Submitted:
09 June 2024
Posted:
11 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Vaccines
2.2. Nasal Devices for Vaccine Delivery
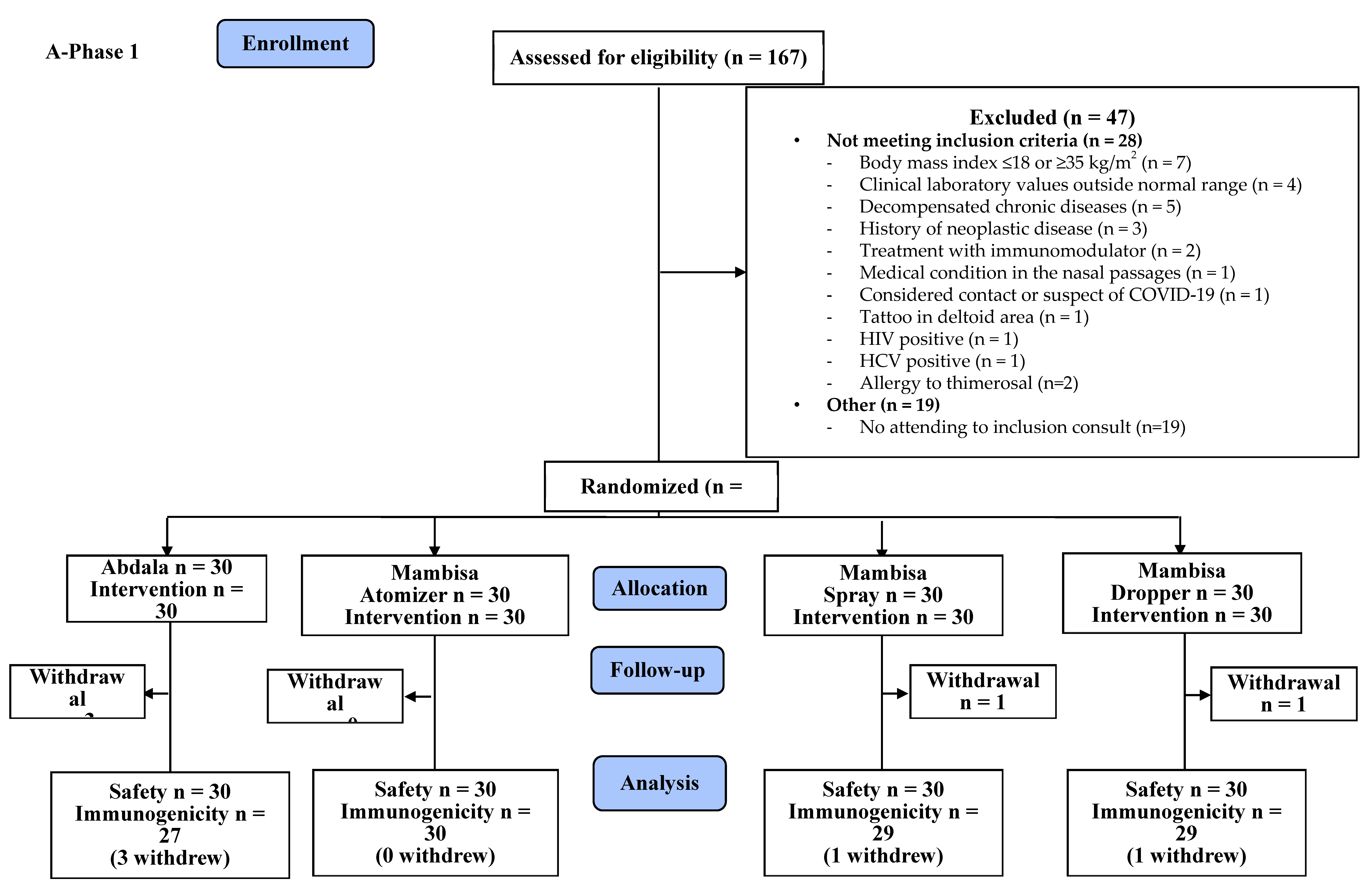
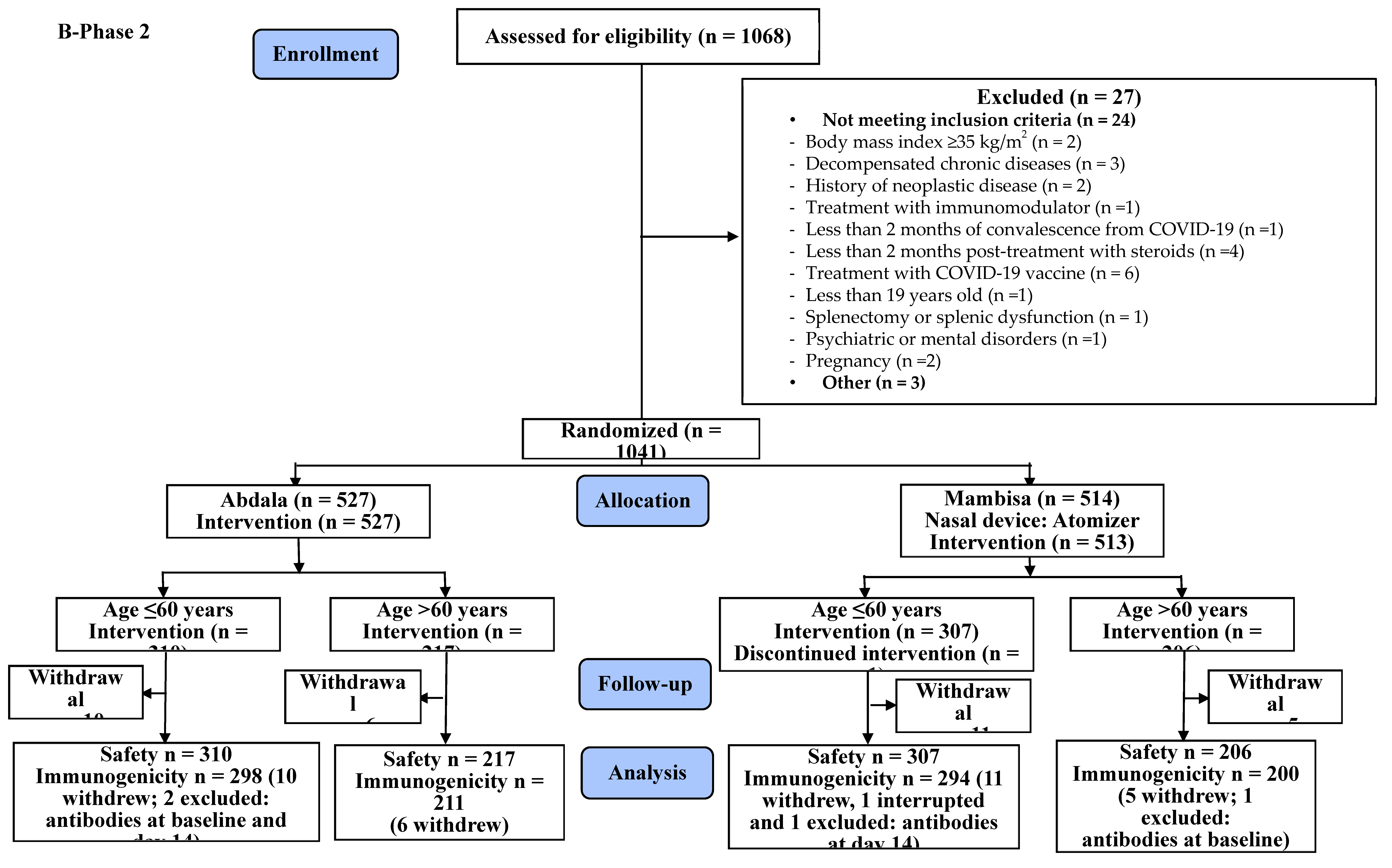
2.3. Trial Design
2.4. Participants
2.5. Variables
2.6. Sample Size
2.7. Randomization
2.8. Intervention
2.9. Outcomes
2.10. Procedures
2.11. Data Management
2.12. Statistical Methods
3. Results
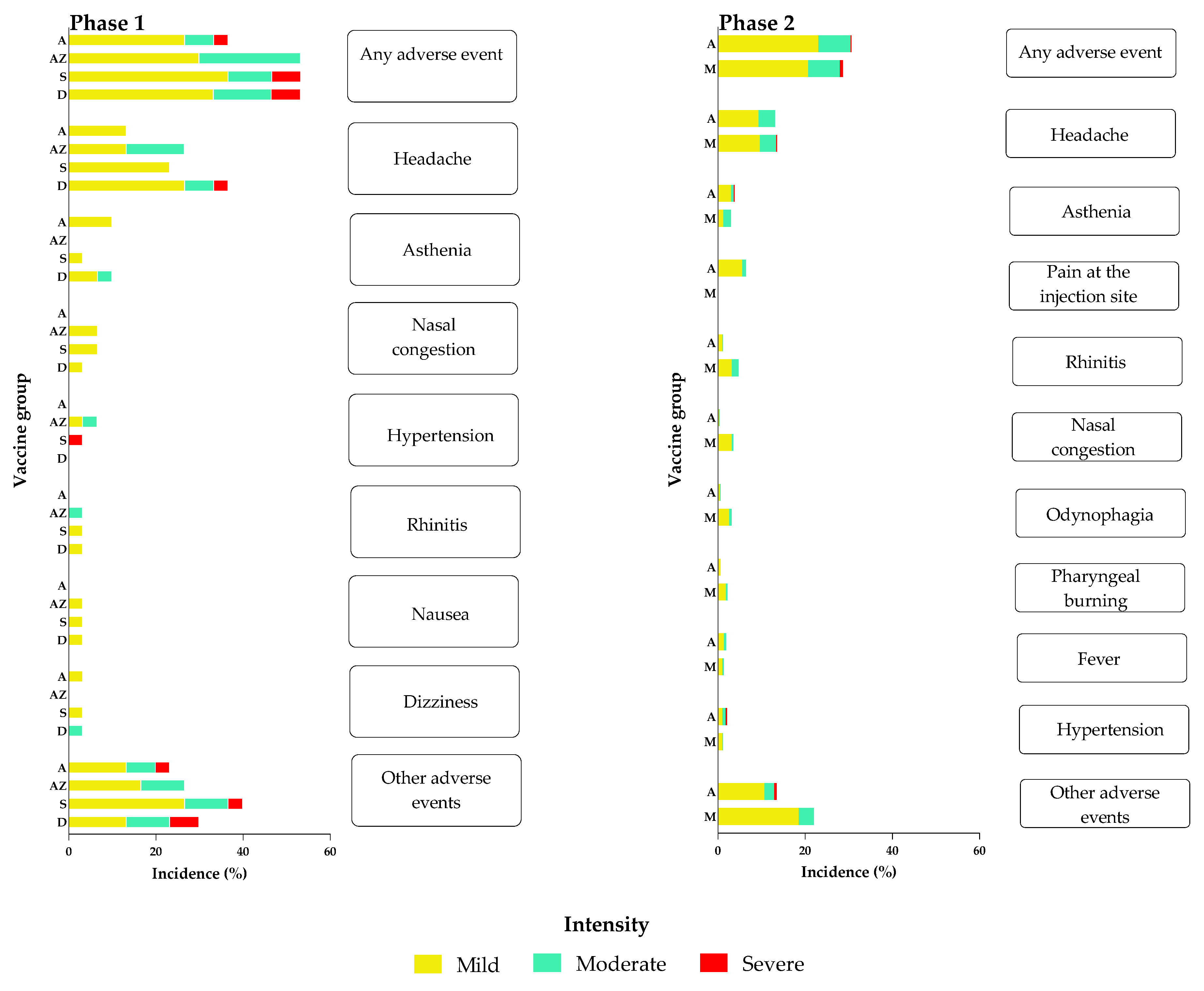
3.1. Safety
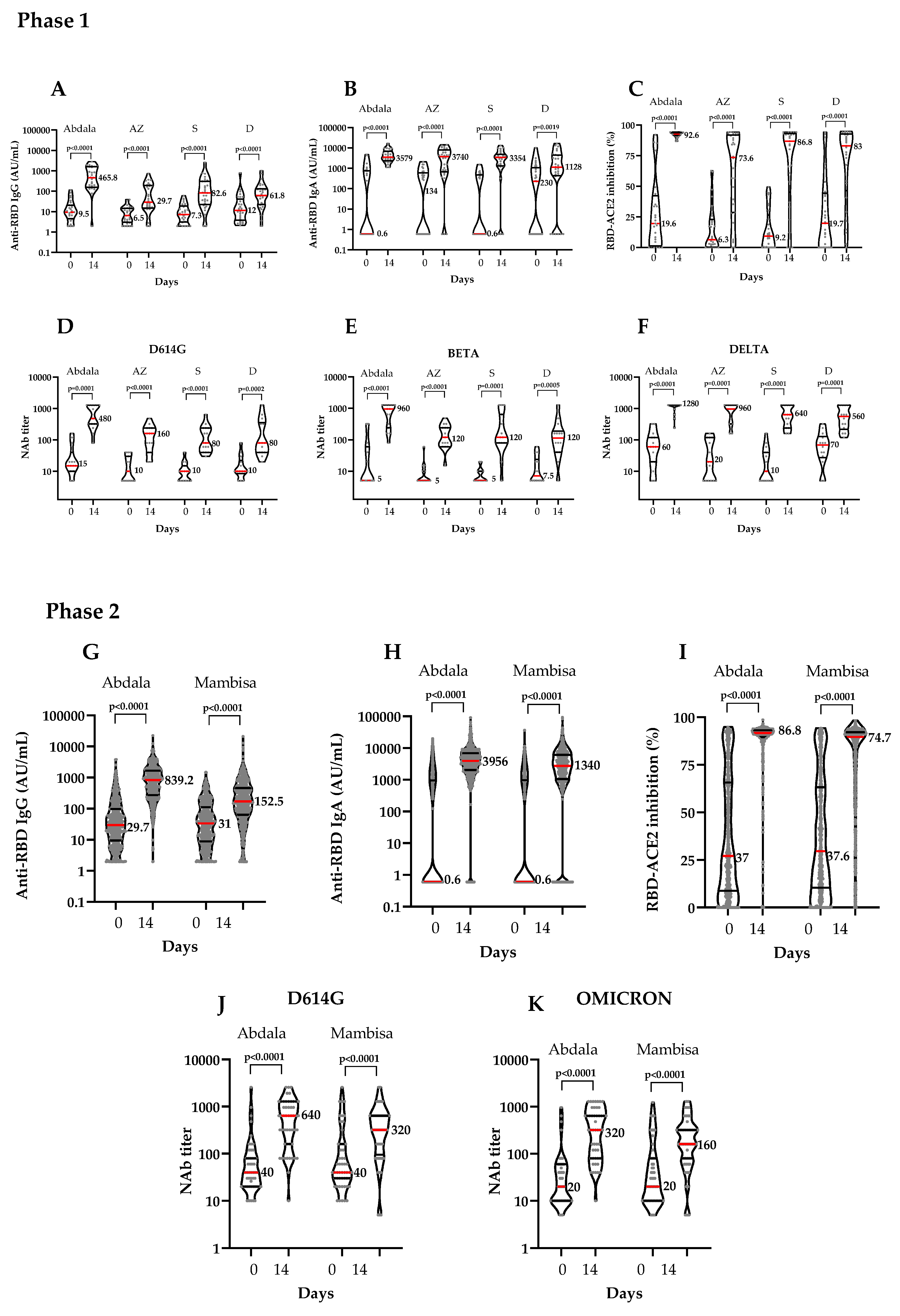
3.2. Immunogenicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. (accessed on 11 March 2020). Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- Rossi, M.A.; Cena, T.; Binala, J.; Alessi, D.; Scotti, L.; Faggiano, F. Evaluation of the risk of SARS-CoV-2 infection and hospitalization in vaccinated and previously infected subjects based on real world data. Sci. Rep. 2023, 13, 1–8. [Google Scholar] [CrossRef]
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ. Res. 2022, 209, 112911. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Hasan, M.R.; Ayoub, H.H.; Qassim, S.; AlMukdad, S.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Benslimane, F.M.; et al. Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. New Engl. J. Med. 2022, 386, 1288–1290. [Google Scholar] [CrossRef]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis 2023, 23, 556–7. [Google Scholar] [CrossRef]
- Gorry, C. Vaccines and Public Trust: Containing COVID-19 in Cuba Verena Muzio-González PhD DSc Director of Clinical Research, Genetic Engineering and Biotechnology Center. MEDICC Rev. 2022, 24, 9–13. [Google Scholar] [CrossRef]
