Submitted:
11 June 2024
Posted:
12 June 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Design of Nirsevimab
3. Definition, Role and Localization of FcRn: Based on our Knowledge of the Role of FcRn, what might be the Consequences of a mAb’s Increased Affinity for this Receptor?
3.1. Transport of Non-Antigen-Bound IgG
3.2. Transport of Protein Antigen-Bound IgG
3.3. Intracellular Transport of Viruses by FcRn
3.4. FcRn Expression in Lung Macrophages
3.5. pH-Dependent Binding of IgG to FcRn on Mucosal Surfaces
4. What are the Mechanisms of ADE in Viral Infections and Following Antiviral Vaccinations, and how might an RSV F-Protein mAb with Increased Affinity to FcRn be Involved? Could mAb Binding to other FcγRs be Involved?
4.1. Immune System Disruption
4.2. Mechanisms of ADE of Viral Infection by Specific Anti-Viral Antibodies
4.3. The Role of FcγRs in ADE
4.4. Role of Complement
4.5. The Same Mechanisms are at Play for ADE in RSV Infection
4.5.1. Involvement of the Monocytic Lineage in ADE
4.5.2. ADE with RSV Antibodies May be Mediated by Macrophages and Phagocytic Cells
4.5.3. Importance of Antibody Levels and Quality: ADE can Occur in the Presence of Low Levels of Strongly Neutralizing RSV Antibodies
5. How Were the Factors Likely to Cause ADE with Nirsevimab Assessed?
5.1. Pharmacokinetics
5.2. Study of Nirsevimab Binding to FcγR In Vitro and Ex-Vivo and of Possible ADE in Animals by Manufacturers
6. Clinical trial Results and 2023-2024 Campaign
6.1. Clinical Trial Results
6.1.1. Phase 1 and 2a
6.1.2. Results of Phase 2b and 3 Trials
6.1.3. Deaths in Trials
6.2. Pharmacovigilance Data
6.3. Results of the 2023-2024 Season Immunization Campaign
6.4. Stillbirths in France
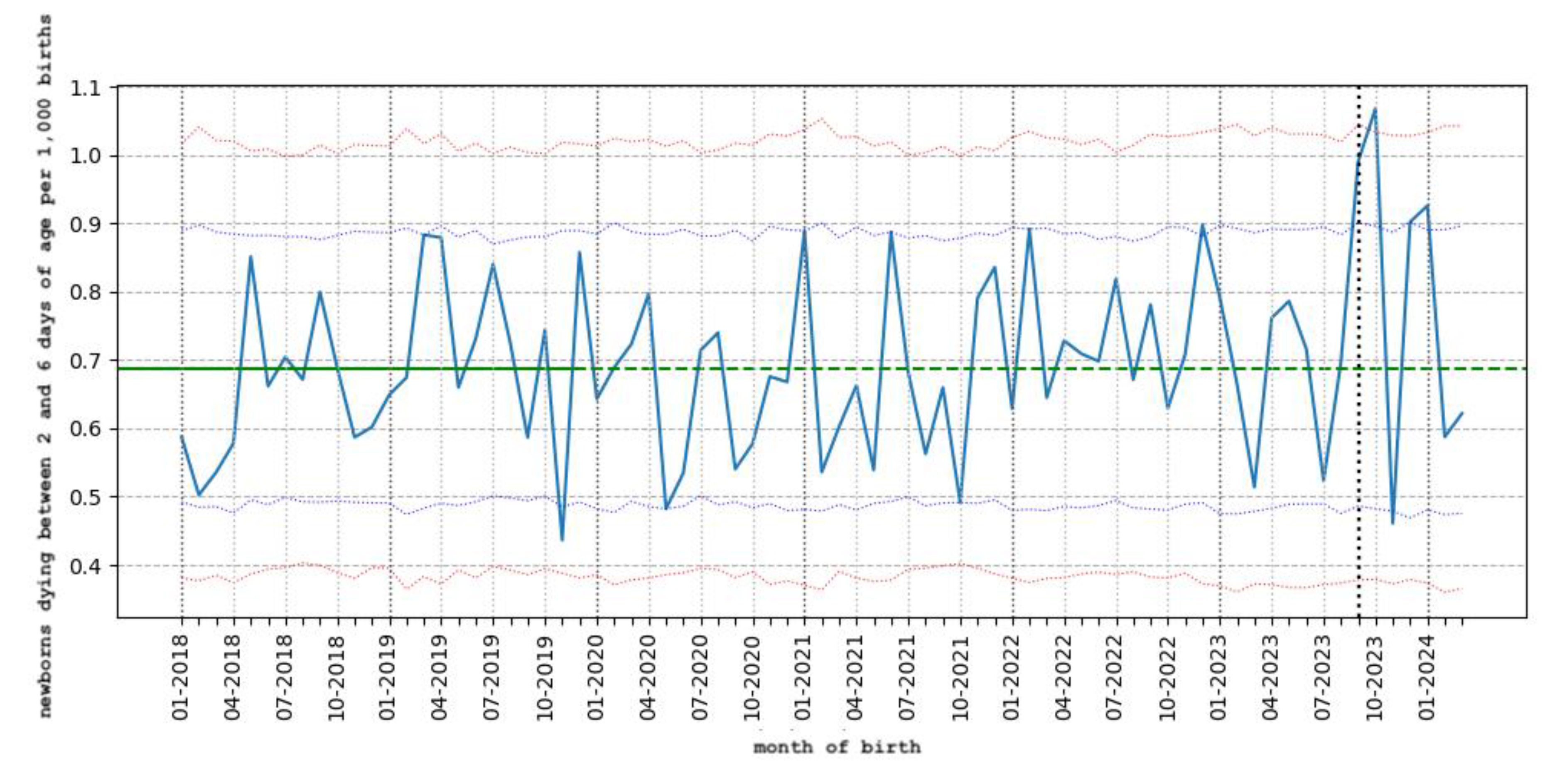
7. Discussion
7.1. Analysis of the Results of the Clinical Trials and the 2023-2024 Beyfortus Immunization Campaign
7.1.1. Clinical Trials
7.1.2. Results of the 2023-2024 Season Immunization Campaign
7.2. Deficiencies in Preclinical In Vitro Exploration of ADE
7.2.1. Gaps Remain in the Study of the Effector Functions of Nirsevimab In Vitro
7.2.2. Animal Studies In Vivo
7.2.3. The Pharmacokinetic Study is Incomplete and Shows Periods when Nirsevimab Levels may be Sub-Neutralizing in Some Individuals
7.3. Economic Benefits of Nirsevimab: Price and All-Cause Hospitalization Rates
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, Madhi SA, Omer SB, Simões EAF, Campbell H, Pariente AB, Bardach D, Bassat Q, Casalegno JS, Chakhunashvili G, Crawford N, Danilenko D, Do LAH, Echavarria M, Gentile A, Gordon A, Heikkinen T, Huang QS, Jullien S, Krishnan A, Lopez EL, Markić J, Mira-Iglesias A, Moore HC, Moyes J, Mwananyanda L, Nokes DJ, Noordeen F, Obodai E, Palani N, Romero C, Salimi V, Satav A, Seo E, Shchomak Z, Singleton R, Stolyarov K, Stoszek SK, von Gottberg A, Wurzel D, Yoshida LM, Yung CF, Zar HJ; Respiratory Virus Global Epidemiology Network; Nair H; RESCEU investigators. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet. 2022 May 28;399(10340):2047-2064. Epub 2022 May 19. PMCID: PMC7613574. https://pubmed.ncbi.nlm.nih.gov/35598608/. [CrossRef] [PubMed]
- Rocca A, Biagi C, Scarpini S, Dondi A, Vandini S, Pierantoni L, Lanari M. Passive Immunoprophylaxis against Respiratory Syncytial Virus in Children: Where Are We Now? Int J Mol Sci. 2021 Apr 2;22(7):3703. [CrossRef] [PubMed]
- Osiowy C, Horne D, Anderson R. Antibody-dependent enhancement of respiratory syncytial virus infection by sera from young infants. Clin Diagn Lab Immunol. 1994 Nov;1(6):670-7. [CrossRef] [PubMed]
- Zhu Q, McLellan JS, Kallewaard NL, Ulbrandt ND, Palaszynski S, Zhang J, Moldt B, Khan A, Svabek C, McAuliffe JM, Wrapp D, Patel NK, Cook KE, Richter BWM, Ryan PC, Yuan AQ, Suzich JA. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med. 2017 May 3;9(388):eaaj1928. [CrossRef] [PubMed]
- Acevedo OA, Díaz FE, Beals TE, Benavente FM, Soto JA, Escobar-Vera J, González PA, Kalergis AM. Contribution of Fcγ Receptor-Mediated Immunity to the Pathogenesis Caused by the Human Respiratory Syncytial Virus. Front Cell Infect Microbiol. 2019 Mar 29;9:75. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6450440/. [CrossRef] [PubMed]
- Diethelm-Varela B, Soto JA, Riedel CA, Bueno SM, Kalergis AM. New Developments and Challenges in Antibody-Based Therapies for the Respiratory Syncytial Virus. Infect Drug Resist. 2023 Apr 8;16:2061-2074. [CrossRef] [PubMed]
- Rigter A, Widjaja I, Versantvoort H, Coenjaerts FE, van Roosmalen M, Leenhouts K, Rottier PJ, Haijema BJ, de Haan CA. A protective and safe intranasal RSV vaccine based on a recombinant prefusion-like form of the F protein bound to bacterium-like particles. PLoS ONE. 2013 Aug 12;8(8):e71072. [CrossRef] [PubMed]
- Kampmann B, Madhi SA, Munjal I, Simões EAF, Pahud BA, Llapur C, Baker J, Pérez Marc G, Radley D, Shittu E, Glanternik J, Snaggs H, Baber J, Zachariah P, Barnabas SL, Fausett M, Adam T, Perreras N, Van Houten MA, Kantele A, Huang LM, Bont LJ, Otsuki T, Vargas SL, Gullam J, Tapiero B, Stein RT, Polack FP, Zar HJ, Staerke NB, Duron Padilla M, Richmond PC, Koury K, Schneider K, Kalinina EV, Cooper D, Jansen KU, Anderson AS, Swanson KA, Gruber WC, Gurtman A; MATISSE Study Group. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. Epub 2023 Apr 5. https://pubmed.ncbi.nlm.nih.gov/37018474/. [CrossRef] [PubMed]
- European Union Risk Management Plan (EU RMP) for Beyfortus® (Nirsevimab) 3 May 2021. Available online: https://www.ema.europa.eu/en/documents/rmp-summary/beyfortus-epar-risk-management-plan_en.pdf (accessed on April 25, 2024).
- Rocca A, Biagi C, Scarpini S, Dondi A, Vandini S, Pierantoni L, Lanari M. Passive Immunoprophylaxis against Respiratory Syncytial Virus in Children: Where Are We Now? Int J Mol Sci. 2021 Apr 2;22(7):3703. [CrossRef] [PubMed]
