1. Introduction
Pigeon pox (PPV) is a poxvirus in the family Poxviridae and genus Avipoxvirus [
1,
2,
3,
4]. The genus is made of three main strains: Fowl pox virus (FPV), Canary pox virus (CPV) and PPV. Pigeon pox virus is a double-stranded enveloped DNA virus causing mild to severe slow-spreading disease in infected pigeons [
5,
6,
7]. Similar to poxviruses in other species, PPV is generally self-limiting, but can cause more severe disease in young or immunocompromised animals [
2].
Transmission of the virus is usually by direct contact through skin abrasions, contaminated feed, water and eggs [
8,
9]. Mosquitoes, other insects and cannibalism have been implicated in mechanical transmission of the virus [
2,
8]. Non-specific clinical signs include dullness, depression, dehydration, emaciation and ruffled feathers, and only a few birds developing lesions at a time [
10]. PPV leads to the formation of visible wart-like lesions known as pox scabs on mucous membranes and non-feathered skin [
10,
11,
12,
13], and rarely neurologic signs [
9]. The scabs can be the source of aerosol infection in poultry houses, resulting in respiratory tract infection [
2]. The lesions may be described as dry/cutaneous or wet/diphtheritic. The cutaneous form is predominant in most outbreaks and is characterized by scabs on non-feathered skin, unthriftiness and reduction in egg production [
10,
11,
12,
14]. The relatively severe diphtheritic form produces firmly attached, caseous pseudomembranous deposits on and in the mucous membranes of the mouth, tongue, entrance to the trachea, eye and/or nasal cavity, thereby interfering with feeding or breathing [
15]. Gross internal pox lesions do not usually appear in pigeons even though the virus may produce a systemic reaction and occasionally results in a viremia [
16,
17]. Latent infection is possible for years with reactivation usually through non-specific stress factors [
4,
14].
Although avian pox has been described worldwide with the exception of Antarctica and Arctic regions [
18,
19], reports of PPV in Africa are rare [
1,
20,
21,
22]. While fowl pox has been documented in Ghana [
23] and is relatively common, PPV has not yet been reported [
19]. Here we describe for the first-time gross necropsy, histopathology, and molecular diagnostic findings of poxvirus in a pigeon (
Columbia livia) in Ghana.
2. Materials and Methods
2.1. Case
A 5-month-old female pigeon weighing 0.14 kg kept in quarantine prior to joining 12 other pigeons, developed bumps, and was presented for veterinary was presented care. All the birds were fed mixed grains of maize, rice, millet, and soya beans. Physical examination revealed multiple nodular lesions of about 0.3 to 1 cm in diameter. The nodules were unevenly distributed chiefly on non-feathered areas including around the eyes and the beak. The lethargic bird had droopy and ruffled feathers, greenish stained vent, and was emaciated. The left eye was completely sealed by periocular nodules. The patient was euthanized using cervical dislocation and a post-mortem examination was performed after duly informing the client of the risk to the other birds, success of treatment and seeking the consent of the owner.
2.2. Postmortem and Histopathology
Necropsy of the bird was performed as described Butcher and Miles [
24] and Dharanesha [
15]. Briefly, the bird was first dipped in water containing disinfectant to reduce the chances of transmission of psittacosis [
24] and then examined externally. The internal organs were then regionally examined; the cranial, thoracic, and then abdominal regions. Images of gross lesions were captured and recorded. Nodules on the head and in the oral cavity was sectioned for histopathological processing [
15]
. 2.3. Sample Collection and Preparation
Portions of the trachea, feather stalk, oesophagus, lung, cutaneous lesions, and gastrointestinal tract were aseptically sampled into sterile tubes and transported on ice to both the Accra Veterinary Laboratory, Veterinary Service Directorate (VSD). Viral inocula were prepared as described by Sultana et al. [
5]. Briefly, a 10 % (w/v) viral suspension was made by adding phosphate buffered saline (PBS) to the ground samples. The viral suspension was then treated with 300µL of gentamicin for an hour and cultured on blood agar for 24 hours. 0.5 ml of the sterile inocula were used for inoculating embryonated eggs for viral extraction while the rest were stored at -20 ℃ for future use.
