1. Introduction
Flowering plants (angiosperms) can be distinguished from gymnosperms by their enclosed ovules before pollination [
1,
2,
3]. But how such a feature came into existence has been a puzzle for botanists for long time. Formerly, carpels in angiosperms were supposed to be derived from megasporophylls that bear ovules along their margins [
4]. But so far there is no fossil evidence favouring this hypothesis. On the contrary, there are increasing evidence rejecting this hypothesis [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18]. To answer this question, fossil evidence is the only reliable source for morphological information of plants in the past. As angiosperms and gymnosperms are distinguished each other by the status of ovules before pollination, being enclosed or naked [
3], therefore seeking an ovule between those two statuses (namely, semi-enclosed) in a fossil plant is apparently more needed than anything else.
2. Materials and Methods
The specimen, including two facing slabs, were collected from the Yixian Formation of Erdaogou, Jianchang, Liaoning (119.7451°E, 40.5288°N). The specimen includes physical connected conifer-like vegetative part and reproductive organs. Specimens were photographed with a Nikon D810 digital camera. Details of the specimens were observed and photographed under a Leica M205A stereomicroscope equipped with a Leica DFC450C digital camera. A seed was removed from the specimen and observed using a Nikon SMZ1500 stereomicroscope equipped with a Nikon DS-Fi1 digital camera and a Leo 1530 scanning electron microscope (SEM). All photographs were saved in TIFF format and organized for publication using a Photoshop 7.0.
3. Results
Shaolinia gen. nov.
Generic diagnosis: Plant woody, including branches, leaves, and connected cone-like organs. Branch rigid, straight, with helically arranged leaves. Leaf Juniperus-like, straight or slightly curving. Cone-like reproductive organs alternately arranged along the branch. Reproductive organ including more than 40 lateral appendages helically arranged around a central axis. Each lateral appendage comprising a single axillary seed and subtending bract wrapping the seed from the bottom and laterals. Bract with a pointed tip, gaping adaxially. Seed round-shaped, with isodiametric sculpture.
Type species: Shaolinia intermedia gen. et sp. nov.
Etymology: Shaolinia dedicated to Dr. Shaolin Zheng, a senior Chinese palaeobotanist.
Stratigraphic horizon: the Yixian Formation.
Shaoliniaintermedia gen. et sp. nov.
Specific diagnosis: the same as the genus.
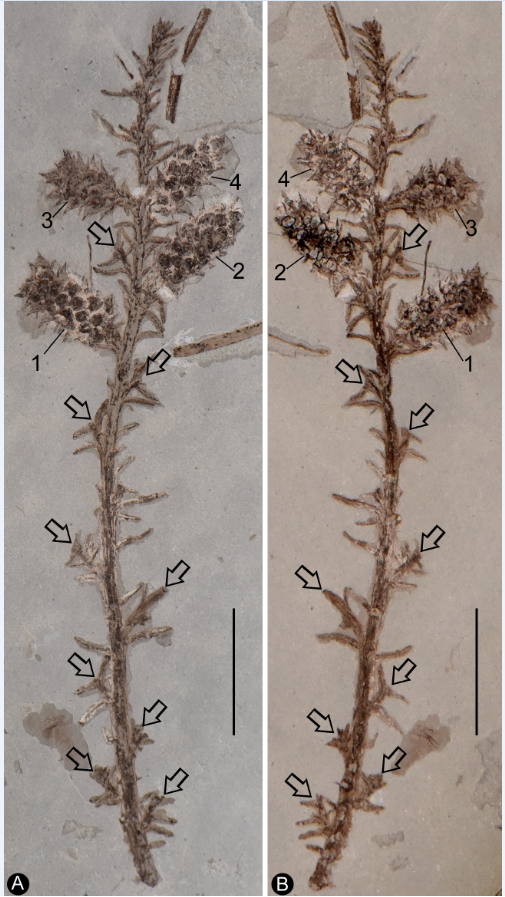
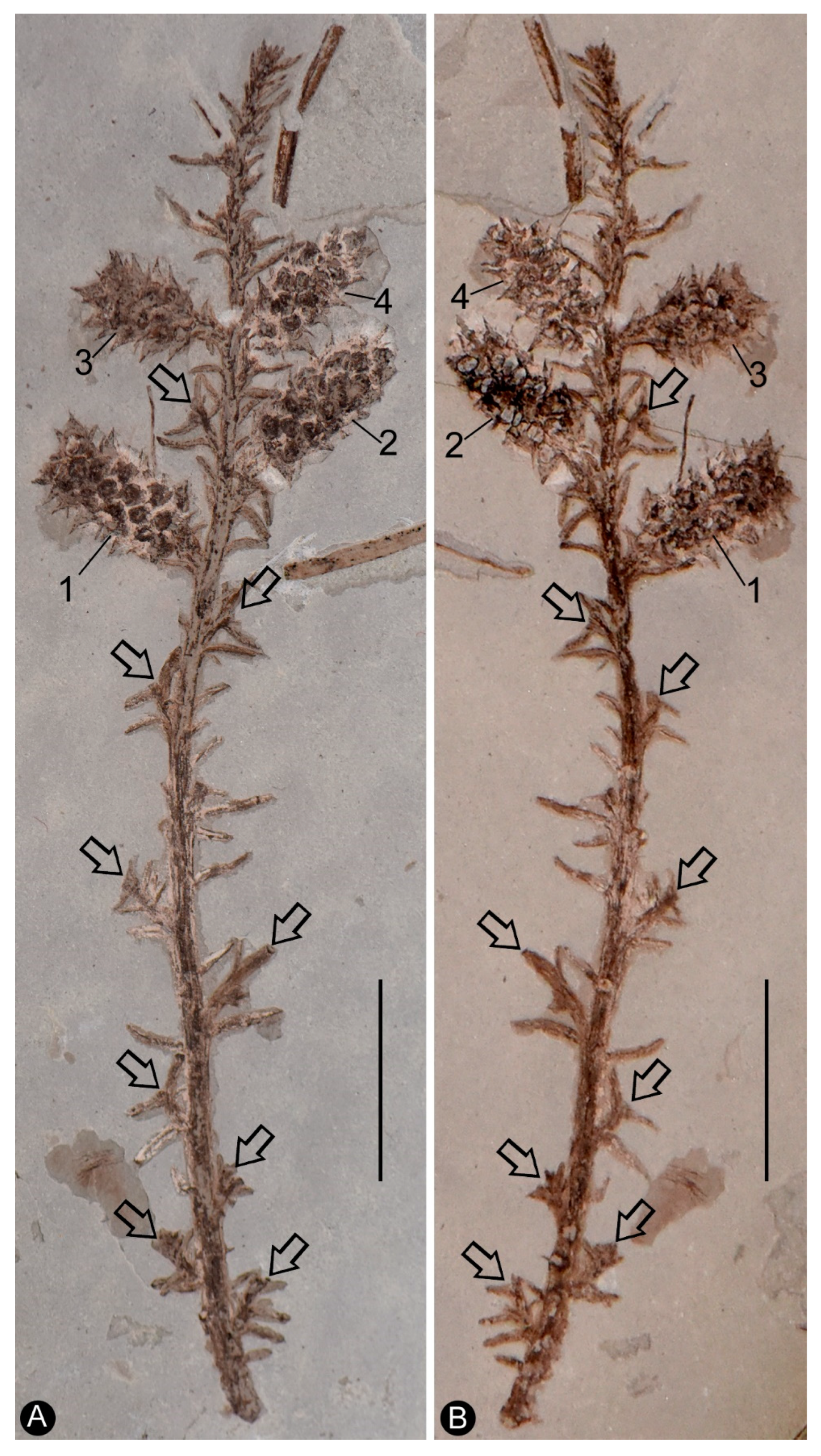