- Sanders SL, Agwan S, Hassan M, van Driel ML, Del Mar CB. Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection. Cochrane Database Syst Rev. 2019. https://doi.org/10.1002/14651858.CD009417.pub2 [PMC free article] [PubMed] [CrossRef] [Google Scholar].
- Resch B. Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection. Hum Vaccin Immunother. 2017 Sep 2;13(9):2138-2149. Epub 2017 Jun 12. PMCID: PMC5612471 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5612471/. [CrossRef] [PubMed]
- Roopenian DC, Akilesh S. FcRn: The neonatal Fc receptor comes of age. Nat Rev Immunol. 2007 Sep;7(9):715-25. Epub 2007 Aug 17. https://pubmed.ncbi.nlm.nih.gov/17703228/. [CrossRef] [PubMed]
- Patel DD, Bussel JB. Neonatal Fc receptor in human immunity: Function and role in therapeutic intervention. J Allergy Clin Immunol. 2020 Sep;146(3):467-478. https://pubmed.ncbi.nlm.nih.gov/32896307/. [CrossRef] [PubMed]
- Dall’Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem. 2006 Aug 18;281(33):23514-24. Epub 2006 Jun 21. PMID: 16793771 https://pubmed.ncbi.nlm.nih.gov/16793771/. [CrossRef]
- Borrok MJ, Wu Y, Beyaz N, Yu XQ, Oganesyan V, Dall’Acqua WF, Tsui P. pH-dependent binding engineering reveals an FcRn affinity threshold that governs IgG recycling. J Biol Chem. 2015 Feb 13;290(7):4282-90. Epub 2014 Dec 23. PMCID: PMC4326836. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4326836/. [CrossRef] [PubMed]
- Qi T, Cao Y. In Translation: FcRn across the Therapeutic Spectrum. Int J Mol Sci. 2021 Mar 17;22(6):3048. PMCID: PMC8002405. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8002405/. [CrossRef] [PubMed]
- Crowe JE Jr. Human Antibodies for Viral Infections. Annu Rev Immunol. 2022 Apr 26;40:349-386. Epub 2022 Feb 3. PMID: 35113730. https://pubmed.ncbi.nlm.nih.gov/35113730/. [CrossRef] [PubMed]
- Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, Lencer WI. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: Functional expression of FcRn in the mammalian lung. J Exp Med. 2002 Aug 5;196(3):303-10. Erratum in: J Exp Med. 2003 Jun 2;197(11):1601. PMCID: PMC2193935 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2193935/. [CrossRef] [PubMed]
- Pyzik M, Sand KMK, Hubbard JJ, Andersen JT, Sandlie I, Blumberg RS. The Neonatal Fc Receptor (FcRn): A Misnomer? Front Immunol. 2019 Jul 10;10:1540. [CrossRef] [PubMed]
- Schlachetzki F, Zhu C, Pardridge WM. Expression of the neonatal Fc receptor (FcRn) at the blood-brain barrier. J Neurochem. 2002 Apr;81(1):203-6. [CrossRef] [PubMed]
- Kim KJ, Fandy TE, Lee VH, Ann DK, Borok Z, Crandall ED. Net absorption of IgG via FcRn-mediated transcytosis across rat alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2004 Sep;287(3):L616-22. Epub 2004 May 28. https://pubmed.ncbi.nlm.nih.gov/15169676/. [CrossRef] [PubMed]
- Pyzik M, Kozicky LK, Gandhi AK, Blumberg RS. The therapeutic age of the neonatal Fc receptor. Nat Rev Immunol. 2023 Jul;23(7):415-432. Epub 2023 Feb 1. PMCID: PMC9891766. https://pubmed.ncbi.nlm.nih.gov/36726033/. [CrossRef] [PubMed]
- Challa DK, Wang X, Montoyo HP, Velmurugan R, Ober RJ, Ward ES. Neonatal Fc receptor expression in macrophages is indispensable for IgG homeostasis. MAbs. 2019 Jul;11(5):848-860. Epub 2019 Apr 30. PMCID: PMC6601554. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6601554/. [CrossRef] [PubMed]
- Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, Lencer WI. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: Functional expression of FcRn in the mammalian lung. J Exp Med. 2002 Aug 5;196(3):303-10. Erratum in: J Exp Med. 2003 Jun 2;197(11):1601. PMCID: PMC2193935 https://pubmed.ncbi.nlm.nih.gov/12163559/. [CrossRef] [PubMed]
- Ye L, Zeng R, Bai Y, Roopenian DC, Zhu X. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat Biotechnol. 2011 Feb;29(2):158-63. Epub 2011 Jan 16. PMCID: PMC3197702. https://pubmed.ncbi.nlm.nih.gov/21240266/. [CrossRef] [PubMed]
- Zhao X, Zhang G, Liu S, Chen X, Peng R, Dai L, Qu X, Li S, Song H, Gao Z, Yuan P, Liu Z, Li C, Shang Z, Li Y, Zhang M, Qi J, Wang H, Du N, Wu Y, Bi Y, Gao S, Shi Y, Yan J, Zhang Y, Xie Z, Wei W, Gao GF. Human Neonatal Fc Receptor Is the Cellular Uncoating Receptor for Enterovirus B. Cell. 2019 May 30;177(6):1553-1565.e16. Epub 2019 May 16. PMCID: PMC7111318. https://pubmed.ncbi.nlm.nih.gov/31104841/. [CrossRef] [PubMed]
- Morosky S, Wells AI, Lemon K, Evans AS, Schamus S, Bakkenist CJ, Coyne CB. The neonatal Fc receptor is a pan-echovirus receptor. Proc Natl Acad Sci U S A. 2019 Feb 26;116(9):3758-3763. Epub 2019 Feb 11. PMCID: PMC6397586. https://pubmed.ncbi.nlm.nih.gov/30808762/. [CrossRef] [PubMed]
- Burstin SJ, Brandriss MW, Schlesinger JJ. Infection of a macrophage-like cell line, P388D1 with reovirus; effects of immune ascitic fluids and monoclonal antibodies on neutralization and on enhancement of viral growth. J Immunol. 1983 Jun;130(6):2915-9. [PubMed]
- Maidji E, McDonagh S, Genbacev O, Tabata T, Pereira L. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am J Pathol. 2006 Apr;168(4):1210-26. PMCID: PMC1606573. [CrossRef] [PubMed]
- Gupta S, Gach JS, Becerra JC, Phan TB, Pudney J, Moldoveanu Z, Joseph SB, Landucci G, Supnet MJ, Ping LH, Corti D, Moldt B, Hel Z, Lanzavecchia A, Ruprecht RM, Burton DR, Mestecky J, Anderson DJ, Forthal DN. The Neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog. 2013;9(11):e1003776. Epub 2013 Nov 21. Erratum in: PLoS Pathog. 2013 Nov;9(11). 10.1371/annotation/31430955-703b-484a-96eb-e180f917d683. PMCID: PMC3836734. [CrossRef] [PubMed]
- Gupta S, Pegu P, Venzon DJ, Gach JS, Ma ZM, Landucci G, Miller CJ, Franchini G, Forthal DN. Enhanced in vitro transcytosis of simian immunodeficiency virus mediated by vaccine-induced antibody predicts transmitted/founder strain number after rectal challenge. J Infect Dis. 2015 Jan 1;211(1):45-52. Epub 2014 May 21. PMCID: PMC4334821. [CrossRef] [PubMed]
