Submitted:
10 June 2024
Posted:
11 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Physical Activity and Sun Exposure Scores Assessment
2.2.1. Physical Activity Assessment
2.2.2. Sun Exposure Assessment
2.3. Anthropometric and Laboratory Assessment
2.3.1. Vitamin D, Lipids Profiles, and Atherogenic Indices Evaluation
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Participants according to 25(OH) D Status
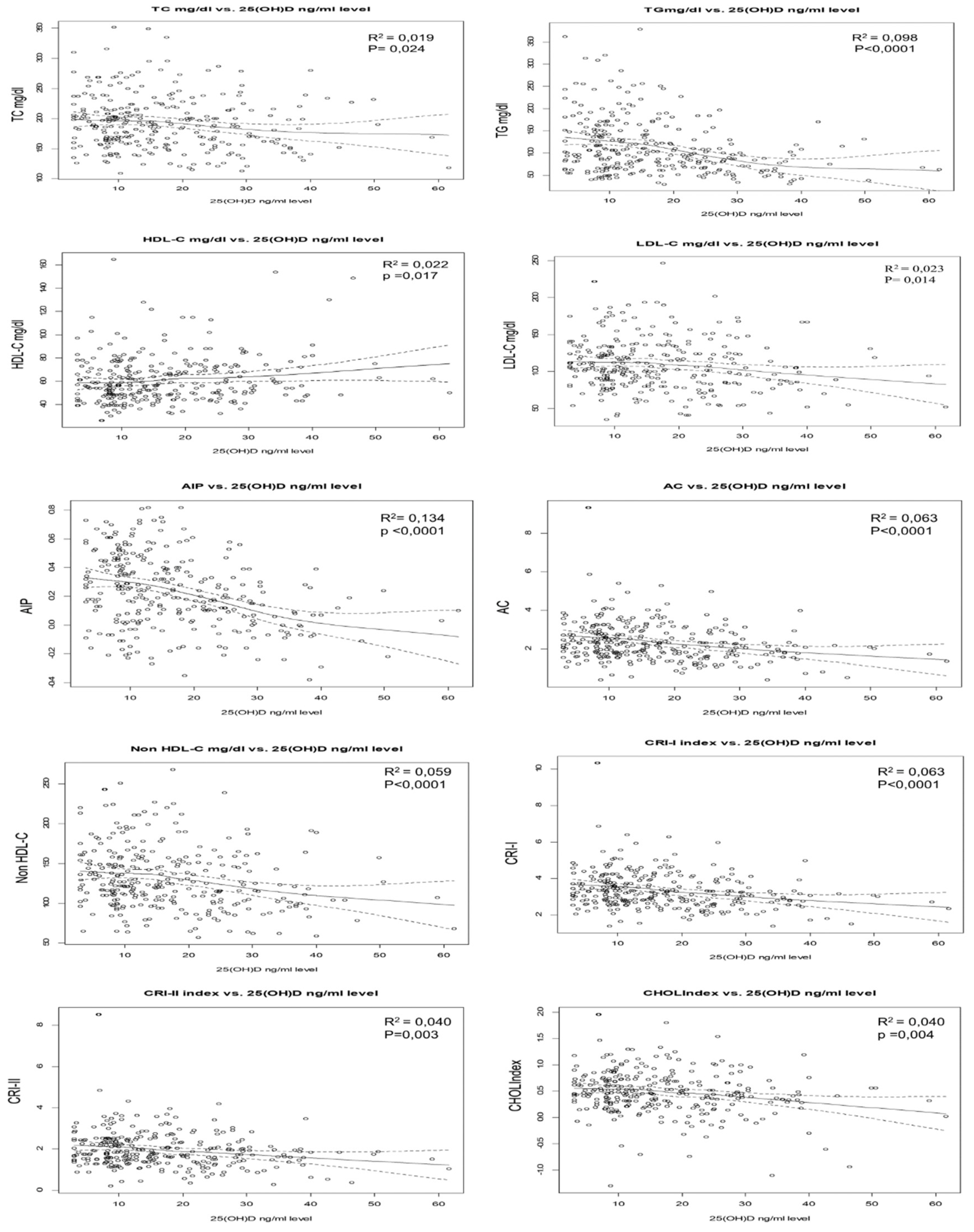
3.2. Association of Lipid Profiles, and Atherogenic Indices with 25(OH) D Concentrations
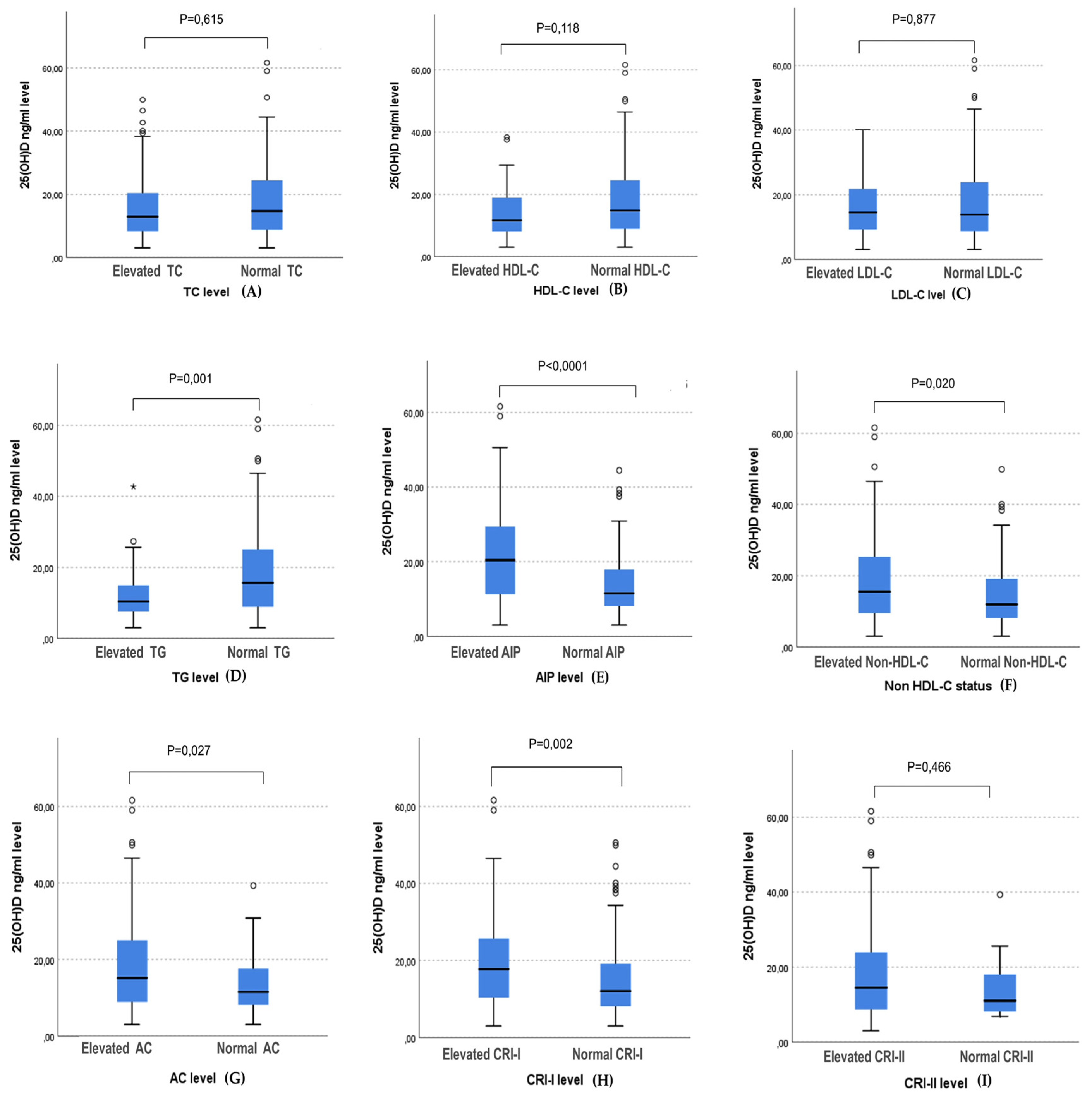
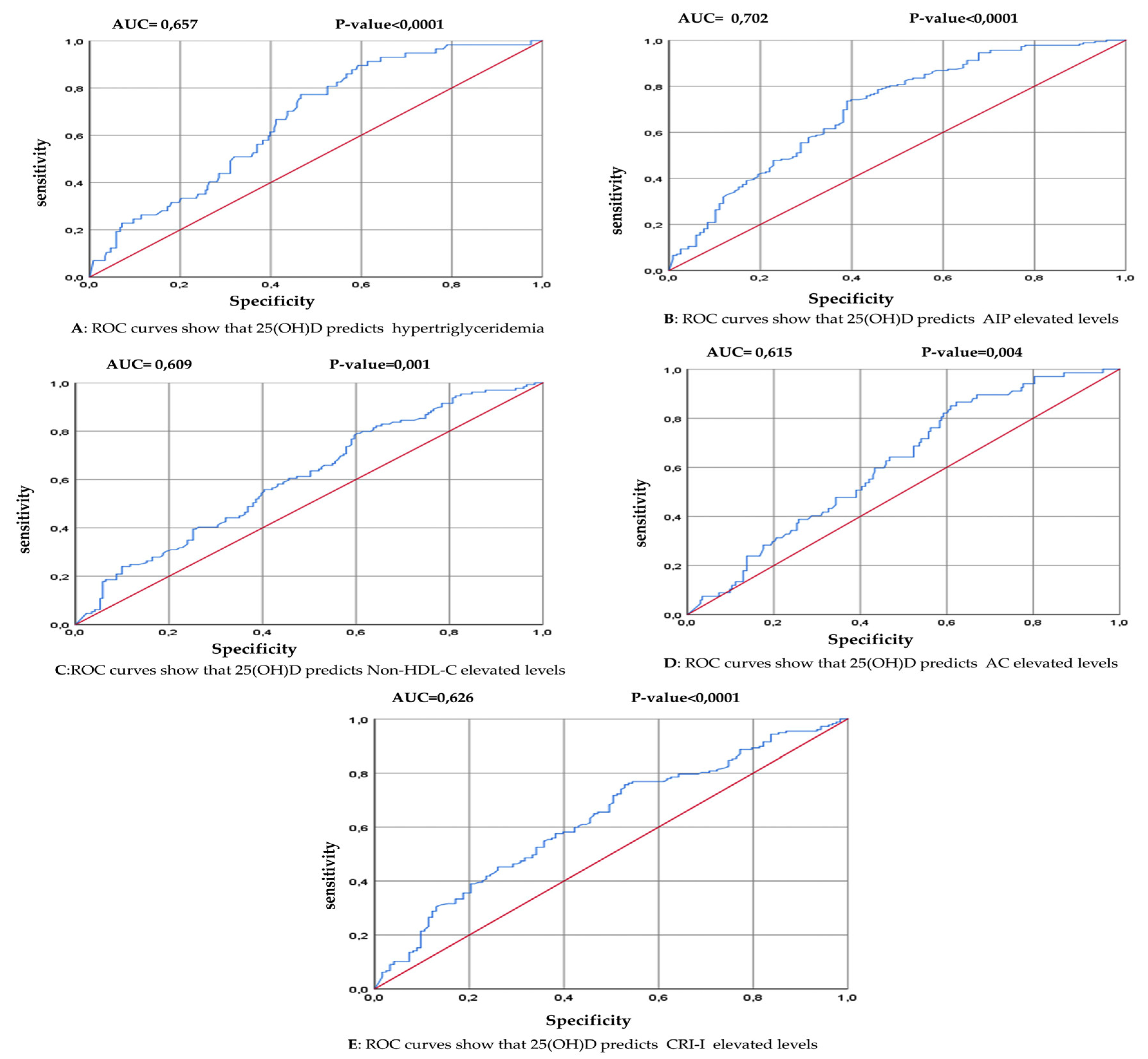
3.3. The Association between 25(OH) D Level and the Occurrences of Hypertriglyceridemia and High Atherogenic Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holick, M.F. The Vitamin D Deficiency Pandemic: Approaches for Diagnosis, Treatment and Prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Schöttker, B.; Haug, U.; Schomburg, L.; Köhrle, J.; Perna, L.; Müller, H.; Holleczek, B.; Brenner, H. Strong Associations of 25-Hydroxyvitamin D Concentrations with All-Cause, Cardiovascular, Cancer, and Respiratory Disease Mortality in a Large Cohort Study. Am. J. Clin. Nutr. 2013, 97, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D Effects on Musculoskeletal Health, Immunity, Autoimmunity, Cardiovascular Disease, Cancer, Fertility, Pregnancy, Dementia and Mortality-A Review of Recent Evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.C.J.; Lazaretti-Castro, M. Vitamin D Metabolism and Extraskeletal Outcomes: An Update. Arch. Endocrinol. Metab. 2022, 66, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Karonova, T.; Grineva, E.; Belyaeva, O.; Bystrova, A.; Jude, E.B.; Andreeva, A.; Kostareva, A.; Pludowski, P. Relationship between Vitamin D Status and Vitamin D Receptor Gene Polymorphisms with Markers of Metabolic Syndrome among Adults. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D Deficiency: A Worldwide Problem with Health Consequences. Am. J. Clin. Nutr. 2008, 87, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Tofano, R.J.; De Campos, A.L.; Rodrigues, A.S.; Quesada, K.; Bechara, M.D.; De Alvares Goulart, R.; Oshiiwa, M. Association between Vitamin D Status and Metabolic Syndrome Risk Factors. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Al-Said, N.H.; Mohamed, N.A.E.G.; Salam, R.F.; Fawzy, M.W. Vitamin D as a Risk Factor for Premature Atherosclerosis in Patients with Type 2 Diabetes. Ther. Adv. Endocrinol. Metab. 2015, 6, 249–257. [Google Scholar] [CrossRef]
- Ali Shah, S.I.; lqbal, S.; Sikandar, M.Z.; Yaqub Qazi, U.; Haq, I. Serum Vitamin D and Cardiometabolic Markers: A Comparative Study in Adult Men Based on Body Mass Index. IIUM Med. J. Malays. 2021, 20, 67–74. [Google Scholar] [CrossRef]
- Alquaiz, A.M.; Kazi, A.; Youssef, R.M.; Alshehri, N.; Alduraywish, S.A. Association between Standardized Vitamin 25(OH)D and Dyslipidemia: A Community-Based Study in Riyadh, Saudi Arabia. Environ. Health Prev. Med. 2020, 25. [Google Scholar] [CrossRef]
- Alyami, A.M.; Lam, V.; Soares, M.J.; Zhao, Y.; Sherriff, J.L.; Mamo, J.C.; James, A.P.; Coombes, F. The Association of Vitamin D Status with Dyslipidaemia and Biomarkers of Endothelial Cell Activation in Older Australians. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Amirkhizi, F.; Pishdadian, A.; Asghari, S.; Hamedi-Shahraki, S. Vitamin D Status Is Favorably Associated with the Cardiovascular Risk Factors in Adults with Obesity. Clin. Nutr. ESPEN 2021, 46, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.A.; Niazi, R.; Arif, S.A. Association of Dyslipidaemia in Patients with Varying Degrees of Vitamin D Deficiency in the Asian Population. J. Pak. Med. Assoc. 2017, 67, 1843–1847. [Google Scholar]
- Baker, J.; Mehta, N.; Baker, D.; Toedter, G.; Shults, J.; Von Feldt, J.; Leonard, M. Vitamin D, Metabolic Dyslipidemia, and Metabolic Syndrome in Rheumatoid Arthritis. Am. J. Med. 2012, 125. [Google Scholar] [CrossRef]
- Cosentino, N.; Campodonico, J.; Milazzo, V.; De Metrio, M.; Brambilla, M.; Camera, M.; Marenzi, G. Vitamin D and Cardiovascular Disease: Current Evidence and Future Perspectives. Nutrients 2021, 13, 3603. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C. Vitamin D and the Renin-Angiotensin System; Fourth Edition.; Elsevier Inc., 2018; Vol. 1; ISBN 978-0-12-809966-7. [CrossRef]