Description: The specimen includes two facing parts of the distal portion of a woody branch, 68 mm long, 17 mm wide, preserved as a partially coalified compression and impressions on yellowish tuffaceous siltstone (
Figure 1a-b). The branch is about 1.1 mm wide in the proximal portion, tapering distally (
Figure 1a-b). There are several small axillary lateral branches up to 4.9 mm long, with a few leaves (
Figure 1a-b). The leaves are
Juniperus-like, 0.9-4.4 mm long and 0.2-0.5 mm wide, straight or slightly curving to the distal of the branch (
Figure 1a-b,
Figure 2a). Four cone-like reproductive organs are inserted alternately along the branch (
Figure 1a-b,
Figure 2b-d). The cone-like organ is 7.4-10.9 mm in length and 4.2-4.7 mm in diameter, with over fifteen lateral appendages helically arranged around a central axis (
Figure 1a-b,
Figure 2b-e). Each lateral appendage is round-triangular in shape in adaxial view, about 2.3 mm long, 0.8 mm high, and 1.5 mm wide, including an axillary seed and a subtending bract, and the latter wraps the former from the bottom and laterals (
Figure 2b-e,
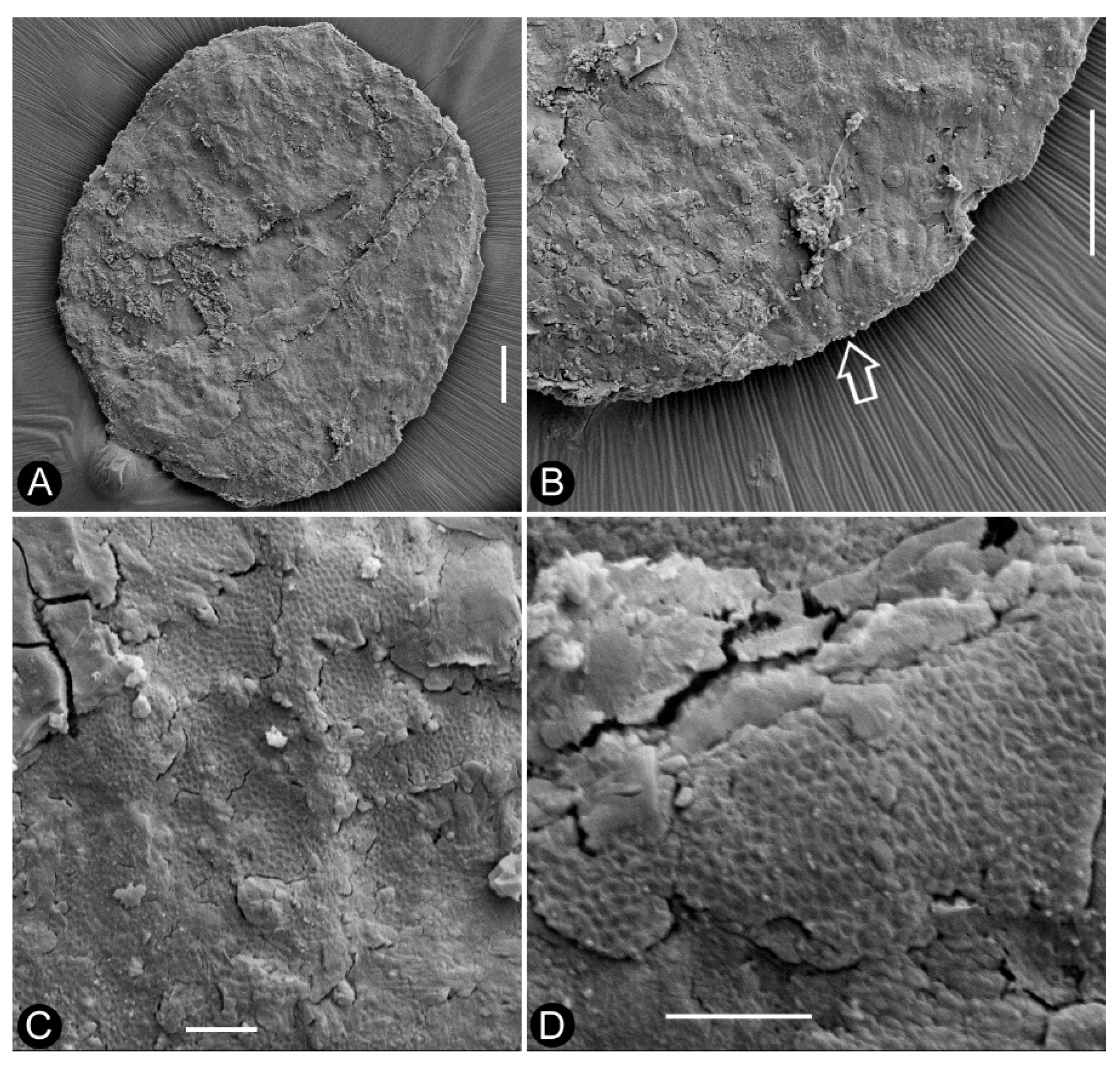
Figure 3a-b). The seed is round in shape, 0.63-0.78 mm x 0.81-1.06 mm, more or less flattened during the fossilization, the seed coat has isodiametric sculpture (
Figure 2d-f,
Figure 3a-b,
Figure 4a-d). The bract has a pointed tip, folding longitudinally and adaxially, gaping adaxially (
Figure 2d-e,
Figure 3a-b).
