Submitted:
08 June 2024
Posted:
11 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Experimental Procedures
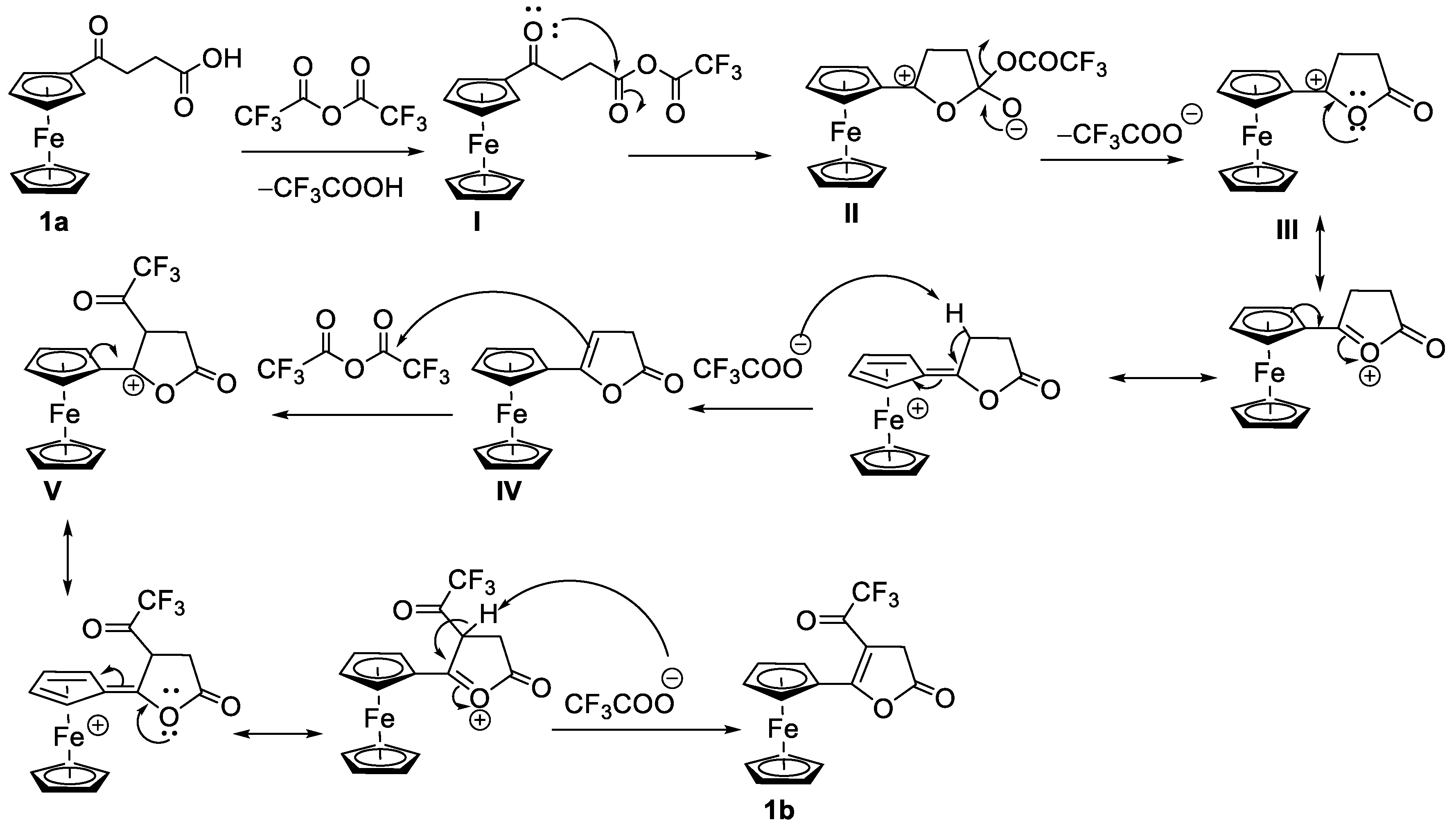
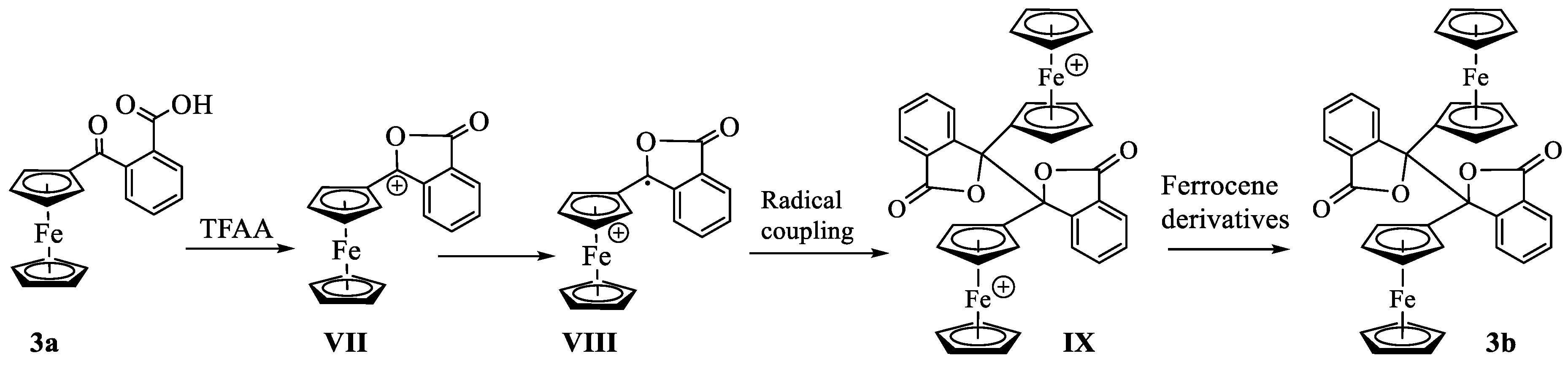
3. Results and Discussion
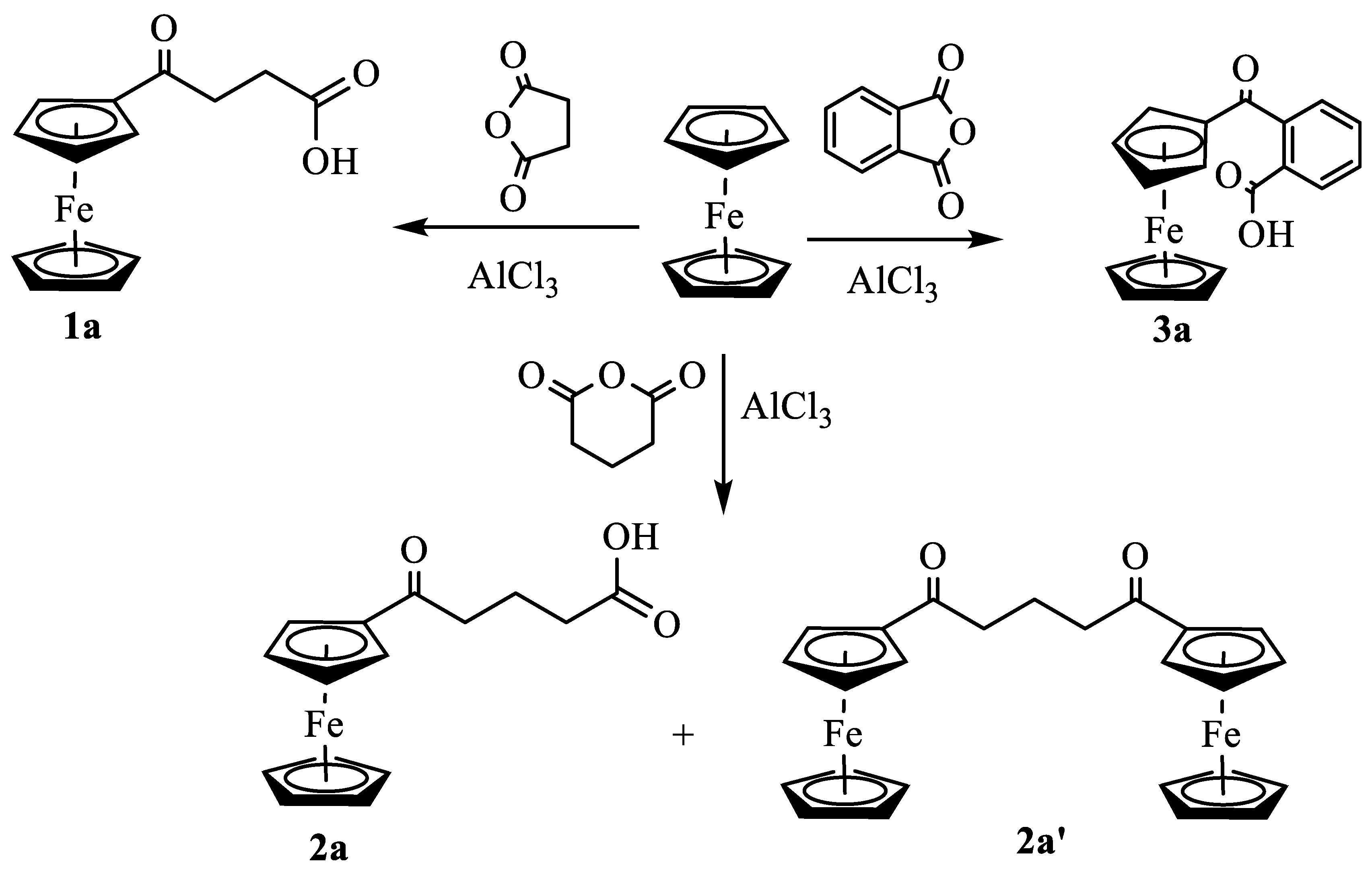
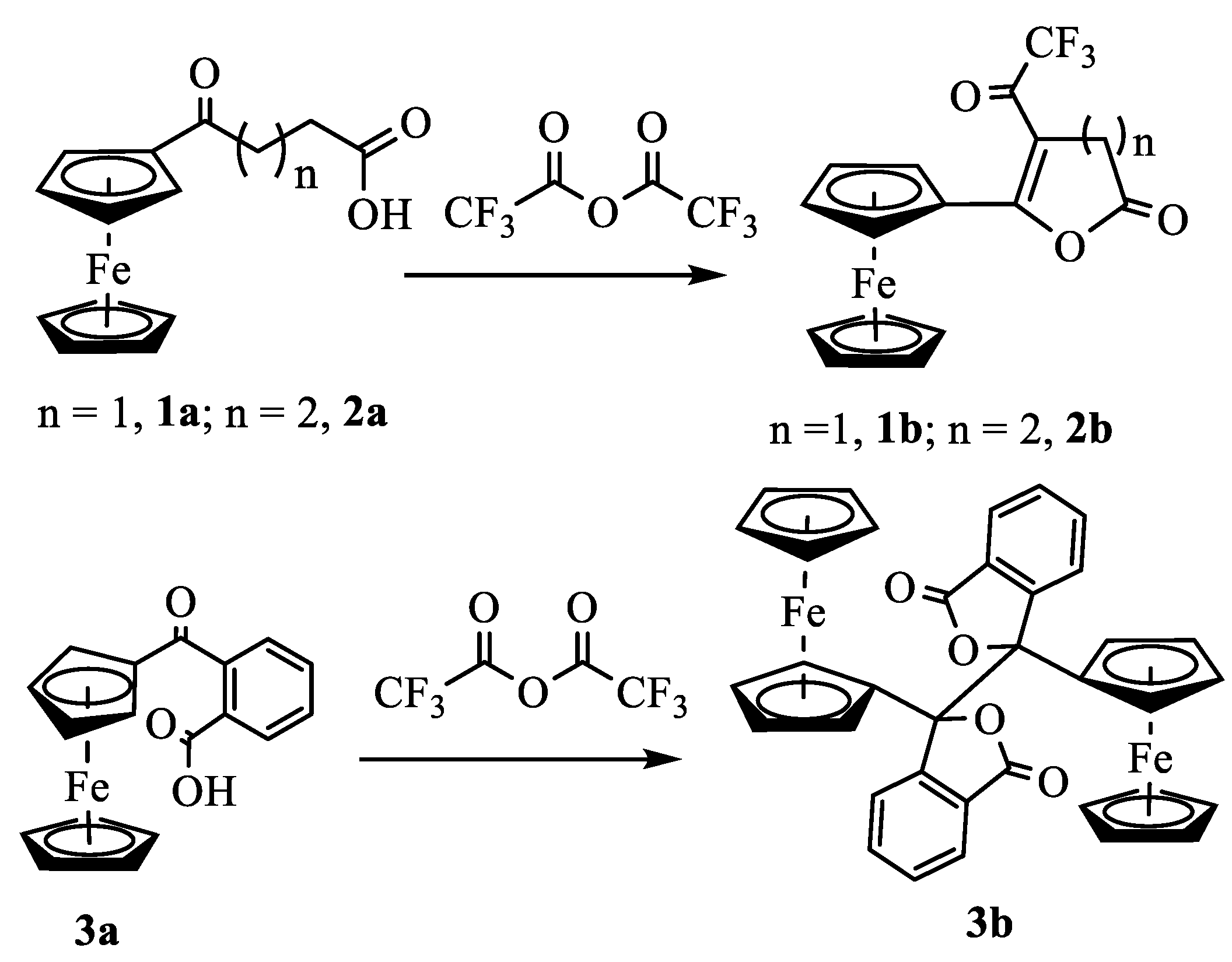
Synthesis and Structural Elucidation of Compounds 2a, 2a’, 1b – 5b and Proposed Mechanisms of Their Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kealy, T.J.; Pauson, P.L. A New Type of Organo-Iron Compound. Nature 1951, 168, 1039–1040. [Google Scholar] [CrossRef]
- Richards, J.H.; Hill, A.A. α-Metallocenyl carbonium ions. J. Am. Chem. Soc. 1959, 81, 3484. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Buchholz, H.; Reddy, V.P.; De Meijere, A.; Olah, G.A. Stable carbocations. 285. 1-Ferrocenyl-1-cyclopropyl cation: the first long-lived cyclopropyl cation. J. Am. Chem. Soc. 1992, 114, 1097. [Google Scholar] [CrossRef]
- Cully, N.; Watts, W.E. Stable carbocations. XX. A kinetic study of the SN1 hydrolysis of aryl(ferrocenyl)methyl acetates. J. Organomet. Chem. 1979, 182, 99–103. [Google Scholar] [CrossRef]
