1. Introduction
Acute myeloid leukemia (AML) is the most common form of acute leukemia among adults and is characterized by the clonal expansion of myeloid blasts in the peripheral blood, bone marrow, or other tissues [
1,
2]. AML is a disease of the elderly, with median age of 67 at diagnosis. Currently 20,380 cases of leukemia in adults per year would be AML, and 11,310 patients die from AML every year [
1].
Current therapeutic options for elderly individuals with AML include intensive chemotherapy with a cytarabine and anthracycline backbone, hypomethylating agents (decitabine and azacitidine), low-dose cytarabine, investigational agents, and supportive care with hydroxyurea and transfusions. Over the last few years, there has been increasing debate regarding the appropriate therapeutic approach to take in older adults given the diversity of the geriatric patient population and heterogeneous AML disease biology including unfavourable cytogenetics and presence of antecedent hematopoietic disorders [
3]. Pharmacokinetic and pharmacodynamic changes in elderly, resulting in decreased drug clearance and in prolonged exposure to chemotherapeutics, reduced immune competence of elderly patients and also psychosocial factors, cognitive decline are some other factors that play into treatment decisions [
4]. Right now, there are limited biomarkers to determine as to which patients will have higher mortality risk or more complications to guide treatment decisions.
Currently, the choice of the best treatment option for AML patients relies on the prognostic risk stratification of the most widely used 2017 European Leukemia Net (2017 ELN), which is mainly based on cytogenetic risk factors and molecular markers [
5]. Although the relationship between some molecular abnormalities and the prognosis of AML has been identified, there remain many molecular abnormalities where relationships with the prognosis of AML that are unclear. Here several other biomarkers such as albumin could be helpful. Additionally, since a wide majority percent of population live in resource poor countries, not all patients will have access to comprehensive diagnostic workup and there will be lack of full genetic and molecular data at diagnosis.
The role of serum albumin as a prognostic factor has been well established in various medical conditions including some hematologic malignancies [
7,
8]. Hypoalbuminemia is so significant in multiple myeloma that it is included as a criteria in the international staging system for multiple myeloma [
8]. At present, there are few validated serum biomarkers that can be used for predicting outcomes in patients with acute myeloid leukemia (AML). Previous works by Khan et al. have investigated the utility of serum albumin in newly diagnosed AML patients who received induction chemotherapy (3+ 7 regimen) and found that hypoalbuminemia was an independent predictor of overall survival with conventional chemotherapy management [
9]. Komrokji et. al determined that hypoalbuminemia was a poor prognostic factor for OS and CR prior to salvage chemotherapy in relapsed and refractory AML [
10].
In this retrospective analysis, we examined the prognostic value of serum albumin at diagnosis prior to any therapy in a cohort of patients with AML irrespective of treatment modalities.
2. Method
We performed a retrospective study of 257 patients with AML, who received treatment between 2002 to 2019 at Augusta University Medical Center. 46 of these patients were lost to follow up and were excluded from the study. The cohort included 1. Patients who received standard 7 + 3 induction, 2. Patients who were not candidates for induction, receiving targeted therapy and 3. Patients who were placed on clinical trials. Patients less than 17 years of age or those who had their initial diagnosis and induction at outside hospital and where laboratory data for initial albumin at diagnosis was not available, and patients who were not identified as Caucasian or African American, were excluded from the study.
Patients were classified as having normal (>3.5 g/dl) albumin or as hypoalbuminemia (<3.5 g/dl). Patient demographics and treatment outcomes were then categorized according to baseline albumin levels. Primary objective of the study was to compare OS between patients with normal albumin and hypoalbuminemia at time of diagnosis. Secondary objective of the study was to determine impact of other demographic factors on overall survival in AML. Statistical analysis was performed using Epi info software. Kaplan- Meier curve was created using SPSS. Chi square test was performed for univariate analysis. Logistic regression was performed for multivariate analysis.
