1. Introduction
Honeybees are one of the most important pollinating insects, providing an irreplaceable service in the maintenance and conservation of ecosystems and biodiversity. However, the complex relationship between honey bees and their environment is fraught with challenges that threaten their delicate balance. The continuing loss of colonies poses serious problems for the integrity of ecosystems, particularly in areas where human intervention is greatest. In recent decades, the number of honeybees has declined due to multiple causes [
1]. Among these, environmental factors and forage quality play critical roles influencing the health and nutritional status of honeybee colonies [
2], as well as the use of pesticides. These factors, in turn, affect the response of bees to disease and other stressors. Additionally, the season is an important factor, as honeybees, known for their adaptability, undergo profound physiological changes to meet the contrasting metabolic demands of summer and winter [
3]. These changes include variations in haemolymph proteins and trace element content in bees, which can be influenced by various factors such as malnutrition, disease, and exposure to pesticides. Trace element content, encompassing a range of essential chemical elements, is emerging as a key player in seasonal adaptation and disease response. For example,
Nosema-infected bees have lower levels of iron, manganese and nickel, which may contribute to higher mortality during the overwintering period [
4].
Among the bee pests,
Varroa destructor and
Nosema ceranae are the most widespread in Italy and currently the most difficult to control, both for the surviving honeybee and for the beekeepers.
V. destructor (Mesostigmata: Varroidae) is a cosmopolitan ectoparasitic mite known for its successful infestation of honeybee colonies worldwide. The
Varroa mite is the leading cause of colony mortality in Europe, causing colony collapse and/or death in highly infested colonies if left untreated [
5].The second most common biological agents associated with worker bee decline are the obligate intracellular microsporidia of the genus
Nosema, mainly
Nosema apis and
Nosema ceranae, both responsible for nosemosis in
Apis spp. In particular,
N. ceranae not only causes severe disease but also increases the susceptibility of honeybees to additional pathogenic infections by inducing immunosuppression [
6,
7,
8]. At the colony level,
N. ceranae infection is associated with a decrease in the size of the adult bee population, impaired brood rearing, reduced honey production, and an increased occurrence of queen replacement, contributing to increased colony losses [
9,
10,
11,
12]. Therefore, it would be interesting to have a tool such as hemolymph protein analysis to assess the nutritional and health status of honeybees in relation to
V. destructor and
Nosema spp. infestation rate.
In this complex scenario, an innovative approach should be introduced. Traditional assessments of colony welfare and performance, such as determining colony size, sealed brood, and stores could be enhanced by implementing molecular diagnostic tools. In a previous study, the authors have suggested that haemolymph total proteins, apolipophorin I and II, vitellogenin, transferrin, and hexamerin 70a may serve as a panel of biomarkers capable of assessing the nutritional and health status at the colony level [
13]. Considering these initial results, the aims of this research are to add other elements to clarify these associations by investigating the possible influence of the environment and the season on these proteins, searching for correlations between these proteins and commonly used measures of colony strength (brood, honey and pollen stores), the prevalent honeybee pathogens (
V. destructor and Nosema spp.), and three essential elements (iron, zinc, and copper).
2. Materials and Methods
2.1. Experimental Design and Colony Management
Four apiaries of Apis mellifera ligustica located in different areas of the province of Bologna (Italy) were selected. The first (identified as A) is located on the hill (San Martino in Pedriolo, municipality of Castel S. Pietro Terme). The environment is characterized by the presence of cereal crops, acacia trees, and wild grasses. The second (identified as B) is located in the plain area of Longara (municipality of Calderara di Reno) in an intensively cultivated landscape with main crops being horticulture and vineyards, both cultivated using conventional methods. The third (identified as C) is an apiary subject to nomadism during the productive season. This apiary was initially set up in June in an agricultural setting in the locality of Gaiana (Municipality of Ozzano Emilia), mainly for the production of seed vegetables such as onions and carrots. It was then moved to Castel Guelfo, on seed alfalfa, and finally finished the season in Farneto (municipality of San Lazzaro di Savena), in a context characterized by abundant late summer flowering of ivy and inula. The fourth (identified as D) is located in Ponte Rizzoli (municipality of Ozzano Emilia) in a mixed landscape characterised by acacia, linden and alfalfa.
For each apiary, 3 hives were randomly selected for the study, for a total of 12 colonies. Samplings were carried out in June, July, August, September and October 2019. Samplings were carried out in June, July, August, September and October 2019. Throughout this period, the beekeepers managed the colonies as usual and also administered Varroa treatments. In apiaries A, B, and D brood blocking was done on the 2nd, 14th and 21st day of July, respectively, followed by oxalic acid treatment 25 days later. In contrast, the colonies in apiary C were treated against Varroa with Apitraz ® (Laboratorios Calier S.A., Barcelona, Spain) from August 25th to October 18th.
None of the colonies exhibited any clinical symptom of disease during the experimental period.
2.2. Monitoring of Colony Performance
Colony performance and health status were checked monthly from June to October. During each visit, the colonies were monitored for functional control. This control was carried out by determining various indices (total brood and adult population, amount of cells occupied by honey and pollen) according to the Liebefeld method, slightly modified [
14]: during the inspection, each side of the frame was divided into six parts (later called "sixths") and the number of sixths covered by each matrix was recorded.
2.3. Varroa Detection
The
Varroa infestation in the colonies was estimated by assessing the natural fall of the mites [
15], using adhesive diagnostic sheets. The sheets were changed three times every three days for a total of nine days of evaluation before each monthly visit. They were examined by counting each individual mite on the sheet, using a mechanical counter. The average daily fall and the total monthly fall were determined.
2.4. Bee Sampling
At each hive and during each sampling time, a minimum of 50 old worker bees were sampled from the outer frames (where foragers predominate). Additionally, 50 young worker bees were sampled between the last brood frame and the stores after a visual inspection to reduce age variability; typically, nursing bees are located on this frame. The bees were carefully transported alive to the laboratory of the Department of Veterinary Medical Sciences.
2.5. Nosema Detection
Thirty old worker bees from each colony were individually tested for
Nosema spp. spores using microscopy. The bees were anesthetised on ice, their guts were extracted and placed in a 1.5 mL sterile Eppendorf tube containing 200 µL of deionised water. Each gut was then gently ground with a small pestle and 20 µL of the resulting suspension was examined under a microscope to assess the presence of
Nosema spp. spores [
11]. This method allows for the detection of an expected 10%prevalence of infection with 95% confidence in each apiary [
16].
2.6. Hemolymph Sampling
From 30 young worker bees anesthetised in ice, 1–2 µL of transparent uncontaminated haemolymph were collected as previously described [
13]. Haemolymph samples collected from bees of the same colony were pooled and stored at -80° C.
2.7. Total Protein Determination
Total protein (TP) concentration was measured using the Bradford method with a commercially available kit (Bradford Reagent, Sigma-Aldrich, MO, USA) following to the manufacturer’s instructions. Bovine serum albumin (Sigma-Aldrich, MO, USA) served as the standard for the calibration curve. Absorbance was measured with a plate reader (Varioskan™ Lux, Thermo Fisher Scientific).