- Han, L.; Xu, X.-J.; Zhang, J.-S.; Liu, H.-M. Association between Vitamin D Deficiency and Levels of Renin and Angiotensin in Essential Hypertension. Int. J. Clin. Pract. 2022, 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Meza, C.A.; Clarke, H.; Kim, J.-S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Polly, P.; Tan, T.C. The Role of Vitamin D in Skeletal and Cardiac Muscle Function. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef]
- Mellenthin, L.; Wallaschofski, H.; Grotevendt, A.; Völzke, H.; Nauck, M.; Hannemann, A. Association between Serum Vitamin D Concentrations and Inflammatory Markers in the General Adult Population. Metabolism. 2014, 63, 1056–1062. [Google Scholar] [CrossRef]
- Karkeni, E.; Bonnet, L.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Ye, J.; Landrier, J.-F. Vitamin D Limits Inflammation-Linked microRNA Expression in Adipocytes in Vitro and in Vivo : A New Mechanism for the Regulation of Inflammation by Vitamin D. Epigenetics 2018, 13, 156–162. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO) WHO List of Priority Medical Devices for Management of Cardiovascular Diseases and Diabetes; 2021; ISBN 9789240027978. https://www.who.int/publications-detail-redirect/9789240027978%0Afiles/1046/9789240027978.html. (Accessed on 15 Septembre 2022).
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Morocco GUIDE D’EVALUATION ET DE PRISE EN CHARGE DU RISQUE CARDIOVASCULAIRE; AZ Edition.; Direction de l’Epidémiologie et de Lutte contre les Maladies, 2019. (Accessed on 15 April 2022). Available online: https://www.sante.gov.ma/Documents/2023/03/Guide risque cardiovasculaire VF AZ 08 juillet 2019.pdf.
- Vogel, B.; Acevedo, M.; Appelman, Y.; Bairey Merz, C.N.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The Lancet Women and Cardiovascular Disease Commission: Reducing the Global Burden by 2030. The Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Stouffer, G.A.; Kucharska-Newton, A.M.; Qamar, A.; Vaduganathan, M.; Pandey, A.; Porterfield, D.; Blankstein, R.; Rosamond, W.D.; Bhatt, D.L.; et al. Twenty Year Trends and Sex Differences in Young Adults Hospitalized With Acute Myocardial Infarction: The ARIC Community Surveillance Study. Circulation 2019, 139, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Gabet, A.; Danchin, N.; Juillière, Y.; Olié, V. Acute Coronary Syndrome in Women: Rising Hospitalizations in Middle-Aged French Women, 2004–14. Eur. Heart J. 2017, 38, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Wilmot, K.A.; O’Flaherty, M.; Capewell, S.; Ford, E.S.; Vaccarino, V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation 2015, 132, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Morocco Enquette Nationale Sur Les Facteurs de Risques Communs Des Maladies Non Transmissibles 2017 - 2018.; 2019; pp. 1–120;. Available online https://www.sante.gov.ma/Documents/2019/05/Rapport%20de%20l%20enqu%C3%AAte%20Stepwise.pdf. (Accessed on ). 15 April; 2022.
- Hedayatnia, M.; Asadi, Z.; Zare-Feyzabadi, R.; Yaghooti-Khorasani, M.; Ghazizadeh, H.; Ghaffarian-Zirak, R.; Nosrati-Tirkani, A.; Mohammadi-Bajgiran, M.; Rohban, M.; Sadabadi, F.; et al. Dyslipidemia and Cardiovascular Disease Risk among the MASHAD Study Population. Lipids Health Dis. 2020, 19, 42. [Google Scholar] [CrossRef]
- Du, Z.; Qin, Y. Dyslipidemia and Cardiovascular Disease: Current Knowledge, Existing Challenges, and New Opportunities for Management Strategies. J. Clin. Med. 2023, 12, 363. [Google Scholar] [CrossRef]
- Wang, Y.; Si, S.; Liu, J.; Wang, Z.; Jia, H.; Feng, K.; Sun, L.; Song, S.J. The Associations of Serum Lipids with Vitamin D Status. PLOS ONE 2016, 11, e0165157. [Google Scholar] [CrossRef]
- Rashidbeygi, E.; Rahimi, M.H.; Mollahosseini, M.; Yekaninejad, M.S.; Imani, H.; Maghbooli, Z.; Mirzaei, K. Associations of Vitamin D Status and Metabolic Dyslipidemia and Hypertriglyceridemic Waist Phenotype in Apparently Healthy Adults. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 985–990. [Google Scholar] [CrossRef]