- Limonta-Fernández, M.; Chinea-Santiago, G.; Martín-Dunn, A.M.; Gonzalez-Roche, D.; Bequet-Romero, M.; Marquez-Perera, G.; González-Moya, I.; Canaan-Haden-Ayala, C.; Cabrales-Rico, A.; Espinosa-Rodríguez, L.A.; et al. An engineered SARS-CoV-2 receptor-binding domain produced in Pichia pastoris as a candidate vaccine antigen. New Biotechnol. 2022, 72, 11–21. [Google Scholar] [CrossRef]
- Hernández-Bernal, F.; Ricardo-Cobas, M.C.; Martín-Bauta, Y.; Navarro-Rodríguez, Z.; Piñera-Martínez, M.; Quintana-Guerra, J.; Urrutia-Pérez, K.; Chávez-Chong, C.O.; Azor-Hernández, J.L.; Rodríguez-Reinoso, J.L.; et al. Safety, tolerability, and immunogenicity of a SARS-CoV-2 recombinant spike RBD protein vaccine: A randomised, double-blind, placebo-controlled, phase 1-2 clinical trial (ABDALA Study). EClinicalMedicine 2022, 46, 101383. [Google Scholar] [CrossRef]
- Registro Público Cubano de Ensayos Clínicos (CU). Ensayo clínico fase I/II adaptativo; aleatorizado; de grupos paralelos; para evaluar la seguridad e inmunogenicidad en adultos de dos candidatos vacunales; basados en subunidades de RBD recombinante para la prevención de COVID-19 en esquemas que emplean la vía de administración nasal. Estudio MAMBISA. https://rpcec.sld.cu/ensayos/RPCEC00000345-Sp (Spanish) [accessed 2 January 2024].
- Registro Público Cubano de Ensayos Clínicos (CU). Evaluación del efecto y la seguridad de una dosis de refuerzo de Mambisa o Abdala contra la COVID-19 (Estudio BACONAO). https://rpcec.sld.cu/ensayos/RPCEC00000398-Sp (Spanish) [accessed 2 January 2024].
- Centro para el Control Estatal de Medicamentos; Equipos y Dispositivos Médicos (CECMED) (CU). ABDALA 50 μg (Vacuna anti COVID-19 de subunidad proteica). Resumen de las Características del Producto. 2021. Havana: CECMED; 2021. https://www.cecmed.cu/registro/rcp/biologicos/abdala-50-mg-vacuna-anti-covid-19-subunidad-proteica. (Spanish) [Accessed 19 July 2023].