2.8. Protein Separation Using SDS-PAGE
Haemolymph proteins were separated using 1D-SDS-PAGE electrophoresis. Diluted hemolymph was loaded onto 4–12% Bis-Tris polyacrylamide gels (NuPage/Thermo Fisher Scientific, Waltham, MA, USA), and electrophoresis was performed in an Xcell SureLock Mini-Cell with 2-(N-morpholino) propanesulfonic acid buffer (MOPS; NuPage/Thermo Fisher Scientific, Waltham, MA, USA) at pH 7.3 containing sodium dodecyl sulfate (SDS). Haemolymph samples were appropriately diluted to obtain 3 µg of total protein for loading onto the gel. Each gel was also loaded with standard proteins of known molecular mass (SeeBlue™ Plus2 Pre-stained Protein Standard, Thermo Fisher Scientific, Waltham, MA, USA). The electrophoresis system was connected to a power supply (Power Pack Basic-Bio-Rad, Hercules, CA, USA) with a constant voltage of 200 V for 40 min. The gels were stained with Coomassie G250 compatible with MS analysis. After staining, each gel was digitised using ChemiDocMP (Bio-Rad, Hercules, CA, USA), and pherograms were generated using ImageLab 5.2.1 software (Bio-Rad, Hercules, CA, USA). To quantify the protein bands of interest, 1 µg of protein, obtained from a solution containing 1 µg/µL of lactate dehydrogenase (LDH), (Sigma-Aldrich/Merck KGaA, Darmstadt, Germany) was added to each sample as a quantitative internal standard. ImageLab software estimated the volume of each protein band based on pixel density. The volume of the band of interest was then compared to that of the internal standard of the same lane, and the protein content was calculated.
2.9. Trace Element Determination Using Atomic Absorption Spectrometry
Trace elements were determined from the remains of both young and old worker honeybees after a careful washing by sequentially rinsing in two separate containers of deionised water. Honeybee pools (300 mg) were placed in individual acid-washed Teflon tubes and digested according to the method described by [
17]. Briefly, samples were mixed with 1–2 mL of 65% HNO
3 and 0.25-0.5 mL of 30% H
2O
2, digested in a microwave oven, transferred to 5-10 mL polyethylene volumetric flasks, and analysed using a flame atomic spectrophotometer (AAnalyst 100, PerkinElmer, Waltham, MA, USA). All the reagents were purchased from Merck (Darmstadt, Germany), with the acids being of Suprapur grade. The accuracy of the method was assessed using ERM®–BB422 fish muscle. The concentrations found with the method used in this study fell within the certified uncertainty interval provided by ERM, corresponding to a 95% confidence level. The detection limits for flame atomic spectrophotometry were 0.09 µg/mL for Fe, 0.04 µg/mL for Zn and 0.01 µg/mL for Cu. Trace element content was expressed as µg/g wet weight.
2.10. Statistical Analysis
Normality of distribution and equality of variances among groups were assessed using the Shapiro-Wilk and Levene tests, respectively [
18,
19]. Quade’s test and Quade’s all-pairs post-hoc test were used to detect differences within the same groups among times [
20,
21]. Differences among groups at the same time, were determined using Kruskal-Wallis and Dunn post-hoc tests for multiple comparisons [
20,
22]. The correlation between values for each month was assessed using Spearman’s correlation [
23]. A full dataset of p-values is reported in supplementary tables S1. A
p-value < 0.05 was considered statistically significant. Statistical analyses were performed using R 4.2.1 (R Foundation for Statistical Computing; Vienna, Austria;
https://www.R-project.org/ accessed 25 August 2023). Data are reported as mean
± SD (standard deviation).
3. Results and Discussion
3.1. Colony Performance
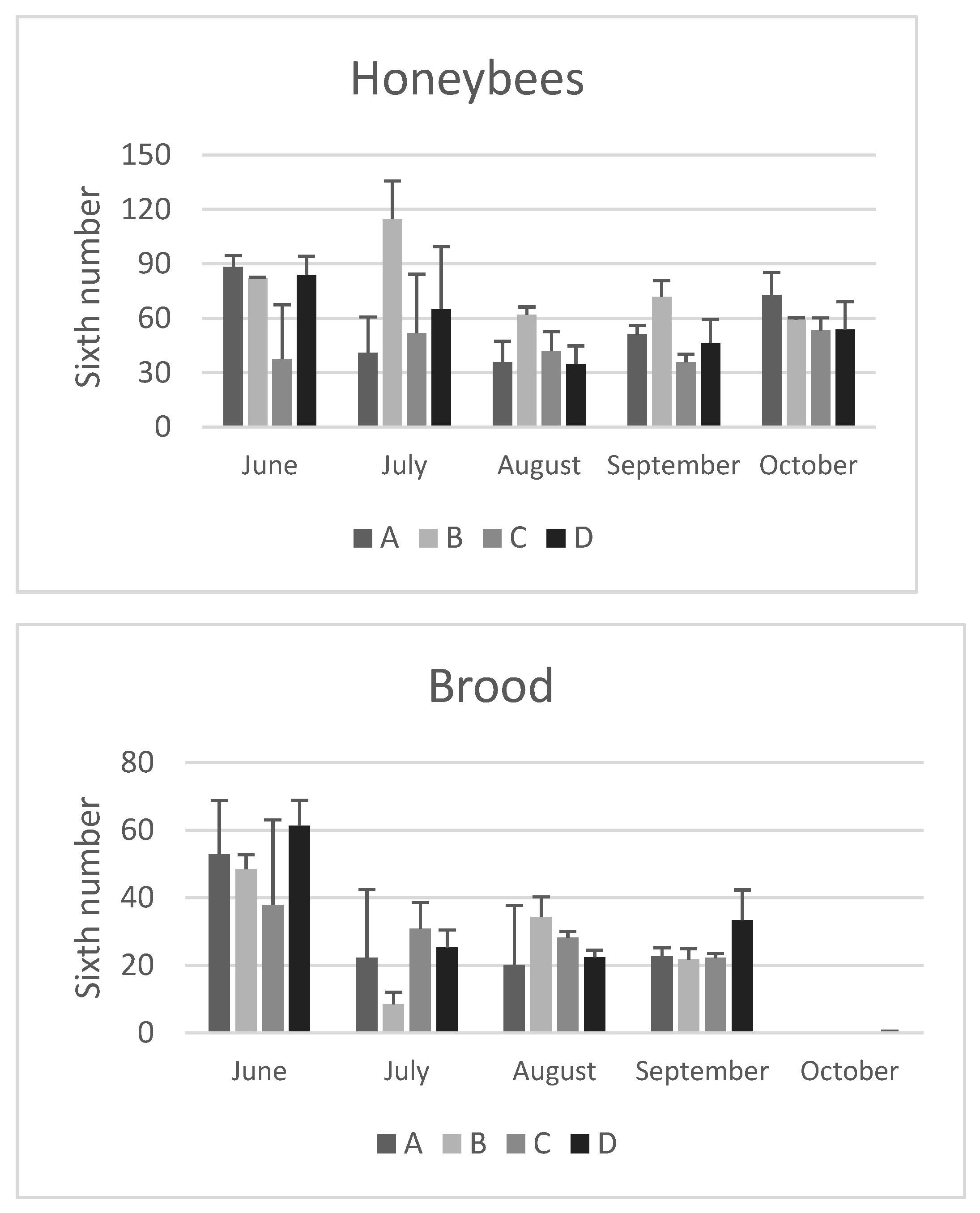
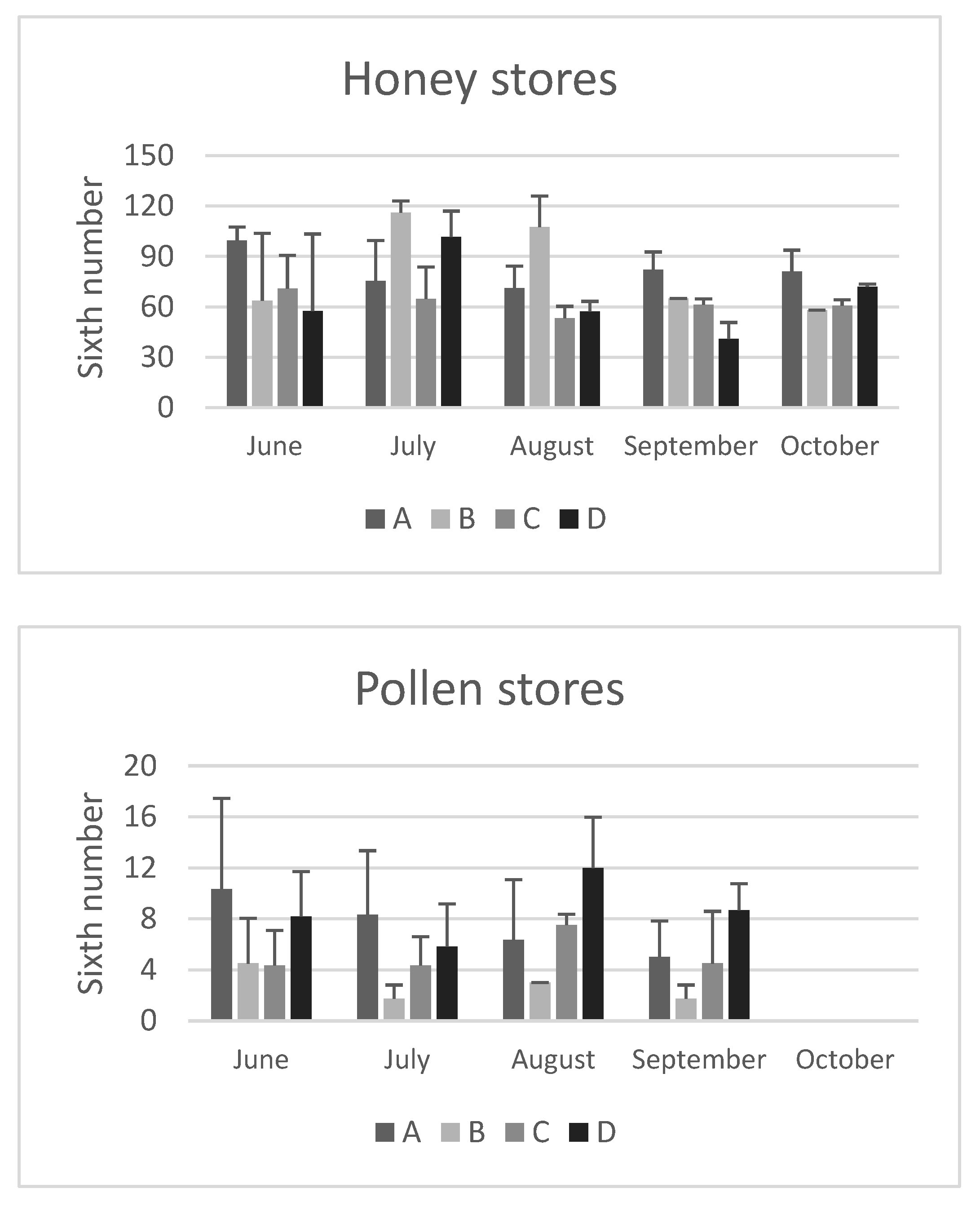
The adult bee population, brood, honey, and pollen stores for each colony were estimated using the sixths method. These data are important for determining colony strength and are shown in
Figure 1 and
Figure 2.
In June, at the beginning of the study, the colonies considered in the different apiaries did not differ significantly from each other in terms of population consistency. Overall, the location of the apiary had no significant effect on the average bee population, with the exception of colonies from apiary B, which had a significantly higher population in September than those of apiary C (
Figure 1).
