Submitted:
10 June 2024
Posted:
11 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Extract of Male Axillary Secretions (EMAS)
2.3. Evaluation of Emotional State of Test Subjects
2.4. Procedure
2.5. Hormone’s Measurements and Further Calculations
2.6. Statistical Analyses
3. Results
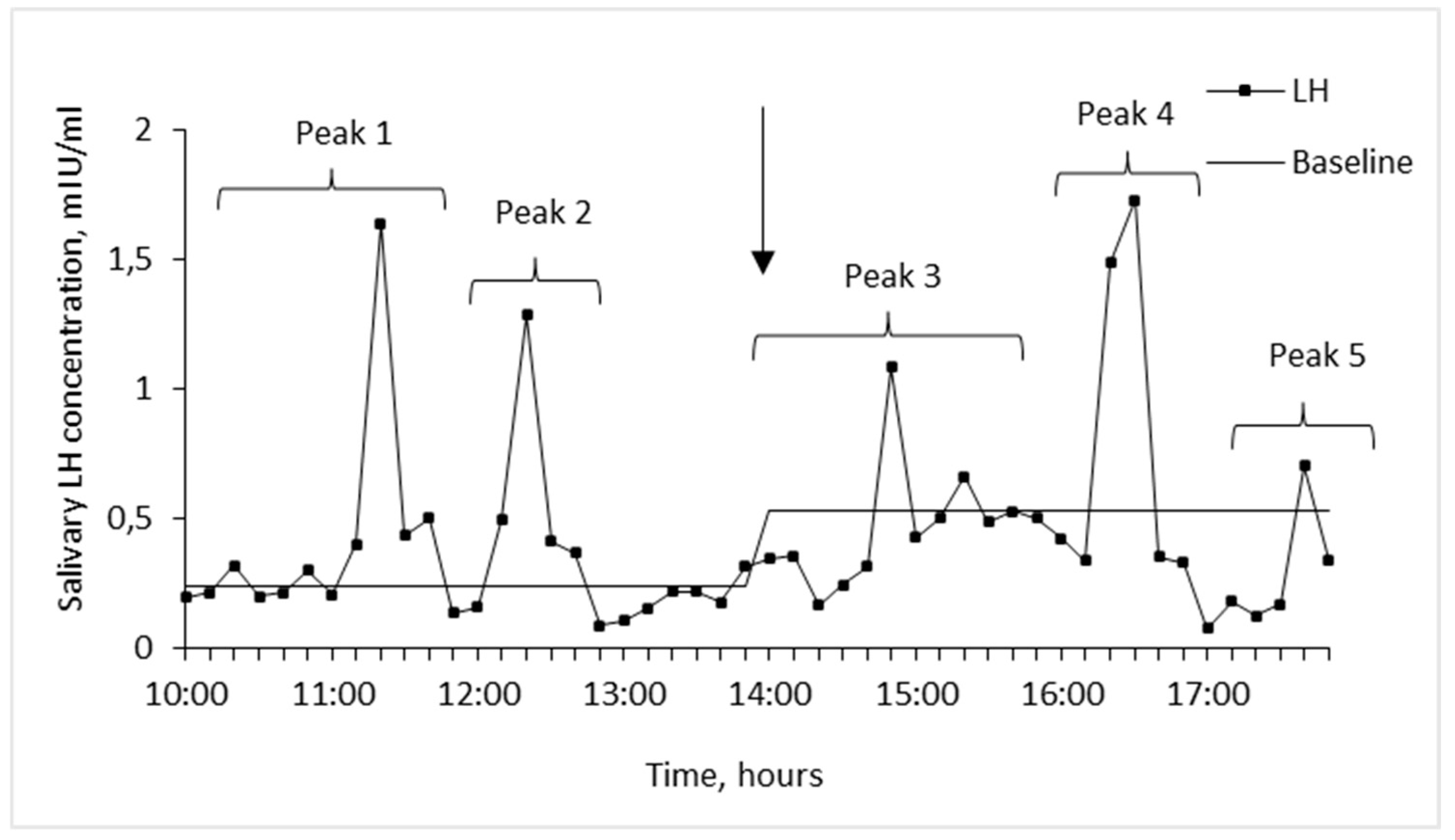
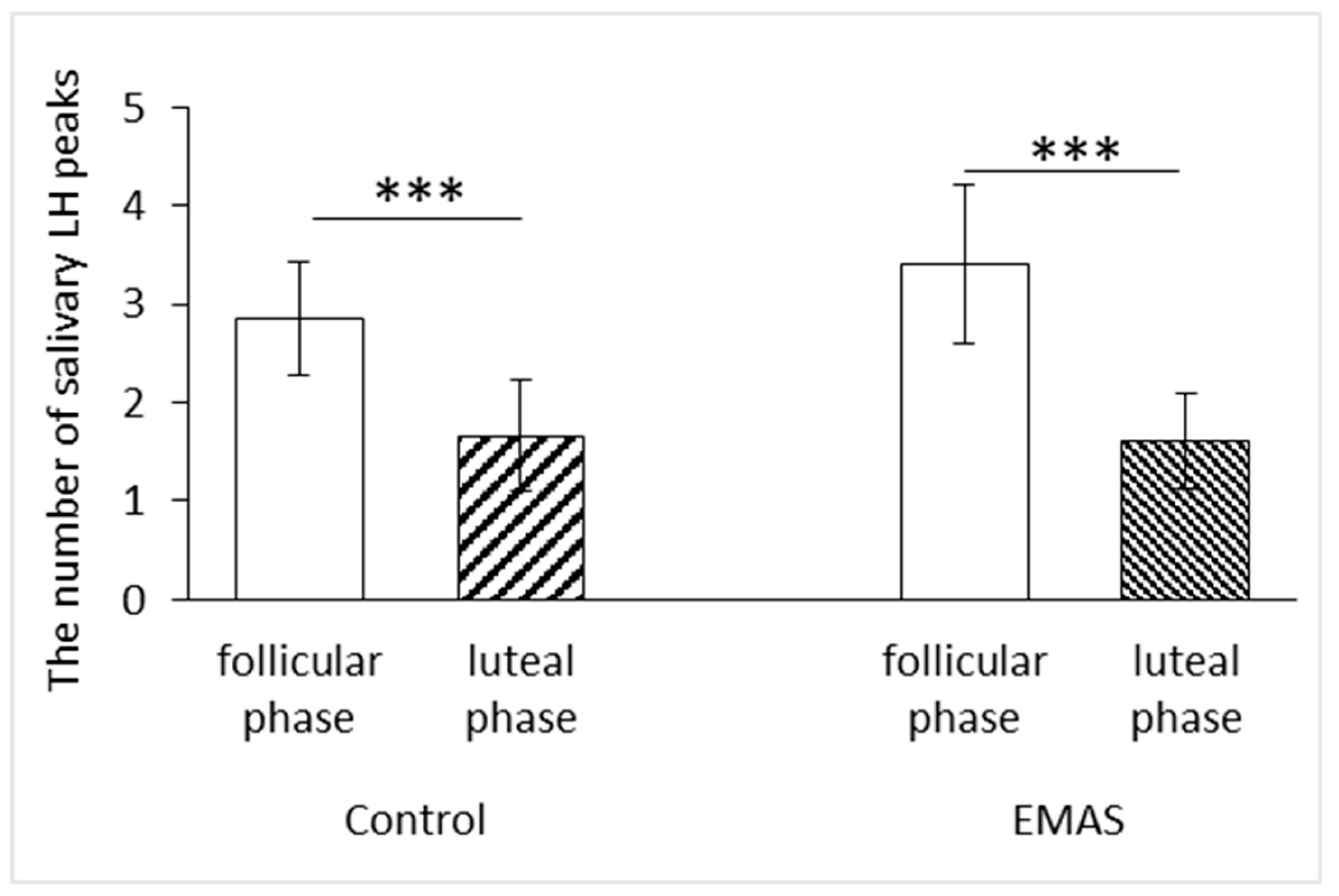
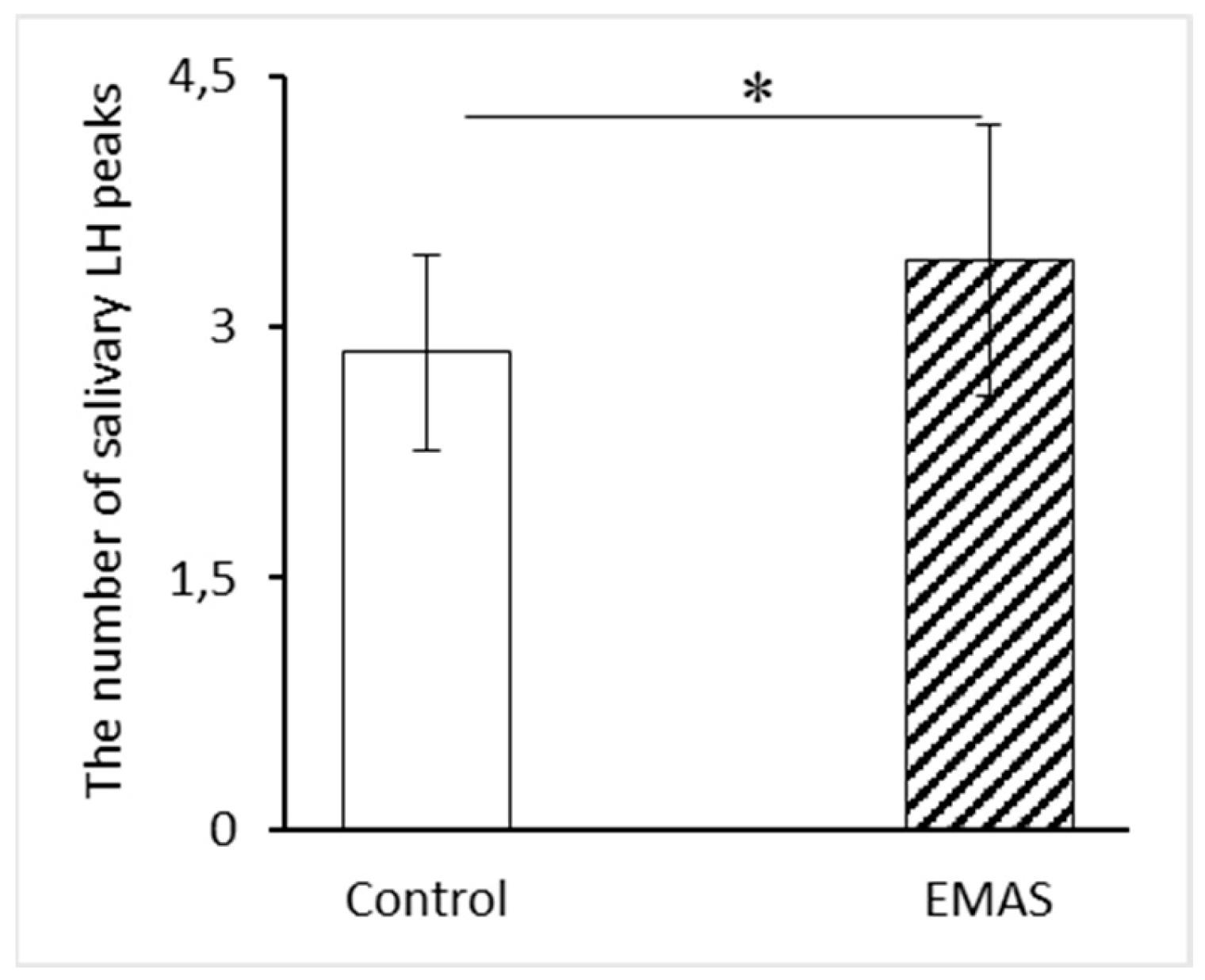
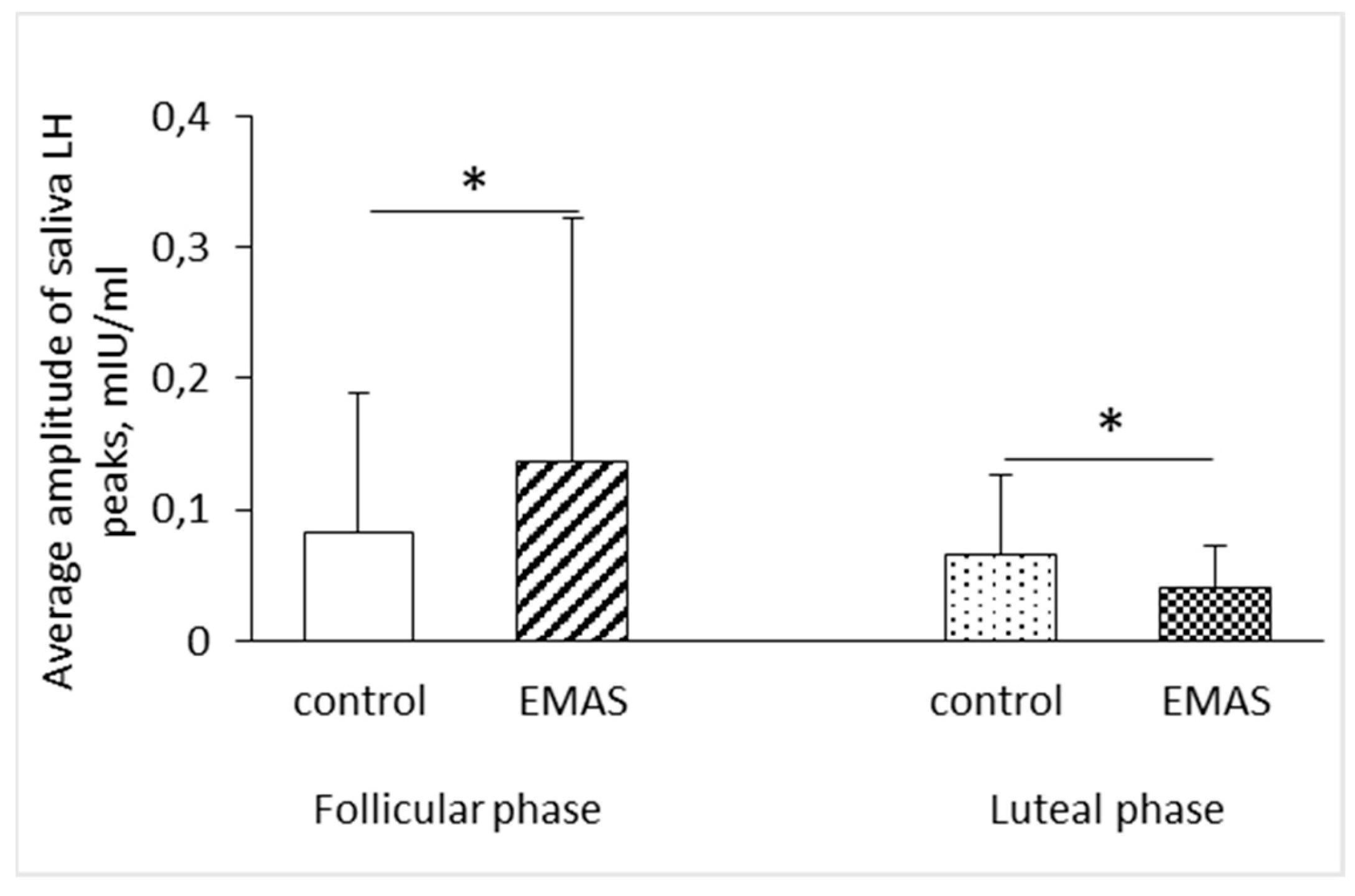
3.1. The Parameters of LH Peaks
3.2. Salivary cortisol
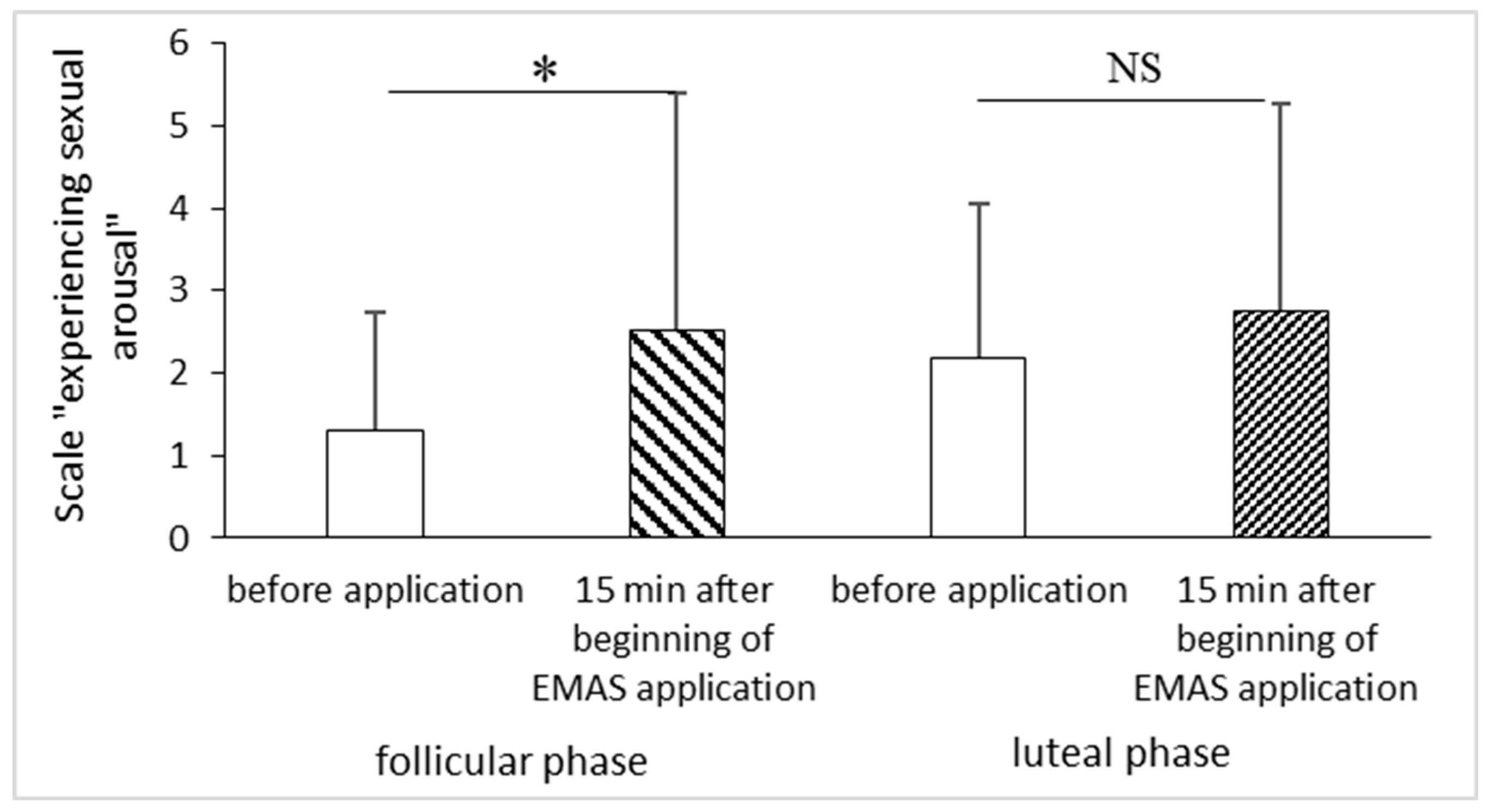
3.3. Emotional State
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGann, J.P. Poor Human Olfaction Is a 19th-Century Myth. Science 2017, 356, eaam7263. [Google Scholar] [CrossRef] [PubMed]
- Mahmut, M.K.; Croy, I. The Role of Body Odors and Olfactory Ability in the Initiation, Maintenance and Breakdown of Romantic Relationships—A Review. Physiology & Behavior 2019, 207, 179–184. [Google Scholar] [CrossRef]
- Loos, H.M.; Schaal, B.; Pause, B.M.; Smeets, M.A.M.; Ferdenzi, C.; Roberts, S.C.; De Groot, J.; Lübke, K.T.; Croy, I.; Freiherr, J.; et al. Past, Present, and Future of Human Chemical Communication Research. Perspect Psychol Sci 2023, 17456916231188148. [Google Scholar] [CrossRef] [PubMed]
- Lundström, J.N.; Olsson, M.J. Functional Neuronal Processing of Human Body Odors. In Vitamins & Hormones; Elsevier, 2010; Vol. 83, pp. 1–23. ISBN 9780123815163. [Google Scholar] [CrossRef]
