1. Introduction
The lymphatic system has long been viewed as a passive component of the immune and circulatory systems. However, in the last 15 years more evidence has emerged to show that the lymphatic system plays an active role in wound healing, cancer, and fibrosis [
1] through regulation and dysregulation of the vasculature. In many of these healing and disease states, the tissue surrounding lymphatic vasculature undergoes dynamic biochemical (e.g., soluble factors) and biophysical (e.g., stiffness) changes that influence cell behavior [
2,
3,
4,
5]. One important biophysical change is increased tissue stiffening that is often associated with chronic fibrosis—a pathological wound healing condition marked by excess tissue deposition and scarring [
6]. Increased tissue deposition and subsequent tissue densification also alters tissue transport properties, which changes the biochemical environment by altering how signaling molecules move through the tissue to reach cells. However, little is known about how tissue stiffening affects lymphatic vessel growth and function, particularly under disease conditions where tissue stiffness is much higher than what is observed in healthy tissue or during lymphatic development. Thus, it is important to leverage modeling approaches that use materials capable of exhibiting mechanical properties of healthy and fibrotic tissue environments to understand the impact of stiffness on lymphatic vasculature in fibrosis and wound healing.
Over the last several years, there has been more interest in investigating the relationship between tissue stiffness and lymphatic vasculature. To date, these studies primarily focus on lymphatic development and/or employ modeling strategies that approximate tissue stiffness levels observed across developmental and growth stages rather than disease. In work from Frye et al. [
7], human dermal lymphatic endothelial cells (HDLEC) were cultured on Softwell™ or Softslip™ dishes (Matrigen) that provided stiff substrates (4 kPa, embryonic cardinal vein; 8 and 12 kPa, muscle; 25 kPa, bone) or soft substrates (0.2 kPa, tissue surrounding cardinal vein)
in vitro. After 24 hours, HDLECs on softer substrates showed upregulation of genes for cell–matrix adhesion, cell migration, new lymphatic vessel growth (e.g., metalloproteinases 1, 2, and 10), and vascular development stages such as valve formation (e.g., GATA2), while genes for cell proliferation were downregulated. They related their results to concurrent
in vivo studies where increased extracellular matrix (ECM) stiffness within the cardinal vein appeared to support HDLECs that were flat and tightly attached to the underlaying basement membrane. Alternatively, HDLECs that migrated outside of the cardinal vein into a softer ECM were more elongated, spindle shaped as a precursor to network formation. Alderfer et al. chose a different approach to study tissue stiffness effects by using thiol-modified hyaluronic acid (HA) hydrogels conjugated with heparin and thiol-modified gelatin [
8]. Tunable matrix stiffness was achieved by varying ratios of polyethylene glycol diacrylate (PEG-DA) to produce soft (30 Pa), medium (300 Pa), and firm (900 Pa) substrates for culturing HDLECs. They identified matrix stiffness as a key player in activating vascular endothelial growth factor receptor (VEGFR)-3, the primary receptor for VEGF-C. On softer matrices, VEGFR-3 activation increased, which enabled more VEGF-C binding and subsequent formation of more extensive cord-like structures when compared to stiffer matrices. Genes for characteristic lymphatic markers (i.e., Prox1) and proteolytic enzymes involved in cell migration and tube formation (i.e., metalloproteinases 2 and 14) were also upregulated on softer matrices, similar to Frye et al. [
7]. Collectively, these studies demonstrate mechanosensing capabilities of HDLECs and highlight the importance of ECM stiffness in directing HDLEC behavior (cell spreading, migration, proliferation) and lymphatic capillary tube formation, and both studies suggest that HDLECs prefer softer substrates [
7,
8]. Stiffer substrates, which appear to be more inhibitory to HDLEC behaviors related to lymphatic capillary growth, have potential to represent stages of fibrosis
in vitro where ECM stiffness increases. However, there are
in vivo situations where fibrotic conditions and increased ECM stiffness support lymphatic capillary growth rather than hinder (e.g., cancer, renal fibrosis), and questions remain on how
in vitro models can be designed to achieve the physical properties necessary to appropriately mimic some of these pathological conditions.
Within the fields of tissue engineering and
in vitro disease modeling, there are a myriad of options for substrates with tunable stiffness properties [
9,
10]. As we consider materials within the context of lymphatic studies, it is also important that they also have physiological relevance to ECM composition to tissues surrounding lymphatic capillaries and higher order lymphatic vessels [
4,
7,
8,
11]. In the previously described
in vitro studies, substrate (or ECM) stiffness differences were achieved in different ways. Softwell™ or Softslip™ dishes have the advantage of defined stiffness properties across a wide stiffness range 0.1-100 kPa. However, they are only suited for 2D monolayer cultures and are unable to support 3D sprouting into the substrate. Moreover, stiffness cannot be tuned outside what is available through the company. HA composite hydrogels offer more room for stiffness tunability with additional physiological relevance as HA directly interacts with HDLECs
in vivo [
12,
13]. Cells can also be cultured on top or within these hydrogels with the latter approach supporting 3D sprouting studies. However, a 30-300 Pa stiffness range for lymphatic studies [
8] is more representative of developmental stages of lymphatic vessel growth, and significantly higher values are needed to study disease states. For instance, in conditions like pancreatic cancer that have intratumoral and peritumoral lymphatic capillaries, tissue stiffness has been measured at 5.46 ± 3.18 kPa compared to 1.06 ± 0.25 kPa for normal pancreatic tissue [
14]. When considering other materials with tunable stiffness capabilities, type I collagen emerges as a potential candidate. Type I collagen is a prominent ECM component in fibrotic tissues, as fibroblasts deposit collagen that undergoes enzymatic crosslinking (i.e., lysyl oxidase) to increase overall ECM stiffness during fibrosis [
15]. Since lymphatic capillaries are not surrounded by a continuous basement membrane or support cells like blood capillaries, they are directly attached to the underlying ECM through adhesion molecules like integrins that bind collagens and fibronectin, as well as elastin microfibril interfacer 1 [
16,
17,
18]. Therefore, changes in type I collagen structure (e.g., crosslinking) and stiffness that occur during fibrosis can be sensed by HDLECs. Moreover, results from HDLEC stiffness studies showed stiffness-mediated changes in matrix metalloproteinases that target a1(I) and a2(I) peptide chains within the type I collagen triple helix (e.g., MMP-1, -2, -14) during cell migration and lymphatic sprouting [
7,
8,
11,
19]. Although these features and outcomes provide rationale for using type I collagen as a substrate for lymphatic studies, traditionally prepared type I collagen hydrogels lack the dynamic stiffness range needed to represent fibrotic tissue stiffness levels.
