1. Introduction
The mammalian ephrin and Eph system includes eight cell surface ephrin ligands (five ephrin-As and three ephrin-Bs) and 14 receptor tyrosine kinases (nine EphA and five EphB receptors) [
1]. Eph receptors make the complex to ephrins with dimerization or oligomerization, which leads to the tyrosine phosphorylation of Eph receptor and ephrin-B [
2]. The phosphorylated tyrosine recruits cytoplasmic effectors containing src-homology 2 (SH2) domains, phosphotyrosine-binding (PTB) domains, and PDZ domains [
3]. Therefore, the Eph receptor and ephrin complexes activate bidirectional (forward and reverse, respectively) signaling, which is essential for communication in the same or different types of cells [
4]. Through bidirectional signaling, the Eph system regulates tissue development, homeostasis, and regeneration; the dysregulation causes many diseases including cancer [
1].
The dysregulation of the Eph system is observed in both tumor cells and tumor microenvironment [
5]. The Eph system plays distinct roles in tumor development and functions as both tumor promoters and suppressors in a cellular context-dependent manner [
5]. EphB2 is overexpressed in several tumors, such as glioblastoma [
6], breast cancer [
7], hepatocellular carcinoma [
8], and malignant mesothelioma [
9], which is associated with poor clinical outcomes. In these tumors, EphB2 promotes the migration and invasion via forward signaling [
10,
11].
In contrast, the expression of EphB2 is downregulated in some tumors such as colorectal cancer [
12]. In the intestinal epithelium, EphB receptors promote stem and progenitor proliferation [
13]. The intestinal epithelial cell migration is deranged in mice lacking EphB2 and EphB3, and the absence of EphB signaling results in ~50% reduction in the number of proliferating cells [
13]. Furthermore, EphB receptor expression is elevated in intestinal adenomas [
14]. In contrast, EphB2 function as tumor suppressors by inhibiting the invasive growth. EphB signaling promotes adherens junction formation of colorectal cancer cells, suppressing cancer progression by inhibiting invasive growth [
15]. EphB2 expression is lost during progression to carcinoma and initiation of invasive growth [
16].
To evaluate the expression of EphB2 and targeting the EphB2-positive cancer cells, the development of monoclonal antibodies (mAbs) against EphB2 is essential. We have developed anti-receptor tyrosine kinase mAbs against human epidermal growth factor (EGFR) (clone EMab-17) [
17], mouse EGFR (EMab-300) [
18], HER2 (H
2Mab-19) [
19], mouse HER2 (H
2Mab-304) [
20], and HER3 (H
3Mab-17) [
21] using the Cell-Based Immunization and Screening (CBIS) method. The CBIS method includes immunizing antigen-overexpressed cells and high-throughput hybridoma screening using flow cytometry. In this study, novel anti-EphB2 mAbs were developed by the CBIS method.
2. Materials and Methods
2.1. Antibodies
OptiBuild™ RB545 mouse anti-human EphB2 mAb (clone 2H9; mouse IgG1, kappa) was purchased from BD Bioscience (Franklin Lakes, NJ). Alexa Fluor 488-conjugated anti-mouse IgG was purchased from Cell Signaling Technology, Inc. (Danvers, MA).
2.2. Preparation of Cell Lines
LS174T (human colorectal cancer), LN229 (human glioblastoma), Chinese hamster ovary (CHO)-K1, and P3X63Ag8U.1 (P3U1) were obtained from the American Type Culture Collection (Manassas, VA).
pCMV6-myc-DDK vector with EphB2 (Catalog No.: RC223882, Accession No.: NM_004442) was purchased from OriGene Technologies, Inc. (Rockville, MD). The EphB2 plasmids were transfected into CHO-K1 and LN229 cells using a Neon transfection system (Thermo Fisher Scientific Inc., Waltham, MA). Stable transfectants were established through cell sorting using the RB545-conjugated anti-human EphB2 (2H9) mAb and a cell sorter (SH800; Sony Corp., Tokyo, Japan), after which cultivation in a medium containing 0.5 mg/mL of G418 (Nacalai Tesque, Inc., Kyoto, Japan) was performed.
CHO-K1, EphB2-overexpressed CHO-K1 (CHO/EphB2), and P3U1 were cultured in a Roswell Park Memorial Institute (RPMI)-1640 medium (Nacalai Tesque, Inc., Kyoto, Japan), with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific Inc.), 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 0.25 μg/mL of amphotericin B (Nacalai Tesque, Inc.). LS174T, LN229, and EphB2-overexpressed LN229 (LN229/EphB2) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Nacalai Tesque, Inc.), supplemented with 10% FBS, 100 units /mL of penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B. All cells were grown in a humidified incubator at 37°C in an atmosphere of 5% CO2 and 95% air.
2.3. Development of Hybridomas
The animal was housed under specific pathogen-free conditions. All animal experiments were approved by the Animal Care and Use Committee of Tohoku University (Permit number: 2022MdA-001).