- Hernández-Bernal, F.; Ricardo-Cobas, M.C.; Martín-Bauta, Y.; Rodríguez-Martínez, E.; Urrutia-Pérez, K.; Quintana-Guerra, J.; Navarro-Rodríguez, Z.; Piñera-Martínez, M.; Rodríguez-Reinoso, J.L.; Chávez-Chong, C.O.; et al. A phase 3, randomised, double-blind, placebo-controlled clinical trial evaluation of the efficacy and safety of a SARS-CoV-2 recombinant spike RBD protein vaccine in adults (ABDALA-3 study). Lancet Reg. Heal. - Am. 2023, 21, 100497. [Google Scholar] [CrossRef]
- Más-Bermejo, P.I.; Dickinson-Meneses, F.O.; Almenares-Rodríguez, K.; Sánchez-Valdés, L.; Guinovart-Díaz, R.; Vidal-Ledo, M.; Galbán-García, E.; Olivera-Nodarse, Y.; Morgado-Vega, I.; Dueñas-Carrera, S.; et al. Cuban Abdala vaccine: Effectiveness in preventing severe disease and death from COVID-19 in Havana, Cuba; A cohort study. Lancet Reg. Heal. - Am. 2022, 16, 100366. [Google Scholar] [CrossRef]
- Cinza-Estévez, Z.; Resik-Aguirre, S.; Figueroa-Baile, N.L.; Oquendo-Martínez, R.; Campa-Legrá, I.; Tejeda-Fuentes, A.; Rivero-Caballero, M.; González-García, G.; Chávez-Chong, C.O.; Alonso-Valdés, M.; et al. Immunogenicity and safety assessment of a SARS-CoV-2 recombinant spike RBD protein vaccine (Abdala) in paediatric ages 3–18 years old: a double-blinded, multicentre, randomised, phase 1/2 clinical trial (ISMAELILLO study). EClinicalMedicine 2023, 63, 102160. [Google Scholar] [CrossRef]
- Aguilar-Estrada, J.A.; Anaya-Herazo, C.A.; Trujillo-Ricaño, M.; Navarro-Marín, E.; Sosa-Leyva, M. COVID-19 y uso de vacunación de emergencia en el embarazo. Rev Cubana Obstetr Ginecol 2022; 48(2):e1131. https://revginecobstetricia.sld.cu/index.php/gin/article/view/1131 (Spanish) [accessed 2 January 2024].
