Submitted:
11 June 2024
Posted:
11 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Modeling
2.2. Material Properties
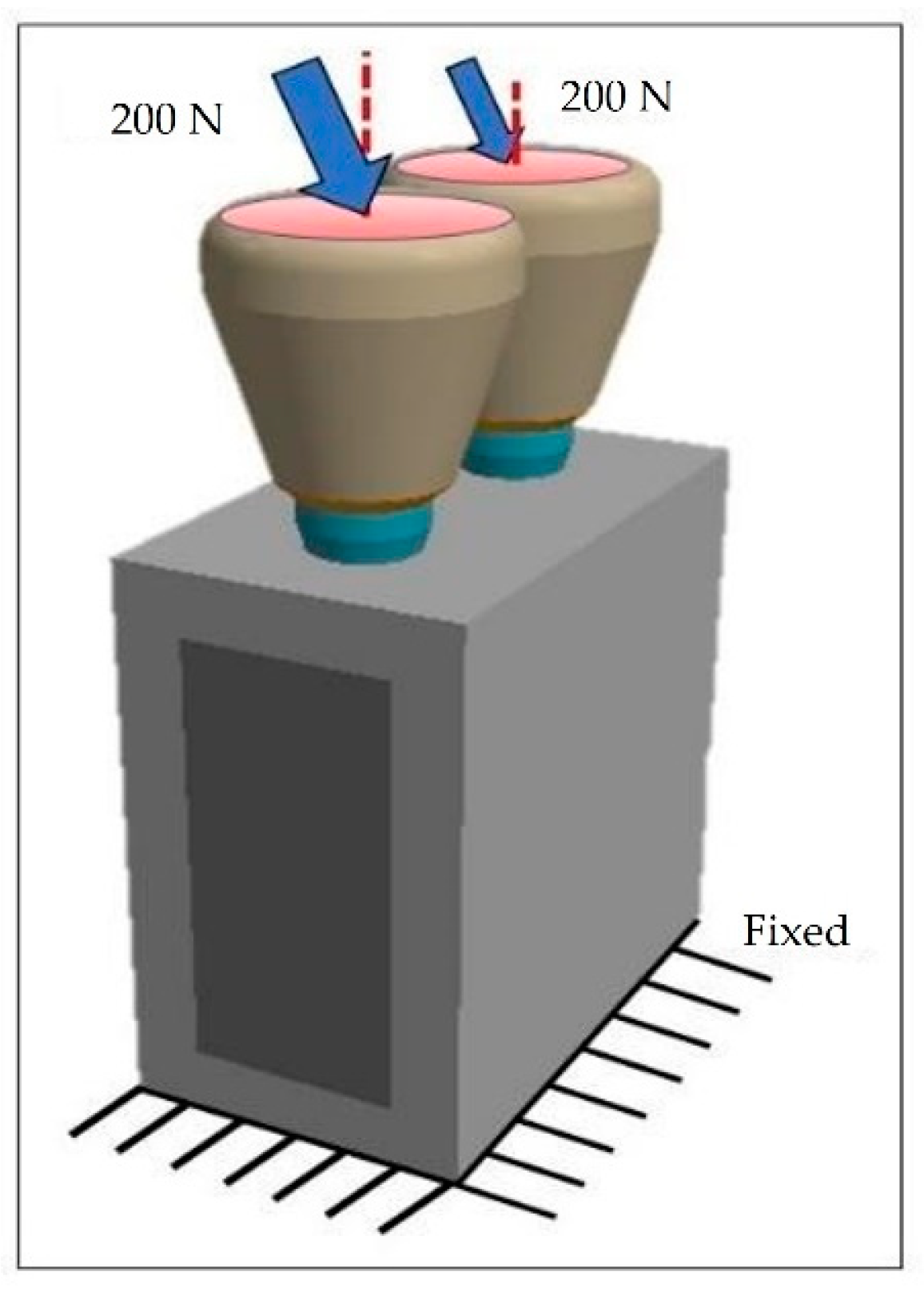
2.3. Constraints and Loading Conditions
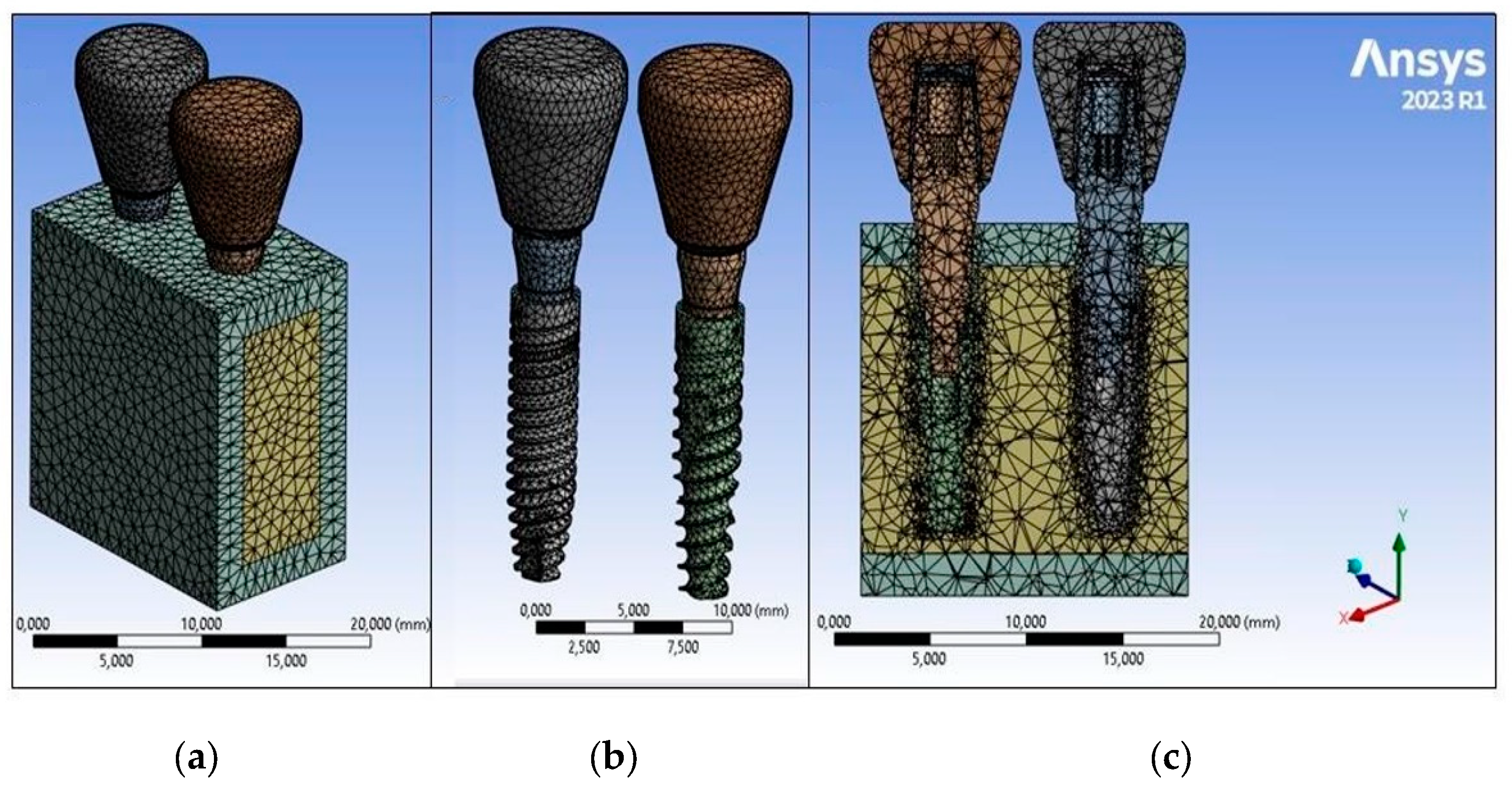
2.4. Finite Element Analysis (FEA)
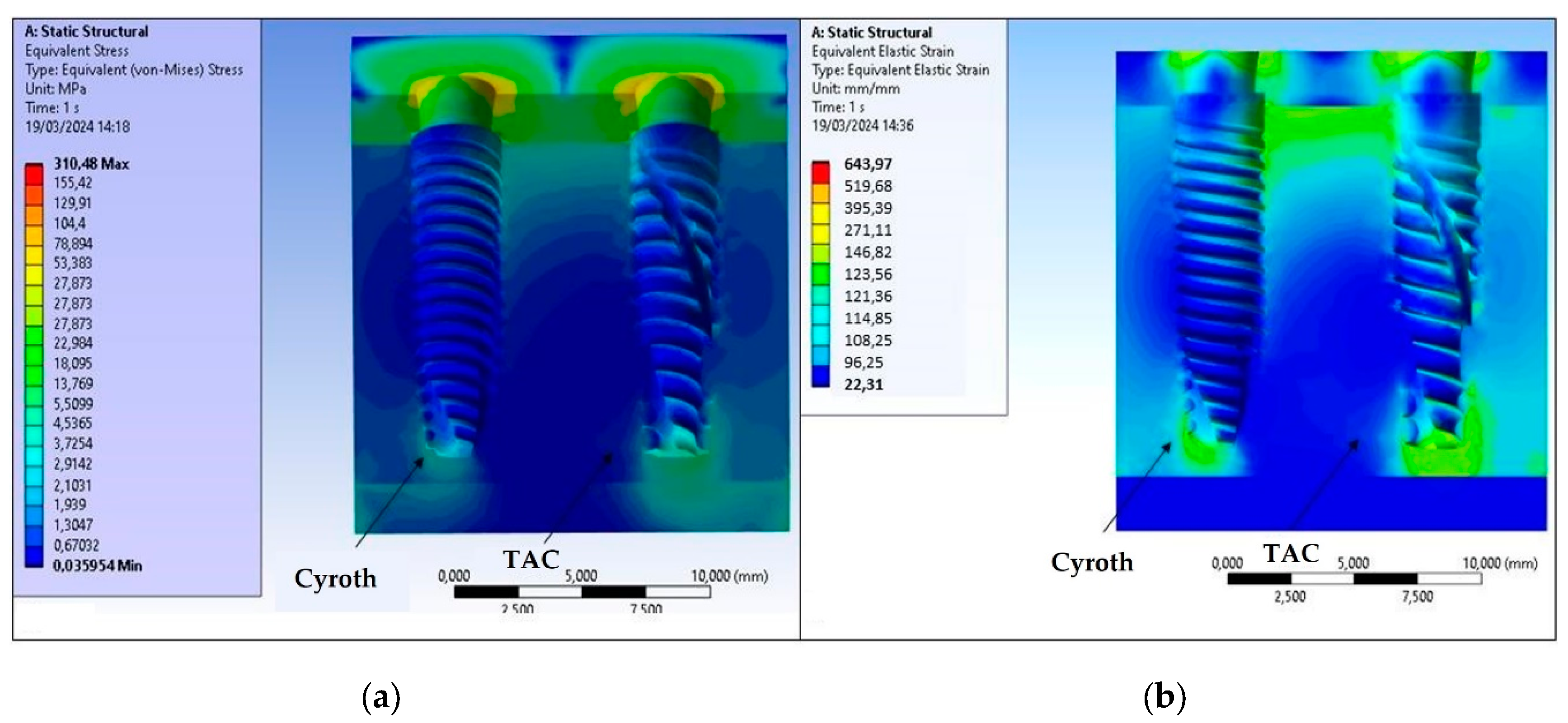
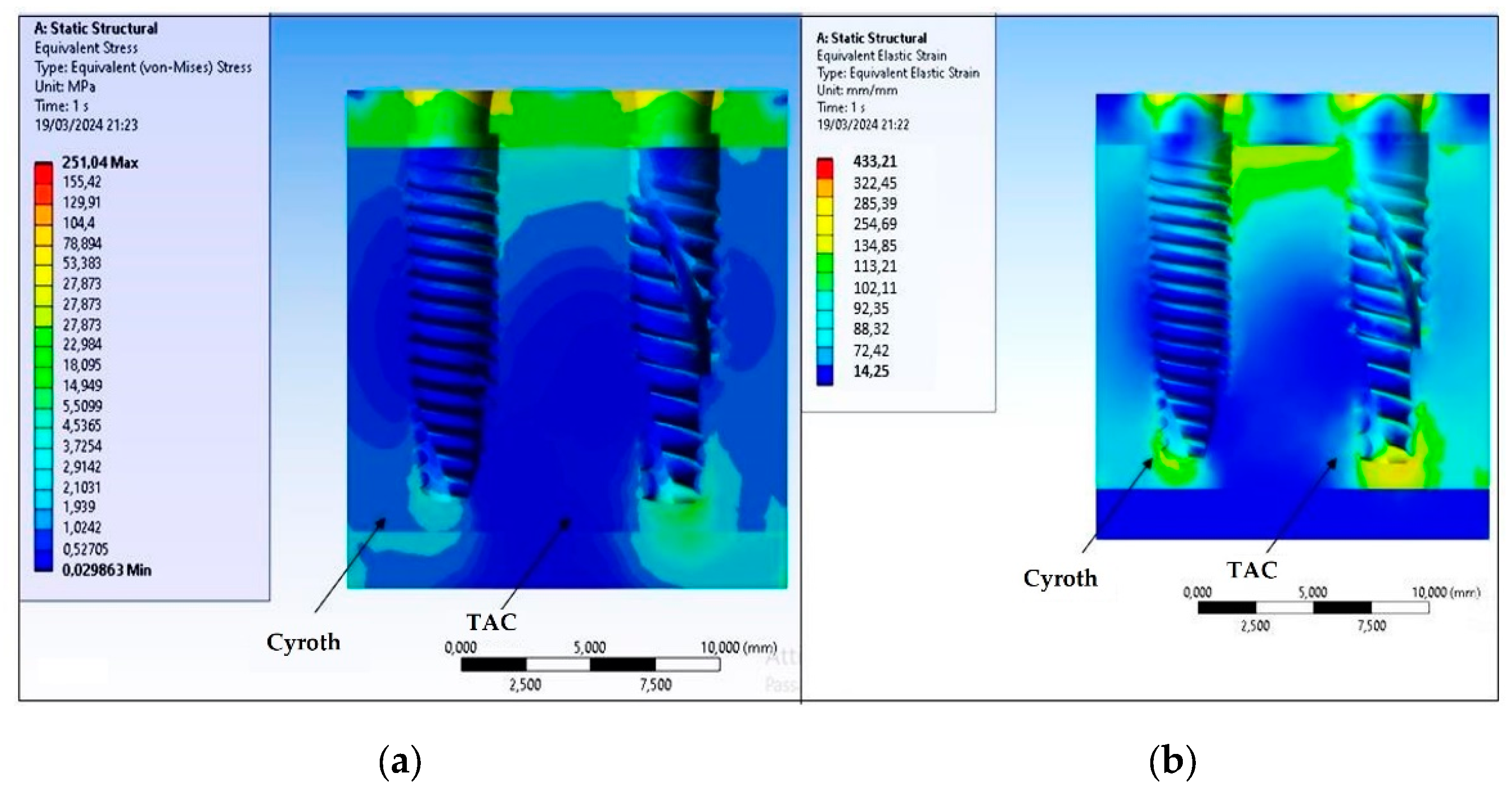
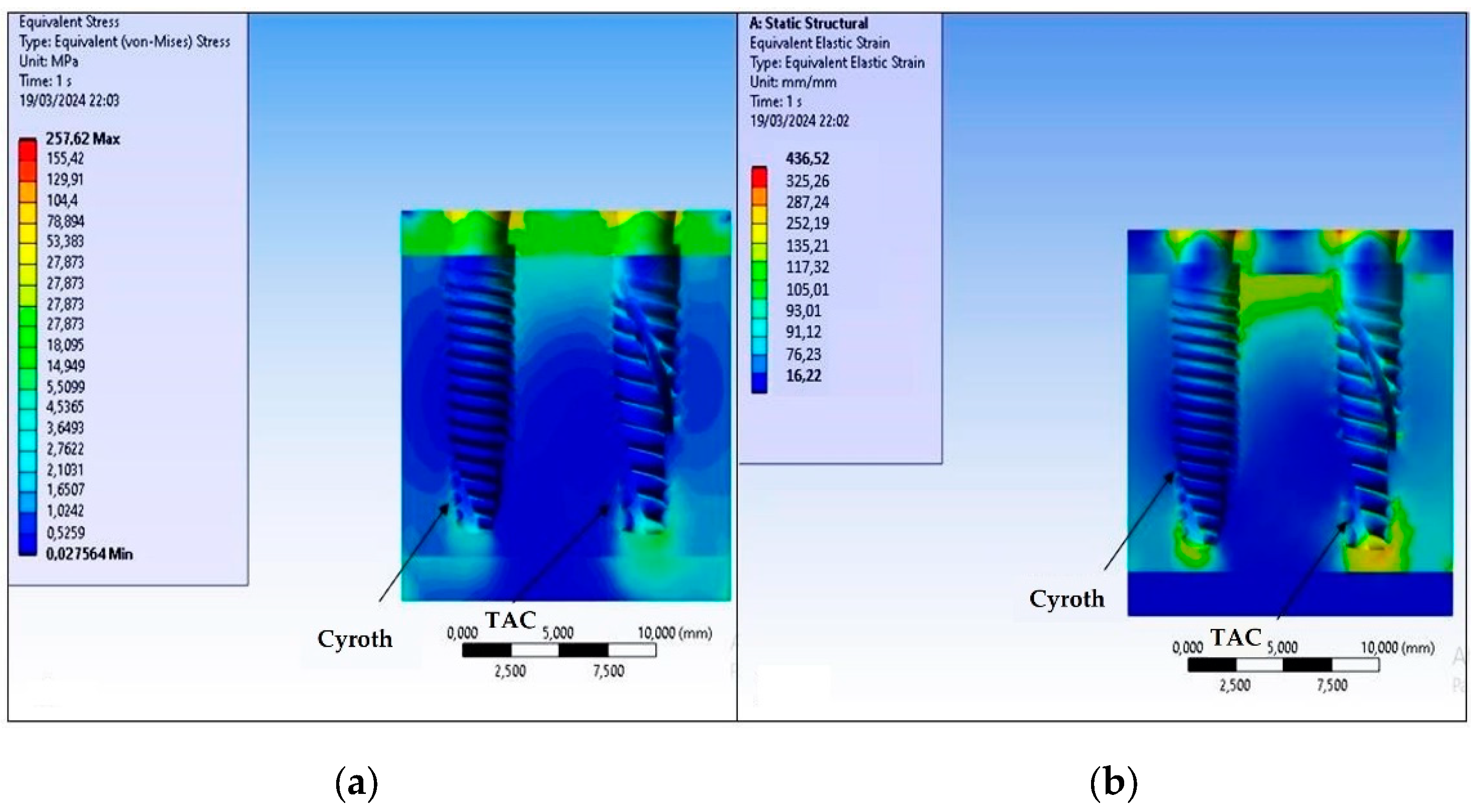
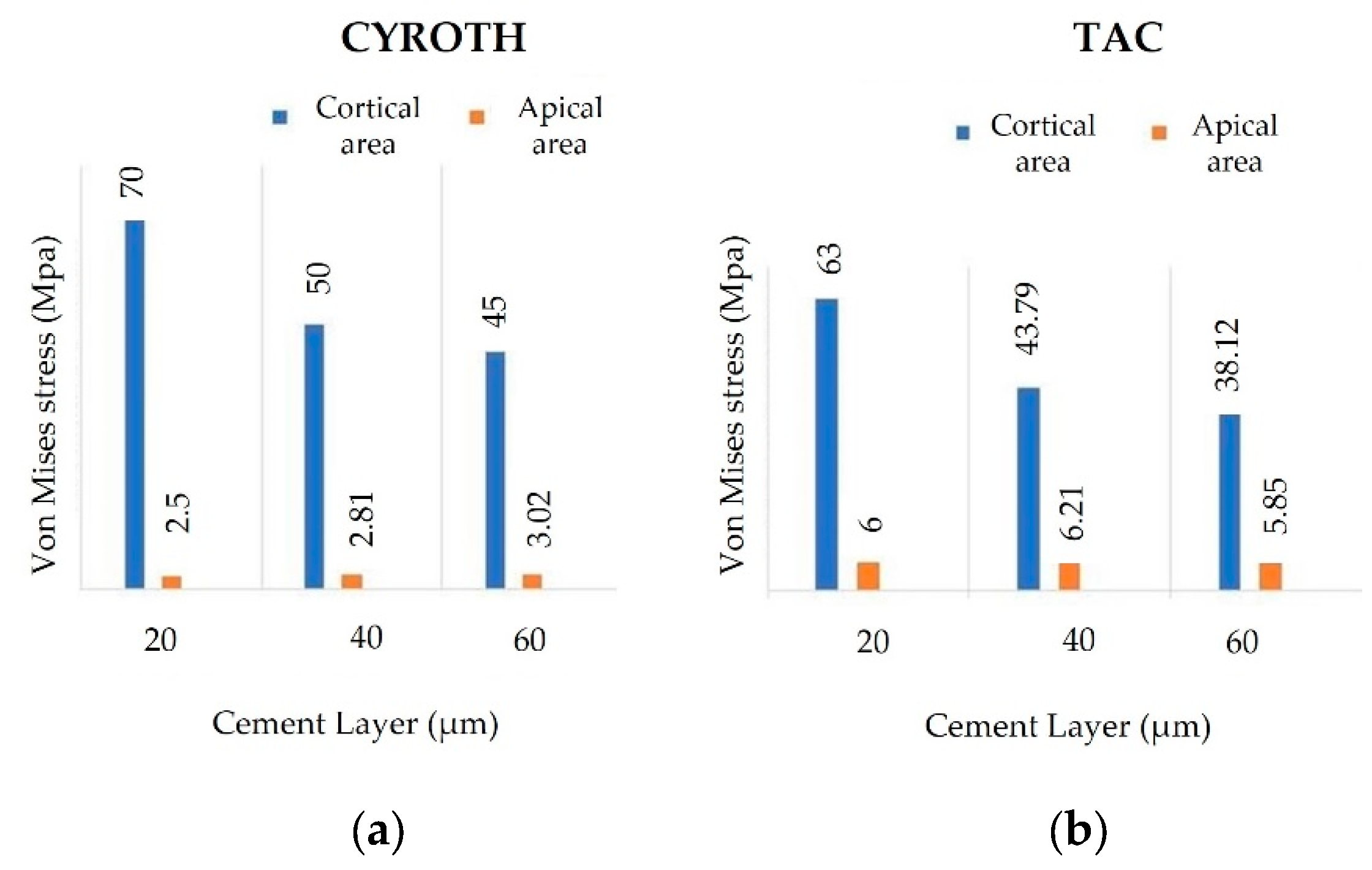
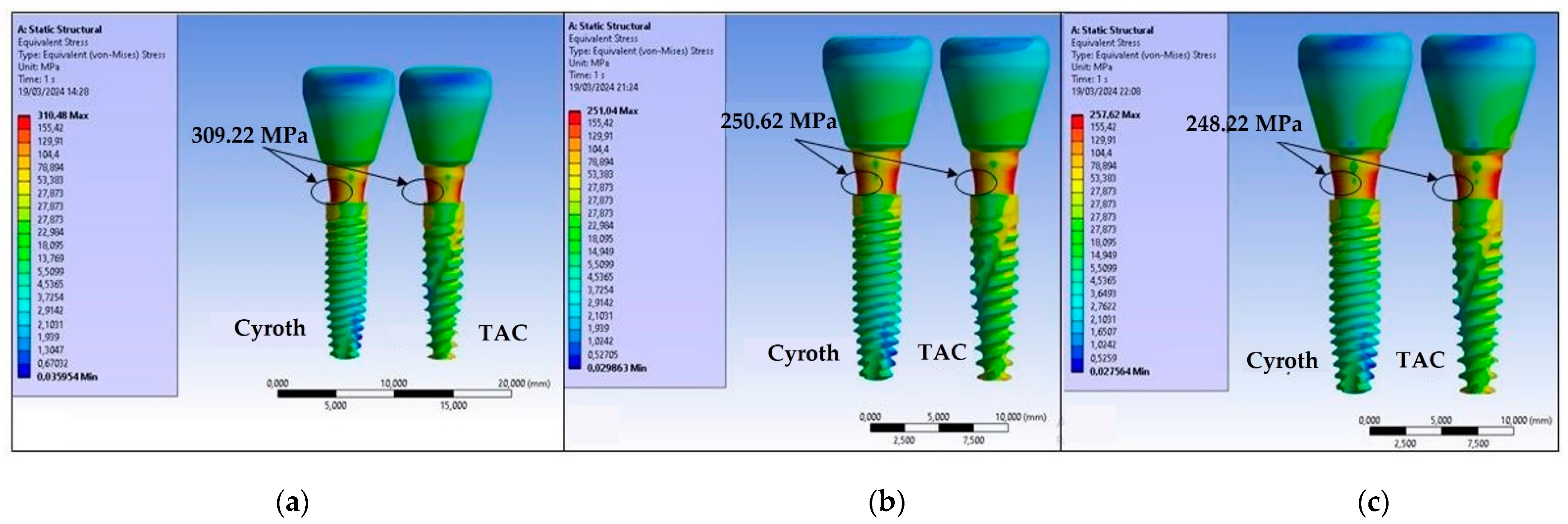
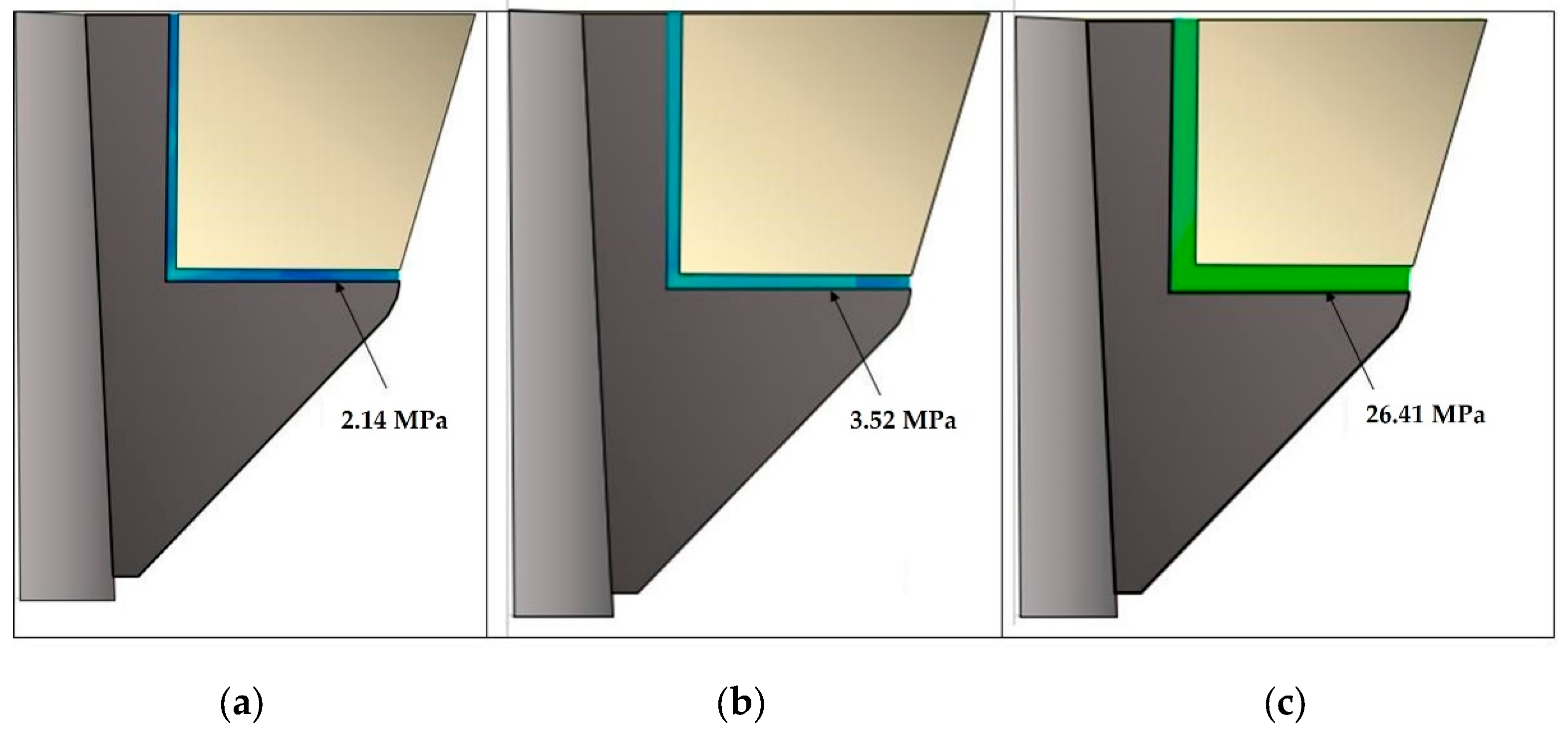
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Zarone, F.; Russo, S.; Sorrentino, R. From porcelain-fused-to-metal to zirconia: clinical and experimental considerations. Dent. Mater. 2011, 27, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Alsarani, M.; Souza, G.; Rizkalla, A.; El-Mowafy, O. Influence of crown design and material on chipping resistance of all-ceramic molar crowns: An in vitro study. Dent. Med. Probl. 2018, 55, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.E.; Ercoli, C.; Rispoli, L.; Maiorana, C.; Esposito, M. Metal-free materials for fixed prosthodontic restorations. Cochrane Database Syst. Rev. 2017, 12, CD009606. [Google Scholar] [CrossRef] [PubMed]
- Anusavice, K.J. Standardizing failure, success, and survival decisions in clinical studies of ceramic and metal-ceramic fixed dental prostheses. Dent. Mater. 2012, 28, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Sadowsky, S.J. Has zirconia made a material difference in implant prosthodontics? A review. Dent. Mater. 2020, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, R.; Triulzio, C.; Tricarico, M.G.; Bonadeo, G.; Gherlone, E.F.; Ferrari, M. In vitro analysis of the fracture resistance of CAD-CAM monolithic zirconia molar crowns with different occlusal thickness. J. Mech. Behav. Biomed. Mater. 2016, 61, 328–333. [Google Scholar] [CrossRef]
