1. Introduction
Liver disease accounts for 4% of all deaths worldwide, and the most common cause is cirrhosis [
1]. Cirrhosis is an advanced stage of liver fibrosis, characterized by the irreversible formation of regenerative nodules with excessive extracellular matrix (ECM) accumulation. Liver fibrogenesis is the key mechanism in the wound-healing process of the liver after inflammation and injury, providing fibril-forming collagen as a scaffold for tissue regeneration [
2]. Various intrinsic and extrinsic stimuli of hepatic inflammation, such as cholecystitis, viral hepatitis, alcohol consumption, and non-alcoholic fatty liver disease (NAFLD), lead to the activation of hepatic stellate cells (HSCs) [
3]. HSCs differentiate from myofibroblasts and have been established as the central driver of liver fibrogenesis in
in vitro and
in vivo experiments [
4]. In the homeostasis of the wound healing process, ECM deposition is a tightly regulated process that is reversible after the stimulant is removed. Transforming growth factor-beta 1 (TGF-β1) is a one of key molecules involved in liver fibrogenesis in HSCs via the stimulation of mothers against decapentaplegic homolog (SMAD) transcription factors [
4]. Patients with chronic liver fibrosis progression are at a higher risk of developing liver cancer such as hepatocellular carcinoma (HCC) [
5]. At present, there is no standard treatment available for liver fibrosis [
6], and novel approaches to treat this condition is a sort-after-goal. Therefore, finding a novel and effective treatment could explore the possibility of a therapeutic approach.
Citrus hystrix DC., also known as Kaffir lime (KL), is a plant belonging to the Rutaceae family that is cultivated mainly in Asiatic countries. The KL is well-known as the source of unique volatile compounds in an ingredient of foods and alternative medicine. Several chemical compounds are responsible for an intense kaffir lemon odor, such as Citronellal, L-Linalool, 1,8-Cineole, and α-Terpeneol [
7]. The extract and active compounds from this plant harbor several pharmacological activities, including anti-inflammatory [
8,
9], antioxidants [
10,
11], anti-microbial [
12,
13], and anti-cancer effects [14-16]. The hepatoprotective effects of KL have been reported in paracetamol-induced liver injury in mice models [
17]. However, the anti-liver fibrosis activity of the KL extract and its derivative compounds remains unexplored.
In this study, we examined the anti-fibrotic effects of the crude extract and β-citronellol, an active compound identified from the KL leaf. β-citronellol is a monoterpenoid that has been previously reported with anti-inflammatory, anti-fungal, and anti-cancer properties [
9,
15,
18]. The anti-liver fibrosis activity of β-citronellol was tested in TGF-β1-induced human HSCs (LX-2 cells). Several hepatic fibrogenesis markers were measured, including genes and protein of HSCs. Proteomics and molecular docking analyses were employed to elucidate the possible β-citronellol mechanisms of action on LX-2 cells. Our study suggested the potential role of KL extract and its compound in preventing liver fibrosis development in the HSCs model, providing a new source for anti-hepatic fibrosis research.
2. Materials and Methods
2.1. Preparation of crude extract and active compound
The initial crude extract of KL leaves employed in this investigation was generated via maceration of KL powder, as detailed in a prior publication [
9]. Briefly, a 1,000-gram sample of KL powder underwent sequential maceration in
n-hexane (absolute), ethyl acetate (95%), and ethanol (95%) for periods of three days each. Following filtration and evaporation of the ethanolic solute, 100.56 grams of crude ethanolic KL leaf extract were obtained. The active compound β-citronellol was acquired from a commercial chemical supplier. Stock solutions of both the crude extract and the active compound were formulated using a solvent mixture of DMSO and Tween-80 (1:1 ratio). Throughout cell treatment procedures, residual solvent concentration was maintained below 0.5%.
2.2. Cell culture
LX-2 cells (an immortalized human hepatic stellate cell line) were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 2% fetal bovine serum (FBS) and 1% antibiotic-antimycotic solution. The cells were maintained in a humidified incubator at 37°C with 5% CO2. This cell line was kindly provided by Dr. Saranyapin Potikanond (Department of Pharmacology, Chiang Mai University, Thailand).
2.3. Cell viability assay
To establish the optimal treatment concentrations of crude Kaffir lime leaf extract (KL) and β-citronellol (β-CIT) on LX-2 cells, a resazurin reduction cytotoxicity assay was conducted. LX-2 cells were seeded (2 × 104 cells/well) in a 96-well plate and treated with varying concentrations of KL and β-CIT (0 – 1,000 µg/mL). Following a 24 h incubation at 37°C, resazurin (25 µg/mL) was added, and fluorescence was measured after 4 h (excitation/emission: 560/590 nm) using an EnSpire® Multimode microplate reader. GraphPad Prism version 8 (GraphPad Software, La Jolla, CA, USA) was used to determine inhibitory concentrations (IC). The IC10 value was selected as the maximum safe concentration for further experimental use.
2.4. RNA extraction and gene expression analysis
To assess the potential anti-fibrotic effects of KL and β-CIT on gene expression, LX-2 cells (2 × 10
5 cells/well) were seeded into 24-well plates and stimulated with TGF-β1 (10 ng/mL) to induce fibrogenesis. Following this, the cells were treated with selected concentrations of both compounds and incubated for 24 hours. After the treatment period, the supernatant was discarded, and RNA extraction was performed using Trizol reagent in adherence to the manufacturer's instructions. RNA concentration and purity were assessed utilizing a Nanodrop spectrophotometer. Subsequently, cDNA synthesis was performed on the extracted RNA from each condition using a Tetro™ cDNA Synthesis Kit. Quantitative real-time polymerase chain reaction (real time qPCR) was employed to evaluate the expression levels of genes implicated in liver fibrosis. Amplification of gene transcripts was achieved using nucleotide primers designed specifically for
ACTA2,
TIMP1,
COL1A1, and
SMAD2 genes. The qPCR reactions were carried out with a SensiFAST™ SYBR® No-ROX Kit on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). The PCR protocol consisted of an initial polymerase activation step (95°C, 1 min), followed by 45 cycles comprising denaturation (95°C, 10 s) and annealing/extension (60°C, 1 min). The 2
(-∆∆Ct) [
19] method was used to determine the relative expression of each target gene, normalized against the internal reference gene,
GAPDH.