When comparing brood, no significant differences were found between the colonies studied. However, a seasonal trend was observed over the months: in June, there was more brood, followed by a decrease in July- to September, and complete absence in October. This trend was consistent across all apiaries, including apiary C, where the brood blocking was not performed. It is possible that this decrease in brood during the summer months is due to a reduction in pollen availability, which is more abundant in the spring, when honeybees primarily forage for nectar. The absence of brood in October is a physiological response to the beginning of the overwintering phase. A similar trend has been reported by other authors (e.g. [
2]), who noted minimal brood from September to January.
On average, honey reserves did not differ significantly between the apiaries (
Figure 2). The only exception was colonies from apiary A, which had significantly higher stores in September compared to apiaries B and D (p<0.05). This difference could be attributed to the less anthropized area of apiary A and the different foraging environment present there.
Pollen is an essential source of proteins, lipids,
vitamins, and minerals for honeybees. It is used directly to feed larvae and consumed by nurse bees that transform it into a jelly in their hypopharyngeal glands. This jelly then is used to feed young larvae in the colony, as well as the queen. Pollen stores were not significantly affected by the season, except for their absence in October, which corresponds to the end of flowering. Regarding the influence of the site, on average there was a significant difference in August (p<0.05): the pollen stores of the colonies in apiary B, located in an intensively cultivated landscape, were lower than those in apiaries C and D. In particular, higher pollen reserves were observed in September in apiary D (
Figure 2). However, these reserves did not seem to affect the size of the colony population or the amount of pollen stored until autumn. This suggests that the bees in site D likely consumed the pollen directly rather than storing it in the colonies. A similar trend in the late summer-early autumn period was also observed by Smart et al. [
2].
3.2. Nosema and Varroa
It has been reported that the percentage of forager bees infected with
N. ceranae is a useful indicator of the extent of disease in the colony [
11]. Although
we have not performed molecular analysis to identify the species involved in
the present study, we have applied this index to Nosema ssp.
Nosema spp. showed low prevalence, ranging from 0 to 7% of infected foragers, with no significant differences between the apiaries. However, there were seasonal variations, with higher prevalence in June in all apiaries, and zero prevalence from July onwards, except in one colony in apiary B. This result is consistent with the findings of other authors. It is well known that the prevalence of
Nosema spp. is highest in winter and spring, very low in the summer due to the increasing honeybee population, and starts to increase again at the end of autumn [
24].
The seasonality of N. apis infection has long been recognised [
25]
, whereas that of N. ceranae infection is still debated. Initial studies conducted in Spain showed no seasonal differences for
Nosema [
26]
, while a recent study performed in Germany showed a pattern essentially identical to that of N. apis [
27]
.
Regarding the
Varroa infestation, starting from a relatively consistent natural mite fall in the different apiaries in June (ranging from 1 to 21 mites), various trends can be observed in July and August, corresponding to the periods of the treatments carried out in the different apiaries (
Table 1). In apiary C, a higher average mite fall was observed in September and October compared to the others, as it was still under treatment with antiparasitic strips. As previously observed, it should be noted that evaluating the natural mite fall is not always indicative of the actual infestation of the colony, as it is strongly influenced by the ratio of adult bees to brood [
15]. In fact, in colonies A and B, a higher number of natural mite falls was observed in October compared to June, which may be related to the lack of brood (
Figure 1), leading to an increase in phoretic
Varroa mites with a subsequent increase in natural fall. On the other hand, in apiary D, a higher number of
Varroa falls was observed in August, after treatment, indicating a higher infestation intensity; nevertheless, the natural fall was lower in September and October when a higher presence of brood was observed respect to the other colonies. This further emphasised the need for winter treatment to start the new season with a sufficiently low number of
Varroa mites [
28].