- Centro para el Control Estatal de Medicamentos; Equipos y Dispositivos Médicos (CECMED) (CU). Mambisa (CIGB 669 y CIGB 66). Aprobación. Ensayo clínico fase I/II adaptativo; aleatorizado; de grupos paralelos; para evaluar la seguridad e inmunogenicidad en adultos de dos candidatos vacunales; basados en subunidades de RBD recombinante para la prevención de COVID-19 en esquemas que emplean la vía de administración nasal. (COVID-19). 2020. https://www.cecmed.cu/covid-19/aprobaciones/mambisa-cigb-669-cigb-66. (Spanish) [Accessed 2 January 2024].
- Lemos-Perez, G.; Chavez-Valdes, S.; Gonzalez-Formental, H.; Freyre-Corrales, G.; Vazquez-Arteaga, A.; Alvarez-Acevedo, B.; Avila-Díaz, L.; Martínez-Rosales, R.U.; Chacon-Quintero, Y.; Coizeau-Rodriguez, E.; et al. Elevated Antibody Titers in Abdala Vaccinees Evaluated by Elecsys® Anti-SARS-Cov-2 S Highly Correlate with UMELISA SARS-Cov-2 ANTI RBD, ACE-2 Binding Inhibition and Viral Neutralization Assays. J. Biotechnol. Biomed. 2022, 05, 151–157. [Google Scholar] [CrossRef]
- Johnson, V.A.; Byington, R.E.; Nara, P.L. Quantitative Assays for Virus Infectivity. In: Aldovini A; Walker BD (eds). Techniques in HIV Research. Chapter 5. London: Palgrave Macmillan; 1990. p. 87‒119. [CrossRef]
- Anand, P.; Stahel, V.P. The safety of Covid-19 mRNA vaccines: a review. Patient Saf. Surg. 2021, 15. [Google Scholar] [CrossRef]
- Mannar, D.; Saville, J.W.; Zhu, X.; Srivastava, S.S.; Berezuk, A.M.; Tuttle, K.S.; Marquez, A.C.; Sekirov, I.; Subramaniam, S. SARS-CoV-2 Omicron variant: Antibody evasion and cryo-EM structure of spike protein–ACE2 complex. Science 2022, 375, 760–764. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef]
- Bertrand, D.; Hamzaoui, M.; Lemée, V.; Lamulle, J.; Laurent, C.; Etienne, I.; Lemoine, M.; Lebourg, L.; Hanoy, M.; Le Roy, F.; et al. Antibody and T-cell response to a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Kidney Int. 2021, 100, 1337–1340. [Google Scholar] [CrossRef]
- Zambrana, J.V.; Saenz, C.; Maier, H.E.; Brenes, M.; Nuñez, A.; Matamoros, A.; Hernández, M.; Dumas, K.; Toledo, C.; Peralta, L.; et al. Comparative Analysis of SARS-CoV-2 Antibody Responses across Global and Lesser-Studied Vaccines. Vaccines 2024, 12, 326. [Google Scholar] [CrossRef]
- Munson, S.; Parker, J.; King, T.H.; Lu, Y.; Kelley, V.; Guo, Z. et al. Coupling innate and adaptive immunity with yeast-based cancer immunotherapy. In: Orentas R; Hodge JW; Johnson BD (eds). Cancer Vaccines and Tumor Immunity. Hoboken; NJ: John Wiley & Sons; 2008. p. 131–49. [CrossRef]
- Butler, S.E.; Crowley, A.R.; Natarajan, H.; Xu, S.; Weiner, J.A.; Bobak, C.A.; Mattox, D.E.; Lee, J.; Wieland-Alter, W.; Connor, R.I.; et al. Distinct Features and Functions of Systemic and Mucosal Humoral Immunity Among SARS-CoV-2 Convalescent Individuals. Front. Immunol. 2021, 11, 618685. [Google Scholar] [CrossRef]
- Alu, A.; Chen, L.; Lei, H.; Wei, Y.; Tian, X.; Wei, X. Intranasal COVID-19 vaccines: From bench to bed. EBioMedicine 2022, 76, 103841. [Google Scholar] [CrossRef]
- Miquel-Clopés, A.; Bentley, E.G.; Stewart, J.P.; Carding, S.R. Mucosal vaccines and technology. Clin. Exp. Immunol. 2019, 196, 205–214. [Google Scholar] [CrossRef]
- Harris, A. Review: Clinical Opportunities Provided by the Nasal Administration of Peptides. J. Drug Target. 1993, 1, 101–116. [Google Scholar] [CrossRef]
- Aguilar, J.C.; Aguiar, J.A.; Akbar, S.M.F. Action Mechanisms and Scientific Rationale of Using Nasal Vaccine (HeberNasvac) for the Treatment of Chronic Hepatitis B. Vaccines 2022, 10, 2087. [Google Scholar] [CrossRef]
- Madhavan, M.; Ritchie, A.J.; Aboagye, J.; Jenkin, D.; Provstgaad-Morys, S.; Tarbet, I.; Woods, D.; Davies, S.; Baker, M.; Platt, A.; et al. Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: An open-label partially-randomised ascending dose phase I trial. EBioMedicine 2022, 85, 104298. [Google Scholar] [CrossRef]
- Singh, C.; Verma, S.; Reddy, P.; Diamond, M.S.; Curiel, D.T.; Patel, C.; Jain, M.K.; Redkar, S.V.; Bhate, A.S.; Gundappa, V.; et al. Phase III Pivotal comparative clinical trial of intranasal (iNCOVACC) and intramuscular COVID 19 vaccine (Covaxin®). npj Vaccines 2023, 8, 125. [Google Scholar] [CrossRef]