- Patzelt, S.B.; Spies, B.C.; Kohal, R.J. CAD/CAM-fabricated implant-supported restorations: A systematic review. Clin. Oral Implant. Res. 2015, 26, 77–85. [Google Scholar] [CrossRef]
- Wasiluk, G.; Chomik, E.; Gehrke, P.; Pietruska, M.; Skurska, A.; Pietruski, J. Incidence of undetected cement on CAD/CAM monolithic zirconia crowns and customized CAD/CAM implant abutments. A prospective case series. Clin. Oral Implant. Res. 2017, 28, 774–778. [Google Scholar] [CrossRef]
- Wittneben, J.G.; Millen, C.; Bragger, U. Clinical performance of screw-versus cement-retained fixed implantsupported reconstructions—A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 84–98. [Google Scholar] [CrossRef]
- Hamed, M.T.; Abdullah, M.H.; Khalid, A.S.; Hossam, H.A.B.; Hussein, N.G. A Systematic Review of Screw versus Cement- Retained Fixed Implant Supported Reconstructions. Clin. Cosmet. Investig. Dent. 2020, 12, 9–16. [Google Scholar] [CrossRef]
- Shadid, R.; Sadaqa, N. A comparison between screw- and cement-retained implant prostheses. A literature review. J. Oral Implant. 2012, 38, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Sailer, I.; Mühlemann, S.; Zwahlen, M.; Hämmerle, C.H.; Schneider, D. Cemented and screw-retained implant reconstructions: A systematic review of the survival and complication rates. Clin. Oral Implant. Res. 2012, 23, 163–201. [Google Scholar] [CrossRef] [PubMed]
- Nissan, J.; Narobai, D.; Gross, O.; Ghelfan, O.; Chaushu, G. Long-term outcome of cemented versus screwretained implant supported partial restorations. Int. J. Oral Maxillofac Implant. 2011, 26, 1102–1107. [Google Scholar] [PubMed]
