Submitted:
10 June 2024
Posted:
11 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Which ML models have been included in the prediction of PE?
- Which ML model demonstrates the highest predictive capability?
- Which features are integrated into the individual ML models?
- Which features did the individual ML model identify to be of high predictive value?
- When are the individual ML models intended to be used during pregnancy?
- How frequently are the individual ML models intended to be deployed throughout pregnancy?
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Search Strategy
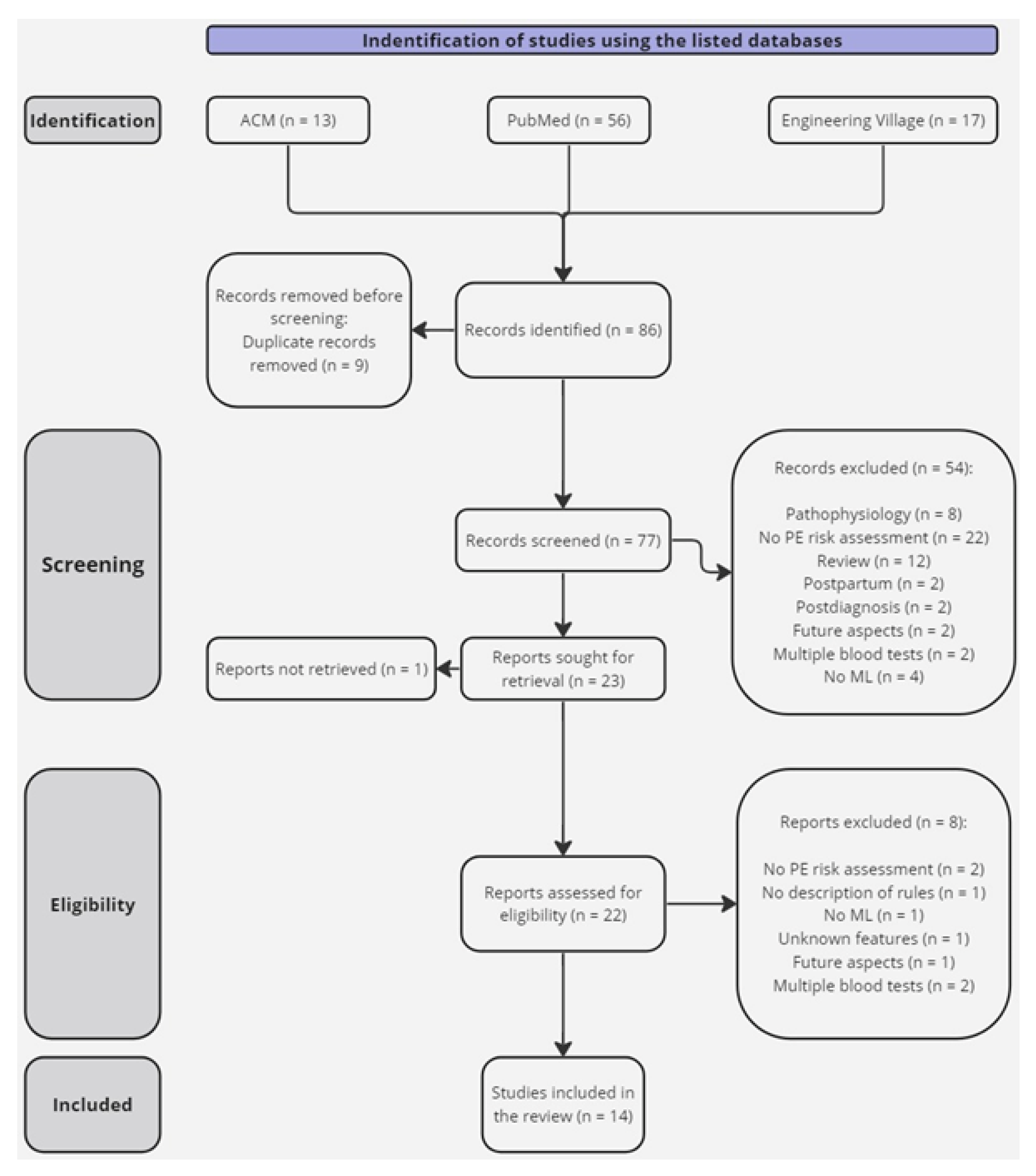
2.4. Selection Process
2.5. Data Collection
- Study characteristics: Study type, year of publication, and country.
- Participant information: Number of participants and the incidence of PE cases used for training, validation, and test sets in the ML models.
- Features: Variables used for training the ML model.
- ML models employed in the study.
- Best performance: Identifying the best-performing ML model and its prediction of PE subgroups. For those studies, where the prediction of PE has not been specified other than predicting PE, it has been denoted as predicting “All PE” within this review to compare across studies. The performance is evaluated using performance metrics (AUC, ROC, accuracy, sensitivity, recall, specificity, precision, F1-score, Brier score, screen positive rate (SPR), true positive (TP), true positive rate (TPR), detection rate (DR), false detection rate (FDR), false negative rate (FNR), and false positive (FP)). Among the listed terms, sensitivity, recall, and TPR refer to the same metric value, describing the prediction of positive cases from all the positive cases within the dataset [11].
- Top predictive features: The top five features identified by the individual ML model to be of high importance for predicting PE among its included features.
- The intended use of the ML model: Is either reported or interpreted from the study. Including the number of times the ML model is intended to be used and which gestational week within the pregnancy, if this has been denoted by the authors.