- Gonzalez OA, Sagar M. Antibodies and Acidic Environment Do Not Enhance HIV-1 Transcytosis. J Infect Dis. 2016 Oct 15;214(8):1221-4. Epub 2016 Aug 4. PMCID: PMC5034959. [CrossRef] [PubMed]
- Pyzik M, Rath T, Lencer WI, Baker K, Blumberg RS. FcRn: The Architect Behind the Immune and Nonimmune Functions of IgG and Albumin. J Immunol. 2015 May https://pubmed.ncbi.nlm.nih.gov/25934922/ 15;194(10):4595-603. [CrossRef] [PubMed]
- Schultz A, Puvvadi R, Borisov SM, Shaw NC, Klimant I, Berry LJ, Montgomery ST, Nguyen T, Kreda SM, Kicic A, Noble PB, Button B, Stick SM. Airway surface liquid pH is not acidic in children with cystic fibrosis. Nat Commun. 2017 Nov 10;8(1):1409. https://pubmed.ncbi.nlm.nih.gov/29123085/. [CrossRef] [PubMed]
- Fischer, H. Function of Proton Channels in Lung Epithelia. Wiley Interdiscip Rev Membr Transp Signal. 2012 May;1(3):247-258. Epub 2011 Oct 25. PMCID: PMC3362208. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3362208/. [CrossRef] [PubMed]
- Abou Alaiwa MH, Beer AM, Pezzulo AA, Launspach JL, Horan RA, Stoltz DA, Starner TD, Welsh MJ, Zabner J. Neonates with cystic fibrosis have a reduced nasal liquid pH; a small pilot study. J Cyst Fibros. 2014 Jul;13(4):373-7. Epub 2014 Jan 11. PMCID: PMC4060428. https://pubmed.ncbi.nlm.nih.gov/24418186/. [CrossRef] [PubMed]
- Garland AL, Walton WG, Coakley RD, Tan CD, Gilmore RC, Hobbs CA, Tripathy A, Clunes LA, Bencharit S, Stutts MJ, Betts L, Redinbo MR, Tarran R. Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):15973-8. Epub 2013 Sep 16. PMCID: PMC3791714. https://pubmed.ncbi.nlm.nih.gov/24043776/. [CrossRef] [PubMed]
- Vanderven HA, Kent SJ. Fc-mediated functions and the treatment of severe respiratory viral infections with passive immunotherapy - a balancing act. Front Immunol. 2023 Nov 22;14:1307398. PMCID: PMC10710136. https://pubmed.ncbi.nlm.nih.gov/38077353/. [CrossRef] [PubMed]
- Vogelzang A, Lozza L, Reece ST, Perdomo C, Zedler U, Hahnke K, Oberbeck-Mueller D, Dorhoi A, Kaufmann SH. Neonatal Fc Receptor Regulation of Lung Immunoglobulin and CD103+ Dendritic Cells Confers Transient Susceptibility to Tuberculosis. Infect Immun. 2016 Sep 19;84(10):2914-21. PMCID: PMC5038074. https://pubmed.ncbi.nlm.nih.gov/27481246/. [CrossRef] [PubMed]
- Cines DB, Zaitsev S, Rauova L, Rux AH, Stepanova V, Krishnaswamy S, Sarkar A, Kowalska MA, Zhao G, Mast AE, Blumberg LJ, McCrae KR, Poncz M, Hubbard JJ, Pyzik M, Blumberg RS. FcRn augments induction of tissue factor activity by IgG-containing immune complexes. Blood. 2020 Jun 4;135(23):2085-2093. PMCID: PMC7273830. https://pubmed.ncbi.nlm.nih.gov/32187355/. [CrossRef] [PubMed]
- Hubbard JJ, Pyzik M, Rath T, Kozicky LK, Sand KMK, Gandhi AK, Grevys A, Foss S, Menzies SC, Glickman JN, Fiebiger E, Roopenian DC, Sandlie I, Andersen JT, Sly LM, Baker K, Blumberg RS. FcRn is a CD32a coreceptor that determines susceptibility to IgG immune complex-driven autoimmunity. J Exp Med. 2020 Oct 5;217(10):e20200359. PMCID: PMC7537387. https://pubmed.ncbi.nlm.nih.gov/32658257/. [CrossRef] [PubMed]
- Taylor, 2015, Taylor A, Foo SS, Bruzzone R, Dinh LV, King NJ, Mahalingam S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol Rev. 2015 Nov;268(1):340-64. [CrossRef] [PubMed]
- Banoun H. Measles and Antibody-Dependent Enhancement (ADE): History and Mechanisms. Explor Res Hypothesis Med. 2022;7(4):246-252. https://www.xiahepublishing.com/2472-0712/ERHM-2022-00018. [CrossRef]
- Tirado SM, Yoon KJ. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16(1):69-86. https://pubmed.ncbi.nlm.nih.gov/12725690/. [CrossRef] [PubMed]
- Bournazos S, Gupta A, Ravetch JV. The role of IgG Fc receptors in antibody-dependent enhancement. Nat Rev Immunol. 2020 Oct;20(10):633-643. Epub 2020 Aug 11. PMCID: PMC7418887. [CrossRef] [PubMed]
- Golebski K, Hoepel W, van Egmond D, de Groot EJ, Amatngalim GD, Beekman JM, Fokkens WJ, van Drunen CM, den Dunnen J. FcγRIII stimulation breaks the tolerance of human nasal epithelial cells to bacteria through cross-talk with TLR4. Mucosal Immunol. 2019 Mar;12(2):425-433. Epub 2019 Jan 21. https://pubmed.ncbi.nlm.nih.gov/30664707/. [CrossRef] [PubMed]
- Acevedo OA, Díaz FE, Beals TE, Benavente FM, Soto JA, Escobar-Vera J, González PA, Kalergis AM. Contribution of Fcγ Receptor-Mediated Immunity to the Pathogenesis Caused by the Human Respiratory Syncytial Virus. Front Cell Infect Microbiol. 2019 Mar 29;9:75. PMCID: PMC6450440. https://pubmed.ncbi.nlm.nih.gov/30984626/. [CrossRef] [PubMed]
- Xu L, Ma Z, Li Y, Pang Z, Xiao S. Antibody dependent enhancement: Unavoidable problems in vaccine development. Adv Immunol 2021;151:99–133. [CrossRef] [PubMed]
- von Kietzell K, Pozzuto T, Heilbronn R, Grössl T, Fechner H, Weger S. Antibody-mediated enhancement of parvovirus B19 uptake into endothelial cells mediated by a receptor for complement factor C1q. J Virol 2014;88(14):8102–8115. [CrossRef] [PubMed]
- Lee CH, Romain G, Yan W, Watanabe M, Charab W, Todorova B, Lee J, Triplett K, Donkor M, Lungu OI, Lux A, Marshall N, Lindorfer MA, Goff OR, Balbino B, Kang TH, Tanno H, Delidakis G, Alford C, Taylor RP, Nimmerjahn F, Varadarajan N, Bruhns P, Zhang YJ, Georgiou G. IgG Fc domains that bind C1q but not effector Fcγ receptors delineate the importance of complement-mediated effector functions. Nat Immunol. 2017 Aug;18(8):889-898. Epub 2017 Jun 12. Erratum in: Nat Immunol. 2017 Sep 19;18(10 ):1173. PMCID: PMC6015732. [CrossRef] [PubMed]
- Polack FP, Teng MN, Collins PL, Prince GA, Exner M, Regele H; et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med (2002) 196(6):859–65. [CrossRef]
- Barr FE, Pedigo H, Johnson TR, Shepherd VL. Surfactant protein-A enhances uptake of respiratory syncytial virus by monocytes and U937 macrophages. Am J Respir Cell Mol Biol. 2000 Nov;23(5):586-92. [CrossRef] [PubMed]
