Submitted:
10 June 2024
Posted:
12 June 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Design, Participants, and Procedure
Measures
Statistical Analysis
Results
Participant Characteristics
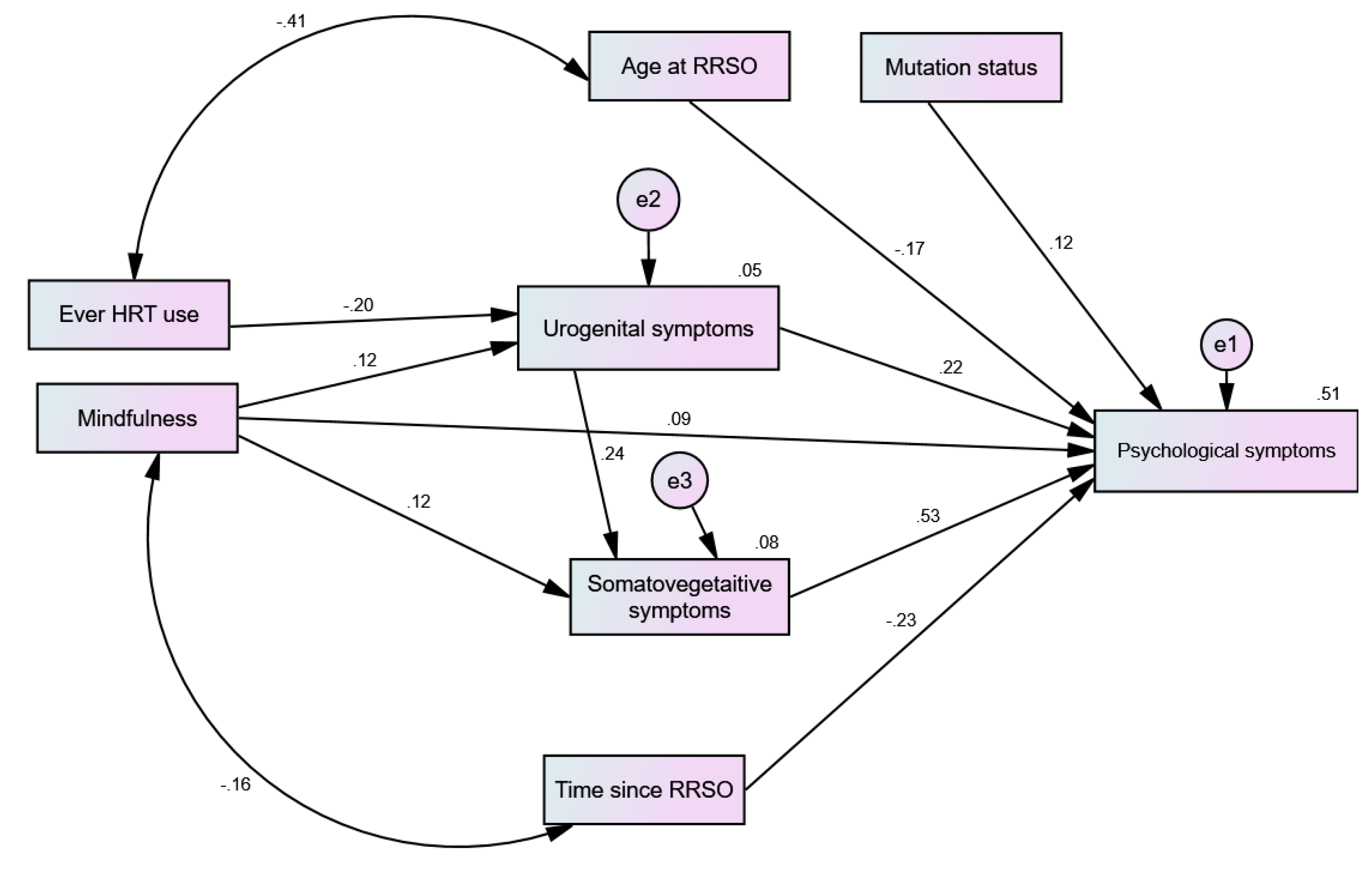
The Effect of HRT and MBSR on Menopausal Symptoms (Path Analysis)
The Effect of HRT and MBSR on Menopausal Symptoms Across Groups (Multigroup Analysis)
Discussion
Strengths, Implications, and Limitations
Conclusions
Ethics approval and consent to participate
Consent for publication
Competing interests
Author Contributions
Funding
Data Availability Statement
References
- Noman, S.; Shahar, H.K.; Abdul Rahman, H.; Ismail, S.; Abdulwahid Al-Jaberi, M.; Azzani, M. The Effectiveness of Educational Interventions on Breast Cancer Screening Uptake, Knowledge, and Beliefs among Women: A Systematic Review. International Journal of Environmental Research and Public Health 2021, 18, 263. [Google Scholar] [CrossRef]
- Ling, J.; Kumar, R. Crosstalk between NFkB and glucocorticoid signaling: A potential target of breast cancer therapy. Cancer Lett 2012, 322, 119–126. [Google Scholar] [CrossRef]
- Fei, F.; Siegal, G.P.; Wei, S. Characterizing Clinicopathologic Features of Estrogen Receptor-Positive/Progesterone Receptor-Negative Breast Cancers. Clin Breast Cancer 2022, 22, e788–e797. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.M.; Fan, S.; Pestell, R.G.; Goldberg, I.D. BRCA1 gene in breast cancer. Journal of Cellular Physiology 2003, 196, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Luo, J.; He, Y.; Jiang, L.; Zhong, L.; Shi, Y. BRCA2 gene mutation in cancer. Medicine (Baltimore) 2022, 101, e31705. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Liu, Q.; Xie, Y.; Shi, X.; Li, Y.; Peng, M.; Guo, H.; Sun, R.; Li, J.; Hong, Y.; et al. Breast cancer susceptibility protein 1 (BRCA1) rescues neurons from cerebral ischemia/reperfusion injury through NRF2-mediated antioxidant pathway. Redox Biology 2018, 18, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Djedovic, V. BRCA1 modulation of glucocorticoid receptor signaling in high-grade serous ovarian cancer cells. University of Toronto, Canada, 2016.
- Stuursma, A.; van Driel, C.M.G.; Wessels, N.J.; de Bock, G.H.; Mourits, M.J.E. Severity and duration of menopausal symptoms after risk-reducing salpingo-oophorectomy. Maturitas 2018, 111, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Tone, A.A.; Virtanen, C.; Shaw, P.; Brown, T.J. Prolonged postovulatory proinflammatory signaling in the fallopian tube epithelium may be mediated through a BRCA1/DAB2 axis. Clin Cancer Res 2012, 18, 4334–4344. [Google Scholar] [CrossRef] [PubMed]
