1. Introduction
Today, a matter of great importance is reducing the impact of sewage sludge on the environment. Due to its content in organic matter and nutrients, the stabilized sludge, also called biosolids, has a high potential for fertilization [
1]. Biosolids containing organic matter and nutrients are organic solids obtained by digestion and stabilization of raw sewage sludge [
2]. If the content of nutrients in biosolids is low, this means that, for production of high-performance organo-mineral fertilizers (OMF), it is necessary, in addition to biosolids, to introduce in their manufacturing formula, fertilizers and mineral compounds. This can be done into a thermo-mechanical process by reactive extrusion, which take place into a reactor extruder with co-rotating screws. That ensures, a very good mixing at the molecular level [
2]. Biosolids used in agriculture resulting from wastewater treatment must have a reduced concentrations of pathogens and toxic chemicals below a certain levels, only then, can be safety used as fertilizers without harming the health of plants, soil and groundwater, and thereby, the health of consumers of agricultural or animal products [
3].
The agronomic performance of a bio-based phosphate (P), fertilizer produced from mineral phosphates (TSP and Gafsa rock phosphate) and filter cake (FC) in two distinct soils such as clayey (CLY) and sandy loam (SDY) different in their particle sizes, was recently studied by Borges and co. [
4]. They uses also biological tests to determine the performance of the organo-mineral fertilizers for sugarcane production versus commercial P sources. In highly weathered agricultural soils the crop production is positively related to P fertilization [
5]. Thus, an insufficient P supply will limits the economically acceptable yields will affect the inputs of other nutrients, in particular the nitrogen (N), that cannot longer be used at his potential, but moreover, will also threatens the food security for the world population [
6]. Organo-mineral fertilizers based on biosolids (OMF-BS) are designed to reduce fertilizer inputs, but in the same time may lead to excessive accumulation of P in soil. The agricultural benefits of biosolids, de-watered sewage sludge and treated to acceptable for agricultural use standard, are widely recognized [
7]. It was shown that BS contained 4.5 % total N was insufficient to meet the crop demands [
8]. In order to meet crop nitrogen (N) demand, urea can be added to BS, producing in this way an organo-mineral fertilizer (OMF). In this way the BS fertilizers provides a slow-release nutrient supply to crops [
9], while the organo-mineral fertilizers provides the additional readily available nutrients from mineral sources [
7]. Therefore, the knowledge of N and P content from organo-mineral fertilizers and the degree of release in situ conditions is an essential information.
Fourier Transform Infrared Spectroscopy (FT-IR) was used, to provide a quick, efficient and relatively inexpensive method for identifying and quantifying of Nitrogen, Phosphorus and Potassium mineral and bio-mineral fertilizers. Honkeldieva and co. identify, by the presence of functional groups in contents of solid fertilizers, that the most used in agriculture are namely: CON2H4, (NH4)2HPO4, MgSO4·7H2O, KCl and KH2PO4 [
10]. Ciampa and co. have combined the magnetic resonance imaging (MRI) and high resolution spectroscopy to study the fertilization effects, mineral (M) and organo-mineral (OM), on metabolome, morphology and yeast community of wine grape berries [
11]. Vinci and co. proposed to replace the mineral phosphorus fertilizers and the new fertilizer based on combined effects of Trichoderma harzianum and compost on Zea mays. They have characterized the new formula by the use of 2D high field 1H NMR and GC-MS metabolomics [
12]. To our knowledge, there are not low field
1H NMR relaxometry or double-quantum study for the characterization of organo-mineral fertilizers based on biosolids (OMF-BS).
The aim of the present study is to assess the efficiency of new methods for characterizing biomaterials such as biosolids obtained by extrusion and used as organo-mineral fertilizers. For that three types of organo-mineral biosolids used as fertilizers are produced and characterized by advanced 1H NMR methods (T2- and T1-distributions and DQ build-up curves) and by FT-IR spectroscopy. Classical methods such as pH, electrical conductivity (EC), totally dissolved solids (TDS), and turbidity are also used. The VIS-nearIR spectroscopy is used for the quantitatively evaluation of nitrogen (N) and phosphorus (P) content for the produced three versions (labeled with V1, V2 and V3) of organo-mineral fertilizers. The measurement of the degree of release of fertilizers will be made by systematically recording the variation in time of electrical conductivity (EC) and pH in solutions with a neutral pH of 200 ml of distilled water and 250 mg fertilizer and the release rate and amount of fertilizer release is evaluated. Finally, the experimental data are discussed in terms of specific components (mainly of mono-ammonium phosphate, mineral fertilizers, hydrolyzed protein, starch, urea, etc.) used in the production of V1, V2 and V3 organo-mineral fertilizers based on biosolids.
2. Materials and Methods
2.1. Materials
The studied organo-mineral fertilizers based on biosolids were produced locally. In the used formulas some components have only a fertilizer role and others, that are absolutely necessary to ensure the matrix that makes possible the processing by reactive extrusion, in addition also have a fertilizer role. The biosolid resulting from composting of sludge from wastewater is an essential component that provides organic matter and partially macro and micronutrients. The biosolid used in the formula was purchased from SEdC Mioveni, Romania. The organic matter content is approximately twice the organic carbon content, therefore the organic matter content in the biosolid is approx. 43 %. This percentage of organic matter, due to their initial structure or due to application of large amounts of mineral fertilizers is very important for the regeneration of soils, especially those poor in this organic matter [
1,
2].
The components introduced in the three versions of formula (V1, V2 and V3) have been starch, protein hydrolysate and molasses. The commercial corn starch was obtained from SC Roquette SA Calafat with a moisture content of 12.01 % and a density of 0.561 g/cm3. Protein hydrolysate was obtained at Cluj Napoca Branch of INMA Bucuresti from a mixture of potassium hydroxide, wool waste and water. This contains 10 – 13 % of proteins, polypeptides and amino acids. Molasses is from sugar beet and was obtained from sugar factory Tereos România S.A. Luduş and contain 1.1 % nitrogen and 3.5 % potassium. The exact contains of these three version are largely presented in references [
1,
2] and therefore not reproduced here.
2.2. The Technological Process
The process of production of the organo-mineral fertilizer based on granular biosolid involves several steps. The first step is the preparation of raw materials consisting of grouping and mixing them into categories: i) mixture of solid components (dry biosolid, monoammonium phosphate/mineral fertilizer NP 20:20, potassium nitrate and starch) and ii) mixture of liquid components (protein hydrolysate in which urea, molasses and microelements in the form of sulphate are dissolved). In the next step these two categories of mixtures supply the extruder via a peristaltic pump of type SP 311/6, manufactured by VELP Scientifica, for liquid dosing which can achieve a flow ranging from 6 to 35 ml/min, and via a feeder for powdered materials. The extrusion was carried out at a temperature in the range 30 – 100 °C and a pressure of 16 bar, with a feed rate of 4.5 kg/h of solids and 0.5 kg/h of liquid components. The granulation was made in a hammer mill and the drying was carried out in an air recirculation oven [
1,
2].
2.3. 1H NMR Relaxometry and Double-Quantum Measurements
The
1H NMR relaxometry and diffusometry measurement were performed using a Bruker Minispec MQ 20 spectrometer working at 19.69 MHz. The NMR
T2 relaxation data were recorded using the classical CPMG pulse sequence [
13,
14,
15] with echo time TE = 70 μs. The repetition time (recycle delay–RD) was set at 0.5 s and a number of 2000 echoes were registered. A number of 256 scans were acquired. The experimental data were processed using a fast inverse Laplace-like transform (ILT) algorithm, developed to analyze multi-exponential decay curves [
16,
17]. The NMR
T1 relaxation data were recorded using the classical saturation recovery pulse sequence [
18]. The repetition time (recycle delay–RD) was set at 0.05 s, numbers of 32 scans were recorded and the recovery time was set between 1 ms and 5000 ms. The DQ five-pulse sequence used to record the build-up curves was presented in many publications [
19,
20]. The tipping pulse length was 8.6 µs and the recycle delay was set 0.5 s. The excitation/evolution period is denoted by and was increased in equal steps up to 5.64 ms. The z-filter tz and the evolution period t0 were kept short of the order of 50 and 20 µs, respectively. The double-quantum (DQ) filtered signals were normalized to the integral intensity of the single-quantum (SQ) signal measured in the same conditions as DQ build-up curves.
2.4. 1H and 13C MASS NMR Spectroscopy
High field measurements were performed at Bruker ULTRASHIELD 600 PLUS NMR spectrometer working at a (hydrogen) frequency of 600.132 MHz, under magic angle sample spinning (MASS) at 20 kHz for 1H and 10 kHz for 13C. For the 1H measurements a number of 1024 points were acquired with a dwell time of 7.6 μs ensuring a spectral width of ~65.79 kHz. The spinning sidebands were excluded thus the 1H NMR spectra were plotted over a range of 45 ppm. A number of 16 scans were accumulated with a recycle delay of 10 s. For the 13C measurements a number of 1024 points were acquired with a dwell time of 7.5 μs ensuring a spectral width of ~ 66.67 kHz (441.75 ppm) at a frequency of 150.916 MHz. The spinning sidebands were also excluded thus the 13C NMR spectra were plotted over a range of 400 ppm. A number of 19589 scans were accumulated with a recycle delay of 30 s. Thus, each measurement took almost a week.
2.5. FT-IR Spectroscopy
The FT-IR spectra were measured using a Jasco 6200 FT-IR spectrometer. For our solid organo-mineral fertilizers based on biosolids, 10-15 mg of samples was mixed with 200 mg of KBr powder in an agate mortar. Then, the resulted powder was transferred into a mold and pressed at 15 mt (metric tons) resulting a 10 mm tablet which can be place in to a designated holder inside FT-IR spectrometer. For background we used a simplest KBr tablet. The spectrometer parameters were set as: the wavenumber
was fixed between 349.053 cm
-1 and 4000.6 cm
-1, a number of 64 scans were accumulated in order to increase the signal to noise ratio (SNR), the resolution was 4 cm
-1, a zero filling followed by a cosine apodization procedure was performed, the auto filter was 10 kHz; and the scanning speed was 2 mm/sec [
21].
2.6. X-Ray Diffraction
X-Ray Diffraction (XRD) is used in the study of solid- crystalline, amorphous and polymeric materials. The powder diffraction was used to obtain information about the crystalline compounds in the analyzed samples. XRD-6000 SHIMADZU diffractometer was used for the XRD analysis performed at room temperature, with a graphite monochromator for the Cu-Kα radiation (λ = 1.54056 Å), at 40 kV and 30 mA, with a step of 0.02° and a speed of 2°/ minute.