Table 1 provides a comprehensive list of the forward and reverse primers used for each gene [
20].
2.5. Measurement of the MMP-9 production
The activation of hepatic stellate cells (HSCs) is characterized by the release of matrix metalloproteinase 9 (MMP-9), a pivotal enzyme in this process. To assess whether KL and β-CIT exhibit MMP-9 inhibitory activity, LX-2 cells (2 x 105 cells/well) were seeded in 24-well plates and stimulated with TGF-β1 (10 ng/mL), either alone or in conjunction with KL and β-CIT, for 24 hours. Quantification of MMP-9 in the collected supernatants was performed using a human MMP-9 ELISA kit, adhering to the manufacturer's protocol. Absorbance readings were analyzed using GraphPad Prism version 8 to determine MMP-9 levels.
2.6. LC-MS/MS analysis
2.6.1. Preparation of protein sample
To rigorously assess the impact of KL and β-CIT on TGF-β1-induced LX-2 cell global protein expression, liquid chromatography with tandem mass spectrometry (LC-MS/MS) was employed. Cellular treatment and lysate extraction procedures were consistent with those outlined in the previous section. LC-MS/MS sample preparation followed an established protocol with minor adjustments to ensure analytical reproducibility [
21]. Protein concentration was attained through the use of a 3 kDa molecular weight cut-off filter. Subsequent precipitation was induced with ice-cold acetone (1:5 v/v). The resulting pellet was then resuspended in a solution of 0.3% RapidGest SF and 2.5 mM ammonium bicarbonate. A 30-µg protein aliquot was subjected to tryptic digestion. To facilitate proteolysis, disulfide bonds were reduced with 1 mM tris(2-carboxyethyl) phosphine (TCEP) at 37 °C for 2 hours, followed by alkylation with 5 mM iodoacetamide (IAA) at room temperature for 50 minutes in a light-protected environment. The sample underwent desalting (Zeba Spin Column) before a second tryptic digestion step (1:40 enzyme-protein ratio) at 37 °C for 6 hours. Finally, the digested peptides were dried and resuspended in 0.1% formic acid in preparation for LC-MS/MS analysis.
2.6.2. Proteomic data analysis
Protein samples underwent analytical scrutiny via a high-fidelity hybrid Quadrupole-Orbitrap system (HF-X) coupled with an EASY-nLC1000 for refined separation. A nano C18 column operating in positive ionization mode facilitated chromatographic resolution. Employing a calibrated gradient of 90% acetonitrile/0.1% formic acid over 135 minutes (300 nL/min), with mobile phase A consisting of 0.1% formic acid in water, optimal separation was achieved. The column was meticulously regenerated and re-equilibrated. Data-dependent acquisition (TopN15) utilizing higher-energy collisional dissociation (29 eV) directed peptide analysis. Proteome Discoverer™ 2.4 software integrated MS parameters and thorough database searches (UniProt Homo sapiens, 14/01/2023). Stringent peptide/protein tolerances, modifications, and a 1% FDR ensured data integrity. After normalization (total intensity count), pathway analysis employed PADOG within Reactome v84 (Homo sapiens, 25/02/2023) [
22].
2.7. Molecular docking analysis
Computational molecular docking analysis was performed to investigate the interaction between β-CIT and selected proteins implicated in HSCs activation, a key process in liver fibrosis. Protein 3D structures associated with HSC activation during hepatic fibrosis were obtained from the RCSB Protein Data Bank (RCSB PDB) [
23,
24] and prepared in PDB format. The chemical structure of β-citronellol (PubChem CID 8842) was retrieved from PubChem [
25]. The protein-ligand blind docking analysis was conducted using the CB-Dock2 server (
https://cadd.labshare.cn/cb-dock2/index.php) [
26]. Prior to docking analysis, both the protein targets and β-citronellol were prepared using the DockPrep tool within UCSF Chimera alpha software version 1.18 [
27]. Post-docking, interactions between the target proteins and ligand were visualized in 2D using BIOVIA Discovery Studio Visualizer version 21.1.0.20298 (San Diego, USA).
2.8. Statistical analysis
Each in vitro experimental procedure was conducted in triplicate. Unless otherwise specified, data representation follows a mean ± SD format. Statistical significance between means was assessed using one-way ANOVA (Tukey's multiple comparison test) within GraphPad Prism software (version 8.0.1). A p-value threshold of < 0.05 denoted statistical significance.