- van Driel, C.; de Bock, G.H.; Schroevers, M.J.; Mourits, M.J. Mindfulness-based stress reduction for menopausal symptoms after risk-reducing salpingo-oophorectomy (PURSUE study): a randomised controlled trial. Bjog 2019, 126, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Royal jelly as an intelligent anti-aging—a focus on cognitive aging and Alzheimer's disease: a review. Antioxidants 2020, 9, E937. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Zuñiga, O.A.; Cruz-Teno, C.; Haro, C.; Quintana-Navarro, G.M.; Camara-Martos, F.; Perez-Martinez, P.; Garcia-Rios, A.; Garaulet, M.; Tena-Sempere, M.; Lopez-Miranda, J.; et al. Differential menopause- versus aging-induced changes in oxidative stress and circadian rhythm gene markers. Mech Ageing Dev 2017, 164, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, M.A.; Zacarías-Flores, M.; Arronte-Rosales, A.; Mendoza-Núñez, V.M. Association between hot flashes severity and oxidative stress among Mexican postmenopausal women: A cross-sectional study. PLoS One 2019, 14, e0214264. [Google Scholar] [CrossRef] [PubMed]
- Shea, A.K.; Sohel, N.; Gilsing, A.; Mayhew, A.J.; Griffith, L.E.; Raina, P. Depression, hormone therapy, and the menopausal transition among women aged 45 to 64 years using Canadian Longitudinal Study on aging baseline data. Menopause 2020. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Ahmed, A.H.; Smail, L. Psychological Climacteric Symptoms and Attitudes toward Menopause among Emirati Women. Int. J. Environ. Res. Public Health 2020, 17, 5028. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.; Díaz, J.; Serrano, E.; Abellán, J.; Carbonell, L.F. Hormone replacement therapy for oxidative stress in postmenopausal women with hot flushes. Obstet Gynecol 2000, 95, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Szabó, R.; Hoffmann, A.; Börzsei, D.; Kupai, K.; Veszelka, M.; Berkó, A.M.; Pávó, I.; Gesztelyi, R.; Juhász, B.; Turcsán, Z.; et al. Hormone Replacement Therapy and Aging: A Potential Therapeutic Approach for Age-Related Oxidative Stress and Cardiac Remodeling. Oxid Med Cell Longev 2021, 2021, 8364297. [Google Scholar] [CrossRef]
- López-Grueso, R.; Gambini, J.; Abdelaziz, K.M.; Monleón, D.; Díaz, A.; El Alami, M.; Bonet-Costa, V.; Borrás, C.; Viña, J. Early, but not late onset estrogen replacement therapy prevents oxidative stress and metabolic alterations caused by ovariectomy. Antioxid Redox Signal 2014, 20, 236–246. [Google Scholar] [CrossRef]
- Geukes, M.; Anema, J.R.; van Aalst, M.P.; de Menezes, R.X.; Oosterhof, H. Improvement of menopausal symptoms and the impact on work ability: A retrospective cohort pilot study. Maturitas 2019, 120, 23–28. [Google Scholar] [CrossRef]
- Gordon, J.L.; Halleran, M.; Beshai, S.; Eisenlohr-Moul, T.A.; Frederick, J.; Campbell, T.S. Endocrine and psychosocial moderators of mindfulness-based stress reduction for the prevention of perimenopausal depressive symptoms: A randomized controlled trial. Psychoneuroendocrinology 2021, 130, 105277. [Google Scholar] [CrossRef]