- Jeenduang, N.; Sangkaew, B. The Association between Serum 25-Hydroxyvitamin D Concentrations and Serum Lipids in the Southern Thai Population. Arch. Med. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Liu, Q.; Zhang, Q.; Wan, Z.; Hu, L.; Xu, R.; Cheng, A.; Lv, Y.; Wang, L. Sex-Specific Association between Serum Vitamin D Status and Lipid Profiles: A Cross-Sectional Study of a Middle-Aged and Elderly Chinese Population. J. Nutr. Sci. Vitaminol. (Tokyo) 2020, 66, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, E.A.; Przychodzeń, S.; Dąbrowski, M. The Effects of Vitamin D on Severity of Coronary Artery Atherosclerosis and Lipid Profile of Cardiac Patients. Arch. Med. Sci. 2016, 6, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.; Musa, N.; Atty, S.; Ibrahem, M.; Wahab, N. Effect of Vitamin D Supplementation on Lipid Profile in Vitamin D-Deficient Children with Type 1 Diabetes and Dyslipidemia. Horm. Res. Paediatr. 2019, 91, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Abid, H.; Abid, Z.; Abid, S. Atherogenic Indices in Clinical Practice and Biomedical Research: A Short Review. Baghdad J. Biochem. Appl. Biol. Sci. 2021, 2, 60–70. [Google Scholar] [CrossRef]
- Afsin, A.; Kaya, H.; Suner, A.; Uzel, K.E.; Bursa, N.; Hosoglu, Y.; Yavuz, F.; Asoglu, R. Plasma Atherogenic Indices Are Independent Predictors of Slow Coronary Flow. BMC Cardiovasc. Disord. 2021, 21, 608. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Bhattacharjee, J.; Bhatnagar, M.K.; Tyagi, S. Atherogenic Index of Plasma, Castelli Risk Index And Atherogenic Coefficient- New Parameters In Assessing Cardiovascular Risk. Int. J. Pharm. Biol. Sci. 2013, 3, 359–364, https://api.semanticscholar.org/CorpusID:2598875. [Google Scholar]
- Olamoyegun, M.; Oluyombo, R.; Asaolu, S. Evaluation of Dyslipidemia, Lipid Ratios, and Atherogenic Index as Cardiovascular Risk Factors among Semi-Urban Dwellers in Nigeria. Ann. Afr. Med. 2016, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Sujatha, R.; Kavitha, S. Atherogenic Indices in Stroke Patients: A Retrospective Study. Iran J Neurol. 2017 Apr 4;16(2):78-82. PMCID: PMC5526781. [PubMed]
- Ulloque-Badaracco, J.R.; Hernandez-Bustamante, E.A.; Alarcon-Braga, E.A.; Mosquera-Rojas, M.D.; Campos-Aspajo, A.; Salazar-Valdivia, F.E.; Valdez-Cornejo, V.A.; Benites-Zapata, V.A.; Herrera-Añazco, P.; Valenzuela-Rodríguez, G.; et al. Atherogenic Index of Plasma and Coronary Artery Disease: A Systematic Review. Open Med. 2022, 17, 1915–1926. [Google Scholar] [CrossRef]
- Cai, G.; Liu, W.; Lv, S.; Wang, X.; Guo, Y.; Yan, Z.; Du, Y.; Zhou, Y. Gender-Specific Associations between Atherogenic Index of Plasma and the Presence and Severity of Acute Coronary Syndrome in Very Young Adults: A Hospital-Based Observational Study. Lipids Health Dis. 2019, 18, 99. [Google Scholar] [CrossRef]
- Fernández-Macías, J.C.; Ochoa-Martínez, A.C.; Varela-Silva, J.A.; Pérez-Maldonado, I.N. Atherogenic Index of Plasma: Novel Predictive Biomarker for Cardiovascular Illnesses. Arch. Med. Res. 2019, 50, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhou, S.; Feng, X.; Yang, J.; Qiao, J.; Zhao, Y.; Shi, D.; Zhou, Y. The Sensibility of the New Blood Lipid Indicator——Atherogenic Index of Plasma (AIP) in Menopausal Women with Coronary Artery Disease. Lipids Health Dis. 2020, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.; Zhao, X.; Jia, Z.; Li, Z.; Chen, S. Association Between Vitamin D Levels and the Atherogenic Index of Plasma Among Chinese with Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2023, Volume 16, 523–531. [Google Scholar] [CrossRef]
- Mahmoodi, M.R.; Najafipour, H. Associations between Serum Vitamin D3, Atherogenic Indices of Plasma and Cardiometabolic Biomarkers among Patients with Diabetes in the KERCADR Study. BMC Endocr. Disord. 2022, 22, 126. [Google Scholar] [CrossRef] [PubMed]
- Ministère de la Santé. ENQUETE NATIONALE SUR LA NUTRITION (ENN 2019-2020). 2022, 1–144, https://www.sante.gov.ma/Documents/2022/07/rapport%20ENN%202019-2020%20ajout%20preface%20.pdf. ( Accessed on ). 15 September; 2022.
- Glenn, D. Israel. Determining Sample Size 1.University of Florida IFAS Extension. Available At. Http://Edis.Ifas.Ufl.Edu. 2022, 1-5.
- Papathanasiou, G.; Georgoudis, G.; Papandreou, M.; Spyropoulos, P.; Georgakopoulos, D.; Kalfakakou, V.; Evangelou, A. Reliability Measures of the Short International Physical Activity Questionnaire (IPAQ) in Greek Young Adults. Hell. J. Cardiol. HJC Hellēnikē Kardiologikē Epitheōrēsē 2009, 50, 283–294. [Google Scholar]