Two five-week-old BALB/cAJcl mice were purchased from CLEA Japan (Tokyo, Japan). To develop mAbs against EphB2, we intraperitoneally immunized the mice with LN229/EphB2 (1 × 108 cells) plus Alhydrogel adjuvant 2% (InvivoGen). The procedure included three additional weekly injections (1 × 108 cells/mouse), followed by a final booster intraperitoneal injection (1 × 108 cells/mouse) two days before harvesting spleen cells. The harvested spleen cells were subsequently fused with P3U1 cells, using PEG1500 (Roche Diagnostics, Indianapolis, IN), after which hybridomas were grown in the RPMI-1640 medium with 10% FBS, 5% Briclone (NICB, Dublin, Ireland), 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 0.25 μg/mL of amphotericin B. For the hybridoma selection, hypoxanthine, aminopterin, and thymidine (HAT; Thermo Fisher Scientific Inc.) were added to the medium. The supernatants were subsequently screened using flow cytometry using CHO/EphB2 and CHO-K1.
The cultured supernatant of Eb2Mab hybridomas was applied to 1 mL of Ab-Capcher (ProteNova, Kagawa, Japan). After washing with phosphate-buffered saline (PBS), the antibodies were eluted with an IgG elution buffer (Thermo Fisher Scientific Inc.). Finally, the eluates were concentrated, and the elution buffer was replaced with PBS using Amicon Ultra (Merck KGaA, Darmstadt, Germany).
2.4. Flow Cytometric Analysis
Cells were harvested after brief exposure to 0.25% trypsin and 1 mM ethylenediaminetetraacetic acid (EDTA, Nacalai Tesque, Inc.). The cells were washed with 0.1% bovine serum albumin (BSA) in PBS (blocking buffer) and treated with 0.01, 0.1, 1, and 10 μg/mL of primary mAbs for 30 