1. Introduction
Functional movement disorders (FMDs) are a group of movement disorders without any organic cause. Among the motor subtypes, positive findings include tremors, dystonia, myoclonus, chorea, and parkinsonism. Therefore, the same patient could exhibit more than one motor disorder [
1]. Beyond motor disorders, patients might also experience non-motor symptoms and complaints. Functional tremor (FT) is the most common FMD subtype [
1]. Sudden onset, changes in frequency and amplitude with cognitive effort or tremor improvement, and recovery periods lasting days or months are important clues for an FT diagnosis [
2,
3].
Essential tremor (ET) is the most common movement disorder, representing a symmetrical tremor of the hand and forearm that occurs during posture and during action. Although hand tremors are the most common, tongue, jaw, head, trunk, and leg tremors have been observed as well [
4]. Half of patients display a positive family history. The worldwide prevalence of ET reportedly falls between 0.41 and 3.92% [
5,
6]. ET pathophysiology-related studies have suggested cerebellar involvement. Although ET had been previously considered as only a motor disorder, recent studies have demonstrated that this disease also exhibits cognitive and psychiatric add-ons. Moreover, social phobias, anxiety disorders, and depression are common in patients with ET [
7,
8,
9].
How the demographic and psychometric characteristics differ between patients diagnosed with ET and FT remains elusive, as do the factors that contribute to the high misdiagnosis rate between these tremor types. Our study uniquely identified and quantified differences in demographic and psychometric profiles between patients with ET and FT, emphasising the need for tailored diagnostic approaches to reduce misdiagnoses and improve treatment outcomes. In this study, we delineated the differences between ET and FT using comprehensive demographic and psychometric analyses, offering novel insights into the differential diagnosis and management of these conditions. ET and FT, often confused due to their overlapping clinical manifestations, require precise diagnostic criteria for patient care and treatment outcome optimisation [
10].
2. Methods
2.1. Study Design
In this study, we included 104 patients diagnosed with ET and FT who visited the movement disorder unit at our neurology outpatient clinic between 2020 and 2024. Each patient underwent a double-blind examination by two doctors specialising in movement disorders. In the case of a difficult diagnosis, the patient underwent electrophysiological tests. We excluded 8 patients as their diagnoses could not be confirmed. This study was approved by the local ethics committee.
2.2. Participants
In our study, we excluded patients with cognitive disorders, additional neurological diseases, potential tremor-inducing drug use (B-adrenergic and antipsychotic drugs), and potential tremor-inducing accompanying diseases from the study, as well as those diagnosed with FT and accompanying movement disorders other than tremors (e.g., myoclonus, dystonia, or chorea). Finally, we included patients diagnosed with FT or ET who provided informed consent in this study. Our study comprised a cohort of 96 patients, and detailed clinical assessments and psychometric testing were performed to identify unique patterns and associations within each tremor type. We meticulously analysed variables such as age, sex, body mass index (BMI), tremor symptom onset, and disease duration. Beyond the distribution of tremors and misdiagnoses, we performed electrophysiological tests on the relevant patients. Additional psychometric assessments included the Mini-mental Status Examination (MMSE), Beck Depression Inventory (BDI), Beck Anxiety Inventory (BAI), Barratt Impulsivity Scale—Short Form (BIS-SF), Buss–Perry Aggression Questionnaire (BPAQ), and Body Image Scale (BIS). The patients’ psychometric test results were then evaluated by a psychiatrist.
2.3. Statistical Analysis
The data were summarised using descriptive statistics. For continuous variables, depending on their distribution, we detailed either the mean ± standard deviation or median and minimum and maximum values in the tables. Categorical variables are presented as counts and percentages. To assess the normality of the numerical variables, we applied Shapiro–Wilk, Kolmogorov–Smirnov, Pearson chi-square, Fisher’s exact, Mann–Whitney U, and Anderson–Darling tests. These tests are crucial for determining the appropriate statistical methods for data analysis, as they help verify whether the data distribution conforms to the assumption of normality, which influences the choice of parametric versus non-parametric tests. In addition, we applied a classification and regression tree (CART) analysis for the model that could separate ET and FT patients. We used age at illness onset and the BDI, BAI, BMI, BPAQ, BIS-SF, and BIS scores as independent variables in the analysis. In the analysis conducted on 96 participants, we considered the prior probabilities for each class to be equal and used the Gini criterion for node division. We determined the model to be the optimal tree within one standard error of the minimum misclassification cost, and validated the model using a 10-fold cross-validation. We performed statistical analyses using Jamovi (version 2.3.28) and JASP (version 0.18.3) software packages and set the significance level at p < 0.05, aligned with standard practices in clinical research to ensure robust and reliable findings.