- Cutler, W.B.; Preti, G.; Krieger, A.; Huggins, G.R.; Garcia, C.R.; Lawley, H.J. Human Axillary Secretions Influence Women’s Menstrual Cycles: The Role of Donor Extract from Men. Hormones and Behavior 1986, 20, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Preti, G.; Wysocki, C.J.; Barnhart, K.T.; Sondheimer, S.J.; Leyden, J.J. Male Axillary Extracts Contain Pheromones That Affect Pulsatile Secretion of Luteinizing Hormone and Mood in Women Recipients1. Biology of Reproduction 2003, 68, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Rozenkrantz, L.; Weissgross, R.; Weiss, T.; Ravreby, I.; Frumin, I.; Shushan, S.; Gorodisky, L.; Reshef, N.; Holzman, Y.; Pinchover, L.; et al. Unexplained Repeated Pregnancy Loss Is Associated with Altered Perceptual and Brain Responses to Men’s Body-Odor. eLife 2020, 9, e55305. [Google Scholar] [CrossRef]
- Prokop-Prigge, K.A.; Greene, K.; Varallo, L.; Wysocki, C.J.; Preti, G. The Effect of Ethnicity on Human Axillary Odorant Production. J Chem Ecol 2016, 42, 33–39. [Google Scholar] [CrossRef]
- Voznessenskaya, V.V.; Laktionova, T.K. Influence of the Male Axillary Extracts on Regulation of Menstrual Cycles in Women. Dokl Biol Sci 2018, 478, 19–21. [Google Scholar] [CrossRef]
- Derntl, B.; Schopf, V.; Kollndorfer, K.; Lanzenberger, R. Menstrual Cycle Phase and Duration of Oral Contraception Intake Affect Olfactory Perception. Chemical Senses 2013, 38, 67–75. [Google Scholar] [CrossRef]
- Yao, F.; Chen, K.; Zhuang, Y.; Shen, X.; Wang, X. Mid-Luteal Olfactory Abilities Reveal Healthy Women’s Emotional and Cognitive Functions. Front. Neurosci. 2022, 16, 826547. [Google Scholar] [CrossRef]
