; Project administration, FC. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
“The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Catholic University of Sacred Heart (protocol code 0023426/20 of 05/06/2020).”
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
All data generated and analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andre F, Slimane K, Bachelot T; et al. Breast cancer with synchronous metastases: Trends in survival during a 14-year period. J Clin Oncol 2004;22:3302-3308. [CrossRef]
- Giordano SH, Buzdar AU, Smith TL; et al. Is breast cancer survival improving? Cancer 2004; 100:44-52. [CrossRef]
- Guckenberger M, Lievens Y, . Bouma AB; et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol 2020; 21:e18-e28. [CrossRef]
- Pagani O, Senkus E, Wood W; et al. International guidelines for management of metastatic breast cancer: Can metastatic breast cancer be cured? J Natl Cancer Inst 2010; 102:456-463. [CrossRef]
- Sofi AA, Mohamed I, Koumaya M; et al. Local therapy in metastatic breast cancer is associated with improved survival. Am J Ther 2013; 20:487-492. [CrossRef]
- Possanzini M, Greco C. Stereotactic radiotherapy in metastatic breast cancer. Breast 2018; 41:57-66. [CrossRef]
- Marazzi F, Orlandi A, Manfrida S; et al. Diagnosis and Treatment of Bone Metastases in Breast Cancer: Radiotherapy, Local Approach and Systemic Therapy in a Guide for Clinicians. Cancers 2020; 12:2390. [CrossRef]
- Hanna GG, Murray L, Patel R; et al. UK Consensus on Normal Tissue Dose Constraints for Stereotactic Radiotherapy. Clin Oncol (R Coll Radiol); 30:5-14. [CrossRef]
- Eisenhauer EA, Therasse P, Bogaerts J; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228-247. [CrossRef]
- Wahl RL, Jacene H, Kasamon Y; et al. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 2009; 50:122S-150S. [CrossRef]
- Nesbit EG, Donnelly ED, J. Strauss B. Treatment Strategies for Oligometastatic Breast Cancer. Curr Treat Options Oncol 2021; 22: 94. [CrossRef]
- Barberi V, Pietragalla A, Franceschini G; et al. Oligometastatic Breast Cancer: How to Manage It? J Pers Med 2021; 11: 532. [CrossRef]
- Trovò M, Furlan C, Polesel J; et al. Radical radiation therapy for oligometastatic breast cancer: Results of a prospective phase II trial,» Radiother Oncol 2018; 126:177-180. [CrossRef]
- Scorsetti M, Franceschini D, De Rose F; et al. Stereotactic body radiation therapy: A promising chance for oligometastatic breast cancer. Breast 2016; 26:11-17. [CrossRef]
- Gerszten PC, Burton SA, Welch WC; et al. Single-fraction radiosurgery for the treatment of spinal breast metastases. Cancer 2005; 104:2244-2254. [CrossRef]
- Milano MT, Zang H, Metcalfe SK; et al. Oligometastatic breast cancer treated with curative-intent stereotactic body radiation therapy. Breast Cancer Res Treat 2009; 115:601-608. [CrossRef]
- Milano MT, Katz AW, Zhang H; et al. Oligometastatic breast cancer treated with hypofractionated stereotactic radiotherapy: Some patients survive longer than a decade. Radiother Oncol 2019; 131:45-51. [CrossRef]
- Cellini F, Manfrida S, Deodato F; et al. Pain REduction with bone metastases STereotactic radiotherapy (PREST): A phase III randomized multicentric trial. Trials 2019; 20:609. [CrossRef]
- Chmura SJ, Winter KA, Woodward WA; et al. NRG-BR002: A phase IIR/III trial of standard of care systemic therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical resection (SR) for newly oligometastatic breast cancer (NCT02364557). J Clin Oncol 2022; 40:1007-1007.
- Jeong J. Local Treatment in ER-positive/HER2-negative Oligo-metastatic Breast Cancer (CLEAR). 23 November 2018. [Online]. Available: https://clinicaltrials.gov/ct2/show/NCT03750396. [Consulted 15 February 2024].
- Roussy Gustave, Cancer Campus, Grand Paris. Trial of Superiority of Stereotactic Body Radiation Therapy in Patients With Breast Cancer (STEREO-SEIN),» 17 March 2014. [Online]. Available: https://clinicaltrials.gov/ct2/show/NCT02089100. [Consulted 23 July 2023].
- Weill Medical College of Cornell University. CIMER: Combined Immunotherapies in Metastatic ER+ Breast Cancer (CIMER),» 7 January 2020. [Online]. Available: https://clinicaltrials.gov/ct2/show/NCT04220476. [Consulted 20 September 2023].
- Mohan R, Wu Q, Manning M; et al. Radiobiological considerations in the design of fractionation strategies for intensity-modulated radiation therapy of head and neck cancers. Int J Radiat Oncol Biol Phys 2000; 46:619-630. [CrossRef]
- Ost P, Speleers B, De Meerleer G; et al. Volumetric arc therapy and intensity-modulated radiotherapy for primary prostate radiotherapy with simultaneous integrated boost to intraprostatic lesion with 6 and 18 MV: A planning comparison study. Int J Radiat Oncol Biol Phys 2011; 79:920-926. [CrossRef]
- Jolly D, Alahakone D, Meyer J. A RapidArc planning strategy for prostate with simultaneous integrated boost. J Appl Clin Med Phys 2010; 12:3320. [CrossRef]
- Lee YK, Bedford JL, McNair HA; et al. Comparison of deliverable IMRT and VMAT for spine metastases using a simultaneous integrated boost,» Br J Radiol 2013; 86:20120466. [CrossRef]
- Sprave T, Welte SE, Bruckner T; et al. Intensity-modulated radiotherapy with integrated-boost in patients with bone metastasis of the spine: Study protocol for a randomized controlled trial. Trials 2018; 19:59. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).