3.3. Hemolymph Proteins
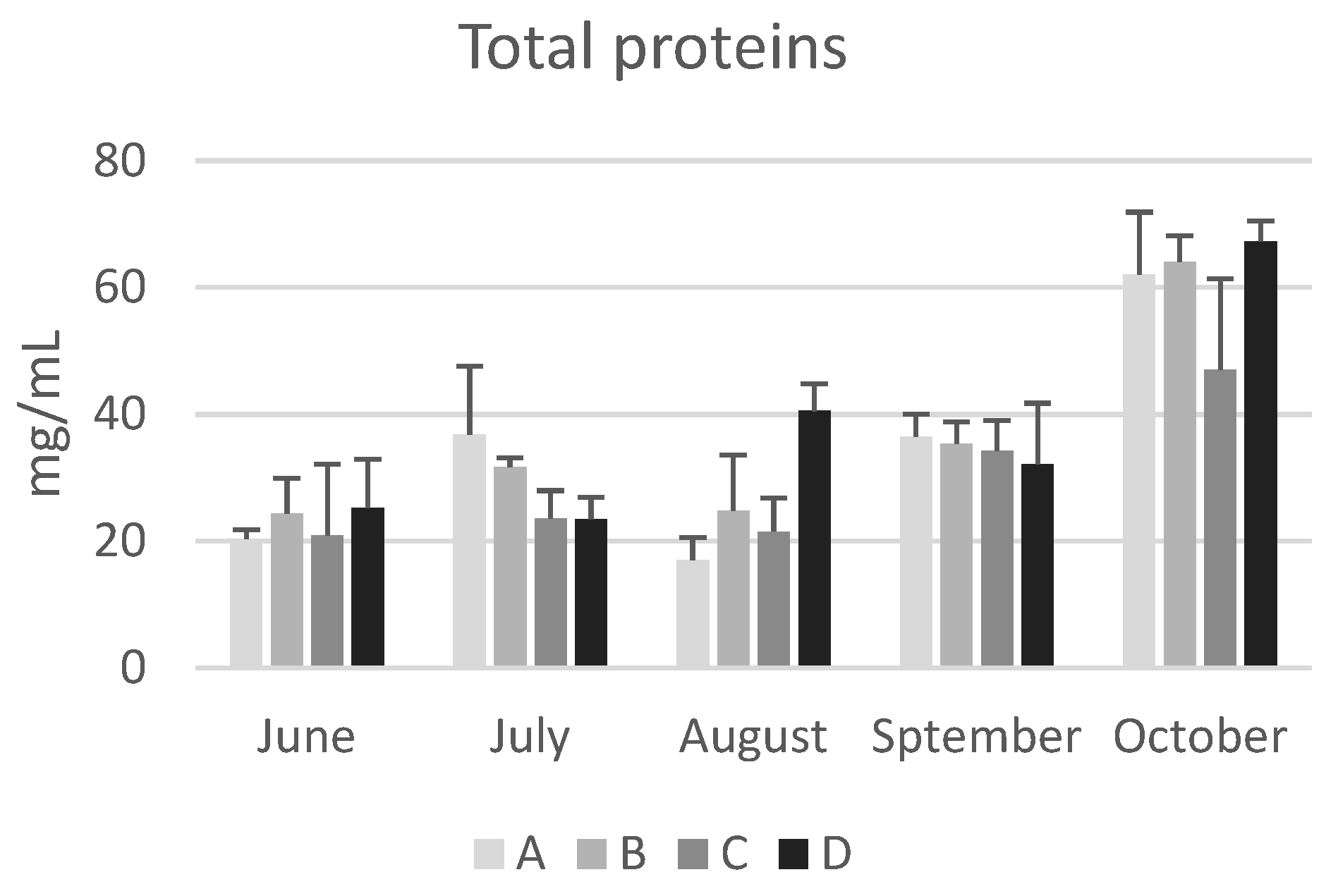
Data of hemolymph total proteins are reported in
Figure 3.
The location of the apiary site did not have a significant impact on the concentration of total protein and the values remained consistent regardless of the environmental characteristics of the apiary areas.
As with seasonal variations, in June the concentration of total proteins in bee haemolymph varied from a minimum of 20.4±1.4 mg/mL (apiary A) to a maximum of 25.3±7,6 mg/mL (apiary D). By the end of October, the values ranged from a minimum of 47.0±14.4 mg/mL (apiary C) to a maximum of 67.3±3.2 mg/mL (apiary D), showing a significant increasing trend (p<0.05) in all colonies except for those from apiary C. These values are consistent with previous reports by Cabbri et al. [
29] and Isani et al. [
13] in bees from the province of Bologna.
In agreement with previous studies [
3,
13,
30], the data reported in this research confirm that long-living winter bees have higher concentrations of haemolymph total proteins compared to summer bees. Kunc et al. [
3] proposed a physiological range of haemolymph total proteins for short-living and long-living bees in the Czech Republic, which can be also applied to honeybees from Emilia Romagna (Italy). The comparison shows a significant overlap of the intervals (
Table 2), except for the upper limit in long-living bees, which is higher in winter bees from the Czech Republic. This could be due to total proteins continuing to increase during the winter, while in Italian bees sample collection ended in October (this study) or November [
13].