- Roberts, S.C.; Třebická Fialová, J.; Sorokowska, A.; Langford, B.; Sorokowski, P.; Třebický, V.; Havlíček, J. Emotional Expression in Human Odour. Evolut. Hum. Sci. 2022, 4, e44. [Google Scholar] [CrossRef] [PubMed]
- De Groot, J.H.B.; Haertl, T.; Loos, H.M.; Bachmann, C.; Kontouli, A.; Smeets, M.A.M. Unraveling the Universality of Chemical Fear Communication: Evidence from Behavioral, Genetic, and Chemical Analyses. Chemical Senses 2023, 48, bjad046. [Google Scholar] [CrossRef] [PubMed]
- Preti, G.; Cutler, W.B.; Garcia, C.R.; Huggins, G.R.; Lawley, H.J. Human Axillary Secretions Influence Women’s Menstrual Cycles: The Role of Donor Extract of Females. Hormones and Behavior 1986, 20, 474–482. [Google Scholar] [CrossRef]
- Zeng, X.-N.; Leyden, J.J.; Lawley, H.J.; Sawano, K.; Nohara, I.; Preti, G. Analysis of Characteristic Odors from Human Male Axillae. J Chem Ecol 1991, 17, 1469–1492. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. Journal of Personality and Social Psychology 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Osin, E.N. Measuring Positive and Negative Affect: Development of a Russian-language Analogue of PANAS: Psychology. Journal of Higher School of Economics 2012, 9, 91–110. (In Russian) [Google Scholar]
- Aitken, R.C. Measurement of Feelings Using Visual Analogue Scales. Proc R Soc Med 1969, 62, 989–993. [Google Scholar]