3. Results
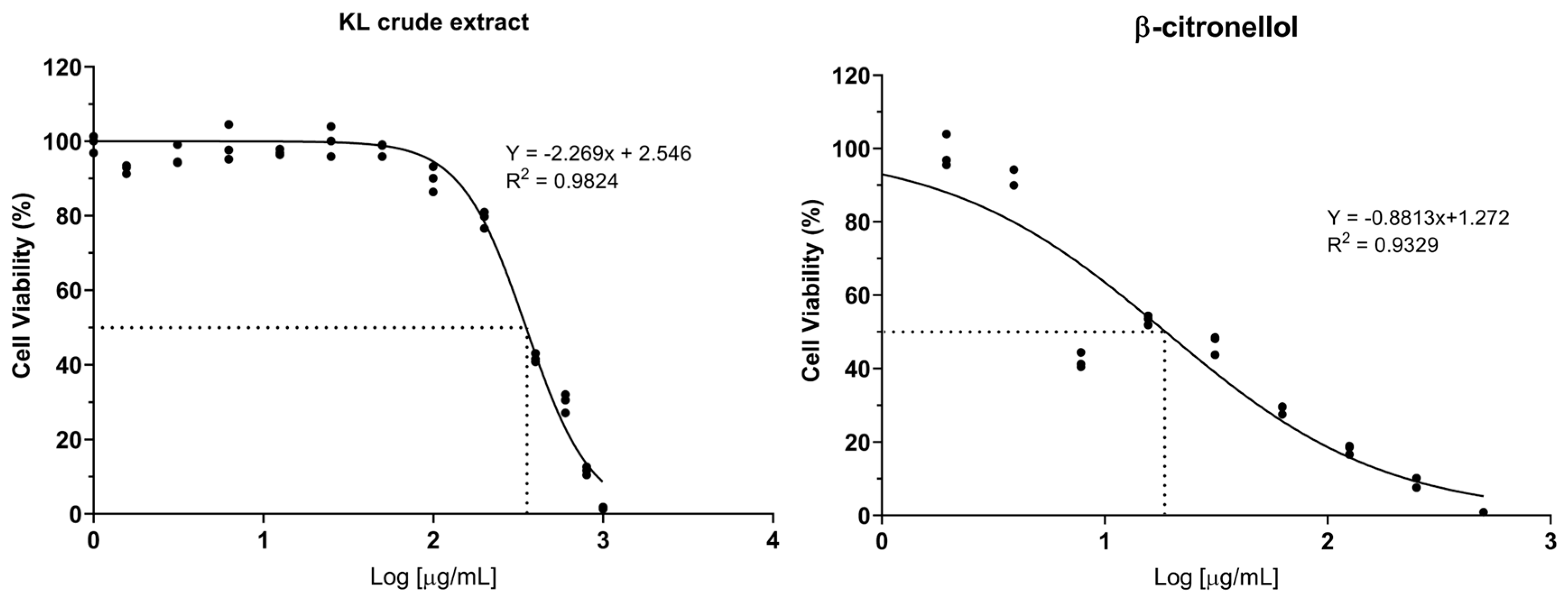
3.1. Cytotoxicity of crude extract and active compound on the LX-2 cell line
The cytotoxic potential of crude KL extract and its constituent compound, β-CIT, was assessed on the LX-2 cell line through the resazurin reduction assay. Dose-response curves were generated to determine IC
5, IC
10, and IC
50 values. Results demonstrated that the crude extract exhibited lower cytotoxicity relative to β-CIT, yielding IC
50 values of 351.80 µg/mL and 18.72 µg/mL respectively (
Figure 1). Furthermore, crude KL extract displayed IC
5 and IC
10 values of 96.03 µg/mL and 133.48 µg/mL, while β-CIT exhibited notably lower values of 0.66 µg/mL and 1.54 µg/mL. Based on these findings, subsequent experiments investigating effects on LX-2 cells were conducted using maximum concentrations of 120 µg/mL for the crude extract and 1 µg/mL for β-CIT.
3.2. The inhibition of activated HSCs genes expression and MMP-9 production
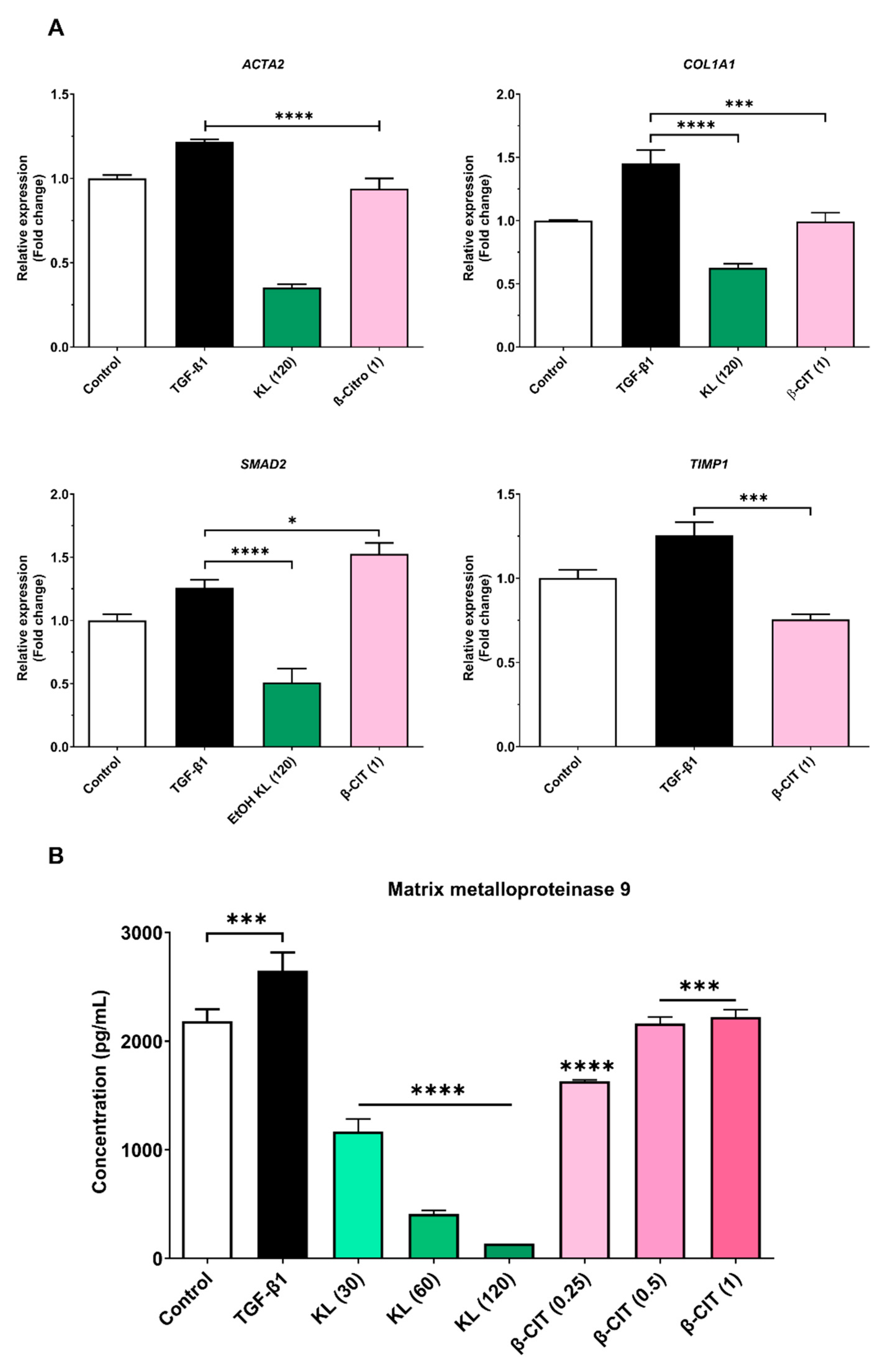
Quantitative real-time polymerase chain reaction (qRT-PCR) elucidated downregulation of key hepatic fibrosis markers (ACTA2, COL1A1, and SMAD2) upon exposure to crude KL extract. Similarly, β-CIT treatment demonstrated inhibition of ACTA2, COL1A1, and TIMP1 gene expression. Analysis of MMP-9 levels via the ELISA kit revealed a marked, dose-dependent reduction following exposure to crude KL extract, with a comparable decrease observed with β-CIT treatment. These findings collectively point towards antifibrotic potential in both compounds, as evidenced by their capacity to attenuate TGF-β1-induced fibrogenesis in the LX-2 cell model.