- Kraus, R.D.; Epprecht, A.; Hämmerle, C.H.F.; Sailer, I.; Thoma, D.S. Cemented vs screw retained zirconiabased single implant reconstructions: A 3-year prospective randomized controlled clinical trial. Clin. Implant. Dent. Relat. Res. 2019, 21, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Wittneben, J.G.; Joda, T.; Weber, H.P.; Brägger, U. Screw retained vs. cement retained implant-supported fixed dental prosthesis. Periodontol 2000 2017, 73, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Zarone, F.; Sorrentino, R.; Traini, T.; Di lorio, D.; Caputi, S. Fracture resistance of implant-supported screw- versus cement-retained porcelain fused to metal single crowns: SEM fractographic analysis. Dent. Mater. 2007, 23, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, M.; Romasco, T.; Comuzzi, L.; Specchiulli, A.; Piattelli, A.; Lamberti, L.; Di Pietro, N.; Trentadue, B. Finite-Element Analysis Study Comparing Titanium and Polyetheretherketone Caps in a Conometric Connection between Implant and Prosthesis. Adv. Eng. Mater. 2024, 2400198. [Google Scholar] [CrossRef]
- Albiero, A.M.; Benato, R.; Momic, S.; Degidi, M. Guided-Welded Approach Planning Using a Computer-Aided Designed Prosthetic Shell for Immediately Loaded Complete-Arch Rehabilitations Supported by Conometric Abutments. J. Prosthet. Dent. 2019, 122, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Antonaya-Martin, J.; Rio-Highsmith, J.D.; Moreno-Hay, I.; Lillo-Rodríguez, J.; Gomez-Polo, M.; CeleminViñuela, A. CAD/CAM Conic Crowns for Predictable Retention in Implant-Supported Prostheses. Int. J. Prosthodont. 2016, 29, 230–232. [Google Scholar] [CrossRef]
- Albiero, A. M.; Benato, R.; Momic, S.; Degidi, M. Computer-Aided Crown Design Using Digital Scanning Technology for Immediate Postextraction Single-Implant Restorations Supported by Conical Indexed Abutments. Int. J. Periodont. Rest. Dent. 2021, 41, 135–140. [Google Scholar] [CrossRef]