min at 4°C. The cells were treated with Alexa Fluor 488-conjugated anti-mouse IgG (1:2,000). The cells were also suspended in 0.01, 0.1, 1, and 10 μg/mL of the RB545-conjugated anti-human EphB2 mAb (2H9). The fluorescence data were collected using the SA3800 Cell Analyzer (Sony Corp), and the data were analyzed using FlowJo (BD Biosciences).
2.5. Determination of Dissociation Constant (KD) by Flow Cytometry
CHO/EphB2 and LS174T cells were suspended in serially-diluted Eb2Mabs for 30 min at 4°C. The cells were treated with Alexa Fluor 488-conjugated anti-mouse IgG (1:200). The cells were also suspended in serially-diluted RB545-conjugated anti-human EphB2 mAb (2H9). The fluorescence data were collected using a FACSLyric (BD Biosciences). The KD was calculated by fitting saturation binding curves to the built-in, one-site binding models in GraphPad PRISM 6 (GraphPad Software, Inc., La Jolla, CA).
3. Results
3.1. Development of Anti-EphB2 mAbs Using the CBIS Method
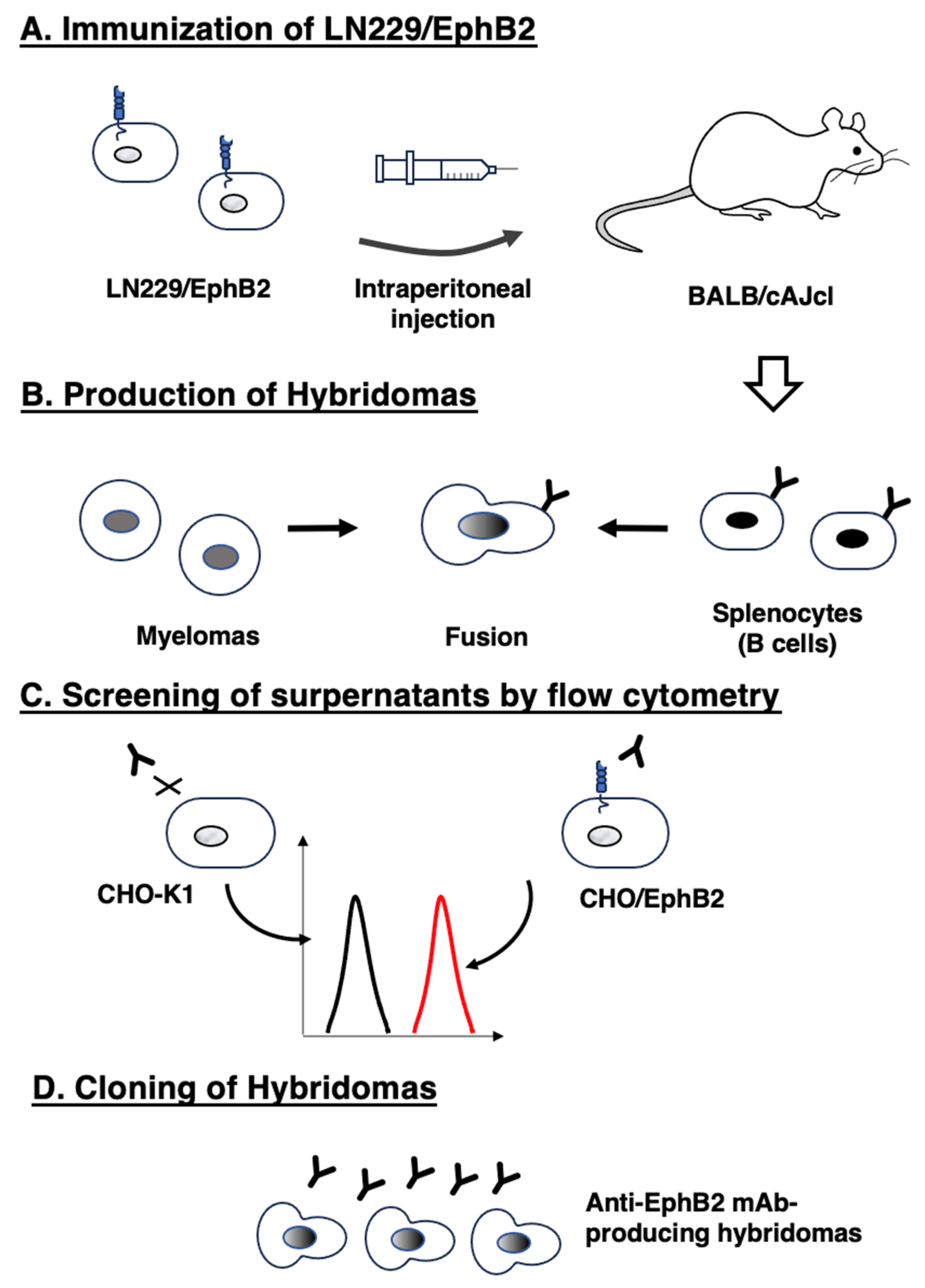
To develop anti-EphB2 mAb, mice were immunized with LN229/EphB2 cells (
Figure 1A). The spleen was then excised from the mice, and splenocytes were fused with myeloma P3U1 cells (
Figure 1B). The developed hybridomas were subsequently seeded into ten 96-well plates and cultivated for six days. The positive wells were screened by selecting CHO/EphB2-reactive and CHO-K1-non-reactive supernatants using flow cytometry (
Figure 1C). We finally obtained 133 positive wells (13.9%) out of 956 wells. After the limiting dilution of the part of positive wells and several additional screenings, twelve clones were finally established (
Figure 1D,