Figure 2.
Crude KL extract and β-CIT attenuate the expression of hepatic fibrosis-associated genes and mitigate MMP-9 production in LX-2 cells challenged with TGF-β1. Following a 24-hour co-treatment protocol, mRNA was extracted and subjected to real-time qRT-PCR analysis. Expression levels were normalized to the housekeeping gene GAPDH (A), revealing a marked downregulation of fibrosis-related genes. Furthermore, a statistically significant reduction in MMP-9 levels was observed in the cell culture supernatant (B). Statistical significance was denoted as follows: p < 0.0332 (*), p < 0.0021 (**), p < 0.0002 (***), p < 0.0001 (****).
Figure 2.
Crude KL extract and β-CIT attenuate the expression of hepatic fibrosis-associated genes and mitigate MMP-9 production in LX-2 cells challenged with TGF-β1. Following a 24-hour co-treatment protocol, mRNA was extracted and subjected to real-time qRT-PCR analysis. Expression levels were normalized to the housekeeping gene GAPDH (A), revealing a marked downregulation of fibrosis-related genes. Furthermore, a statistically significant reduction in MMP-9 levels was observed in the cell culture supernatant (B). Statistical significance was denoted as follows: p < 0.0332 (*), p < 0.0021 (**), p < 0.0002 (***), p < 0.0001 (****).
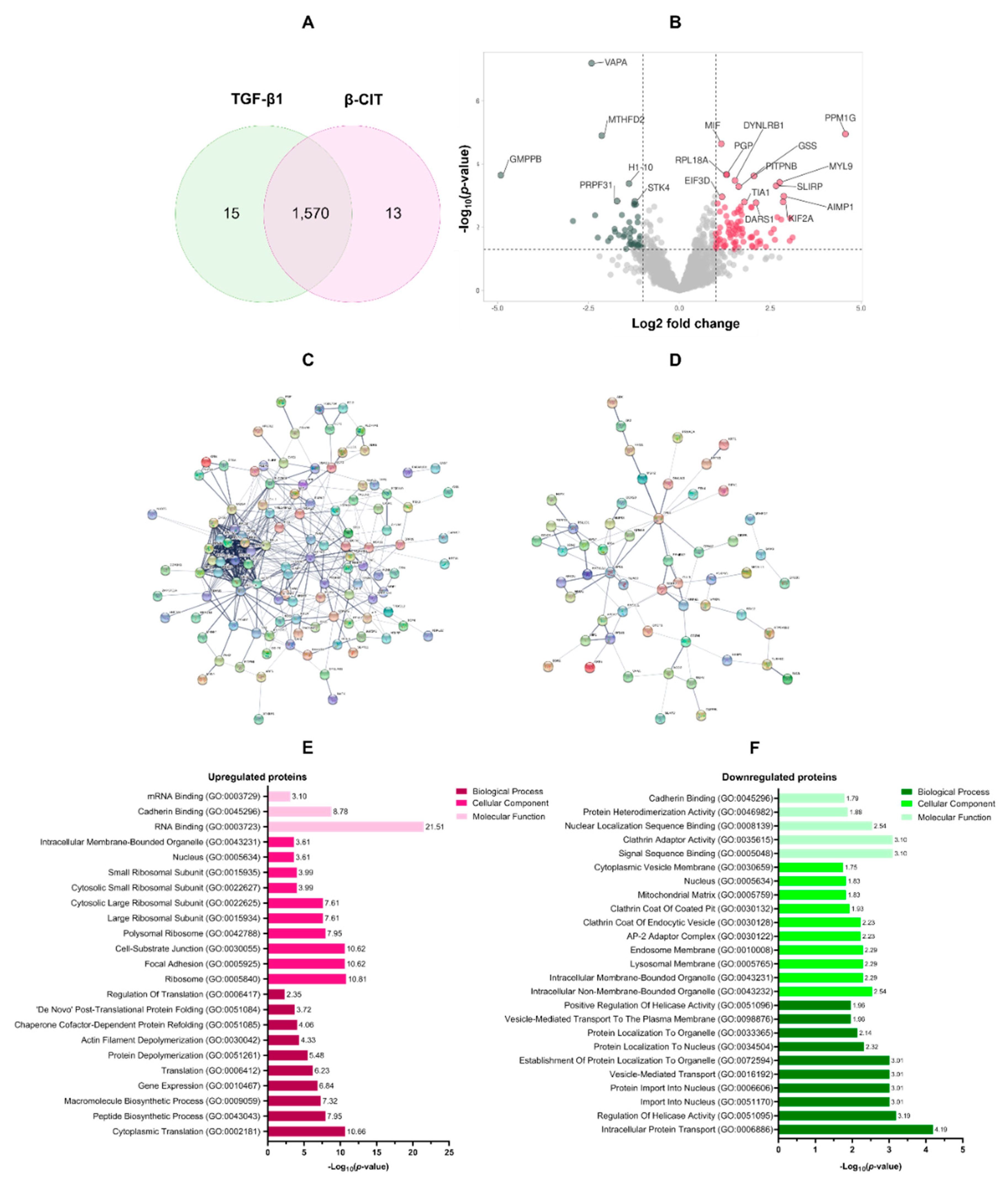
3.3. Proteomic analysis of the effect of β-citronellol on LX-2 cell
To elucidate the impact of β-CIT on TGF-β1-induced LX-2 cells, a 48-hour treatment regimen with β-CIT was implemented. Subsequent protein extraction and LC-MS/MS analysis yielded a total of 1,570 overlapping proteins present in both TGF-β1 treatment alone and β-CIT co-treatment conditions (
Figure 3A). Rigorous filtering parameters were applied (log2 fold change of ±1.5 and
p-value < 0.05) resulting in the identification of 125 upregulated and 65 downregulated proteins (
Figure 3B). The list of DEPs was summarized in
Table 2. Further analysis of these differentially expressed proteins (DEPs) was conducted utilizing protein-protein interaction (PPI) network visualization, revealing intricate relationships among the DEPs (
Figure 3C,D).