- Polack FP, Alvarez-Paggi D, Libster R, Caballero MT, Blair RV, Hijano DR, de la Iglesia Niveyro PX, Menendez DR, Gladwell W, Avendano LM, Velozo L, Wanek A, Bergel E, Prince GA, Kleeberger SR, Johnson J, Pociask D, Kolls JK. Fatal enhanced respiratory syncytial virus disease in toddlers. Sci Transl Med. 2021 Oct 20;13(616):eabj7843. Epub 2021 Oct 20. PMCID: PMC10712289 https://pubmed.ncbi.nlm.nih.gov/34669442/. [CrossRef] [PubMed]
- Halstead SB, Venkateshan CN, Gentry MK, Larsen LK. Heterogeneity of infection enhancement of dengue 2 strains by monoclonal antibodies. J Immunol. 1984 Mar;132(3):1529-32. https://pubmed.ncbi.nlm.nih.gov/6607288/. [PubMed]
- Thomas S, Smatti MK, Ouhtit A, Cyprian FS, Almaslamani MA, Thani AA, Yassine HM. Antibody-Dependent Enhancement (ADE) and the role of complement system in disease pathogenesis. Mol Immunol. 2022 Dec;152:172-182. Epub 2022 Nov 10. PMCID: PMC9647202 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9647202/. [CrossRef] [PubMed]
- Tirado SM, Yoon KJ. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16(1):69-86. https://pubmed.ncbi.nlm.nih.gov/12725690/. [CrossRef] [PubMed]
- Gimenez HB, Keir HM, Cash P. In vitro enhancement of respiratory syncytial virus infection of U937 cells by human sera. J Gen Virol. 1989 Jan;70 ( Pt 1):89-96. https://pubmed.ncbi.nlm.nih.gov/2732688/. [CrossRef] [PubMed]
- Krilov LR, Anderson LJ, Marcoux L, Bonagura VR, Wedgwood JF. Antibody-mediated enhancement of respiratory syncytial virus infection in two monocyte/macrophage cell lines. J Infect Dis. 1989 Nov;160(5):777-82. https://pubmed.ncbi.nlm.nih.gov/2809253/. [CrossRef] [PubMed]
- Osiowy C, Horne D, Anderson R. Antibody-dependent enhancement of respiratory syncytial virus infection by sera from young infants. Clin Diagn Lab Immunol. 1994 Nov;1(6):670-7. [CrossRef] [PubMed]
- Gómez RS, Ramirez BA, Céspedes PF, Cautivo KM, Riquelme SA, Prado CE, González PA, Kalergis AM. Contribution of Fcγ receptors to human respiratory syncytial virus pathogenesis and the impairment of T-cell activation by dendritic cells. Immunology. 2016 Jan;147(1):55-72. Epub 2015 Nov 6. PMCID: PMC4693880 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4693880/. [CrossRef] [PubMed]
- Cirino NM, Panuska JR, Villani A, Taraf H, Rebert NA, Merolla R, Tsivitse P, Gilbert IA. Restricted replication of respiratory syncytial virus in human alveolar macrophages. J Gen Virol. 1993 Aug;74 ( Pt 8):1527-37. [CrossRef] [PubMed]
- Wang Y, Zheng J, Wang X, Yang P, Zhao D. Alveolar macrophages and airway hyperresponsiveness associated with respiratory syncytial virus infection. Front Immunol. 2022 Oct 20;13:1012048. [CrossRef] [PubMed]
- Makris S, Bajorek M, Culley FJ, Goritzka M, Johansson C. Alveolar Macrophages Can Control Respiratory Syncytial Virus Infection in the Absence of Type I Interferons. J Innate Immun. 2016;8(5):452-63. Epub 2016 Jul 16. PMCID: PMC5322584. [CrossRef] [PubMed]
- Kecse-Nagy C, Szittner Z, Papp K, Hegyi Z, Rovero P, Migliorini P, Lóránd V, Homolya L, Prechl J. Characterization of NF-κB Reporter U937 Cells and Their Application for the Detection of Inflammatory Immune-Complexes. PLoS ONE. 2016 May 27;11(5):e0156328. [CrossRef] [PubMed]
- Gimenez HB, Chisholm S, Dornan J, Cash P. Neutralizing and enhancing activities of human respiratory syncytial virus-specific antibodies. Clin Diagn Lab Immunol. 1996 May;3(3):280-6. PMCID: PMC170331. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC170331/. [CrossRef] [PubMed]
- Thomas S, Smatti MK, Ouhtit A, Cyprian FS, Almaslamani MA, Thani AA, Yassine HM. Antibody-Dependent Enhancement (ADE) and the role of complement system in disease pathogenesis. Mol Immunol. 2022 Dec;152:172-182. Epub 2022 Nov 10. PMCID: PMC9647202 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9647202/. [CrossRef] [PubMed]
- Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Balmaseda A, Harris E. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017 Nov 17;358(6365):929-932. Epub 2017 Nov 2. PMCID: PMC5858873. https://pubmed.ncbi.nlm.nih.gov/29097492/. [CrossRef] [PubMed]
- van Mechelen L, Luytjes W, de Haan CA, Wicht O. RSV neutralization by palivizumab, but not by monoclonal antibodies targeting other epitopes, is augmented by Fc gamma receptors. Antiviral Res. 2016 Aug;132:1-5. Epub 2016 May 13. https://pubmed.ncbi.nlm.nih.gov/27185625/. [CrossRef] [PubMed]
- Rigter A, Widjaja I, Versantvoort H, Coenjaerts FE, van Roosmalen M, Leenhouts K, Rottier PJ, Haijema BJ, de Haan CA. A protective and safe intranasal RSV vaccine based on a recombinant prefusion-like form of the F protein bound to bacterium-like particles. PLoS ONE. 2013 Aug 12;8(8):e71072. PMCID: PMC3741363. https://pubmed.ncbi.nlm.nih.gov/23951084/. [CrossRef] [PubMed]
- Jang MJ, Kim YJ, Hong S, Na J, Hwang JH, Shin SM, Ahn YM. Positive association of breastfeeding on respiratory syncytial virus infection in hospitalized infants: A multicenter retrospective study. Clin Exp Pediatr. 2020 Apr;63(4):135-140. Epub 2019 Nov 12. PMCID: PMC7170789. [CrossRef] [PubMed]
- Pasittungkul S, Thongpan I, Vichaiwattana P, Thongmee T, Klinfueng S, Suntronwong N, Wanlapakorn N, Vongpunsawad S, Poovorawan Y. High seroprevalence of antibodies against human respiratory syncytial virus and evidence of respiratory syncytial virus reinfection in young children in Thailand. Int J Infect Dis. 2022 Dec;125:177-183. Epub 2022 Nov 1. [CrossRef] [PubMed]
- Griffin MP, Khan AA, Esser MT, Jensen K, Takas T, Kankam MK, Villafana T, Dubovsky F. Safety, Tolerability, and Pharmacokinetics of MEDI8897, the Respiratory Syncytial Virus Prefusion F-Targeting Monoclonal Antibody with an Extended Half-Life, in Healthy Adults. Antimicrob Agents Chemother. 2017 Feb 23;61(3):e01714-16. [CrossRef] [PubMed]
- EMA-EPAR Beyfortus-Nirsevimab, Available on line : https://www.ema.europa.eu/en/medicines/human/EPAR/beyfortus#assessment-history, https://web.archive.org/web/20240105135355/https://www.ema.europa.eu/en/medicines/human/EPAR/beyfortus#assessment-history (accessed on April 15, 2024).
