1. Introduction
The demand for fresh-cut vegetables is increasing, especially in metropolitan cities. They are perishable products with a short shelf life. Physiological decomposition, microbial growth, and sensory deterioration are the main reasons of quality reduction and shelf life shortening. The industry is constantly launching new species and varieties to meet consumer demands for convenience, freshness, safety, and health-promoting values. Increasing consumption of fresh-cut fruits and vegetables is associated with numerous reports of foodborne disease outbreaks [
1,
2,
3,
4,
5]. Therefore, the need to optimize anti-contamination conditions for produce has become a global problem [
6,
7,
8].
Currently, chlorine is the most widely used disinfectant in light processing of horticultural products [
7,
9,
10,
11]; however, the use of synthetic additives to reduce spoilage of fresh-cut vegetables has raised consumer concerns due to chemical residues and their effect on human health. In contrast, the technologies based on natural additives to reduce losses and ensure microbiological safety are widely accepted. Aromatic herbs have been used for centuries for seasoning of dishes because of their distinctive flavor and properties that improve well-being and reduce the risk of developing diseases. Santos et al. [
12] stated that from a global standpoint, the addition of fresh-cut aromatic herbs to vegetable salads seems to be a good direction because of the incremental nutritional value and antimicrobial activity. Most vegetables compose very well with fresh herbs, so they can be considered as added value with culinary seasoning and natural preservative properties. Freshly chopped broccoli, cabbage, carrots, onions, cucumber, and tomatoes can be mixed with aromatic species, such as: thyme, oregano, rosemary, cilantro, ginger, bay, and garlic [
13]. Santos et al. [
12] stated that there were only minor changes in phytonutrient content in fresh-cut herbs, like chives, cilantro, mint, and parsley, over 10 d at 3 °C. In addition, mint had retained its fresh green color, while cilantro and parsley had become slightly discolored, and the chives had turned yellow. The addition of 1.5 % marjoram to a salad containing 37.5 % lettuce and 61 % tomatoes achieved a 4-fold increase in oxygen radical absorbance capacity [
14]. In a study by Scollard et al. [
15], shredded, rosemary was found to have anti-listeria effects, while shredded, fresh thyme and oregano did not show these properties.
The aim of this study was to determine the health-promoting properties, microbiological quality and storage ability of mixes of fresh-cut iceberg lettuce with fresh herbs. An important challenge was to develop a ready-to-eat product with improved nutritional and health properties, as well as high sensory qualities acceptable to consumers. Estimating the storage life of lettuce-herbs mixes was also an important part of this study. Improving the storage life of fresh-cut horticultural products is still a big challenge for food technologists and nutritional researchers.
2. Material and Methods
2.1. Plant Material
The study was conducted on iceberg lettuce (Lactuca sativa L. var. capitata) obtained from a commercial farm near Skierniewice (Poland). The lettuce cv. Ice Wave F1 was grown for summer harvest. The herbs that were used as admixtures to fresh-cut lettuce were cultivated in the experimental field of the National Institute of Horticultural Research in Skierniewice. There were two perennial species, peppermint (Mentia piperita) and oregano (Origanum vulgare), and three annuals, red basil (Ocinum basilicum purpurascens), green basil (Ocimum basilicum L.), and flat-leaf parsley (Petroselinum crispum). Immediately after harvest, the iceberg lettuce and herbs were transported to the storage laboratory and stored at 0–1 °C.
2.2. Setting Up Storage Experiments
Storage experiments were set up one day after the harvesting of lettuce and herbs. The lettuce was transferred from cold storage to room temperature (16–20 °C), and the outer and damaged leaves were removed. Next, the lettuce was cut into strips 0.5–1.0 cm wide. The herbs were also acclimated to room temperature and washed in tap water that was 5 °C warmer than the temperature of the herbs. After washing, the herbs were placed on special sieves to dry and then cut into 2–3 mm strips. Lettuce-herb mixes were prepared separately for each sample, weighing the appropriate amount of cut lettuce and cut herbs. The amount of herbs added to the iceberg lettuce was previously determined by the sensory panel based on a comparison of the taste and aroma of mixes containing 3 % to 10 % herbal additives. Thyme, rosemary, and marjoram were eliminated from these studies due to unacceptable sensory quality (unpleasant taste in the mixture with lettuce). Finally, for the storage experiments, the following lettuce-herb mixtures were prepared:
− mix with red basil: 95 % fresh-cut lettuce and 5 % fresh-cut red basil
− mix with green basil: 97 % fresh-cut lettuce and 3 % fresh-cut green basil
− mix with peppermint: 95 % fresh-cut lettuce and 5 % fresh-cut peppermint
− mix with oregano: 97 % fresh-cut lettuce and 3 % fresh-cut oregano
− mix with leaf parsley (90 %): 90 % fresh-cut lettuce and 10 % fresh-cut leaf parsley
− mix with leaf parsley (95 %): 95 % fresh-cut lettuce and 5 % fresh-cut leaf parsley
− control: 100 % fresh-cut lettuce
The overall weight of each sample was 100 g. The mixes were packed into transparent polypropylene boxes recommended for food products, including fruit, vegetables, and salads. The boxes were 154 mm x 98 mm x 70 mm (L x W x H) with a capacity of 1.0 L (GUILLIN W1/059C). The boxes were covered with lids (GUILLIN W2/001) with 77 punctures for air exchange.