- Abram, T.S.; Watts, W.E. Stable carbocations. Part 12. Generation, observation, and properties of ferrocenyl-stabilized vinyl cations. J. Chem. Soc., Perkin Trans. 1. [CrossRef]
- Cerichelli, G.; Floris, B.; Ortaggi, G. Ferrocenyl carbocations. Ionization of ferrocenyl alcohols in aqueous sulfuric acid. J. Organomet. Chem. 1974, 78, 241. [Google Scholar] [CrossRef]
- Natsume, S.; Kurihara, H.; Yamaguchi, T.; Erabi, T.; Wada, M. Stability and reactivity of ferrocenyl(2,4,6-trimethoxyphenyl)carbenium salts. J. Organomet. Chem. 1999, 574, 86–93. [Google Scholar] [CrossRef]
- Korb, M.; Mahrholdt, J.; Liu, X.; Lang, H. Reactivity of Planar-Chiral α-Ferrocenyl Carbocations towards Electron-Rich Aromatics. Eur. J. Inorg. Chem. 2019, 2019, 973–987. [Google Scholar] [CrossRef]
- Rubalcava, H.E.; Thomson, J.B. A spectroscopic study of the protonation of acylferrocenes. Spectrochim. Acta 1962, 18, 449–459. [Google Scholar] [CrossRef]
- Olah, G.A.; Mo, Y.K. Organometallic chemistry: V. Protonation of acylferrocenes under stable ion conditions in fso3h-so2c1f solution. J. Organomet. Chem. 1973, 60, 311–321. [Google Scholar] [CrossRef]