- Haraya, K., Tachibana, T. Translational Approach for Predicting Human Pharmacokinetics of Engineered Therapeutic Monoclonal Antibodies with Increased FcRn-Binding Mutations. BioDrugs 37, 99–108 (2023). [CrossRef]
- Domachowske JB, Khan AA, Esser MT, Jensen K, Takas T, Villafana T, Dubovsky F, Griffin MP. Safety, Tolerability and Pharmacokinetics of MEDI8897, an Extended Half-life Single-dose Respiratory Syncytial Virus Prefusion F-targeting Monoclonal Antibody Administered as a Single Dose to Healthy Preterm Infants. Pediatr Infect Dis J. 2018 Sep;37(9):886-892. [CrossRef] [PubMed]
- Hammitt LL, Dagan R, Yuan Y, Baca Cots M, Bosheva M, Madhi SA, Muller WJ, Zar HJ, Brooks D, Grenham A, Wählby Hamrén U, Mankad VS, Ren P, Takas T, Abram ME, Leach A, Griffin MP, Villafana T; MELODY Study Group. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N Engl J Med. 2022 Mar 3;386(9):837-846. https://pubmed.ncbi.nlm.nih.gov/35235726/. [CrossRef] [PubMed]
- Simões EAF, Madhi SA, Muller WJ, Atanasova V, Bosheva M, Cabañas F, Baca Cots M, Domachowske JB, Garcia-Garcia ML, Grantina I, Nguyen KA, Zar HJ, Berglind A, Cummings C, Griffin MP, Takas T, Yuan Y, Wählby Hamrén U, Leach A, Villafana T. Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: A pooled analysis of randomised controlled trials. Lancet Child Adolesc Health. 2023 Mar;7(3):180-189. Epub 2023 Jan 9. PMCID: PMC9940918. https://pubmed.ncbi.nlm.nih.gov/36634694/. [CrossRef] [PubMed]
- Munoz FM, Cramer JP, Dekker CL, Dudley MZ, Graham BS, Gurwith M, Law B, Perlman S, Polack FP, Spergel JM, Van Braeckel E, Ward BJ, Didierlaurent AM, Lambert PH; Brighton Collaboration Vaccine-associated Enhanced Disease Working Group. Vaccine-associated enhanced disease: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021 May 21;39(22):3053-3066. Epub 2021 Feb 23. PMCID: PMC7901381. https://pubmed.ncbi.nlm.nih.gov/33637387/. [CrossRef] [PubMed]
- Dall’Acqua WF, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, Wu H, Kiener PA, Langermann S. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: Biological consequences. J Immunol. 2002 Nov 1;169(9):5171-80. https://pubmed.ncbi.nlm.nih.gov/12391234/. [CrossRef] [PubMed]
- Cingoz O. Motavizumab. MAbs. 2009 Sep-Oct;1(5):439-42. https://doi.org/10.4161/mabs.1.5.9496. Epub 2009 Sep 10. Erratum in: MAbs. 2010 Sep-Oct;2(5):591. PMID: 20065632; PMCID: PMC2759493.
- Brady T, Cayatte C, Roe TL, Speer SD, Ji H, Machiesky L, Zhang T, Wilkins D, Tuffy KM, Kelly EJ. Fc-mediated functions of nirsevimab complement direct respiratory syncytial virus neutralization but are not required for optimal prophylactic protection. Front Immunol. 2023 Oct 11;14:1283120. https://doi.org/10.3389/fimmu.2023.1283120. PMID: 37901217; PMCID: PMC10600457 https://pubmed.ncbi.nlm.nih.gov/37901217/.
- Booth BJ, Ramakrishnan B, Narayan K, Wollacott AM, Babcock GJ, Shriver Z, Viswanathan K. Extending human IgG half-life using structure-guided design. MAbs. 2018 Oct;10(7):1098-1110. https://doi.org/10.1080/19420862.2018.1490119. Epub 2018 Jul 26. PMID: 29947573; PMCID: PMC6204840. https://pubmed.ncbi.nlm.nih.gov/29947573/.
- US-FDA FDA Approves New Drug to Prevent RSV in Babies and Toddlers, Available on line : https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers. https://web.archive.org/web/20230801224229/https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers accessed April 15,2024.
- Jones JM, Fleming-Dutra KE, Prill MM; et al. Use of Nirsevimab for the Prevention of Respiratory Syncytial Virus Disease Among Infants and Young Children: Recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72:920–925. https://doi.org/.
- HAS nirsevimab Transparency Committee, 19 July 2023, accessed April 22, 2024. https://www.has-sante.fr/plugins/ModuleXitiKLEE/types/FileDocument/doXiti.jsp?id=p_3476336. https://web.archive.org/web/20240104100601/https://www.has-sante.fr/upload/docs/application/pdf/2023-11/beyfortus_19072023_summary_ct20356_en.pdf.
- Sánchez Luna M, Fernández Colomer B, Couce Pico ML; en representación de la Junta Directiva de la Sociedad española de Neonatología SENEO Comisión de Infecciones SENEO y Comisión de Estándares de SENEO. Recommendations of the Spanish Society of Neonatology for the prevention of severe respiratory syncytial virus infections with nirsevimab, for the 2023-2024 season. An Pediatr (Engl Ed). 2023 Oct;99(4):264-265. https://doi.org/10.1016/j.anpede.2023.09.005. Epub 2023 Sep 20. PMID: 37739825. https://pubmed.ncbi.nlm.nih.gov/37739825/.
- New immunisation to protect newborns and young children against bronchiolitis, The Luxembourg Governement press release, September 22, 2023 accessed May 17, 2024, https://gouvernement.lu/en/actualites/toutes_actualites/communiques/2023/09-septembre/22-immunisation-bronchiolite-nourrissons.html. https://web.archive.org/web/20230922222249/https://gouvernement.lu/en/actualites/toutes_actualites/communiques/2023/09-septembre/22-immunisation-bronchiolite-nourrissons.html.
- Domachowske JB, Khan AA, Esser MT, Jensen K, Takas T, Villafana T, Dubovsky F, Griffin MP. Safety, Tolerability and Pharmacokinetics of MEDI8897, an Extended Half-life Single-dose Respiratory Syncytial Virus Prefusion F-targeting Monoclonal Antibody Administered as a Single Dose to Healthy Preterm Infants. Pediatr Infect Dis J. 2018 Sep;37(9):886-892. https://doi.org/10.1097/INF.0000000000001916. PMID: 29373476; PMCID: PMC6133204.