2.7. SEM-EDX Analysis
The morphology of bio-solid fertilizer surface was examined using a scanning electron microscope (SEM) JEOL JSM 5600LV. Images were captured at an accelerating voltage of 15 kV, utilizing the secondary electron signal. Before analysis, the samples underwent gold coating via a sputtering device. The analysis focused on examining the sample's morphology, with particular attention to surface details. To evaluate the chemical composition, the Energy dispersive X-ray spectrometry (EDX) method was used. The Energy spectrums were recorded using the UltimMAX65 (Oxford Instruments) detector coupled with the SEM equipment.
2.8. Physical-Chemical Characterization
For the measurement of the physical-chemical properties a Hanna Edge multi-parameter device vas used to measure the pH and electric conductivity (EC). For dynamic measurement of release amount of the three fertilizers, 200 ml of distillated water Hanna Edge multi-parameter was set to read the measured pH and EC with a ratio of 1 minute, up to 120 minutes. In order to quantitatively assess the N and P content Hanna instruments HI3896N-0 Nitrogen Reagent and HI3896P-0 Phosphorus Reagent were used in contact with 2.5 ml of distillated water in which the three bio-solids fertilizer samples were dissolved. The samples were placed into a UV-VIS cuvette and a Pasco VIS-nearIR spectrometer was used for a quantitatively assessment of N and P content.
3. Results and Discussions
3.1. 1H NMR T1 Distributions
Nuclear magnetic resonance (NMR) is a powerful technique for characterizing materials, especially those of organic origin. Our formulas from organic biosolid fertilizer based on organic solids, fit very well in this category. One of NMR method uses the saturation recovery pulses sequence, to determine the distributions of spin-network (longitudinal) relaxation times, T1. Unlike many other characterization methods, NMR can give us distributions of the measured parameters and not just a value that characterizes the samples globally.
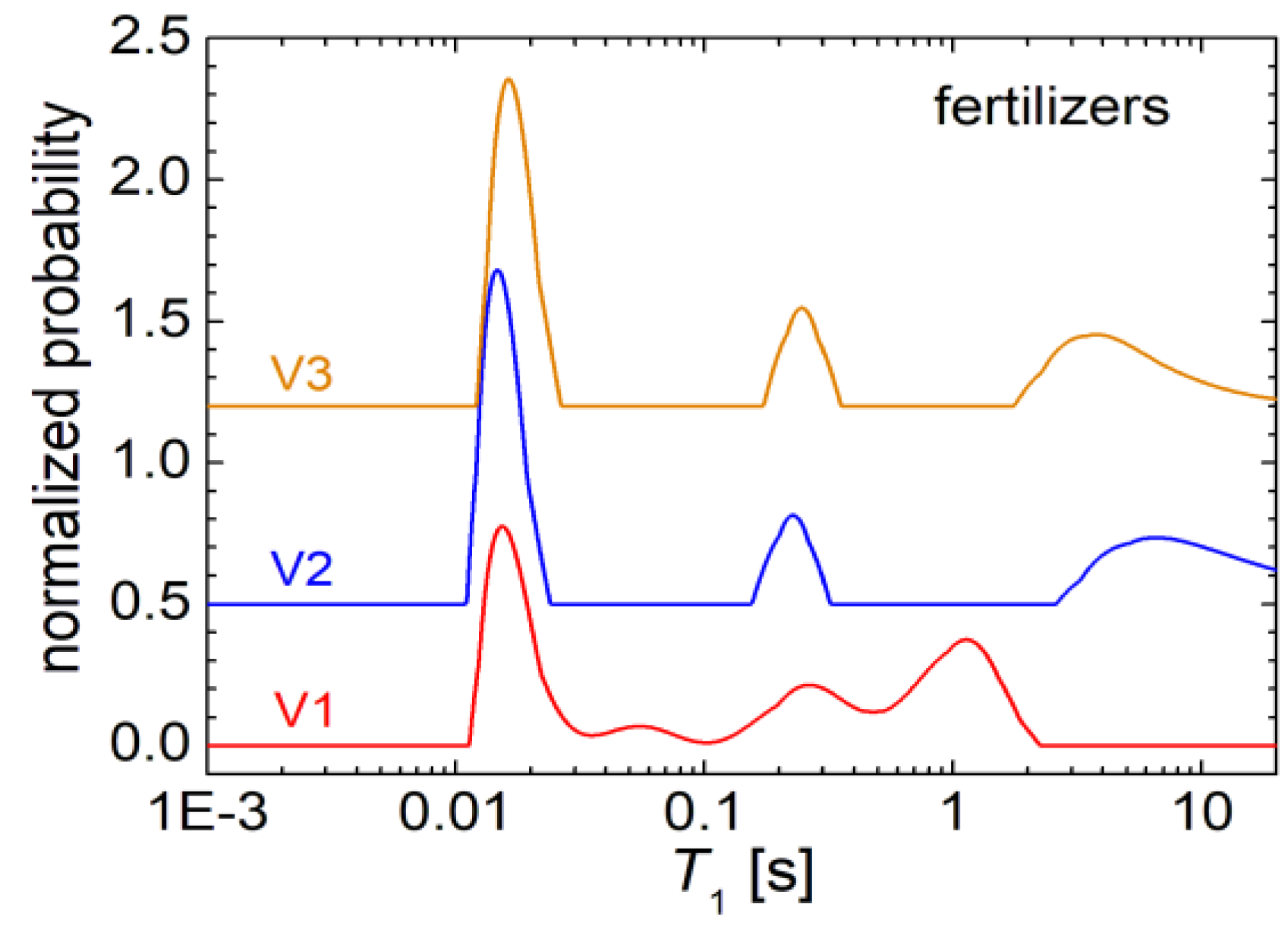
In
Figure 1 the distributions of the longitudinal relaxation times
T1 for the three fertilizers V1, V2 and V3 are presented. One can observe that the
T1-distributions presents similarities for the fertilizers samples V2 and V3, while V1 shown a different distribution of
T1 relaxation time. Thus, in the distribution of
T1 measured for sample V1 one can observe four relatively well resolved peaks. A main peak is located at about 18 ms and which describes a fast relaxation specific to rigid components of V1 biosolid. A significant amount of hydrogen is characterized by this type of relaxation (see the red curve in
Figure 1). Then, a peak with small amplitude (small integral area) is observed, located at about 55 ms. This is followed by a peak with an higher integral area located at about 250 ms. The last two peaks can be associated with water molecules, probably with increased mobility, interacting more difficult with the network leading to a longer longitudinal relaxation time. Another peak is observed at about 1 s, which can be most probably associated with free water. The
T1-distributions measured for formulas V2 and V3, presents only three peaks. The main peak is located as in the case of the
T1-distribution measured for the V1 fertilizer, at approximately 15-18 ms. This is followed by a well-defined peak centered at about 250 ms, corresponding to the third peak in the V1 fertilizer
T1-distribution. Then there is a wide peak not very well defined at high values of
T1 relaxation time and which could be associated with free water that can be found in the pores of these fertilizers.
3.2. 1H NMR T2 Distributions
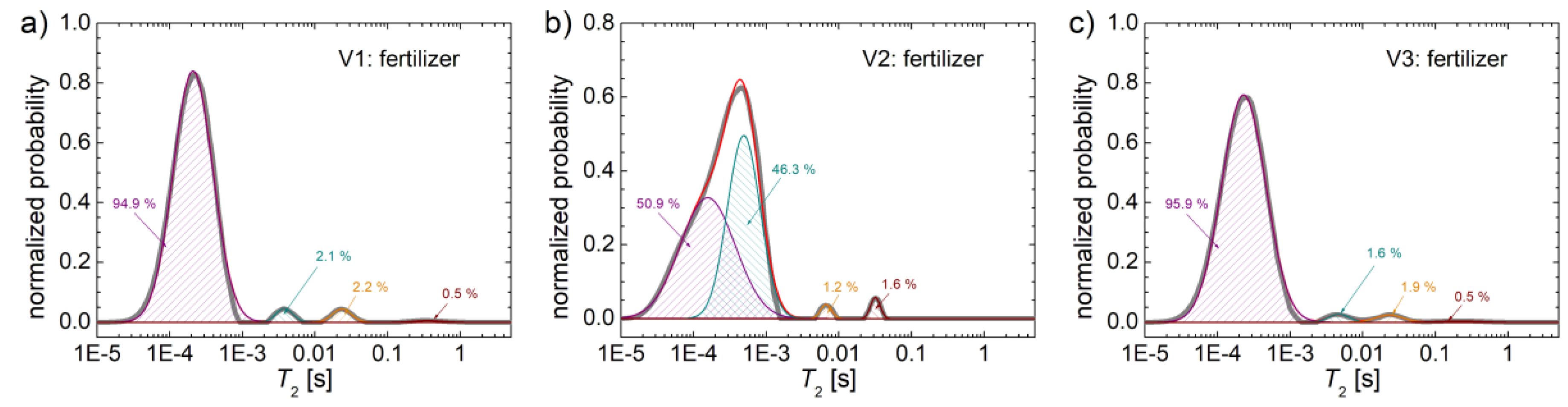
In
Figure 2 the distributions of spin-spin relaxation time,
T2 measured for fertilizers with formulas V1, V2 and V3 are presented. Specific variations and differences compare to the distributions obtained for
T1 relaxation times are observed. The main peaks, localized at low
T2 values, can be associated with rigid components of these fertilizers containing hydrogen, with reduce mobility. As the
T2 relaxation time increases, and the mobility of these components increases also, one can expect that the hydrogen to belong to water molecules (moisture absorbed from air) from various types of pores of these fertilizers. With the increase of the pore diameter, the location of the associated peak is more to the right, i.e., the higher is the value of
T2. Thus, the very high values of the relaxation time
T2, can be associated with the very mobile water.
If we compare the distributions of
T2 obtained for the three fertilizers, we notice that the
T2-distribution measured for V1 (see the broad gray line in
Figure 2a) and V3 (see the broad gray line in
Figure 2c) are quite similar. Instead the
T2-distribution measured for the fertilizer V2 (see the broad gray line in
Figure 2b) is slightly different. All three distributions have a large peak, located at low
T2-values. For fertilizer V1 the value of
T2 at which the maximum of main peak appears is about 200 μs (see
Figure 2a), for V3 this maximum appear at about 250 μs (see
Figure 2c), and for V2 this one appear at about 500 μs (see
Figure 2b). In the measured
T2 -distribution for fertilizer V2 it is observed that another peak located also at lower
T2-values appears as a left shoulder. Taking a look in the list of components (see Table 1 from Cota et al., 2019) used for the production of the three variants of fertilizers V1, V2 and V3 we notice that the mineral fertilizers that could contain (among others) nitrogen and phosphorus are found only in variant V2.