3. Results
The median age of the participants was 33.0 years, and 41.7% (
n = 40) of them were males and 58.3% (
n = 56) were females. The misdiagnosis rate was 14.5% (
n = 14). In the pairwise comparisons, the proportion of males in the ET group was significantly higher than that in the FT group (
p = 0.015). In addition, the BMI and age at disease onset were significantly lower in the ET group than in the FT group (
p = 0.050 and 0.023, respectively). The disease duration was significantly longer in the ET group than in the FT group (
p < 0.001).
Table 1 summarises the demographic and clinical results.
Pairwise comparisons revealed that the MMSE and BIS scores were higher in ET patients than in FT patients, whereas the BDI, BAI, BPAQ, and BIS-SF scores were significantly lower in ET patients than in FT patients (
p < 0.05).
Table 2 summarises the psychometric data.
Univariate analyses of demographic and clinical variables indicated that female gender increased the risk of FT by 3.11-fold (95% CI: 1.32–7.34,
p = 0.010), and each one-year increase in the age of onset raised the risk of FT by 5% (95% CI: 1.01–1.09,
p = 0.020). An analysis of psychometric variables indicated that each one-unit increase in BDI, BAI, BPAQ, and BIS-SF scores increased the risk of FT by 13% (95% CI: 1.05–1.21,
p < 0.001), 11% (95% CI: 1.05–1.18,
p < 0.001), 11% (95% CI: 1.06–1.15,
p < 0.001), and 6% (95% CI: 1.03–1.09,
p < 0.001), respectively. Conversely, each one-unit increase in BIS score decreased the risk of FT by 3% (95% CI: 0.95–0.98,
p < 0.001). In multivariate analyses, each one-unit increase in BPAQ and BIS-SF scores was associated with 8% (95% CI: 1.03–1.12,
p < 0.001) and 4% (95% CI: 1.01–1.07,
p = 0.009) increases in the risk of FT, respectively.
Table 3 summarises the clinical and psychometric variables.
The confusion matrix we used to evaluate the performance of the model revealed that in the training data, 45 of 52 ET cases were correctly predicted, resulting in an accuracy of 86.5%. Similarly, 34 of 44 FT cases were correctly predicted with an accuracy of 77.3%, leading to an overall accuracy of 82.3%. In the test data, 44 and 32 of the 52 and 44 ET and FT cases were correctly predicted (accuracies of 84.6% and 72.7%), respectively, with an overall accuracy of 79.2%. The sensitivities of the model were 86.5% and 84.6% for training and test data, respectively. The false positive rate was 22.7% and 27.3% in the training and test data, respectively. The false negative rate was 13.5% and 15.4% in the training and test data, respectively. The true negative rate (specificity) was 77.3% and 72.7% in the training and test data, respectively. These findings are summarised in
Table 4.
We evaluated the performance of the CART model using several statistical methods. The mean log-likelihood values were 0.4668 and 0.5287 for the training and test data, respectively. The area under the ROC curve (AUC) values were 0.8191 for training and 0.7340 for testing, with 95% confidence intervals of 0.06453–1 and 0.6257–0.8423 for the training and testing, respectively. We calculated the lift values to be 1.5105 and 1.2088 for training and testing, respectively. The misclassification costs were 0.3619 and 0.4266 for training and testing, respectively. These measurements indicate that the model performed better on the training than on the test data. Although the decrease in performance indicators on the test data suggests certain limitations in the model’s generalisability, the obtained values were still within acceptable limits.