Methacrylated type I collagen offers an alternative to produce collagen-based hydrogels with a wide dynamic stiffness range that can be defined “on demand” for lymphatic studies. Free amines on the type I collagen molecule are modified with methacrylate groups, and when modified collagen is combined with a photoinitiator, light irradiation promotes crosslinking via methacrylamide polymerization (i.e., photo-crosslinking) [
20,
21]. With this modification and photo-crosslinking, methacrylated type I collagen stiffness can increase from approximately 0.5 kPa(uncrosslinked) up to 8 kPa [
20] while maintaining its higher order fibrillar microstructure, which is advantageous for recreating
in vivo-like tissue. Other methacrylated materials such as gelatin, HA, and various polysaccharides (e.g., dextran, alginate, chitosan) can achieve high stiffness and are routinely used cell and tissue culture as substrates, but they lack fibrillar structures on their own [
22,
23]. Studies have shown that photo-crosslinked methacrylated collagens from human, bovine, and rat tissue sources have a robust mechanical response and support cell viability with photoinitiators lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) and Irgacure 2959 (IRG) [
20,
21,
24,
25]. While stiffness and lack of toxicity are important to establish, there has been little work exploring other characteristics that impact cell behavior (e.g., permeability, diffusivity). Studies have been performed to measure transport of soluble factors like dextran and bovine serum albumin through non-methacrylated type I collagens. Hsu et al. showed an inverse relationship between permeability speed and size of soluble factors but not within the context of changing concentration or crosslinking [
26]. They also investigated transport across a fibroblast barrier. Chen et al. looked at the interplay between concentration and crosslinking to establish release characteristics of methacrylated collagen hydrogels photo-crosslinked with IRG [
25]. Using 70 kDa FITC-labeled dextran, diffusion was observed to be more dependent on collagen concentration rather than photo-crosslinking with the highest diffusion rate observed in uncrosslinked collagen at low concentration. They cited increased fibril density from higher collagen concentration as the driving factor in hindering diffusion rather than increased stiffness from photo-crosslinking (~40 Pa vs. 200 Pa). These studies provide some insight into molecular transport through methacrylated collagen gels, but more information is needed for a broader range of molecular sizes and matrix properties (e.g., stiffness, microstructure) that are more representative of fibrotic tissues. However, they represent progress for establishing potential for methacrylated type I collagen to be used in disease models to study the interplay between cells and their microenvironment.