- Yazdani Aliabadi, M.; Javadnoori, M.; Saki Malehi, A.; Aslani, K. A study of mindfulness-based stress-reduction training effects on menopause-specific quality of life in postmenopausal women: A randomized controlled trial. Complement Ther Clin Pract 2021, 44, 101398. [Google Scholar] [CrossRef]
- Stuursma, A.; Wessels, N.; Bock, G.H.d.; Mourits, M.; Driel, C.V. Data for: Severity and duration of menopausal symptoms after risk-reducing salpingo-oophorectomy. Mendeley Data 2018, Version 1, doi:10.17632/6wtd46ry6s.1. 1. [CrossRef]
- Ali, A.M.; Hendawy, A.O.; Al-Amer, R.; Shahrour, G.; Ali, E.M.; Alkhamees, A.A.; Ibrahim, N.; Lamadah, S.M.T.; Ahmed, A.H. Psychometric evaluation of the Depression Anxiety Stress Scale 8 among women with chronic non-cancer pelvic pain. Scientific Reports 2022, 12, 20693. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Al-Amer, R.; Kunugi, H.; Stănculescu, E.; Taha, S.M.; Saleh, M.Y.; Alkhamees, A.A.; Hendawy, A.O. The Arabic version of the Impact of Event Scale – Revised: Psychometric evaluation in psychiatric patients and the general public within the context of COVID-19 outbreak and quarantine as collective traumatic events. Journal of Personalized Medicine 2022, 12, 681. [Google Scholar] [CrossRef] [PubMed]
- Stanisz, M.; Panczyk, M.; Kurzawa, R.; Grochans, E. The Effect of Prophylactic Adnexectomy on the Quality of Life and Psychosocial Functioning of Women with the BRCA1/BRCA2 Mutations. International Journal of Environmental Research and Public Health 2019, 16, 4995. [Google Scholar] [CrossRef] [PubMed]
- Borreani, C.; Manoukian, S.; Bianchi, E.; Brunelli, C.; Peissel, B.; Caruso, A.; Morasso, G.; Pierotti, M.A. The psychological impact of breast and ovarian cancer preventive options in BRCA1 and BRCA2 mutation carriers. Clin Genet 2014, 85, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Poggio, F.; Del Mastro, L.; Bruzzone, M.; Ceppi, M.; Razeti, M.G.; Fregatti, P.; Ruelle, T.; Pronzato, P.; Massarotti, C.; Franzoi, M.A.; et al. Safety of systemic hormone replacement therapy in breast cancer survivors: a systematic review and meta-analysis. Breast Cancer Res Treat 2022, 191, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.R.; Lee, A.M.; Mills, L.J.; Thuras, P.D.; Eum, S.; Clancy, D.; Erbes, C.R.; Polusny, M.A.; Lamberty, G.J.; Lim, K.O. Methylation of FKBP5 and SLC6A4 in Relation to Treatment Response to Mindfulness Based Stress Reduction for Posttraumatic Stress Disorder. Front Psychiatry 2018, 9, 418. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Aliberti, S.M.; Barbieri, A.; Pentangelo, P.; Bisogno, I.; D'Arena, G.; Cianciola, E.; Caraglia, M.; Capunzo, M. Potential Mechanisms by which Glucocorticoids Induce Breast Carcinogenesis through Nrf2 Inhibition. Front Biosci (Landmark Ed) 2022, 27, 223. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.M.; Kannai, A.; Sasano, H. Possible roles for glucocorticoid signalling in breast cancer. Mol Cell Endocrinol 2018, 466, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Halbert, C.H.; Schwartz, M.D.; Wenzel, L.; Narod, S.; Peshkin, B.N.; Cella, D.; Lerman, C. Predictors of Cognitive Appraisals Following Genetic Testing for BRCA1 and BRCA2 Mutations. J Behav Med 2004, 27, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W.; Dougall, A.L.; Posluszny, D.M.; Somers, T.J.; Rubinstein, W.S.; Baum, A. Psychological distress and quality of life associated with genetic testing for breast cancer risk. Psychooncology 2008, 17, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Shanmugan, S.; Sammel, M.D.; Loughead, J.; Ruparel, K.; Gur, R.C.; Brown, T.E.; Faust, J.; Domchek, S.; Epperson, C.N. Executive function after risk-reducing salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: does current mood and early life adversity matter? Menopause 2020, 27, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Woods-Burnham, L.; Stiel, L.; Martinez, S.R.; Sanchez-Hernandez, E.S.; Ruckle, H.C.; Almaguel, F.G.; Stern, M.C.; Roberts, L.R.; Williams, D.R.; Montgomery, S.; et al. Psychosocial Stress, Glucocorticoid Signaling, and Prostate Cancer Health Disparities in African American Men. Cancer Health Disparities 2020, 4, e1–e30. [Google Scholar]
- Halbert, C.H.; Jefferson, M.S.; Danielson, C.; Froeliger, B.; Giordano, A.; Thaxton, J.E. An observational study and randomized trial of stress reactivity in cancer disparities. Health Psychol 2020, 39, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Akter, N.; Kulinskaya, E.; Steel, N.; Bakbergenuly, I. The effect of hormone replacement therapy on the survival of UK women: a retrospective cohort study 1984−2017. BJOG 2022, 129, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Sim, K.-S.; Heo, W.; Kim, J.-H. Protective Effects of Coumestrol on Metabolic Dysfunction and Its Estrogen Receptor-Mediated Action in Ovariectomized Mice. Nutrients 2023, 15, 954. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Wu, L.; Fu, L.; Wang, B.; Zhou, H. Unifying mechanism in the initiation of breast cancer by metabolism of estrogen (Review). Mol Med Rep 2017, 16, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Faria, C.C.; Peixoto, M.S.; Carvalho, D.P.; Fortunato, R.S. The Emerging Role of Estrogens in Thyroid Redox Homeostasis and Carcinogenesis. Oxid Med Cell Longev 2019, 2019, 2514312. [Google Scholar] [CrossRef] [PubMed]