- Roberts, R.M.G.; Silver, J.; Wells, A.S. Mössbauer and NMR studies of protonated acyl diphosphaferrocenes. Inorganica Chim. Acta. 1986, 119, 171–176. [Google Scholar] [CrossRef]
- Saric, A.; Vrcek, V.; Buehl, M. Density functional study of protonated formylmetallocenes. Organometallics 2008, 27, 394–401. [Google Scholar] [CrossRef]
- Bunnett, J.F. Nucleophilic Reactivity. Annu. Rev. Phys. Chem. 1963, 14, 271–290. [Google Scholar] [CrossRef]
- Jaramillo, P.; Pérez, P.; Fuentealba, P. Relationship between basicity and nucleophilicity. Journal of Physical Organic Chemistry 2007, 20, 1050–1057. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect A: Foundations and Advances 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Structural Chemistry 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, U.R.; Daigle, D.P.; Naquin, S.D.; Engeron, G.S.; Lo, M.A.; Fronczek, F.R. Synthesis, Crystal Structure, and Electrochemistry of Mono- and Bis-Homoannular Ferrocene Derivatives. Crystals 2024, 14, 141. [Google Scholar] [CrossRef]
- Wieczorek, A.; Blauz, A.; Makal, A.; Rychlik, B.; Plazuk, D. Synthesis and evaluation of biological properties of ferrocenyl-podophyllotoxin conjugates. Dalton Trans. 2017, 46, 10847–10858. [Google Scholar] [CrossRef]