Etymology: intermedia, Latin for the morphology of the lateral appendages that falls between carpels in some angiosperms and lateral appendages of conifers.
Holotype: JCEDG0001.
Depository: National Orchid Conservation Center of China and Orchid Conservation and Research Center of Shenzhen, Shenzhen, China.
Remarks: We refrain us from using the terms like “carpels” and “flowers” in our description as they would make as if we preferred to treat Shaolinia as an angiosperms, although we cannot rule out the possibility that later evolution Shaolinia may turn into a true angiosperm bearing flowers and carpels.
The term “bract” is equivalent to that in conifers, and the “axillary seed” is equivalent to and comparable to the ovuliferous scale more reduced (reduced into a single seed) than in conifers.
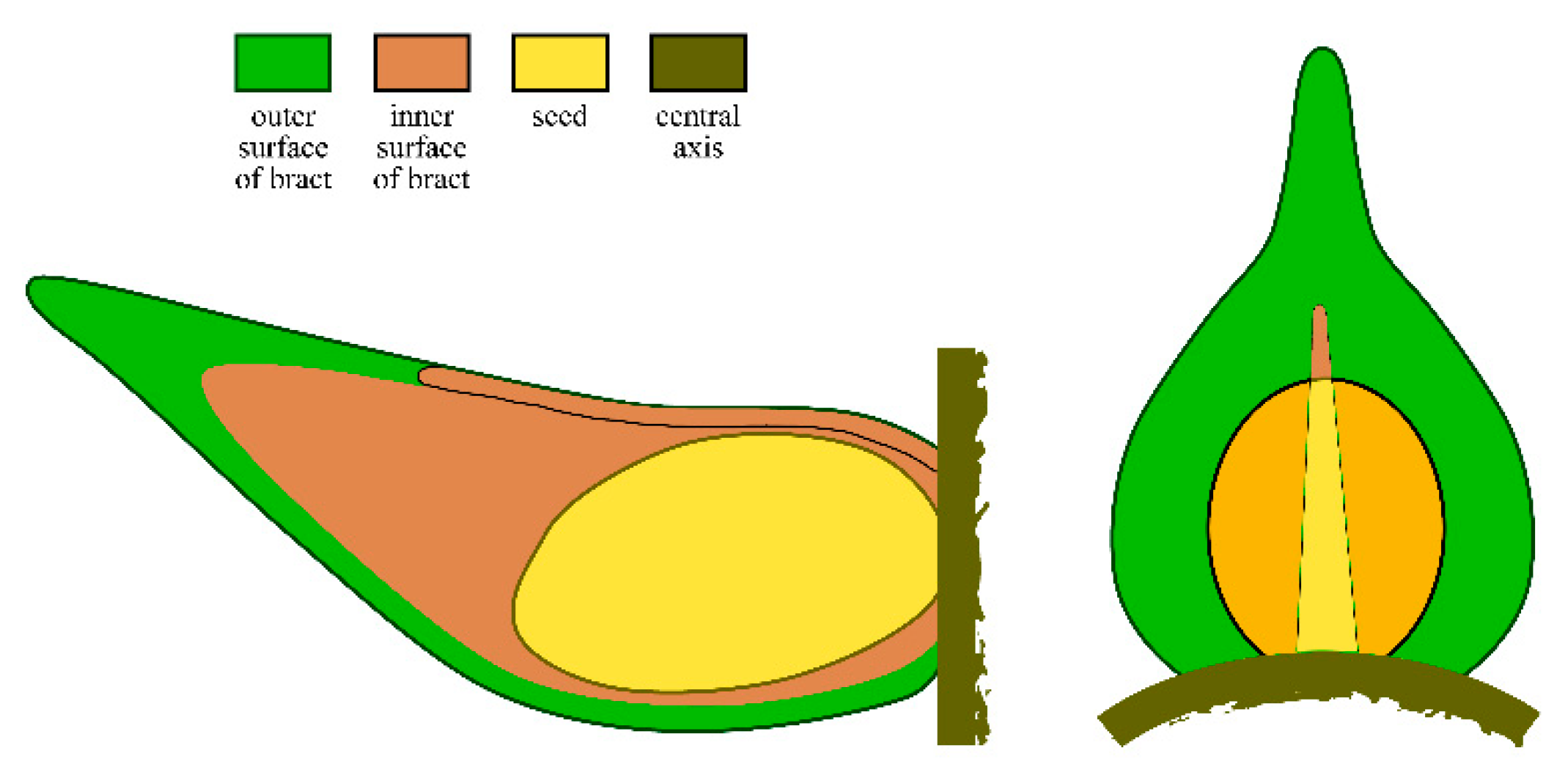
Figure 5.