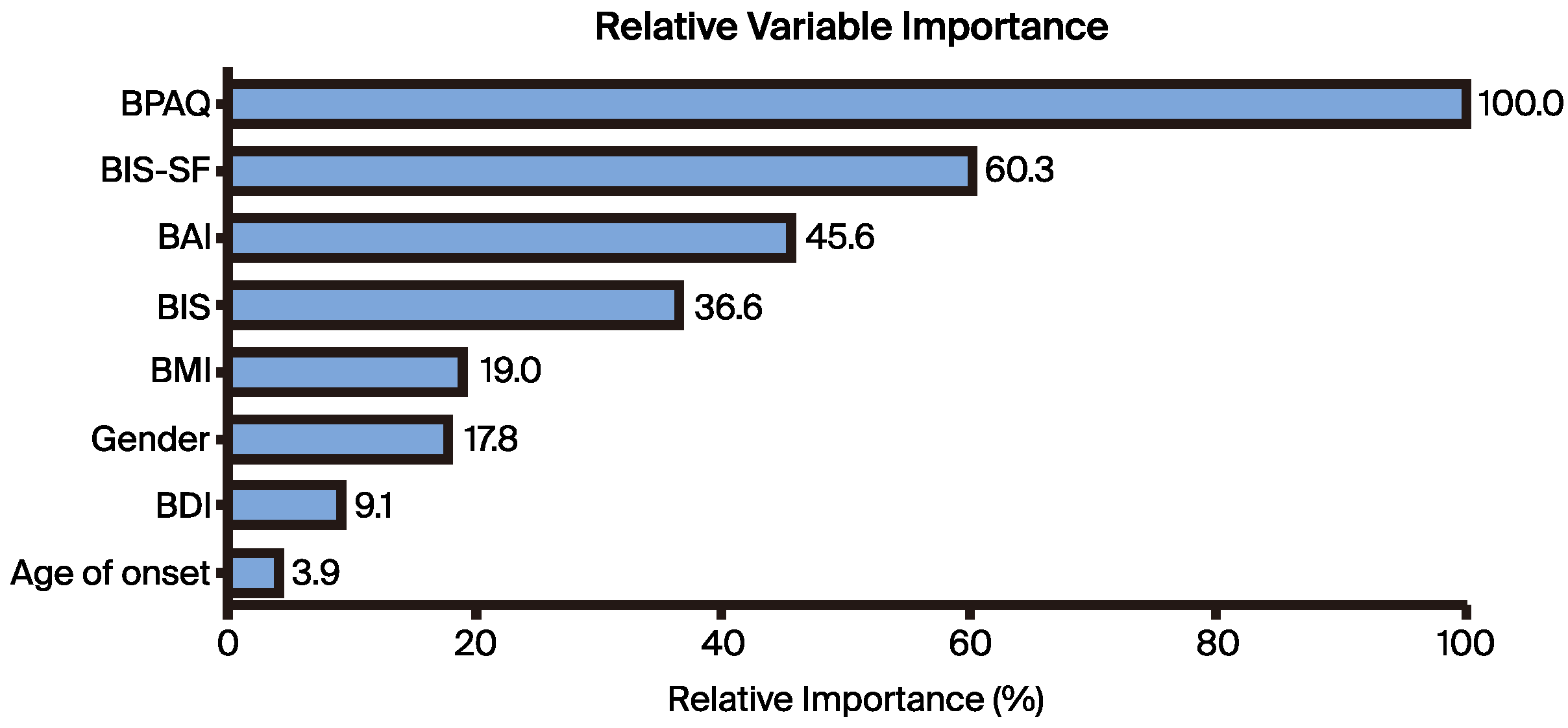
We expressed the relative importance of the predictors as a percentage, with the most effective predictor being rated at 100%. According to our analysis, the BPAQ scores displayed the highest importance at 100%, followed by BIS, BDI, BIS, BMI, sex, BDI, and age at illness onset at 60.3%, 45.6%, 36.6%, 19.0%, 17.8%, 9.1%, and 3.9%, respectively (
Figure 1).