- Evans, J.P.; Zeng, C.; Carlin, C.; Lozanski, G.; Saif, L.J.; Oltz, E.M.; Gumina, R.J.; Liu, S.-L. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci. Transl. Med. 2022, 14, eabn8057. [Google Scholar] [CrossRef]
- Guzmán, M.G.; Pérez, L.; Tejero, Y.; Mederos, D.; Aguado, M.E.; Pintos, Y. et al. Emergence and evolution of SARS-CoV-2 genetic variants during the Cuban epidemic. J Clin Virol Plus 2022; 2:100104. [CrossRef]
- Cromer, D.; Steain, M.; Reynaldi, A.; E Schlub, T.; Wheatley, A.K.; A Juno, J.; Kent, S.J.; A Triccas, J.; Khoury, D.S.; Davenport, M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe 2022, 3, e52–e61. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Efficacy of COVID-19 vaccine booster doses in older people. Eur. Geriatr. Med. 2022, 13, 275–278. [Google Scholar] [CrossRef]
- Henry, C.; Zheng, N.-Y.; Huang, M.; Cabanov, A.; Rojas, K.T.; Kaur, K.; Andrews, S.F.; Palm, A.-K.E.; Chen, Y.-Q.; Li, Y.; et al. Influenza Virus Vaccination Elicits Poorly Adapted B Cell Responses in Elderly Individuals. Cell Host Microbe 2019, 25, 357–366. [Google Scholar] [CrossRef]
- Renia, L.; Goh, Y.S.; Rouers, A.; Le Bert, N.; Ni Chia, W.; Chavatte, J.-M.; Fong, S.; Chang, Z.W.; Zhuo, N.Z.; Tay, M.Z.; et al. Lower vaccine-acquired immunity in the elderly population following two-dose BNT162b2 vaccination is alleviated by a third vaccine dose. Nat. Commun. 2022, 13, 4615. [Google Scholar] [CrossRef]
- Jeffery-Smith, A.; Burton, A.R.; Lens, S.; Rees-Spear, C.; Davies, J.; Patel, M.; Gopal, R.; Muir, L.; Aiano, F.; Doores, K.J.; et al. SARS-CoV-2–specific memory B cells can persist in the elderly who have lost detectable neutralizing antibodies. J. Clin. Investig. 2022, 132, e152042. [Google Scholar] [CrossRef]
- 39. World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
| Phase 1 | ||||||||
| Group | ||||||||
| Variable | Abdala | Mambisa (three nasal application devices) | ||||||
| Atomizer | Spray | Dropper | Subtotal | Total | ||||
| N | 30 | 30 | 30 | 30 | 90 (100) | 120 (100) | ||
| Sex – n (%) | Female | 18 (60.0) | 14 (46.7) | 15 (50.0) | 23 (76.7) | 52 (57.8) | 70 (58.3) | |
| Male | 12 (40.0) | 16 (53.3) | 15 (50.0) | 7 (23.3) | 38 (42.2) | 50 (41.7) | ||
| Age | Years | 47.9 ± 15.1 | 50.9 ± 13.1 | 49.5 ± 17.1 | 48.6 ± 15.7 | 49.1 ± 14.5 | 49.2 ± 15.2 | |
| Skin color – n (%) | White | 19 (63.3) | 18 (60.0) | 17 (56.7) | 20 (66.7) | 55 (61.1) | 74 (61.7) | |
| Mulatto | 3 (10.0) | 8 (26.7) | 4 (13.3) | 4 (13.3) | 16 (17.8) | 19 (15.8) | ||
| Black | 8 (26.67) | 4 (13.3) | 9 (30.0) | 6 (20.0) | 19 (21.1) | 27 (22.5) | ||
| BMI ± SD | kg/m2 | 26.5 ± 4.0 | 27.4 ± 3.7 | 26.6 ± 3.0 | 27.2 ± 4.0 | 26.7 ± 3.6 | 26.9 ± 3.7 | |
| Age group – n (%) | ≤ 60 years | 24 (80.0) | 25 (83.3) | 23 (76.7) | 23 (76.7) | 71 (78.9) | 95 (79.2) | |
| > 60 years | 6 (20.0) | 5 (16.7) | 7 (23.3) | 7 (23.3) | 19 (21.1) | 25 (20.8) | ||
| p-value | 0.9065 | |||||||
| COVID-19 symptoms – n (%) | Asymptomatic | 9 (30.0) | 8 (26.7) | 10 (33.3) | 7 (23.3) | 25 (27.8) | 34 (28.3) | |
| Symptomatic | 21 (70.0) | 22 (73.3) | 20 (13.3) | 23 (76.7) | 65 (72.2) | 86 (71.7) | ||
| p-value | 0.8445 | |||||||
| COVID-19 disease severity – n (%) | Severe | 2 (9.5) * | 1 (4.5) * | 2 (10.0) * | 5 (21.7) * | 8 (8.9) * | 10 (11.6) * | |
| Mild | 12 (57.4) * | 19 (86.4) * | 11 (55.0) * | 12 (52.2) * | 42 (46.7) * | 54 (62.8) * | ||
| Moderate | 7 (33.3) * | 2 (9.1) * | 7 (35.0) * | 6 (26.1) * | 25 (27.8) * | 22 (25.6) * | ||
| p-value | 0.1582 | |||||||
| Phase 2 | ||||||||
| Group | ||||||||
| Variable | Abdala | Mambisa | Total | |||||
| N | 527 | 514 | 1041 (100) | |||||
| Sex – n (%) | Female | 370 (70.2) | 364 (70.8) | 734 (70.5) | ||||
| Male | 157 (29.8) | 150 (29.2) | 307 (29.5) | |||||
| Age | Years | 55.3 ± 13.7 | 55.0 ± 14.1 | 55.1 ± 13.9 | ||||
| Skin color – n (%) | White | 397 (75.3) | 384 (74.7) | 781 (75.0) | ||||
| Mulatto | 49 (10.0) | 56 (26.7) | 105 (10.1) | |||||
| Black | 81 (26.67) | 74 (13.3) | 155 (14.9) | |||||
| BMI ± SD | kg/m2 | 26.9 ± 4.2 | 26.6 ± 4.1 | 26.7 ± 4.1 | ||||
| Age group – n (%) | ≤ 60 years | 310 (58.8) | 308 (59.9) | 618 (59.4) | ||||
| > 60 years | 217 (41.2) | 206 (40.1) | 423 (40.6) | |||||
| p-value | 0.3830 | |||||||
| COVID-19 symptoms – n (%) | Asymptomatic | 27 (5.1) | 35 (6.8) | 62 (6.0) | ||||
| Symptomatic | 500 (94.9) | 479 (93.2) | 979 (94.0) | |||||
| p-value | 0.1543 | |||||||