Sketches of lateral appendages of Shaolinia intermedia. A. Longitudinal radial profile of a lateral appendage showing a bract and an adaxial seed attached to the central axis and partially wrapped. B. Adaxial view of a lateral appendage showing a bract and a partially wrapped adaxial seed inside. Note the bract gapes adaxially.
Figure 5.
Sketches of lateral appendages of Shaolinia intermedia. A. Longitudinal radial profile of a lateral appendage showing a bract and an adaxial seed attached to the central axis and partially wrapped. B. Adaxial view of a lateral appendage showing a bract and a partially wrapped adaxial seed inside. Note the bract gapes adaxially.
4. Discussion
Angiosperms can be distinguished from their gymnosperm peers by their enclosed ovules (angio-ovuly) before pollination [
1,
2,
3], a character that can ensure the pollination to be carried out in an angiospermous (not gymnospermous) way. However, how such a feature is derived from former gymnosperms with naked ovules is a crucial question of interest in botanical studies on the origin of angiosperms, because the answer to this question will found the relationship between angiosperms and gymnosperms and unite both into an integral system of seed plants. To answer this question, a fossil plant with semi-enclosed ovule is of key importance. Although conifer-like in vegetative morphologies,
Shaolinia has its seed wrapped by its subtending bract. Despite the seed/ovule is not fully enclosed as in typical angiosperms, the seed-wrapping tendency of
Shaolinia is intriguing because it is not hard to extrapolate further evolution from conifers and
Shaolinia to fully-enclosed ovules/seeds in angiosperms.
Shaolinia is not alone in term of such an implication, as the recently reported
Combina triassica has given exactly the same implication [
19]. Such a congruous ovule-enclosing tendency in conifer-like
Shaolinia and
Combina makes it intriguing to relate conifers with angiosperms, an evolutionary scenario anticipated by some botanists [
3].
Axillary branching is a pattern frequently seen in angiosperms and many gymnosperms. Its history can be dated at least back to the Middle Pennsylvanian [
20], and is the Bau-plan underlying the lateral appendages of conifer cones. As proven by Florin’s works [
21,
22,
23,
24], the lateral appendages in the reproductive organs of Cordaitales and Coniferales are compound organs comprising an axillary ovule-bearing branch and a subtending bract. Various metamorphisms of these two parts have given rise to diverse cones/gynoecia in these two groups. This information has been well known for long time. However, its implication for the homology of carpels has never been explored before, most likely due to the dominance of Arber and Parkin’s hypothesis [
4], in which a carpel was assumed derived from a so-called “megasporophyll”, which, however, has been proven non-existent [
9,
10]. Most botanists have been misled by such a groundless speculation. The first obvious light elucidating the homology of carpels emerged more than 20 years ago when Roe et al. [
5] demonstrated that the ovules in
Arabidopsis are independent of the carpel wall and borne on a branch. Morphologically, the most significant progress was made recently when Zhang et al. [
6] revealed that the carpels in
Michelia figo (Magnoliaceae) are composite organs derived from a former axillary ovule-bearing branch and its subtending foliar part. The varying morphology of carpels in a single tree of
Michelia figo [
6] demonstrates a great resemblance to the lateral appendages of
Shaolinia in term of axillary seeds and subtending foliar parts. Such observations make it obvious that axillary branching frequently seen in many seed plants is a feature shared by conifers (besides others) and angiosperms, and a carpel may well be derived from an axillary ovule-bearing branch (equivalent to a placenta) and its subtending foliar part (equivalent to ovarian wall). Configuration and organization of lateral appendages in
Shaolinia is comparable the carpels in
Illicium lanceolatum (Schisandraceae) [
25], and the only difference between these two lies in the extent the carpel wall (= bract) is closed. This speculation has been favoured by a Triassic conifer-like reproductive organ,
Combina triassica [
19]. Now the occurrence of
Shaolinia seems to reinforce and strengthen the evidence chain for the derivation of carpels in angiosperms from bract-scale complexes in conifers, as suggested by Tomlinson [
3], and solves the recalcitrant problem of origin of angiosperms and their carpels.