The storage experiment was set up in four replications. Additional samples were prepared for microbiological, chemical, and sensory analyses. All samples were stored at 5 °C. The experiments were repeated three times each.
2.3. Quality Assessment
Quality assessment was performed every two days during the storage up to 6 d. The following vegetable characteristics were visually assessed: wilting/softening, browning/discoloration of cut surface, rotting, and marketable value. The evaluation was conducted on the basis of the 9-grade scoring scale:
− wilting/softening: 1 - no signs of wilting and softening, 3 – light, 5 – medium, 7 – strong, 9 – very strong
− browning/discoloration of the cut surface: 1– no signs of discoloration, 3 – very beginning of discolouration, 5 – light brown, 7 – medium brown, 9 – brown
− rotting: 1 - no signs of rotting, 3 – up to three small spots, 5 – quite strong, 7 – strong, 9 – very strong
− marketable value: 1 – no marketable value, 3 – limited, 5 - fairy (marketability threshold), 7 – good, 9 – excellent.
The visual quality of lettuce and herbs was evaluated separately. The weight loss of whole sample was calculated based on the difference between the initial weight and the weight measured every two days during storage. The difference was divided by the initial weight and expressed as a percentage.
2.4. Chemical Analysis
Chemical analyses were performed on whole samples after 3 d of storage at 5 °C. The chlorophyll and carotenoids were measured by a spectrophotometric method according to Lichtenthaler and Wellburn [
16] with modifications by Sumanta et al. [
17].The plant samples were homogenized with cold 80 % acetone and centrifuged at 8,500 relative centrifugal force (RCF) at 4 °C for 15 min. The supernatant (0.5 mL) was mixed with 4.5 ml of 80 % acetone. The absorbance was measured using a Cary 300 Bio UV-Vis spectrophotometer at the following wavelengths: 646.8 nm for chlorophyll a, 663.2 nm for chlorophyll b, and 470 nm for carotenoids. Total chlorophyll is the sum of chlorophyll a and chlorophyll b. Results were expressed in mg kg
-1 fresh weight of the analyzed sample.
The polyphenol content was measured by a modified spectrophotometric method of Tsao and Yang [
18]. The plant samples were homogenized with 70 % ethanol and centrifuged at 20,000 RCF for 10 min. Diluted phenolic extracts (0.4 mL), were mixed with 1.6 mL of sodium carbonate solution (7.5 %). Then, Folin–Ciocalteu’s phenol reagent (2 mL) was added and the mixture was shaken. After 30 min., the absorbance was read against the prepared blank at 765 nm at an ambient temperature in dark conditions. The polyphenol content was expressed as mg of gallic acid equivalents per mg kg
-1 fresh weight of the analyzed plant material.
The ascorbic acid content was determined by high-performance liquid chromatography Agilent 1200 HPLC system, equipped with a diode array detector (DAD), using two Supelcosil LC-18 columns (250 mm x 4.6 mm; 5 µm) with a precolumn according to IFU (Instructions for Use) [
19] procedures. A phosphate-buffered solution (1 % KH
2PO
4; pH 2.5) was used as the mobile phase. The column temperature was kept at 30 °C with a flow rate of 0.8 mL/min. Detection of ascorbic acid was performed by absorbance at 244 nm wavelength. Samples for acid determinations were dissolved in 6 % HPO
3. The results were expressed as mg kg
-1 fresh weight.
2.5. Sensory Analysis
Sensory analyses were performed on whole samples after 4 d of storage at 5 °C. The evaluation was carried out according to the accredited quantitative descriptive analysis (QDA) method in accordance with the implementation procedure described in ISO 13299:2016 [
20]. The following quality attributes were selected and defined: color, crispness, lettuce flavor, herbal aroma, sweet flavor, overall quality, and consumer acceptance. An unstructured linear scale was used with marked threshold values from 0 to 10 contract units (CU), where 0 meant no intensity and 10 meant high intensity of the attribute. Analsens computer software was used during the evaluations. The evaluations were carried out at the sensory analysis laboratory, which meets all the requirements specified in PN-EN ISO 8589:2010/A1:2014-07 [
21] for sensory analysis laboratories. Sensory characterization of samples was carried out in two independent repetitions by a 10-member evaluation team. The judges have received methodological training in sensory methods and are qualified as expert evaluators in accordance with PN-EN ISO 8586:2014-3 [
22]. Unit samples of the test product were placed in coded plastic containers (250 mL) covered with lids. Non-carbonated water was used between the evaluated samples as a taste neutralizer.