2.4. Virus Isolation
The virus was propagated by inoculating the prepared inocula into 10 -12 days old embryonated chicken hen through the chorioallantoic membrane (CAM) route as described by Rahman et al. [
25]. That is, 0.5 ml of the sterile inocula was inoculated into the centre of the CAM using sterile 1 ml, 1.5 inches needle tuberculin syringe, and the opening of the air sac and shell sealed with melted wax. The inoculated eggs were placed in trays with the CAM upwards and incubated at 37 ℃ for 5 days in an egg incubator. The inoculated eggs were then chilled at 4 – 8 ℃ for 2 hours. Afterwards, the inoculated eggs were coated with iodine tincture, cracked open and the thickened CAM harvested for preparation of inocula as described above. The viral concentration was increased by performing three passages and the CAMs with pock lesions collected and transported to the Virology Laboratory at the West African Centre for Cell Biology of Infectious Pathogens (WACCBIP) for further analysis.
2.5. DNA Extraction, PCR and Gel Electrophoresis
DNA of the virus was extracted using Quick-gDNA™ Miniprep Kit as per the instruction of the manufacturer. PCR technique targeting pox virus p4b gene with the primer set p2fPF- 5’ CAGCAGGTGCTAAACAAACAAA 3’ and p2fPR- 5’ CGGTAGCTTAACGCCGAATA 3’ with an amplification size of 578 bp [
1,
18] was employed. Over here, PCR reaction mixture containing 12.5 µL One Taq 2X master mix, 1 µL each of forward and reverse primers, 6.5 µL Nuclease-free water and 4 µL DNA template was prepared. PCR was done in PCRmax alpha cycler with cycling conditions as follows: initial denaturation at 94 ℃ for 5 min, followed by 35 cycles of denaturation at 94 ℃ for 45 sec, annealing at 48 ℃ for 1.5 min, elongation at 60 ℃ for 2 min, and final extension at 60 ℃ for 10 min. Then, a 1.5% agarose gel was prepared, and electrophoresis of the PCR products was done with a follow-up visualization using Amersham Imager 600.