Therefore, one can consider that this mineral fertilizer is the decisive elements that lead to the variations of T2-distribution measured for V2 compared to the measured T2-distribution for V1 and V3. Most likely, the similarities between the T2-distributions measured for V1 and V3 are due to similar contents of many components mainly of mono-ammonium phosphate. Specifically, the difference in the T2-distributions of V1 and V3 compared to V2 are given by exactly these two components, namely: i) the presence of 24 % of mineral fertilizer in variant V2 and the lack of these mineral fertilizers for variants V1 and V3 and ii) the presence of mono-ammonium phosphate in variant V1 and V3 at approximately 24.5 – 25 % and the lack of this ammonium phosphate in V2.
For a better characterization of the
T2 relaxation time distributions, a deconvolution procedure was applied on these curves. The deconvolution and the percentage in which each component occur are shown explicitly in
Figure 2. Thus, it is observed that for the V1 fertilizer, the highest amount of hydrogen (approximately 94.9 %) is found in rigid components. Then, a small amount of hydrogen (2.1 %) most likely in the form of water in small pores (semi-rigid component) can be associated with the second peak into. A similar amount of hydrogen can be found in medium pores, (2.2 %), and a tiny amount (0.5 %) of
1H is found in large pores, connected in the
T2-distributions with the peak centered at about 300 ms (see
Figure 2a). For the
T2-distributions measured for fertilizer V2, this numerical deconvolution procedure makes sense, since the wide peak located between 10 μs and about 1.5 ms is actually composed of two peaks one wide centered at about 100 - 150 μs, and most likely is due to the presence of hydrogen in very rigid components. These could be found in urea, protein hydrolysate, starch, or, as moisture in dry biosolids. Then one can observe a narrow peak centered at about 400 μs, corresponding to an amount of 46.3 % hydrogen. The origin of this hydrogen could be the
1H from polymer chains of hydrolyzed proteins or starch, or could originate form water in extremely small pores. There is also a peak for which the amount of hydrogen is only 1.2 % of the total amount of measured hydrogen and which could be associated with small to medium pores. For that, see the peaks located at about 7 ms. A slightly increased amount of hydrogen for the V2 fertilizer (as indicated by the integral area below the peak of 1.6 % from the total amount of measured hydrogen) is found in medium pores.
The T2-distribution measured for V3 fertilizer is most similar to the T2-distributions measured for V1 fertilizer. One can observe also that a significant amount of hydrogen (95.9 %) was measured for extremely rigid components which can be found in the dry components (protein hydrolysate or probably starch backbone chain). Then, one can notice a smaller amount of water, of about 1.6 % from the total amount of measured hydrogen for V3 sample, is found in small to medium pores, as can be observed from the peak located at about 3.5 ms. A comparable amount of 1.9 % hydrogen is found in medium pores and a small amount of water (about 0.5 %) is found in large pores. If we compare the T2-distributions measured for V1 with V3 one can say that for the V3 fertilizer a slightly higher amount of hydrogen is found in extremely rigid components. In medium pores and in large pores the amount of water is found to be slightly smaller for V3 than for V1. In large pores, about the same extremely small amount (of about 0.5 %) of water was measured for both V1 and V3.
3.3. 1H NMR Double-Quantum (DQ) Measurements
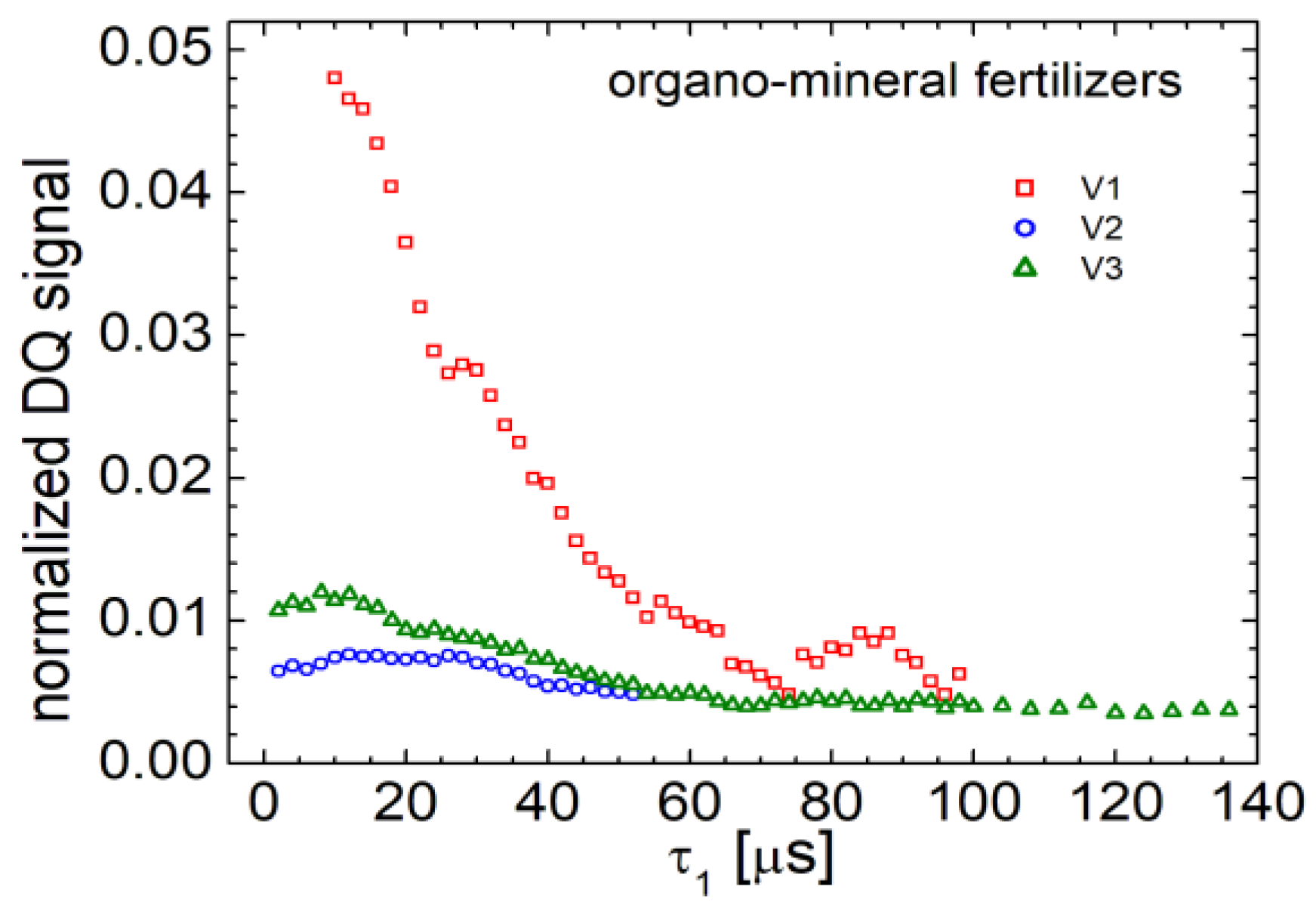
An advanced method in NMR characterization uses the signal from multi-quantum coherences. The most commonly used are double-quantum (DQ) coherences. These require at least two nuclear spins of hydrogen (the measured spin) to be coupled. In this way, the double quantum measurements act as a filter for the coupled spines and which must be located in rigid components. Nuclear spines of hydrogen from free water are not able to provide a double quantum NMR signal. This is due to the fact that the water molecules can perform translational but especially rotational kind of motion. In
Figure 3 the double quantum build-up curves recorded for organo-mineral fertilizers are presented.
From the analysis of T2-distributions (see the previous section), it was found that a large part of hydrogen from these organo-mineral fertilizers it is located in rigid components. Unfortunately, this large amount of hydrogen (about 94-95 %) into extremely rigid components is characterized by a very short relaxation time T2 of only few hundred of milliseconds. This short relaxation time leads also to a fast relaxation of the double-quantum signal. In order to record it, the pulse sequence needed to be improved by adding a 180o refocusing pulse between the two 90o pulses, during the excitation of DQ and reconversion of double-quantum to a single quantum (SQ). In addition, it was necessary to record a number of 8000 scans to gain a good signal-to-noise ratio. Usually, the double-quantum curves start from a small value of the NMR signal which increases until a maximum is reached. After that, a decay is obtained due to the spin-spin relaxation mechanisms. In the case of our fertilizers (V1, V2 and V3), due to the fact that the specific relaxation times T2 are extremely short, the initial regime of the double-quantum curve is also extremely short leading to a maximum that appear very fast (early in time). Thus, for the V1 fertilizer, the build-up part of the double-quantum curves was not even recorded since the maximum was measured at about 10 μs. For fertilizer V2, of which amplitude is the smallest, the maximum of DQ build-up curve was obtained at about 12 μs. This curve is characterized by a second maximum at about 35 μs, which indicates that the hydrogen is coupled by extremely strong dipole couplings. The same behavior presenting two maximum can be observed also for the measure double-quantum build-up curves for fertilizer V3. This indicates that the distributions of dipole couplings in these fertilizers have different origins, in the raw materials used in this compounds. Each of them, for example hydrolyzed protein or starch, has different values of dipolar couplings.