- Gundogdu, M.; Aladag, L.I. Effect of adhesive resin cements on bond strength of ceramic core materials to dentin. Niger. J. Clin. Pract. 2018, 213, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.H.; Lepe, X.; Patterson, A.; Schäfer, O. Simplified cementation of lithium disilicate crowns: Retention with various adhesive resin cement combinations. J. Prosthet. Dent. 2018, 1195, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Dressano, D.; Salvador, M.V.; Oliveira, M.T.; Marchi, G.M.; Fronza, B.M.; Hadis, M.; Palin, W.M.; Lima, A.F. Chemistry of novel and contemporary resin-based dental adhesives. J. Mech. Behav. Biomed. Mater. 2020, 110, 103875. [Google Scholar] [CrossRef] [PubMed]
- Carville, R.; Quinn, F. The selection of adhesive systems for resin-based luting agents. J. Ir. Dent. Assoc. 2008, 54, 218–222. [Google Scholar] [PubMed]
- Wilson, A.D. Glass-ionomer cement origins, development and future. Clin. Mater. 1991, 7, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Moshaverinia, M.; Roohpour, N.; Chee, W.W.L.; Schricker, S.R.; Moshaverinia, A. Properties of a proline-containing glass ionomer dental cement. J. Prosthet. Dent. 2013, 110, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Bahsi, E.; Sagmak, S.; Dayi, B.; Cellik, O.; Akkus, Z. The evaluation of microleakage and fluoride release of different types of glass ionomer cements. Niger. J. Clin. Pract. 2019, 22, 961. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.H.; Croll, T.P. Glass ionomer restorative cement systems: An update. Pediatr. Dent. 2015, 37, 116–124. [Google Scholar] [PubMed]
- Powers, J.M. Self-adhesive Resin Cements: Characteristics, Properties, and Manipulation. AEGIS Dental Network. Funct. Esthet. Restor. Dent. 2008, 2, 34–40. [Google Scholar]
- Weiser, F.; Behr, M. Self-Adhesive Resin Cements: A Clinical Review. J. Prosthodont. 2015, 24, 100–108. [Google Scholar] [CrossRef]