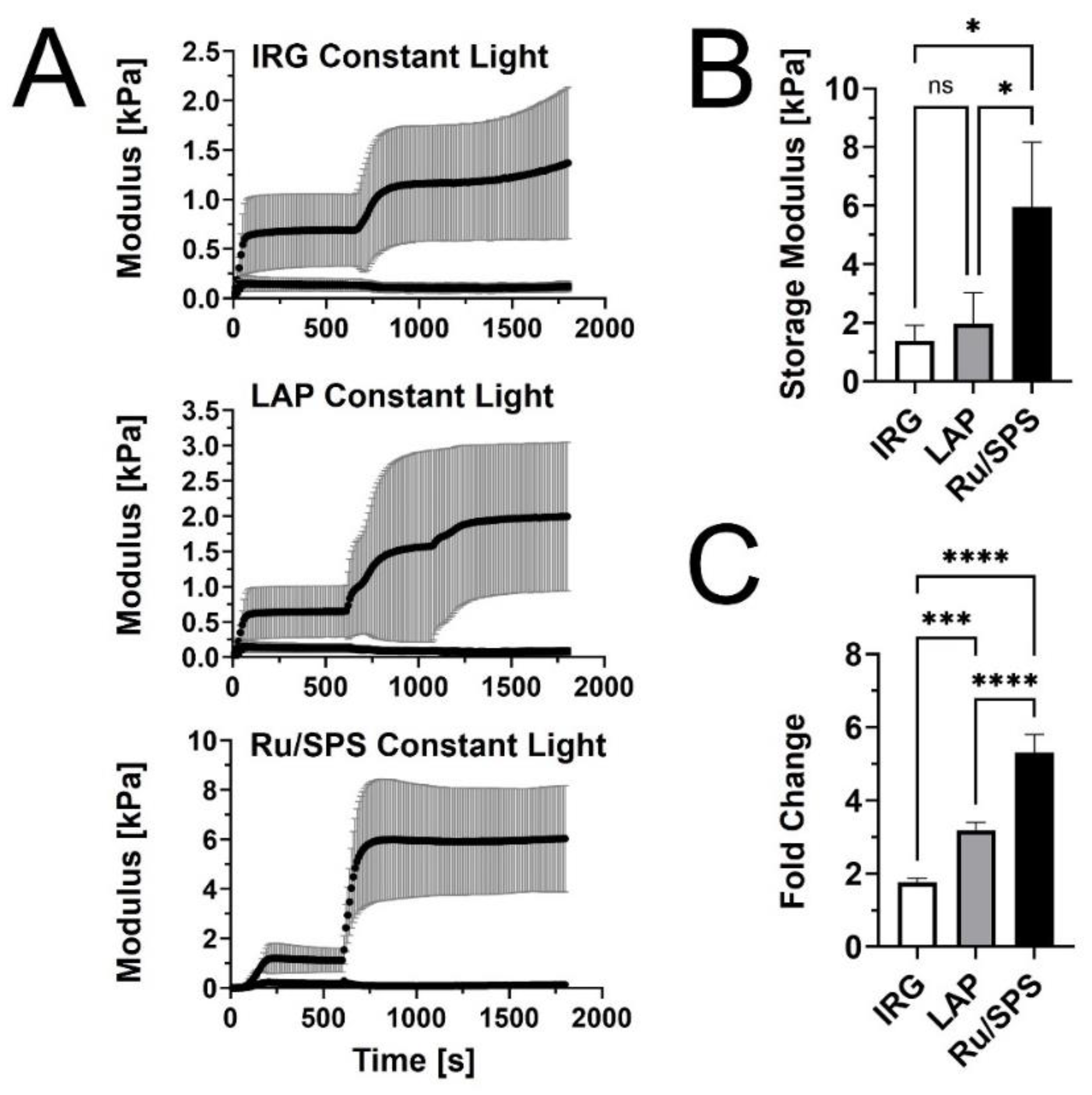
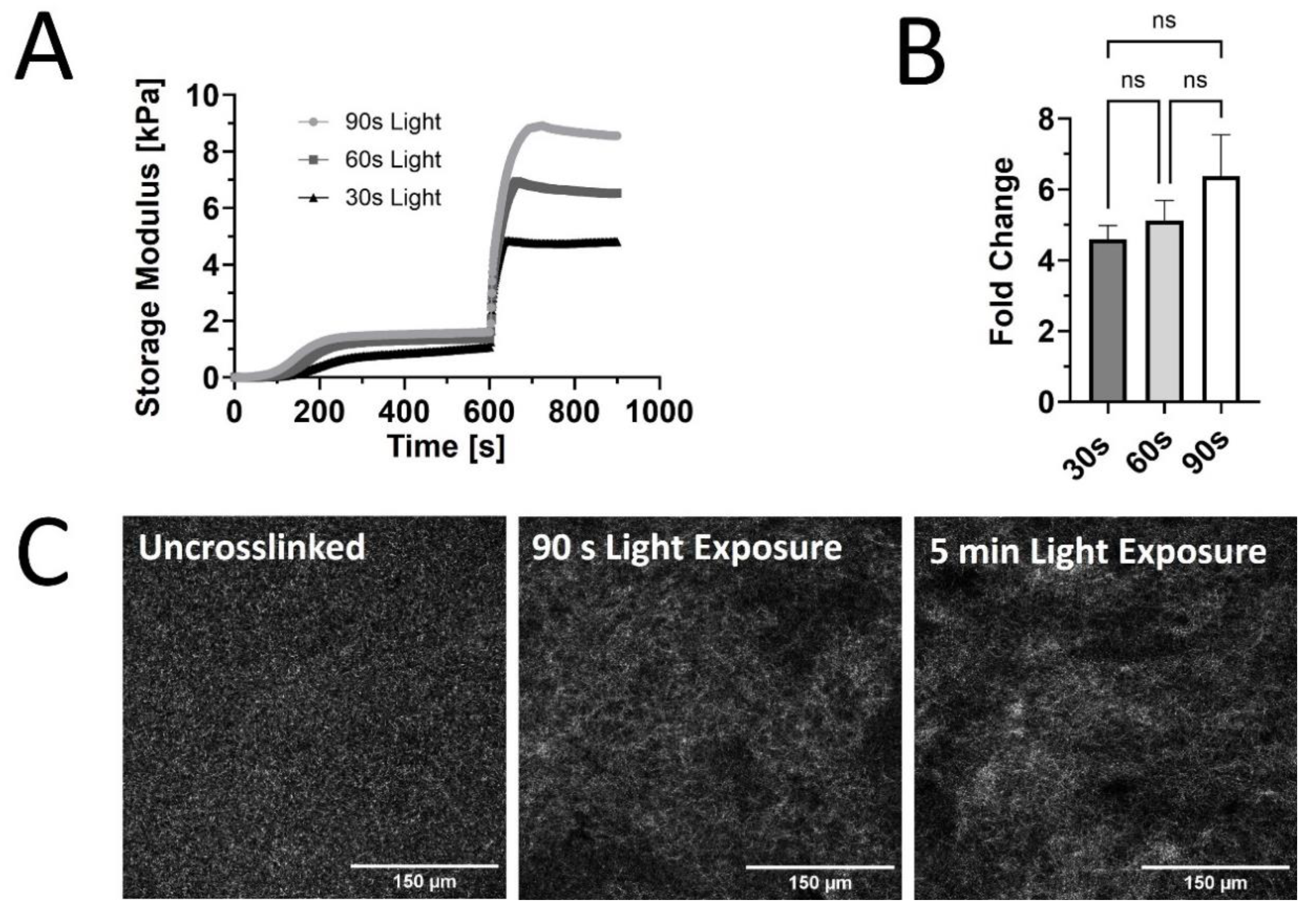
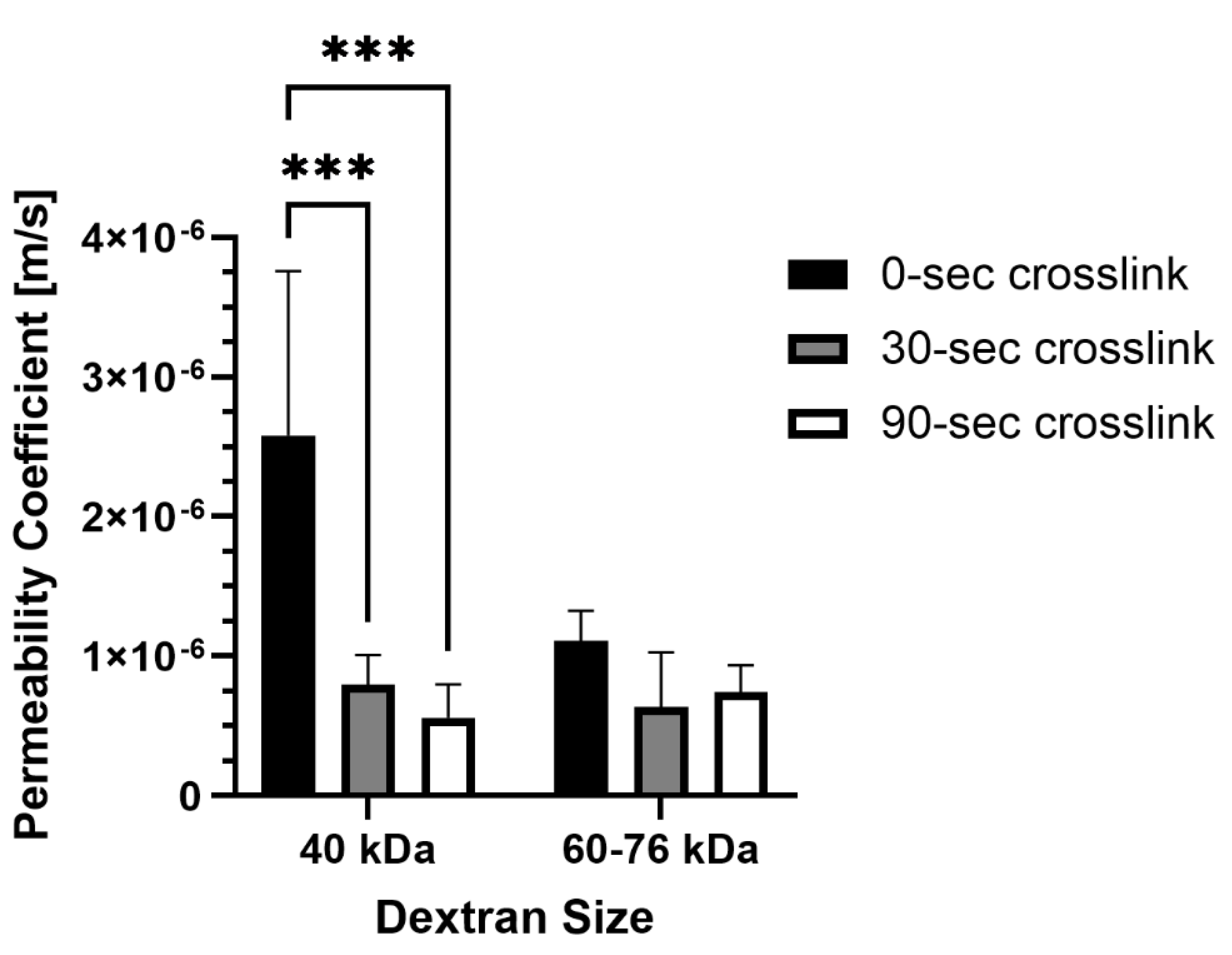
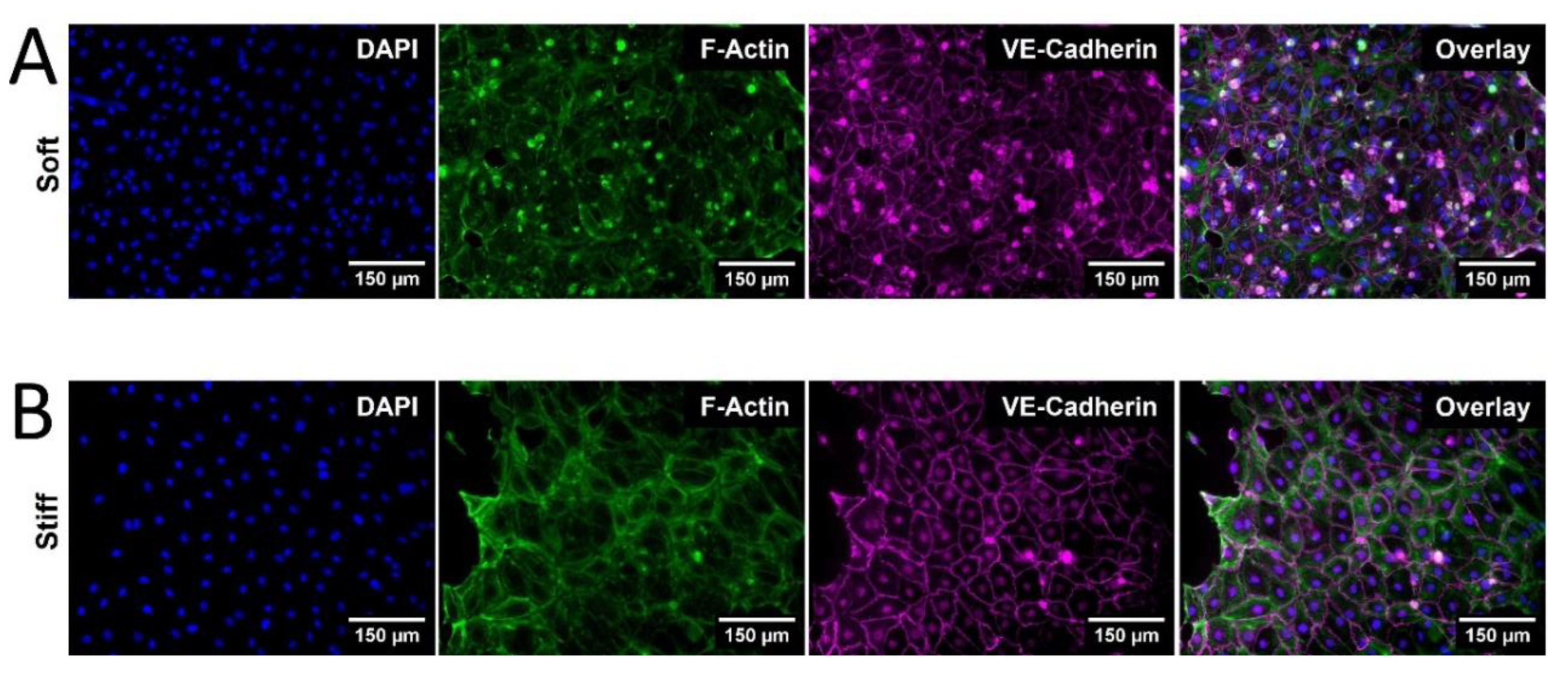
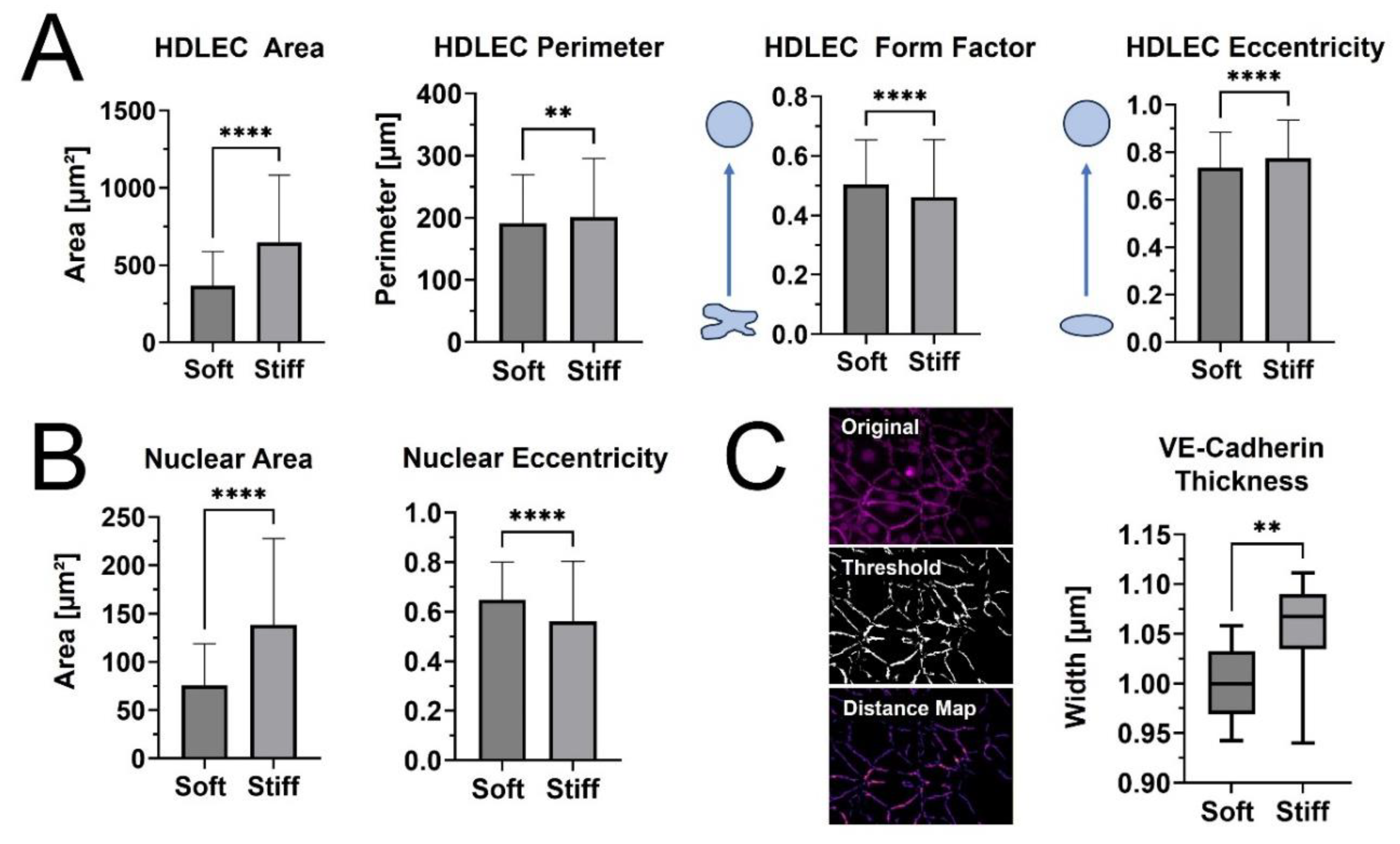
In the current study, PhotoCol®, a methacrylated type I collagen formulation (Advanced BioMatrix, Inc., Carlsbad, CA) was characterized on its “on demand” stiffening properties with LAP, IRG, and Ruthenium/Sodium Persulfate (Ru/SPS) photoinitiators. Irradiation with constant light exposure (20 minutes) and burst light exposure (30-, 60-, and 90 seconds) of 395-405 nm was used to measure maximum and intermediate shear storage modulus values (i.e., stiffness). Results showed that Ru/SPS yielded photo-crosslinked samples with the greatest dynamic stiffness range (0.5 - 6 kPa), and stiffness values were similar when exposed to burst light exposures. Permeability studies showed a decrease in overall permeability with 40 kDa dextran for 30- and 90 second photo-crosslinked PhotoCol® compared to uncrosslinked, but no difference was observed with 60-76 kDa dextran. Stiffer hydrogels were hypothesized to induce a phenotype in LECs indicative of fibrosis response, i.e. thicker and zipper like cellular junctions. When HDLECs were cultured on fibronectin-coated PhotoCol® with Ru/SPS at low stiffness (0.5 kPa) and high stiffness (6 kPa), results showed thicker cell-cell junctions (i.e., VE-Cadherin) and increased average cell area and shape irregularity on stiffer samples. Overall, we demonstrated that PhotoCol® with Ru/SPS induces morphological changes in HDLECs when photo-crosslinked to produce stiffness levels that are consistent with fibrotic tissues. Moreover, we established that PhotoCol©, with its “on demand” stiffening capability and fibrillar microstructure, is a viable substrate for in vitro models of lymphatic vasculature that focus on modeling fibrotic disease states.
3. Discussion
Recently, there has been increased focus on the relationship between tissues stiffness and lymphatic vessel response, primarily with lymphatic capillaries and LECs [
6,
7,