2.6. Risk of Bias
3. Results
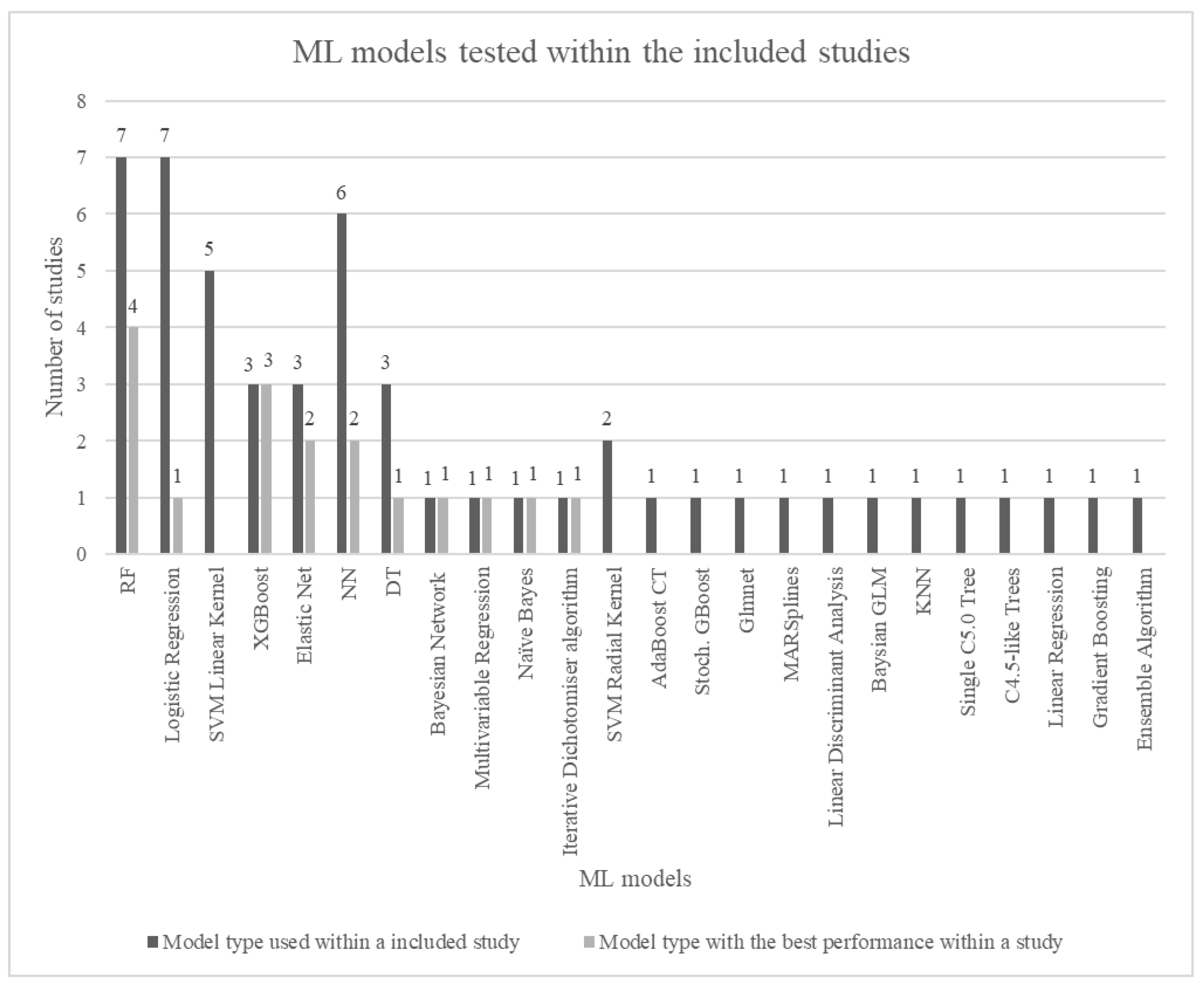
3.1. Performance of Machine Learning Models
4. Discussion
4.1. Best-Performing Machine Learning Model
4.2. Feature Selection
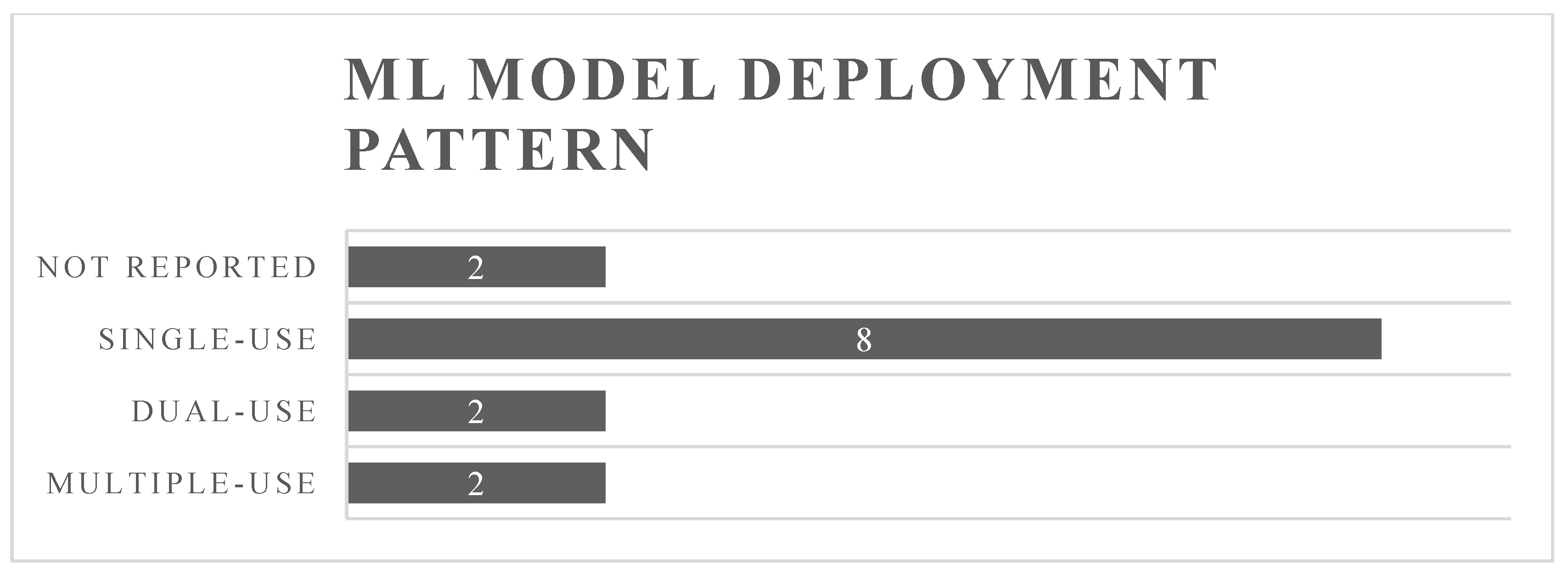
4.3. Machine Learning Deployment Pattern
4.4. Limitations
4.5. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Study | Features used in the machine learning model |
|---|---|
| FMF competing risk model [5] | Maternal factors: Age Heigh Weight Racial origin Conception method Smoking Chronic hypertension Diabetes mellitus Systemic lupus erythematosus Antiphospholipid syndrome Mother of the pregnant woman’s history of PE Parity Previous had PE Gestational age at prior birth Birthweight of the baby in last pregnancy Years between birth Estimated conception data MAP UtA-PI PlGF PAPP-A |
| A predictive Bayesian network model for home management of preeclampsia [12] | Values taken at each of the following gestational week: 12, 16, 20, 24, 28, 32, 36, 38, 40, and 42: Age Smoking Obese Chronic hypertension Parity-history PE Treatment Systolic BP Diastolic BP Hemoglobin Creatinine Protein/creatinine |
| Machine learning approach for preeclampsia risk factors association [13] | Duration of completed pregnancy in weeks. Toxemia Education (completed years of schooling) Highest completed year school or degree Pregnancy outcome Labor force status Poverty Water retention/edema Race Anemia Sex Birth order Birth weight One-minute and five-minute APGAR scores Month of pregnancy when prenatal care began Number of prenatal visits Weight gained during pregnancy Medical risk factors for the pregnancy Obstetric procedures performed Delivery complications Congenital anomalies and abnormalities Mother's marital status Number of live births now living The parents' age Hispanic origin State/country of birth |
| Preeclampsia Prediction Using machine learning and Polygenic Risk Scores From Clinical and Genetic Risk Factors in Early and Late Pregnancies [14] | Maternal age at delivery Self-reported race Relf-reported ethnicity (Hispanic or non-Hispanic) Hospital (tertiary or community) Gravidity Parity Gestational age at delivery Gestational age at preeclampsia diagnosis Last BMI before pregnancy BMI at delivery Maximal diastolic BP during pregnancy Maximal systolic BP during pregnancy Family history of chronic hypertension Family history of preeclampsia Interpregnancy interval In vitro fertilization Multiple gestation Smoking before pregnancy Drugs of abuse before pregnancy Drugs of abuse during pregnancy Alcohol use before pregnancy High-risk pregnancy Maximal BMI before pregnancy Mean BMI in the period 0-14 gestational weeks Systolic BP at first prenatal visit Diastolic BP at first prenatal visit History of pregestational diabetes History of kidney disease before pregnancy History of gestational diabetes in a prior pregnancy History of a prior high-risk pregnancy History of autoimmune disease History of preeclampsia in a prior pregnancy Family history of hypertension Family history of PE Minimal platelet count in the period 0-14 gestational weeks and in pregnancy before preeclampsia diagnosis or delivery Maximal uric acid in the period 0-14 gestational weeks and in pregnancy before preeclampsia diagnosis or delivery Presence of proteinuria in the period 0-14 gestational weeks and in pregnancy before preeclampsia diagnosis or delivery Systolic BP polygenic risk score Small for gestational age or intrauterine growth restriction Last BMI during pregnancy before preeclampsia diagnosis or delivery Maximal BMI before pregnancy Prescription of antihypertensive medication during pregnancy Diagnosis of gestational hypertension during pregnancy |
| Performance of a machine learning approach for the prediction of pre-eclampsia in a middle-income country [21] |
Maternal age Nulliparity Spontaneous pregnancy Induction of ovulation In-vitro fertilization Gestation age at screening Smoker Alcohol intake Other drugs (heroin or cocaine) Pre-existing diabetes Chronic hypertension Lupus Antiphospholipid syndrome Polycystic ovary syndrome Hypothyroidism Congenital heart disease PE in a previous pregnancy Fetal growth restriction in a previous pregnancy Mother of the patient had PE BMI MAP MAP (MoM) UtA-PI UtA-PI (MoM) PlGF PlGF (MoM) PAPP-A Gestational age at delivery |
| Validation of machine-learning model for first-trimester prediction of pre-eclampsia using cohort from PREVAL study. Based on the machine learning model trained by Ansbacher-Feldman et al. [23] | Maternal age Maternal weight Maternal height Gestation age at screening Racial origin Medical history: Chronic hypertension Diabetes type I Diabetes type II Systemic lupus erythematosus/antiphospholipid syndrome Smoker Family history of PE Method of conception: Spontaneous In-vitro fertilization Use of ovulation drugs Obstetric history: Nulliparous Parous, no previous PE Parous, previous PE Interpregnancy interval Aspirin MAP UtA-PI Serum concentration of pregnancy-associated plasma protein-A (PAPP-A) Serum concentration of PlGF |