3. Results
3.1. Baseline Characteristics
Out of 211 patients, median age was 59.4 years. There was no significant age difference between normal albumin group and hypoalbuminemia group. (Median age 59 and 59.7 respectively)(p=0.854). Out of 211 patients, 105 were male and 106 were female, 38 males had hypoalbuminemia, and 35 women had hypoalbuminemia at diagnosis. No statistical significant difference with respect to sex and hypoalbuminemia could be identified (33 % in females, 36.2% in males) (p=0.560). With regards to race, more African Americans had hypoalbuminemia compared to Caucasians (46 percent in African Americans versus 30 percent in Caucasians) (p=0.027).
3.2. Survival
Of the overall cohort, 171 of 211 patients survived at the end of 6 months. Patients with hypoalbuminemia had an overall survival rate of 72.2 percent at 6 months and patients with normal albumin had OS rate of 85.1 percent (p=0.027) at 6 months. 185 patients could be traced till 1 year period. OS at 1 year in hypoalbuminemia group was 62.5 percent and OS in normal albumin group was 72.7 percent (p=0.18). Further results can be found in
Table 1. Multivariate logistic regression analysis including age, race, sex and albumin levels depicted that age, sex and albumin levels were statistically significant important predictors of survival at 6 months (as in
Table 2). It showed that age was inversely associated with survival at 6 months (p=0.003) and males were more likely to survive than females (p=0.034).
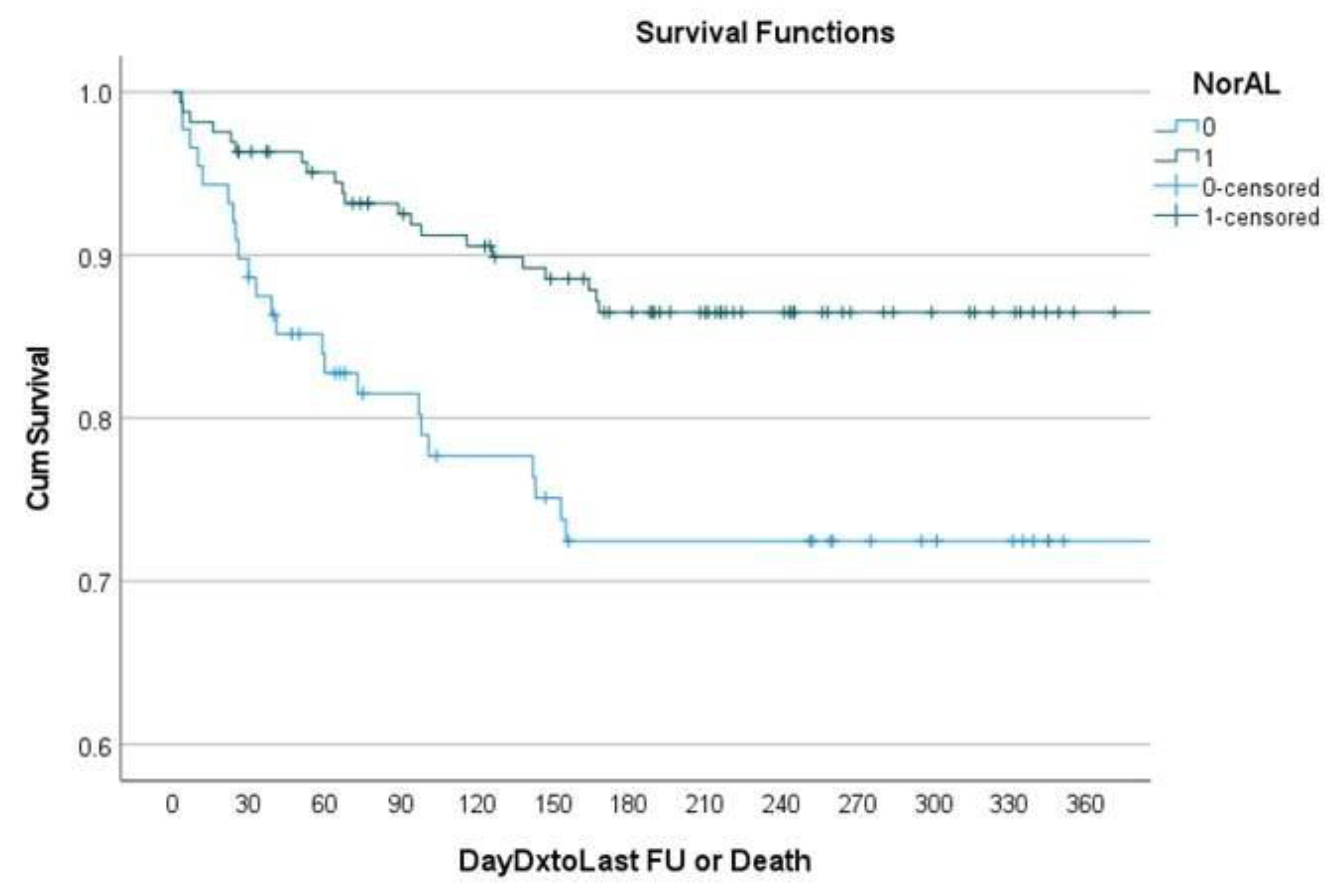
Patients with albumin >=3.5 were more likely to survive at 6 months controlling for age, race and sex (OR 2.16, p=0.044). A Kaplan-Meier curve [
Figure 1] demonstrates that at 180 days patients with hypoalbuminemia had significantly decreased survival compared to patients with normal albumin.
Logistic regression showing sex, albumin levels and age at diagnosis as important predictors of survival in AML
4. Discussion
In this cohort of newly diagnosed, relapsed and refractory AML patients, treated with various regimens, we found that hypoalbuminemia was an independent predictor of overall survival. Serum albumin < 3.5 was associated with significantly decreased overall survival at 6 months. We additionally found that age and sex were also independent predictors of survival. This data suggests that hypoalbuminemia has prognostic utility in AML patients.