2.6. Microbiological Analysis
Microbiological analyses were conducted on samples of lettuce-herb mixes after 0 and 3 d of storage. The analyses were performed according to standard methodologies: PN-EN ISO 4833-2:2013-12 [
23] and PN-ISO 21527-1:2009 [
24]. Twenty-five grams of analyzed material were transferred into 225 mL of peptone water in sterile stomacher filter bags (400 mL). The samples were homogenized in a stomacher BagMixer
® 400 P with a fixed speed of 8 stroke/s for 10 min. Further, decimal dilutions were made with the same diluent and analyzed for aerobic mesophilic bacteria, yeasts and molds. Mesophilic aerobic bacteria were enumerated on plate count agar (PCA, Merck) after incubation at 30 °C. Yeasts and molds were counted on yeast extract glucose chloramphenicol agar (YGC agar, Merck) after incubation at 25 °C. The results were expressed as colony-forming units per gram of plant material (CFU/g) and transformed for statistical calculations to decimal logarithm (log
10).
2.7. Data Analysis
The experiments were set up in a one factorial design in the study, considering the herbal additive as differentiating factor of the experimental objects. The results were statistically analyzed using one-factor analysis of variance (ANOVA). The obtained mean values were compared using the Tukey HSD (Honestly Significant Difference) procedure at p = 0.05. Sensory characteristics of the evaluated plant material were described using principal component analysis (PCA) based on a correlation matrix. Calculations were carried out in the statistical package STATISTICA 13 (Dell Inc.).
3. Results
3.1. Weight Loss and Storage Ability of Mixes of Fresh-Cut Iceberg Lettuce with Fresh-Cut Herbs
Weight losses of the lettuce-herb mixes were very low. After 2 d, it ranged from 0.01 % to 0.04 %, after 4 d from 0.04 % to 0.12 %, and after 6 d from 0.06 % to 0.16 %. The differences after 2 and 4 d were statistically insignificant. After 6 d, the statistically lowest weight loss was found for the mix of lettuce with red basil (0.06 %), while the highest was found for the mix with oregano (0.16 %).
During 6-d storage, no signs of wilting or rotting were observed on either lettuce or herbs. The decrease in the marketable value of lettuce was due to browning of the cutting surface. After the first two days, the lettuce presented as high quality (
Table 1). However, significantly greater browning and the lowest marketable value was estimated for control lettuce, while the lowest browning and highest marketable value was found for the lettuce mixed with oregano. Extending the storage to 4 d, the greatest browning was found for lettuce mixed with green basil, which also translated into the lowest quality of this object, albeit not statistically significant. Over the next two days, browning of the cut surface in all objects increased, reaching an almost light brown color, and the marketable value dropped close to the limit of commercial suitability. In the last days of storage, the quality of lettuce from all mixes reached statistically equal marks for both browning and marketable value.
Discoloration was also the main reason for the decrease in quality of the fresh-cut herbs (
Table 2). There were significant differences among the tested species in susceptibility to discoloration during storage. Green basil and oregano already showed clear discoloration after two days, lowering the quality from excellent to good. These two herbs stood out from the others with the greatest discoloration and the lowest marketable value throughout the entire storage period. The specifics of these discolorations varied because green basil discolored all over, while oregano only discolored on the cut edges. The darkening of the cut surface of the peppermint contributed to lowering its quality to above good after 4 d and to below good after 6 d. Fresh-cut red basil and parsley showed the best appearance during storage. These herbs showed no discoloration and maintained excellent quality up to 4 d. After another two days, the quality of the red basil continued to be excellent, while the freshly cut parsley showed very slight signs of yellowing. After 6 d of storage at 5 °C, the freshly cut herbs in mixes with iceberg lettuce presented with very different quality: red basil was excellent, parsley was very good, and peppermint was between good and fair, while green basil and oregano were fair.
It turned out that red basil, peppermint and parsley retained better quality than lettuce during short-term storage, while green basil and oregano maintained worse quality than lettuce (Tables 1, 2). Despite the small addition of herbs, they were clearly visible in the mixes, and especially red basil and parsley throughout the storage period added to the visual attractiveness of the obtained product.
Values are means of 12 samples ± standard deviation (SD). Means followed by the different letter within columns are significantly different (p<0.05, Tukey test). For browning “a” means the lowest intensity and further letters of the alphabet - higher intensity. For marketable value “a” means the lowest score and further letters of the alphabet - higher score.
Scoring scales for plant material quality evaluation: browning of the cut surface: 1– no signs of discoloration, 3 – very beginning of discolouration, 5 – light brown, 7 – medium brown, 9 – brown; marketable value: 1 – no marketable value, 3 – limited, 5 – fairy (marketability threshold), 7 – good, 9 – excellent
Values are means of 12 samples ± standard deviation (SD). Means followed by the different letter within columns are significantly different (p<0.05, Tukey test). For discoloration “a” means the lowest intensity and further letters of the alphabet - higher intensity. For marketable value “a” means the lowest score and further letters of the alphabet - higher score.