2.6. Sequencing and Sequence Analysis
PCR products were purified with Wizard® SV Gel and PCR Clean-up System. Sequencing was performed using MinION (nanopore) sequencing technology and long reads were obtained. Base calling and demultiplexing was performed using guppy basecaller and barcoder respectively. Quality control was assessed, and samples were trimmed using porechop before being mapped to the PPV isolate (FeP2) reference sequence, downloaded from NCBI (NC_024447.1:193678-195654), representing the region amplified by the PCR primers. Minimap2 was using for mapping and bcftools (mpileup), vcfutils.pl, and seqtk were used to map the fastq files and generate consensus sequences for the virus isolates. Alignment was performed using mafft and phylogenetic analysis using MEGA. A maximum likelihood tree was generated using 100 bootstraps and gamma distributed invariant sites.
3. Results
3.1. Postmortem
Physical examination revealed multiple nodular lesions of about 0.3 to 1 cm in diameter distributed primarily in non-feathered regions of the bird (
Figure 1A), prominent keel bone and congested breast muscles (
Figure 1B) and diphtheritic nodules in the oral cavity (
Figure 1C). Other observed lesions were greenish pasty vent, air sacculitis and petechial haemorrhages on kidneys.
3.2. Histopathology
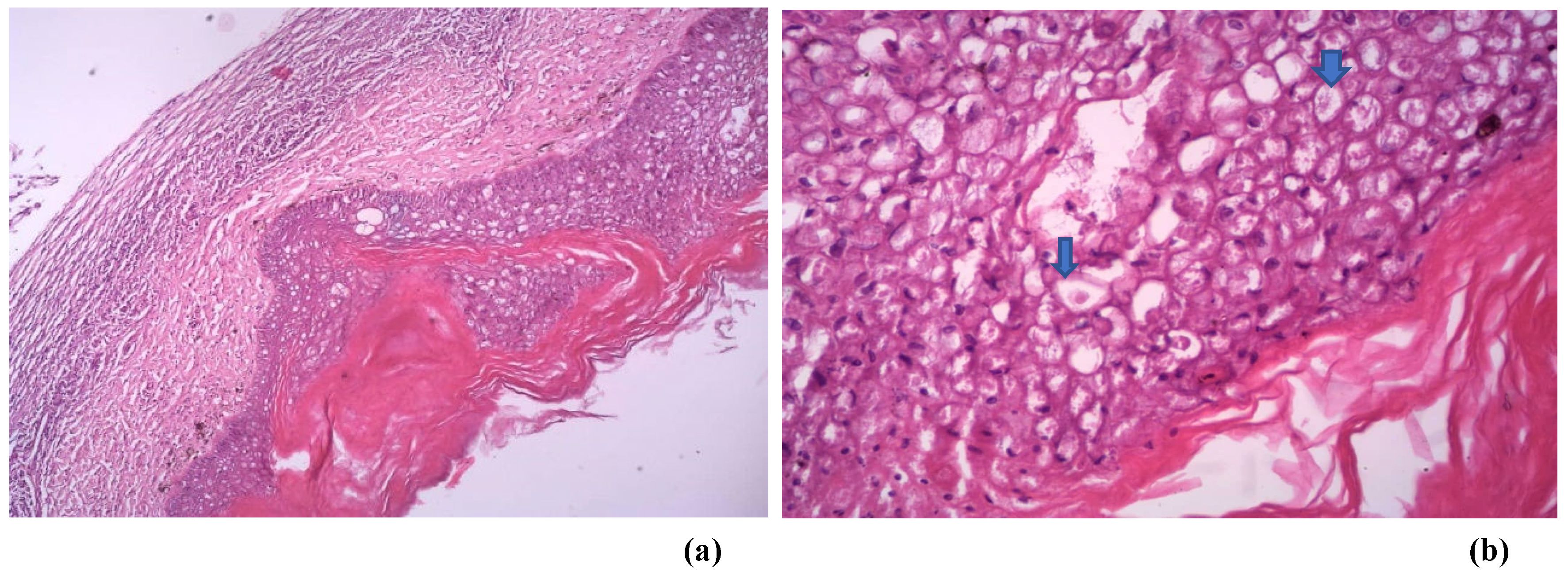
Infiltrating the epidermis were mixed inflammatory cells (macrophages and heterophils) and areas of necrosis. There is hyperkeratosis with the presence of eosinophilic intracytoplasmic inclusion bodies (Bollinger bodies) in markedly distended and vacuolated keratinocytes with pleomorphic and hyperchromatic nuclei. The presence of Bollinger bodies is an indication that the lesions were caused by a pox virus.
3.3. PCR and Gel Electrophoresis
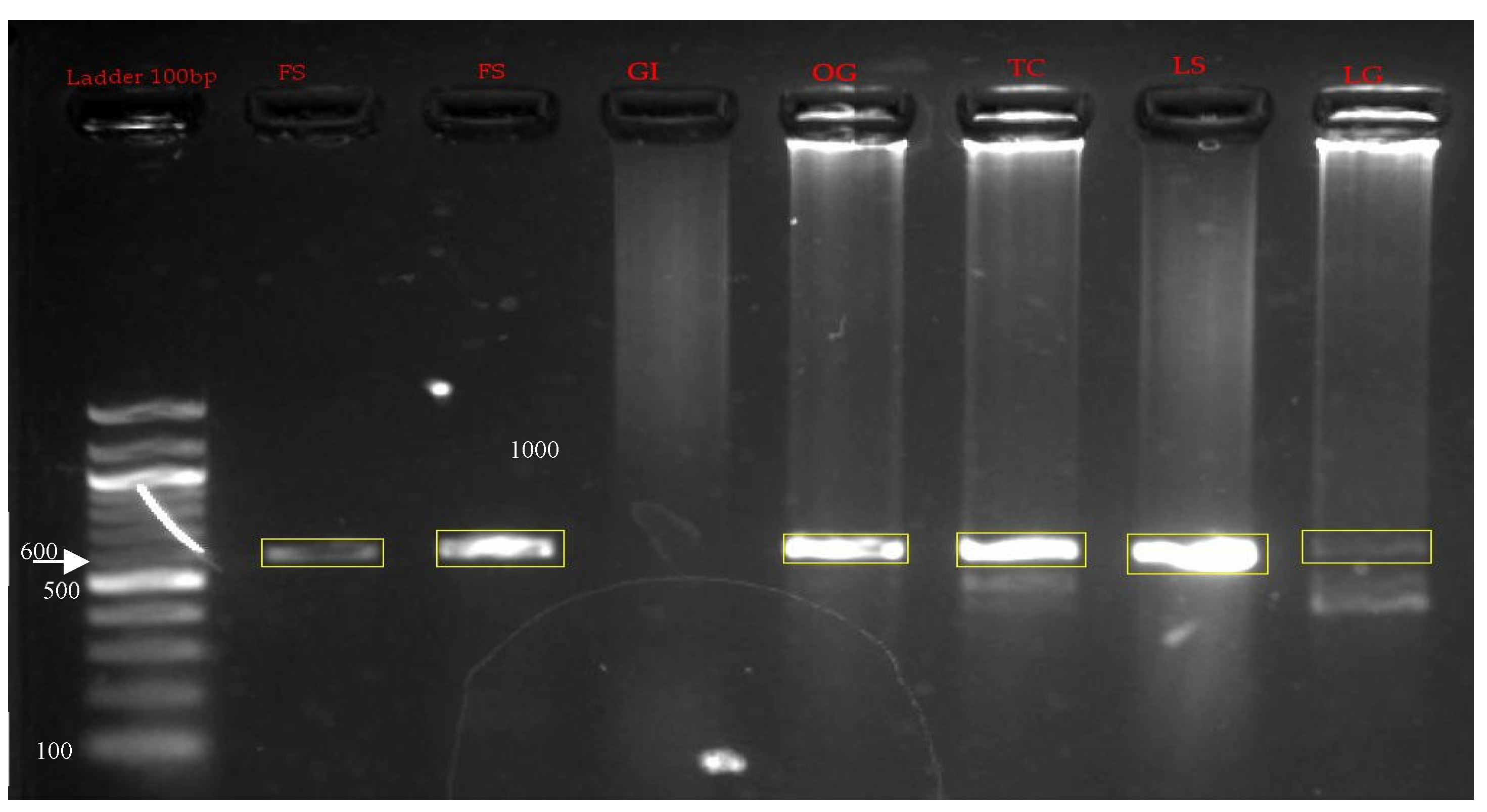