- Makkar, S.; Malhotra, N. Self-adhesive resin cements: A new perspective in luting technology. Dent. Update 2013, 40, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Marghalani, H.Y. Sorption and solubility characteristics of self-adhesive resin cements. Dent. Mater. 2012, 28, e187–e198. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.K. Glass-ionomer cement restorative materials: A sticky subject? Aust. Dent. J. 2011, 56 (Suppl. S1), 23–30. [Google Scholar] [CrossRef] [PubMed]
- Walia, T.; Brigi, C.; Ziadkhani, M.M.; Khayat, A.A.; Tabibzadeh, Z. Retention Force of Glass Ionomer Based Luting Cements with Posterior Primary Zirconium Crowns—A Comparative in Vitro Study. J. Clin. Pediatr. Dent. 2021, 45, 259–264. [Google Scholar] [CrossRef] [PubMed]
- El-Anwar, M.; Tamam, R.A.; Fawzy, U.M.; Yousief, S.A. The effect of luting cement type and thickness on stress distribution in upper premolar implant restored with metal ceramic crowns. Tanta Dental Journal. 2015, 77. [Google Scholar] [CrossRef]
- Shillingburg, H.T.; Sather, D.A. Fundamentals of fixed prosthodontics, 4th ed.; Quintessence Pub: Chicago, 2012. [Google Scholar]
- Anusavice, K.J.; Hojjatie, B. Stress distribution in metal-ceramic crowns with a facial porcelain margin. J. Dent. Res. 1987, 66, 1493–1498. [Google Scholar] [CrossRef]
- Chai, J.Y.; Steege, J.W. Effects of labial margin design on stress distribution of a porcelain-fused-to-metal crown. J. Prosthodont. 1992, 1, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, N.; Ceddia, M.; Romasco, T.; De Bortoli, J.N.; Mello, B.F.; Tumedei, M.; Specchiulli, A.; Piattelli, A.; Trentadue, B. Appl. Sci. 2023, 13, 8147. [CrossRef]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Bai, J.; Zeng, X.; Zhou, Y. Comparison of isotropic and orthotropic material property assignments on femoral finite element models under two loading conditions. Med. Eng. Phys. 2006, 28, 227–233. [Google Scholar] [CrossRef]