| COVID-19 disease severity – n (%) | Severe | 38 (7.6) * | 50 (10.4) * | 88 (9.0) * | ||||
| Mild | 305 (61.0) * | 276 (57.6) * | 581 (59.3) * | |||||
| Moderate | 157 (31.4) * | 153 (31.9) * | 310 (31.7) * | |||||
| p-value | 0.2608 | |||||||
| Phase 1 | |||||||||
| Group | |||||||||
| Variable | Abdala | Mambisa (three nasal application devices) | |||||||
| Atomizer | Spray | Dropper | |||||||
| Age | ≤ 60 years | > 60 years | ≤ 60 years | > 60 years | ≤ 60 years | > 60 years | ≤ 60 years | > 60 years | |
| N | 22 | 5 | 25 | 5 | 22 | 7 | 22 | 7 | |
| Asymptomatic - n (%) | 8 (36.4) | 0 (0.0) | 8 (32.0) | 0 (0.0) | 7 (31.8) | 3 (42.8) | 7 (31.8) | 0 (0.0) | |
| Symptomatic - n (%) | 14 (63.6) | 5 (100.0) | 17 (68.0) | 5 (100.0) | 15 (57.1) | 4 (57.1) | 15 (68.2) | 7 (100.0) | |
| p-value | 0.2798 | 0.2868 | 0.6647 | 0.1470 | |||||
| COVID-19 disease severity - n (%) | Severe | 1 (7.1) * | 1 (20.0) * | 1 (5.9) * | 0 (0.0) * | 1 (6.7) * | 1 (25.0) * | 2 (13.3) * | 3 (42.8) * |
| Mild | 10 (71.4) * | 1 (20.0) * | 15 (88.2) * | 4 (80.0) * | 10 (66.7) * | 1 (25.0) * | 9 (60.0) * | 2 (28.6) * | |
| Moderate | 3 (21.4) * | 3 (60.0) * | 1 (5.9) * | 1 (20.0) * | 4 (26.7) * | 2 (50.0) * | 4 (26.7) * | 2 (28.6) * | |
| p-value | 0.4213 | >0.9999 | 0.3860 | 0.2743 | |||||
| Phase 2 | |||||||||
| Variable | Group | ||||||||
| Abdala | Mambisa | ||||||||
| Age | ≤ 60 years | > 60 years | ≤ 60 years | > 60 years | |||||
| N | 298 | 211 | 294 | 200 | |||||
| Asymptomatic - n (%) | 19 (6.4) | 7 (3.3) | 21 (7.1) | 12 (6.0) | |||||
| Symptomatic - n (%) | 279 (93.6) | 204 (96.6) | 273 (92.8) | 188 (94.0) | |||||
| p-value | 0.1534 | 0.7149 | |||||||
| COVID-19 disease severity - n (%) | Severe | 22 (7.9) * | 15 (7.3) * | 25 (9.1) * | 21 (11.2) * | ||||
| Mild | 171 (61.3) * | 123 (60.3) * | 170 (62.3) * | 97 (51.6) * | |||||
| Moderate | 86 (30.8) * | 66 (32.3) * | 78 (28.6) * | 70 (37.2) * | |||||
| p-value | 0.9276 | 0.0726 | |||||||
| Phase 1 | |||||||
| Group | |||||||
| Variable | Abdala | Mambisa (three nasal delivery devices) | TOTAL | ||||
| Atomizer (AZ) | Spray (S) | Dropper (D) | |||||
| ITT population | |||||||
| Responders-n/N (%; 95% CI) | 25/30 (83.3; 65.2‒94.3) |
25/30 (83.3; 65.2-94.3) |
25/30 (83.3; 65.2-94.3) |
22/30 (73.3; 54.1-87.7) |
97/120 (80.8; 72.6-87.4) |
||
| p-value | 0.0009 | 0.0009 | 0.0009 | 0.0218 | <0.0001 | ||
| PP population | |||||||
| Responders-n/N (%; 95% CI) | 25/27 (92.6; 75.7-99.1) |
25/30 (83.3; 65.2-94.3) |
25/29 (86.2; 68.3-96.1) |
22/29 (80.0; 61.0-92.4) |
97/115 (84.3; 76.4-90.4) |
||
| p-value | 0.0000 | 0.0009 | 0.0007 | 0.0119 | <0.0001 | ||
| Phase 2 | Group | ||||||
| Variable | Abdala | Mambisa | TOTAL | ||||
| ITT population | |||||||
| Responders-n/N (%; 95% CI) | 451/527 (85.6; 82.3- 88.5) |
366/514 (71.2; 67.1-75.1) |
817/1041 (78.5; 75.7-81.0) |
||||
| p-value | <0.0001 | 0.2753 | <0.0001 | ||||
| PP population | |||||||
| Responders-n/N (%; 95% CI) | 451/509 (88.6; 85.5-91.2) |
366/494 (74.1; 70.0-77.9) |
817/1003 (81.4; 78.9-83.8) |
||||
| p-value | <0.0001 | 0.0237 | <0.0001 | ||||
| Age | ≤60 years | ||||||
| Responders-n/N (%; 95% CI) | 263/298 (88.2; 84.0-91.6) |
222/294 (75.5; 70.2-80.3) |
485/592 (81.9; 78.6-84.9) |
||||
| p-value | <0.0001 | 0.0196 | <0.0001 | ||||
| Age | >60 years | ||||||
| Responders-n/N (%; 95% CI) | 188/211 (89.1; 84.1-93.0) |
144/200 (72.0; 65.2-78.1) |
332/411 (80.8; 76.6-84.5) |
||||
| p-value | <0.0001 | 0.2685 | <0.0001 | ||||
| Phase 1 | |||||||||||||||||||||||||||
| Group | |||||||||||||||||||||||||||
| Variable | Abdala | Mambisa (three nasal application devices) | |||||||||||||||||||||||||
| Atomizer | Spray | Dropper | |||||||||||||||||||||||||
| Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | ||||||||||||||||||||
| Anti-RBD IgG | |||||||||||||||||||||||||||
| GMT (95% CI) | 11.0 (7.0-17.2) | 399 (219.6-724.9) | 6.79 (4.8-9.5) | 41.4 (22.5-76.2) | 7.9 (5.3-11.7) | 72.6 (37.7-139.9) | 13.6 (7.9-23.5) | 59.84 (34.5-103.8) | |||||||||||||||||||
| Seroconversion rate-n/N (%; 95% CI) | 25/27 (92.6; 75.7-99.1) |
21/30 (70; 50.6-85.3) |
20/29 (68.9; 49.1-84.7) |
15/29 (51.7; 32.5-70.5) |
|||||||||||||||||||||||
| p-value | 0.2646 | ||||||||||||||||||||||||||