PCR products were observed in all samples except gastrointestinal tract indicating the presence or distribution of pox viruses in the trachea, lungs, cutaneous lesion, feathered stalk, and oesophagus. Band sizes of about 600 bp were observed. This was congruent with the band size expected for the p4b gene of pox virus.
Figure 3.
Agarose gel electrophoresis of the PCR products analysis of the P4b gene amplified from the isolated viruses. Lanes FS -feathered stock, GI- Gastrointestinal tract, OG- Oesophagus, TC- Trachea, LS- cutaneous lesions and LG- Lungs.
Figure 3.
Agarose gel electrophoresis of the PCR products analysis of the P4b gene amplified from the isolated viruses. Lanes FS -feathered stock, GI- Gastrointestinal tract, OG- Oesophagus, TC- Trachea, LS- cutaneous lesions and LG- Lungs.
3.4. Sequencing Data
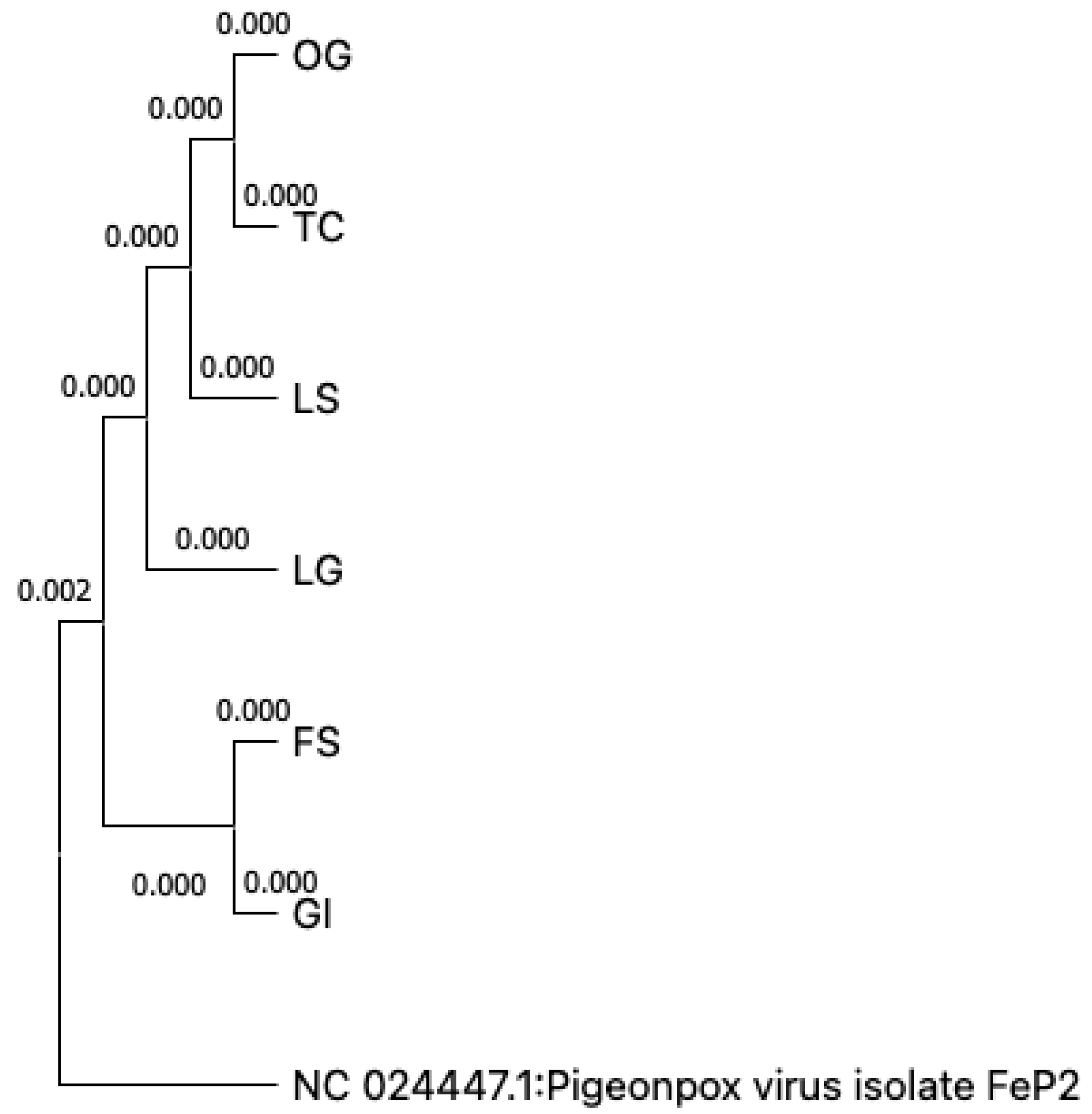
Seven samples were sequenced, and they all mapped 100% to the NC_024447.1:193678-195654 Feral Pigeonpox isolate FeP2 sequence (
Table 1). The sequences are phylogenetically related to the reference sequence (
Figure 5).
4. Discussion
This is the first confirmation of pigeon pox virus in Ghana. The tentative diagnosis was made using observation of clinical signs coupled with the presence of Bollinger bodies and the hyperkeratosis or hyperplasia of the basal epithelium is diagnostic of avian pox [
15,
26]. Definitive diagnosis of PPV was by PCR confirmation as recommended [
1,
7,
20,
22,
27]. Even though no specific lesions, as described by Audarya et al. [
12], where observed from the CAM, the gel images confirmed the PCR amplification of the P4b gene. An identical observation was reported by Abd El-Samie et al. [
1]. The infected pigeon presented with both the cutaneous and diphtheritic forms. While it relatively common for the cutaneous form to occur [
2,
6,
7,
11,
12,
21,
25,
28,
29] and the diphtheritic form is uncommon and usually associated with young animals [
27,
30,
31]. The occurrence of both forms in a bird is a rarity [
17,
32].