Scoring scales for plant material quality evaluation: discoloration: 1– no signs of discoloration, 3 – very beginning of discolouration, 5 – light brown, 7 – medium brown, 9 – brown; marketable value: 1 – no marketable value, 3 – limited, 5 – fairy (marketability threshold), 7 – good, 9 – excellent
3.2. Effect of Herbal Additives on the Chemical Composition of Mixes with Iceberg Lettuce
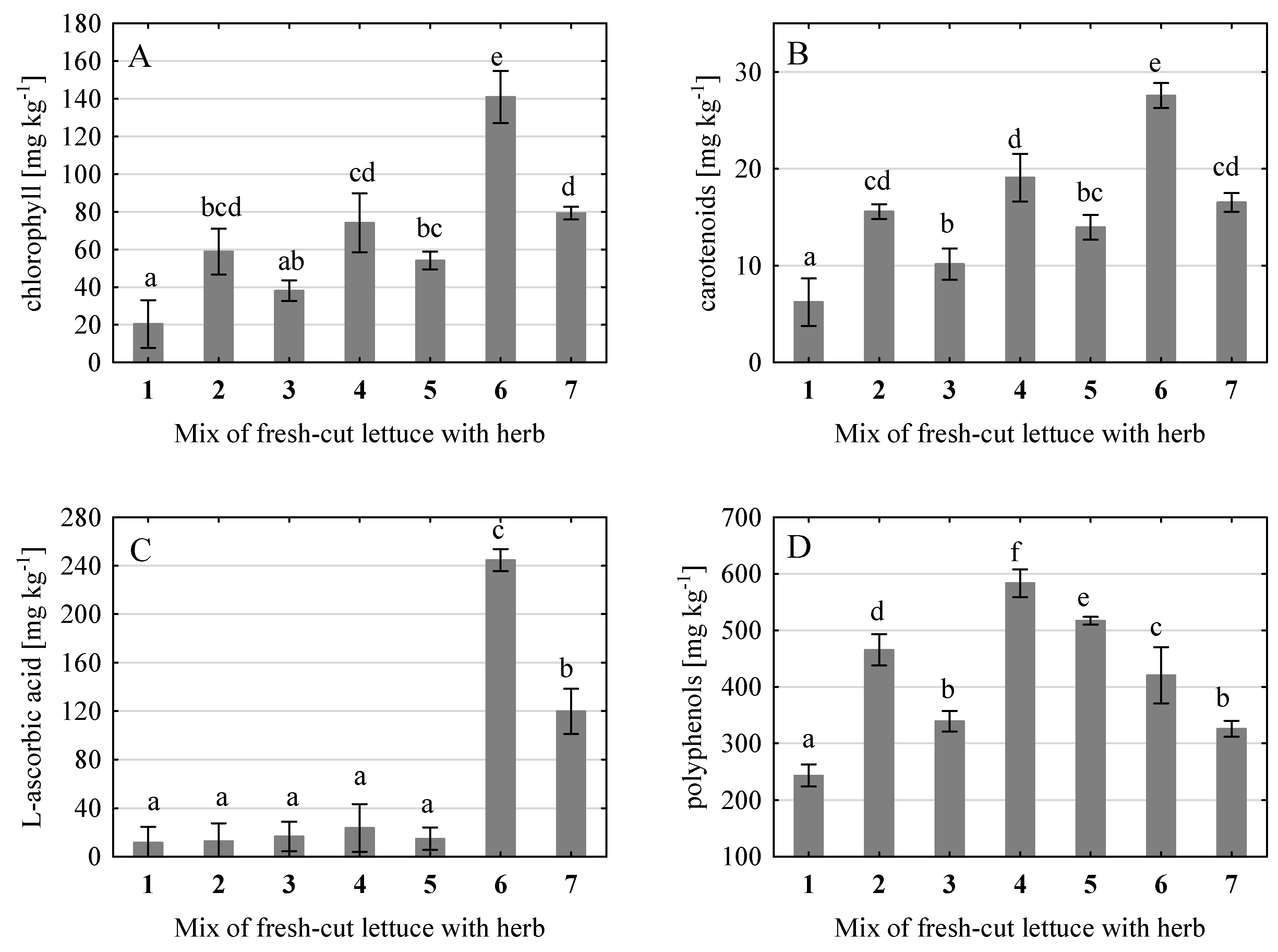
The small addition of herbs to the fresh-cut lettuce had a very significant effect on improving the health-promoting properties of the created salads (
Figure 1). The increase in chlorophyll content depended on the admixed herb and its proportion in the mix (
Figure 1A). The smallest increase of almost 86 % (not statistically significant compared to control) was found in the mix with green basil (3 % share). In the other mixes, the increase was statistically significant. Mixes with 5 % added herbs (red basil, peppermint, and leaf parsley) had 3–4-fold more chlorophyll than the control samples. The mixture with 10 % of leaf parsley contained the most chlorophyll - 7-fold higher than the control lettuce. A very similar trend regarding the effect of herbs on chlorophyll content was also found for carotenoids (
Figure 1B). Comparing the experimental objects, the 3 % portion of green basil contributed the least to the increase in carotenoid content in mixes with iceberg lettuce, although the increase was 65 % and was statistically significant. Oregano, also applied at 3 %, contributed to an increase of 126 %. Higher growth of carotenoids was found in mixes with 5 % herbs, and the highest in the mix with 10 % of leaf parsley (3.5-fold).