- Scabia, V.; Ayyanan, A.; De Martino, F.; Agnoletto, A.; Battista, L.; Laszlo, C.; Treboux, A.; Zaman, K.; Stravodimou, A.; Jallut, D.; et al. Estrogen receptor positive breast cancers have patient specific hormone sensitivities and rely on progesterone receptor. Nature Communications 2022, 13, 3127. [Google Scholar] [CrossRef] [PubMed]
- Kunugi, H.; Ali, A.M. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci 2019, 20, 4662. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Lu, T.; Pan, J.; Guo, L.; Pang, Y.; Liu, P. Association between BRCA mutations and endometrial carcinoma: a systematic review with meta-analysis. Arch Gynecol Obstet 2021, 303, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Quick, S.K.; Shields, P.G.; Nie, J.; Platek, M.E.; McCann, S.E.; Hutson, A.D.; Trevisan, M.; Vito, D.; Modali, R.; Lehman, T.A.; et al. Effect modification by catalase genotype suggests a role for oxidative stress in the association of hormone replacement therapy with postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 2008, 17, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Ali, E.M.; Mousa, A.A.; Ahmed, M.E.; Hendawy, A.O. Bee honey and exercise for improving physical performance, reducing fatigue, and promoting an active lifestyle during COVID-19. Sports Med Health Sci 2021, 3, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Physical frailty/sarcopenia as a key predisposing factor to coronavirus disease 2019 (COVID-19) and its complications in older adults. BioMed 2021, 1, 11–40. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Apitherapy for age-related skeletal muscle dysfunction (sarcopenia): A review on the effects of royal jelly, propolis, and bee pollen. Foods 2020, 9, E1362. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, J.V.; Stovall, D.W.; Kightlinger, R.S. Advances in the treatment of menopausal symptoms. Womens Health (Lond) 2009, 5, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Alkhamees, A.A.; Al-Dwaikat, T.N.; Khatatbeh, H.; A1-Dossary, S.A. The Depression Anxiety Stress Scale 8: Investigating its cutoff scores in relevance to loneliness and burnout among dementia family caregivers. Research Square 2023, V1. [Google Scholar] [CrossRef]
| Predictors | Type of Mutation | Type of Effect | Outcome Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urogenital | Somatic-Vegetative | Psychological | |||||||||
| β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | |||
| MBSR | BRCA1 BRCA2 |
Direct Indirect Direct Indirect |
-0.006 0.304 |
0.905 0.001 |
-0.200 to 0.160 0.157 to 0.439 |
0.164 -0.001 0.112 0.066 |
0.039 0.892 0.259 0.052 |
0.010 to 0.330 -0.058 to 0.045 -0.073 to 0.311 -0.001 to 0.172 |
0.089 0.085 0.116 0.172 |
0.263 0.071 0.158 0.010 |
-0.065 to 0.230 -0.006 to 0.173 -0.039 to 0.285 0.042 to 0.305 |
| HRT | BRCA1 BRCA2 |
Direct Indirect Direct Indirect |
-0.160 -0.291 |
0.119 0.019 |
-0.342 to 0.031 0.498 to -0.049 |