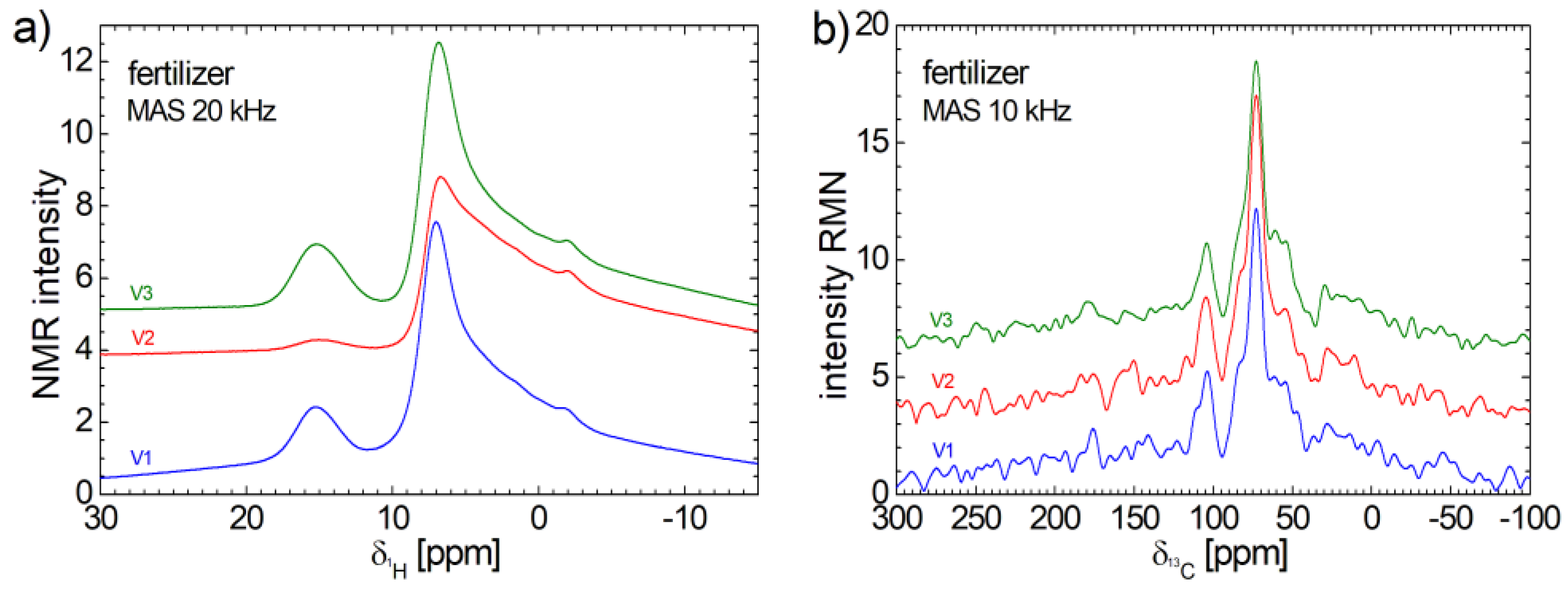
3.4. 1H and 13C NMR Spectra
The
1H and
13C NMR spectra measured for V1, V2 and V3 organo-mineral fertilizers recorded under MAS condition were presented in
Figure 4. As a general characteristic one can observe the similarities among the measured spectra, indicating a similar molecular structure. The
1H NMR spectra presents a good signal to noise ratio. These consist of a small and relative broad peak centered at ~15.27 ppm. The amplitude of this peak measured for V2 fertilize is much smaller compares to the amplitude of peak measured for V1 and V3. Then one can observe a relative narrow peak centered at ~6.89 ppm with a long tail extended up to approximately – 15 ppm. A small shoulder appears for all three spectra at approximately - 1.93 ppm. Despite the long acquisition time (almost a week) the
13C NMR spectra are characterized by very small signal to noise ratio which make difficult the spectra assignment. Nevertheless, one can observe a high and narrow peak located at ~72.9 ppm overlapping a broad peak which appears as left and right shoulder. One can clearly observe another narrow peak located at 102.5 ppm. The rest of peaks have the height comparable with the noise level, and then, are hard to be discriminated from these. The main conclusion from these
13C NMR spectra is that the all V1, V2, V3 organo-mineral fertilizers contain a very small quantity of carbon.
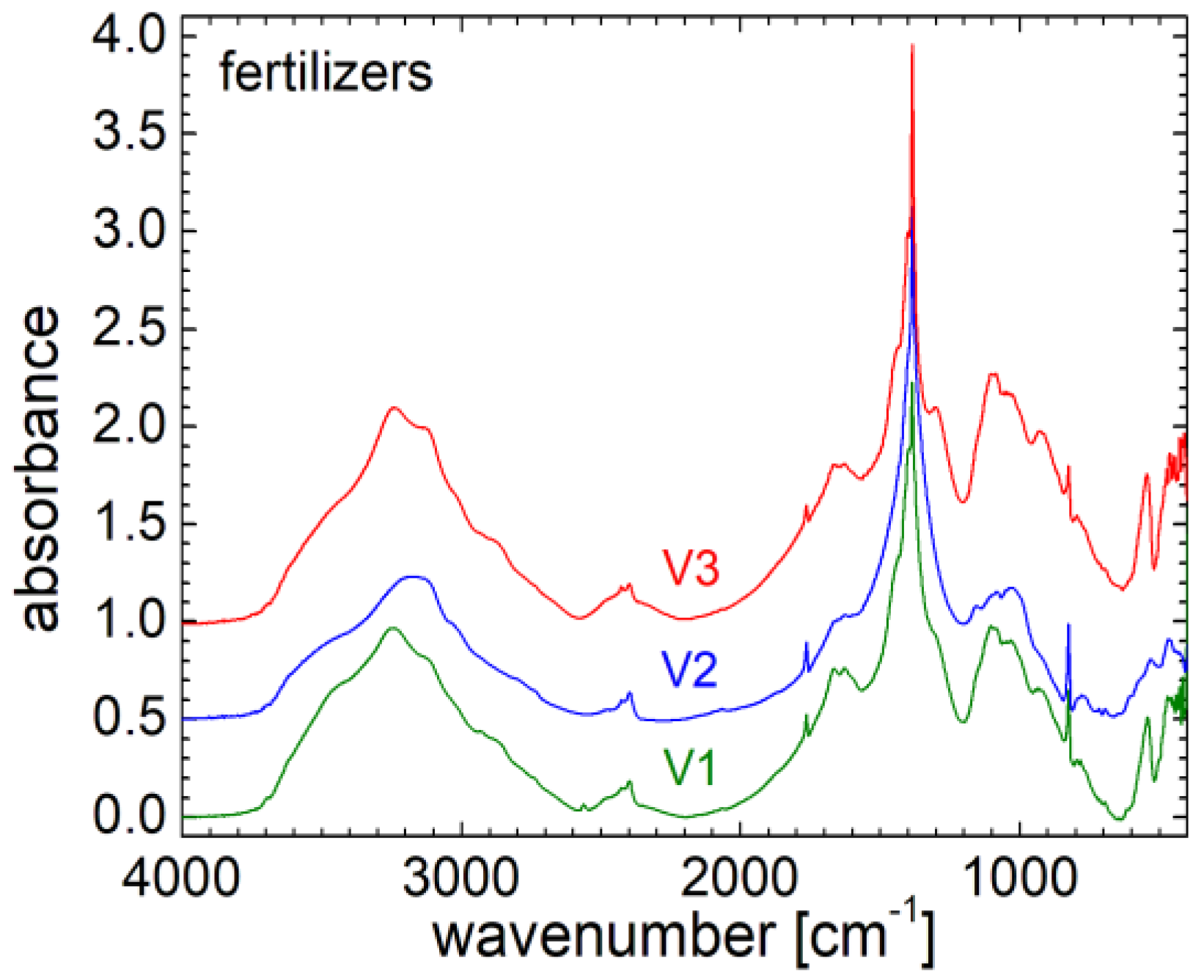
3.5. FT-IR Spectra
Fourier transform infrared spectroscopy (FT-IR) is a modern method capable of identifying the structure of simple samples, or if the measured sample is more complex, to provide complex information about the type of chemical bonds. In
Figure 5 the infrared FT-IR spectra measured for the three fertilizers are presented. One can observe high similarity between these spectra, but there are also major specific differences between them. Thus, for the analysis of FT-IR spectra, the focus is on two areas of interest. The first area is in the range from about 2600 to 3800 cm
-1, where a wide peak associated with the water in the samples it is located, and presenting some small maximums at about 2737-2741 cm
-1 (associated with stretching N-H (
)), 2873-, 320 cm
-1 (associated with ν
s CH
3, stretching C-H, N-H), 2933-2945 cm
-1 (associated with C-H stretching bands, ν
as CH
2), 3124-3127 cm
-1 and 3241-3244 (associated with symmetric stretching of O-H belonging to water that is intrinsically found in the measured samples, or can be hygroscopic absorbed as moisture from air). For V1 samples a prominent peak located at about 3452 cm
-1 (associated with asymmetric stretching of O-H) [
22] is observed. From a structural point of view, one can say that the FT-IR spectrum measured for V1 resembles most with that measured for fertilizer V3, and this is most likely a consequence of the fact that for the two fertilizer variants there are no minerals in composition. Contrary, for variant V2, these minerals are found in proportion of 24 %. Instead the ammonium monophosphate is missing from variant V2 but this can be found in proportion of 24.5 – 25 % in variants V1 and V3.
The most important features are obtained in the range of wave numbers from 350 to about 2000 cm
-1. At a visual inspection, the spectra measured for V1 and V2 are similar to each other, and somehow different from the spectrum measured for fertilizer V3. Thus, these spectra present solved peaks and bands for all three fertilizers: i) a doublet, located at wave numbers between 468-473 cm
-1 (associated with C
α = C
α torsion and C-OH
3 torsion of methoxy group) and 541-545 cm
-1 (associated with C
α = C
α torsion and ring torsion of phenyl); ii) a narrow peak centered at about 824-825 cm
-1 (associated with deformation of CH ring) presenting a higher amplitude for V1 and V2 and a smaller amplitude for the V3 fertilizer; iii) a wide band centered at about 1093 cm
-1 for V1 and V3, and at about 1082 cm
-1 for V2 and which can be associated with symmetric stretching of
(phosphate II) for V1 and V3 and with symmetric
phosphate band for V2. There are also some small shoulders located at about 924, 932, 1028 cm
-1 and well resolved peak located at about 1155 cm
-1 (stretching vibrations of hydrogen-bonding C-OH groups, stretching vibration C-O) for V2 and which barely appear as a left shoulder for V1 and V3; iv) an extremely high peak located at about 1384 cm
-1 (associated with δCH
3, stretching C-O, deformation C-H, deformation N-H), accompanied by small peaks appearing as left and right shoulders and located at about 1399-1401 cm
-1 (associated with CH
3 symmetric deformation, symmetric CH
3 bending modes of the methyl groups, symmetric δ[(CH
3)] and symmetric δ [C(CH
3)
2], symmetric vibrational stretching of COO
- group), at about 1299-1301 cm
-1 (associated with Deformation N-H) only for V1 and V3, at about 1435 cm
-1 (associated with δCH
2) which appears for all three fertilizers; v) a barely defined doublet located at about 1628 cm
-1 and 1664 cm
-1 (associated with Amide I region) very similar for the spectra measured for fertilizer V1 and V2 but diminished for fertilizer V2; vi) a narrow peak with a small amplitude located at about 1764 cm
-1 associated with ν(C=C) visible for all three fertilizers, [
22].