| An interpretable longitudinal preeclampsia risk prediction using machine learning [15] | Maternal age Self-reported race Self-reported ethnicity (Hispanic or non-Hispanic) Private insurance Public insurance Alcohol use history Smoking history Illicit drugs history Gravidity Parity In vitro fertilization Nulliparous Interpregnancy interval Multiple gestation Maximal systolic BP: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Maximal diastolic BP: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Maximal heart rate: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Maximal BMI: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Maximal weight: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Family history of chronic hypertension Family history of preeclampsia Family history of diabetes Family history of heart disease Family history of hyperlipidemia Family history of stroke Past history of diabetes Past history of gestational diabetes Past history of cesarean delivery Past history of preterm birth Past history of gynecologic surgery Past history of asthma Past history of chronic hypertension Past history of gestational hypertension Past history of high-risk pregnancy Past history of hyperemesis gravidarum Past history of migraine Past history of obesity Past history of PE Past history of pregnancy related fatigue Past history of sexually transmitted disease Chronic hypertension Anemia during pregnancy Headaches during pregnancy Autoimmune disease High risk pregnancy Hyperemesis gravidarum Pregnancy related fatigue Oligohydramnios: At week 39 and admission Proteinuria: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Maximal aspartate transferase: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Maximal white blood count: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Maximal alanine transaminase: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Maximal serum calcium: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Maximal serum creatinine: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Maximal eosinophils: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Maximal serum glucose: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Maximal hemoglobin: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Maximal lymphocytes: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Maximal platelets: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Minimal red blood count: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks - admission Antihypertensive medications: 0-14 weeks 0-20 weeks 0-24 weeks 0-28 weeks 0-32 weeks 0-36 weeks 0-39 weeks 0 weeks – admission |
| Predictive Performance of machine learning-Based Methods for the Prediction of Preeclampsia-A Prospective Study [24] | Maternal age BMI Medium: Urban Rural Parity: Nulliparity Multiparity Smoking status during pregnancy The use of assisted reproductive technologies Personal or family history of PE Personal history of hypertension Personal history of renal disease Personal history of diabetes Personal history of systemic lupus erythematosus/antiphospholipid syndrome Hyperglycemia in pregnancy Obesity Interpregnancy interval MAP (MoM) UtA-PI (MoM) PAPP-A (MoM) PLGF (MoM) Placental protein-13 (MoM) |
| Dynamic gestational week prediction model for pre-eclampsia based on ID3 algorithm [16] | Static parameters: Multiple births Spontaneous miscarriage history History of hypertension in pregnancy History of diabetes mellitus Family history of hypertension Preconception BMI Dynamic parameters: Gestational week BMI during pregnancy Systolic BP Diastolic BP Pulse pressure MAP Pulse waveform area parameters Cardiac output Cardiac index Total peripheral resistance Hematocrit Mean platelet volume Platelet count Alanine aminotransferase Aspartate aminotransferase Creatinine Uric acid PlGF |
| Development of a prediction model on preeclampsia using machine learning-based method: a retrospective cohort study in China [17] | Maternal age Height Weight BMI Parity Method of conception Previous diagnosis of hypertension History of diabetes mellitus History of gestational diabetes History of PE History of fetal growth restriction MAP β-human chorionic gonadotropin PAPP-A Gestational age at screening Chronic hypertension Left uterine artery PI Right uterine artery PI Mean uterine artery PI |