There is no known treatment for pigeon pox disease and attempts at treatment is usually not recommended since it might involve the disruption of the lesions which aids the spread of the virus [
2,
33]. Generally, attempts at treating birds with diphtheritic forms of pox are not successful. Wet forms of pigeon pox are complicated with inanition due to lesions in the oral cavity and respiratory tract [
9]. The accompanying dysphagia associated with formation of the diphtheritic nodules in the oral cavity makes it impractical to give oral medicines. The combination of the digestive and respiratory factors result in higher mortality in pigeons with wet forms [
11]. Euthanasia is recommended in severe cases, most instances of wet forms and in high-density colony situations as a means of preventing the spread of the disease [
2]. The severity of the case, the possibility of spread to the other birds, and the admittance of the owner not to be able to follow through with the treatment regimen influenced the decision to euthanize the pigeon. Thus, the euthanasia allowed for the disinfection of the premises with the aim of preventing the perpetuation of a rather environmentally stable virus [
34].
However, in mild cases administration of supportive and preventive treatment including disinfection and administration of electrolytes, multivitamin and antibiotics have been found to reduce mortality [
32]. For instance, the use of acyclovir (80mg/kg QID PO 8-10 days) [
10] , azithromycin (20mg/kg BID) [
10,
11], or enrofloxacin (10 mg/kg IM for 14 days) [
29] in conjunction with vitamin supplementation and topical application of turmeric and neem leave paste on the cutaneous lesions or liver tonic [
10,
11,
14,
35] proved useful in treating the cutaneous form of pigeon pox disease. To ensure the elimination of the viruses from the environment, 1% KOH, 2% NaOH or 5% phenol have been recommended [
9]. This helps reduce the rate of transmission to naïve birds on the farm during an epornitic.