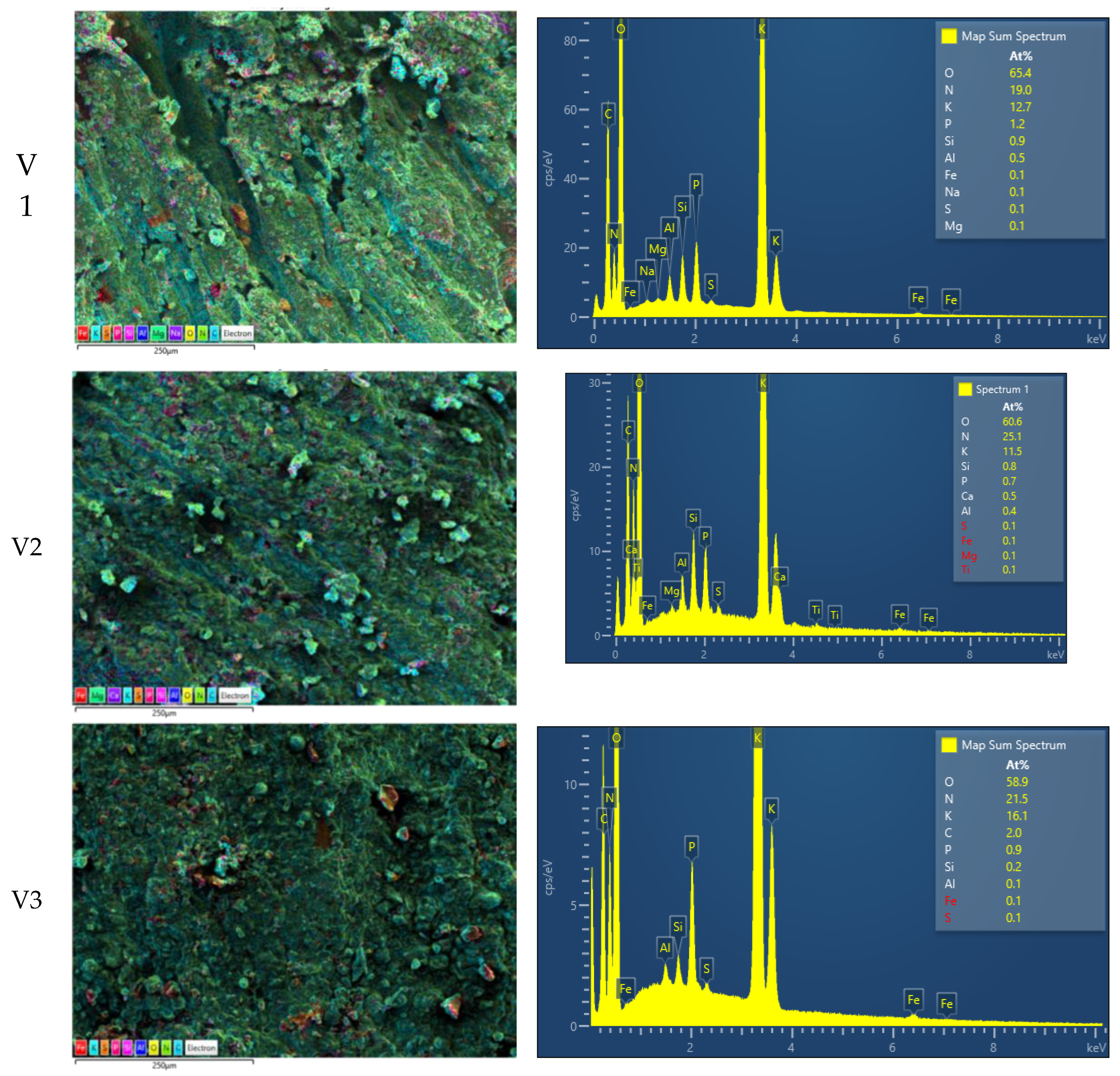
3.6. SEM and EDX Analysis and XRD Pattern
SEM and EDX
If FT-IR analysis can identify structures at molecular level (via chemical bonds), scanning electron microscopy (SEM) and X-Ray diffraction pattern can offer information about the structural organization at nano-to-micro metric level. Thus, SEM-EDX (energy dispersive spectroscopy localized by SEM) images are presented in
Figure 6 for all three fertilizer V1 (top), V2 (middle) and V3 (bottom). A colored map overlapping the SEM images of a submillimeter surface of fertilizers are presented in the left column. The colored pixels indicate the presence of particular elements as follow: i) iron (Fe) red; ii) Potassium (K-
kalium) cyan; iii) Sulfur (S) orange; iv) Phosphorus (P) red; v) Silicon (Si) pink; vi) Aluminum (Al) blue; vii) Oxygen (O) yellow; viii) Nitrogen (N) green; ix) Carbon (C) light-blue. The EDX spectrums for the left images are presented on the right column. According to the measured energy (position on the horizontal axis) one can identify the presence of particular elements on the localized area of each bio-solid fertilizer sample. The relative height of the peaks can be inferred with the chemical content of the analyzed surface. The samples were measured in triplicate (for each formulation three different areas were measured, but there are presented only one) and it was find out that: i) the Oxygen is the most abundant element in the ranges of 63.7-65.6 % for V1 of 60.6-62.0 % for V2 and of 58.2-58.9 % for V3; ii) second abundant element is Sodium in the ranges of 17.8-19.0 % for V1 of 24.7-25.1 % for V2 and of 20.2-21.8 % for V3; iii) the third abundant element is Potassium in the ranges of 12.7-14.4 % for V1 of 10.5-12.4 % for V2 and of 14.5-16.1 % for V3; iv) the rest of elements are found in percentages under 2 %; v) for V1 fertilizer Phosphorus is the forth element with an average of 1.4 % and Silicone can be found in percentage around 1 %; vi) for V2 fertilizer Phosphorus is only the fifth element with an average of 0.63 % while Silicone can be found in percentage around 0.7 %; vii) for fertilizer V3 the Phosphorus is also found on the fifth place with an average of 1.03 % while Carbon can be found in percentage around 3.03 %. Additionally to the element content, the SEM images provide information related to the surface aspect of the samples (
Figure 6 – left column). The most rugose surface is presented by V1 fertilizer and the most fine (among these three measured) surface is presented by V3 fertilizer. For V1 sample one can see deep gaps which may originate from extrusion process. The same process may be guessed also for V2 sample from the diagonal traces, while the V3 fertilizer seems do not be affected by extrusion. Nevertheless, all samples present a series of agglomerates on surface mainly constituted by particular elements.
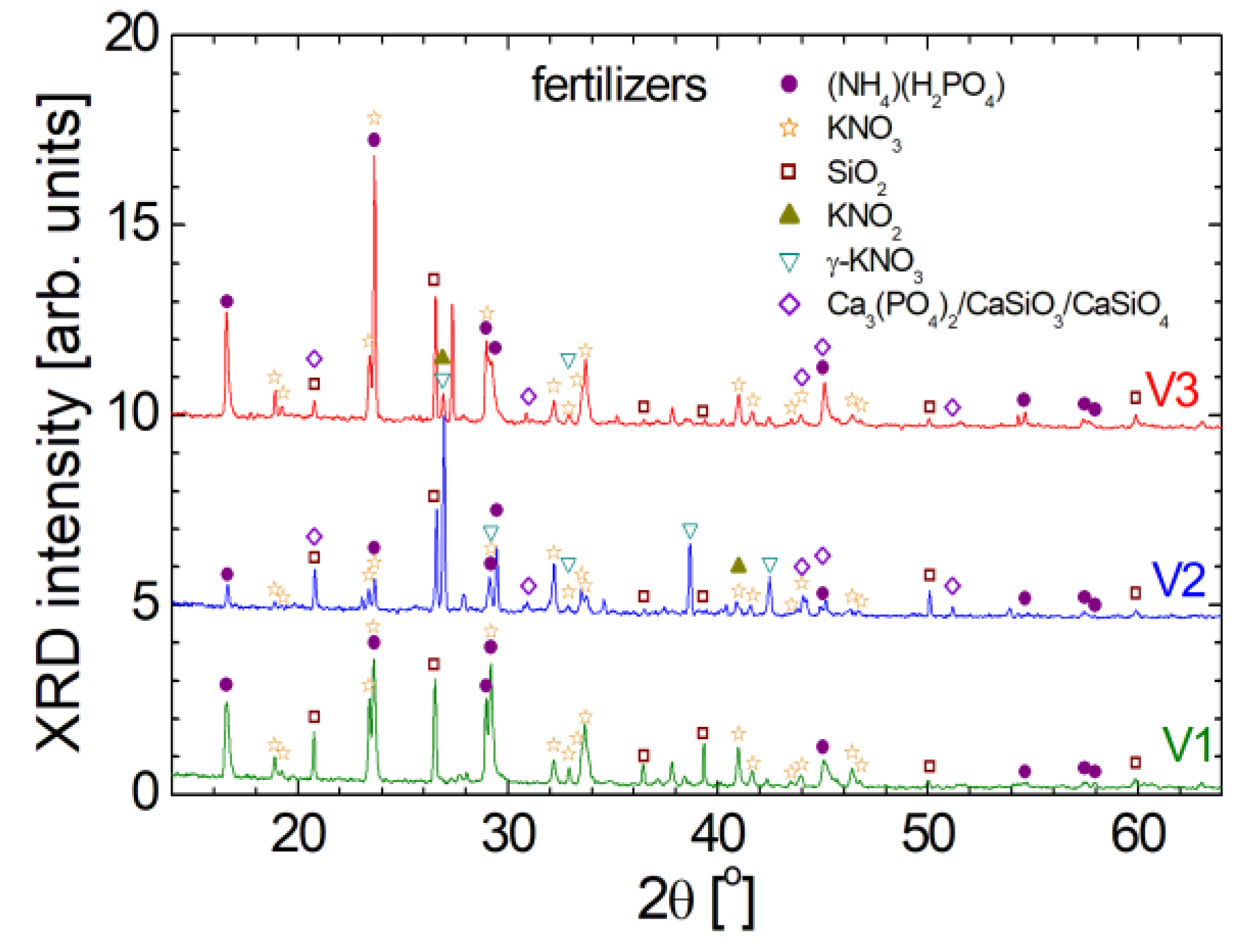
As it was shown early, the
1H and
13C NMR spectra (
Figure 4) show that at molecular level the measured V1, V2 and V3 organo-mineral fertilizers are similar; the FT-IR spectra show only small differences at chemical bond level. Nevertheless, from the point of view of information about the crystalline structure (interatomic distances, crystalline orientation, relative concentration of different phases, and the presence of any crystal defects or distortions) provided by the XRD patterns one can conclude that the measured fertilizers are quite different, as can be observed from
Figure 7. The narrow and high peaks indicate a well-organized crystalline structure of organo-mineral fertilizers. The similarities reside in the fact that for all three spectra of V1, V2 and V3 fertilizers the significant peaks appear for all of them at the same 2θ angle, but in many cases with different amplitude. For the interpretation of diffractogram it was considered an inter-planar spacing which was found between d
hkl = 5.33 Å for (2θ ≈ 16.62
o) and goes down below d
hkl = 1.819 Å for (2θ ≈ 50.11
o). The automated assignment procedure lead to the following five major crystalline components: i) (NH
4)(H
2PO
4) labeled with purple filled circle; ii) KNO
3 labeled with orange open star; iii) SiO
2 labeled with wine open square; iv) KNO
2 with dark yellow filed up-triangle; v) γ-KNO
3 with dark cyan open dawn-triangle and vi) a mixture of Ca
3(PO
4)
2/CaSiO
3/CaSiO
4 labeled with violet open diamond. Additional to these, other crystalline structures can be found in relative small amount. Thus, for example (NH
4)(H
2PO
4) or ammonium phosphate, is also known as monoammonium phosphate being a colorless salt, soluble in water, and is used in various applications such as direct fertilization in agriculture being a reach source of nitrogen and phosphorus in plant nutrition. It is an efficient source of nitrogen in the ammoniacal form and phosphorus in the soluble form, which can be easily assimilated by plants. Nitrogen is a critical limiting factor in crop production across tropical and temperate regions. Plants require it in a significant quantities but nitrogen's availability can vary. While nitrogen (N
2) constitutes the most abundant gas in the atmosphere, its absorption by plants occurs primarily in the form of ammonium or nitrate [
23]. In agriculture, KNO
2 is generally not used directly as a fertilizer since is toxic to plants and animals at high concentrations. KNO
3 known as potassium nitrate (also known as saltpeter) is used in a variety of applications, including agriculture as a fertilizer. Potassium is crucial for optimal plant functioning, including root development, stomatal regulation, and energy transfer. In agriculture, the gamma form (γ-KNO
3) of potassium nitrate, is widely used as a fertilizer being water-soluble and therefore can be applied directly to the soil or through irrigation systems. The most problematic was the assignment of the last series of peaks marked with violet open diamond since no compound was found in our database. For fertilizer V2 the highest peak is marked with this symbol. And the peaks series have no correspondent in diffractogram measured for V1 fertilizer but may be found into a small amount in V3 formulation. The first action was to measure the fertilizer biosolids by SEM-EDX. The results were discussed in the previous paragraph. Taking a look to the list of elements one can observe that Calcium appear only in the V2 formulation of biosolid fertilizer. Therefore we have assumed that must be a Calcium based compound. Indeed, we found that some impure calcium phosphate and calcium silicate mixture (Ca
3(PO
4)
2/CaSiO
3/CaSiO
4) may be responsible with these peaks [
24].