- Pas-a-Pas research group; Arija, V. ; Villalobos, F.; Pedret, R.; Vinuesa, A.; Timón, M.; Basora, T.; Aguas, D.; Basora, J. Effectiveness of a Physical Activity Program on Cardiovascular Disease Risk in Adult Primary Health-Care Users: The “Pas-a-Pas” Community Intervention Trial. BMC Public Health 2017, 17, 576. [Google Scholar] [CrossRef]
- Lhilali, I.; Zouine, N.; Menouni, A.; Godderis, L.; Kestemont, M.-P.; El Midaoui, A.; El Jaafari, S.; Filali-Zegzouti, Y. Sun Exposure Score and Vitamin D Levels in Moroccan Women of Childbearing Age. Nutrients 2023, 15, 688. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I Through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimationof the Concentrationof Low-Density LipoproteinCholesteroiln Plasma,Without Useof the PreparativeUltracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- IOM (Institute of Medicine) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; A. Catharine Ross, Christine L. Taylor, Ann L. Yaktine, Valle, H.B.D., Eds.; Washington (DC): National Academies Press (US), 2011; ISBN 978-0-309-16394-1. [Google Scholar]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef] [PubMed]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 2002, 106, 3143–3421. [CrossRef]
- Xavier, H.T.; Izar, M.C.; Faria Neto, J.R.; Assad, M.H.; Rocha, V.Z.; Sposito, A.C.; Fonseca, F.A.; Santos, J.E.D.; Santos, R.D.; Bertolami, M.C.; et al. V Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose. Arq. Bras. Cardiol. 2013, 101, 01–22. [Google Scholar] [CrossRef]
- Molani Gol, R.; Rafraf, M.; Asghari Jafarabadi, M. Assessment of Atherogenic Indices and Lipid Ratios in the Apparently Healthy Women Aged 30–55 Years. Arter. Hypertens. 2022, 25, 172–177. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Casp. J. Intern. Med. 2013, 4, 627–635. [Google Scholar] [PubMed] [PubMed Central]
- Anouti, F.A.; Ahmed, L.A.; Riaz, A.; Grant, W.B.; Shah, N.; Ali, R.; Alkaabi, J.; Shah, S.M. Vitamin D Deficiency and Its Associated Factors among Female Migrants in the United Arab Emirates. Nutrients 2022, 14, 1074. [Google Scholar] [CrossRef] [PubMed]
- Dadda, S.; Azekour, K.; Sebbari, F.; El Houate, B.; El Bouhali, B. Sun Exposure, Dressing Habits, and Vitamin D Status in Morocco. E3S Web Conf. 2021, 319, 01097. [Google Scholar] [CrossRef]
- Dimakopoulos, I.; Magriplis, E.; Mitsopoulou, A.V.; Karageorgou, D.; Bakogianni, I.; Micha, R.; Michas, G.; Chourdakis, M.; Ntouroupi, T.; Tsaniklidou, S.M.; et al. Association of Serum Vitamin D Status with Dietary Intake and Sun Exposure in Adults. Clin. Nutr. ESPEN 2019, 34, 23–31. [Google Scholar] [CrossRef]
- Santana, K.V. de S. de; Oliver, S.L.; Mendes, M.M.; Lanham-New, S.; Charlton, K.E.; Ribeiro, H. Association between Vitamin D Status and Lifestyle Factors in Brazilian Women: Implications of Sun Exposure Levels, Diet, and Health. eClinicalMedicine 2022, 47, 101400. [Google Scholar] [CrossRef]
- Mehdad, S.; Belghiti, H.; Zahrou, F.E.; Guerinech, H.; Mouzouni, F.Z.; El hajjab, A.; El Berri, H.; El Ammari, L.; Benaich, S.; Benkirane, H.; et al. Vitamin D Status and Its Relationship with Obesity Indicators in Moroccan Adult Women. Nutr. Health 2022, 1–9. [Google Scholar] [CrossRef]
- Zouine, N.; Lhilali, I.; Menouni, A.; Godderis, L.; El Midaoui, A.; El Jaafari, S.; Zegzouti Filali, Y. Development and Validation of Vitamin D- Food Frequency Questionnaire for Moroccan Women of Reproductive Age: Use of the Sun Exposure Score and the Method of Triad’s Model. Nutrients 2023, 15, 796. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Thayakaran, R.; Gittoes, N.; Hewison, M.; Thomas, G.N.; Scragg, R.; Nirantharakumar, K. Non-Linear Associations of 25-Hydroxyvitamin D Concentrations with Risk of Cardiovascular Disease and All-Cause Mortality: Results from The Health Improvement Network (THIN) Database. J. Steroid Biochem. Mol. Biol. 2019, 195, 105480. [Google Scholar] [CrossRef]