- Misch, C.E.; Qu, Z.; Bidez, M.W. Mechanical properties of trabecular bone in the human mandible: implications for dental implant treatment planning and surgical placement. J. Oral Maxillofac. Surg. 1999, 57, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jansen, J.A.; Walboomers, X.F.; van den Beucken, J.J. Mechanical aspects of dental implants and osseointegration: A narrative review. J. Mech. Behav. Biomed. Mater. 2020, 103, 103574. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, J.L.; Capozza, R.F.; Mondelo, N.; Zanchetta, J.R. Interrelationships between densitometric, geometric, and mechanical properties of rat femora: inferences concerning mechanical regulation of bone modeling. J. Bone Miner. Res. 2020, 8, 1389–1396. [Google Scholar] [CrossRef]
- Ramos, A.; Nyashin, Y.; Mesnard, M. Influences of geometrical and mechanical properties of bone tissues in mandible behaviour–experimental and numerical predictions. Comput. Methods Biomech. Biomed. Engin. 2017, 20, 1004–1014. [Google Scholar] [CrossRef]
- Azcarate-Velázquez, F.; Castillo-Oyagüe, R.; Oliveros-López, L.G.; Torres-Lagares, D.; Martínez-González, Á.J.; Pérez-Velasco, A.; Lynch, C.D.; Gutiérrez-Pérez, J.L.; Serrera-Figallo, M.Á. Influence of bone quality on the mechanical interaction between implant and bone: A finite element analysis. J Dent. 2019, 88, 103161. [Google Scholar] [CrossRef]
- Oddbratt, E.; Hua, L.; Chrcanovic, B.R.; Papia, E. Bond strength of zirconia- or polymer-based copings cemented on implant-supported titanium bases - an in vitro study. Biomater. Investig. Dent. 2021, 8, 129–136. [Google Scholar] [CrossRef]
- Ceddia, M.; Lamberti, L.; Trentadue, B. FEA Comparison of the Mechanical Behavior of Three Dental Crown Materials: Enamel, Ceramic, and Zirconia. Materials (Basel). 2024, 17, 673. [Google Scholar] [CrossRef]