Gene ontology (GO) annotation of differentially expressed proteins (DEPs) revealed distinct functional profiles for those exhibiting upregulation versus downregulation. Biological process enrichment demonstrated a clear emphasis on protein synthesis within the upregulated group, including cytoplasmic translation (GO:0002181), peptide biosynthesis (GO:0043043), macromolecule biosynthesis (GO:0009059), gene expression (GO:0010467), and overall translation (GO:0006412). Conversely, downregulated proteins displayed association with processes governing intracellular transport (GO:0006886), helicase regulation (GO:0051095), nuclear import (GO:0051170 & GO:0006606), and vesicle-based transport (GO:0016192). Cellular component analysis further elucidated this contrast. Upregulated proteins were overrepresented in ribosomal structures (GO:0005840, GO:0042788, GO:0015934), alongside focal adhesions (GO:0005925) and cell-substrate junctions (GO:0030055). Downregulated proteins were preferentially associated with both membrane-bound (GO:0043231) and non-membrane-bound (GO:0043232) intracellular organelles, as well as components of the lysosomal (GO:0005765), endosomal (GO:0010008), and AP-2 adaptor complex (GO:0030122). Molecular function categories mirrored these distinctions. Upregulated proteins exhibited enrichment for various RNA binding functions (GO:0003723, GO:0003729), cadherin binding (GO:0045296), signal sequence binding (GO:0005048), and activities related to clathrin adaptor function (GO:0035615). Downregulation encompassed proteins involved in nuclear localization sequence binding (GO:0008139), protein heterodimerization (GO:0046982), and cadherin binding (GO:0045296). These findings are summarized in
Figure 3E,F.
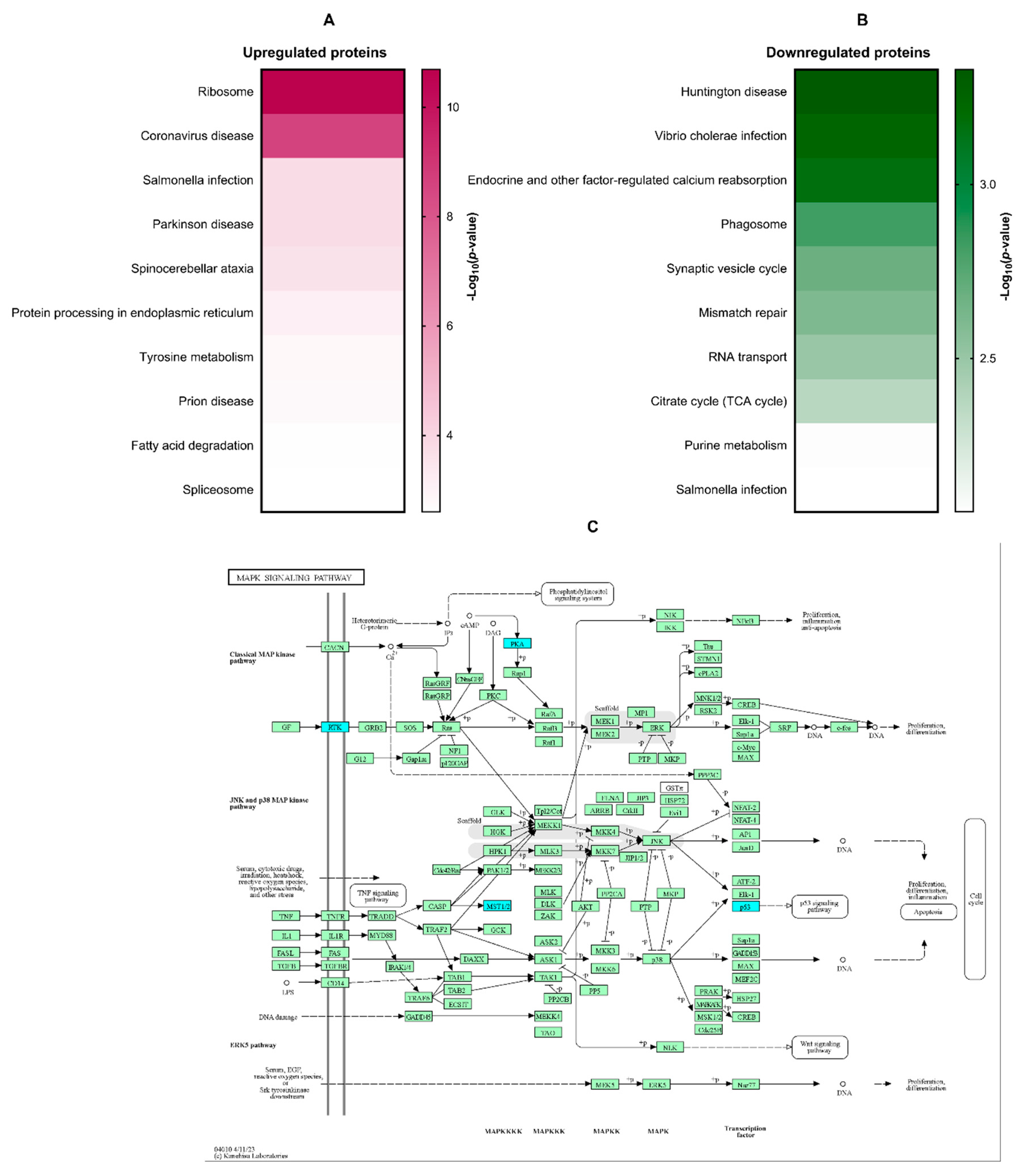
KEGG pathway enrichment analysis was conducted to identify signaling cascades potentially modulated by β-citronellol treatment in LX-2 cells. Enriched pathways included those associated with ribosome function, coronavirus disease, Salmonella infection, Parkinson's disease, and spinocerebellar ataxia (
Figure 4A). Downregulated proteins showed significant involvement in Huntington's disease, Vibrio cholerae infection, endocrine-regulated calcium reabsorption, phagosome formation, and synaptic vesicle cycling (
Figure 4B). The MAPK signaling pathway (
Figure 4C), a known contributor to hepatic fibrosis, was a focus of further examination, with downregulated proteins highlighted in blue.