| Novel electronic health records applied for prediction of pre-eclampsia: Machine-learning algorithms [18] | All features: Maternal age BMI Mean BP Maternal abdominal circumference Gravidity Parity PE in a previous pregnancy Prior cesarean delivery Pregnancy interval Nulliparity Multifetal gestations Assisted reproductive technology Pre-pregnancy diabetes Heart disease Thyroid disease Renal disease Autoimmune diseases Mental disorder Uterine leiomyoma Adenomyosis Uterine malfunctions History of seizure disorder Family history of hypertension Hemoglobin White blood cell count Platelet counts Direct bilirubin Total bilirubin Alanine aminotransferase Γ-glutamyl transferase Total protein Albumin Globulin Fasting plasma glucose Total bile acid Creatinine Serum urea nitrogen Serum uric acid Baseline risk features: Nulliparity Multifetal gestations PE in a previous pregnancy Pre-gestational diabetes BMI Maternal age Assisted reproductive technology Kidney diseases Autoimmune diseases Questionnaire features: Family history of hypertension Nulliparity Prior cesarean delivery Pregnancy interval Multifetal gestations Assisted reproductive technology Gravidity Parity Pre-gestational diabetes Heart disease Thyroid disease Renal disease Autoimmune diseases Mental disorder Uterine leiomyoma Adenomyosis Uterine malfunctions History of seizure disorder Maternal age BMI |
| Early prediction of preeclampsia via machine learning [25] | Maternal age Height weight Blood pressure: Mean systolic Mean diastolic Maximum systolic Maximum diastolic Race Ethnicity: Hispanic Non-Hispanic unknown Gravida: Nulliparous Multiparous Number of babies Medical history: PE Assisted reproductive treatment Chronic hypertension Diabetes (type I or type II) Obesity Renal disease Autoimmune conditions: Systemic lupus erythematosus Discoid lupus erythematosus Systemic sclerosis Rheumatoid arthritis Dermatomyositis Polymyositis Undifferentiated connective tissue disease Celiac disease Antiphospholipid syndrome Sexually transmitted diseases (human papillomavirus, chlamydia, genital herpes) Hyperemesis gravidarum Headache Migraine Poor obstetrics history Poor obstetrics history Medical history at 17 weeks of gestation: Gestational diabetes Anemia High-risk pregnancy Routine prenatal laboratory results: Protein from urine Glucose from urine Platelet count Red blood cells White blood cells Creatinine Hemoglobin Hematocrit Monocytes Lymphocytes Eosinophils Neutrophils Basophils Blood type with Rh Uric acid Rubella Varicella Hepatitis B Syphilis Chlamydia Gonorrhea Intake of medication: Aspirin Nifedipine Aldomet Labetalol Insulin Glyburide Prednisone Azathioprine Plaquenil Heparin Levothyroxine Doxylamine Acyclovir |
| Clinical risk assessment in early pregnancy for preeclampsia in nulliparous women: A population based cohort study [26] | Multivariable regression model: Family history of PE Country of birth Method of conception Gestational length Maternal age Height Weight Smoking in early pregnancy Pre-existing diabetes mellitus Chronic hypertension Systemic lupus erythematosus MAP Backward selection model and RF model: Gestational length first examination in weeks Maternal age BMI MAP Capillary glucose Protein in urine Hemoglobin Previous miscarriage Previous ectopic pregnancy Infertility duration Family situation: Single Living together with partner Other Region of birth: Sweden Nordic countries (except Sweden) Europe (except of Nordic countries) Africa North America South America Asia Oceania Smoking 3 months before pregnancy Smoking at registration Snuff 3 months before pregnancy Snuff at registration Alcohol consumption three months before registration Alcohol consumption at registration Family history of PE Family history of hypertension Infertility: Without treatment Ovary simulation In-vitro fertilization Cardiovascular disease Endocrine disease Pre-existing diabetes Thrombosis Psychiatric disease systemic lupus erythematosus Epilepsy Chronic hypertension Morbus Chron/Ulcerous colitis Lung disease or asthma Chronic kidney disease Hepatitis Gynecological disease or operation Recurrent urinary tract infections Blood group |
| Artificial intelligence-assisted prediction of preeclampsia: Development and external validation of a nationwide health insurance dataset of the BPJS Kesehatan in Indonesia [19] | Demographic: Age Marriage Family role Member strata Member type International Classification of Diseases 10th Revision coded diagnoses: A codes B codes C codes D codes E codes F codes G codes H codes I codes J codes K codes L codes M codes N codes Infection-related codes: G0, H00, H01, H10, H15, H16, H20, H30, H60, H65, H66, H67, H68, H70, I0, J0, J1, J2, J40, J41, J42, J85, J86, K12, K2, K35, K36, K37, K5, K65, K67, K73, K80, K81, L0, M00, M01, M02, N7 Immune-related codes: B20, D8, E10, G35, G61, G70, I0, J30, J31, J32, J35, J45, L2, L50, M04, M05, M06, M15, M16, M17, M18, M19, M3, M65, N00, N01, N03, N04 Nervous system-related codes: A8, C7, G Eye-related codes: C69, H0, H1, H2, H3, H4, H5 Ear-related codes: C30, D02, H6, H7, H8, H9 Heat-related codes: C38, I2, I3, I4, I5 Respiratory system-related codes: A1, C0, C3, J Digestive system-related codes: A0, C0, C1, K0, K1, K3, K4, K5, K6 Skin and subcutaneous-related codes: B0, B1, B8, C43, C44, L Musculoskeletal system-related codes: C40, C41, M Urinary system-related codes: C64, C65, C66, C67, C68, N0, N1, N2, N3 Reproduction system-related codes: A5, A60, A61, A62, A63, A64, C51, C52, C53, C54, C55, C56, C57, C58, N7, N8 Liver and pancreas-related codes: B15, B16, B17, B19, C22, C23, C24, C25, K7, K8 Breast-related codes: C50, N6 Vascular-related codes: I1, I7, I8 |