Based on comparison of gynoecium organization in early angiosperms [
13,
14,
15,
16,
17,
18,
26,
27,
28,
29,
30,
31,
32,
33], it is obvious that the gynoecia of these early angiosperms are diversified in morphology and organization. Although gynoecia in Magnoliales and Amborellales cannot be excluded from the list of ancestral taxa, there are other types of gynoecia that may be plesiomorphic and derived independently in the geological history. For example, the Early Jurassic
Nanjinganthus is hard to accept for many because of its “noncarpellate” inferior ovary [
30,
31,
34], a feature that used to be thought much derived in angiosperm systematics. It appears more plausible that the gynoecia in angiosperms were derived from different taxa independently (
33-35), an evolutionary scenario unexpected previously.
5. Conclusions
The new fossil sheds a new light on the evolution of female reproductive organs, suggesting potential homology between some conifers and angiosperms. In line with previous fossil reproductive morphology, there appear to be various independent ways to derive the reproductive organs (flowers) in angiosperms.
Author Contributions
L.-J. C. collected the specimens; X.W. carried out the observation and photography; X.W. drafted the manuscript. L.-J. C. and X.W. modified and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the National Natural Science Foundation of China (42288201).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We thank Dr. Yan Fang for help with SEM during this research.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tomlinson, P.B.; Takaso, T. Seed cone structure in conifers in relation to development and pollination: a biological approach. Canadian Journal of Botany 2002, 80, 1250–1273. [Google Scholar] [CrossRef]
- Wang, X. The Dawn Angiosperms, 2nd ed.; Springer: Cham, Switzerland, 2018; p. 407. [Google Scholar]
- Tomlinson, P.B. Rescuing Robert Brown-The origins of angio-ovuly in seed cones of conifers. Botanical Review 2012, 78, 310–334. [Google Scholar] [CrossRef]
- Arber, E.A.N.; Parkin, J. On the origin of angiosperms. Journal of the Linnean Society of London, Botany 1907, 38, 29–80. [Google Scholar] [CrossRef]
- Roe, J.L.; Nemhauser, J.L.; Zambryski, P.C. TOUSLED participates in apical tissue formation during gynoecium development in Arabidopsis. Plant Cell 1997, 9, 335–353. [Google Scholar]
- Zhang, X.; Liu, W.; Wang, X. How the ovules get enclosed in magnoliaceous carpels. PLOS ONE 2017, 12, e0174955. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-M.; Xiao, X.; Wang, G.-X.; Gao, R.-F. Vascular Anatomy of Kiwi Fruit and its Implications for the origin of carpels. Frontiers in Plant Science 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-Z.; Hilu, K.; Wang, Y.-L. From leaf and branch into a flower: Magnolia tells the story. Botanical Studies 2014, 55, 28. [Google Scholar] [CrossRef]
- Miao, Y.; Liu, Z.J.; Wang, M.; Wang, X. Fossil and living cycads say "No more megasporophylls". Journal of Morphology and Anatomy 2017, 1, 107. [Google Scholar]
- Wang, X.; Luo, B. Mechanical pressure, not genes, makes ovulate parts leaf-like in Cycas. American Journal of Plant Sciences 2013, 4, 53–57. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.-J.; Liu, W.; Zhang, X.; Guo, X.; Hu, G.; Zhang, S.; Wang, Y.; Liao, W. Breaking the stasis of current plant systematics. Science AND Technology Review 2015, 33, 97–105. [Google Scholar] [CrossRef]
- Fu, Q.; Diez, J.B.; Pole, M.; Garcia-Avila, M.; Liu, Z.-J.; Chu, H.; Hou, Y.; Yin, P.; Zhang, G.-Q.; Du, K.; et al. <italic>Nanjinganthus</italic>: An unexpected flower from the Jurassic of China. Biorxiv 2017. [Google Scholar] [CrossRef]
- Han, G.; Fu, X.; Liu, Z.-J.; Wang, X. A new angiosperm genus from the Lower Cretaceous Yixian Formation, Western Liaoning, China. Acta Geologica Sinica (English edition) 2013, 87, 916–925. [Google Scholar]