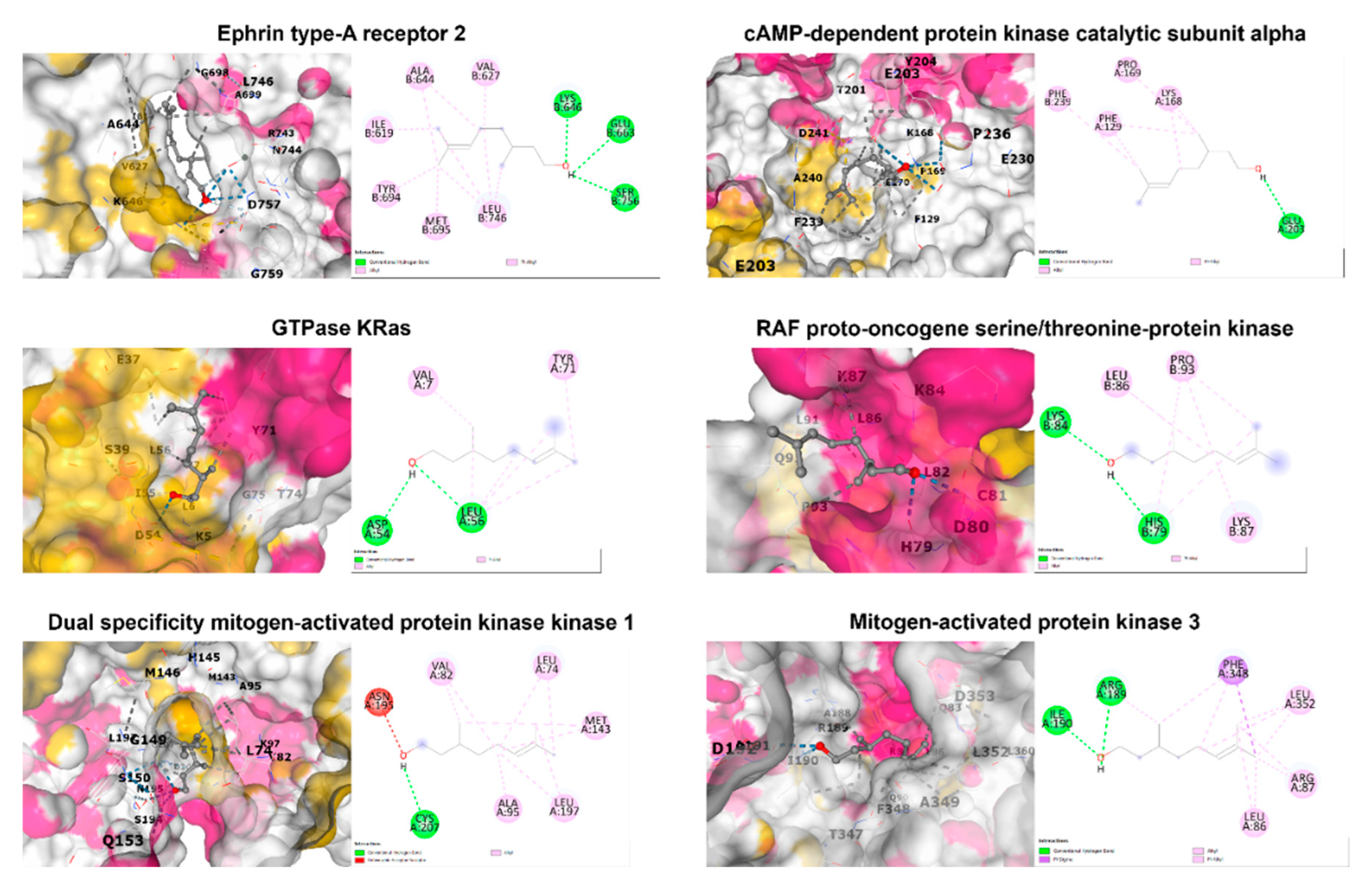
3.4. In silico molecular docking analysis
Binding interactions between β-citronellol and potential protein targets were examined using the cavity detection-guided blind docking capabilities of the CB-Dock2 web-based platform. Detailed results, including Autodock Vina binding scores and the amino acids involved in the interactions, are outlined in
Table 3. Visualization of the protein-ligand interactions in 2D and 3D formats can be found in
Figure 5. Of particular interest, β-citronellol displayed favorable binding scores (-4 to -6 Kcal/mol) with target proteins known to participate in the MAPK signaling pathway.
4. Discussion
Considered the antecedent stage of irreversible cirrhosis, liver fibrosis arises from sustained liver injury and predisposes individuals to grave complications such as portal hypertension, hepatic encephalopathy, and hepatocellular carcinoma. The asymptomatic presentation of hepatic fibrosis, coupled with diagnostic challenges, frequently allows for unchecked progression, even though the condition may be reversible upon removal of causative factors. Furthermore, the lack of a singular, targeted medication for liver fibrosis highlights the critical need for continued research in the development of effective anti-fibrotic agents.