At best, the complete relatedness of all the isolates to the feral pigeon pox virus isolate (FeP2) which has been isolated globally including in South Africa [
22,
36,
37], Egypt [
31], India [
38] and the general ubiquity of avipoxviruses [
18,
19,
31,
39] indicates the possible endemicity of the virus in Ghana. Furthermore, the isolation of other PPV in Nigeria [
31,
32], the only West African country with data on PPV [
31], also gives some credence to the endemic hypothesis if not denote the risk of sharing the virus based on the fluid animal movement and trade between Ghana and Nigeria.
The impact of this discovery on the general avian population in Ghana is uncertain but appears to have a potential to negatively impact the pigeon population in the country. While pigeon farming is in its budding stage, it has been shown to provide a source of income to some individuals in the Northern Regions of Ghana [
40] and a pigeon pox epornitic could be devastating to such an industry. Furthermore, most farmers prefer to keep other non-commercial birds including pigeons on their poultry farms [
23]. The pigeons on such farms can be sources of other avipoxviruses to the commercial birds [
8,
41]. The established mantra that PPV is host specific incapable of infecting or causing disease in other avian species [
7,
31,
37,
42,
43,
44] might require a second look. This is due to the lack of a clear pattern of infection regarding species-specificity among avipoxviruses [
38]. The existence of evidence of avipoxviral infection in not classically considered their host [
6,
18,
30,
45] hint at this possibility of a cross infections. Increasing evidence for the high relatedness of PPV to some avipoxviruses [
31,
36,
37] and the establishment of a serological, antigenic and pathogenic relationship between PPV, FPV and CPV [
46] also raise concern for a potential spill over into other potential avian hosts. Finally, chicken vaccinated with psittacine pox were shown to be susceptible to both fowl and pigeon pox [
47] suggesting that under the right conditions, PPV can precipitate a disease in other avian species not considered the classical host of the virus. Hence, while this could be an isolated case, it highlights the need for surveillance in determining the distribution and potential hazard of the PPV viruses to pigeons and other avian species, especially, those of production significance to Ghana.
Author Contributions
Conceptualization, K.K.A, B.E, T.O and RKA; methodology, R.K.A, B.E, and T.O; validation, O.Q; formal analysis, C.M, R.K.A, and B.E; investigation, K.K.A, S.M.A, P.T.A, P.A.B, and T.Y.N.Y; resources, T.O, R.K.A, B.E and O.Q; data curation, K.K.A, B.E, and R.K.A; writing—original draft preparation, B.E and R.K.A; writing—review and editing, G.D, T.O, and O.Q; visualization, R.K.A, C.M.; supervision, T.O, R.S and O.Q; funding acquisition, T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was primarily funded by the Accra Veterinary Laboratory and received additional support from the School of Veterinary Medicine and funds from a World Bank African Centres of Excellence grant (WACCBIP+NCDs: Awandare) and a DELTAS Africa grant (DEL-22-014: Awandare).
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
the authors are grateful to the owner of the pigeonIn this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abd El-Samie, H. A., Mohamed, H. S., Al-Bakry, I. & Manal, A. Genomic characterisation of pigeon pox virus in Egypt. Zagazig Vet. J. 43, 94–101 (2015).
- Hibl, B. M., Blackwood, R. S., Simons, B. W. & Collins, D. E. Poxvirus Infection in a Colony of Laboratory Pigeons (Columba livia). Comp. Med. 69, 179–183 (2019). [CrossRef]
- Radwan, A. & Mikhael, C. Comparative Evaluation On The Efficacy Of Embryonated Chicken Egg Adapted And Tissue Culture Pigeon Pox Vaccines Against The Local Virulent Strain. J. Appl. Vet. Sci. 5, 87–93 (2020). [CrossRef]
- Boyle, D. B. Genus Avipoxvirus. Poxviruses 217–251 (2007). [CrossRef]
- Sultana, R. et al. Isolation and molecular detection of Fowl pox and Pigeon pox viruses for the development of live attenuated vaccine seeds from the local isolates. J. Bangladesh Agric. Univ. 17, 211–219 (2019). [CrossRef]
- Khaleefah, I. A. et al. Clinical and molecular detection of fowl pox in domestic pigeons in Basrah Southern of Iraq. Korean J. Vet. Res. 64, e7 (2024). [CrossRef]
- Kabir, L., Haque, E. & Borty, S. C. Isolation and Molecular Detection of Fowl Pox and Pigeon Pox Viruses From Recent Outbreak in Bangladesh. Indian J. Life Sci. 5, 1–7 (2015).