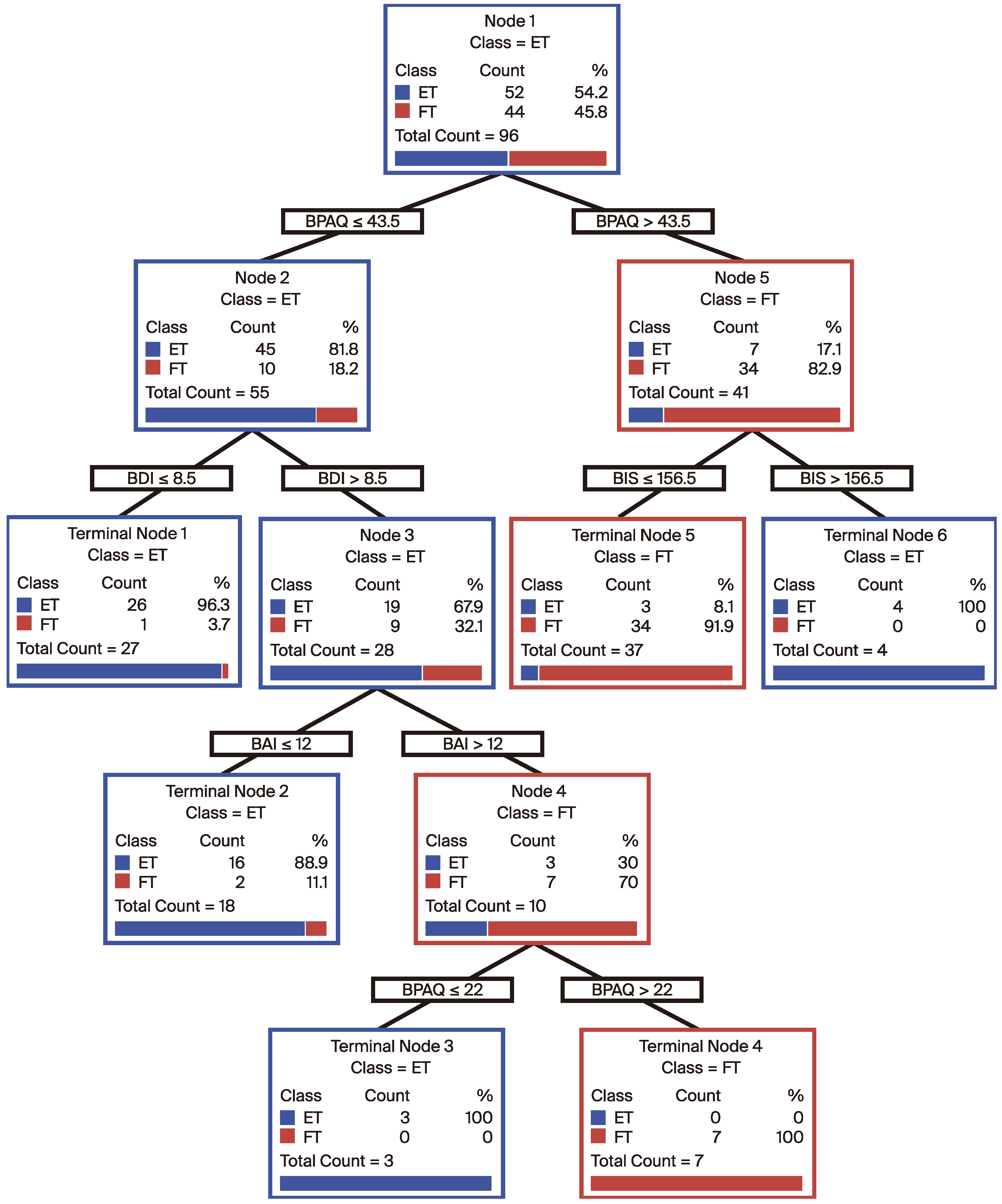
In our analysis, we used the CART methodology to divide patients into two main groups based on various clinical and demographic parameters: ET and FT. We identified key features (such as age at onset of illness, BDI, BAI, BMI, BPAQ, BIS-SF, and BIS) as the primary predictors for classifying the patients. The results showed that the patients in the dataset were grouped according to their risk profiles, initially based on BIS-SF values. The first division was made at BIS-SF ≤ 43.5 and BIS-SF > 43.5, followed by further subdivisions into more detailed subgroups based on BDI, BAI, and BIS-SF values.