Green and red basil, like peppermint and oregano, appeared to be poor in ascorbic acid, and their addition to fresh-cut lettuce did not affect the content of this bioactive compound (
Figure 1C). Leaf parsley greatly enriched the created mixes in vitamin C. The addition of 5 % resulted in about a 10.5-fold increase, and the addition of 10 % resulted in an approximately 21-fold increase.
The polyphenol content of lettuce-herb mixes was significantly higher than in lettuce alone (
Figure 1D). The mix with peppermint added at 5 % was the richest in polyphenols, while the mix with oregano (3 %) was in second place. In the case of the addition of green basil (3 %) and parsley (5 %), the increase in polyphenols in salads was the smallest in comparison with other mixes (about 34 %).
3.3. Sensory Evaluation of Mixes of Fresh-Cut Lettuce with Fresh Herbs
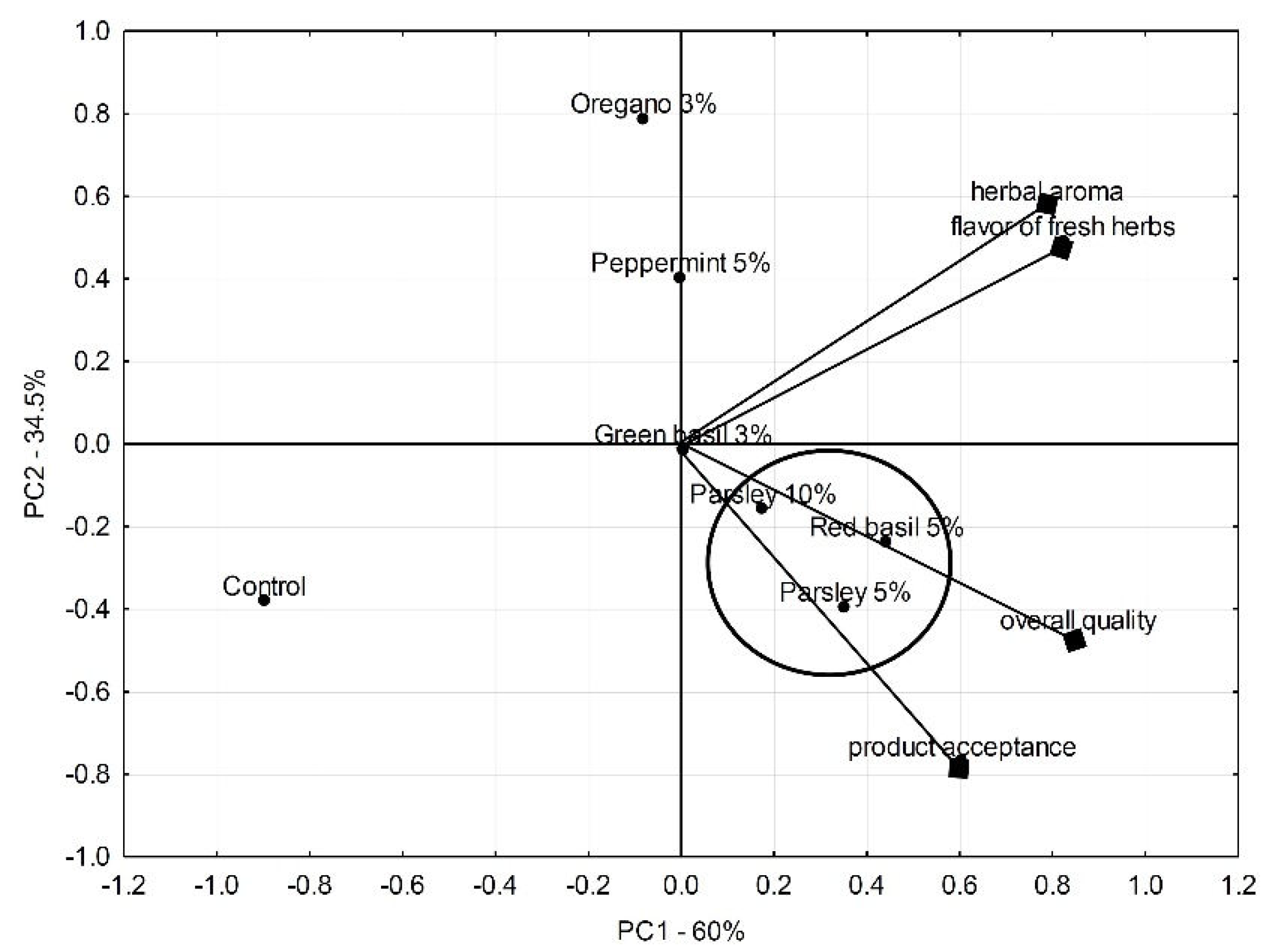
The sensory quality of the evaluated lettuce with fresh herbs is shown in
Figure 2. The map space was defined by the first two principal components, explaining 60 % and 34.5% of the overall variation, respectively. Overall quality was positively related to the product acceptance score, while the herbal aroma was correlated with the flavor of fresh herbs (vectors aligned in the same direction and in close proximity). The mixes of lettuce with parsley and red basil were in the closest position in the vectors of overall quality and product acceptability ratings, which indicates their high sensory scores. Comparing salads with parsley, higher acceptability was obtained when the admixture was 5 % rather than 10 %. The lettuce mixed with oregano was at the furthest distance and the opposite side of the vectors of overall quality and consumer acceptability, indicating its low sensory score.