Supplementary Table S1).
3.2. Flow Cytometric Analysis Using Anti-EphB2 mAbs
We next focus on eight mouse IgG
1 clones (Eb
2Mab-1, 2, 3, 4, 7, 8, 10, and 12) and purified the mAbs from supernatants (
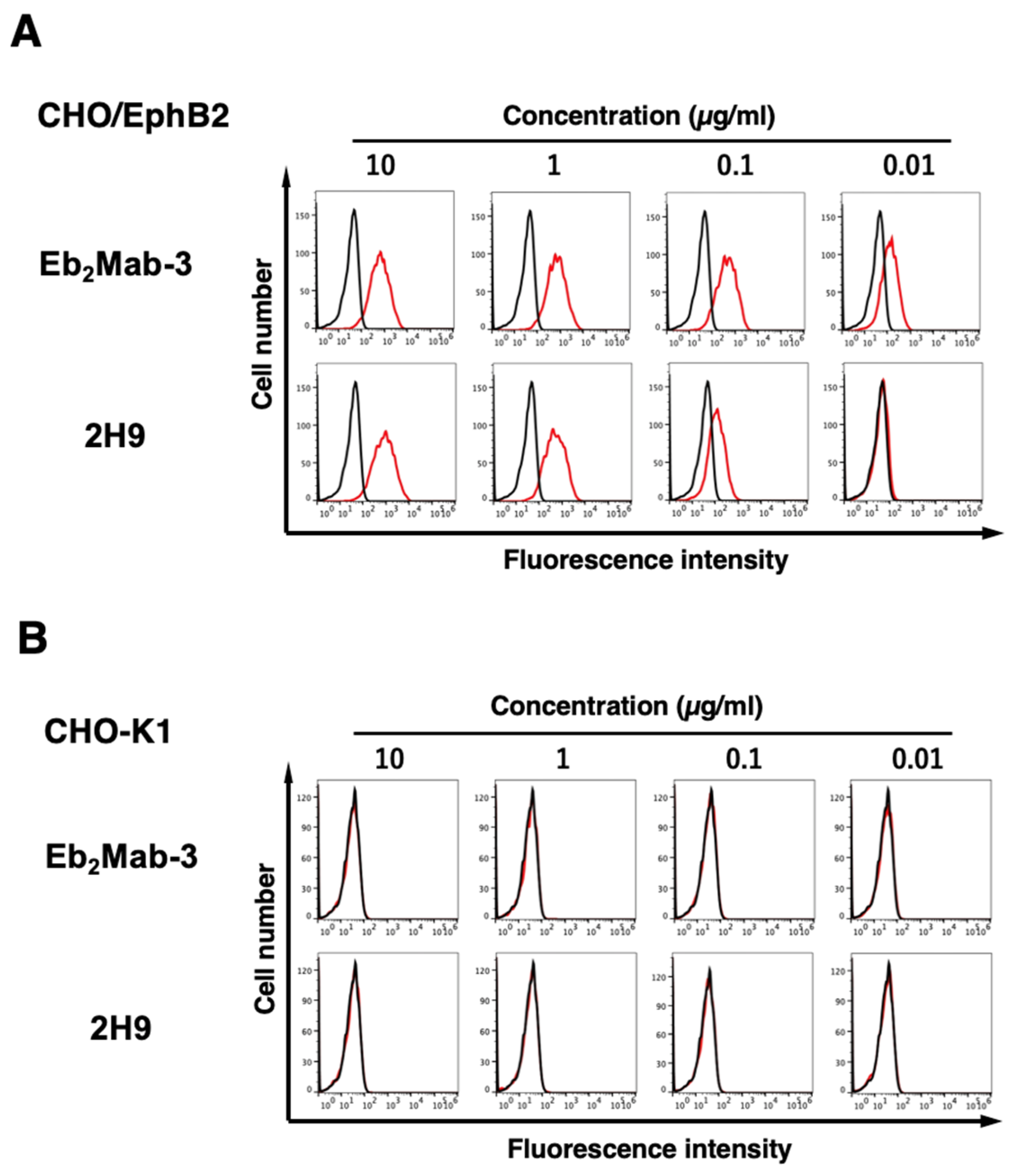
Supplementary Table S1). We conducted flow cytometry using the Eb
2Mabs and 2H9 (from BD Biosciences) against CHO/EphB2, CHO-K1, LN229/EphB2, and LN229 cells. Eb
2Mabs recognized CHO/EphB2 cells dose-dependently at 10, 1, 0.1, and 0.01 μg/mL (
Supplementary Figure S1). Among Eb
2Mabs, Eb
2Mab-3 showed the highest reactivity (
Figure 2A). 2H9 also recognized CHO/EphB2 cells dose-dependently at 10, 1, 0.1, and 0.01 μg/mL, which are less effective compared to Eb
2Mab-3 (
Figure 2A). Parental CHO-K1 cells were not recognized even at 10 μg/mL of Eb
2Mab-3 and 2H9 (
Figure 2B). The superior reactivity of Eb
2Mab-3 compared to 2H9 was also observed in LN229/EphB2 and LN229 cells (
Supplementary Figure S2). The weak expression of endogenous EphB2 in LN229 was previously confirmed by quantitative PCR and western blot analyses [
22].
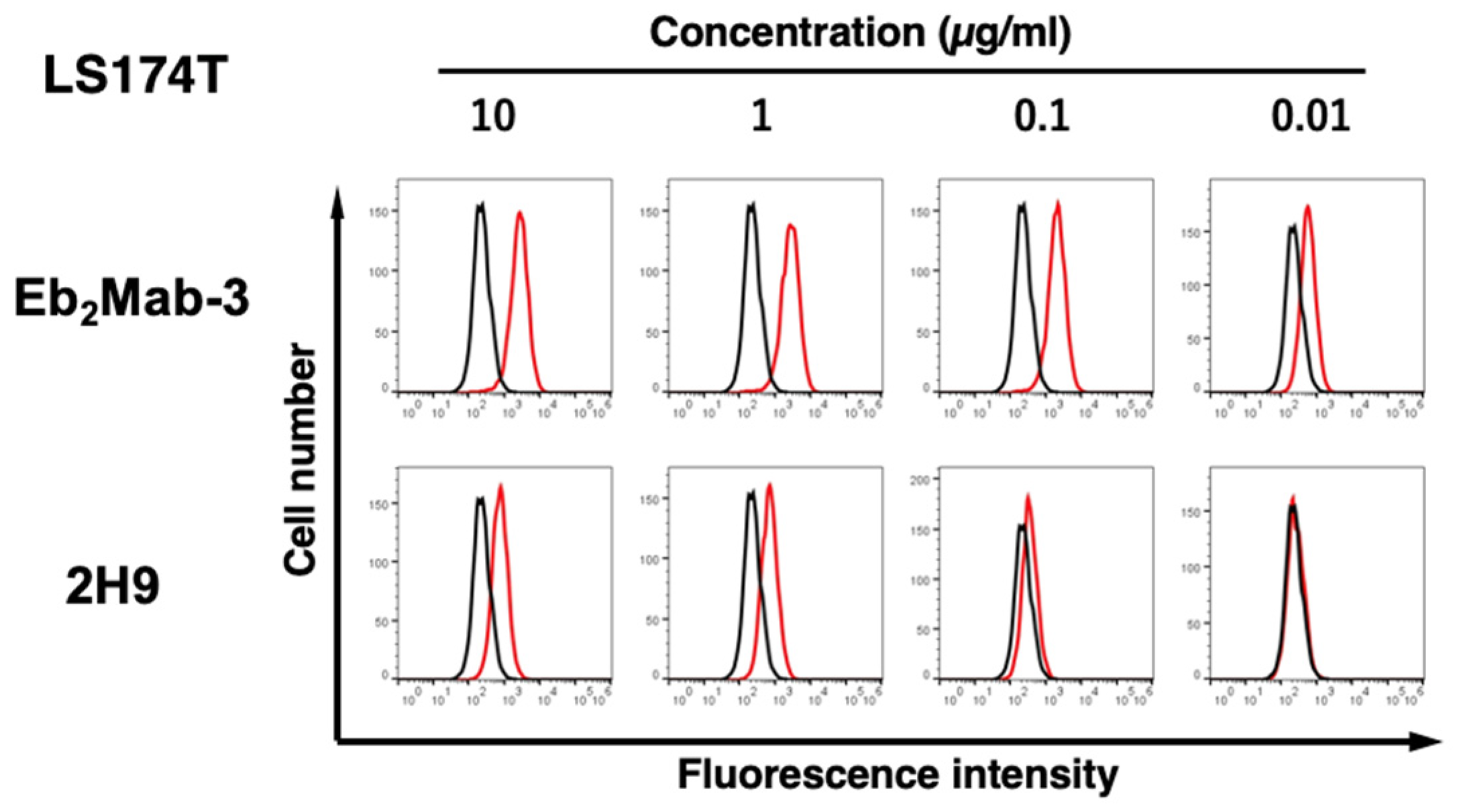
We next investigated the reactivity of Eb
2Mabs and 2H9 against an endogenous EphB2-expressing colorectal cancer cell line, LS174T [
23]. Eb
2Mabs recognized LS174T cells dose-dependently at 10, 1, 0.1, and 0.01 μg/mL (
Supplementary Figure S3). Among Eb
2Mabs, Eb
2Mab-3 also showed the highest reactivity (
Figure 3). In contrast, 2H9 could react with LS174T cells at more than 0.1 μg/mL (
Figure 3). These results suggest that Eb
2Mab-3 specifically recognizes EphB2, and is also helpful in detecting endogenous EphB2 by flow cytometry.