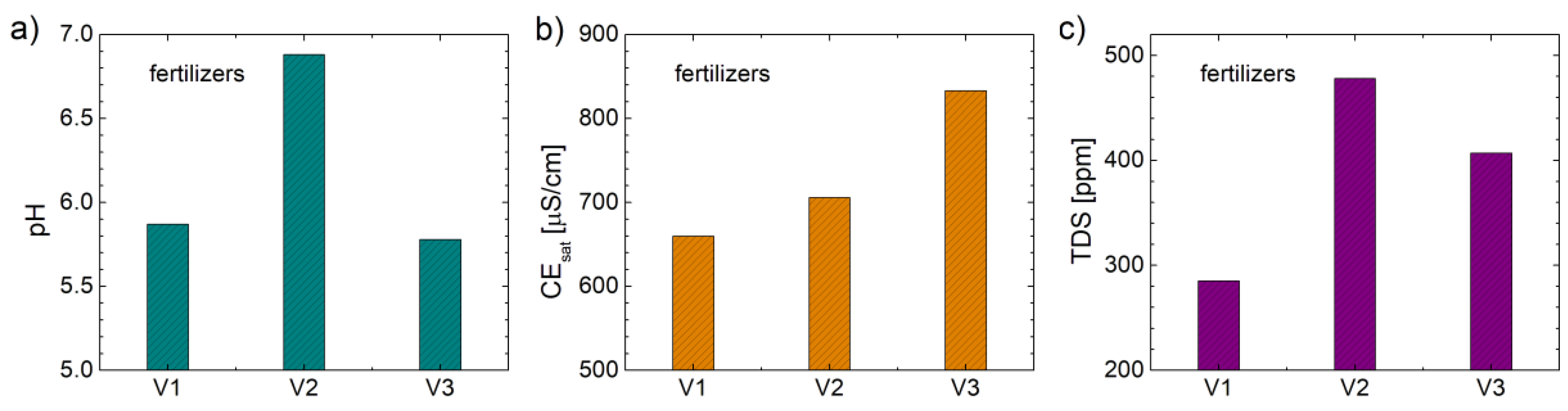
3.7. Physical-Chemical Characterization
Usually, the soil pH can have a great influence on the agricultural plant culture and must have values between 5 and 7.5. Therefore, any fertilizers must not change drastically the soil pH. In
Figure 8a) the values of pH, measured for a solution of 200 ml of distillated water and 250 mg of biosolids fertilizes, are presented. One can observe that the samples V2 present the highest value of pH (6.88) while the samples V1 and V2 present a similar value of pH (5.87 and 5.78 for V1 and V3, respectively).
Another parameter that describes the properties of distilled water solutions with biosolids from V1 to V3 is the electrical conductivity (EC).
Figure 8b) shows the values of maximum electrical conductivity (measured for a time of dissolution of 120 min). Relatively high values (over 660 µS/cm) are observed for all samples. The highest value (883 µS/cm) was measured for sample V3 containing orthophosphoric acid (H
2PO
4). Another explanation for the high EC value measured for the V3 sample could be a higher capacity to be dissolved in distilled water.
The parameter that represents the totally dissolved solids (TDS) is usually well correlated with the electrical conductivity of the solutions. In our case the non-correlations of the two parameters (EC and TDS) is most probably due to the presence of different substances in the electrolytes that conduct differently the electric current (probably due to the presence of electrical charges in different quantities).
Figure 8c) shows the TDS values measured for distilled water solutions with our three biosolids. It is observed that the measured quantities have average values (higher than 280 ppm and lower than 500 ppm). As in the case of EC, the lowest value (285 ppm) is measured for V1, while the highest value (478 ppm) is found in sample V2. In conclusion it can be said that the high EC measured for sample V3 (see
Figure 8c)) is not due to the dissolution of a larger amount of V3 sample compare to the amount dissolved for V1 and V2, but rather due to a better conductive properties of the electric current in the dissolved solids from V3, most likely due to orthophosphoric acid.
Most often the turbidity of a solution is given by the amount of undissolved solids.
Figure 9 shows the measured turbidity values for distilled water solutions with biosolids after a sufficiently long time (greater than 120 minutes) needed for a solution to reach the saturation. It is observed that the measured values are high (higher than 495 ntu), and the highest value is presented by the V1 sample (~ 660 ntu).
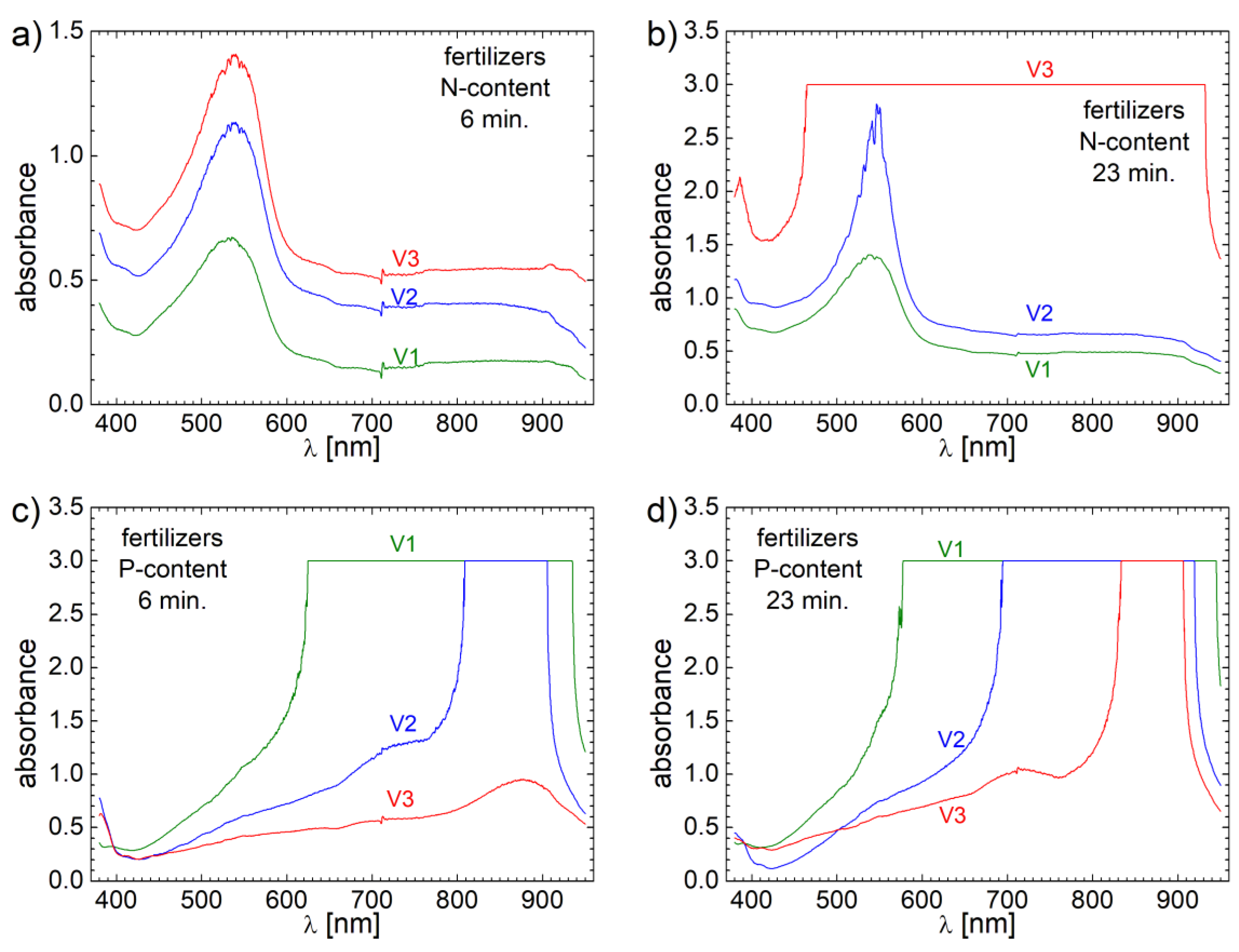
Among Potassium, two of the very important parameters of a fertilizer are the Nitrogen (N) and Phosphorus (P) contents. In order to quantitatively asses the capacity of fertilizers to release N and P, 2.5 ml of distillated water in which all 3 biosolids fertilizer samples were dissolved for 6 and 23 minutes was mixed with the specific reagents. Then the solution with each reactive was sacked for one minutes and then placed on a holder for sedimentation. The supernatant was transferred into a VIS-nearIR cuvette and another 2.5 ml of distillated water was added to fill the cuvette. The measured VIS-nearIR spectra are presented in
Figure 10.