3.4. Microbiological Assessment of Fresh-Cut Iceberg Lettuce with Additives of Fresh Herbs
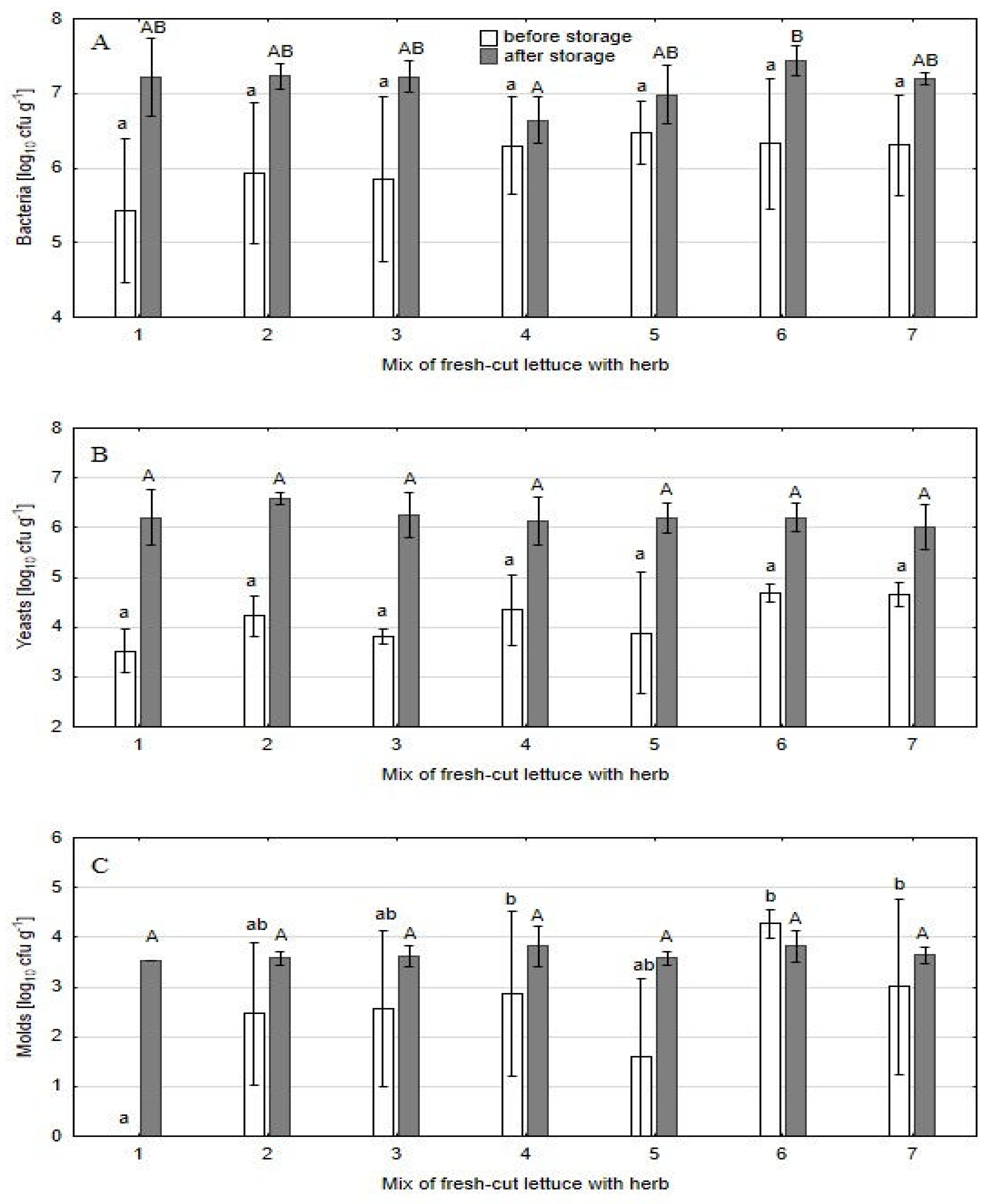
The addition of herbs to fresh-cut lettuce enhanced the microbial load in the mixes compared to control lettuce samples; however, the differences were significant only in the case of molds (
Figure 3). The highest contamination with molds was noted for the parsley additive, especially when it was used in a dose of 10 % (4.3 log
10 cfu g
-1) (
Figure 3 C). The lowest contamination of the mix was noted with the addition of oregano (1.6 log
10 log
10 cfu g
-1).