3.3. Determination of the Binding Affinity of Eb2Mabs and 2H9 Using Flow Cytometry
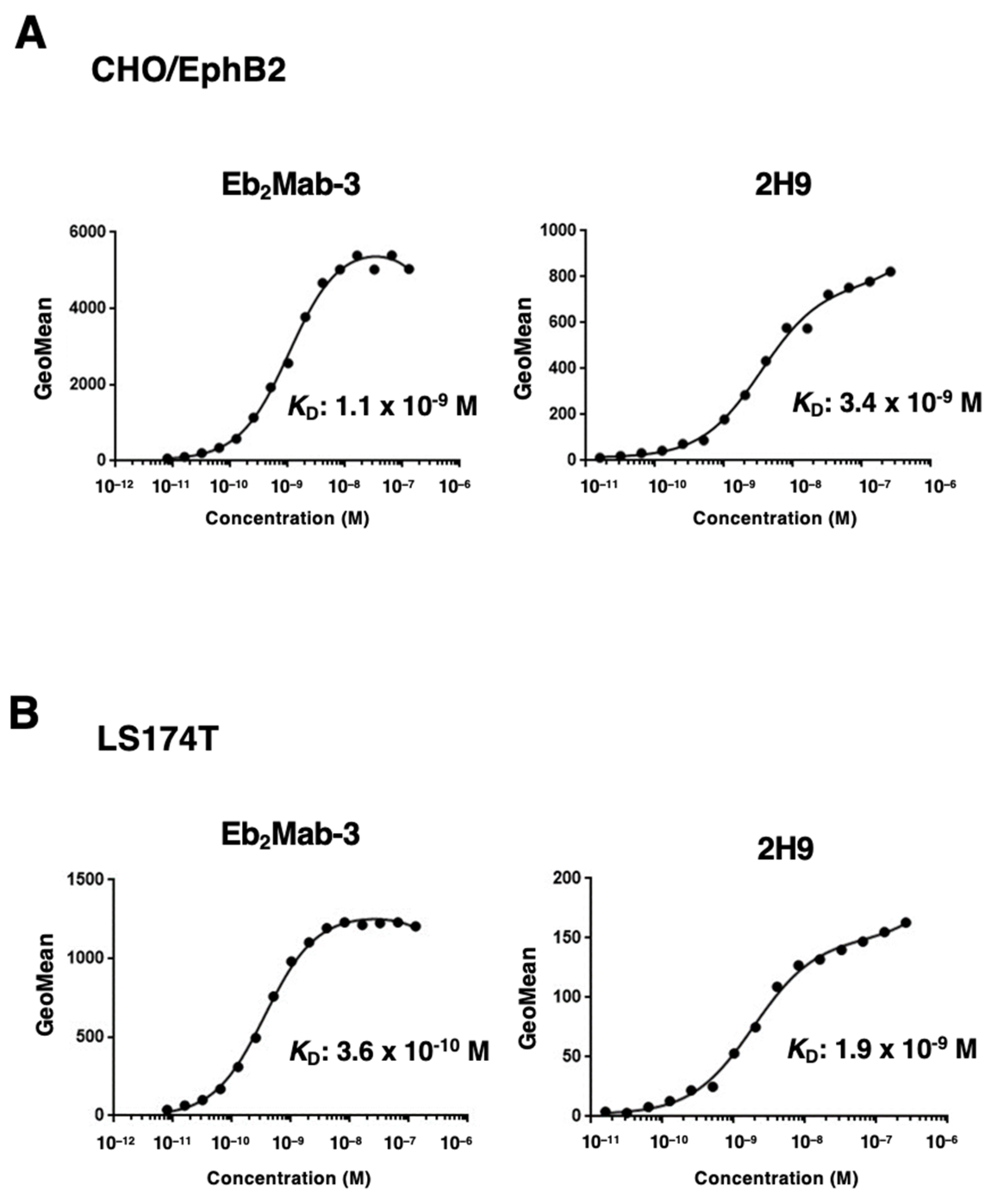
To determine the
KD values of Eb
2Mabs and 2H9, we conducted flow cytometry, and the geometric mean of the fluorescence intensity was plotted versus the concentration of mAbs. The
KD values of Eb
2Mab-3 and 2H9 for CHO/EphB2 were determined as 1.1 × 10
−9 M and 3.4 × 10
−9 M, respectively (
Figure 4A). Although we also determined the
KD values of other Eb
2Mabs, Eb
2Mab-3 exhibited the highest affinity (
Supplementary Table S1). We next determined the
KD values of Eb
2Mab-3 and 2H9 for LS174T as 3.6 × 10
−10 M and 1.9 × 10
−9 M, respectively (
Figure 4B). These results indicate that Eb
2Mab-3 possesses the superior affinity to CHO/EphB2 and LS174T compared to that of 2H9.