- Nakamura, T.; Wakabayashi, K.; Kinuta, S.; Nishida, H.; Miyamae, M.; Yatani, H. Mechanical properties of new self-adhesive resin-based cement. J. Prosthodont. Res. 2010, 54, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Ouldyerou, A.; Merdji, A.; Aminallah, L.; Roy, S.; Mehboob, H.; Özcan, M. Biomechanical performance of Ti-PEEK dental implants in bone: An in-silico analysis. J. Mech. Behav. Biomed. Mater. 2022, 134, 105422. [Google Scholar] [CrossRef]
- Jafariandehkordi, A.; Jafariandehkordi, Z. A finite element optimization of the design variables of a dental implant screw based on the Mechanostat Theory. Computer Methods and Programs in Biomedicine Update 2021, 1, 100033. [Google Scholar] [CrossRef]
- Lee, A.; Okayasu, K.; Wang, H.L. Screw- versus cement-retained implant restorations: Current concepts. Implant. Dent. 2010, 19, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gapski, R.; Neugeboren, N.; Pomeranz, A.Z.; Reissner, M.W. Endosseous implant failure influenced by crown cementation: A clinical case report. Int. J. Oral Maxillofac. Implant. 2008, 23, 943–946. [Google Scholar] [PubMed]
- Chee, W.; Jivraj, S. Screw versus cemented implant-supported restorations. Br. Dent. J. 2006, 201, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Michalakis, K.X.; Hirayama, H.; Garefis, P.D. Cement-retained versus screw-retained implant restorations: A critical review. Int. J. Oral Maxillofac. Implant. 2003, 18, 719–728. [Google Scholar] [PubMed]
- Lee, J.-H.; Jang, H.Y.; Lee, S.Y. Finite Element Analysis of Dental Implants with Zirconia Crown Restorations: Conventional Cement-Retained vs. Cementless Screw-Retained. Materials 2021, 14, 2666. [Google Scholar] [CrossRef] [PubMed]
- Rangert, B.; Gunne, J.; Glantz, P.O.; Svensson, A. Vertical load distribution on a three-unit prosthesis supported by a natural tooth and a single Brånemark implant. An in vivo study. Clin. Oral Implant. Res. 1995, 6, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Nardi, D.; Sighinolfi, G.; Degidi, D. The