| Ethnicity as a Factor for the Estimation of the Risk for Preeclampsia: A Neural Network Approach [20] | MAP Uterine Pulsatility index PAPP-A Ethnicity Weight Height Smoking Alcohol consumption Previous PE Conception: Spontaneous Ovulation drug In-vitro fertilization Medical condition of pregnant woman Drugs taken by the pregnant woman Gestation age Crown rump length Mother had PE |
References
- L. Duley, “The Global Impact of Pre-eclampsia and Eclampsia,” Seminars in Perinatology, vol. 33, no. 3. pp. 130–137, Jun. 2009. [CrossRef]
- L. A. Magee, K. H. Nicolaides, and P. von Dadelszen, “Preeclampsia,” New England Journal of Medicine, vol. 386, no. 19, pp. 1817–1832, May 2022. [CrossRef]
- R. van Doorn et al., “Dose of aspirin to prevent preterm preeclampsia in women with moderate or high-risk factors: A systematic review and meta-analysis,” PLoS One, vol. 16, no. 3 March, Mar. 2021. [CrossRef]
- M. Hackelöer, L. Schmidt, and S. Verlohren, “New advances in prediction and surveillance of preeclampsia: role of machine learning approaches and remote monitoring,” Archives of Gynecology and Obstetrics, vol. 308, no. 6. Springer Science and Business Media Deutschland GmbH, pp. 1663–1677, Dec. 01, 2023. [CrossRef]
- D. Wright, A. Syngelaki, R. Akolekar, L. C. Poon, and K. H. Nicolaides, “Competing risks model in screening for preeclampsia by maternal characteristics and medical history,” Am J Obstet Gynecol, vol. 213, no. 1, pp. 62.e1-62.e10, Jul. 2015. [CrossRef]
- D. Wright, D. M. Gallo, S. Gil Pugliese, C. Casanova, and K. H. Nicolaides, “Contingent screening for preterm pre-eclampsia,” Ultrasound in Obstetrics and Gynecology, vol. 47, no. 5, pp. 554–559, May 2016. [CrossRef]
- A. Ranjbar, F. Montazeri, S. R. Ghamsari, V. Mehrnoush, N. Roozbeh, and F. Darsareh, “Machine learning models for predicting preeclampsia: a systematic review,” BMC Pregnancy Childbirth, vol. 24, no. 1, p. 6, Jan. 2024. [CrossRef]
- V. B. Brunelli and F. Prefumo, “Quality of first trimester risk prediction models for pre-eclampsia: A systematic review,” BJOG, vol. 122, no. 7, pp. 904–914, Jun. 2015. [CrossRef]
- Bertini, R. A. Salas, S. Chabert, L. Sobrevia, and F. Pardo, “Using Machine Learning to Predict Complications in Pregnancy: A Systematic Review,” Frontiers in Bioengineering and Biotechnology, vol. 9. Frontiers Media S.A., Jan. 19, 2022. [CrossRef]
- M. J. Page et al., “The PRISMA 2020 statement: an updated guideline for reporting systematic reviews,” Syst Rev, vol. 10, no. 1, Dec. 2021. [CrossRef]
- C. Ao, W. Zhou, L. Gao, B. Dong, and L. Yu, “Prediction of antioxidant proteins using hybrid feature representation method and random forest,” Genomics, vol. 112, no. 6, pp. 4666–4674, Nov. 2020. [CrossRef]
- M. Velikova, P. J. F. Lucas, and M. Spaanderman, “A Predictive Bayesian Network Model for Home Management of Preeclampsia,” in Peleg, M., Lavrač, N., Combi, C. (eds) Artificial Intelligence in Medicine. AIME 2011. Lecture Notes in Computer Science(LNAI,volume 6747), Springer, Berlin, Heidelberg, 2011, pp. 179–183. [CrossRef]
- A. Martínez-Velasco, L. Martínez-Villaseñor, and L. Miralles-Pechuán, “Machine learning approach for pre-eclampsia risk factors association,” in GOODTECHS 2018 – 4th EAI International Conference on Smart Objects and Technologies for Social Good, Association for Computing Machinery, Nov. 2018, pp. 232–237. [CrossRef]
- V. P. Kovacheva, B. W. Eberhard, R. Y. Cohen, M. Maher, R. Saxena, and K. J. Gray, “Preeclampsia Prediction Using Machine Learning and Polygenic Risk Scores from Clinical and Genetic Risk Factors in Early and Late Pregnancies,” Hypertension, vol. 81, no. 2, pp. 264–272, Feb. 2024. [CrossRef]
- B. W. Eberhard, R. Y. Cohen, J. Rigoni, D. W. Bates, K. J. Gray, and V. P. Kovacheva, “An Interpretable Longitudinal Preeclampsia Risk Prediction Using Machine Learning.,” medRxiv, Aug. 2023. [CrossRef]
- Z. Li et al., “Dynamic gestational week prediction model for pre-eclampsia based on ID3 algorithm,” Front Physiol, vol. 13, Oct. 2022. [CrossRef]
- M. Liu et al., “Development of a prediction model on preeclampsia using machine learning-based method: a retrospective cohort study in China,” Front Physiol, vol. 13, Aug. 2022. [CrossRef]
- Y. xin Li et al., “Novel electronic health records applied for prediction of pre-eclampsia: Machine-learning algorithms,” Pregnancy Hypertens, vol. 26, pp. 102–109, Dec. 2021. [CrossRef]
- H. Sufriyana, Y. W. Wu, and E. C. Y. Su, “Artificial intelligence-assisted prediction of preeclampsia: Development and external validation of a nationwide health insurance dataset of the BPJS Kesehatan in Indonesia,” EBioMedicine, vol. 54, Apr. 2020. [CrossRef]
- C. Neocleous, K. Nicolaides, K. Neokleous, and C. Schizas, “Ethnicity as a Factor for the Estimation of the Risk for Preeclampsia: A Neural Network Approach,” Springer-Verlag, 2010.