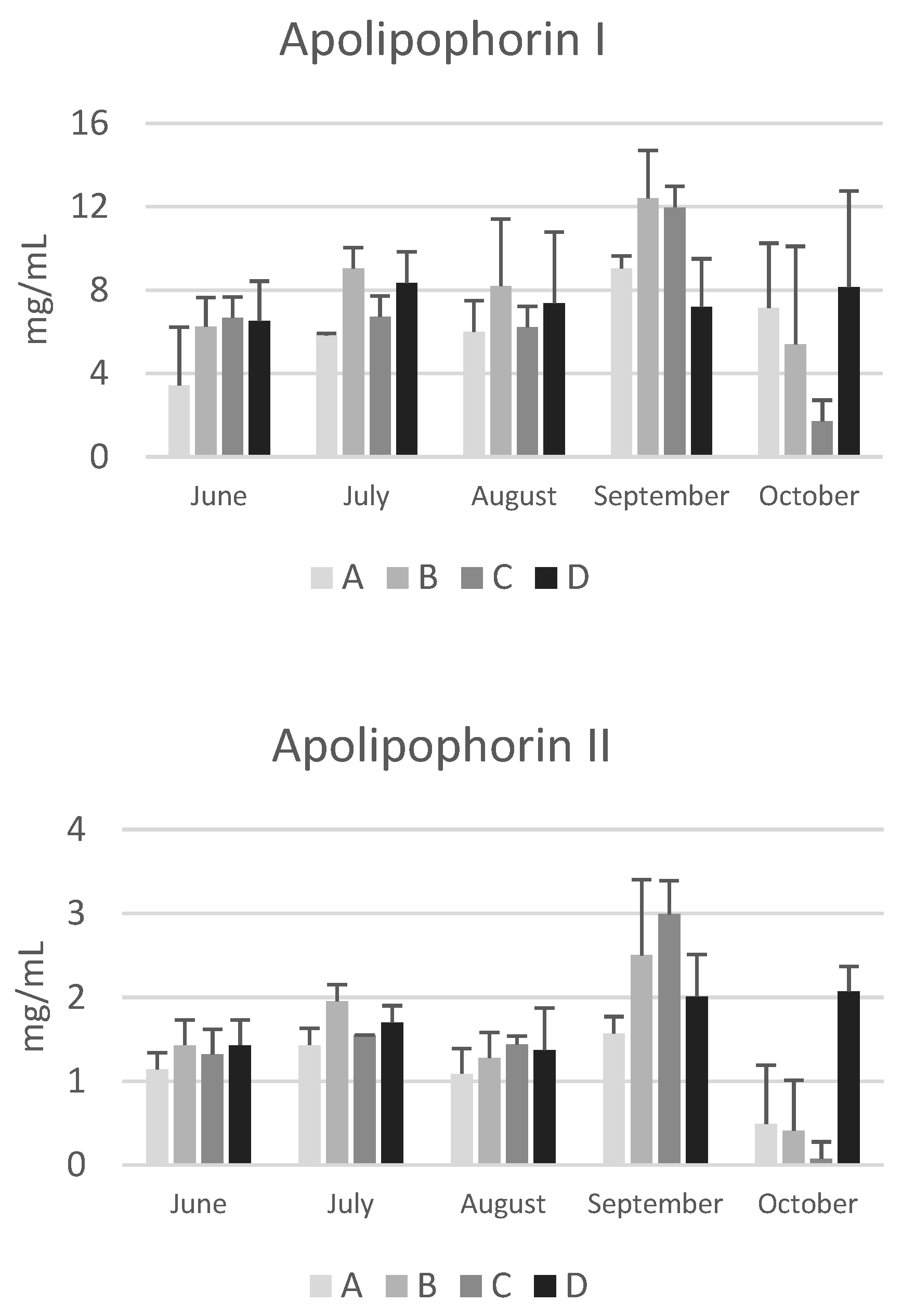
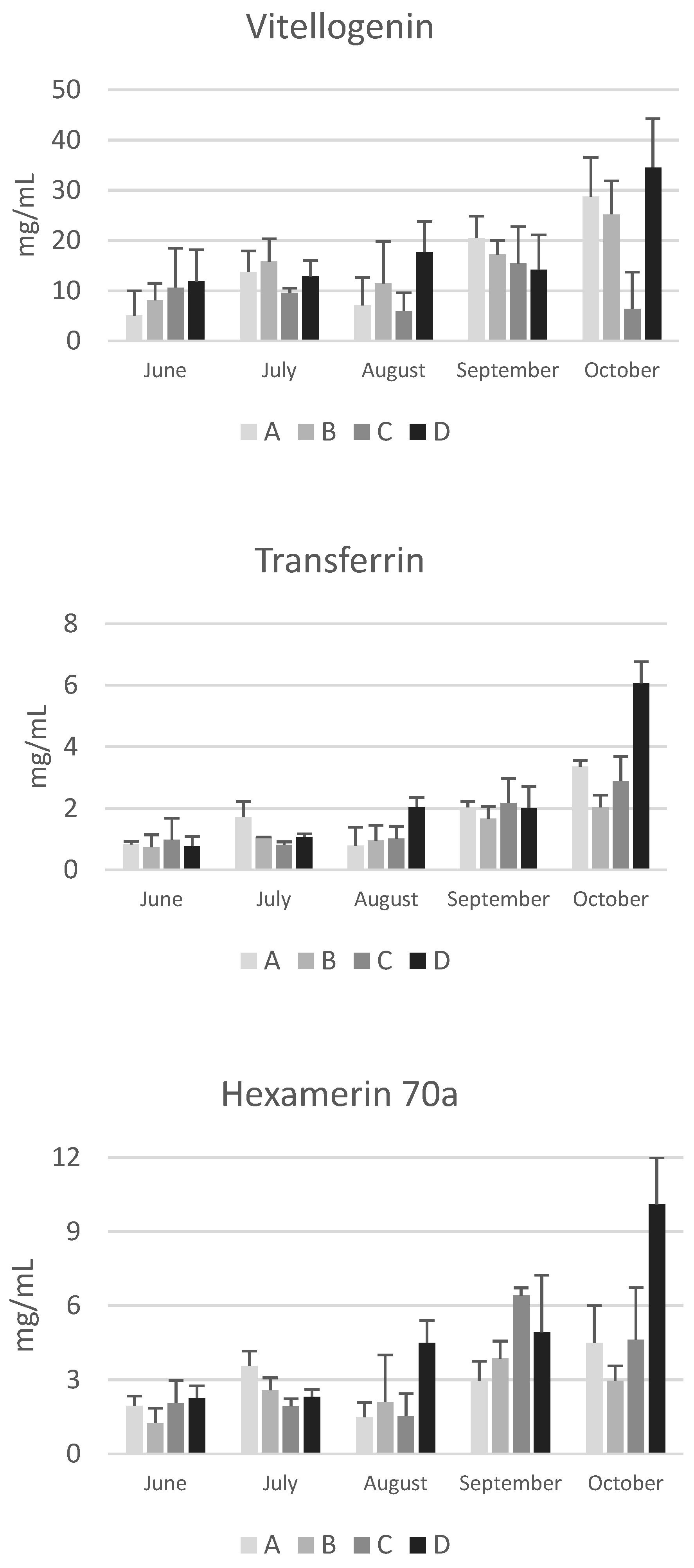
Data on vitellogenin, apolipophorins, transferrin, and hexamerin 70a are reported in
Figure 4.
Although post-hoc tests did not reveal significant differences between apiaries due to the high variability among the colonies of the same apiary, in October the concentrations of apolipophorins, vitellogenin, transferrin and hexamerin 70a are higher in bees from apiary D, particularly compared to those from apiary C, whose colonies are subject to nomadism (
Figure 4). It is known that foraging environments with intensive agriculture negatively affect the nutritional status, the resistance to oxidative stress and immune response [
31], while areas characterized by a higher abundance of non-cultivated landscapes favor a higher success during the overwintering period. Smart et al. [
32] reported that bees located in apiaries surrounded by greater floral abundance had higher levels of vitellogenin transcripts and experienced higher survival rate during the winter than bees living in environments characterized by fewer blossoms and smaller foraging areas. Accordingly, Ricigliano et al. [
31] reported that colonies in the proximity of less cultivated environments showed elevated vitellogenin gene expression, suggesting an improved nutritional condition in these colonies compared to those living in more intensively cultivated lands. The data reported in the present study are particularly interesting due to the low concentration of vitellogenin and apolipophorin I and II (
Figure 4) in October in colonies from apiary C. Despite an area characterized by abundant late summer flowering of ivy and inula the colonies of this nomadic apiary have very low concentrations of these proteins. This suggests that the management practice is not optimal and leads to a poor nutritional status of the bees at the end of the production season.
Regarding seasonal variations, an increasing trend from June to October was observed for vitellogenin, transferrin, and hexamerin 70a. This trend is in line with the increase in total proteins and confirms the finding from another study carried out in different apiaries of the Emilia Romagna region [
13]. Due to variability among colonies within the same apiary, significant increases were only found in the colonies of apiary D for vitellogenin and transferrin (p<0.01). Conversely, a significant decrease was observed in colonies of apiary C for apolipophorin I and II (p<0.05). Apolipophorins are lipid transport proteins, and their concentration in the haemolymph is related to the lipid mobilization from the fat body [
33]. Interestingly, the seasonal pattern of apolipophorin I and II differs from that of the other haemolymph proteins. These two lipid transport proteins did not show the increasing trend from June to October; notably, in October, the concentration of apolipophorin II was low in the colonies of apiaries A, B, and C, suggesting a reduced mobilisation of lipids from the fat body to the tissues.