conometric concept for the definitive rehabilitation of a single posterior implant by using a conical indexed abutment: A technique. J. Prosthet. Dent. 2020, 123, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Lupi, S.M.; Todaro, C.; De Martis, D.; Blasi, P.; Rodriguez y Baena, R.; Storelli, S. The Conometric Connection for the Implant-Supported Fixed Prosthesis: A Narrative Review. Prosthesis 2022, 4, 458–467. [Google Scholar] [CrossRef]
- Elbieh, A.; Othman, H.; Haggag, K. Effect of Cement Gap on the Retention of Zirconia Crowns. Al-Azhar J. Dent. Sci. 2020, 23, 235–240. [Google Scholar] [CrossRef]
- Chen, X.; Mao, B.; Zhu, Z.; Yu, J.; Lu, Y.; Zhang, Q.; Yue, L.; Yu, H. A three-dimensional finite element analysis of mechanical function for 4 removable partial denture designs with 3 framework materials: CoCr, Ti-6Al-4V alloy and PEEK. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Frost, H.M. Perspectives: bone’s mechanical usage windows. Bone Miner. 1992, 19, 257–271. [Google Scholar] [CrossRef] [PubMed]
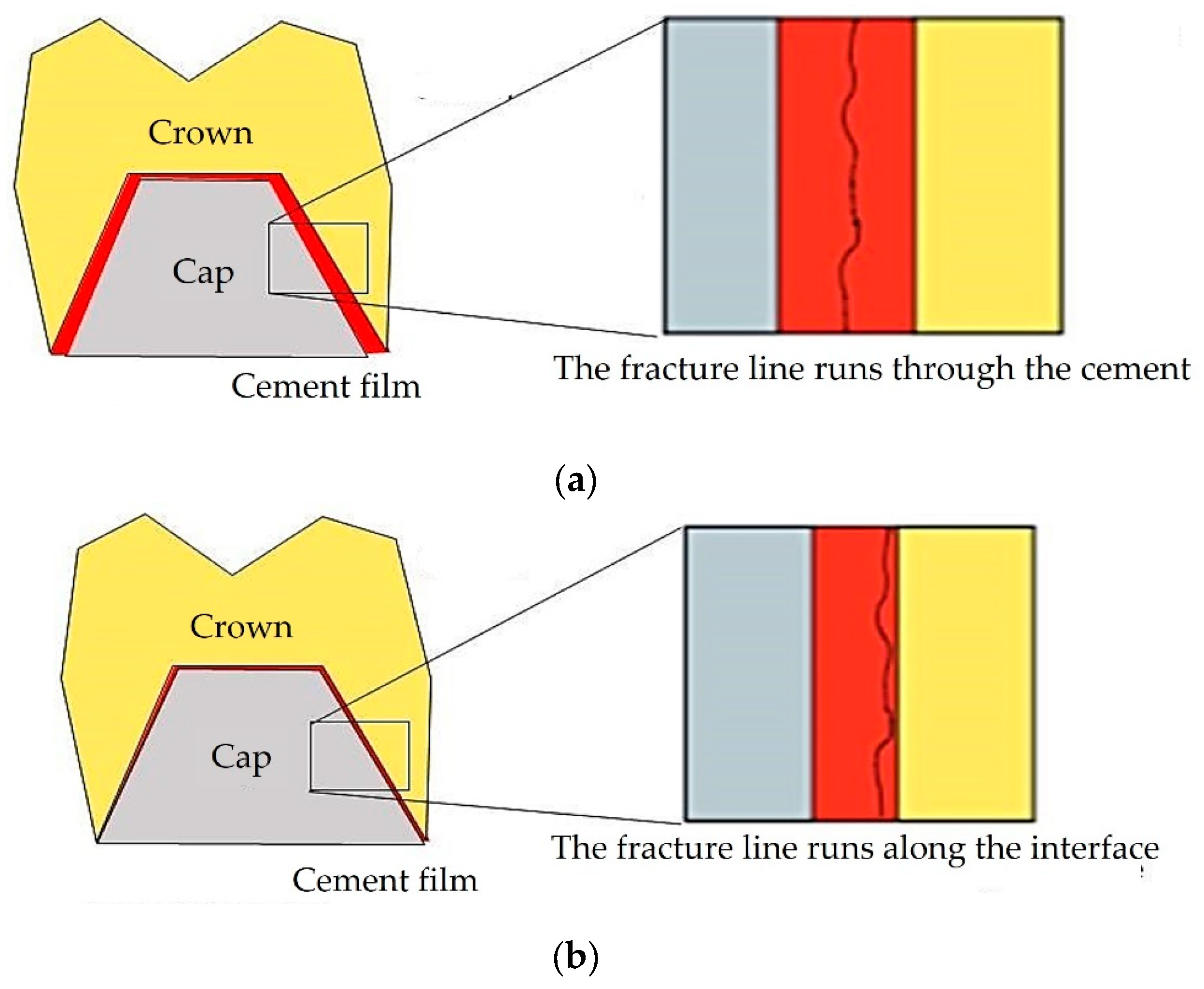
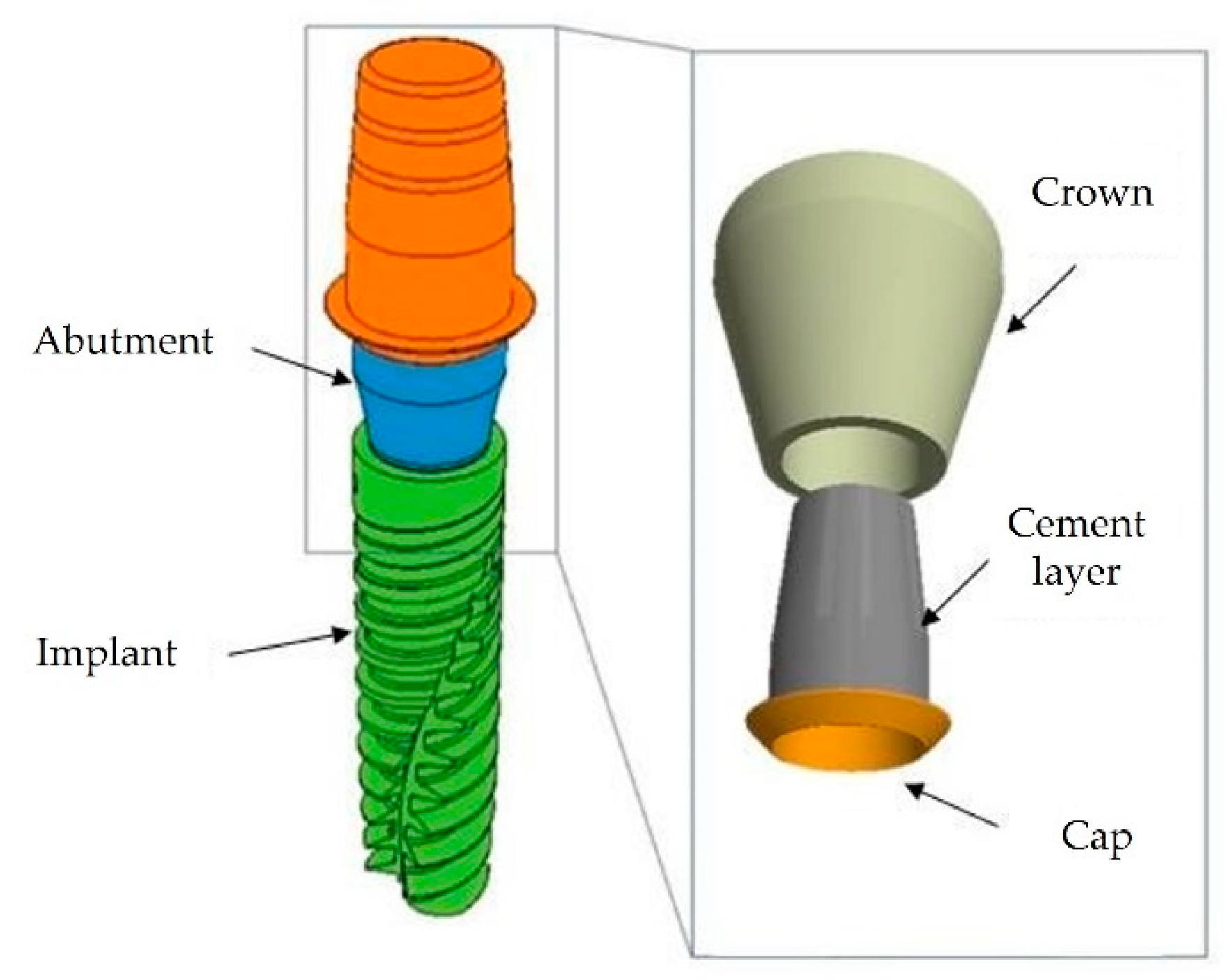
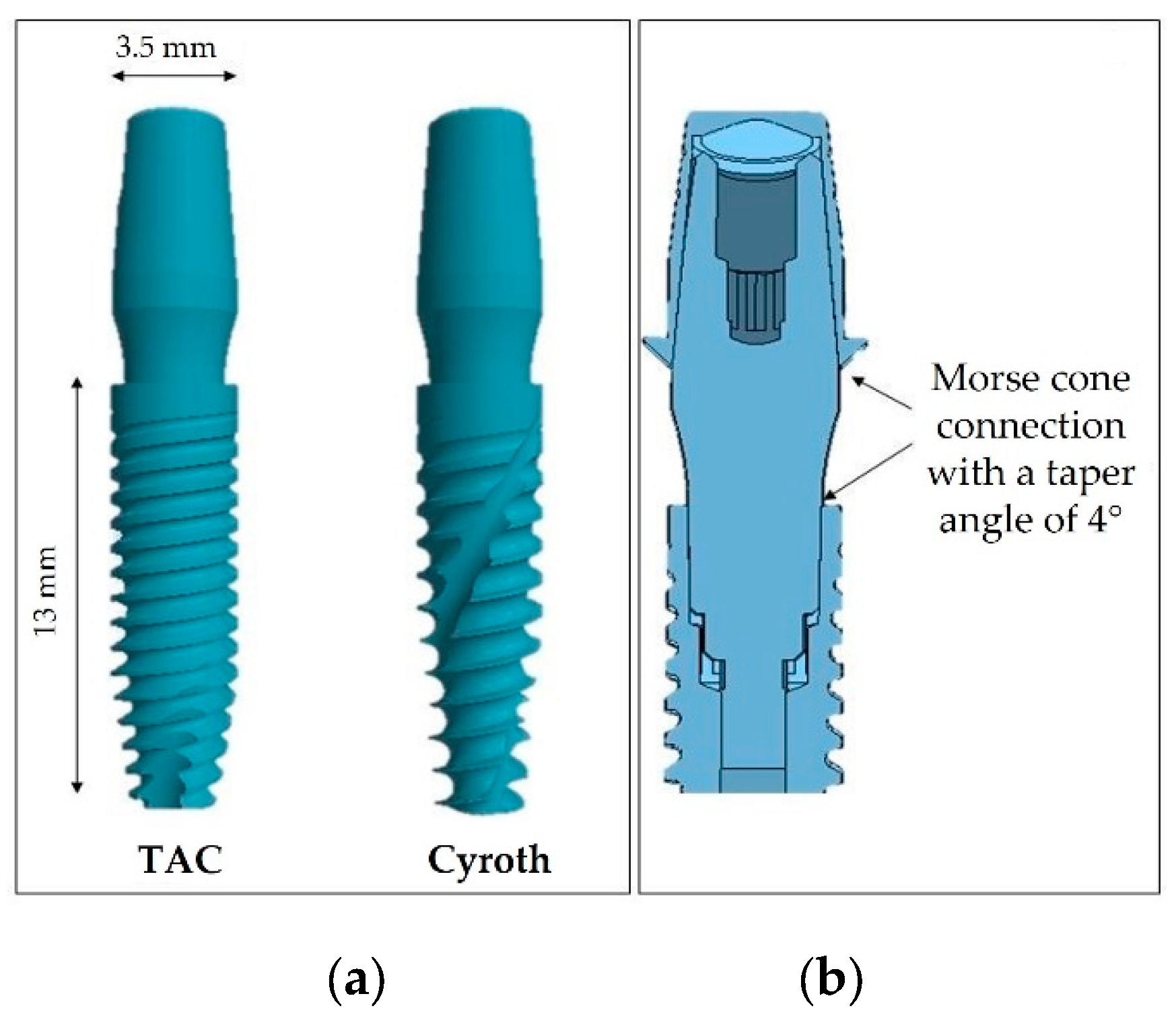
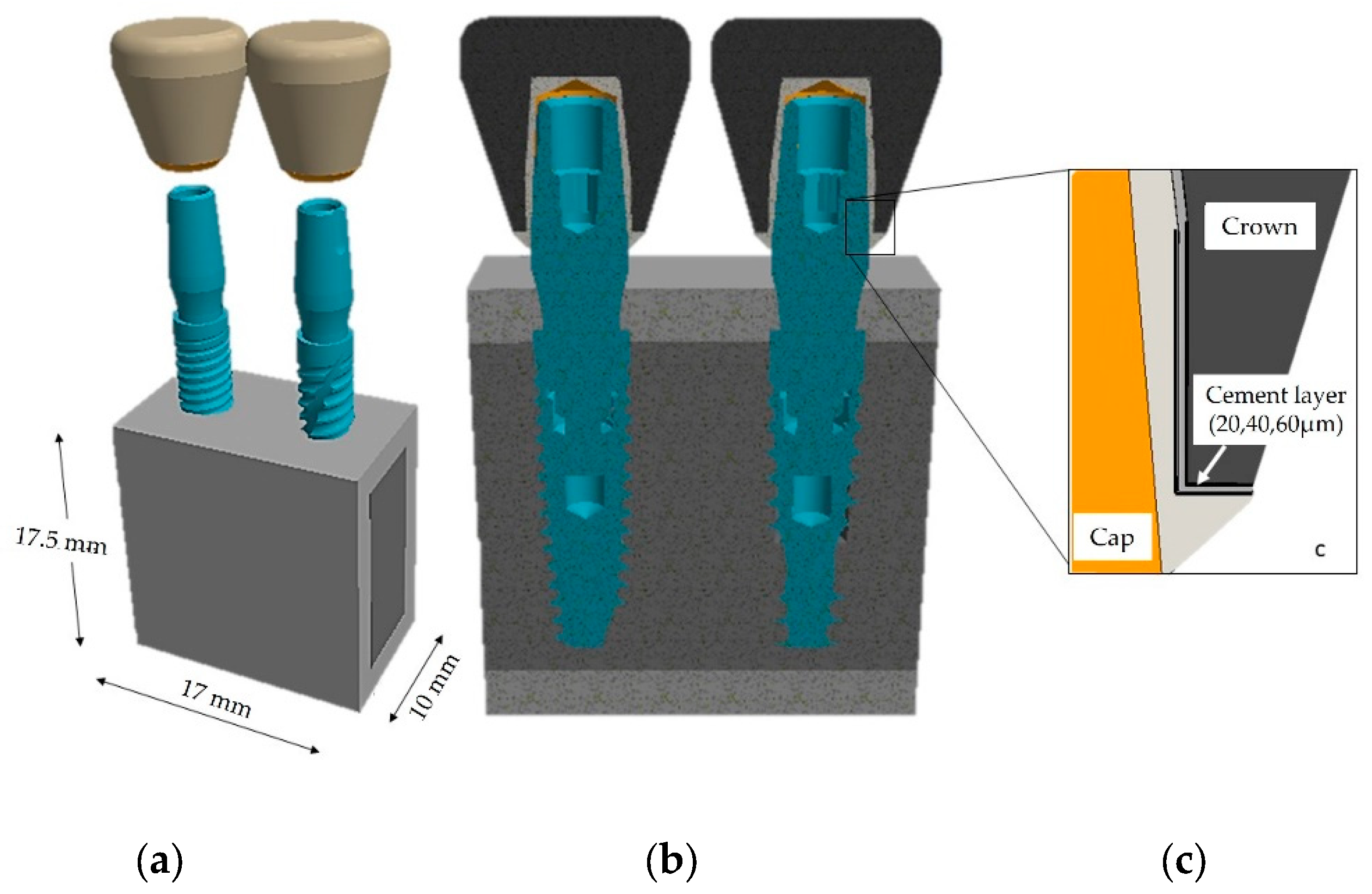
| Cement | Type / Curing | Composition |
|---|---|---|
| Multilink Hybrid Abutment (MHA) |
Resin-based cement/ Auto-polymerization | Dimethacrylate, HEMA1, fillers (barium glass, ytterbium (III) fluoride, spheroid mixed oxides, titanium dioxide), MMA2, PMMA3, dimethacrylates, initiators |
| Model | Material | Young’s Modulus (GPa) | Poisson’s ratio (ν) |
|---|---|---|---|
| Crown | Zirconia | 205 | 0.34 |
| Cement | Resin-based cement (MHA) | 6.3 | 0.25 |
| Cap | Ti6Al4V | 110 | 0.35 |
| Abutment | Ti6Al4V | 110 | 0.35 |
| Implant | Ti6Al4V | 110 | 0.35 |
| Jaw bone 1 | Spongy | 1.37 | 0.30 |
| Jaw bone 2 | Cortical | 13.7 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