- Woltersdorf, M.; Kranich, R.; Schmalz, H.-G. Enantioselective synthesis of new C2-symmetric ferrocenylalkylamines via sonochemical amination of 1-ferrocenylalkyl acetates. Tetrahedron 1997, 53, 7219–7230. [Google Scholar] [CrossRef]
- Pokharel, U.R.; Bergeron, J.T.; Fronczek, F.R. Synthesis and crystal structures of 2-(ferrocenylcarbonyl)benzoic acid and 3-ferrocenylphthalide. Acta Crystallogr., Sect. E 2020, 76, 1163–1167. [Google Scholar] [CrossRef]
- Goldberg, S.I.; Breland, J.G. Ferrocene studies. XIX. Synthesis of 1,2-terferrocene. J. Org. Chem. 1971, 36, 1499–1503. [Google Scholar] [CrossRef]
- Tombul, M.; Gemici, S.; Bulut, A. Alkyl Lewis Acid Catalyzed Syntheses of Dicarbonyl Ferrocenes. Asian J. Chem. 2010, 22, 7070. [Google Scholar]
- Tombul, M.; Bulut, A.; Guven, K.; Buyukgungor, O. 1,4-Diferrocenylbutane-1,4-dione. Acta Crystallogr., Sect. E 2008, 64, m444–m445. [Google Scholar] [CrossRef] [PubMed]
- Cremer, D.t.; Pople, J. General definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Cais, M.; Modiano, A.; Raveh, A. Organometallic Studies. XVII.1 A Novel Approach to the Synthesis of the Benzopentalene System2. J. Am. Chem. Soc. 1965, 87, 5607–5614. [Google Scholar] [CrossRef]