8]. For our study, we focused on tissue stiffness levels that are found in fibrotic disease conditions, since tissue stiffening from excess ECM deposition and crosslinking are major features of fibrosis that alter the biophysical and biochemical microenvironments surrounding lymphatic vasculature. Methacrylated type I collagen, combined with photoinitiators (LAP, IRG, or Ru/SPs) and light exposure (356-405 nm), was utilized as an “on-demand” stiffened material for fibrotic tissue disease modeling. The three photoinitiations were tested for how they changed the stiffening profiles of PhotoCol®, and Ru/SPS showed the highest average maximum stiffness, highest fold change after photo-crosslinking (i.e., dynamic range), and the quickest time to stiffness plateau. Importantly, the maximum stiffness of Ru/SPS PhotoCol® reached an average of approximately 6 kPa, which is within the stiffness range of some diseased fibrotic tissue [
29,
30]. When Ru/SPS PhotoCol® was tested for stiffness tunability using burst light exposures, a range of 5-9 kPa was achieved, but the fold change of the burst exposure was not statistically different despite an observable trend of increasing stiffness with light exposure. There was also a visual difference in how the PhotoCol® fibrils formed under different stiffening conditions with longer, less densely packed fibrils with increased photo-crosslinking. Permeability of 40 kDa dextran was statistically different for photo-crosslinked versus uncrosslinked samples, but these effects were not significant for 60-76 kDa dextran. This result demonstrated that as the physical environment changes due to stiffness, the soluble factor signaling may vary depending on the size of the molecule impacting cellular response. LEC morphology was also significantly affected by substrate stiffness with increased cell area and irregularity observed on stiffer PhotoCol©. This results was similar to results found by Frye et.al., where soft substrates induced smaller and more elongated cells, and stiff substrates induced flattened and spread-out morphologies [
7]. Notably, our study also quantified the thickness differences in VE-Cadherin cell junctions, which is known to be related to healthy and diseased LEC states [
28].
Similar to other studies, our results show that HDLECs are responsive to differences in stiffness of the underlying substrate. However, we add to the field by using methacrylated type I collagen (PhotoCol®) at higher stiffness levels that are more representative of tissues undergoing fibrosis rather than development. Our results also show the impact of photo-crosslinking methacrylated collagen to fibrotic stiffness levels on molecular transport and extend the analysis of HDLEC response to quantify morphological characteristics to include shape metrics for cells and nuclei, along with VE-Cadherin thickness. The morphological assessment is particularly helpful, because morphology is less commonly used to describe changes in lymphatic vasculature outside of flow-based studies that assess changes in elongation and alignment [