- Seubert, J.; Gregory, K.M.; Chamberland, J.; Dessirier, J.-M.; Lundström, J.N. Odor Valence Linearly Modulates Attractiveness, but Not Age Assessment, of Invariant Facial Features in a Memory-Based Rating Task. PLoS ONE 2014, 9, e98347. [Google Scholar] [CrossRef]
- Brown, J.L.; Schmitt, D.L.; Bellem, A.; Graham, L.H.; Lehnhardt, J. Hormone Secretion in the Asian Elephant (Elephas Maximus): Characterization of Ovulatory and Anovulatory Luteinizing Hormone Surges. Biology of Reproduction 1999, 61, 1294–1299. [Google Scholar] [CrossRef]
- Bruin, P.R.D.; Medger, K.; Bennett, N.C.; Ganswindt, A. Assessment of Reproductive Function in Southern African Spiny Mice ( Acomys Spinosissimus ) Using Faeces as Hormone Matrix. African Zoology 2014, 49, 44–53. [Google Scholar] [CrossRef]
- Kvasha, I.G.; Laktionova, T.K.; Voznessenskaya, V.V. The Presentation Rate of Chemical Signals of the Domestic Cat Felis Catus Affects the Reproductive Status of the House Mouse. Biol Bull Russ Acad Sci 2018, 45, 278–283. [Google Scholar] [CrossRef]
- Gingold, J.A.; Jain, M.; Jalai, C. Hypothalamic-Pituitary-Ovarian Axis and Control of the Menstrual Cycle. In Clinical Reproductive Medicine and Surgery; Falcone, T., Hurd, W.W., Eds.; Springer International Publishing: Cham, 2022; pp. 1–22. ISBN 9783030995959 9783030995966. [Google Scholar] [CrossRef]
- Mezzullo, M.; Fanelli, F.; Fazzini, A.; Gambineri, A.; Vicennati, V.; Di Dalmazi, G.; Pelusi, C.; Mazza, R.; Pagotto, U.; Pasquali, R. Validation of an LC–MS/MS Salivary Assay for Glucocorticoid Status Assessment: Evaluation of the Diurnal Fluctuation of