- Zhang, R.; Li, B.; Gao, X.; Tian, R.; Pan, Y.; Jiang, Y.; Gu, H.; Wang, Y.; Wang, Y.; Liu, G. Serum 25-Hydroxyvitamin D and the Risk of Cardiovascular Disease: Dose-Response Meta-Analysis of Prospective Studies. Am. J. Clin. Nutr. 2017, 105, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Sastry, K.; Chouchane, A. Role of Vitamin D Beyond the Skeletal Function: A Review of the Molecular and Clinical Studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Allabhakshu, S.; Prabhu, K. Does Serum Vitamin D Affect Lipid Profile? Biomed. India 2021, 41, 787–792. [Google Scholar] [CrossRef]
- Yassir, S.; Ramarajan, M.G.; Patil, S.; Shaikh, S.B.; Abdulla, Y.M.; Manjrekar, P.A. Impact of Serum 25 Hydroxyvitamin D Deficiency on Lipid Biomarkers in Established Coronary Artery Disease. Turk. J. Biochem. 2022, 47, 79–84. [Google Scholar] [CrossRef]
- Zittermann, A.; Gummert, J.; Borgermann, J. The Role of Vitamin D in Dyslipidemia and Cardiovascular Disease. Curr. Pharm. Des. 2011, 17, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Conghui Guan; Songbo Fu; Donghu Zhen; Xuehong Li; Jinglei Niu; Jianguo Cheng; Nan Zhao; Jinjin Liu; Hongtao Yin; Xulei Tang Correlation of Serum Vitamin D with Lipid Profiles in Middle-Aged and Elderly Chinese Individuals. Asia Pac. J. Clin. Nutr. 2020, 29. [CrossRef] [PubMed]
- Lupton, J.R.; Faridi, K.F.; Martin, S.S.; Sharma, S.; Kulkarni, K.; Jones, S.R.; Michos, E.D. Deficient Serum 25-Hydroxyvitamin D Is Associated with an Atherogenic Lipid Profile: The Very Large Database of Lipids (VLDL-3) Study. J. Clin. Lipidol. 2016, 10, 72–81.e1. [Google Scholar] [CrossRef]
- Muñoz-Aguirre, P.; Flores, M.; Macias, N.; Quezada, A.D.; Denova-Gutiérrez, E.; Salmerón, J. The Effect of Vitamin D Supplementation on Serum Lipids in Postmenopausal Women with Diabetes: A Randomized Controlled Trial. Clin. Nutr. 2015, 34, 799–804. [Google Scholar] [CrossRef]
- Stellaard, F. From Dietary Cholesterol to Blood Cholesterol, Physiological Lipid Fluxes, and Cholesterol Homeostasis. Nutrients 2022, 14, 1643. [Google Scholar] [CrossRef]
- Han, Y.-Y.; Hsu, S.H.-J.; Su, T.-C. Association between Vitamin D Deficiency and High Serum Levels of Small Dense LDL in Middle-Aged Adults. Biomedicines 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Vaskonen, T. Dietary Minerals and Modification of Cardiovascular Risk Factors. J. Nutr. Biochem. 2003, 14, 492–506. [Google Scholar] [CrossRef]
- Mousa, A.; Naderpoor, N.; De Courten, M.P.J.; Scragg, R.; De Courten, B. 25-Hydroxyvitamin D Is Associated with Adiposity and Cardiometabolic Risk Factors in a Predominantly Vitamin D-Deficient and Overweight/Obese but Otherwise Healthy Cohort. J. Steroid Biochem. Mol. Biol. 2017, 173, 258–264. [Google Scholar] [CrossRef]
- Öztürk Özkan, G. Does Vitamin D Level Affect Beta Cell Activity? Bagcilar Med. Bull. 2021, 6, 397–406. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Śliwińska, A. Analysis of Association between Vitamin D Deficiency and Insulin Resistance. Nutrients 2019, 11, 794. [Google Scholar] [CrossRef] [PubMed]
- Larrick, B.M.; Kim, K.-H.; Donkin, S.S.; Teegarden, D. 1,25-Dihydroxyvitamin D Regulates Lipid Metabolism and Glucose Utilization in Differentiated 3T3-L1 Adipocytes. Nutr. Res. 2018, 58, 72–83. [Google Scholar] [CrossRef]
- Farrar, M.D.; Webb, A.R.; Kift, R.; Durkin, M.T.; Allan, D.; Herbert, A.; Berry, J.L.; Rhodes, L.E. Efficacy of a Dose Range of Simulated Sunlight Exposures in Raising Vitamin D Status in South Asian Adults : Implications for Targeted Guidance on Sun Exposure 1 – 3. 2013; 25, 1216. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal Relationship between Obesity and Vitamin D Status: Bi-Directional Mendelian Randomization Analysis of Multiple Cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef] [PubMed]
- Savastano, S.; Barrea, L.; Savanelli, M.C.; Nappi, F.; Di Somma, C.; Orio, F.; Colao, A. Low Vitamin D Status and Obesity: Role of Nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 215–225. [Google Scholar] [CrossRef]
- Song, K.; Park, G.; Choi, Y.; Oh, J.; Choi, H.; Suh, J.; Kwon, A.; Kim, H.; Chae, H. Association of Vitamin D Status and Physical Activity with Lipid Profile in Korean Children and Adolescents: A Population-Based Study. Child.-BASEL 2020, 7. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Barreto Junior, W. dos R. Association between Physical Activity and Vitamin D: A Narrative Literature Review. Rev. Assoc. Médica Bras. 2017, 63, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143. [Google Scholar] [CrossRef] [PubMed]