31,
32,
33]. Moreover, as we move toward more mechanistic studies, we can relate alterations in mechanosensing pathways to morphological changes for more robust characterization of our model systems. For instance, the YAP/TAZ pathway is notable for its mechanosensing capability LECs when exposed to oscillatory shear and varied ECM stiffness [
7,
8,
34]. Increased substrate stiffness has been shown to promote YAP/TAZ expression and translocation to the nucleus, which blocks Prox-1 and its downstream targets, VEFGR-3 and matrix metalloproteinase-14. Without robust activation of those two targets, lymphatic sprouting and subsequent formation of cord-like structures decreases. However, studies have not investigated whether this signaling pathway has any impact on cell morphology. Less is also known about relationships between VE-Cadherin and either YAP/TAZ or ECM stiffness. Almost all lymphatic studies stain for VE-Cadherin and many assess VE-Cadherin expression via western blot, but very few studies measure VE-Cadherin morphology as we did in the current study [
35]. Moreover, the driving factors behind VE-Cadherin expression are not typically investigated. In our study, we not only observed thicker VE-Cadherin junctions formed in HDLECs on stiff photo-crosslinked samples, but we also noted potential association between VE-Cadherin junctions and F-actin formation. A similar observation was noted in HDLECs on viscoelastic substrates [
13]. Although neither study investigated the link between the two molecules within the context of HDLECs and stiffness, there are established connections between the VE-Cadherin/Catenin complex with actin [
36]. These molecules are integrated through the processes of junction formation and maturation and remain associated during junction remodeling and maintaining junction integrity. Moreover, additional molecules such as the ARP2/3 complex, α-catenin, and p120
ctn also help coordinate interactions. It will be important to investigate these mechanisms further within the context of lymphatic vasculature and ECM stiffness. Materials like methacrylated collagen will be helpful in these pursuits, because they retain collagen fibrillar microstructure that is important for cell attachment and appropriate force balance between cells and the surrounding ECM.