Cortisol and Cortisone and of Their Association within and between Serum and Saliva. The Journal of Steroid Biochemistry and Molecular Biology 2016, 163, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; Schoenfeld, D.A.; Martin, K.A.; Crowley, W.F. Hypothalamic Gonadotropin-Releasing Hormone Secretion and Follicle-Stimulating Hormone Dynamics during the Luteal-Follicular Transition. The Journal of Clinical Endocrinology & Metabolism 1992, 74, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Loewit, K.K.; Kraft, H.-G.; Ortlieb, A. Measurement of LH in Saliva; a New Approach to Ovulation Detection. In Future Aspects in Contraception; Runnebaum, B., Rabe, T., Kiesel, L., Eds.; Springer: Dordrecht, Netherlands, 1985; pp. 31–47. ISBN 9789401086783 9789400949164. [Google Scholar] [CrossRef]
- Saibaba, G.; Srinivasan, M.; Priya Aarthy, A.; Silambarasan, V.; Archunan, G. Ultrastructural and Physico-Chemical Characterization of Saliva during Menstrual Cycle in Perspective of Ovulation in Human. DD&T 2017, 11, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Urban, R.J.; Johnson, M.L.; Veldhuis, J.D. In Vivo Biological Validation and Biophysical Modeling of the Sensitivity and Positive Accuracy of Endocrine Peak Detection. I. The LH Pulse Signal*. Endocrinology 1989, 124, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Rossmanith, W.G.; Lauritzen, C. The Luteinizing Hormone Pulsatile Secretion: Diurnal Excursions in Normally Cycling and Postmenopausal Women. Gynecological Endocrinology 1991, 5, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Klingman, K.M.; Marsh, E.E.; Klerman, E.B.; Anderson, E.J.; Hall, J.E. Absence of Circadian Rhythms of Gonadotropin Secretion in Women. The Journal of Clinical Endocrinology & Metabolism 2011, 96, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Van Kerkhof, L.W.M.; Van Dycke, K.C.G.; Jansen, E.H.J.M.; Beekhof, P.K.; Van Oostrom, C.T.M.; Ruskovska, T.; Velickova, N.; Kamcev, N.; Pennings, J.L.A.; Van Steeg, H.; et al. Diurnal Variation of Hormonal and Lipid Biomarkers in a Molecular Epidemiology-Like Setting. PLoS ONE 2015, 10, e0135652. [Google Scholar] [CrossRef]
- Rahman, S.A.; Grant, L.K.; Gooley, J.J.; Rajaratnam, S.M.W.; Czeisler, C.A.; Lockley, S.W. Endogenous Circadian Regulation of Female Reproductive Hormones. The Journal of Clinical Endocrinology & Metabolism 2019, 104, 6049–6059. [Google Scholar] [CrossRef]
- Ozgocer, T.; Ucar, C.; Yildiz, S. Daily Cortisol Awakening Response and Menstrual Symptoms in Young Females. Stress and Health 2022, 38, 57–68. [Google Scholar] [CrossRef]
- Vining, R.F.; McGinley, R.A.; Maksvytis, J.J.; Ho, K.Y. Salivary Cortisol: A Better Measure of Adrenal Cortical Function than Serum Cortisol. Ann Clin Biochem 1983, 20, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Wyart, C.; Webster, W.W.; Chen, J.H.; Wilson, S.R.; McClary, A.; Khan, R.M.; Sobel, N. Smelling a Single Component of Male Sweat Alters Levels of Cortisol in Women. J. Neurosci. 2007, 27, 1261–1265. [Google Scholar] [CrossRef]