Thus, in
Figure 10 a) the VIS-nearIR spectra sensitive to N content are presented for all V1, V2 and V3 samples. One can observe that all spectra present a broad absorbance band centered at about 531 nm for V1, 537 nm for V2 and 539 nm for V3. One can observe also a beginning of absorption in ultra-violet (UV) for all samples. There is also relative broad pallier (a constant absorption value) between 710-900 nm. The most important aspect is the total integral area of these spectra which are proportional with the N content. One can observe that the integral area of VIS-nearIR spectrum measured for V3 is larger than the corresponding VIS-nearIR spectra measured for V1 and V2, which are only 31.13 % for V1 and 75.33 % for V2, respectively. Therefore, one can assume that the content of Nitrogen in fertilizer V3 is highest or at least is released faster. In order to discriminate between these two hypotheses another measurement was performed for samples collected after 23 minutes of dissolution of biosolids fertilizers samples in distillated water. The corresponding spectra are presented in
Figure 10 b). One can observe that the absorbance of all spectra increases, which is an indication of a continuous release of fertilizers in distillated water. The spectra structure for V1 and V2 remains the same but the VIS-nearIR spectrum measured for V3 present a total absorbance in the range of wavelength, λ from 465 – 931 nm. Due to this inconvenient one can no longer estimate correctly the amount of Nitrogen for samples V1 and V2 reported to V3 samples but one can say that the Nitrogen content in sample V1 is 71.45 % from N content in sample V2. Nevertheless, is clear that the highest content of Nitrogen is found in fertilizer V3 and the smallest content is found in a fertilizer V1.
Unfortunately, the quantitative assessment of released phosphorus content cannot be done by this spectroscopic method due to the highest content of phosphorus in solution with samples V1 and V2 even at small time for dissolution of fertilizers in distillated water, where the VIS-nearIR spectra presents a total absorption (see
Figure 10c). Only VIS-nearIR spectrum measured for V3 presents absorbance values smaller than 1.0 spectrum for which present a broad absorption band centered at about 878 nm. Nevertheless, from the range in which of total absorbance appear for samples V1 and V2 one can say that the largest amount of P is found in fertilizer V1 then in fertilizer V2, while the smallest amount of P is found in fertilizer V3. This observation is validated by the VIS-nearIR spectra measured for all 3 fertilizers from samples collected at 23 minutes of fertilizers dissolution in distillated water (see
Figure 10d).
3.7. Evaluation of Fertilization Properties
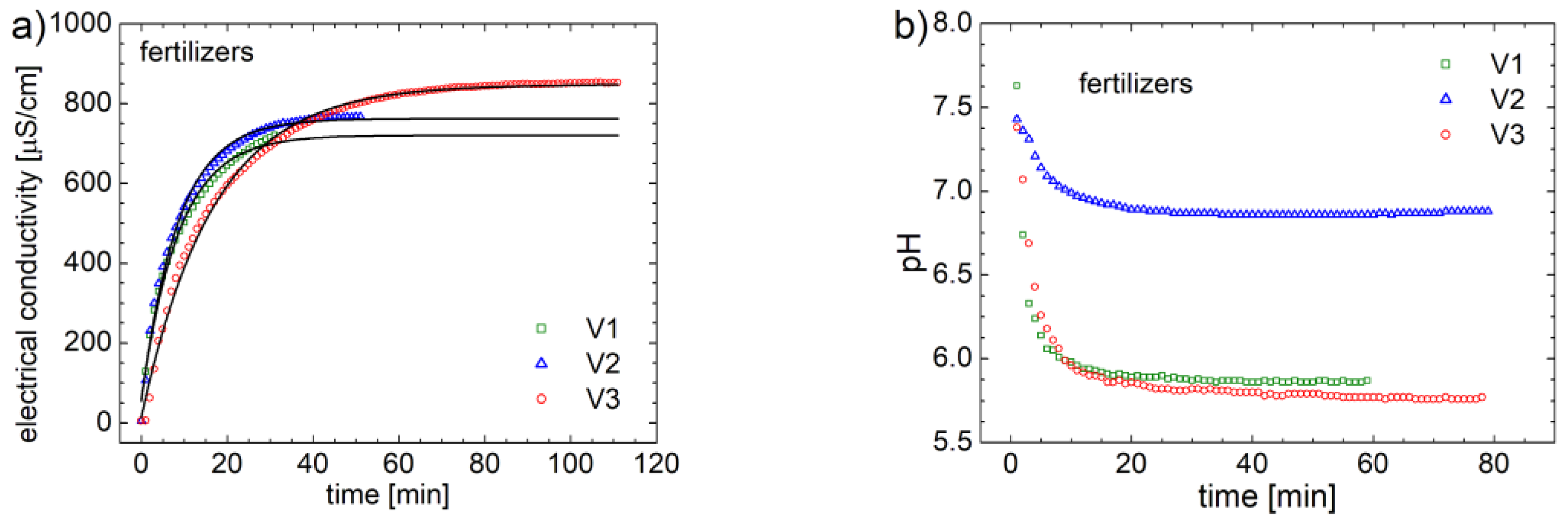
The final goal of this study is to evaluate the fertilizing properties for three organo-mineral fertilizers based on biosolids. For that, systematic electrical conductivity (EC) measurements were performed over time, for a solution of 0.25 g of fertilizer in 200 ml of distilled water. The measurements were performed with a Hanna-EDGE instrument that automatically records at each minute the value of the electrical conductivity. The curves of variation of the electrical conductivity for the three variants of fertilizer V1, V2 and V3 are presented in
Figure 11a. The electric conductivity of distillated water is near to zero. During dissolution of fertilizers, conductive components are released in water therefore the EC increases. One can observe that, after a certain time, the value of electrical conductivity reaches saturation (see
Figure 11a). In order to characterize both, the degree of release (
and the rate of release (
) of fertilizers, the experimental data were approximated with an ad hoc curve of exponential type,
For each data, the fitting curve is presented with a black line. One can observe that the fertilizer V3 releases the largest amount of active fertilizer, but the release time is the longest. The degree and the rate of release will be discussed latter.
Another parameter that can monitor the degree of nutrient release is the pH (which changes when fertilizer it is released) of the solution with distillated water and fertilizers. Thus, in 200 ml of distilled water, 0.25 g of formula V1, V2 or V3 were immersed. The initial pH of the water was around 7.5, as can be seen in
Figure 11b. This figure shows the pH variation curves for the three fertilizers. One can observe that after about 20 – 40 minutes the pH values reach a saturation value. From these measurements it is observed that the smallest variation of the pH value is obtained for the fertilizer V2, whose pH decay below 7. While, for fertilizers V1 and V2 the final pH value is below 6. This is most likely due to the different amount of mineral fertilizers, but also due to ammonium monophosphate. Ammonium is known to be a substance that can lead to major changes in pH, and the lack of this ammonium monophosphate in the formula for fertilizer V2 is reflected in a small variation of pH of the solution of distilled water with V2 fertilizer.
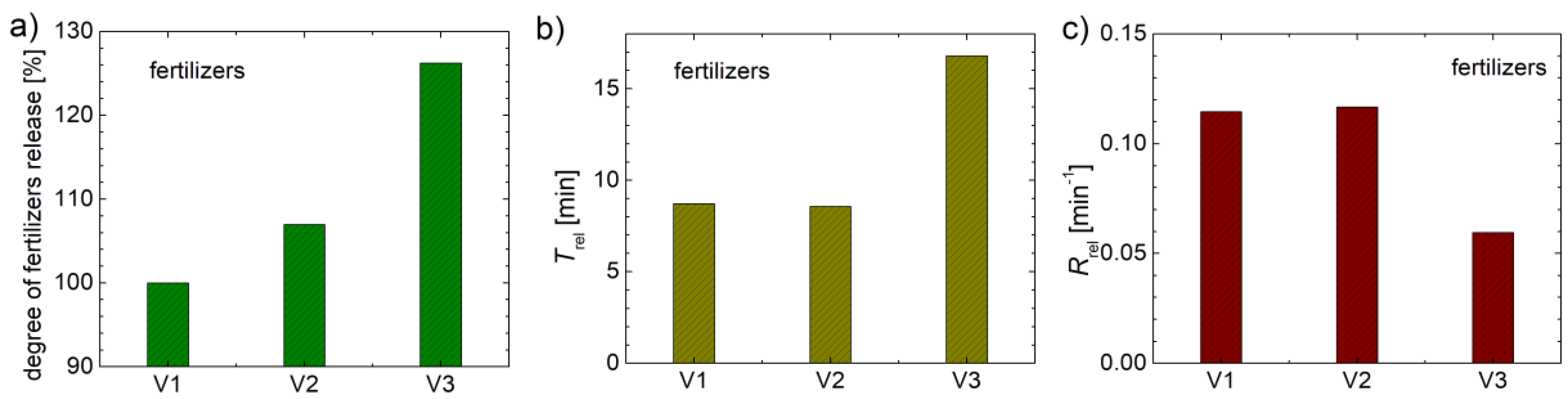
From the curves of variation of electrical conductivity (see
Figure 11a) and approximated using eq. 1, information related to electrical conductivity at saturation (
and release time (
) can be extracted. By normalization, the degree of release of mineral organ fertilizers can be comparatively estimated. The normalization procedure assumed that the saturation concentration was divided by the measured value for fertilizer V1, and the percentage results are shown in
Figure 12a. Thus, one can observe that the fertilizer V2 it releases only 6.9 % more active agents than the fertilizer obtained by formula V1. The fertilizer V3, with a probably improved recipe, releases with 26.2 % more active agent than the fertilizer V1. In addition to the amount of active substance released by each organo-mineral fertilizers based on biosolids discussed in this study, another important fertilization property is the rate at each active substance is released. Thus, in distilled water with a slightly basic pH, the release rate of fertilizers is observed to be of the order of a few minutes. The specific release times,
of the fertilizers obtained by fitting the variation curve of EC (see
Figure 11a) with eq. 1 are presented in
Figure 12b. For the fertilizer V3 the specific release time,
is about 16.8 minutes. The fertilizers V1 and V2 are characterized by a
of 8.73 and 8.57 minutes, respectively. The inverse of this release time indicates the release rate (
). Thus, V1 and V2 are the organo-mineral fertilizers based on biosolids that release the active specific substance fastest, while V3 releases this active substance at almost half the rate. The measurements were performed in laboratory conditions while the substances were completely immersed in distillated water. Under normal conditions, for example, as a protective film on the ground, we expect that this release rate to be greatly different. Subsequent studies could also focus on the pH of the solution in which fertilizers to be released, and which could be correlated, for example, with the soil’s pH.