- J. Torres-Torres et al., “Performance of a machine learning approach for the prediction of pre-eclampsia in a middle-income country,” Ultrasound in Obstetrics & Gynecology, Sep. 2023. [CrossRef]
- M. M. Gil et al., “Validating a machine-learning model for first-trimester prediction of pre-eclampsia using the cohort from the PREVAL study,” Ultrasound in Obstetrics & Gynecology, Jan. 2023. [CrossRef]
- Z. Ansbacher-Feldman, A. Syngelaki, H. Meiri, R. Cirkin, K. H. Nicolaides, and Y. Louzoun, “Machine-learning-based prediction of pre-eclampsia using first-trimester maternal characteristics and biomarkers,” Ultrasound in Obstetrics and Gynecology, vol. 60, no. 6, pp. 739–745, Dec. 2022. [CrossRef]
- A. S. Melinte-Popescu, I. A. Vasilache, D. Socolov, and M. Melinte-Popescu, “Predictive Performance of Machine Learning-Based Methods for the Prediction of Preeclampsia—A Prospective Study,” J Clin Med, vol. 12, no. 2, Jan. 2023. [CrossRef]
- Marić et al., “Early prediction of preeclampsia via machine learning,” Am J Obstet Gynecol MFM, vol. 2, no. 2, May 2020. [CrossRef]
- A. Sandström, J. M. Snowden, J. Höijer, M. Bottai, and A. K. Wikström, “Clinical risk assessment in early pregnancy for preeclampsia in nulliparous women: A population based cohort study,” PLoS One, vol. 14, no. 11, Nov. 2019. [CrossRef]
- G. James, D. Witten, T. Hastie, and R. Tibshirani, “Statistical Learning,” in An Introduction to Statistical Learning with Applications in R, Second Edition., Springer, 2023, pp. 15–58.
- G. James, D. Witten, T. Hastie, and R. Tibshirani, “Linear Regression,” in An Introduction to Statistical Learning with Applications in R, Second Edition., Springer, 2023, pp. 59–128.
- M. Lazdam et al., “Unique Blood Pressure Characteristics in Mother and Offspring After Early Onset Preeclampsia,” pp. 1338–1345, 2012. [CrossRef]
- C. Macdonald-Wallis, D. A. Lawlor, A. Fraser, M. May, S. M. Nelson, and K. Tilling, “Preeclampsia Blood Pressure Change in Normotensive, Gestational Hypertensive, Preeclamptic, and Essential Hypertensive Pregnancies,” 2012. [CrossRef]
| Study (reference) | Author (Year of publication) |
Study type (country) |
Type of dataset: Participants (PE %) |
Features used for training. (full list is available in Appendix A) |
ML models included in the study |
Best performing ML model: Group of PE predicted: Time of use: listed performance metrics |
The ML models top five included features with high predictive value | Bias of the study | Deployment pattern of the ML model: when in pregnancy is the model described to be deployed |
|---|---|---|---|---|---|---|---|---|---|
| A predictive Bayesian network model for home management of preeclampsia [12] | Velikova M. et al. (2011) | Retrospective research (Netherlands) |
Training set: Using incidence rates and prior probabilities from literature, for risk factors, and gynecologist estimated measurements and research studies was used for measurements. Test set: 417 (7.9% PE) |
112 features from 10 checkups including: Age, smoking, obese, chronic hypertension, parity history of preeclampsia, blood pressure, hemoglobin, protein, and creatinine | Temporal Bayesian Network Model |
Temporal Bayesian Network Model: All PE: GA week 12: True-positive: 82% False-positive: 54% GA week 16: True-positive: 73% False-positive: 39% |
Not specified | Not listed all 112 features used for training the model. Data was not consistent, which lead to some missing values. Small test set. Not divided PE into subgroups. Retrospective study. |
Two times: GA week 12 and 16 Intended to be multiple times: not specified which gestations weeks |
| Machine Learning Approach for Pre-Eclampsia Risk Factors Association [13] |
Martínez-Velasco A. et al. (2018) | Retrospective cohort (Italy) |
Training and validation set: 1,634 (16.46% PE) |
25 features including poverty status, highest education, pregnancy in weeks, and water retention | RF AdaBoost CT Stoch. GBoost Glmnet MAR-Splines Linear Discriminant Analysis Bayesian GLM NN with Feature Extraction SVM Radial Kernel SVM Linear Kernel KNN Single C5.0 Tree Boosted Logistic Regression C4.5-like Trees |
RF: All PE: Not specified: ROC: 0.85 Accuracy: 85% Sensitivity: 68% Specificity: 86% Precision: 20% F1: 0.31 |
1. Gestation weeks completed 2. Poverty 3. Water retention/edema 4. Toxemia 5. Highest educational degree |
Not tested on a new dataset. Missing values were replaced by the specific features mean value. Not divided PE into subgroups. Retrospective study. |
One time: Not specified when |
| Preeclampsia Prediction Using machine learning and Polygenic Risk Scores From Clinical and Genetic Risk Factors in Early and Late Pregnancies [14] | Kovacheva VP. Et al. (2024) | Retrospective study (United States) |
Training and validation set: 1,125 (7.8% PE) |
Routinely collected features at first hospital visit: Demographic, smoking/drug use/alcohol use before pregnancy, BMI, systolic and diastolic BP. Additional feature: a hypertension genetic risk score. |
Logistic Regression XGBoost |
XGBoost without the additional feature: All PE: Before gestation week 14: AUC: 0.74 Accuracy: 91% Sensitivity: 97% Specificity: 26% Precision: 41% Before birth: AUC: 0.91 Accuracy: 93% Sensitivity: 97% Specificity: 43% Precision: 57% |
Shapley: 1. History of PE 2. Mean diastolic BP (<14 weeks) 3. Mean systolic BP (first prenatal visit) 4. History of renal disease 5. BMI |
Not divided PE into subgroups. Retrospective study. Not specified how the data set was used for training and what the performance is based on. |
Two times: one model for first prenatal visit and one model for before the delivery admission (not specified further) |
| An interpretable longitudinal preeclampsia risk prediction using machine learning1 [15] | Eberhard BW. Et al. (2023) | Cohort Retrospective (United States) |
Training set: 98,241 Test set: 22,511 External validation set: 7,705 Total: 120,752 (5.7% PE) |