4. Discussion
An anti-EphB2 mAb, clone 2H9, was extensively characterized and developed for tumor therapy as an antibody-drug conjugate [
24], The 2H9 was established by the immunization of mice with the EphB2 ectodomain produced by baculovirus expression system [
24]. In this study, we established anti-EphB2 mAbs using the CBIS method (
Figure 1). Among the established mAbs, Eb
2Mab-3 exhibited the superior reactivity compared to 2H9 in CHO/EphB2 (
Figure 2), LN229/EphB2 (Supplemental
Figure S2), and LS174T (
Figure 3) cells. Furthermore, Eb
2Mab-3 possesses a higher affinity to CHO/EphB2 and LS174T than that of 2H9 (
Figure 4).
The 2H9 effectively blocked the interaction of EphB2 with the ligands and inhibited the autophosphorylation of EphB2 [
24]. However, 2H9 did not affect the proliferation of EphB2-positive tumor cells [
24]. The identification of the epitope is essential to assess the properties of Eb
2Mab-3 and 2H9. We have developed the RIEDL insertion for epitope mapping (REMAP) and PA insertion for epitope mapping (PAMAP) methods to determine the conformational epitopes of mAbs. The epitopes of anti-EGFR mAbs (EMab-51 and EMab-134) [
25,
26] and anti-CD44 mAbs (C
44Mab-5 and C
44Mab-46) [
27,
28] could be determined using the REMAP method. Furthermore, the epitopes of anti-mouse CD39 mAb (C
39Mab-1) could be determined using both REMAP and PAMAP methods [
29]. Therefore, further studies are required to determine the epitope and biological activities of Eb
2Mab-3.
We investigated the expression of EphB2 in more than 100 cell lines using flow cytometry. LS174T exhibited the highest expression (
Figure 3). Since LS174T is a transplantable cancer cell line in BALB/c nude mice [
30],
in vivo antitumor effects of Eb
2Mab-3 could be evaluated. To perform this, converting Eb
2Mab-3 (mouse IgG
1) to mouse IgG
2a subclass is essential for enhancing effector activation ability. We previously produced recombinant mAbs, which were converted into the mouse IgG
2a subclass from mouse IgG
1. Furthermore, we produced defucosylated IgG
2a mAbs using fucosyltransferase 8-deficient CHO-K1 cells to enhance the antibody-dependent cellular cytotoxicity and
in vivo antitumor effect in mouse xenograft models [
31,
32,
33]. Therefore, a class-switched and defucosylated type of Eb
2Mab-3 could contribute to the treatment of EphB2-positive tumors in preclinical studies.
We have developed cancer-specific mAbs (CasMabs) against HER2 (H
2Mab-214 and H
2Mab-250) [
34,
35], podocalyxin (PcMab-6 and PcMab-60) [
36,
37], and podoplanin (LpMab-2 and LpMab-23) [
38,
39] and revealed that these mAbs react with cancer cells, but not normal cells in flow cytometry. The strategy used in this study is also applicable to select anti-EphB2 CasMabs from Eb
2Mabs (
Supplementary Table S1) or clones from remaining positive wells. We have confirmed that EphB2 is detected in some normal epithelial cells and will screen the anti-EphB2 CasMabs. The unique property of H
2Mab-250 could contribute to developing HER2-targeting chimeric antigen receptor (CAR)-T cells (now in a clinical phase I study in the US). Therefore, developing Eb
2Mabs for CAR-T would be necessary for treating EphB2-positive tumors.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Flow cytometry of EphB2-expressed CHO-K1 cells using Eb2Mabs and 2H9.; Figure S2: Flow cytometry of EphB2-expressed LN229 cells using Eb2Mab-3 and 2H9.; Figure S3: Flow cytometry of endogenous EphB2-expressing cells using Eb2Mabs and 2H9.; Table S1: Determination of KD of Eb2Mabs (IgG1 isotype) using flow cytometry.
Author Contributions
R.U., H.Suzuki., M.H., H.Satofuka, and T.T. performed the experiments. M.K.K. and Y.K. designed the experiments. R.U. and H.Suzuki. analyzed the data. H.Suzuki. and Y.K. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding Information
This research was supported in part by Japan Agency for Medical Research and Development (AMED) under Grant Numbers: JP23ama121008 (to Y.K.), 23bm1123027 (to Y.K.), and JP23ck0106730 (to Y.K.), and by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) grant nos. 22K06995 (to H.S.) and 22K07224 (to Y.K.).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of Tohoku University (Permit number: 2022MdA-001) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data and methods are presented in this paper. Additional inquiries should be addressed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest involving this article.