- Casper, L.A.; Ebel, V.; Linseis, M.; Winter, R.F. Five shades of green: substituent influence on the (spectro-) electrochemical properties of diferrocenyl(phenyl)methylium dyes. Dalton Trans. 2021, 50, 15336–15351. [Google Scholar] [CrossRef] [PubMed]
- Nesmeyanov, A.N.; Vil'chevskaya, V.D.; Kochetkova, N.S. Reactions of ο-carboxybenzoylferrocene. Dokl. Akad. Nauk SSSR 1965, 165, 835–837. [Google Scholar]
- Gleiter, R.; Seeger, R. The Structure of the Ferrocenyl-Methyl Cation. Preliminary communication. Helv. Chim. Acta 1971, 54, 1217–1220. [Google Scholar] [CrossRef]
- Cais, M.; Eisenstadt, A. Organometallic Studies. X.1a Reductive Dimerization of α-Metallocenylcarbonium Ions. I1b. J. Org. Chem. 1965, 30, 1148–1154. [Google Scholar] [CrossRef]
- Fedin, E.I.; Blumenfeld, A.L.; Petrovskii, P.V.; Kreindlin, A.Z.; Fadeeva, S.S.; Rybinskaya, M.I. Conversion of the diamagnetic nonamethylferrocenylcarbenium salts into the paramagnetic salts of bis(nonamethylferroceniumyl)ethane. J. Organomet. Chem. 1985, 292, 257–268. [Google Scholar] [CrossRef]