Through photo-crosslinking, we produced elastic hydrogels with covalent crosslinks that achieve static stiffness. However, there are temporal elements to the fibrotic process as excess ECM is deposited and crosslinked over time. Beyond stiffness, the biochemical environment also shifts with changes in ECM permeability that affect the movement of soluble factors and altered biodegradation that impacts tissue remodeling. Moreover, temporal changes in tissue stiffness and remodeling also affect ECM viscoelasticity. Fan et al. recently looked beyond static ECM stiffness and identified viscoelasticity as an important ECM property that impacts lymphatic morphogenesis and tube formation [
13]. They combined supramolecular and covalent crosslinking to create dynamic HA hydrogels with tunable viscoelasticity that is spatially controlled with UV light exposure. Although the hydrogels exhibit the same maximum shear storage modulus (~1500 Pa) before and after UV irradiation, they differ in stress-relaxation behavior from static stiffness hydrogels (fully covalently crosslinked and elastic). In standard and photo-patterned viscoelastic HA substrates, HDLECs showed evidence of a higher degree of cell spreading and migration with increased F-actin stress fiber formation and focal adhesion assembly. We also observed greater cell area and F-actin formation on our photo-crosslinked samples, even though our stiffness levels were ~4 times higher than Fan et al. HDLECs on viscoelastic HA hydrogels also formed a more extensive and branched lymphatic tube network compared to elastic (static) hydrogels with increased expression of characteristic lymphatic markers (LYVE-1, Prox1, podoplanin, VEGFR-3). Matrix metalloproteinases 1, 2, and 14, which are involved in ECM degradation and remodeling events necessary for tube formation, also increased on viscoelastic hydrogels. Interestingly, these are some of the same markers that others observed as being expressed at higher levels on softer substrates (30-200 Pa) [
7,
8]. Collectively, these observations and study results provide rationale for why viscoelasticity and temporal stiffening are important factors to consider beyond static stiffness when modeling dynamic processes like fibrosis. These differences may also be a contributing factor to why
in vitro lymphatic capillaries do not match the dense, non-functional capillary beds seen
in vivo in disease.
Even though the current work focuses on a new material approach to investigate the lymphatic response to ECM stiffness, we recognize other work within the field that is making progress toward improved understanding of the lymphatic system. Beyond our work and other work with natural materials that were previously discussed, Hooks et al. recently used a synthetic poly(ethylene glycol) (PEG) hydrogel functionalized with four maleimide groups (PEG-4MAL) and binding arginylglycylaspartic acid (RGD) ligands to observe the relationship between matrix elasticity (stiffness), ligand binding density, and degradability on lymphatic sprouting [
37]. The study was designed to target one of the drawbacks of collagen, being that ligand density and stiffness profiles of gels are not independent when using collagen concentration to alter collagen stiffness. They were able to control matrix elasticity via PEG weight percentage without altering the RGD ligand density and generate matrices at 680 Pa with comparable ligand density to 2 mg/mL collagen at 20 Pa. Moreover, they showed successful lymphatic sprouting
in vitro and functional grafting into host vasculature
in vivo. Although they were able maintain ligand density at increased stiffness levels, methacrylated collagen behaves similarly by achieving multiple stiffness levels via photo-crosslinking at a single collagen concentration [
20]. Methacrylated collagens are also capable of being integrated into lymphatic-on-a-chip models, which are popular models for studying lymphatic sprouting and growth [
32,
38,
39,
40,
41,
42]. Lymphatic capillaries sprout into ECM materials from LEC-lined channels, usually guided by a gradient of growth factors such as VEGF-C and sphingosine 1 phosphate [
31]. Disease is currently a part of lymphatic-on-a-chip modeling; however, the focus tends to be on using soluble factors and co-cultures to recreate a disease environment. As with most lymphatic capillary models, ECM-based features of fibrosis have not been included in lymphatic-on-a-chip models. Therefore, there is an opportunity to merge the two approaches to advance the field of lymphatic modeling to include more robust disease models with altered stiffness and transport properties that impact LEC behavior.
4. Materials and methods
Table 1.
Materials, Cells, Software, Catalog Number, and Company.
Table 1.
Materials, Cells, Software, Catalog Number, and Company.