Finally, Kunc et al. [
3] have proposed physiological intervals for vitellogenin in short-living and long-living bees in the Czech Republic. As for total proteins, physiological intervals can also be proposed for vitellogenin in short-living summer bees and long-living winter bees in Emilia Romagna (
Table 3). Despite yearly variability, the data confirm a higher concentration of vitellogenin in winter bees, in accordance to the absence of brood.
3.4. Essential Trace Elements
Iron, zinc, and copper, the most abundant essential trace elements in living organisms, were measured in honeybee sampled in August, September, and October. Data on trace element content in honeybees are given in
Table 4.
Significant differences between apiaries were found for iron and copper. In August, iron levels were significantly higher (p<0.05) in the colonies of apiary A than in those of apiaries C and D. In October, significantly higher levels of iron and copper were found in colonies of apiary B than in those of apiaries C and D (p<0.05).
The seasonal influence was evident for iron and copper with lower levels in winter bees, although the decrease from August to October was significant only for iron.
It is well known that the environment and the season influence the elemental content of honeybees, as reported in the literature [
33,
34]. However, the values found in this study are similar to those reported by Goretti et al. [
33] (who found average values of 125.84 μg/g dry weight for iron, 132.47 μg/g dry weight for zinc and 14.39 μg/g dry weight for copper in bees from apiaries in different locations in Umbria), Ćirić et al. [
35] (who found values from 47.79 to 64.50 μg/g for iron, from 29.14 to 47.56 μg/g for zinc and from 6.27 to 7.67 μg/g for copper in worker honeybees collected from different locations in Serbia), and Zarić et al. [
34] (who reported intervals of 102 to 265 μg/g dry weight for iron, 60-142 μg/g dry weight for zinc, and 15-31 μg/g dry weight for copper, in individual honeybees from Serbia and Austria), assuming a mean value of dry weight equal to 28% of wet weight [
36].
In August, the great homogeneity of the values obtained, except for the iron value of 79.2 found in the colonies of apiary A, regardless of the location and the management of the apiaries, suggests that these values may represent the physiological levels of iron (between 46.7 and 49.2 µg/g), zinc (between 32.3 and 36.1 µg/g) and copper (between 8.41 and 8.89 µg/g) in healthy summer bees. For winter bees, there was a higher variability between apiaries, with values ranging from 23.3 to 38.5 μg/g wet weight for iron, from 28.2 to 41.5 μg/g wet weight for zinc, and from 5.7 to 10.6 μg/g wet weight for copper.
In winter bees the level of zinc was higher than that of iron. Vitellogenin is the primary circulating zinc-carrying protein in honeybees. A recent study has shown that this protein can bind on average 3 Zn
2+ ions/molecule [
37]. As a consequence, the increase in zinc in winter bees may be related to the increase in vitellogenin. Due to their involvement in the immune defense system, this could be a physiological adaptation to cope with the cold season with a higher potential to induce an immune response The significant decrease in iron in winter bees may be related to several causes: reduced activity and opportunities to feed due to lack of flowering, and the need to reduce a possible source of oxidative stress to increase the longevity. This finding is consistent with data reported by Ilijec et al. [
38], while Ptaszyńska et al. [
4] observed the highest iron content during winter in honeybees from Poland.
3.5. Correlations between Biochemical and Functional Parameters and Parasites
An analysis was conducted to determine possible correlations between biochemical, functional parameters and parasites. The p values of Spearman correlation coefficients are reported in supplementary table S1.
In August, a significant negative correlation was found between total brood and pollen (p<0.05), vitellogenin (p<0.05), transferrin (p<0.01), and hexamerin 70a (p<0.05). It is logical that the more brood there is to feed the fewer pollen stores there are. By October there is no brood left (
Figure 1), no pollen stores remaining (
Figure 2) and the winter bees, whose haemolymph is rich in vitellogenin and hexamerin 70a, have become living protein stores.
The amount of pollen stores in June and August showed a significant positive correlation with total proteins (p<0.05; p<0.05), transferrin (p<0.01; p<0.01), and hexamerin 70a (p<0.05; p<0.05). This correlation is particularly interesting in August, as the quantity and quality of pollen reserves accumulated during the late summer are crucial for successful overwintering. Similarly, Di Pasquale et al. [
39] reported that vitellogenin and transferrin gene expression were significantly higher in bees fed with pollen than in bees that did not receive pollen. Furthermore, these authors also focused on the effects of different types of pollen, suggesting that not only the availability but also the quality of pollen is important for bee health.
In the present study, no correlation was found between the presence of Nosema spp. and biochemical or functional parameters. However, the prevalence of infected bees in the apiaries studied was rather low and limited to the first observation period.
It has been described in the literature that honeybees from colonies with low levels of
N. ceranae infection have been found to produce more vitellogenin than bees from colonies with high levels, and this finding has been associated with colony resistance to
N. ceranae [
40]. Given that pollen diversity and nutritional quality can influence bee health, the diet may also influence the tolerance to
N. ceranae [
39,
41,
42]
. In fact, healthy and N. ceranae-infected honeybees survive longer when they eat pollen, and the quality of the pollen strongly influences how the infection affects the bees [
39]
.
Conversely, many significant correlations were found between the number of
Varroa mites and haemolymph components. Honeybees usually overwinter with very low or no pollen reserves, as was the case with the colonies in this study (
Figure 2). The haemolymph proteins of winter bees are crucial for survival and for brood nutrition in spring. In June,
Varroa infestation was positively correlated with total proteins (p<0.05) and transferrin (p<0.05), while in October it was negatively correlated with total proteins (p<0.05), vitellogenin (p<0.01), apolipophorin II (p<0.01), transferrin (p<0.01) and hexamerin 70a (p<0.01). This last finding suggests a reduced storage capacity and a reduced immune response in infested bees, mainly due to the lower concentration of vitellogenin, the most abundant protein in honeybee haemolymph, which is also involved in immunity. Accordingly, it has been reported that
Varroa infestation affects the nutritional status of honeybees [
43,
44]
and compromises their normal polyethism by altering the interaction between vitellogenin and juvenile hormone, two essential physiological components [
45]
. In addition, parasitisation reduces the life expectancy and impairs immune system function, increasing the risk of viral infections. The negative correlation between Varroa infestation and vitellogenin is consistent with data previously reported by Kunc et al. [
46]. These authors found that vitellogenin gene expression was significantly downregulated and haemolymph vitellogenin was lower in the
Varroa-parasitized (1.19 ± 1.17 mg/mL) bees as compared to non-parasitised bees (7.75 ± 3.30 mg/mL). The negative effects of
Varroa are due to the mite feeding on the fat body and haemolymph of immature and adult bees [
47]. Low concentrations of total and specific haemolymph proteins during the critical pre-winter period may be a relevant factor in determioning colony success or collapse after winter.