- Williams, R. A. J., Truchado, D. A. & Benitez, L. A Review on the Prevalence of Poxvirus Disease in Free-Living and Captive Wild Birds. Microbiol. Res. (Pavia). 12, 403–418 (2021). [CrossRef]
- Greenacre, C. B. Viral diseases of companion birds. Vet. Clin. North Am. Exot. Anim. Pract. 8, 85–105 (2005). [CrossRef]
- Somasundaram, C., Raj, A. & Rangasamy, A. Therapeutic Management of Cutaneous Form of Pigeon Pox in Coimbatore District of Tamil Nadu. Int. J. Livest. Res. 1 (2020). [CrossRef]
- Sudhakara, R. S., Sivajothi, S., Reddy B, S. & S, S. Therapeutic management of cutaneous form of pox in pigeons with azithromycin. Int. Int. J. Avian Wildl. Biol. 3, 2017–2019 (2018).
- Audarya, S. D. D. et al. Molecular Diagnosis of a Cutaneous Form of Pox in Pigeons at Mhow in Madhya Pradesh. Int. J. Curr. Microbiol. Appl. Sci. 7, 1318–1323 (2018). [CrossRef]
- Haller, S. L., Peng, C., McFadden, G. & Rothenburg, S. Poxviruses and the evolution of host range and virulence. Infect. Genet. Evol. 21, 15–40 (2014). [CrossRef]
- Tripathy, D. N. & Reed, W. M. Pox. in Diseases of Poultry 333–349 (Wiley, 2013). [CrossRef]
- Dharanesha, N. K., Baghel, K. R., Pathak, M. & Saminathan, M. Gross and histopathology of avian pox in a feral pigeon (Columba spp) - A case report. Indian J. Vet. Pathol. 44, 268–270 (2020). [CrossRef]
- David C. Tudor. Pigeon Pox: a continuing problem. (1978). doi:https://www.google.com/url?sa=i&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=0CBsQw7AJahcKEwjYw9ff38eBAxUAAAAAHQAAAAAQAw&url=https%3A%2F%2Fjournals.tdl.org%2Fwatchbird%2Findex.php%2Fwatchbird%2Farticle%2Fview%2F2952%2F2936&psig=AOvVaw1eIk55IS8XGCBhigdbTKB5&ust=1695799548451951&opi=89978449.
- Akanbi, O. B., Rimfa, A. G. & Okewole, P. A. Comparative study on diphtheritic, cutaneous and systemic forms of natural avipoxvirus infection in chickens. J. World Poult. Res. 6, 117–120 (2016).
- Sharma, B. et al. Occurrence and phylogenetic analysis of avipoxvirus isolated from birds around Jammu. VirusDisease 30, 288–293 (2019). [CrossRef]
- van Riper III, C. & Forrester, D. Avian pox. in Infectious diseases of wild birds (eds. Thomas, N. J., Hunter, D. B. & Atkinson, C. T.) 131–167 (Wiley, 2007). [CrossRef]
- Abd El Hafez, M. S., Shosha, E. A. E. M. & Ibrahim, S. M. Isolation and molecular detection of pigeon pox virusin Assiut and New Valley governorates. J. Virol. Methods 293, 114142 (2021).
- Bwala, D. G., Fasina, F. O. & Duncan, N. M. Avian poxvirus in a free-range juvenile speckled (rock) pigeon (Columba guinea). J. S. Afr. Vet. Assoc. 86, e1–e4 (2015). [CrossRef]
- Offerman, K., Carulei, O., van der Walt, A. P., Douglass, N. & Williamson, A.-L. The complete genome sequences of poxviruses isolated from a penguin and a pigeon in South Africa and comparison to other sequenced avipoxviruses. BMC Genomics 15, 463 (2014). [CrossRef]
- Ouma, E. A. et al. Poultry health constraints in smallholder village poultry systems in Northern Ghana and Central Tanzania. Front. Vet. Sci. 10, 1–13 (2023). [CrossRef]
- Butcher, G. D. & Miles, R. D. Avian Necropsy Techniques. https://edis.ifas.ufl.edu/publication/VM009 (2019).
- OIE. OIE Terrestial Manual; Fowl Pox. in OIE Terrestial Manual (2018).
- Pandey, M. et al. Detection of Pox virus in domestic pigeon. Indian J. Vet. Pathol. 38, 139 (2014). [CrossRef]
- Mohan, M. & Fernandez, T. F. A case report of Pigeon Pox-Histopathologic Diagnosis. Vet. World 1, 117–118 (2008).