- Han, G.; Liu, Z.; Wang, X. A Dichocarpum-like angiosperm from the Early Cretaceous of China. Acta Geologica Sinica (English edition) 2017, 90, 1–8. [Google Scholar] [CrossRef]
- Han, G.; Liu, Z.-J.; Liu, X.; Mao, L.; Jacques, F.M.B.; Wang, X. A whole plant herbaceous angiosperm from the Middle Jurassic of China. Acta Geologica Sinica (English edition) 2016, 90, 19–29. [Google Scholar]
- Liu, Z.-J.; Hou, Y.-M.; Wang, X. Zhangwuia: An enigmatic organ with bennettitalean appearance and enclosed ovules. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 2019, 108, 419–428. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Wang, X. A perfect flower from the Jurassic of China. Historical Biology 2016, 28, 707–719. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Wang, X. Yuhania: a unique angiosperm from the Middle Jurassic of Inner Mongolia, China. Historical Biology 2017, 29, 431–441. [Google Scholar] [CrossRef]
- Santos, A.A.; Wang, X. Pre-Carpels from the Middle Triassic of Spain. Plants 2022, 11, 2833. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.N.; Taylor, E.L.; Krings, M. Paleobotany: the biology and evolution of fossil plants, 2nd ed.; Elsevier: Amsterdam, 2009; p. 1230. [Google Scholar]
- Florin, R. The morphology of the female fructifications in cordaites and conifers of Palaeozoic age. Botaniska Notiser 1939, 36, 547–565. [Google Scholar]
- Florin, R. Die Koniferen des Oberkarbons und des unteren Perms. Paläontographica B 1944, 85, 457–654. [Google Scholar]
- Florin, R. Die Koniferen des Oberkarbons und des unteren Perms. Paläontographica B 1945, 85, 655–729. [Google Scholar]
- Florin, R. Evolution in cordaites and conifers. Acta Horti Bergiani 1951, 15, 285–388. [Google Scholar]
- Zhang, X. Floral ontogeny of Illicium lanceolatum (Schisandraceae) and its implications on carpel homology. Phytotaxa 2019, 416, 200–210. [Google Scholar] [CrossRef]
- Han, G.; Wang, X. A new infructescence of angiosperms from the Early Cretaceous of China. Acta Geologica Sinica (English edition) 2020, 94, 1711–1713. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Chen, L.-J.; Wang, X. A whole-plant monocot from Lower Cretaceous. Palaeoworld 2021, 30, 169–175. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Wang, X. A novel angiosperm from the Early Cretaceous and its implications on carpel-deriving. Acta Geologica Sinica (English edition) 2018, 92, 1293–1298. [Google Scholar] [CrossRef]
- Liu, X.; Ma, L.; Liu, B.; Liu, Z.-J.; Wang, X. A novel angiosperm including various parts from the Early Cretaceous sheds new light on flower evolution. Historical Biology 2021, 33, 2706–2714. [Google Scholar] [CrossRef]
- Fu, Q.; Diez, J.B.; Pole, M.; Garcia-Avila, M.; Liu, Z.-J.; Chu, H.; Hou, Y.; Yin, P.; Zhang, G.-Q.; Du, K.; et al. An unexpected noncarpellate epigynous flower from the Jurassic of China. eLife 2018, 7, e38827. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Diez, J.B.; Pole, M.; García-Ávila, M.; Wang, X. Nanjinganthus is an angiosperm, isn't it? China Geology 2020, 3, 359–361. [Google Scholar] [CrossRef]
- Friis, E.M.; Crane, P.R.; Pedersen, K.R. The early flowers and angiosperm evolution; Cambridge University Press: Cambridge, 2011; p. 596. [Google Scholar]
- Mendes, M.M.; Grimm, G.W.; Pais, J.; Friis, E.M. Fossil Kajanthus lusitanicus gen. et sp. nov. from Portugal: floral evidence for Early Cretaceous Lardizabalaceae (Ranunculales, basal eudicot). Grana 2014, 53, 283–301. [Google Scholar] [CrossRef]
- Fu, Q.; Hou, Y.; Yin, P.; Diez, J.B.; Pole, M.; García-Ávila, M.; Wang, X. Micro-CT results exhibit ovules enclosed in the ovaries of Nanjinganthus. Scientific Reports 2023, 13, 426. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).