As suggested earlier, current prognostication in AML is primarily by the ELN criteria, which relies heavily on the molecular and genetic characteristics of leukemia [
5]. In addition to disease related factors, patient related factors including age, other comorbidities and overall functional status become important, especially in elderly population [
4]. Further, in resource poor settings, complete molecular and genetic data may not be available at the time of diagnosis [
14]. Hence in these cases, clinical characteristics may help guide treatment decisions and help indicate further prognosis. Serum albumin level can be used as one such tool.
Khan et al demonstrated serum albumin as a prognostic marker in newly diagnosed patients with AML receiving 7 plus 3 induction chemotherapy [
9]. Wang et al. studied albumin as a prognostic marker in patients with de novo AML, treated with induction and HSCT (hematopoetic stem cell transplant), and concluded albumin could serve as an important and cheap prognostic marker to determine long term survival [
11]. Dou et al suggested use of albumin and CRP and determined CRP albumin ratio, which through a retrospective study was associated with worse CR and worse OS rates, particularly patients with intermediate risk stratification and those who did not undergo HSCT [
13]. Heini et al investigated use of index incorporating CRP, albumin and fibrinogen, via a retrospective study as a prognostic marker in AML [
12]. Douglas et al, demonstrated scoring system based integrating clinical characteristics (albumin, age and WBC counts) with genetic risks comparable with ELN prognostication, especially when molecular data were unavailable or not fully available [
14].
There are certain limitations in our study including its retrospective nature, limited data regarding cause of death, limited data on fitness of the patients, limited data on the type of treatment modality used and genetic and molecular classification of the AML. Furthermore, serum albumin may not be a causal factor in survival and may merely be a marker of inflammation, disease activity or overall functional, nutritional and physiological status.