References
- Pasquale, E.B. Eph receptors and ephrins in cancer progression. Nat Rev Cancer 2024, 24, 5–27. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol 2005, 6, 462–475. [Google Scholar] [CrossRef]
- Arora, S.; Scott, A.M.; Janes, P.W. Eph Receptors in Cancer. Biomedicines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer 2010, 10, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, C.; Li, J.; Fang, J. The Roles of EphB2 in Cancer. Front Cell Dev Biol 2022, 10, 788587. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, B.; Wang, H.; Hu, L.; Zhang, J.; Yu, T.; Xu, X.; Tian, W.; Zhao, C.; Zhu, H.; et al. circMELK promotes glioblastoma multiforme cell tumorigenesis through the miR-593/EphB2 axis. Mol Ther Nucleic Acids 2021, 25, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; Wiercinska, E.; Teunisse, A.F.; Lodder, K.; ten Dijke, P.; Jochemsen, A.G. Wild-type p53 inhibits pro-invasive properties of TGF-β3 in breast cancer, in part through regulation of EPHB2, a new TGF-β target gene. Breast Cancer Res Treat 2014, 148, 7–18. [Google Scholar] [CrossRef]
- Leung, H.W.; Leung, C.O.N.; Lau, E.Y.; Chung, K.P.S.; Mok, E.H.; Lei, M.M.L.; Leung, R.W.H.; Tong, M.; Keng, V.W.; Ma, C.; et al. EPHB2 Activates β-Catenin to Enhance Cancer Stem Cell Properties and Drive Sorafenib Resistance in Hepatocellular Carcinoma. Cancer Res 2021, 81, 3229–3240. [Google Scholar] [CrossRef] [PubMed]
- Goparaju, C.; Donington, J.S.; Hsu, T.; Harrington, R.; Hirsch, N.; Pass, H.I. Overexpression of EPH receptor B2 in malignant mesothelioma correlates with oncogenic behavior. J Thorac Oncol 2013, 8, 1203–1211. [Google Scholar] [CrossRef]
- Nakada, M.; Niska, J.A.; Miyamori, H.; McDonough, W.S.; Wu, J.; Sato, H.; Berens, M.E. The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res 2004, 64, 3179–3185. [Google Scholar] [CrossRef]
- Nakada, M.; Niska, J.A.; Tran, N.L.; McDonough, W.S.; Berens, M.E. EphB2/R-Ras signaling regulates glioma cell adhesion, growth, and invasion. Am J Pathol 2005, 167, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.Q.; Wu, X.S.; Wei, B.; Chen, L. Eph receptors and ephrins as targets for cancer therapy. J Cell Mol Med 2012, 16, 2894–2909. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, J.; Genander, M.; Halford, M.M.; Annerén, C.; Sondell, M.; Chumley, M.J.; Silvany, R.E.; Henkemeyer, M.; Frisén, J. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell 2006, 125, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Henderson, J.T.; Beghtel, H.; van den Born, M.M.; Sancho, E.; Huls, G.; Meeldijk, J.; Robertson, J.; van de Wetering, M.; Pawson, T.; et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 2002, 111, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Cortina, C.; Palomo-Ponce, S.; Iglesias, M.; Fernández-Masip, J.L.; Vivancos, A.; Whissell, G.; Humà, M.; Peiró, N.; Gallego, L.; Jonkheer, S.; et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet 2007, 39, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Jubb, A.M.; Zhong, F.; Bheddah, S.; Grabsch, H.I.; Frantz, G.D.; Mueller, W.; Kavi, V.; Quirke, P.; Polakis, P.; Koeppen, H. EphB2 is a prognostic factor in colorectal cancer. Clin Cancer Res 2005, 11, 5181–5187. [Google Scholar] [CrossRef] [PubMed]
- Takei, J.; Kaneko, M.K.; Ohishi, T.; Kawada, M.; Harada, H.; Kato, Y. A novel anti-EGFR monoclonal antibody (EMab-17) exerts antitumor activity against oral squamous cell carcinomas via antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. Oncol Lett 2020, 19, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.; Suzuki, H.; Tanaka, T.; Ishikawa, K.; Ouchida, T.; Kaneko, M.K.; Kato, Y. EMab-300 Detects Mouse Epidermal Growth Factor Receptor-Expressing Cancer Cell Lines in Flow Cytometry. Antibodies (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Kato, Y.; Ohishi, T.; Sano, M.; Asano, T.; Sayama, Y.; Takei, J.; Kawada, M.; Kaneko, M.K. H(2)Mab-19 Anti-Human Epidermal Growth Factor Receptor 2 Monoclonal Antibody Therapy Exerts Antitumor Activity in Pancreatic Cancer Xenograft Models. Monoclon Antib Immunodiagn Immunother 2020, 39, 61–65. [Google Scholar] [CrossRef]
- Ouchida, T.; Suzuki, H.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Development of Highly Sensitive Anti-Mouse HER2 Monoclonal Antibodies for Flow Cytometry. International Journal of Translational Medicine 2023, 3, 310–320. [Google Scholar] [CrossRef]
- Asano, T.; Ohishi, T.; Takei, J.; Nakamura, T.; Nanamiya, R.; Hosono, H.; Tanaka, T.; Sano, M.; Harada, H.; Kawada, M.; et al. Anti-HER3 monoclonal antibody exerts antitumor activity in a mouse model of colorectal adenocarcinoma. Oncol Rep 2021, 46. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Song, S.; Chen, W.; Zhang, J.; Yang, H.; Chen, Y. Hypoxia-induced EPHB2 promotes invasive potential of glioblastoma. Int J Clin Exp Pathol 2019, 12, 539–548. [Google Scholar] [PubMed]