Hepatic stellate cell (HSC) activation is a pivotal factor in the pathogenesis of liver fibrosis. Transforming growth factor beta-1 (TGF-β1) serves as a highly effective activator of hepatic stellate cells (HSCs) via dual signaling mechanisms: the canonical (Smad-dependent) pathway and the non-canonical (non-Smad) pathway. The TGF-β1/Smad signaling cascade represents a well-documented process central to HSC activation during liver damage. Upon binding to its specific receptor, TGF-β type II receptor (TβRII), TGF-β1 initiates the recruitment of the TGF-β type I receptor (TβRI). TβRII subsequently activates TβRI through phosphorylation of serine residue, facilitating the phosphorylation of substrates like Smad2 and Smad3. Phosphorylated Smad2 and Smad3 then complex with Smad4, translocating into the nucleus. This Smad2/3/4 complex directly interacts with DNA, modulating the expression of key genes such as
ACTA2,
COL1A1, and
TIMP1 [
28]. Our findings demonstrate that treatment of HSCs with crude KL extract and β-CIT effectively attenuates the transcriptional expression of these markers, implying a direct interference with the canonical pathway of TGF-β1-driven signaling cascade.
Proteomic analysis elucidated proteins and signaling pathways potentially mediating inhibitory effect of β-CIT on TGF-β1-induced HSCs. The KEGG database identified several signaling pathway, especially the downregulated pathways, including ribosome function, coronavirus disease, Salmonella infection, Parkinson's disease, and spinocerebellar ataxia. The mitogen-activated protein kinase (MAPK) signaling pathway is one of the non-canonical pathways in the HSC activation by the TGF-β1 [
29]. The MAPK pathway is known to regulate diverse cellular functions such as proliferation, differentiation, survival, apoptosis, and inflammation. This pathway comprises three signaling cascades: ERK, JNK, and p38. Extensive research implicates this pathway in hepatic fibrosis through its influence on HSC behavior. MAPK signaling modulates proliferation, migration, and ECM synthesis/deposition following stimulation by growth factors like TGF-β1 via tyrosine kinases (RTKs) [
30]. Our proteomic analysis demonstrated downregulation of the RTK proteins, Eph receptor A2 (
EPHA2), alongside the MAPK signaling proteins kinase cAMP-activated catalytic subunit alpha (
PRKACA). The Eph receptors are the largest known receptor tyrosine kinases in mammals, regulating vast biological activity of the cells, including cell adhesion, migration, proliferation, and immune cell activation [
31]. The expression of Eph receptors has been linked to the progression of liver fibrosis [
32].
PRKACA is a member of the PKA family, has a role to transfer phosphate groups to serine or threonine residues on substrate proteins, which can alter their function and activity. It is involved in various cellular processes, including metabolism, gene expression, and cell proliferation. Some studies have revealed that
PRKACA exhibits hyperkinase activity in fibrotic liver samples from both humans and mice. This suggests that
PRKACA may play a significant role in the development and progression of hepatic fibrosis [
33]. Molecular binding analysis indicated favorable affinities between these proteins and β-CIT. These findings suggest anti-hepatic fibrosis potential of β-CIT may involve targeting the MAPK signaling pathway, though further studies are necessary to confirm the precise mechanism.
5. Conclusions
This study presents the first evidence of the potential anti-hepatic fibrotic activity of β-CIT. We demonstrate that β-CIT inhibits hepatic stellate cell activation induced by the TGF-β1 model. Mechanistically, β-CIT appears to target the canonical TGF-β1/Smad signaling pathway, along with reducing MMP-9 production—all major contributors to extracellular matrix (ECM) deposition. Further proteomic analysis and molecular docking suggest a potential additional mode of action involving MAPK tyrosine kinase signaling proteins. While in vitro results are promising, further in vivo investigations are required to fully understand therapeutic potential of β-CIT for liver fibrosis.
Author Contributions
This research was contributed by authors “conceptualization, K.U. and W.B.; methodology, W.B.; formal analysis, W.B., S.K. and Y.Y; investigation, W.B.; resources, P.P., and K.D.; data curation, K.U., and Y.T.; writing—original draft preparation, W.B.; writing—review and editing, W.B., A.F. and K.U.; visualization, K.U. and W.B.; supervision, K.U.; funding acquisition, K.U. All authors have read and agreed to the published version of the manuscript.
Funding
This work was support by Thailand Science Research and Innovation, and Naresuan University (R2567B041) and “the APC was funded by Reinventing University Program 2023, The Ministry of Higher Education, Science, Research and Innovation (MHESI), Thailand (R2566A056).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Naresuan University (IRB No. P1-0133/2565, approved on October 21, 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data presented in the study are openly available in ProteomeXchange Consortium via the PRIDE partner repository at
http://www.ebi.ac.uk/pride/archive/, PXD052806. The following username and password can be used to access the dataset by logging in to the PRIDE website. Username: reviewer_pxd052806@ebi.ac.uk. Password: 24jgZXwu1IAN
Acknowledgments
The authors gratefully acknowledge Associate Professor Dr. Saranyapin Potikanond (Department of Pharmacology, Faculty of Medicine, Chiang Mai University) for the generous contribution of the LX-2 cells used in this research.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J Hepatol 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Luangmonkong, T.; Parichatikanond, W.; Olinga, P. Targeting collagen homeostasis for the treatment of liver fibrosis: Opportunities and challenges. Biochem Pharmacol 2023, 215, 115740. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Chouhan, K.; Weiskirchen, S.; Weiskirchen, R. Cellular Mechanisms of Liver Fibrosis. Front Pharmacol 2021, 12, 671640. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of liver fibrosis and its role in liver cancer. Exp Biol Med 2020, 245, 96–108. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Liu, S.; Yang, M. Treatment of liver fibrosis: Past, current, and future. World J Hepatol 2023, 15, 755–774. [Google Scholar] [CrossRef] [PubMed]