Future studies validating serum albumin as a prognostic biomarker for morbidity and mortality, especially with regards to specific treatments should be considered. Once validated, serum albumin can be incorporated into prognostication tools and decision-making tools for guiding treatment regimens based on predicted outcomes.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Siegel, RL, Miller, KD, Wagle, NS, Jemal, A. Cancer statistics, 2023. CA Cancer J Clin. 2023; 73( 1): 17- 48. [CrossRef]
- Pollyea, D. A., Bixby, D., Perl, A., Bhatt, V. R., Altman, J. K., Appelbaum, F. R., de Lima, M., Fathi, A. T., Foran, J. M., Gojo, I., Hall, A. C., Jacoby, M., Lancet, J., Mannis, G., Marcucci, G., Martin, M. G., Mims, A., Neff, J., Nejati, R., Olin, R., Percival, M., Prebet, T., Przespolewski, A., Rao, D., Ravandi-Kashani, F., Shami, P. J., Stone, R. M., Strickland, S. A., Sweet, K., Vachhani, P., Wieduwilt, M., Gregory, K. M., Ogba, N., & Tallman, M. S. (2021). NCCN Guidelines Insights: Acute Myeloid Leukemia, Version 2.2021, Journal of the National Comprehensive Cancer Network, 19(1), 16-27.
- Eunice S. Wang; Treating acute myeloid leukemia in older adults. Hematology Am Soc Hematol Educ Program 2014; 2014 (1): 14–20. [CrossRef]
- Gert Ossenkoppele, Bob Löwenberg; How I treat the older patient with acute myeloid leukemia. Blood 2015; 125 (5): 767–774. [CrossRef]
- Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Löwenberg B, Bloomfield CD. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017 Jan 26;129(4):424-447. [CrossRef]
- Komrokji RS, Corrales- Yepez M, Kharfan-Dabaja MA, et al. Hypo-albuminemia is an independent prognostic factor for overall survival in myelodysplastic syndromes.Am J Hematol.2012;87(11):1006-1009. [CrossRef]
- Greipp PR,San Miguel J,Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol 2005; 23: 3412–3420. 2. [CrossRef]
- Komrokji RS, Corrales-Yepez M, Kharfan-Dabaja MA, et al. Hypoalbuminemia is an independent prognostic factor for overall survival in myelodysplastic syndromes. Am J Hematol. 2012;87(11):1006-1009. [CrossRef]
- Khan AM, Lancet JE, Kharfan-Dabaja MA, Al Ali NH, List AF, Komrokji RS. Albumin is a prognostic factor for overall survival in newly diagnosed patients with acute myeloid leukemia (AML). Blood. 2011; 118(21): 4253- 4253. [CrossRef]
- Komrokji RS, Kharfan-Dabaja MA, Price SL, et al. Albumin is a prognostic factor for response and overall survival in relapsed or refractory acute myeloid leukemia (AML). Blood. 2009; 114(22): 4685- 4685. [CrossRef]
- Wang N, Desai A, Ge B, et al. Prognostic value of hypoalbuminemia at diagnosis in de novo non-M3 acute myeloid leukemia. Leuk Lymphoma, 2020;61(3):641-649. [CrossRef]
- Heini AD, Hugo R, Berger MD, Novak U, Bacher U, Pabst T. Simple acute phase protein score to predict long-term survival in patients with acute myeloid leukemia. Hematol Oncol. 2020; 38(1): 74- 81. [CrossRef]
- Dou L, Shi M, Song J, Niu X, Niu J, Wei S, Li D, Bai Y, Sun K. The Prognostic Significance of C-Reactive Protein to Albumin Ratio in Newly Diagnosed Acute Myeloid Leukaemia Patients. Cancer Manag Res. 2022 Jan 25;14:303-316. [CrossRef]
- Douglas R. A. Silveira, Lynn Quek, Itamar S. Santos, Anna Corby, Juan L. Coelho-Silva, Diego A. Pereira-Martins, Grant Vallance, Benjamin Brown, Luciana Nardinelli, Wellington F. Silva, Elvira D. R. P. Velloso, Antonio R. Lucena-Araujo, Fabiola Traina, Andy Peniket, Paresh Vyas, Eduardo M. Rego, Israel Bendit, Vanderson Rocha; Integrating clinical features with genetic factors enhances survival prediction for adults with acute myeloid leukemia. Blood Adv 2020; 4 (10): 2339–2350. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).