- Banide, E.V.; Ortin, Y.; Chamiot, B.; Cassidy, A.; Niehaus, J.; Moore, A.; Seward, C.M.; Müller-Bunz, H.; McGlinchey, M.J. Syntheses, Structures, and Dimerizations of Ferrocenyl- and Fluorenylideneallenes: Push−Pull Multiple Bonds? Organometallics 2008, 27, 4173–4182. [Google Scholar] [CrossRef]
- Casper, L.A.; Deuter, K.L.; Rehse, A.; Winter, R.F. Dimerization of 9-Phenyl-ferroceno [2,3]indenylmethyl Radicals: Electrochemical and Spectroelectrochemical Studies. ACS Org. & Inorg. Au. [CrossRef]
- Casper, L.A.; Linseis, M.; Demeshko, S.; Azarkh, M.; Drescher, M.; Winter, R.F. Tailoring Valence Tautomerism by Using Redox Potentials: Studies on Ferrocene-Based Triarylmethylium Dyes with Electron-Poor Fluorenylium and Thioxanthylium Acceptors. Chem. Eur. J. 2021, 27, 10854–10868. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.W.; Rabb, D.J. 1,2-(α-Oxotetramethylene)ferrocene. J. Org. Chem. 1961, 26, 3588. [Google Scholar] [CrossRef]
- Sugiyama, N.; Suzuki, H.; Shioura, Y.; Teitei, T. Reaction of ferrocene with acyl chlorides. Bull. Chem. Soc. Jpn. 1962, 35, 767. [Google Scholar] [CrossRef]