- Domachowske J, Madhi SA, Simões EAF, Atanasova V, Cabañas F, Furuno K, Garcia-Garcia ML, Grantina I, Nguyen KA, Brooks D, Chang Y, Leach A, Takas T, Yuan Y, Griffin MP, Mankad VS, Villafana T; MEDLEY Study Group. Safety of Nirsevimab for RSV in Infants with Heart or Lung Disease or Prematurity. N Engl J Med. 2022 Mar 3;386(9):892-894. https://doi.org/10.1056/NEJMc2112186. PMID: 35235733. https://pubmed.ncbi.nlm.nih.gov/35235733/.
- Drysdale SB, Cathie K, Flamein F, Knuf M, Collins AM, Hill HC, Kaiser F, Cohen R, Pinquier D, Felter CT, Vassilouthis NC, Jin J, Bangert M, Mari K, Nteene R, Wague S, Roberts M, Tissières P, Royal S, Faust SN; HARMONIE Study Group. Nirsevimab for Prevention of Hospitalizations Due to RSV in Infants. N Engl J Med. 2023 Dec 28;389(26):2425-2435. https://doi.org/10.1056/NEJMoa2309189. PMID: 38157500. https://pubmed.ncbi.nlm.nih.gov/38157500/.
- Griffin MP, Yuan Y, Takas T, Domachowske JB, Madhi SA, Manzoni P, Simões EAF, Esser MT, Khan AA, Dubovsky F, Villafana T, DeVincenzo JP; Nirsevimab Study Group. Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N Engl J Med. 2020 Jul 30;383(5):415-425. https://doi.org/10.1056/NEJMoa1913556. Erratum in: N Engl J Med. 2020 Aug 13;383(7):698. PMID: 32726528. https://pubmed.ncbi.nlm.nih.gov/32726528/.
- FDA Biologics License Application (BLA) 761328 Nirsevimab Antimicrobial Drugs Advisory Committee Meeting June 8, 2023 Division of Antivirals, Office of Infectious Diseases Center for Drug Evaluation and Research, accessed April 13,2024 https://www.fda.gov/media/169322/download. https://web.archive.org/web/20230613110427/.
- EudraVigilance European database of suspected adverse drug reaction reports. https://www.adrreports.eu/fr/eudravigilance.html accessed 15 April 2024.
- Pharmacovigilance d'Île de France, campagne d'immunisation contre le VRS-Beyfortus, 31 janvier 2024, accessed April 22,2024 https://www.pharmacovigilance-iledefrance.fr/d%C3%A9tails-dune-br%C3%A8ve/campagne-dimmunisation-contre-le-vrs-beyfortus-nirs%C3%A9vimab. https://web.archive.org/web/20240223011807/https://www.pharmacovigilance-iledefrance.fr/détails-dune-brève/campagne-dimmunisation-contre-le-vrs-beyfortus-nirsévimab.
- CDC, Nirsevimab Coverage, Children 0 to 19 months, United States. Data are current through February 29, 2024.accessed April 15,2024 https://www.cdc.gov/vaccines/imz-managers/coverage/rsvvaxview/nirsevimab-coverage-children-0-19months.html.
- RSV-NET Interactive Dashboard, CDC, accessed April15,2024 https://www.cdc.gov/rsv/research/rsv-net/dashboard.html.
- Moline HL et al., Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024 https://www.cdc.gov/mmwr/volumes/73/wr/mm7309a4.htm?s_cid=mm7309a4_w.
- Ernst C, Bejko D, Gaasch L, Hannelas E, Kahn I, Pierron C, Del Lero N, Schalbar C, Do Carmo E, Kohnen M, Andlauer E, Hublart P, Masi S, de la Fuente Garcia I, Vergison A, Mossong J. Impact of nirsevimab prophylaxis on paediatric respiratory syncytial virus (RSV)-related hospitalisations during the initial 2023/24 season in Luxembourg. Euro Surveill. 2024 Jan;29(4):2400033. https://doi.org/10.2807/1560-7917.ES.2024.29.4.2400033. PMID: 38275017; PMCID: PMC10986653.
- DGS-URGENT 9, 08/24/2023, DGS-URGENT N°2023_14, PREVENTION MEDICAMENTEUSE DES BRONCHIOLITES A VRS A PARTIR DE SEPTEMBRE accessed August 30, 2023, available on line :.https://sante.gouv.fr/IMG/pdf/dgs-urgent_2023-14_-_traitement_preventif_vrs.pdf. https://web.archive.org/web/20240202032041/https://sante.gouv.fr/IMG/pdf/dgs-urgent_2023-14_-_traitement_preventif_vrs.pdf.
- HAS Bronchiolite : La HAS publie des réponses rapides pour accompagner l’administration du Beyfortus® Communiqué de presse - 14 sept. 2023, available on line: https://www.has-sante.fr/jcms/p_3461146/fr/bronchiolite-la-has-publie-des-reponses-rapides-pour-accompagner-l-administration-du-beyfortus accessed April 22,2024. https://web.archive.org/web/20240104084538/https://www.has-sante.fr/jcms/p_3461146/fr/bronchiolite-la-has-publie-des-reponses-rapides-pour-accompagner-l-administration-du-beyfortus.
- HAS Assessing health technologies, summary, nirsevimab, Original French opinion adopted by the Transparency Committee on 19 July 2023, available on line : https://www.has-sante.fr/plugins/ModuleXitiKLEE/types/FileDocument/doXiti.jsp?id=p_3476336. https://web.archive.org/web/20240104100601/https://www.has-sante.fr/upload/docs/application/pdf/2023-11/beyfortus_19072023_summary_ct20356_en.pdf accessed april 22, 2024.
- DGS Beyfortus, fin de la campagne de distribution, 12/26/2023, reference MARS n° 2023-23, limited diffusion. https://sante.gouv.fr/professionnels/article/dgs-urgent accessed January 12,2024.
- INSEE Bilan démographique 2023, January 16,2024, available on line https://www.insee.fr/fr/statistiques/7750004 accessed on June 4, 2024.
- Bulletin Infections Respiratoires Aigües, publié le 17 avril 2024, https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/grippe/documents/bulletin-national/infections-respiratoires-aigues-grippe-bronchiolite-covid-19-.-bilan-de-la-saison-2023-2024, accessed April 22,2024.
- Bronchiolite : Bilan de la surveillance hivernale 2022-2023, Santé Publique France, July 19, 2023, accessed May 1, 2024. https://www.santepubliquefrance.fr/les-actualites/2023/bronchiolite-bilan-de-la-surveillance-hivernale-2022-2023.
- Paireau J., Durand C., Raimbault S., Cazaubon J., Mortamet G.; et al.. Nirsevimab effectiveness against cases of respiratory syncytial virus bronchiolitis hospitalised in pediatric intensive care units in France, September 2023 -January 2024. 2024. pasteur-04501286 https://hal.science/pasteur-04501286 March 12, 2024.
- La sanidad pública paga 209 euros por cada vacuna infantil contra la bronquiolitis, Civio, October 30, 2023, available on line : https://civio.es/medicamentalia/2023/10/30/nirsevimab-beyfortus-precio-virus-respiratorio-sincitial/. https://web.archive.org/web/20231103052634/https://civio.es/medicamentalia/2023/10/30/nirsevimab-beyfortus-precio-virus-respiratorio-sincitial/ accessed April 23,2024.
- Servicio Andaluz de Salud, El Distrito Serranía recibe un reconocimiento por la cobertura de la vacuna combinada frente a difteria, tétanos, tosferina y poliomielitis 30 January 2024, Available on line : https://www.sspa.juntadeandalucia.es/servicioandaluzdesalud/todas-noticia/el-distrito-serrania-recibe-un-reconocimiento-por-la-cobertura-de-la-vacuna-combinada-frente https://web.archive.org/web/20240602170353/https://www.sspa.juntadeandalucia.es/servicioandaluzdesalud/todas-noticia/el-distrito-serrania-recibe-un-reconocimiento-por-la-cobertura-de-la-vacuna-combinada-frente accessed February 10 2024.