| MD(95% CI) | 463.8 (173.2-1473) | 22.0 (11.4-122.3) | 57.5 (28.6-186.3) | 45.5 (12.7-89.89) | |||||||||||||||||||||||
| Anti-RBD IgA | |||||||||||||||||||||||||||
| GMT (95% CI) | 12.3 (2.8-54.3) | 3937 (2997-5172) | 19.8 (5.2-75.5) | 1244 (433.1-3571) | 7.9 (2.2-28.9) | 1262 (439.8- 3620) | 26.2 (6.2-110.9) | 389.2 (101.9-1486) | |||||||||||||||||||
| Seroconversion rate-n/N (%; 95% CI) | 23/27 (85.2; 66.3-95.8) |
22/30 (73.3; 54.1-87.7) |
24/29 (82.7; 64.2-94.1) |
14/29 (48.3;29.5-67.9) |
|||||||||||||||||||||||
| p-value | 0.0142 | ||||||||||||||||||||||||||
| MD (95% CI) | 3333 (2357-6104) | 2635 (813.9-5947) | 2825 (1345-4245) | 650.4 (0.0-2929) | |||||||||||||||||||||||
| Inhibition of the RBD-ACE2 binding | |||||||||||||||||||||||||||
| Mean (95% CI) | 27.5 (16.0-38.9) | 92.16 (91.3-93) | 15.1 (8.2- 22) | 60.36 (47.5-73.2) | 14.5 (8.3-20.7) | 68.5 (55.8-81.2) | 27.7 (16.1-39.2) | 67.94(55.8-80) | |||||||||||||||||||
| Seroconversion rate-N/n (%, 95% CI) | 23/27 (85.2; 66.3-95.8) |
23/30 (84.1; 66.2-94.8) |
24/29 (82.7; 64.2-94.1) |
18/29 (62.1; 42.3-79.3) |
|||||||||||||||||||||||
| p-value | 0.1826 | ||||||||||||||||||||||||||
| MD (95% CI) | 70.3 (59.0-84.9) | 53.0 (30.7-65.6) | 57.7 (40.1-75.5) | 40.8 (4.1-73.9) | |||||||||||||||||||||||
| Neutralizing antibodies to live SARS-CoV-2 variants | |||||||||||||||||||||||||||
| VOC | D614G | ||||||||||||||||||||||||||
| GMT (95% CI) | 20.9 (11.8- 37.1) | 526 (317.2-872.3) | 11.3 (6.9-18.4) | 107.2 (62.0-185.5) | 9367 (6.5-13.4) | 106 (63.1-178.1) | 12.9 (8.1-20.7) | 111.8 (52.8-236.5) | |||||||||||||||||||
| Seroconversion rate-n/N (%; 95% CI) | 14/15 (93.3; 68.1-99.8) |
14/15 (93.3; 68.1-99.8) |
14/15 (93.3; 68.1-99.8) |
12/14 (85.7; 57.2-98.2) |
|||||||||||||||||||||||
| p-value | 0.7152 | ||||||||||||||||||||||||||
| MD (95% CI) | 475 (305-1240) | 155 (30-220) | 75 (35-230) | 57.5 (20-475) | |||||||||||||||||||||||
| VOC | Beta | ||||||||||||||||||||||||||
| GMT (95% CI) | 15.6 (6.5-37.5) | 576.9 (340.7-976.9) | 7.5 (5.0-11.1) | 122.7 (74.1-203.3) | 7.2 (5.4-9.6) | 147 (61.9-349.2) | 10.8 (6.3-18.5) | 100.4 (45.4-222.1) | |||||||||||||||||||
| Seroconversion rate-n/N (%; 95% CI) | 13/15 (86.7; 59.6-98.3) |
14/15 (93.3; 68.1-99.8) |
14/15 (93.3; 68.1-99.8) |
11/14 (78.5;49.1-95.3) |
|||||||||||||||||||||||
| p-value | 0.3561 | ||||||||||||||||||||||||||
| MD (95% CI) | 955 (235-1260) | 115 (55-235) | 115 (70-620) | 87.5 (15-280) | |||||||||||||||||||||||
| VOC | Delta | ||||||||||||||||||||||||||
| GMT (95% CI) | 47.9 (22.9-100.5) | 1004 (763.2-1322) | 21.3 (9.7-46.5) | 713.1 (462.4-1100) | 15.3 (7.7-30.3) | 540.4 (352-829.6) | 52.1 (25.2-107.6) | 469.8 (288.8-764.3) | |||||||||||||||||||
| Seroconversion rate-n/N (%; 95% CI) | 15/15 (100; 78.2-100) |
13/15 (86.7; 59.6-98.3) |
15/15 (100; 78.2-100) |
12/14 (85.7; 57.2-98.2) |
|||||||||||||||||||||||
| p-value | 0.3201 | ||||||||||||||||||||||||||
| MD (95% CI) | 1160 (800-1260) | 955 (315-1260) | 560 (235-1220) | 360 (155-1160) | |||||||||||||||||||||||
| Phase 2 | |||||||||||||||||||||||||||
| Group | |||||||||||||||||||||||||||
| Variable | Abdala | Mambisa | |||||||||||||||||||||||||
| Day 0 | Day 14 | Day 0 | Day 14 | ||||||||||||||||||||||||
| Anti-RBD IgG | |||||||||||||||||||||||||||
| GMT (95% CI) | 31.6 (27.4-36.3) | 647.9 (578.1-726.1) | 31.0 (27.0-35.7) | 152.5 (132.3- 175.7) | |||||||||||||||||||||||
| Seroconversion rate-N/n (%; 95% CI) | 408/509 (80.1; 76.4-83.5) | 261/494 (52.1; 48.3-57.3) | |||||||||||||||||||||||||
| MD (95% CI) | 667.0 (519.4-804.0) | 95.8 (78.0-125.0) | |||||||||||||||||||||||||
| Anti-RBD IgA | |||||||||||||||||||||||||||
| GMT (95% CI) | 11.0 (7.9-15.4) | 3086 (2666-3573) | 10.0 (7.2-14) | 1340 (1053-1706) | |||||||||||||||||||||||
| Seroconversion rate-N/n (%; 95% CI) | 387/509 (76.0; 72.1-79.7) | 326/494 (66.0; 61.8-70.4) | |||||||||||||||||||||||||
| MD (95% CI) | 3416 (3080-3803) | 2143 (1658-2485) | |||||||||||||||||||||||||
| Inhibition of the RBD-ACE2 binding | |||||||||||||||||||||||||||
| Mean (95% CI) | 37.1 (34.3-39.8) | 86.8 (85.4-88.2) | 37.6 (34.9-40.3) | 74.8 (72.4-77.1) | |||||||||||||||||||||||
| Seroconversion rate-N/n (%; 95% CI) | 381/509 (74.8; 70.8-78.5) | 317/494 (64.2; 48.5-57.5) | |||||||||||||||||||||||||
| MD (95% CI) | 59.0 (53.0-63.4) | 34.5 (29.3-38.1) | |||||||||||||||||||||||||