| Materials (Cells and Cell Culture) |
Catalog Number |
Company |
| Human Dermal Lymphatic Endothelial Cells (HDLEC), Adult, Cyropreserved |
#C-12217 |
PromoCell |
| MV2 Media |
#C-39221 |
PromoCell |
| Materials (Reagents and Antibodies) |
Catalog Number |
Company |
| PhotoCol® Methacrylated Collagen |
#5198-100MG |
Advanced Biomatrix |
| LAP (405nm) |
#5269 |
Advanced Biomatrix |
| Irgacure 2959 (365nm) |
#5200 |
Advanced Biomatrix |
| Tuthenium (400-450nm) |
#5248 |
Advanced Biomatrix |
| Fibronectin Lyophilized (Human) |
#5080 |
Advanced Biomatrix |
| PBS, Phosphate Buffered Saline, 10X Solution |
#BP3991 |
Fisher Bioreagents |
| Fluorescein isothiocyanate–dextran, 40kDa |
#FD40S-250MG |
Sigma Aldrich |
| Fluorescein isothiocyanate–dextran, 60-76kDa |
#FD70S-250MG |
Sigma Aldrich |
| Pierce™ 16% Formaldehyde (w/v), Methanol-free |
#28908 |
ThermoFisher Scientific |
| Triton™ X-100 |
T8787-50ML |
Sigma Aldrigh |
| Alexa Fluor™ 488 Phalloidin |
#A12379 |
ThermoFisher Scientific |
| DAPI (4',6-diamidino-2-phenylindole, dihydrochloride) |
#62247 |
ThermoFisher Scientific |
| Bovine Albumin Fraction V (7.5% solution) |
#15260037 |
ThermoFisher Scientific |
| Rabbit Anti-Human VE-cadherin Polyclonal Antibody |
#PA5-19612 |
ThermoFisher Scientific |
| Rat Anti-Mouse LYVE1 Monoclonal Antibody (ALY7) |
#MA5-32512 |
ThermoFisher Scientific |
| Mouse Anti-Human VEGF Receptor 3 Monoclonal Antibody (4H4) |
#MA5-15651 |
ThermoFisher Scientific |
| Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor™ Plus 647 |
# A32795 |
ThermoFisher Scientific |
| Materials (Disposables) |
Catalog Number |
Company |
| Nunc™ Lab-Tek™ II Chambered Coverglass |
#155360 |
ThermoFisher Scientific |
| Costar® 24-well Clear TC-treated Multiple Well Plates, Individually Wrapped, Sterile |
#3524 |
Corning |
| Falcon® Permeable Support for 24-well Plate with 8.0 µm Transparent PET Membrane, Sterile |
#393097 |
Corning |
| Corning® 96-well Flat Clear Bottom Black Polystyrene TC-treated Microplates, Individually Wrapped, with Lid, Sterile |
#3603 |
Corning |
| Fisherbrand™ Surface Treated Sterile Tissue Culture Flasks, Vented Cap |
#FB012937 |
Fisher Scientific |
| 96 Well glass bottom plate with high performance #1.5 cover glass |
#P96-1.5H-N |
Cellvis |
| Materials (Equipment and Software) |
Catalog/Part Number |
Company |
| MCR 302e WESP rheometer |
#241353 |
Anton Paar |
| UV Mounted LED |
#M365LP1 |
Thorlabs |
| UV Mounted LED |
#M405LP1 |
Thorlabs |
| Leica TCS SP5 Spectral Confocal Microscope |
|
Leica Microsystems |
| Victor Nivo 6T Multimode Plate Reader |
#HH35000500 |
Revvity Health Sciences |
| Keyence All-in-One Fluorescence Microscope |
#BZ-X810 |
KEYENCE Corp. of America |
| GraphPad Prism |
|
GraphPad Software Inc. |
4.1. Preparation of Methacrylated Collagen Hydrogels (PhotoCol®)
Lyophilized methacrylated type I collagen (PhotoCol®, Advanced BioMatrix, Carlsbad, California) was solubilized on a rotator at 4°C in sterile 20 mM acetic acid to a final concentration of 8 mg/mL. Three photoinitiators were prepared according to manufacturer (Advanced BioMatrix) protocols: Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP, 17 mg/mL stock volume), Irgacure 2959 (IRG, 10% stock), and Ruthenium (Ru)/Sodium Persulfate (SPS) (Ru at 37.4 mg/mL and SPS at 119mg/mL stock volume). Neutralization solution (Advanced BioMatrix) at 8% (v/v) (7.407 mg/mL) and photoinitiator at 2% (v/v) were mixed with PhotoCol® to make the final hydrogel mixture for each photoinitiator (LAP - 7.262 mg/mL; IRG - 7.334 mg/mL; and Ru/SPS - 7.122 mg/mL). Neutralized PhotoCol® hydrogels with photoinitiator were incubated at 37°C for a minimum of 30 minutes for hydrogel self-assembly prior to photo-crosslinking in subsequent studies.
4.2. Rheological Assessment of PhotoCol® Viscoelasticity
Matrix viscoelastic properties (shear storage modulus, G’; shear loss modulus, G”) were measured using a MCR 302e WESP rheometer (Anton Paar, Graaz, Austria) with a quartz stage to photo-crosslink PhotoCol® hydrogel samples (365-405 nm) during testing. Frequency and strain sweeps were conducted in oscillatory shear on fully crosslinked and un-crosslinked hydrogels to determine the linear viscoelastic region (data not shown). Temporal changes in stiffness in response to photo-crosslinking were conducted in time sweeps (constant: 0.1% strain, 1 hz). Hydrogel samples were allowed to self-assemble for 10 minutes at 37°C before exposure to one of two light exposure conditions using 365 or 405 nm UV mounted LED (Thorlabs, Newton, NJ) —constant and burst expsoure. Constant light was applied for 20 minutes after self-assembly for complete photo-crosslinking and maximum stiffness (G’). Burst light exposures consisted of a single light pulse of 30-, 60-, or 90-seconds after self-assembly with continued measurement for 10 minutes after light exposure.
Using MATLAB (Mathworks, Natick, MA), a locally weighted regression was used on each replicate to create a fitted curve that could be analyzed. The first derivate was taken of these regressions to determine rate of change in stiffness. A threshold of 3 Pa/s rate was used to determine the regions of plateau and regions of stiffening. Average stiffness values of the plateau regions before and after light exposure, within the regions of stiffening, were used to calculate fold change by the ratio of each replicate’s post-stiffening average to the same replicate’s pre-stiffening average. The post-stiffening averages of each replicate were then averaged to give each group’s average maximum stiffness. The average number of seconds the rate of change of stiffening was above the 3 Pa/s threshold was used to calculate the amount of time to stiffness plateau.
4.3. Qualitative Microstructural Assessment of PhotoCol® Hydrogels
Confocal laser scanning microscopy in reflectance mode was used to obtain images of fibrillar collagen microstructure for qualitative assessment. All samples were prepared in Nunc™ Lab-Tek™ II Chambered Coverglass #1.5 slides (ThermoFisher Scientific, Waltham, Massachusetts). Briefly, PhotoCol® hydrogels with Ru/SPS were neutralized and allowed to self-assemble for 30 minutes at 37°C as previously described. Photo-crosslinked samples were exposed to 405 nm light for 90-seconds (longest burst exposure) or 5 minutes (sufficient time to reach maximum G’) using a 405 nm UV mounted LED (Thorlabs). Images were collected with a Leica TCS SP5 Spectral Confocal Microscope (Leica Microsystems, Wetzlar, Germany) using a 40X oil immersion objective (2D scan).