- Ugwuja, E.; Ogbonna, N.; Nwibo, A.; Onimawo, I. Overweight and Obesity, Lipid Profile and Atherogenic Indices among Civil Servants in Abakaliki, South Eastern Nigeria. Ann. Med. Health Sci. Res. 2013, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and Dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.; Cabezas, M. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.B. Sunlight Has Cardiovascular Benefits Independently of Vitamin D. Blood Purif. 2016, 41, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Fleury, N.; Geldenhuys, S.; Gorman, S. Sun Exposure and Its Effects on Human Health: Mechanisms through Which Sun Exposure Could Reduce the Risk of Developing Obesity and Cardiometabolic Dysfunction. Int. J. Environ. Res. Public. Health 2016, 13, 999. [Google Scholar] [CrossRef] [PubMed]
- Zand, J.; Lanza, F.; Garg, H.K.; Bryan, N.S. All-Natural Nitrite and Nitrate Containing Dietary Supplement Promotes Nitric Oxide Production and Reduces Triglycerides in Humans. Nutr. Res. 2011, 31, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, E.; Gustafsson, S.; Ingelsson, E. Associations of Fitness, Physical Activity, Strength, and Genetic Risk With Cardiovascular Disease: Longitudinal Analyses in the UK Biobank Study. Circulation 2018, 137, 2583–2591. [Google Scholar] [CrossRef]
- Chomistek, A.K.; Henschel, B.; Eliassen, A.H.; Mukamal, K.J.; Rimm, E.B. Frequency, Type, and Volume of Leisure-Time Physical Activity and Risk of Coronary Heart Disease in Young Women. Circulation 2016, 134, 290–299. [Google Scholar] [CrossRef]
- Gersh, B.J. Dose Response Between Physical Activity and Risk of Coronary Heart Disease: A Meta-Analysis. Yearb. Cardiol. 2012, 2012, 271–273. [Google Scholar] [CrossRef]
- Silva, R.C.D.; Diniz, M.D.F.H.S.; Alvim, S.; Vidigal, P.G.; Fedeli, L.M.G.; Barreto, S.M. Physical Activity and Lipid Profile in the ELSA-Brasil Study. Arq. Bras. Cardiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Maciel, E.D.S.; Silva, B.K.R.; Figueiredo, F.W.D.S.; Pontes-Silva, A.; Quaresma, F.R.P.; Adami, F.; Fonseca, F.L.A. Physical Inactivity Level and Lipid Profile in Traditional Communities in the Legal Amazon: A Cross-Sectional Study: Physical Inactivity Level in the Legal Amazon. BMC Public Health 2022, 22, 542. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Cho, Y.K.; Kim, Y.-J.; Jung, C.H.; Lee, W.J.; Park, J.-Y.; Huh, J.H.; Kang, J.G.; Lee, S.J.; Ihm, S.-H. Association of the Atherogenic Index of Plasma with Cardiovascular Risk beyond the Traditional Risk Factors: A Nationwide Population-Based Cohort Study. Cardiovasc. Diabetol. 2022, 21, 81. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhou, Y.; Sun, J.; Zhu, Z.; Xing, Z.; Zhou, S.; Wang, Y.; Tai, S. Atherogenic Index of Plasma Is Associated with Major Adverse Cardiovascular Events in Patients with Type 2 Diabetes Mellitus. Cardiovasc. Diabetol. 2021, 20, 201. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; Backer, G.G.D.; Delgado, V.; Ference, B.A.; Graham, I.M.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. European Heart Journal 2019, 1–78. [Google Scholar] [CrossRef] [PubMed]
- Izadi, A.; Aliasghari, F.; Gargari, B.P.; Ebrahimi, S. Strong Association between Serum Vitamin D and Vaspin Levels, AIP, VAI and Liver Enzymes in NAFLD Patients. Int. J. Vitam. Nutr. Res. 2020, 90, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, S.; Giri, N.; Pokhrel, R.; Pardhe, B.; Lamichhane, A.; Chaudhary, A.; Bhatt, M. Vitamin D Deficiency and Cardiovascular Risk in Type 2 Diabetes Population. OPEN LIFE Sci. 2021, 16, 464–474. [Google Scholar] [CrossRef]
- Faridi, K.F.; Zhao, D.; Martin, S.S.; Lupton, J.R.; Jones, S.R.; Guallar, E.; Ballantyne, C.M.; Lutsey, P.L.; Michos, E.D. Serum Vitamin D and Change in Lipid Levels over 5 y: The Atherosclerosis Risk in Communities Study. Nutrition 2017, 38, 85–93. [Google Scholar] [CrossRef]
- Lee, M.; Ebert, J.R.; Kadakia, M.P.; Zhang, J.; Czerwinski, S.A. Inverse Associations between Cardiometabolic Risk Factors and 25-Hydroxyvitamin D in Obese American Children and Adolescents. Am. J. Hum. Biol. 2016, 28, 736–742. [Google Scholar] [CrossRef]