- Husain, A.; Khan, S.A.; Iram, F.; Iqbal, M.A.; Asif, M. Insights into the chemistry and therapeutic potential of furanones: A versatile pharmacophore. Eur. J. Med. Chem. 2019, 171, 66–92. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.S.; Rather, M.A.; Maqbool, M.; Lah, H.U.; Yousuf, S.K.; Ahmad, Z. α-pyrones: Small molecules with versatile structural diversity reflected in multiple pharmacological activities-an update. Biomedicine & Pharmacotherapy 2017, 91, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, K.L., Jr.; Curby, R.J., Jr. Ferrocene bridging and homoannular cyclizations. J. Am. Chem. Soc. 1957, 79, 3290. [Google Scholar] [CrossRef]
- Rinehart, K.L., Jr.; Curby, R.J., Jr.; Gustafson, D.H.; Harrison, K.G.; Bozak, R.E.; Bublitz, D.E. Organic chemistry of ferrocene. V. Cyclization of ω-ferrocenylaliphatic acids. J. Am. Chem. Soc. 1962, 84, 3263. [Google Scholar] [CrossRef]
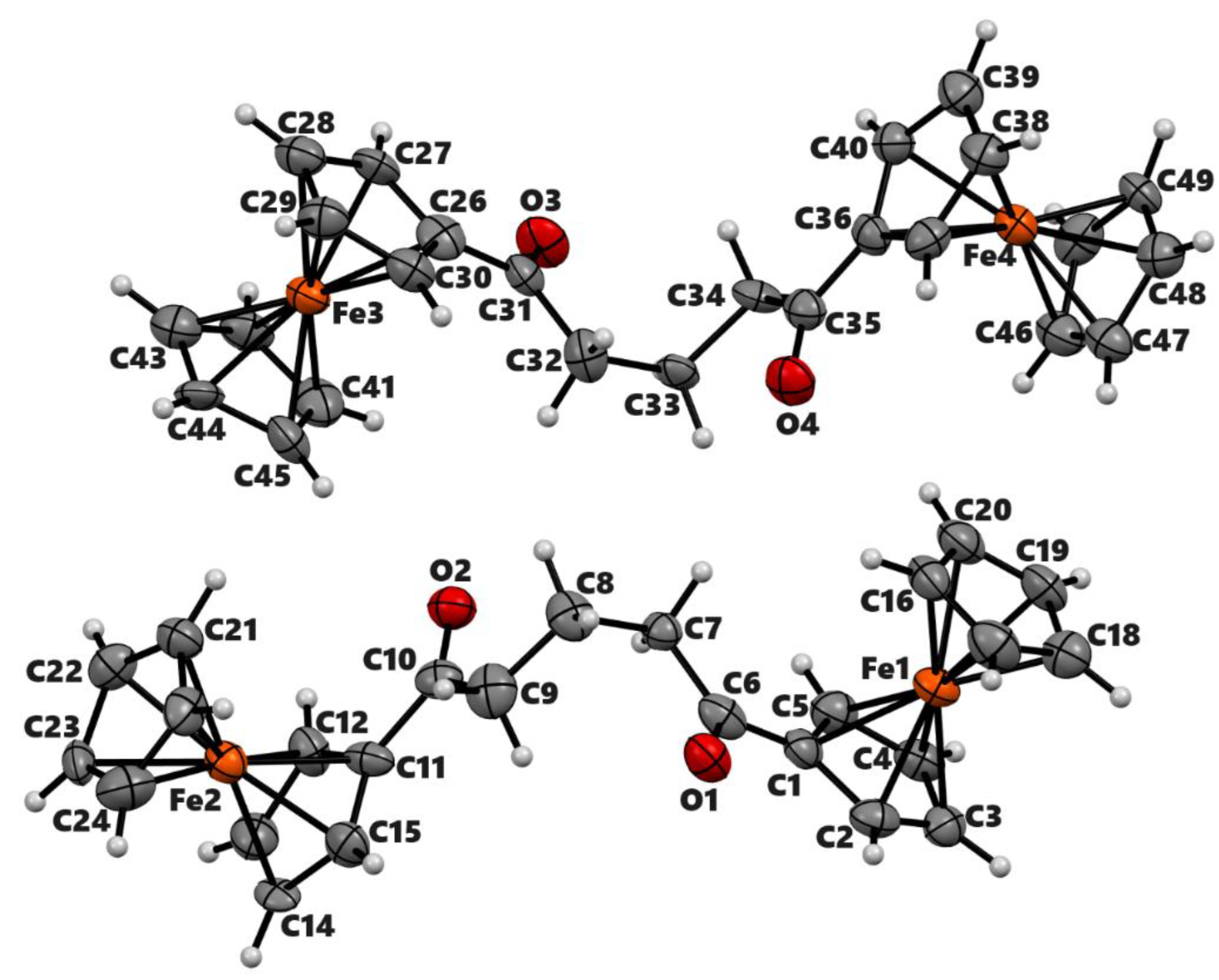
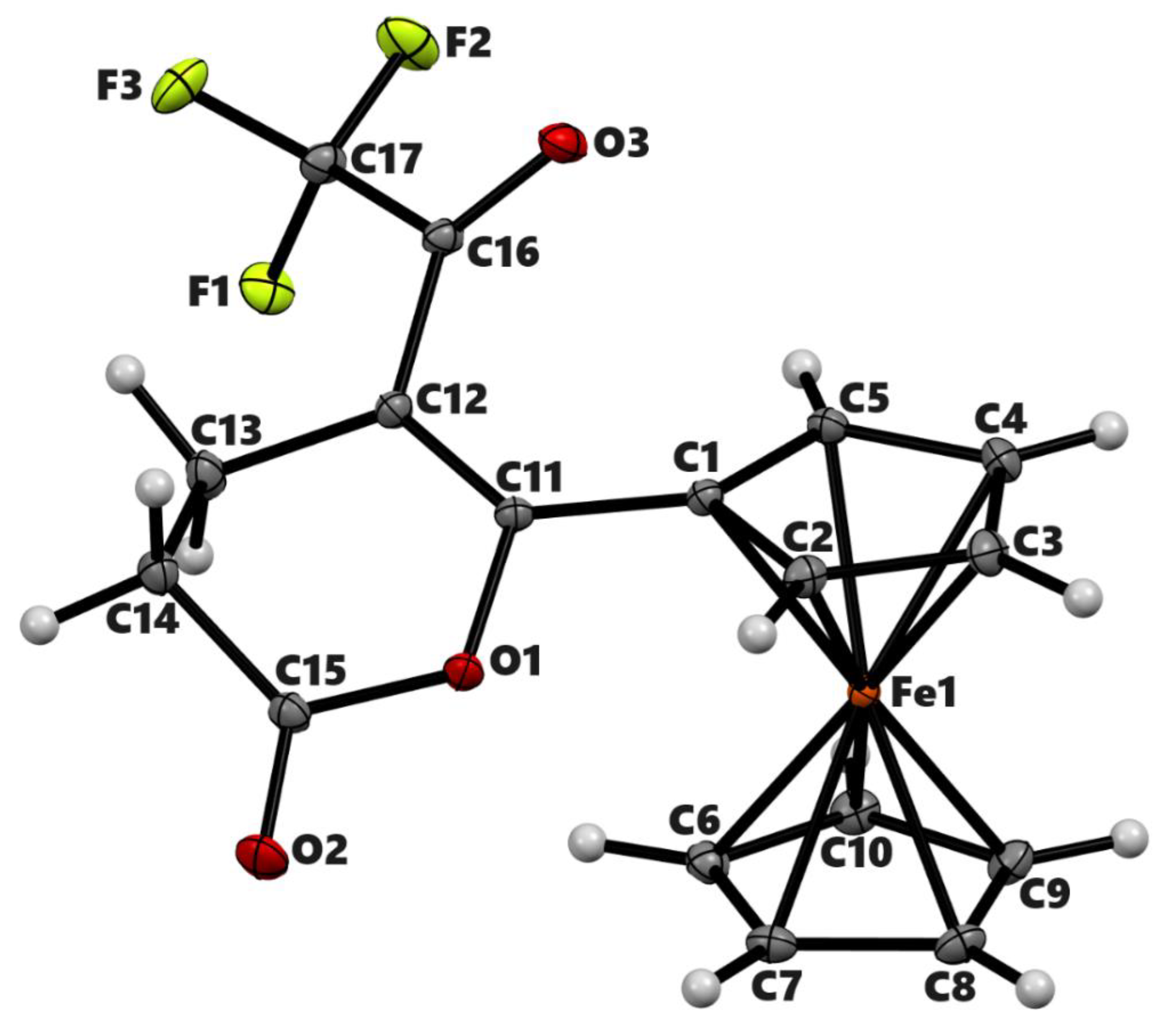
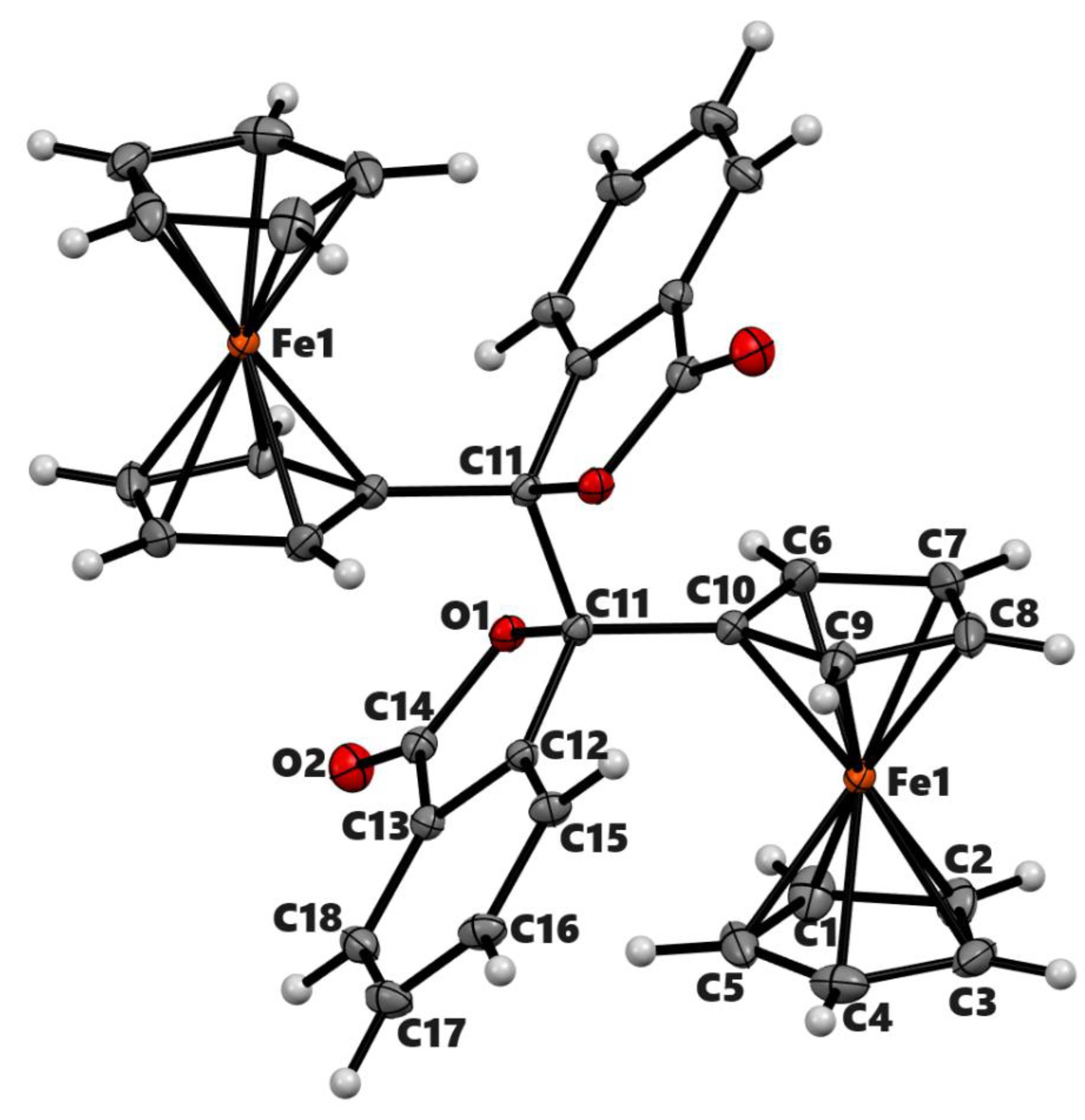


| 2a’ | 2b | 3b | |
| Chemical formula | C25H24Fe2O2 | C17H13F3FeO3 | C36H26Fe2O4 |
| Mr | 468.14 | 378.12 | 634.27 |
| Deposition number | CCDC 2355187 | CCDC 2355188 | CCDC 2355189 |
| Crystal system, space group | Monoclinic, P21 | Monoclinic, P21/n | Monoclinic, P21/c |