-0.039 -0.063 |
0.078 0.063 |
-0.124 to 0.003 -0.200 to 0.003 |
-0.049 -0.102 |
0.089 0.018 |
-0.133 to 0.005 -0.257 to -0.011 |
| Predictors | Breast Cancer History | Type of Effect | Outcome Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urogenital | Somatic-Vegetative | Psychological | |||||||||
| β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | |||
| MBSR | Yes No |
Direct Indirect Direct Indirect |
0.105 0.156 |
0.191 0.082 |
-0.073 to 0.227 -0.018 to 0.283 |
0.211 -0.004 0.086 0.049 |
0.004 0.565 0.175 0.054 |
0.054 to 0.362 -0.049 to 0.020 -0.038 to 0.222 -0.001 to 0.117 |
0.163 0.113 0.054 0.108 |
0.045 0.004 0.351 0.012 |
0.003 to 0.311 0.038 to 0.216 -0.063 to 0.171 0.019 to 0.189 |
| HRT | Yes No |
Direct Indirect Direct Indirect |
0.169 -0.191 |
0.024 0.030 |
0.026 to 0.277 -0.351 to -0.022 |
-0.006 -0.060 |
0.701 0.019 |
-0.052 to 0.034 -0.133 to -0.010 |
0.008 -0.078 |
0.634 0.022 |
-0.039 to 0.060 -0.158 to -0.012 |
| Predictors | Menopausal Status | Type of Effect | Outcome Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urogenital | Somatic-Vegetative | Psychological | |||||||||
| β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | |||
| MBSR | Menopausal Pre-menopausal |
Direct Indirect Direct Indirect |
0.201 0.134 |
0.038 0.124 |
0.009 to 0.375 -0.032 to 0.263 |
0.234 0.045 0.141 0.032 |
0.017 0.080 0.031 0.082 |
0.033 to 0.470 -0.004 to 0.156 0.011 to 0.276 -0.003 to 0.091 |
0.059 0.202 0.111 0.120 |
0.626 0.006 0.099 0.002 |
-0.179 to 0.306 0.046 to 0.383 -0.018 to 0.229 0.041 to 0.204 |
| HRT | Menopausal Pre-menopausal |
Direct Indirect Direct Indirect |
-0.081 -0.212 |
0.575 0.016 |
-0.461 to 0.199 -0.366 to -0.032 |
-0.018 -0.051 |
0.396 0.016 |
-0.170 to 0.036 -0.123 to -0.007 |
-0.037 -0.069 |
0.472 0.010 |
-0.270 to 0.080 -0.147 to -0.012 |
| Predictors | Body Mass Index | Type of Effect | Outcome Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urogenital | Somatic-Vegetative | Psychological | |||||||||
| β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | |||
| MBSR | ≤25 >25 |
Direct Indirect Direct Indirect |
0.203 -0.008 |
0.001 0.919 |
0.104 to 0.303 -0.213 to 0.164 |
0.075 0.042 0.179 -0.002 |
0.217 0.031 0.020 0.920 |
-0.044 to 0.212 0.004 to 0.098 0.023 to 0.339 -0.063 to 0.054 |
0.140 0.098 0.039 0.099 |
0.084 0.006 0.510 0.073 |
-0.021 to 0.285 0.024 to 0.180 -0.084 to 0.152 -0.009 to 0.205 |
| HRT | ≤25 >25 |
Direct Indirect Direct Indirect |
-0.068 -0.351 |
0.489 0.003 |
-0.540 to -0.125 -0.366 to -0.032 |
-0.014 -0.104 |
0.355 0.003 |
-0.083 to 0.013 -0.217 to -0.028 |
-0.020 -0.130 |
0.415 0.001 |
-0.094 to 0.030 -0.258 to -0.041 |
| Predictors | Physical Activity Level | Type of Effect | Outcome Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urogenital | Somatic-Vegetative | Psychological | |||||||||