- Censani, M.; Hammad, H.T.; Christos, P.J.; Schumaker, T. Vitamin D Deficiency Associated With Markers of Cardiovascular Disease in Children With Obesity. Glob. Pediatr. Health 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.R.; Jeong, S.J. Relationship between Vitamin D Level and Lipid Profile in Non-Obese Children. Metabolites 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Zhao, X.Y.; Hong, W.; Hou, D.Q.; Yu, Z.C.; Wang, L.G.; Wang, H.J.; Gao, A.Y.; Cheng, H.; Mi, J. A prospective cohort study on the associations between vitamin D nutritional status and cardiometabolic abnormities in children. Zhonghua Liu Xing Bing Xue Za Zhi Zhonghua Liuxingbingxue Zazhi 2020, 41, 2059–2065. [Google Scholar] [CrossRef] [PubMed]
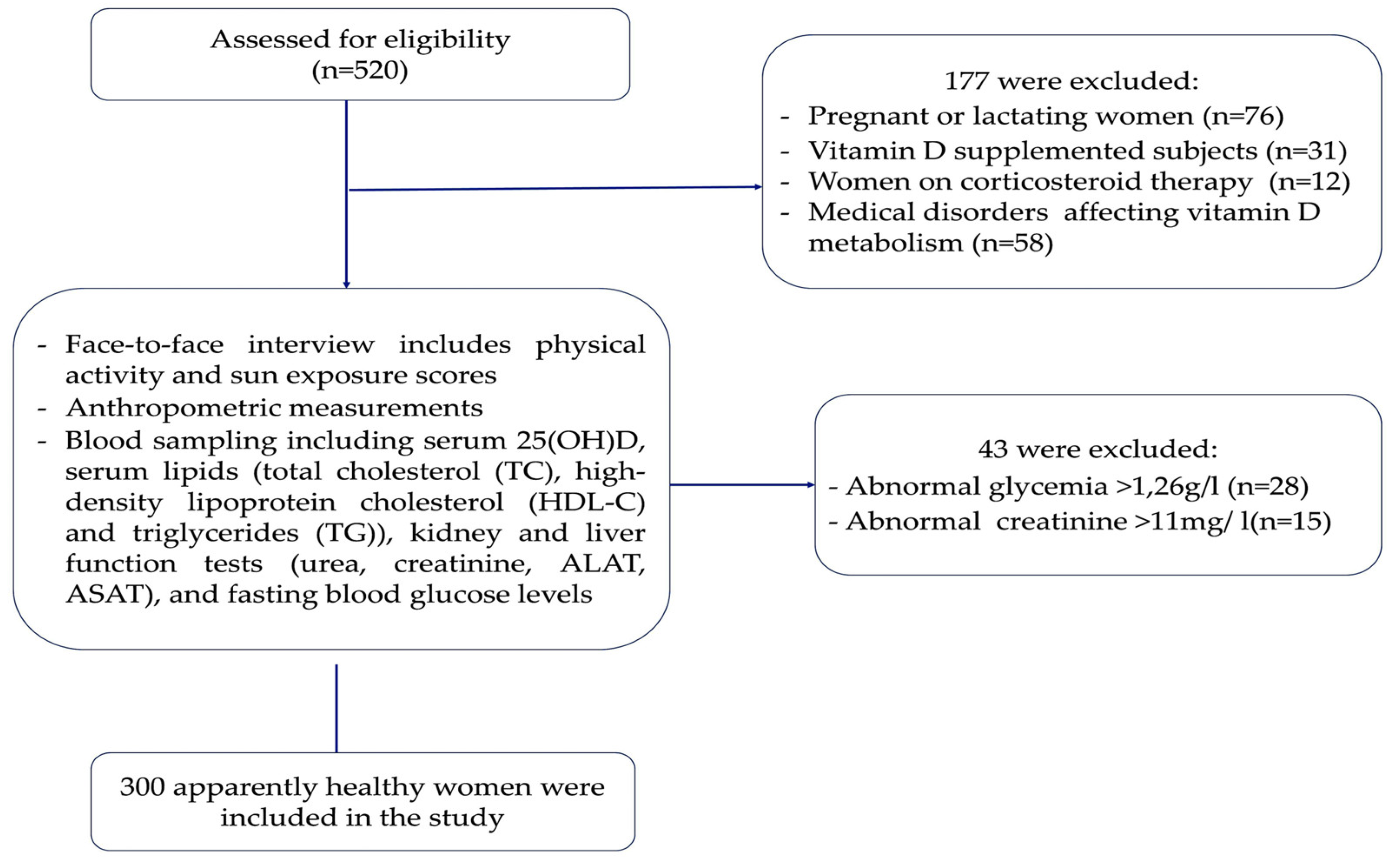
| Participants characteristics | Total = 300 |
25(OH)D <12 ng/ml N= 129 |
25(OH)D (12-20ng/ml) N= 73 |
25(OH)D > 20ng/ml N= 97 |
P-value |
|---|---|---|---|---|---|
| Clinical characteristics N (%) | |||||
|
Age (yrs) * |
38 ± 18 (18 – 50) |
33 ± 20 (20 – 50) |
38 ± 18 (18 – 50) |
41 ± 14 (19 – 50) |
0.006b |
| 18-28 | 96 (32) | 53 (40.8) | 23 (31.5) | 20 (20.6) | 0.020c |
| 29-39 | 89 (29.7) | 37 (28.5) | 22 (30.1) | 30 (30.9) | |
| 40-50 | 115 (38.3) | 40 (30.8) | 28 (38.4) | 47 (48.5) | |
| BMI ( (kg/m2) * | 25.74 ± 4.81 (14.53 – 38.10) |
26.45 ± 5.92 (17.47 – 38.10) |
26.35 ± 4.60 (14.53 – 36.65) |
24.84 ± 4.08 (14.53 – 32.39) |
0.008b |
| Normal weight | 122 (40.7) | 45 (34.6) | 25 (34.2) | 52 (53.6) | 0.001c |
| Overweight | 124 (14.3) | 51 (39.2) | 34 (46.6) | 39 (40.2) | |
| Obese | 54 (18) | 34 (26.2) | 14 (19.2) | 6 (6.2) | |
| Sun exposure score * | 15.25 ± 6.86 |
13.21 ± 5.75 (5.06 -23.06) |
14.75 ± 8.09 (7.5 – 31) |
18.35 ± 8.99 (8.5 – 34.50) |
<0.0001b |
| Physical activity score (MET-min/week)* | 2038.8 ± 1838.7 (519 – 4458) |
1778.4 ± 1721.1 (519.6 – 4458) |
1597.6 ± 1318.8 (519 – 4197.8) |
2896.6 ±1300.3 (799 – 4197) |
<0.0001b |
|
low physical activity score < 600MET-min/week |
19 (6.3) | 9 (6.9) | 10 (13.7) | 0 (0) | <0.0001c |
| Moderate physical activity score ≥600–2999 METs/min/week | 213 (71) | 97 (74.6) | 54 (74) | 62 (63.9) | |
|
High physical activity score ≥ 3000 MET-min/week |
68 (22.7) | 24 (18.5) | 09 (12.3) | 35 (36.1) | |
| Biochemical Characteristics N (%) | |||||
| 25(OH) D (ng/ml) * | 13.97 ± 15.17 (3 – 61.60) |
8.30 ± 3.43 (3 – 11.89) |
15.70 ± 4.04 (1.02 – 19.80) |
27.84 ± 10.52 (20.10 – 61.60) |
<0.0001b |
| TC (mg/dL) * | 188 ± 55 (109 – 352) |
195 ± 49 (109 – 352) |
190 ± 65 (109 – 349) |
177 ± 48 (113 – 287) |
0.153b |
| Elevated TC (mg/dL) | 91 (30.3) | 41 (31.5) | 26 (35.6) | 24 (24.7) | 0.288 c |
| HDL-C (mg/dl) * | 56 ± 23 (0.26 – 165) |
55 ± 24 (26 – 165) |
56 ± 21 32 – 128) |
60 ± 24 (32 – 154) |
0.153 b |
| Low HDL-C (mg/dl) | 56 (18.7) | 29 (22.3) | 15 (20.5) | 12 (12.4) | 0.147 c |
| LDL-C (mg/dl) * | 104 ± 044 (35 – 247) |
107 ± 36 (35 – 222) |
107 ± 48 (58 – 247) |
96 ± 44 (41 – 202) |
0.334 b |
| Elevated LDL-C (mg/dl) | 50 (16.7) | 21 (16.2) | 15 (20.5) | 14 (14.4) | 0.559 c |
| TG (mg/dl) * | 97 ± 75 (29 – 379) |
116 ± 86 (40 – 320) |
109 ± 87 (29 – 379) |
81 ± 42 (30 – 227) |
<0.0001b |
| Elevated TG (mg/dl) | 57 (19%) | 33 (25.4) | 19 (26) | 5 (5.2) | <0.0001c |
| Atherogenic indices characteristics N (%) | |||||
| AIP (N”) | 0.23 ± 0.25 (-0.38 – 0.82) |
0.30 ± 0.25 (-0.23 – 0.81) |
0.28 ± 0.25 (-0.35 – 0.82) |
0.11 ± 0.25 (-0.38 – 0.58) |
<0.0001a |
| Elevated AIP | 182 (60.7) | 96 (73.8) | 50 (68.5) | 36 (37.1) | <0.0001c |
| Non- HDL-C mg/dl * | 123 ± 50 (57–268) |
129 ± 46 (64 – 251) |
130 ± 50 (72 – 268) |
110 ± 50 (57 – 239) |
0.004b |
| Elevated Non-HDL-C | 129 (43) | 65 (50) | 36 (49.3) | 28 (28.9) | 0.003c |
| AC * | 2.19 ±1.17 (0.39 – 9.35) |
2.35 ± 1.29 (0.39 – 9.35) |
2.32 ± 1.32 (0.56 – 5.29) |
1.82 ± 0.91 (0.40 – 4.98) |
<0.0001b |
| Elevated AC | 67 (22.3) | 35 (26.9) | 23 (31.5) | 9 (9.3) | 0.001c |
| CRI-I * | 3.19 ±1.17 (1.39 – 10.35) |
3.35 ± 1.29 (1.39 – 10.35) |
3.30 ± 1.33 (1.56 – 6.29) |
2.82 ± 0.91 (1.40 – 5.98) |
<0.0001b |
| Elevated CRI-I | 177 (59) | 88 (67.7) | 48 (65.8) | 41 (42.3) | <0.0001c |
| CRI-II * | 1.79 ±0.98 (0.21–8.54) |
1.91 ± 0.99 (0.21 – 8.54) |
1.98 ± 1.05 (0.45 – 3.97) |
1.63 ± 0.81 (0.29 – 4.21) |
0.035b |
| Elevated CRI-II | 18 (6) | 10 (7.7) | 5 (6.8) | 3 (3.4) | 0.332 c |
| CHOLIndex (CI) * | 0.45 ± 0.44 (-1.30 – 1.96) |
0.52 ± 0.43 (-1.30 – 1.96) |
0.53 ± 0.47 (-0.70 – 1.80) |
0.38 ±0.41 (-1.10 – 1.54) |