- Hamilton, L.D.; Rellini, A.H.; Meston, C.M. Cortisol, Sexual Arousal, and Affect in Response to Sexual Stimuli. The Journal of Sexual Medicine 2008, 5, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Lundström, J. Psychological Effects of Subthreshold Exposure to the Putative Human Pheromone 4,16-Androstadien-3-One. Hormones and Behavior 2003, 44, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Gulyás, B.; Kéri, S.; O’Sullivan, B.T.; Decety, J.; Roland, P.E. The Putative Pheromone Androstadienone Activates Cortical Fields in the Human Brain Related to Social Cognition. Neurochemistry International 2004, 44, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Marazziti, D.; Masala, I.; Baroni, S.; Polini, M.; Massimetti, G.; Giannaccini, G.; Betti, L.; Italiani, P.; Fabbrini, L.; Caglieresi, C.; et al. Male Axillary Extracts Modify the Affinity of the Platelet Serotonin Transporter and Impulsiveness in Women. Physiology & Behavior 2010, 100, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, T.D. Pheromones and Animal Behavior: Chemical Signals and Signatures, 2nd edition; Cambridge University Press: Cambridge, 2014; ISBN 9780521112901 9780521130196. [Google Scholar]
- Ferdenzi, C.; Razafindrazaka, H.; Baldovini, N.; Poupon, D.; Pierron, D.; Bensafi, M. Influence of Gender and Culture on the Perception of Acidic Compounds of Human Body Odor. Physiology & Behavior 2019, 210, 112561. [Google Scholar] [CrossRef] [PubMed]