4.4. Permeability Assessment of PhotoCol® Hydrogels
A transwell membrane insert system protocol adapted from Hsu et al. [
26] was used to characterize permeability of PhotoCol® hydrogels with Ru/SPS photoinitiator. Neutralized PhotoCol® (60 µL; 7.122mg/mL) was polymerized for 60 min at 37°C on a 8.0 µm PET membrane 24-well transwell insert (Corning, Corning, New York) within a tissue-cultured treated 24-well plate (Corning). For photo-crosslinking, transwell inserts containing PhotoCol® were removed and exposed to 405 nm near UV light for 30- or 90-seconds. Lyophilized human fibronectin (Advanced BioMatrix) was reconstituted in Milli-Q water and diluted in 1X PBS (Fisher Bioreagents, Waltham, Massachusetts) to achieve 5 ug/cm
2 (0.03 mg/mL) on the PhotoCol® surface. Samples were then incubated at 37°C for 1 hour to establish a fibronectin coating and submerged in 1X PBS (overnight at 37°C) to remove any excess Ru/SPS from the PhotoCol® gels. A 2 mg/mL solution of fluorescein isothiocyanate (FITC)-labeled dextran at 40 kDa or 60-76 kDa (Sigma Aldrich, St. Louis, Montanna) was added on top of the fibronectin-coated PhotoCol® samples within the inserts as the donor solution, while 1X PBS was pipetted into the outer well to serve as the acceptor solution. Solution volumes were controlled to be at equal heights to minimize flow due to hydrostatic pressure. PhotoCol® with FITC-dextran was incubated at 37°C, and 20 µL was removed from the acceptor solution at one-hour increments for a total of 3-5 hours. The sampled solution was then diluted at 1:25 in 1X PBS and transferred to a black walled polystyrene 96-well plate (Corning) to be read on a PerkinElmer VICTOR Nivo plate reader (Revvity, Waltham, Massachusetts) (480 nm excitation/530 nm emission). A standard curve (0-0.0125 mg/mL of FITC-dextran) was used to calculate the concentration of measured samples. To calculate the permeability coefficient, Fick’s second law was used using the dimensions of the PhotoCol® and the change in concentration of the acceptor solution in the linear region of permeability.
4.5. Lymphatic Endothelial Cell Culture
Human Dermal Lymphatic Endothelial Cells (HDLEC) isolated from adult skin (PromoCell, Heidelberg, Germany) were maintained according to manufacturer instructions in endothelial growth media with MV2 growth supplements (PromoCell): Fetal calf serum (5% v/v), Epidermal growth factor (recombinant human, 5 ng/mL), Basis fibroblast growth factor (recombinant human, 10 ng/mL), Insulin-like growth factor (Long R3 IGF, recombinant human, 20 ng/mL), Vascular endothelial growth factor 165 (recombinant human, 0.5 ng/mL), Ascorbic acid (1 µg/mL), Hydrocortisone (0.2 µg/mL) (PromoCell). All cell culture surfaces were coated with human fibronectin (3.5 µg/cm2; Advanced BioMatrix) prior to HDLEC seeding to promote cell attachment. Cells were passaged and maintained in Fisherbrand™ Surface Treated Sterile Tissue Culture Flasks, Vented Cap (Fisher Scientific, Waltham, Massachusetts) until ready for experimental use. Cells were maintained at 37°C in a humidified incubator (5% CO2) and passaged at 70–90% confluency. All cells were used between passages 6 and 12 for experiments.
4.6. Morphological Assessment of Lymphatic Endothelial Cell Stiffness Response
HDLECs were characterized on their morphological response to hydrogels at different stiffness levels (low and high G'). Cells were seeded in 15 well µ-slide (Ibidi, Gräfelfing, Germany) at 10,000 cells/cm2 on top of fibronectin-coated PhotoCol® prepared with Ru/SPS (photo-crosslinking, 90 seconds of light exposure high G') and without photoinitiator (uncrosslinked, no light exposure, low G') for a total culture time of 3 days (37°C, 5% CO2). To evaluate any impact of light exposure, HDLECs were also seeded on fibronectin-coated 96 well glass bottom plate with high performance #1.5 cover glass plates (Cellvis, Mountain View, California) and exposed to the 90-seconds of light (not shown). Additional HDLECs on coated wells with no light exposure served as 2D controls (not shown). All groups were fixed with 4% paraformaldehyde (ThermoFisher Scientific) in 1X PBS for 15 minutes at 37°C and permeabilized using 0.1% of Triton™ X-100 (Sigma Aldrich) diluted in 1X PBS. Samples were stained with Alexa Fluor™ 488 Phalloidin (1:400, ThermoFisher Scientific) to visualize F-actin and counterstained with 4′,6-diamidino-2-phenylindole (300 nM DAPI; ThermoFisher Scientific) to visualize the nuclei. For immunostaining of characteristic HDLEC markers, fixed samples were blocked with bovine albumin fraction V (7.5% solution; ThermoFisher Scientific) diluted in 1X PBS to 1% and incubated with primary antibodies for VE-Cadherin (1:1000 ThermoFisher Scientific). Samples were then rinsed and incubated with Alexa Fluor™ Plus 647-conjugated secondary antibody donkey anti-rabbit IgG (H+L) (1:200, ThermoFisher Scientific). All samples were imaged with a Keyence BZX810 All-in-One Fluorescence Microscope (KEYENCE Corp. of America, Itasca, IL). Images were collected at 20X magnification for cellular and nuclear area, cellular and nuclear eccentricity, cellular form factor, and VE-Cadherin thickness, utilizing the Keyence BZ-H4A analysis software to create full focus images from z-stacks. The threshold of these images was obtained utilizing CellProfiler™, removing signal from cell nuclei and noise before obtaining the threshold image of just the VE-Cadherin cellular outline. Primary (nuclei) and secondary objects (cell body) were obtained and quantified using CellProfiler™ as well. Threshold images were then quantified using distance map and Vessel Analysis plugins. Due to the visual similarity between threshold images of vasculature and threshold images of cell boundaries, the Vessel Analysis plugin was able to accurately obtain average vessel diameters of four random regions of interest in each image (n=4, N=3).
4.7. Statistical Analysis
Statistical analysis was carried out using GraphPad Prism 10.0 (GraphPad Software Inc., La Jolla, CA). Stiffness data was expressed as mean storage modulus + standard deviation (SD) without removal of outliers. Permeability data was expressed as a mean ± SD after outliers were removed using Grubbs test (α = 0.05). Morphological values were expressed as mean ± SD without removal of outliers. Normality was verified through a Shapiro-Wilk normality test using α = 0.05. Multiple comparisons were performed using one-way or a two-way analysis of variance (ANOVA), and parametric data were analyzed using the Tukey post hoc method (significance: *p<0.05, **p<0.001, ***p<0.0001, ****p<0.00001). Nonparametric data were analyzed with the Mann-Whitney U test (significance: (**p<0.005 and ****p<0.0001).