0.011b |
| Elevated CI | 0% | - | - | - | |
| Age (yr) | BMI(kg/m2) | Sun exposure score | Physical activity score (MET-min/week) | ||
|---|---|---|---|---|---|
| TC (mg/dL) | Rho | 0.085 | 0.288 | -0.027 | -0.072 |
| P-value | 0.141 | <0.0001 | 0.639 | 0.215 | |
| HDL-C (mg/dL) | Rho | 0.054 | 0.071 | 0.204 | 0.226 |
| P-value | 0.350 | 0.220 | <0.0001 | <0.0001 | |
| LDL-C (mg/dL) | Rho | 0.106 | 0.196 | -0.034 | -0.060 |
| P-value | 0.068 | 0.001 | 0.559 | 0.299 | |
| TG (mg/dL) | Rho | -0.50 | 0.266 | -0.232 | -0.371 |
| P-value | 0.385 | <0.0001 | <0.0001 | <0.0001 | |
| AIP | Rho | -0.052 | 0.226 | -0.306 | -0.432 |
| P-value | 0.369 | <0.0001 | <0.0001 | <0.0001 | |
| AC | Rho | 0.032 | 0.141 | -0.206 | -0.264 |
| P-value | 0.579 | 0.015 | <0.0001 | <0.0001 | |
| Non-HDL-C (mg/dL) | Rho | 0.060 | 0253 | -0.091 | -0.147 |
| P-value | 0.303 | <0.0001 | 0.116 | 0.011 | |
| CRI-I | Rho | 0.032 | 0.141 | -0.206 | -0.264 |
| P-value | 0.579 | 0.015 | <0.0001 | <0.0001 | |
| CRI-II | Rho | 0.078 | 0.096 | -0.157 | -0.194 |
| P-value | 0.180 | 0.097 | 0.006 | 0.001 | |
| CHOLIndex | Rho | 0.091 | 0.136 | -0.106 | -0.133 |
| P-value | 0.117 | 0.019 | 0.067 | 0.021 | |
| Dependent variables | 25(OH)D ng/ml | |||
|---|---|---|---|---|
| B | B (95% CI) | Beta | P-value | |
| TC | -0.555 | -0.701 to -0.414 | -0.130 | 0.024 |
| HDL-C | 0.216 | 0.159 to 0.272 | 0.137 | 0.017 |
| LDL-C | -0.484 | -0.569 to -0.349 | -0.144 | 0.014 |
| TG | -1.528 | -1.688 to -1.362 | -0.314 | P < 0.0001 |
| AIP | -0.009 | -0.011 to -0.006 | -0.366 | P < 0.0001 |
| AC | -0.021 | -0.024 to -0.018 | -0.261 | P < 0.0001 |
| Non-HDL-C | -0.817 | -0.930 to-0.707 | -0.231 | P < 0.0001 |
| CRI-I | -0.021 | -0.024 to -0.018 | -0.261 | P < 0.0001 |
| CRI-II | -0.012 | -0.014 to-0.01 | -0.176 | 0.003 |
| CHOLIndex | -0.006 | -0.007 to -0.005 | -0.169 | 0.004 |
| Dependent variables a | 25(OH)D ng/ml | 25(OH) D status b | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | Wald | OR (95% CI) | P-value | B | Wald | OR (95% CI) | P-value | ||
| TG | Unadjusted | -0.071 | 13.490 | 0.931(0.897–0.967) | <0.0001 | 1.846 | 14.40 | 6.336(2.442–16.443) | <0.0001 |
| Model 1 | -0.056 | 8.086 | 0.946(0.910–0.983) | 0.004 | 1.590 | 10.28 | 4.904(1.856–12.959) | 0.001 | |
| Model 2 | -0.047 | 5.164 | 0.954(0.916–0.993) | 0.023 | 1.355 | 7.065 | 3.876(1.427–10.525) | 0.008 | |
| AIP | Unadjusted | -0.078 | 34.926 | 0.924(0.901–0.949) | <0.0001 | 1.468 | 31.427 | 4.340(2.598–7.251) | <0.0001 |
| Model 1 | -0.072 | 28.495 | 0.931(0.906–0.956) | <0.0001 | 1.291 | 23.111 | 3.637(2.149–6.158) | <0.0001 | |
| Model 2 | -0.062 | 20.811 | 0.940(0.915–0.965) | <0.0001 | 1.069 | 14.642 | 2.912(1.684–5.035) | <0.0001 | |
| AC | Unadjusted | -0.050 | 9.753 | 0.951(0.921-0.981) | 0.001 | 1.364 | 12.687 | 3.911(1.847–8.284) | <0.0001 |
| Model 1 | -0.047 | 7.885 | 0.954(0.924–0.986) | 0.005 | 1.278 | 10.765 | 3.589(1.673–7.700) | 0.001 | |
| Model 2 | -0.042 | 4.924 | 0.958(0.922–0.995) | 0.026 | 1.103 | 7.511 | 3.190(1.463–6.957) | 0.006 | |
| Non-HDL-C | Unadjusted | -0.041 | 11.325 | 0.959(0.937–0.983) | 0.001 | 0.892 | 11.382 | 2.440(1.453–4.097) | 0.001 |
| Model 1 | -0.32 | 6.381 | 0.969(0.945–0.993) | 0.012 | 0.729 | 7.154 | 2.074(1.215–3.540) | 0.007 | |
| Model 2 | -0.038 | 6.497 | 0.962(0935–0.991) | 0.011 | 0.716 | 6.318 | 2.047(1.171–3.578) | 0.012 | |
| CRI-I | Unadjusted | -0.039 | 12.10 | 0.961(0.939–0.982) | <0.0001 | 1.020 | 16.116 | 2.772(1.685–4.561) | <0.0001 |
| Model 1 | -0.034 | 8.264 | 0.967(0.944–0.989) | 0.004 | 0.905 | 11.998 | 2.481(1.481–4.123) | 0.001 | |
| Model 2 | -0.029 | 5.727 | 0.971(0.948–0.994) | 0.017 | 0.823 | 9.098 | 2.278(1.334–3.888) | 0.003 | |
| Variable | AUC (95% CI) | P-value | Youden Index | Cutoff (ng/ml) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| TG> 150 mg/dL | 0.657 (0.586 – 0.727) | <0.0001 | 0.294 | ≤ 15.15 | 77.19 | 52.26 |
| AIP > 0.15 | 0.702 (0.641– 0.764) | <0.0001 | 0.346 | ≤ 17.5 | 73.63 | 61.02 |
| AC > 3.0 | 0.615 (0.545 – 0.685) | 0.004 | 0.243 | ≤ 19.8 | 86.57 | 37.77 |
| Non-HDL-C > 130 mg/dL | 0.609 (0.545 – 0.672) | 0.001 | 0.188 | ≤ 20.1 | 79.07 | 39.77 |
| CRI-I >3.0 | 0.626 (0.562 – 0.690) | <0.0001 | 0.228 | ≤ 19.5 | 75.71 | 47.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