- Natsch, A.; Emter, R. The Specific Biochemistry of Human Axilla Odour Formation Viewed in an Evolutionary Context. Philosophical Transactions of the Royal Society B: Biological Sciences, 2020; 375, 20190269. [Google Scholar] [CrossRef]
- Moller, A.P.; Pomiankowski, A. Why Have Birds Got Multiple Sexual Ornaments? Behav Ecol Sociobiol 1993, 32. [Google Scholar] [CrossRef]
- Thornhill, R. The Scent of Symmetry: A Human Sex Pheromone That Signals Fitness? Evolution and Human Behavior 1999, 20, 175–201. [Google Scholar] [CrossRef]
- Grammer, K.; Fink, B.; Juette, A.; Ronzal, G.; Thornhill, R. Female Faces and Bodies: N-Dimensional Feature Space and Attractiveness. In Facial attractiveness: Evolutionary, cognitive, and social perspectives; Advances in visual cognition, vol. 1.; Ablex Publishing: Westport, CT, US, 2002; pp. 91–125. ISBN 9781567506365 9781567506372. [Google Scholar]
- Třebický, V.; Delplanque, S.; Ferdenzi, C.; Fink, B.; Jelínková, L.; Pátková, Ž.; Roberts, S.C.; Röder, S.; Saxton, T.K.; Schwambergová, D.; et al. Cross-Modal Associations of Human Body Odour Attractiveness with Facial and Vocal Attractiveness Provide Little Support for the Backup Signals Hypothesis: A Systematic Review and Meta-Analysis. Evolution and Human Behavior 2023, 44, 19–29. [Google Scholar] [CrossRef]
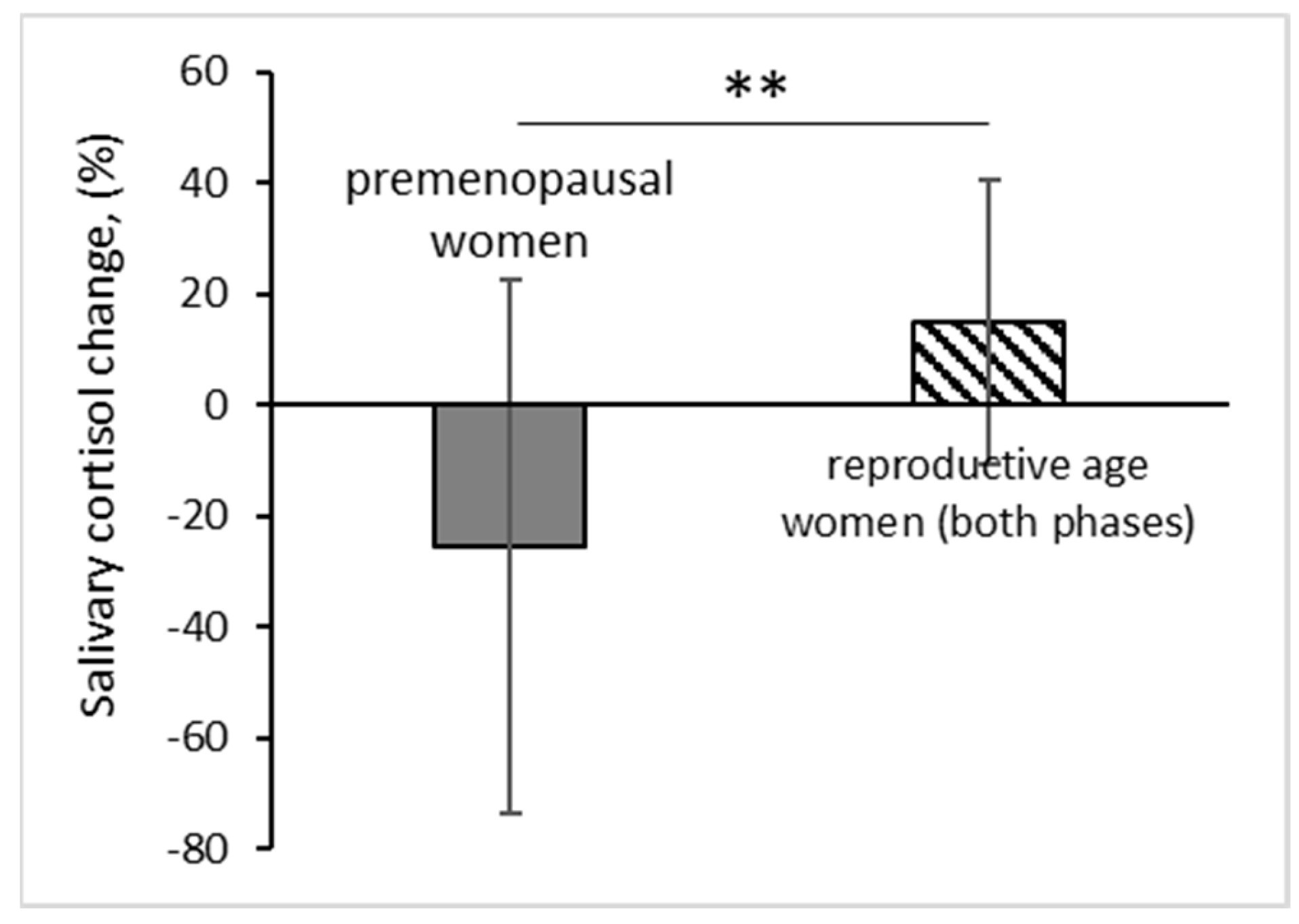
| Component 1: | |||||
|---|---|---|---|---|---|
| Df | R Sum Sq | R Mean Sq | Iter | Pr(Prob) | |
| age group | 1 | 10697 | 10696.9 | 5000 | 0.0032 ** |
| Residuals | 27 | 32726 | 1212.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