The homeostasis of essential elements is related to the health status of an organism. A close relationship between
Varroa infestation and iron metabolism was evidenced by the negative correlation between them in August, suggesting a possible negative effect of the parasite on iron homeostasis. To the author’s knowledge, there are no data in the literature on the effect of
Varroa on iron levels in honeybees, whereas
N. ceranae infection has been found to cause iron deficiency in honeybees and to increase the production of transferrin, which binds and transports iron [
48]
. These findings suggest that N. ceranae may have developed a method of scavenging iron from the host, and that N. ceranae parasitism may lead to a significant reduction in total body iron reserves. Furthermore, Rodríguez-García et al. [
48] reported that
N. ceranae infection caused upregulation of the transferrin gene to counteract iron depletion by the pathogen and Ptaszyńska et al. [
4] suggested that the decrease in Fe, Mn, Ni, and Na observed in
Nosema-infected bees compared to uninfected bees could be the reason for the higher mortality of
Nosema-infected bees during the overwintering period.
Finally, Spearman correlation analysis also revealed many significant correlations among haemolymph proteins. From June to October, a significant positive correlation was observed between vitellogenin and total proteins as well as between vitellogenin and transferrin and hexamerin 70a. This suggests an interplay between these multitasking haemolymph proteins related to brood nutrition and immunity.
4. Conclusions
In this study, the traditional assessment of colony welfare and performance was conducted by relating colony size, sealed brood, honey and pollen stores, and the presence of Nosema and Varroa pathogens with molecular diagnostic parameters.
The location of the apiary and the surrounding environment are crucial factors for the success of a colony, as reported in numerous publications. Despite variations in locations and foraging environments, which can be considered representative of the landscape of the Emilia-Romagna region, the four apiaries selected for this study showed few significant differences. The management of the colonies appears to have a greater impact on determining the trophic state. The haemolymph proteins indicate that the colonies from apiary D are in a better trophic condition, whereas the colonies of apiary C, which are subject to nomadism, are in a poorer nutritional condition.
Biomarkers measured in honeybee haemolymph have proven to be useful for monitoring changes in trophic conditions during summer and early autumn. In October, the absence of brood and the high concentrations of total proteins in the haemolymph indicate the presence of winter bees. Since long-living bees are the ones that must survive the winter, their nutritional and health status is critical for the success of the colony. These bees have a high concentration of proteins, particularly vitellogenin, as well as transferrin and hexamerin 70a, which may play a key role in the physiology of winter bees.
Several interesting correlations were found between the biomarkers analysed and the traditional performance indices. In particular, in June and August a significant positive correlation was observed between pollen stores and total proteins, transferrin and hexamerin 70a, indicating a beneficial effect of pollen integration to prepare the colonies for the winter phase with sufficient reserves. On the other hand, in October, a negative correlation was observed between the Varroa infestation and the concentration of different haemolymph proteins highlighting the importance of combating this parasite in the autumn and winter. In addition, a significant negative correlation between the Varroa infestation and the iron content was found, suggesting a deterimental effect of the parasite also on iron metabolism with possible implications for immunity and orientation, which merits further studies in the future.
Finally, the data reported in this study confirm the abundance of vitellogenin, which is the major component of the haemolymph proteome, and consequently the importance of quantifying this protein as a nutritional biomarker also at the colony level. The proposed intervals for total proteins and vitellogenin could be considered as a first attempt to define physiological ranges in healthy summer and winter honeybees in Italy. In addition, other important proteins, including transferrin and hexamerin 70a are certainly of great interest and deserve attention in future studies.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org, Table S1: p values of Spearman correlation.
Author Contributions
C.R. investigation, writing—original draft; G.I. conceptualization, validation, writing—original draft, writing—review and editing, project administration, and funding acquisition; R.C. investigation, statistical analysis; T.D. software and statistical analysis; G.A. investigation, writing—review and editing; L.F. conceptualization, writing—review and editing; R.G. investigation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Regione Emilia-Romagna: BEE-RER-3 project—CUP E37G22000030007—del Regolamento (UE) no. 1308/2013—(OCM Apicoltura) and by Agritech National Research Center and received funding from the European Union Next-Generation EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)–MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4–D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions; neither the European Union nor the European Commission can be considered responsible for them.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data reported in this study are available upon request.
Acknowledgments
We would like to thank Dr. Thomas Dalmonte for his help and support with the statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Steinhauer N, Kulhanek K, Antúnez K; et al. Drivers of colony losses. Curr Opin insect Sci. 2018;26:142-148. [CrossRef]
- Smart M, Pettis J, Rice N, Browning Z, Spivak M. Linking Measures of Colony and Individual Honey Bee Health to Survival among Apiaries Exposed to Varying Agricultural Land Use. PLoS ONE. 2016;11(3):e0152685. [CrossRef]
- Kunc M, Dobeš P, Hurychová J; et al. The Year of the Honey Bee (Apis mellifera L.) with Respect to Its Physiology and Immunity: A Search for Biochemical Markers of Longevity. Insects. 2019;10(8). [CrossRef]
- Ptaszyńska AA, Gancarz M, Hurd PJ; et al. Changes in the bioelement content of summer and winter western honeybees (Apis mellifera) induced by Nosema ceranae infection. PLoS ONE. 2018;13(7):e0200410. [CrossRef]
- Brodschneider R, Gray A, Adjlane N; et al. Multi-country loss rates of honey bee colonies during winter 2016/2017 from the COLOSS survey. J Apic Res. 2018;57(3):452-457. [CrossRef]
- Antúnez K, Martín-Hernández R, Prieto L, Meana A, Zunino P, Higes M. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ Microbiol. 2009;11(9):2284-2290. [CrossRef]
- Costa C, Tanner G, Lodesani M, Maistrello L, Neumann P. Negative correlation between Nosema ceranae spore loads and deformed wing virus infection levels in adult honey bee workers. J Invertebr Pathol. 2011;108(3):224-225. [CrossRef]
- Zheng H-Q, Gong H-R, Huang S-K, Sohr A, Hu F-L, Chen YP. Evidence of the synergistic interaction of honey bee pathogens Nosema ceranae and Deformed wing virus. Vet Microbiol. 2015;177(1):1-6. [CrossRef]
- Higes M, Martín-Hernández R, Garrido-Bailón E; et al. Honeybee colony collapse due to Nosema ceranae in professional apiaries. Environ Microbiol Rep. 2009;1(2):110-113. [CrossRef]
- Botías C, Martín-Hernández R, Barrios L, Meana A, Higes M. Nosema spp. infection and its negative effects on honey bees (Apis mellifera iberiensis) at the colony level. Vet Res. 2013;44(1):25. [CrossRef]
- Higes M, Martín-Hernández R, Botías C; et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ Microbiol. 2008;10(10):2659-2669. [CrossRef]
- VanEngelsdorp D, Evans JD, Saegerman C; et al. Colony collapse disorder: A descriptive study. PLoS ONE. 2009;4(8):e6481.
- Isani G, Bellei E, Rudelli C, Cabbri R, Ferlizza E, Andreani G. SDS-PAGE-Based Quantitative Assay of Hemolymph Proteins in Honeybees: Progress and Prospects for Field Application. Int J Mol Sci. 2023;24(12). [CrossRef]
- Delaplane KS, van der Steen J, Guzman-Novoa E. Standard methods for estimating strength parameters of Apis mellifera colonies. J Apic Res. 2013;52(1):1-12. [CrossRef]
- Branco MR, Kidd NAC, Pickard RS. A comparative evaluation of sampling methods for Varroa destructor (Acari: Varroidae) population estimation. Apidologie. 2006;37(4):452-461. [CrossRef]
- Pirk CWW, de Miranda JR, Kramer M; et al. Statistical guidelines for Apis mellifera research. J Apic Res. 2013;52(4):1-24. [CrossRef]
- Andreani G, Ferlizza E, Cabbri R, Fabbri M, Bellei E, Isani G. Essential (Mg, Fe, Zn and Cu) and Non-Essential (Cd and Pb) Elements in Predatory Insects (Vespa crabro and Vespa velutina): A Molecular Perspective. Int J Mol Sci. 2021;22(1). [CrossRef]
- Ahad N, Sin Yin T, Othman AR, Yaacob C. Sensitivity of Normality Tests to Non-normal Data. Sains Malaysiana. 2011;40:637-641.