| Neutralizing antibodies to live SARS-CoV-2 variants | |||||||||||||||||||||||||||
| VOC | D614G | ||||||||||||||||||||||||||
| GMT (95% CI) | 50.0 (35.6-70.3) | 428.7 (300.6-611.3) | 68.5 (46.0-101.9) | 262 (176-390) | |||||||||||||||||||||||
| Seroconversion rate-N/n (%; 95% CI) | 43/50 (86.0; 64.0-88.5) | 30/52 (57.7; 41.3-69.5) | |||||||||||||||||||||||||
| MD (95% CI) | 480.0 (280.0-630.0) | 220.0 (50.0-310.0) | |||||||||||||||||||||||||
| VOC | Omicron (BA.5) | ||||||||||||||||||||||||||
| GMT (95% CI) | 25.6 (18.1-36.2) | 261.5 (182.6-374.0) | 34.7 (23.2-51.9) | 156.3 (108-226.4) | |||||||||||||||||||||||
| Seroconversion rate-N/n (%; 95% CI) | 39/50 (78.0; 64.0-88.5) | 34/52 (65.4; 48.9-76.3) | |||||||||||||||||||||||||
| MD (95% CI) | 280.0 (140.0-580.0) | 150.0 (60.0-240.0) | |||||||||||||||||||||||||
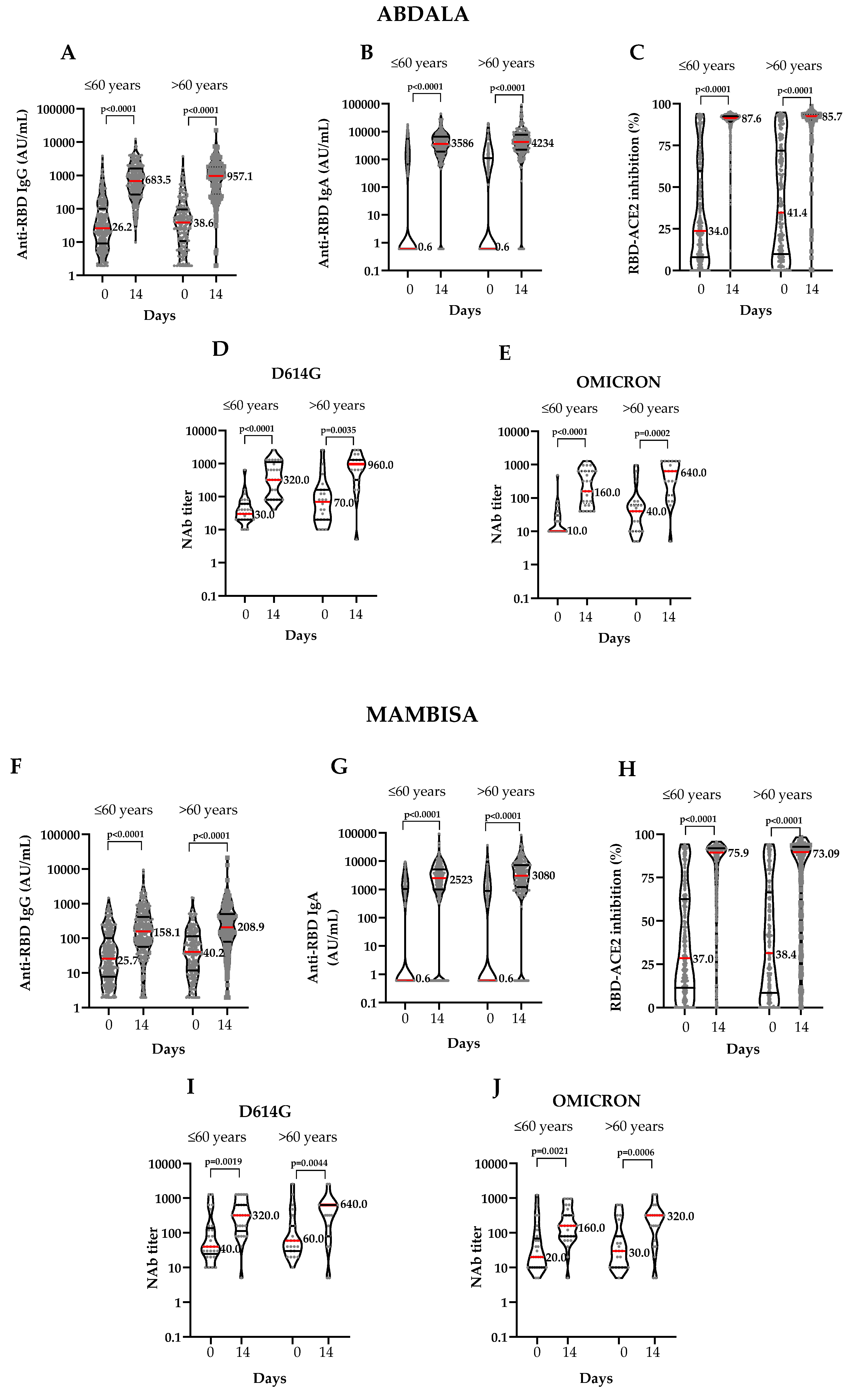
| Phase 2 immunogenicity results according to age groups | |||||||||||||||||||||||||||
| Group | |||||||||||||||||||||||||||
| Variable | Abdala | Mambisa | |||||||||||||||||||||||||
| Age | ≤60 years | >60 years | ≤60 years | >60 years | |||||||||||||||||||||||
| Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | ||||||||||||||||||||
| Anti-RBD IgG | |||||||||||||||||||||||||||
| GMT (95% CI) | 30.1 (25.1-36.1) | 616.2 (536-708.4) | 33.7 (27.0-42.0) | 695.4 (573.5-843.3) | 27.6 (22.9-33.2) | 134.6 (112-161.8) | 36.9 (29.9-45.6) | 183.2 (146.7-228.6) | |||||||||||||||||||
| Seroconversion rate-N/n (%; 95% CI) | 223/298 (74.8, 69.5-79.7) |
173/211 (81.9; 73.1-86.9) |
148/294 (50.3; 44.4-56.2) |
101/200 (50.5; 43.4-57.6) |
|||||||||||||||||||||||
| p-value | 0.0656 | >0.9999 | |||||||||||||||||||||||||
| MD (95% CI) | 541.0 (445.9-720.5) | 845.0 (645.4-966.2) | 79.3 (58.6-106.9) | 125.7 (89.8-195.0) | |||||||||||||||||||||||
| Anti-RBD IgA | |||||||||||||||||||||||||||
| GMT (95% CI) | 6.9 (4.5-10.5) | 2891 (2399-3484) | 20.8 (12.2-35.3) | 3385 (2672-4288) | 9.0 (5.8-13.8) | 1052 (754.9-1465) | 11.8 (6.9-20.1) | 1915 (1363-2690) | |||||||||||||||||||
| Seroconversion rate-N/n (%; 95% CI) | 234/298 (78.5; 73.3-83.0) |
153/211 (72.5; 66.0-78.4) |
189/294 (64.3; 58.5-69.7) |
137/200 (68.5; 61.4-74.9) |
|||||||||||||||||||||||
| p-value | 0.1399 | 0.3840 | |||||||||||||||||||||||||
| MD (95% CI) | 3212 (2712-3604) | 3680 (3146-4167) | 1893 (1435-2376) | 2460 (1811-3133) | |||||||||||||||||||||||
| Inhibition of the RBD-ACE2 binding | |||||||||||||||||||||||||||
| Mean (95% CI) | 34.05 (30.6-37.5) | 87.6 (86.2-89.0) | 41.4 (37.0-45.8) | 85.7 (82.8-88.6) | 37.0 (33.6-40.5) | 75.9 (73.0-78.8) | 38.4 (34-42.9) | 73.1 (69.1-77.1) | |||||||||||||||||||
| Seroconversion rate-N/n (%; 95% CI) | 236/298 (79.2; 74.1-83.7) |
145/211 (68.7; 62.0-74.9) |
197/294 (67.0; 60.6-71.7) |
120/200 (60.0; 52.8-66.8) |
|||||||||||||||||||||||
| p-value | 0.0094 | 0.1262 | |||||||||||||||||||||||||
| MD (95% CI) | 63.5 (58.9-66.7) | 44.0 (31.3-58.2) | 35.4 (29.6-45.5) | 31.8 (24.1-38.1) | |||||||||||||||||||||||
| Neutralizing antibodies to live SARS-CoV-2 variants | |||||||||||||||||||||||||||
| VOC | D614G | ||||||||||||||||||||||||||
| GMT (95% CI) | 37.3 (26.6-52.3) | 340.4 (218.4-530.5) | 68.5 (35.5-132.1) | 570.4 (297.8-1092) | 61.9 (36.0-106.5) | 264.3 (164-426) | 77.8 (41.6-145.8) | 259.1 (126.9-529.3) | |||||||||||||||||||
| Seroconversion rate-N/n (%; 95% CI) | 27/29 (93.1; 77.2-99.1) |
16/21 (76.2; 52.8-91.8) |
24/29 (82.7; 64.2-94.1) |
16/23 (69.6; 47.1-86.8) |
|||||||||||||||||||||||
| p-value | 0.1153 | 0.3290 | |||||||||||||||||||||||||
| MD (95% CI) | 294.0 (120.0-620.0) | 610.0 (40.0-1240) | 200.0 (40.0-310.0) | 290.0 (0.0-560.0) | |||||||||||||||||||||||
| VOC | Omicron (BA.5) | ||||||||||||||||||||||||||
| GMT (95% CI) | 18.8 (13.3-26.7) | 208.6 (133-327.1) | 37.8 (20.1-71.3) | 345.7 (181-660.2) | 31.7 (18.2-55.2) | 151.4 (96.0 -238.7) | 38.8 (20.6-73.1) | 162.8 (84.9-312.5) | |||||||||||||||||||
| Seroconversion rate-N/n (%; 95% CI) | 25/29 (86.2; 68.3-96.1) |
14/21 (66.7; 43.1-85.4) |
20/29 (69.0; 49.2-84.7) |
14/23 (60.9; 38.6-80.3) |
|||||||||||||||||||||||
| p-value | 0.1658 | 0.5711 | |||||||||||||||||||||||||
| MD (95% CI) | 150.0 (60.0-600.0) | 320.0 (80.0-950.0) | 120.0 (50.0-260.0) | 150.0 (20.0-290.0) | |||||||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