Maternal risk factors, medications, insurance, vital signs, and procedural information data. All information that is routinely collected at week 14, 20, 24, 28, 32, 36, and 39. | XGBoost Deep NN Elastic Net RF Linear Regression |
XGBoost: External validation set: All PE: Gestation week 14 AUC: 0.63 Specificity: 78% Sensitivity: 62% PPV: 13% NPV: 95% Accuracy: 77% F1-score: 0.24 Gestation week 20: AUC: 0.64 Specificity: 79% Sensitivity: 64% PPV: 12% NPV: 0.96% Accuracy: 78 F1-score: 0.25 Gestation week 24: AUC: 0.67 Specificity: 85% Sensitivity: 37% PPV: 13% NPV: 96% Accuracy: 82% F1-score: 0.19 Gestation week 28: AUC: 0.69 Specificity: 84% Sensitivity: 40% PPV: 13% NPV: 96% Accuracy: 81% F1-score: 0.2 Gestation week 32: AUC: 0.71 Specificity: 83% Sensitivity: 44% PPV: 14% NPV: 96% Accuracy: 81% F1-score: 0.21 Gestation week 36: AUC: 0.76 Specificity: 85% Sensitivity: 49% PPV: 17% NPV: 96% Accuracy: 83% F1-score: 0.25 Gestation week 39: AUC: 0.86 Specificity: 88% Sensitivity: 66% PPV: 25% NPV: 98% Accuracy: 86% F1-score: 0.36 On admission: AUC: 0.9 Specificity: 88% Sensitivity: 75% PPV: 28% NPV: 98% Accuracy: 87% F1-score: 0.41 |
Shapley: 1. Diastolic and systolic BP 2. Maternal age 3. Insurance 4.Interpregnancy interval 5. chronic and gestational hypertension |
Not divided PE into subgroups. Retrospective study. | Multiple times: week 14, 20, 24, 28, 32, 36, 39, and on admission. They made a model for each time point. |
| Dynamic gestational week prediction model for pre-eclampsia based on ID3 algorithm [16] | Li Z. et al. (2023) | Case-control retrospective (China) |
Training set: 1,272 (18% PE) Test set: 546 (26% PE) Total: 1818 (20.4% PE) |
Maternal risk factors. Dynamic parameters include among others: gestational week, BMI, systolic and diastolic BP, pulse, MAP, hematocrit, platelet count, creatinine, uric acid, and PlGF. | Iterative Dichotomiser algorithm |
Iterative Dichotomiser algorithm: All PE: Macro average: Precision: 76% Recall: 73% F1-score: 75% Weighted average: Precision: 88% Recall: 89% F1-score: 89% |
Not specified | Not clarified which data set is used to evaluate the performance. Not clarified how the data sets are constructed with a study population of 932. Retrospective study. Not divided the prediction into PE subgroups. |
Multiple times: At prenatal visits at different gestational weeks. (not specified further) |
| Development of a prediction model on preeclampsia using machine learning-based method: a retrospective cohort study in China [17] |
Liu M. et al. (2022) | Cohort Retrospective study (China) |
Training set: 9,945 Test set: 1,105 Total: 11,050 (1.3% PE) |
Maternal risk factors, MAP, PAPP-A, β-human chorionic gonadotropin, and UtA-PI. Collected at first prenatal visit and at 6 weeks of gestation. | Deep Artificial NN DT Logistic Regression RF SVM Linear kernel |
RF: All PE: AUC: 0.86 Accuracy: 74% Precision: 82% Recall: 42% F1-score: 0.56 Brier score: 0.17 |
Not specified | Low number of PE cases. Not divided PE into subgroups. Retrospective study. |
One time: first prenatal visit (not specified further) |
| Novel electronic health records applied for prediction of pre-eclampsia: Machine-learning algorithms [18] | Li Y-x. et al. (2021) | Retrospective cohort study (China) |
Total: 3,759 (5.08% PE) |
38 features: demographics (age, BMI, mean BP), pregnancy history (parity, PE history), medical conditions (diabetes, hypertension history), and laboratory tests (hemoglobin, platelet counts, MAP) at early second trimester A simple model using 8 questions with 18 binary features (hypertension history, parity, diabetes) and 2 continuous features (maternal age and BMI) |
RF SVM Linear versus radial kernel XGBoost Logistic Regression |
XGBoost: All PE: All features: AUC: 0.96 Accuracy: 92 % F1-score: 0.57 Simple model: AUC: 0.84 Accuracy: 83% F1-score: 0.34 |
1. Fasting plasma glucose 2. Mean BP 3. BMI 4. Maternal abdominal circumference5. Serum uric acid |
Not divided PE into subgroups. Retrospective study. Not specified the sizes of the training, internal validation, and temporal validation sets. |
One time: early second trimester (not specified further) |
| Artificial intelligence-assisted prediction of preeclampsia: Development and external validation of a nationwide health insurance dataset of the BPJS Kesehatan in Indonesia [19] | Sufriyana H. et al. (2020) | Retrospective case-control study (Indonesia) |
Internal validation set: Cases 3,054, controls 17,921 External validation with geographic split: Cases 145, controls 1,177 External validation with temporal split: Cases 119, controls 785 Total: 23,201 (14,3% PE) |
Health insurance data: Demographic (age, family role, labor-type) and diagnoses (causes of disease and organ-related diseases) from one year before PE development and during gestation. For those with event times in 2015, diagnoses within 2 years prior the event was included together with the feature of time to event. |
Logistic Regression DT Artificial NN RF SVM Ensemble algorithm |
RF: All PE: External validation with geographical split: AUC: 0.76 Precision: 82% with FPR of 10% External validation with temporal split: AUC: 0.70 Precision: 78% with FPR of 10% External validation in subgroup geographical split (approximation from study figure): AUC 12-24 months before PE: 0.77 AUC 9-<12 months before PE: 0.88 AUC 6-<9 months before PE: 0.78 AUC 2 days – 6 months before PE: 0.75 External validation in subgroup temporal split (approximation from study figure): AUC 12-24 months before PE: 0.76 AUC 9-<12 months before PE: 0.86 AUC 6-<9 months before PE: 0.68 AUC 2 days – 6 months before PE: 0.67 |
Not specified | Demands health information that might not be available in the same databases. Retrospective study |
Not specified |
| Ethnicity as a Factor for the Estimation of the Risk for Preeclampsia: A Neural Network Approach. [20] | Neocleous KC et al. (2010) | Prospective study (England) |
Training set: 6793 (1,7% PE) Test set: 36 (44% PE) Verification set: 9 (56% PE) Total: 6838 (1.99% PE) |