After three days of storage at 5 °C, the densities of microorganisms, estimated in lettuce without additives and in lettuce mixed with herbs, were comparable and reached an average of 7.1 log
10 cfu g
-1 for bacteria, 6.2 log
10 cfu g
-1 for yeasts, and 3.7 log
10 cfu g
-1 for molds (
Figure 3). The differences between salads were not significant, except for peppermint’s effect on bacteria. The addition of this herbal plant to lettuce lowered microbial growth rate during storage. This was particularly evident in the case of mold, as the growth in the control object was increased 3.52 log
10 cfu g
-1, while in the mixtures it ranged from -0.45 log
10 cfu g
-1 to 1.99 log
10 cfu g
-1 (
Figure 3C). Parsley inhibited molds growth to the greatest extent. In combination with 10 % parsley, there was the decrease of 0.46 log
10 cfu g
-1 compared to initial molds number estimated in the mix, while with 5 % parsley, the increase was only 0.64 log
10 cfu g
-1. Trends toward slower bacterial and yeast growth were also noted (although statistically unproven) (
Figure 3 A, B). Bacterial growth over 3 d of storage in the control object was 1.79 log
10 cfu g
-1, while in the mixes it was 0.34–1.37 log
10 cfu g
-1. Yeast growth in the control was 2.68 log
10 cfu g
-1, while in the mixes it was 1.35–2.43 log
10 cfu g
-1.
4. Discussion
The launch of mixes of fresh-cut vegetables with fresh herbs is in line with the market trend of expanding ready-to-eat products. Among minimally processed vegetables and fruits (MPVF), lettuces and other leafy vegetables occupy a prominent place as important ingredients of salad. The lettuce-herb mixes meet the requirements for convenience food, which is characterized by increased health-promoting properties compared to lettuce alone. This coincides with dietary recommendations and is becoming increasingly important in the era of fast-food bars and the growing problem of the overweight population. Mixes are still a perishable product due to rapid spoilage, but in the current study, the main ingredient, which was fresh-cut iceberg lettuce, showed slightly less discoloration of the cutting surface at the very beginning of storage compared to lettuce without the herb additive. This proved the positive effect of herbs on inhibiting physiological changes in lettuce. This effect was attributed to the action of volatile substances secreted by the herbs. Similar to the results obtained by Lopresti and Tomkins [
25] and Cantwell and Reid [
26] the herbs differed in their shelf life during storage. The riskiest is the use of green basil and oregano due to their tendency to discolor quickly. The green basil darkening mostly occurred due to the susceptibility to chilling injury (CI). A temperature of 5 °C was too low for this species, as it is a plant of tropical origin and exposure to temperatures below 12 °C create brown spots and black necrosis on the leaves [
27] In the case of oregano, one of the reasons for discoloration can be chilling sensibility, although Cantwell and Reid [
28] suggest that leaf discoloration only occurs in plant material cultivated under a cool climate. Another reason may be relatively high ethylene production, which can eventually lead to aging, senescence, and tissue browning. A distinctly different behavior was exhibited by red basil, which maintained its proper color, gloss, and fresh appearance during short-term storage. This was indicative of resistance to low-temperature stress, which was most likely due to high anthocyanin content. In the study of Baczek-Kwinta et al. [
29] red basil “Rubin” contained 10-fold more anthocyanins than green-leaf plants, and this may be a stress protection factor. Parsley showed relatively good shelf life, with a slight decline in quality at the end of storage due to yellowing. Cantwell and Reid [
28] also reported a decrease in chlorophyll content in parsley leaves during storage. The above authors emphasize the poorer shelf life of mint than parsley, which also appeared in the current study.
Very low weight loss showed that both lettuce and herbs maintained good firmness, and there was no shrinkage of leaf tissue. The plastic boxes used to package lettuce with herbs protected the plant material from moisture loss.