- Administradas 452.000 vacunas por virus respiratorios, Ondacero.es ; April 8,2024, Available on line :. https://www.ondacero.es/emisoras/asturias/noticias/administradas-452000-vacunas-virus-respiratorios_202404086613a4ec099903000111cfb3.html. https://web.archive.org/web/20240602170546/https://www.ondacero.es/emisoras/asturias/noticias/administradas-452000-vacunas-virus-respiratorios_202404086613a4ec099903000111cfb3.html accessed April 23, 2024.
- La Comunidad de Madrid reduce un 90% los ingresos hospitalarios de menores de un año tras incorporar la vacuna contra la bronquiolitis, Communiqué de presse de la Comunidad de Madrid, April 16,2024, Available on line : https://www.comunidad.madrid/notas-prensa/2024/04/16/comunidad-madrid-reduce-90-ingresos-hospitalarios-menores-ano-incorporar-vacuna-bronquiolitis. https://web.archive.org/web/20240602170719/https://www.comunidad.madrid/notas-prensa/2024/04/16/comunidad-madrid-reduce-90-ingresos-hospitalarios-menores-ano-incorporar-vacuna-bronquiolitis accessed April 23,2024.
- Ministerio de ciencia, innovacion y universidades, Gobierno de Espana, Informes semanales de vigilancia centinela de IRAs y de IRAG: Gripe, Covid-19 y otros virus respiratorios, Available on line : https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/VIGILANCIA-CENTINELA-DE-INFECCION-RESPIRATORIA-AGUDA.aspx. https://web.archive.org/web/20240122052000/https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/GRIPE/Informes%20semanales/Temporada_2023-24/Informe%20semanal_SiVIRA_022024.pdf. https://web.archive.org/web/20230316142333/https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/GRIPE/Informes%20semanales/Temporada_2022-23/Informe%20semanal_SiVIRA_102023.pdf accessed April 23, 2024.
- FOLLOW-UP REPORT ON IMMUNIZATION WITH NIRSEVIMAB IN GALICIA Dirección Xeral de Saúde Pública Data up to week 13, 2024 (31-03-2024), Available on line : https://www.nirsegal.es/en. https://web.archive.org/web/20240409062929/https://assets-global.website-files.com/65774b0d3a50ee58b24dba82/660e95b8142542e4cae669d2_Report_RSV_week13.pdf accessed April 23, 2024.
- Ares-Gómez S, Mallah N, Santiago-Pérez MI, Pardo-Seco J, Pérez-Martínez O, Otero-Barrós MT, Suárez-Gaiche N, Kramer R, Jin J, Platero-Alonso L, Alvárez-Gil RM, Ces-Ozores OM, Nartallo-Penas V, Mirás-Carballal S, Piñeiro-Sotelo M, Malvar-Pintos A, González-Pérez JM, Rodríguez-Tenreiro-Sánchez C, Rivero-Calle I, Salas A, Durán-Parrondo C, Martinón-Torres F; NIRSE-GAL study group. Effectiveness and impact of universal prophylaxis with nirsevimab in infants against hospitalisation for respiratory syncytial virus in Galicia, Spain: Initial results of a population-based longitudinal study. Lancet Infect Dis. 2024 Apr 30:S1473-3099(24)00215-9. https://doi.org/10.1016/S1473-3099(24)00215-9. Epub ahead of print. PMID: 38701823. https://pubmed.ncbi.nlm.nih.gov/38701823/.
- Evaluation of the Effectiveness and Impact of Nirsevimab Administered as Routine Immunization (NIRSE-GAL), accessed May 10, 2024, https://classic.clinicaltrials.gov/ct2/show/NCT06180993. https://web.archive.org/web/20240602172604/https://classic.clinicaltrials.gov/ct2/show/NCT06180993.
- Ezpeleta G, Navascués A, Viguria N, Herranz-Aguirre M, Juan Belloc SE, Gimeno Ballester J, Muruzábal JC, García-Cenoz M, Trobajo-Sanmartín C, Echeverria A, Martínez-Baz I, Vera-Punzano N, Casado I, López-Mendoza H, Ezpeleta C, Castilla J. Effectiveness of Nirsevimab Immunoprophylaxis Administered at Birth to Prevent Infant Hospitalisation for Respiratory Syncytial Virus Infection: A Population-Based Cohort Study. Vaccines (Basel). 2024 Apr 4;12(4):383. https://doi.org/10.3390/vaccines12040383. PMID: 38675765; PMCID: PMC11054679. https://pubmed.ncbi.nlm.nih.gov/38675765/.
- Boletin de Salud Publica de Navarra, n°126, September 2023, Available on line http://www.navarra.es/home_es/Gobierno+de+Navarra/Organigrama/Los+departamentos/Salud/Organigrama/Estructura+Organica/Instituto+Navarro+de+Salud+Publica/Publicaciones/Publicaciones+profesionales/Epidemiologia/Boletin+ISP.htm. https://web.archive.org/web/20231129071427/http://www.navarra.es/NR/rdonlyres/AECCD760-AB2A-4841-818A-FA53478FD6DC/488194/BOL126INT1.pdf accessed May 1, 2024.
- DREES, La Naissance : Caractéristiques des accouchements, Available on line: https://drees.solidarites-sante.gouv.fr/sites/default/files/2021-07/Fiche%2024%20-%20La%20naissance%20-%20caract%C3%A9ristiques%20des%20accouchements.pdf. https://web.archive.org/web/20231206003500/https://drees.solidarites-sante.gouv.fr/sites/default/files/2021-07/Fiche%2024%20-%20La%20naissance%20-%20caractéristiques%20des%20accouchements.pdf accessed April 23, 2024.
- INSEE Décès et Mortalité, Available on line : https://www.insee.fr/fr/statistiques/7767420?sommaire=7764286. et https://www.insee.fr/fr/statistiques/6959517?sommaire=4487854 , accessed April 30, 2024.
- Nombre mensuel de naissances (de janvier 2015 à octobre 2023), Available on line : https://www.insee.fr/fr/statistiques/7758827?sommaire=5348638 et https://www.insee.fr/fr/statistiques/8064935?sommaire=7944361 , accessed April 30,2024.
- Article 14(9) EC-726/2004 of the European Parliament and of the Council of 31 March 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency, Available on line : http://data.europa.eu/eli/reg/2004/726/oj. https://web.archive.org/web/20231030191458/https://eur-lex.europa.eu/eli/reg/2004/726/oj accessed May 15,2024.
- Nair H, Simões EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JSF, Feikin DR, Mackenzie GA, Moiïsi JC, Roca A, Baggett HC, Zaman SM, Singleton RJ, Lucero MG, Chandran A, Gentile A, Cohen C, Krishnan A, Bhutta ZA, Arguedas A, Clara AW, Andrade AL, Ope M, Ruvinsky RO, Hortal M, McCracken JP, Madhi SA, Bruce N, Qazi SA, Morris SS, El Arifeen S, Weber MW, Scott JAG, Brooks WA, Breiman RF, Campbell H; Severe Acute Lower Respiratory Infections Working Group. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: A systematic analysis. Lancet. 2013 Apr 20;381(9875):1380-1390. https://doi.org/10.1016/S0140-6736(12)61901-1. Epub 2013 Jan 29. PMID: 23369797; PMCID: PMC3986472. https://pubmed.ncbi.nlm.nih.gov/23369797/.
- Del Riccio M, Spreeuwenberg P, Osei-Yeboah R, Johannesen CK, Fernandez LV, Teirlinck AC, Wang X, Heikkinen T, Bangert M, Caini S, Campbell H, Paget J; RESCEU Investigators. Burden of Respiratory Syncytial Virus in the European Union: Estimation of RSV-associated hospitalizations in children under 5 years. J Infect Dis. 2023 Nov 28;228(11):1528-1538. https://doi.org/10.1093/infdis/jiad188. PMID: 37246724; PMCID: PMC10681872 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10681872/.
- Suss RJ, Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024 Apr 1;7(4):e247125. https://doi.org/10.1001/jamanetworkopen.2024.7125. PMID: 38635270. https://pubmed.ncbi.nlm.nih.gov/38635270/.