MAP, Uterine pulsatility index (UPI), PAPP-A, weight, ethnicity, height, smoking, alcohol, drugs, conception, crown rump length, mother had PE, medical condition, previous PE, and gestation age | NN |
NN: All PE: Training set with ethnicity: Cases predicted: 45% PE cases predicted: 84% Training set without ethnicity: PE cases predicted: 85% Test set with ethnicity: Cases predicted: 72% PE cases predicted: 94% Test set without ethnicity: PE cases predicted: 100% Verification set with ethnicity: Cases predicted: 78% PE cases predicted: 100% Verification set without ethnicity: PE cases predicted: 100% |
Not specified | Did not use common performance metric values to evaluate the model. | Not specified |
| Performance of a machine learning approach for the prediction of pre-eclampsia in a middle-income country [21] |
Torres-Torres J. et al. (2023) | Prospective cohort study (Mexico) |
Training set: 1,068 Validation set: 914 Test set: 1,068 Total: 3,050 (4.07% PE) |
Maternal characteristics (age, smoking, other drugs (heroin or cocaine), alcohol intake, BMI, congenital heart disease, hypothyroidism, polycystic ovary syndrome, and PE in previous pregnancy), MOM of: MAP, UtA-PI, and PlGF. | Elastic Net |
Elastic Net: All PE: AUC: 0.78 DR: 50% at 10% FPR Early-onset (<34 gestation weeks): AUC: 0.96 DR: 88% at 10% FPR Pre-term PE (<37 gestation weeks): AUC: 0.90 DR: 77% at 10% FPR |
Regularization Coefficient: 1. PlGF 2. MAP 3. UtA-PI 4. BMI 5. APS |
Only including high risk patients who did not adhere to aspirin treatment | One time: first trimester (not specified further) |
| Validation of machine-learning model for first-trimester prediction of pre-eclampsia using cohort from PREVAL study [22] | Gil MM. et al. (2024) | Validation using prospective cohort data (Spain) |
Training set: 30,352 Validation set: 10,000 Test set: 20,352 External test set (PREVAL): 10,110 (2.27% PE) |
Maternal risk factors, MAP, UtA-PI, PlGF, and PAPP-A. Using the raw data and not MoM. | Feed-Forward NN with two hidden layers compared to FMF |
NN: All PE: AUC: 0.85 DR: 56% at 10% SPR (without PAPP-A) Early-onset PE (<34 gestation weeks): AUC: 0.92 DR: 84% at 10% SPR (without PAPP-A) Pre-term PE (<37 gestation weeks): AUC: 0.91 DR: 78% at 10% SPR (without PAPP-A) |
Not specified by Gil et al. According to the developer of the ML model Ansbacher-Feldman et al.[23] using Shapley: 1. MAP 2. UtA-PI 3. PlGF 4. Racial origin 5. Chronic hypertension |
Small number of PE cases. 6% of the patients took aspirin. Similar or less detection rate compared to FMF. |
One time: first prenatal visit specified by Ansbacher-Feldman et al. [23]. (not specified further) |
| Predictive Performance of machine learning-Based Methods for the Prediction of Preeclampsia-A Prospective Study [24] |
Melinte-Popescu A-S et al. (2023) | Prospective case-control study (Romania) |
Training set: 163 Test set: 70 Total: 233 (50% PE) |
Maternal risk factors (age, BMI, community (urban or rural), personal history of renal disease, obesity, and hyperglycemia) and MoM of: MAP, UtA-PI, PAPP-A, PlGF, and Placental protein-13 collected at first trimester. | DT Naïve Bayes SVM with Linear Kernel RF |
Naïve Bayes: All PE: AUC: 0.98 Accuracy: 99% Precision: 96% TPR: 96% FNR: 4% PPV: 96.4% FDR: 4% F1: 0.98 Recall: 96% DT: Early-onset (<34 gestation weeks): AUC: 0.95 Accuracy: 94% Precision: 93% TPR: 93% FNR: 7% PPV: 75% FDR: 25% F1: 0.86 Recall: 75% RF: Late-onset PE (>34 gestation weeks): AUC: 0.84 Accuracy: 88% Precision: 93% TPR: 67% FNR: 33% PPV: 92,9% FDR: 7% F1: 0.93 Recall: 93% DT: Moderate PE (Not specified): AUC: 0.80 Accuracy: 82% Precision: 85% TPR: 75% FNR: 25% PPV: 92% FDR: 8% F1: 0.88 Recall: 92% RF: Severe PE (when certain criteria are present): AUC: 0.76 Accuracy: 77% Precision: 33% TPR: 86% FNR: 14% PPV: 33% FDR: 67% F1: 0.33 Recall: 33% |
Not specified | Small dataset. | One time: First prenatal visit (not specified further) |
| Early prediction of preeclampsia via machine learning [25] | I. et al. (2020) | Retrospective cohort study (United States) |
Total: 5,245 (10.7 % PE) |
Maternal characteristics (age, height, weight), mean and max of systolic and diastolic BP, history of PE, other medical diseases (diabetes, autoimmune conditions), urine glucose and protein, platelet count, and medications (aspirin, insulin). | Elastic Net Gradient Boosting Multiple Logistic Regression |
Elastic Net: All PE: AUC: 0.79 TPR: 45% FPR: 8% Early-onset (<34 gestation weeks): AUC: 0.89 TPR: 72% FPR: 9% |
All PE: 1. Hypertension 2. History of PE 3. insulin 4. Mean systolic BP 5. Number of babies Early-onset (<34 gestation weeks): 1. Hypertension 2. Number of babies 3. History of PE 4. Protein 3+ 5. Anemia |
Data set contains missing values. Retrospective study. Not specified the sizes of the training set and test set. |
One time: week 16 of gestation |
| Clinical risk assessment in early pregnancy for preeclampsia in nulliparous women: A population based cohort study [26] | Sandström A. et al. (2019) | Retrospective cohort study (Sweden) | Total: 62,562 (4.4% PE) | Gestational length, age, BMI, MAP, capillary glucose, protein in urine, hemoglobin, infertility duration, region of birth, smoking, alcohol, family history, and diseases | RF Backward selection model on multivariable logistic regression Multivariable regression model using FMF variables |
Multivariable regression model: Early-onset (<34 gestation weeks): AUC: 0.68 Sensitivity: 31% for 10% FPR. Preterm PE (<37 gestation weeks): AUC: 0.68 Sensitivity: 29% for 10% FPR. Backward selection model: Term PE (≥37 gestation weeks): AUC: 0.67 Sensitivity: 28% with 10% FPR |
Not specified | Missing values in the data set. Not specified the sizes of the training and test sets. No external validation. Retrospective study. |
One time: first prenatal visit (not specified further) |
| 1 | This study is a pre-print and has not been peer-reviewed. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