Including culinary herbs in meals is a big nutritional advantage, considering the metabolites with health-promoting properties. Santos et al. [
12] also showed high levels of health-promoting components in fresh-cut chives, cilantro, mint, and parsley. As in the current study, cut parsley was found to be particularly rich in vitamin C and peppermint was found to be rich in polyphenols, while the carotene content of both herbs was similar. The assertion by Martinez-Gracia et al. [
30], that small amounts of herbs and spices do not contribute to the food, has not been confirmed. The results of our study showed that the addition of herbs at a dose of 3 % – 10 % considerably enriched the salads in vitamins and other important nutrients. Vitamin C is a stimulator of immune system activity in the human body [
31]. Carotenoids contribute to increased protection of the skin from UV radiation. They also protect against diabetes, cancer, and inflammatory diseases [
32]. Polyphenols have the ability to prevent lipid overproduction, liver dysfunction, inflammation, infections, and cancer [
33].
The addition of fresh-cut culinary herbs to fresh vegetables increases the variety of flavors and expands the market offering in the ready-to-eat salad department [
30] Toivonen and Brummell [
34] have paid attention to an important feature, which is the sensory quality of the products on the market. In the current study, the mixes of iceberg lettuce with the addition of leaf parsley and red basil in the amounts of 5 % – 10 % and 5 %, respectively, gained high sensory evaluation, so they should be accepted by consumers. According to Pollack [
35], fresh-cut vegetables are generally used as an accompaniment to other dishes or as ready-to-eat salads with dressing, so the addition of fresh herbs can further enhance the flavor of the meal.
In our study, the addition of herbs increased the microbial load in the mixes. However, in the case of bacteria and yeasts the increase was not significant compared to control. The strongest increase after herbs application was observed for molds. Kowalska and Szczech [
36] reported that among the leafy green vegetables (rocket, spinach, lamb’s lettuce, iceberg lettuce, celery, chive, dill, and parsley) the most contaminated vegetables with molds were parsley and rocket, but the least contaminated was iceberg lettuce. This was related to the structure of the plants, as the inner leaves in lettuce heads are less likely to be colonized by microorganisms [
37] than the leaves of plants with a spreading, open habit (like parsley and other herbs). Straight after mixing, the bacteria counts in salads did not excided the value of 10
7 cfu g
-1, which is in line with the results previously obtained for fresh leafy greens [
38,
39,
40]. During storage the number of microorganisms has risen in all objects, what is usually observed in minimally processed leafy products. Jackson et al. [
41] have found that total cultured bacteria in lettuce and spinach, stored in modified chilled atmosphere increased to 5.5 x 10
8 cfu g
-1. Kowalska and Szczech [
36] reported that in leafy vegetables sold in supermarkets, the mesophilic bacteria was the dominant group in all products and reached values as high as 8.4 log
10 cfu g
-1, and for iceberg lettuce the bacterial load ranged from 2.08 to 7.16 log
10 cfu g
-1. Similarly Sun et al. [
42] have found that after storage for 3 days the number of bacteria in lettuce increased more than 5 log
10 cfu g
-1. The other dominant group of microorganisms were yeasts. According to Kowalska and Szczech [
36] their number in iceberg lettuce ranged from 3.4 to 4.0 log
10 cfu g
-1, while in other leafy greens the value was higher – about 6.0 log
10 cfu g
-1, and these findings are in accordance with presented data. In the case of molds, their number increased during storage especially in control lettuce. In the mixes with herbs the increase in molds numbers was markedly less intensive than in lettuce alone. It is also worth noting, that during storage, there was a general tendency for a greater increase in the number of microorganisms in lettuce alone than in mixes with herbs. This indicates a slowdown in the growth of microorganisms during storage with fresh herbs. Thus, the reports of Holley and Patel [
43] about the antimicrobial effect of herbs were confirmed.
5. Conclusions
Iceberg lettuce is a very popular vegetable around the world, and there is a high demand for both whole heads and fresh-cut versions. As a minimally processed food, it should meet the requirements of convenience, sanitary safety, nutrition, and good taste and smell. The addition of fresh herbs significantly improves the health-promoting properties of the new mixes. Depending on the herb, the contents of chlorophyll, carotenes, vitamin C, and polyphenols were even several times higher in the mixes than in the lettuce itself. Of the tested herbs (peppermint, oregano, green basil, red basil, and leaf parsley), red basil and parsley proved to be the best additions in terms of sensory and storage qualities. It is also important, that addition of herbs does not significantly raise the contamination of lettuce-herb mixes by bacteria and yeasts, and the microbial load did not excided the level typical for marketable products. Moreover the growth rate of molds during storage of the mixes, especially with parsley, was lower than in control lettuce samples. This shows the great potential for launching a new, valuable, ready-to-eat product - lettuce mixes with herbs.
Author Contributions
Conceptualization, M.G.; investigation, M.G., M.S., B.K., A.W., M.M-F.; methodology, M.G., M.S., B.K., A.W., M.M-F.; writing original draft, M.G.; resources, T.S.; writing-review and editing, M.G. All authors have read and agreed to published version of the manuscript.