- Fox Jhoon. Applied_Regression_Analysis_and_Generali. 2015;3. https://books.google.es/books?id=3wrwCQAAQBAJ&dq=Applied+Regression+Analysis+and+Generalized+Linear+Models,+Third+Edition.+Sage.&lr=&hl=es&source=gbs_navlinks_s.
- Hollander M, Wolfe DA, Chicken E. Nonparametric Statistical Methods. Wiley; 2013. https://books.google.it/books?id=Y5s3AgAAQBAJ.
- Quade, D. Using Weighted Rankings in the Analysis of Complete Blocks with Additive Block Effects. J Am Stat Assoc. 1979;74(367):680-683. [CrossRef]
- Dunn, OJ. Multiple Comparisons Using Rank Sums. Technometrics. 1964;6(3):241-252. [CrossRef]
- Lin LI-K. A Concordance Correlation Coefficient to Evaluate Reproducibility. Biometrics. 1989;45(1):255-268. [CrossRef]
- Emsen B, De la Mora A, Lacey B; et al. Seasonality of Nosema ceranae Infections and Their Relationship with Honey Bee Populations, Food Stores, and Survivorship in a North American Region. Vet Sci. 2020;7(3). [CrossRef]
- Bailey L, Ball B V. 6 - MICROSPORA AND PROTOZOA. In: Bailey L, Ball BVBT-HBP (Second E, eds. Academic Press; 1991:64-77. [CrossRef]
- Martín-Hernández R, Meana A, Prieto L, Salvador AM, Garrido-Bailón E, Higes M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl Environ Microbiol. 2007;73(20):6331-6338. [CrossRef]
- Gisder S, Schüler V, Horchler LL, Groth D, Genersch E. Long-Term Temporal Trends of Nosema spp. Infection Prevalence in Northeast Germany: Continuous Spread of Nosema ceranae, an Emerging Pathogen of Honey Bees (Apis mellifera), but No General Replacement of Nosema apis. Front Cell Infect Microbiol. 2017;7:301. [CrossRef]
- van Dooremalen C, Gerritsen L, Cornelissen B, van der Steen JJM, van Langevelde F, Blacquière T. Winter Survival of Individual Honey Bees and Honey Bee Colonies Depends on Level of Varroa destructor Infestation. PLoS ONE. 2012;7(4):e36285. [CrossRef]
- Cabbri R, Ferlizza E, Nanetti A; et al. Biomarkers of nutritional status in honeybee haemolymph: Effects of different biotechnical approaches for Varroa destructor treatment and wintering phase. Apidologie. 2018;49(5):606-618. [CrossRef]
- Dostálková S, Dobeš P, Kunc M; et al. Winter honeybee (Apis mellifera) populations show greater potential to induce immune responses than summer populations after immune stimuli. J Exp Biol. 2021;224(Pt 3). [CrossRef]
- Ricigliano VA, Mott BM, Maes PW; et al. Honey bee colony performance and health are enhanced by apiary proximity to US Conservation Reserve Program (CRP) lands. Sci Rep. 2019;9(1):4894. [CrossRef]
- Smart MD, Otto CR V, Lundgren JG. Nutritional status of honey bee (Apis mellifera L.) workers across an agricultural land-use gradient. Sci Rep. 2019;9(1):16252. [CrossRef]
- Goretti E, Pallottini M, Rossi R; et al. Heavy metal bioaccumulation in honey bee matrix, an indicator to assess the contamination level in terrestrial environments. Environ Pollut. 2020;256:113388. [CrossRef]
- Zarić NM, Brodschneider R, Goessler W. Honey bees as biomonitors - Variability in the elemental composition of individual bees. Environ Res. 2022;204(Pt C):112237. [CrossRef]
- Ćirić J, Spirić D, Baltić T; et al. Honey Bees and Their Products as Indicators of Environmental Element Deposition. Biol Trace Elem Res. 2021;199(6):2312-2319. [CrossRef]
- Brodschneider R, Riessberger-Gallé U, Crailsheim K. Flight performance of artificially reared honeybees (Apis mellifera). Apidologie. 2009;40:441-449. [CrossRef]
- Leipart V, Enger Ø, Turcu DC; et al. Resolving the zinc binding capacity of honey bee vitellogenin and locating its putative binding sites. Insect Mol Biol. 2022;31(6):810-820. [CrossRef]
- Ilijević K, Vujanović D, Orčić S; et al. Anthropogenic influence on seasonal and spatial variation in bioelements and non-essential elements in honeybees and their hemolymph. Comp Biochem Physiol C Toxicol Pharmacol. 2021;239:108852. [CrossRef]
- Di Pasquale G, Salignon M, Le Conte Y; et al. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE. 2013;8(8):e72016. [CrossRef]
- Antúnez K, Mendoza Y, Santos E, Invernizzi C. Differential expression of vitellogenin in honey bees (Apis mellifera) with different degrees of Nosema ceranae infection. J Apic Res. 2013;52:227-234. [CrossRef]
- Jack CJ, Uppala SS, Lucas HM, Sagili RR. Effects of pollen dilution on infection of Nosema ceranae in honey bees. J Insect Physiol. 2016;87:12-19. [CrossRef]
- Tritschler M, Vollmann JJ, Yañez O, Chejanovsky N, Crailsheim K, Neumann P. Protein nutrition governs within-host race of honey bee pathogens. Sci Rep. 2017;7(1):14988. [CrossRef]
- Aronstein KA, Saldivar E, Vega R, Westmiller S, Douglas AE. How Varroa Parasitism Affects the Immunological and Nutritional Status of the Honey Bee, Apis mellifera. Insects. 2012;3(3):601-615. [CrossRef]
- Frizzera D, Andreuzza L, Boaro G; et al. The Interaction between a Parasite and Sub-Optimal Temperatures Contributes to Honey Bee Decline.; 2021. [CrossRef]
- Zanni V, Değirmenci L, Annoscia D, Scheiner R, Nazzi F. The reduced brood nursing by mite-infested honey bees depends on their accelerated behavioral maturation. J Insect Physiol. 2018;109:47-54. [CrossRef]
- Kunc M, Dobeš P, Ward R; et al. Omics-based analysis of honey bee (Apis mellifera) response to Varroa sp. parasitisation and associated factors reveals changes impairing winter bee generation. Insect Biochem Mol Biol. 2023;152:103877. [CrossRef]
- Ramsey SD, Ochoa R, Bauchan G; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc Natl Acad Sci U S A. 2019;116(5):1792-1801. [CrossRef]
- Rodríguez-García C, Heerman MC, Cook SC; et al. Transferrin-mediated iron sequestration suggests a novel therapeutic strategy for controlling Nosema disease in the honey bee, Apis mellifera. PLoS Pathog. 2021;17(2):1-30. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).