- Genander, M.; Halford, M.M.; Xu, N.J.; Eriksson, M.; Yu, Z.; Qiu, Z.; Martling, A.; Greicius, G.; Thakar, S.; Catchpole, T.; et al. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell 2009, 139, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Luis, E.; Ross, S.; Silva, J.; Tan, C.; Crowley, C.; Chui, C.; Franz, G.; Senter, P.; Koeppen, H.; et al. EphB2 as a therapeutic antibody drug target for the treatment of colorectal cancer. Cancer Res 2004, 64, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Kaneko, M.K.; Aasano, T.; Kato, Y. Epitope Mapping of an Antihuman EGFR Monoclonal Antibody (EMab-134) Using the REMAP Method. Monoclon Antib Immunodiagn Immunother 2021, 40, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Nanamiya, R.; Sano, M.; Asano, T.; Yanaka, M.; Nakamura, T.; Saito, M.; Tanaka, T.; Hosono, H.; Tateyama, N.; Kaneko, M.K.; et al. Epitope Mapping of an Anti-Human Epidermal Growth Factor Receptor Monoclonal Antibody (EMab-51) Using the RIEDL Insertion for Epitope Mapping Method. Monoclon Antib Immunodiagn Immunother 2021, 40, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Kaneko, M.K.; Takei, J.; Tateyama, N.; Kato, Y. Epitope Mapping of the Anti-CD44 Monoclonal Antibody (C(44)Mab-46) Using the REMAP Method. Monoclon Antib Immunodiagn Immunother 2021, 40, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Epitope Mapping System: RIEDL Insertion for Epitope Mapping Method. Monoclon Antib Immunodiagn Immunother 2021, 40, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Suzuki, H.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Epitope Mapping of an Anti-Mouse CD39 Monoclonal Antibody Using PA Scanning and RIEDL Scanning. Monoclon Antib Immunodiagn Immunother 2024, 43, 44–52. [Google Scholar] [CrossRef]
- Sharkey, R.M.; Govindan, S.V.; Cardillo, T.M.; Donnell, J.; Xia, J.; Rossi, E.A.; Chang, C.H.; Goldenberg, D.M. Selective and Concentrated Accretion of SN-38 with a CEACAM5-Targeting Antibody-Drug Conjugate (ADC), Labetuzumab Govitecan (IMMU-130). Mol Cancer Ther 2018, 17, 196–203. [Google Scholar] [CrossRef]
- Li, G.; Suzuki, H.; Ohishi, T.; Asano, T.; Tanaka, T.; Yanaka, M.; Nakamura, T.; Yoshikawa, T.; Kawada, M.; Kaneko, M.K.; et al. Antitumor activities of a defucosylated anti-EpCAM monoclonal antibody in colorectal carcinoma xenograft models. Int J Mol Med 2023, 51. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ohishi, T.; Kaneko, M.K.; Takei, J.; Mizuno, T.; Kawada, M.; Saito, M.; Suzuki, H.; Kato, Y. Defucosylated Mouse-Dog Chimeric Anti-EGFR Antibody Exerts Antitumor Activities in Mouse Xenograft Models of Canine Tumors. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Takei, J.; Kaneko, M.K.; Ohishi, T.; Hosono, H.; Nakamura, T.; Yanaka, M.; Sano, M.; Asano, T.; Sayama, Y.; Kawada, M.; et al. A defucosylated anti-CD44 monoclonal antibody 5-mG2a-f exerts antitumor effects in mouse xenograft models of oral squamous cell carcinoma. Oncol Rep 2020, 44, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.K.; Suzuki, H.; Kato, Y. Establishment of a Novel Cancer-Specific Anti-HER2 Monoclonal Antibody H(2)Mab-250/H(2)CasMab-2 for Breast Cancers. Monoclon Antib Immunodiagn Immunother 2024, 43, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Arimori, T.; Mihara, E.; Suzuki, H.; Ohishi, T.; Tanaka, T.; Kaneko, M.K.; Takagi, J.; Kato, Y. Locally misfolded HER2 expressed on cancer cells is a promising target for development of cancer-specific antibodies. Structure 2024. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ohishi, T.; Tanaka, T.; Kaneko, M.K.; Kato, Y. A Cancer-Specific Monoclonal Antibody against Podocalyxin Exerted Antitumor Activities in Pancreatic Cancer Xenografts. Int J Mol Sci 2023, 25. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.K.; Ohishi, T.; Kawada, M.; Kato, Y. A cancer-specific anti-podocalyxin monoclonal antibody (60-mG(2a)-f) exerts antitumor effects in mouse xenograft models of pancreatic carcinoma. Biochem Biophys Rep 2020, 24, 100826. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Ogasawara, S.; Kaneko, M.K.; Kato, Y. LpMab-23: A Cancer-Specific Monoclonal Antibody Against Human Podoplanin. Monoclon Antib Immunodiagn Immunother 2017, 36, 72–76. [Google Scholar] [CrossRef]
- Kato, Y.; Kaneko, M.K. A cancer-specific monoclonal antibody recognizes the aberrantly glycosylated podoplanin. Sci Rep 2014, 4, 5924. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).