| β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | |||
| MBSR | Yes No |
Direct Indirect Direct Indirect |
0.134 0.090 |
0.145 0.301 |
-0.058 to 0.275 -0.077 to 0.233 |
0.132 0.046 0.129 0.016 |
0.076 0.080 0.061 0.198 |
-0.015 to 0.291 -0.007 to 0.125 -0.009 to 0.274 -0.008 to 0.065 |
0.052 0.120 0.112 0.099 |
0.548 0.042 0.043 0.006 |
-0.113 to 0.211 0.004 to 0.230 0.003 to 0.221 0.022 to 0.182 |
| HRT | Yes No |
Direct Indirect Direct Indirect |
-0.162 -0.201 |
0.205 0.019 |
-0.390 to 0.082 -0.363 to -0.022 |
-0.056 -0.035 |
0.129 0.061 |
-0.171 to 0.018 -0.102 to 0.001 |
-0.070 -0.059 |
0.176 0.012 |
-0.199 to 0.029 -0.137 to -0.008 |
| Predictors | Smoking | Type of Effect | Outcome Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urogenital | Somatic-Vegetative | Psychological | |||||||||
| β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | |||
| MBSR | Yes No |
Direct Indirect Direct Indirect |
0.292 0.086 |
0.002 0.193 |
0.099 to 0.554 -0.050 to 0.189 |
-0.289 0.202 0.161 0.018 |
0.029 0.002 0.001 0.108 |
-0.526 to -0.013 0.057 to 0.479 0.055 to 0.272 -0.004 to 0.055 |
0.097 0.008 0.098 0.114 |
0.462 0.983 0.074 0.001 |
-0.182 to 0.438 -0.282 to 0.264 -0.009 to 0.200 0.049 to 0.182 |
| HRT | Yes No |
Direct Indirect Direct Indirect |
0.035 -0.247 |
0.876 0.004 |
-0.403 to 0.502 -0.381 to -0.067 |
0.024 -0.051 |
0.857 0.006 |
-0.308 to 0.322 -0.111 to -0.011 |
0.019 -0.084 |
0.829 0.002 |
-0.246 to 0.256 -0.152 to -0.030 |
| Predictors | Groups | β | p | 95% CI |
|---|---|---|---|---|
|
Age at RRSO Time since RRSO |
Type of mutation BRCA1 BRCA2 BRCA1 BRCA2 |
-0.224 -0.122 -0.210 -0.084 |
0.002 0.234 0.003 0.316 |
-0.393 to -0.077 -0.351 to 0.083 -0.342 to -0.067 -0.258 to 0.081 |
|
Age at RRSO Time since RRSO |
Menopausal status Yes No Yes No |
-0.010 0.064 -0.176 0.053 |
0.937 0.001 0.215 0.001 |
-0.307 to 0.251 -0.357 to -0.151 -0.458 to 0.087 -0.379 to -0.135 |
|
Age at RRSO Time since RRSO |
Body mass index ≤25 >25 ≤25 >25 |
-0.189 -0.142 -0.154 -0.309 |
0.006 0.120 0.031 0.001 |
-0.331 to -0.052 -0.307 to 0.034 -0.278 to -0.014 -0.438 to -0.171 |
|
Age at RRSO Time since RRSO |
Cancer history Yes No Yes No |
-0.065 -0.234 -0.275 -0.239 |
0.475 0.001 0.003 0.001 |
-0.245 to 0.109 -0.368 to -0.104 -0.454 to -0.097 -0.346 to -0.124 |
|
Age at RRSO Time since RRSO |
Current smoking Yes No Yes No |
-0.243 -0.156 -0.133 -0.221 |
0.327 0.011 0.507 0.001 |
-0.518 to 0.307 -0.271 to -0.036 -0.451 to 0.266 -0.320 to -0.117 |
|
Age at RRSO Time since RRSO |
Physical activity 0 to 4 days > 4 days 0 to 4 days > 4 days |
-0.095 -0.185 -0.311 -0.162 |
0.256 0.005 0.001 0.013 |
-0.266 to 0.072 -0.316 to -0.055 -0.452 to -0.166 -0.278 to -0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