4. Conclusions
New methods for characterizing biomaterials such as biosolids obtained by extrusion and used as organo-mineral fertilizers were proposed and developed. In this study 3 types of biosolids used as fertilizers were measured by advanced 1H NMR methods (T1, T2 distributions and DQ curves) and by FT-IR spectroscopy, pH, electrical conductivity (EC), totally dissolved solids (TDS), and turbidity. The nitrogen and phosphorus content for the 3 versions of organo-mineral fertilizers were evaluated quantitatively by VIS-nearIR spectroscopy. The measurement of the degree of release of fertilizers was made by dynamically recording the time variation of electrical conductivity in solutions with a neutral pH of 200 ml of distilled water and 0.25 g fertilizer. The experimental data were approximated with a function whose parameters allowed us the extraction of a specific release time which characterizes the release rate for V1, V2 and V3 organo-mineral fertilizers based on biosolids. We have observed some similarities between the measured T2-distributions for V1 and V3 which were attributed to similar contents of many components, mainly of mono-ammonium phosphate at approximately 24.5 – 25 % which is not found in formula V2. The difference in the T2-distributions of V1 and V3 compared to V2 are given by the lack of this ammonium phosphate in V2 and by the presence of 24 % of mineral fertilizer in variant V2 and the lack of these mineral fertilizers for variants V1 and V3. The double-quantum build-up curves presents two maximum, fact that indicates distributions of dipole couplings with different origins, for example hydrolyzed protein or starch. From a structural point of view the measured FT-IR spectrum for V1 resembles most with that measured for fertilizer V3, most likely as consequence of the absence of minerals form their composition. Relatively high values of electric conductivity (over 660 µS/cm) are observed for all samples. The highest value of EC measured for sample V3 was associated with the presence of orthophosphoric acid and to a higher dissolution capacity in distilled water. The Nitrogen content in fertilizer V3 is highest and it is released faster, while largest amount of Phosphorus is found in fertilizer V1. The fertilizer V2 release 6.9 % more active agents than the fertilizer obtained V1, while the fertilizer V3 release 26.2 % more active agent. Finally, we shown that the organo-mineral fertilizers based on biosolids V1 and V2 are releasing faster the active specific substance, while V3 release this active substance in slightly basic pH of distillated water at almost half of the rate.
Author Contributions
Conceptualization, R.C. and R.F.; methodology, R.C. and R.F.; software, R.C. and R.F.; validation, R.C. and R.F.; formal analysis, R.C. and R.F.; investigation, R.C., E.M.N., N.C., M.V., P.P., F.P. and R.F.; resources, E.M.N., N.C.; data curation, R.C. and R.F.; writing—original draft preparation, R.C. and R.F.; writing—review and editing, R.C., E.M.N., N.C., M.V., P.P., F.P. and R.F.; visualization, R.C. and R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Special thanks are addressed by authors to Dr. Nicolae Cioica from National Institute of Research-Development for Machines and Installations Designed to Agriculture and Food Industry – INMA Bucureşti – Cluj-Napoca Branch for many discussions and constant support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coța, C. , Cioica, N., Nagy, E. M., Gyorgy, Z., Fechete, R. Research Regarding the Realisation of Granular Organo-Mineral Fertilizers Based on Biosolids, INTERNATIONAL SYMPOSIUM, INMA TECH 2019.
- Cioica, N. , Coța, C., Nagy, E. M., Gyorgy, Z., Nicola, C. Reactive Extrusion Processing of Nutrient-Enriched Biosolids. INTERNATIONAL SYMPOSIUM, INMA TECH 2020.
- Pöykiö, R.; Watkins, G.; Dahl, O. Characterisation of Municipal Sewage Sludge as a Soil Improver and a Fertilizer Product. Ecol. Chem. Eng. S 2019, 26, 547–557. [Google Scholar] [CrossRef]
- Borges, B.M.M.N.; Abdala, D.B.; de Souza, M.F.; Viglio, L.M.; Coelho, M.J.A.; Pavinato, P.S.; Franco, H.C.J. Organomineral phosphate fertilizer from sugarcane byproduct and its effects on soil phosphorus availability and sugarcane yield. Geoderma 2018, 339, 20–30. [Google Scholar] [CrossRef]
- Lu, C.; Tian, H. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: shifted hot spots and nutrient imbalance. Earth Syst. Sci. Data 2017, 9, 181–192. [Google Scholar] [CrossRef]
- FAO. Efficiency of Soil and Fertilizer Phosphorus Use. 2008.
- Pawlett, M. , Deeks, L. K., Sakrabani, R. Nutrient potential of biosolids and urea derived organo-mineral fertilizers in a field scale experiment using ryegrass (Lolium perenne L.). Field Crops Research 2015, 175, 56–63. [Google Scholar]
- Antille, D.L. Formulation, Utilization and Evaluation of Organo-mineral Fertilizers. Cranfield University 2011, Ph.D. dissertation. https://dspace.lib.cranfield.ac.uk/handle/1826/6853.
- Gendebien, A. , Davis, B., Hobson, J., Palfrey, R., Palfrey, R., Pitchers, R., Rumsby, P., Carlton-Smith, C., Middleton, J. Environmental, Economic and Social Impacts of the Use of Sewage Sludge on Land. Final Report. Project III. Project Interim Reports. 2008, EC DG ENV.G.4/ETU/2008/0076r.
- Honkeldieva, M.T.; Li, H.; Bukhorov, K.X.; A Ahmedov, H.; Yulbarsova, M.V. Fourier transformation of infrared spectroscopy and X-ray diffraction analyses of NPK mineral and biomineral fertilizers. IOP Conf. Series: Earth Environ. Sci. 2021, 868, 012042. [Google Scholar] [CrossRef]
- Ciampa, A.; Dell'Abate, M.T.; Florio, A.; Tarricone, L.; Di Gennaro, D.; Picone, G.; Trimigno, A.; Capozzi, F.; Benedetti, A. Combined magnetic resonance imaging and high resolution spectroscopy approaches to study the fertilization effects on metabolome, morphology and yeast community of wine grape berries, cultivar Nero di Troia. Food Chem. 2018, 274, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Vinci, G. , Cozzolino, V., Mazzei, P., Monda, H., Spaccini, R., Piccolo, A. An alternative to mineral phosphorus fertilizers: The combined effects of Trichoderma harzianum and compost on Zea mays, as revealed by 1H NMR and GC-MS metabolomics. PLoS One 2018, 13(12): e0209664.
- Borgia, G.; Brown, R.; Fantazzini, P. Uniform-Penalty Inversion of Multiexponential Decay Data. J. Magn. Reson. 1998, 132, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Crainic, R.; Fechete, R. Advanced monitoring of a laboratory scale modular green roof model. 12TH INTERNATIONAL CONFERENCE OF PROCESSES IN ISOTOPES AND MOLECULES (PIM 2019). LOCATION OF CONFERENCE, RomaniaDATE OF CONFERENCE; p. 030004.
- Drăgan, L.R.; Crainic, R.; Fechete, R. A Comparative Study for Natural Degradation of Three Local Anesthetic Drugs for Human use by 1H NMR Relaxometry and FT-IR Spectroscopy. 2018, 63, 61–73. [CrossRef]
- Venkataramanan, L.; Song, Y.Q.; Hurlimann, M.D. Solving Fredholm integrals of the first kind with tensor product structure in 2 and 2.5 dimensions. IEEE Trans. Signal Process. 2002, 50, 1017–1026. [Google Scholar] [CrossRef]
- Fechete, R.; Morar, I.A.; Moldovan, D.; Chelcea, R.I.; Crainic, R.; Nicoară, S.C. Fourier and Laplace-like low-field NMR spectroscopy: The perspectives of multivariate and artificial neural networks analyses. J. Magn. Reson. 2021, 324, 106915. [Google Scholar] [CrossRef] [PubMed]
- Cioica, N. , Fechete, R., Filip, C., Cozar, I. B., Cota, C., Nagy, E. M. NMR and SEM Investigation of Extruded Native Corn Starch with Plasticizers, Rom. J. of Phys 2015, 60 (3-4), 512-520.
- Fechete, R. , Demco, D. E., Blümich B. Enhanced sensitivity to residual dipolar couplings by high-order multiple-quantum NMR. J. Magn. Reson. 2004, 169, 19–26. [Google Scholar]
- Moldovan, D.; Fechete, R. Bimodal 1H Double Quantum Build-Up Curves by Fourier and Laplace-like Transforms on Aged Cross-Linked Natural Rubber. Polymers 2021, 13, 3523. [Google Scholar] [CrossRef] [PubMed]
- Crainic R, Drăgan, L. R., Fechete, R. 1H NMR relaxometry and ATR-FT-IR spectroscopy used for the assessment of wastewater treatment in slaughterhouse. Stud. Univ. Babes-Bolyai, Phys. 2018, 63, 49–60.
- Movasaghi, Z. , Rehman, S. , Rehman, I.U., Fourier Transform Infrared (FT-IR) Spectroscopy of Biological Tissues, Applied Spectroscopy Reviews 2008, 43, 134–179. [Google Scholar]
- Rakhmad, F.; Suwardi; Dyah, T. S. Release pattern of ammonium, nitrate, and potassium from Slow-Release Fertilizer (SRF) in the Soil. IOP Conf. Series: Earth Environ. Sci. 2019, 383, 012037. [Google Scholar] [CrossRef]
- Jin, J.; Zhou, W.; Sun, Y.; Han, Y.; Li, Y. Reaction Characteristics and Existing Form of Phosphorus during Coal-Based Reduction of Oolitic Iron Ore. Minerals 2021, 11, 247. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).