- Sullivan K, Sullivan B. Does nirsevimab prevent lower respiratory infections caused by respiratory syncytial virus? J Perinatol. 2024 May;44(5):767-769. https://doi.org/10.1038/s41372-024-01970-y. Epub 2024 Apr 18. PMID: 38637681; PMCID: PMC11090781. https://pubmed.ncbi.nlm.nih.gov/38637681/.
- Vaux S, Viriot D, Forgeot C, Pontais I, Savitch Y, Barondeau-Leuret A, Smadja S, Valette M, Enouf V, Parent du Chatelet I. Bronchiolitis epidemics in France during the SARS-CoV-2 pandemic: The 2020-2021 and 2021-2022 seasons. Infect Dis Now. 2022 Sep;52(6):374-378. https://doi.org/10.1016/j.idnow.2022.06.003. Epub 2022 Jun 23. PMID: 35753628; PMCID: PMC9222408. https://pubmed.ncbi.nlm.nih.gov/35753628/.
- Delestrain C, Danis K, Hau I, Behillil S, Billard MN, Krajten L, Cohen R, Bont L, Epaud R. Impact of COVID-19 social distancing on viral infection in France: A delayed outbreak of RSV. Pediatr Pulmonol. 2021 Dec;56(12):3669-3673. Epub 2021 Sep 2. PMID: 34473914; PMCID: PMC8662089. https://pubmed.ncbi.nlm.nih.gov/34473914/. [CrossRef]
- International Classification of Diseases, Tenth Revision, International Statistical Classification of Diseases and Related Health Problems 10th Revision latest version 2019, WHO, Available on line : https://icd.who.int/browse10/2019/en accessed May 12, 2024.
- Journal Officiel, France, 12/231/2023, available on line : https://www.senat.fr/questions/jopdf/2023/2023-12-21_seq_20230050_0001_p000.pdf, accessed on June 3, 2024.
- EPI-PHARE, Epidémiologie des produits de santé, GIS ANSM-CNAM, Rapport d’étude d’utilisation du Nirsévimab (Beyfortus®) en ville en France lors de la première campagne de prévention (saison 2023/2024) 8 avril 2024, available on line : https://www.epi-phare.fr/rapports-detudes-et-publications/utilisation-beyfortus/ https://web.archive.org/web/20240603171635/https://www.epi-phare.fr/rapports-detudes-et-publications/utilisation-beyfortus/ accessed on June 3, 2024.
- Dekkers G, Treffers L, Plomp R, Bentlage AEH, de Boer M, Koeleman CAM, Lissenberg-Thunnissen SN, Visser R, Brouwer M, Mok JY, Matlung H, van den Berg TK, van Esch WJE, Kuijpers TW, Wouters D, Rispens T, Wuhrer M, Vidarsson G. Decoding the Human Immunoglobulin G-Glycan Repertoire Reveals a Spectrum of Fc-Receptor- and Complement-Mediated-Effector Activities. Front Immunol. 2017 Aug 2;8:877. https://doi.org/10.3389/fimmu.2017.00877. PMID: 28824618; PMCID: PMC5539844.
- Hiatt A, Bohorova N, Bohorov O, Goodman C, Kim D, Pauly MH, Velasco J, Whaley KJ, Piedra PA, Gilbert BE, Zeitlin L. Glycan variants of a respiratory syncytial virus antibody with enhanced effector function and in vivo efficacy. Proc Natl Acad Sci U S A. 2014 Apr 22;111(16):5992-7. https://doi.org/10.1073/pnas.1402458111. Epub 2014 Apr 7. PMID: 24711420; PMCID: PMC4000855.
- Zheng K, Bantog C, Bayer R. The impact of glycosylation on monoclonal antibody conformation and stability. MAbs. 2011 Nov-Dec;3(6):568-76. https://doi.org/10.4161/mabs.3.6.17922. Epub 2011 Nov 1. PMID: 22123061; PMCID: PMC3242843.
- Liu L. Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins. J Pharm Sci. 2015 Jun;104(6):1866-1884. https://doi.org/10.1002/jps.24444. Epub 2015 Apr 14. PMID: 25872915.
- Mokhtary 2022 Mokhtary P, Pourhashem Z, Mehrizi AA, Sala C, Rappuoli R. Recent Progress in the Discovery and Development of Monoclonal Antibodies against Viral Infections. Biomedicines. 2022 Aug 2;10(8):1861. PMCID: PMC9405509. https://pubmed.ncbi.nlm.nih.gov/36009408/. [CrossRef] [PubMed]
- Taylor G. Animal models of respiratory syncytial virus infection. Vaccine. 2017 Jan 11;35(3):469-480. https://doi.org/10.1016/j.vaccine.2016.11.054. Epub 2016 Nov 29. PMID: 27908639; PMCID: PMC5244256.
- FDA full prescribing information Beyfortus, Reference ID : 5203445, Available on line : https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761328s000lbl.pdf. https://web.archive.org/web/20230804203015/https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761328s000lbl.pdf accessed May 28, 2024.
- van Erp EA, Luytjes W, Ferwerda G, van Kasteren PB. Fc-Mediated Antibody Effector Functions During Respiratory Syncytial Virus Infection and Disease. Front Immunol. 2019 Mar 22;10:548. https://doi.org/10.3389/fimmu.2019.00548. PMID: 30967872; PMCID: PMC6438959. https://pubmed.ncbi.nlm.nih.gov/30967872/.
- Shoukat A, Abdollahi E, Galvani AP, Halperin SA, Langley JM, Moghadas SM. Cost-effectiveness analysis of nirsevimab and maternal RSVpreF vaccine strategies for prevention of Respiratory Syncytial Virus disease among infants in Canada: A simulation study. Lancet Reg Health Am. 2023 Nov 9;28:100629. PMID: 38026446; PMCID: PMC10663690. https://pubmed.ncbi.nlm.nih.gov/38026446/. [CrossRef]
- Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI) Statement on the prevention of respiratory syncytial virus (RSV) disease in infants: Supplementary systematic review of economic evidence, Available on line : https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/vaccines-immunization/national-advisory-committee-immunization-statement-prevention-respiratory-syncytial-virus-disease-infants-supplementary-systematic-review-economic-evidence/naci-appendix-2024-05-17.pdf. https://web.archive.org/web/20240521111714/https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/vaccines-immunization/national-advisory-committee-immunization-statement-prevention-respiratory-syncytial-virus-disease-infants-supplementary-systematic-review-economic-evidence/naci-appendix-2024-05-17.pdf accessed May 26, 2024.
- Neumann S, Alverson B. Nirsevimab: The Hidden Costs. Hosp Pediatr. 2024 May 9;14(6):e2024007739. PMID: 38721666. https://pubmed.ncbi.nlm.nih.gov/38721666/. [CrossRef]
- AAP news, Sanofi raising price of RSV immunization nirsevimab April 12, 2024, Available on line : https://publications.aap.org/aapnews/news/28657/Sanofi-raising-price-of-RSV-immunization. https://web.archive.org/web/20240530142639/https://publications.aap.org/aapnews/news/28657/Sanofi-raising-price-of-RSV-immunization accessed April 20, 2024.
- BEYFORTUS 50 mg sol inj ser préremplie, Vidal, Available on line : https://www.vidal.fr/medicaments/beyfortus-50-mg-sol-inj-ser-preremplie-242583.html. https://web.archive.org/web/20240526204604/https://www.vidal.fr/medicaments/beyfortus-50-mg-sol-inj-ser-preremplie-242583.html accessed May 26, 2024.
- Circuit de prescription du Nirsevimab (BEYFORTUS ®) ARS de Bretagne, Available on line: https://www.bretagne.ars.sante.fr/system/files/2023-09/Presentation%20Dr%20Lefevre.pdf. https://web.archive.org/web/20240329150151/https://www.bretagne.ars.sante.fr/system/files/2023-09/Presentation%20Dr%20Lefevre.pdf accessed October 10, 2023.
- Gebremedhin B. Gebretekle, Man Wah Yeung, Raphael Ximenes, Alexandra Cernat, Alison E. Simmons, April Killikelly, Winnie Siu, Ellen Rafferty, Nicholas Brousseau, Matthew Tunis, Ashleigh R. Tuite medRxiv 2024.03.21.24304675. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