- Agouillal, F.; Taher, Z.; Moghrani, H.; Nasrallah, N.; El Enshasy, H. A Review of Genetic Taxonomy, Biomolecules Chemistry and Bioactivities of Citrus hystrix DC. Biosci Biotechnol Res Asia 2017, 14, 285–305. [Google Scholar] [CrossRef]
- Kidarn, S.; Saenjum, C.; Hongwiset, D.; Phrutivorapongkul, A. Furanocoumarins from Kaffir lime and their inhibitory effects on inflammatory mediator production. Cogent Chem 2018, 4, 1529259. [Google Scholar] [CrossRef]
- Buakaew, W.; Pankla Sranujit, R.; Noysang, C.; Thongsri, Y.; Potup, P.; Nuengchamnong, N.; Suphrom, N.; Usuwanthim, K. Phytochemical Constituents of Citrus hystrix DC. Leaves Attenuate Inflammation via NF-κB Signaling and NLRP3 Inflammasome Activity in Macrophages. Biomolecules 2021, 11, 105. [Google Scholar] [CrossRef]
- Ratanachamnong, P.; Chunchaowarit, Y.; Namchaiw, P.; Niwaspragrit, C.; Rattanacheeworn, P.; Jaisin, Y. HPLC analysis and in vitro antioxidant mediated through cell migration effect of C. hystrix water extract on human keratinocytes and fibroblasts. Heliyon 2023, 9, 13068. [Google Scholar] [CrossRef]
- Abirami, A.; Nagarani, G.; Siddhuraju, P. In vitro antioxidant, anti-diabetic, cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and C. maxima fruits. Food Sci Hum Wellness 2014, 3, 16–25. [Google Scholar] [CrossRef]
- Srifuengfung, S.; Bunyapraphatsara, N.; Satitpatipan, V.; Tribuddharat, C.; Junyaprasert, V.B.; Tungrugsasut, W.; Srisukh, V. Antibacterial oral sprays from kaffir lime (Citrus hystrix DC.) fruit peel oil and leaf oil and their activities against respiratory tract pathogens. J Tradit Complement Med 2020, 10, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Sreepian, P.M.; Rattanasinganchan, P.; Sreepian, A. Antibacterial efficacy of Citrus hystrix (makrut lime) essential oil against clinical multidrug-resistant methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates. Saudi Pharm J 2023, 31, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Tunjung, W.A.S.; Cinatl, J.; Michaelis, M.; Smales, C.M. Anti-Cancer Effect of Kaffir Lime (Citrus Hystrix DC) Leaf Extract in Cervical Cancer and Neuroblastoma Cell Lines. Procedia Chem 2015, 14, 465–468. [Google Scholar] [CrossRef]
- Ho, Y.; Suphrom, N.; Daowtak, K.; Potup, P.; Thongsri, Y.; Usuwanthim, K. Anticancer Effect of Citrus hystrix DC. Leaf Extract and Its Bioactive Constituents Citronellol and, Citronellal on the Triple Negative Breast Cancer MDA-MB-231 Cell Line. Pharmaceuticals 2020, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Abolmaesoomi, M.; Mat Junit, S.; Mohd Ali, J.; Chik, Z.B.; Abdul Aziz, A. Effects of polyphenolic-rich extracts from Citrus hystrix on proliferation and oxidative stress in breast and colorectal cancer. Turk Biyokim Derg 2023, 48, 110–118. [Google Scholar] [CrossRef]
- Abirami, A.; Nagarani, G.; Siddhuraju, P. Hepatoprotective effect of leaf extracts from Citrus hystrix and C. maxima against paracetamol induced liver injury in rats. Food Sci Hum Wellness 2015, 4, 35–41. [Google Scholar] [CrossRef]
- Buakaew, W.; Pankla Sranujit, R.; Noysang, C.; Krobthong, S.; Yingchutrakul, Y.; Thongsri, Y.; Potup, P.; Daowtak, K.; Usuwanthim, K. Proteomic Analysis Reveals Proteins Involved in the Mode of Action of β-Citronellol Identified From Citrus hystrix DC. Leaf Against Candida albicans. Front Microbiol 2022, 13, 894637. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2 -ΔΔCT method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath 2013, 3, 71–85. [Google Scholar]
- Robert, S.; Gicquel, T.; Bodin, A.; Lagente, V.; Boichot, E. Characterization of the MMP/TIMP Imbalance and Collagen Production Induced by IL-1β or TNF-α Release from Human Hepatic Stellate Cells. PLoS One 2016, 11, 153118. [Google Scholar] [CrossRef]
- Krobthong, S.; Yingchutrakul, Y.; Samutrtai, P.; Hitakarun, A.; Siripattanapipong, S.; Leelayoova, S.; Mungthin, M.; Choowongkomon, K. Utilizing Quantitative Proteomics to Identify Species-Specific Protein Therapeutic Targets for the Treatment of Leishmaniasis. ACS Omega 2022, 7, 12580–12588. [Google Scholar] [CrossRef] [PubMed]
- Griss, J.; Viteri, G.; Sidiropoulos, K.; Nguyen, V.; Fabregat, A.; Hermjakob, H. ReactomeGSA - Efficient Multi-Omics Comparative Pathway Analysis. Mol Cell Proteomics 2020, 19, 2115–2125. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat Struct Mol Biol 2003, 10, 980–980. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res 2023, 51, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.-X.; Cao, Y. CB-Dock2: improved protein–ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res 2022, 50, 159–164. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J Comput Chem 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl, B.; Nadja. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis—Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Y.; Sun, B. The Molecular Mechanisms of Liver Fibrosis and Its Potential Therapy in Application. Int J Mol Sci 2022, 23, 12572. [Google Scholar] [CrossRef]
- Westenberger, G.; Sellers, J.; Fernando, S.; Junkins, S.; Han, S.M.; Min, K.; Lawan, A. Function of Mitogen-Activated Protein Kinases in Hepatic Inflammation. J Cell Signal 2021, 2, 172–180. [Google Scholar]
- Darling, T.K.; Lamb, T.J. Emerging Roles for Eph Receptors and Ephrin Ligands in Immunity. Front Immunol 2019, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- Mekala, S.; Dugam, P.; Das, A. Ephrin–Eph receptor tyrosine kinases for potential therapeutics against hepatic pathologies. J Cell Commun Signal 2023, 17, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Creeden, J.F.; Kipp, Z.A.; Xu, M.; Flight, R.M.; Moseley, H.N.B.; Martinez, G.J.; Lee, W.H.; Alganem, K.; Imami, A.S.; McMullen, M.R.; et al. Hepatic kinome atlas: An in-depth identification of kinase pathways in liver fibrosis of humans and rodents. Hepatology 2022, 76, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).