- Rahman, S., Islam, M., Islam, M., Nazir, K. & Khan, M. Isolation and molecular detection of Avipox virus from field outbreaks in Mymensingh, Bangladesh. J. Adv. Vet. Anim. Res. 7710, 1 (2019). [CrossRef]
- Smriti, S., Upasana, V., Rs, S. & Sb, M. Efficacy of enrofloxacin for the therapeutic management of cutaneous form of pigeon pox. Pharma Innov. J. 12, 2766–2768 (2023).
- Hartati, S., Untari, T., Nuraini, A. L. & Nururrozi, A. A Case Report of Outbreak Avian Pox Virus from Layer Chickens and a Pigeon in Yogyakarta, Indonesia. Adv. Anim. Vet. Sci. 9, 1559–1563 (2021). [CrossRef]
- Lebdah, M., Hassanin, O. & Ali, A. Avipoxvirus in Egypt and African continent: A review. Zagazig Vet. J. 47, 364–377 (2019). [CrossRef]
- Wazari, M. I. & Saidu, L. CUTENEOUS AND DIPHTHERITIC FORMS OF AVIAN POX INFECTIONS IN A JUVENILE OSTRICH. Niger. Vet. J. 43, 76–83 (2022). [CrossRef]
- Ha, H. J., Alley, M., Howe, L. & Gartrell, B. Evaluation of the pathogenicity of avipoxvirus strains isolated from wild birds in New Zealand and the efficacy of a fowlpox vaccine in passerines. Vet. Microbiol. 165, 268–274 (2013). [CrossRef]
- Martain, A. R. Avian Poxvirus. https://www.wildlifecenter.org/avian-poxvirus (2023).
- Alexander, D. J., Calnek, B. W., Barnes, H. J., Beard, C. W. & McDougald, L. R. Diseases of poultry. Dis. Poult. 11, 1281/1423 (2003).
- He, L. et al. A novel pathogenic avipoxvirus infecting oriental turtle dove (Streptopelia orientalis) in China shows a high genomic and evolutionary proximity with the pigeon avipoxviruses isolated globally. Microbiol. Spectr. 11, (2023).
- Offerman, K. et al. Six host-range restricted poxviruses from three genera induce distinct gene expression profiles in an in vivo mouse model. BMC Genomics 16, 1–20 (2015). [CrossRef]
- Pravas Sahu, B. et al. First complete genome characterization of an Indian pigeon pox virus directly 1 from a clinical sample. BioRix Prepr. (2023). [CrossRef]
- Pandey, M. M. et al. Detection of Pox virus in domestic pigeon. Indian J. Vet. Pathol. 38, 139 (2014). [CrossRef]
- Husein, S. M. A., Agbolosu, A. A. & Ishaq, N. Characterization of Pigeon ( columba livia domestica ) Production Systems and Marketing in Northern Ghana. Res. Rev. A J. Vet. Sci. Technol. 12, 35–42 (2023).
- Landolt, M. & Kocan, R. M. Transmission of avian pox from starlings to Rothchild’s mynahs. J. Wildl. Dis. 12, 353–356 (1976). [CrossRef]
- Weli, S. C., Traavik, T., Tryland, M., Coucheron, D. H. & Nilssen, Ø. Analysis and comparison of the 4b core protein gene of avipoxviruses from wild birds: Evidence for interspecies spatial phylogenetic variation. Arch. Virol. 149, 2035–2046 (2004). [CrossRef]
- Mosad, S. Conventional and Molecular Detection of Avipoxviruses from Chickens, Pigeons and Turkeys. Mansoura Vet. Med. J. 20, 85–91 (2019).
- Sharma, B., Nashiruddullah, N., Ahmed, J. A., Sharma, S. & Ahamad, D. B. Host susceptibility of different Avipoxvirus isolates. J. Entomol. Zool. Stud. 7, 437–440 (2019).
- Abdallah, F. M. & Hassanin, O. Detection and molecular characterization of avipoxviruses isolated from different avian species in Egypt. Virus Genes 46, 63–70 (2013). [CrossRef]
- Mohammed, S. K. Pathogenic, antigenic and serological relationship between fowl pox, pigeon pox and canary pox viruses. (University of Khartoum, 2005).
- Boosinger, T. R., Winterfield, R. W., Feldman, D. S. & Dhillon, A. S. Psittacine pox virus: virus isolation and identification, transmission, and cross-challenge studies in parrots and chickens. Avian Dis. 26, 437–444 (1982). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).