| Temperature (K) | 90 | 90 | 90 |
| a, b, c (Å) | 5.7838 (5), 11.8770 (9), 28.071 (2) | 10.6270 (2), 9.7968 (2), 14.9960 (3) | 9.8279 (8), 13.3787 (10), 10.8256 (8) |
| β (°) | 93.773 (6) | 110.1183 (9) | 111.064 (4) |
| V (Å3) | 1924.2 (3) | 1465.98 (5) | 1328.29 (18) |
| Z | 4 | 4 | 2 |
| Radiation type | Cu Kα | Mo Kα | Mo Kα |
| µ (mm−1) | 12.24 | 1.08 | 1.14 |
| Crystal size (mm) | 0.15 × 0.04 × 0.01 | 0.38 × 0.30 × 0.13 | 0.22 × 0.15 × 0.14 |
| Diffractometer | Bruker Kappa APEX-II DUO | Bruker Kappa APEX-II DUO | Bruker Kappa APEX-II DUO |
| Absorption correction | Multi-scan | Multi-scan | Multi-scan |
| Tmin, Tmax | 0.564, 0.887 | 0.764, 0.873 | 0.811, 0.857 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 12160, 5225, 3851 | 74201, 13090, 12014 | 65909, 9349, 7963 |
| Rint | 0.074 | 0.019 | 0.031 |
| (sin θ/λ)max (Å−1) | 0.568 | 1.086 | 0.945 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.068, 0.178, 1.04 | 0.021, 0.059, 1.05 | 0.031, 0.086, 1.05 |
| No. of reflections | 5225 | 13090 | 9349 |
| No. of parameters | 524 | 244 | 190 |
| Δρmax, Δρmin (e Å−3) | 1.01, −0.55 | 0.88, −0.48 | 1.02, −0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