Each terminal node contains the final classification of the model, including the number of patients and the percentage distribution within these groups. The high accuracy rates observed at these terminal nodes demonstrate the efficacy of the model in predicting the disease states. Notably, one terminal node, which included 26 patients in the ET group, correctly classified 96% of patients (
Figure 2).
4. Discussion
Our study, which focused on differentiating the clinical and psychometric characteristics of ET and FT, contributes significantly to our understanding of these conditions in a clinical setting. By assessing variables such as sex, BMI, age of onset, misdiagnosis rates, and various psychometric assessments, we performed a comprehensive analysis that aligns with and extends the current research on tremor pathology and diagnosis.
Our study identified a notably higher proportion of males in the ET than FT group. This sex disparity is not only significant in understanding the demographic distribution of tremor types, but also suggests that inherent differences could exist in how these conditions manifest across sexes. This finding is consistent with the results of Govorova et al. (2023), who explored how the clinical features of ET vary among different ethnic groups, suggesting that genetic or environmental factors might influence the disease phenotype [
11,
12]. The variation in the clinical ET features, as noted by Govorova et al., underscores ET’s complexity as a neurodegenerative condition influenced by various factors, including sex [
11,
12,
13]. In summary, our findings on the sex-related ET distribution not only corroborate the observations made by Govorova et al. concerning ET variability across different groups, but also emphasise the need for further studies focusing on the biological underpinnings that contribute to these differences [
11,
13]. Such studies could ultimately lead to more effective and personalised therapeutic interventions, enhancing the outcomes of patients with tremor-related disorders [
14].
In our study, the misdiagnosis rate of 14.5% emphasises that accurately distinguishing between ET and FT is a complex challenge. This high misdiagnosis rate reflects the subtleties in the clinical presentations of tremor syndromes, which can often overlap and lead to diagnostic errors. Misdiagnosis delays appropriate treatment, potentially leading to ineffective therapy, underscoring the need for enhanced diagnostic criteria and methods [
3,
4]. Our findings resonate with those of Peng et al. (2022), who highlighted the critical need for the reassessment and reclassification of patients with tremor syndromes to enhance diagnostic precision and treatment outcomes [
10]. Peng et al. argued that the current classification systems might not suffice, suggesting that a more nuanced approach, possibly integrating more sophisticated diagnostic tools such as advanced imaging techniques, enhanced neurophysiological assessments or new criteria, would be required. This recommendation aligns with our observed misdiagnosis rates, suggesting that both studies recognised the significant limitations in the current clinical framework for tremor diagnosis [
10]. Such advancements could reduce misdiagnosis rates by providing clearer distinctions between ET and FT at earlier stages of patient evaluation [
15,
16,
17,
18].
In our study, we observed notable differences in the psychometric profiles between patients with ET and those with FT, providing crucial insights into the distinct nature of these conditions. These differences were particularly evident in the assessment scores of psychological scales, such as depression and anxiety, which were significantly higher in FT patients than in ET patients. In addition, body image scales were distorted and the rate of impulsivity and aggression was high in patients with FT. Such findings are crucial, as they not only help in differentiating between these tremor types, but also in understanding the broader psychosocial impacts of these conditions on patients [
19,
20,
21,
22]. This aspect of our study aligns with the observations of S. Lidstone and A. Lang (2020), who explored the diagnostic criteria for functional tremors, emphasising the role of clinical and examination features, including psychometric evaluations, in their diagnosis [
3]. This is particularly relevant, as psychometric evaluations could reveal underlying psychological factors that might not be immediately apparent through physical examinations alone [
19,
20,
21,
22]. The significant psychometric differences identified underline the necessity of incorporating comprehensive psychological assessments into the diagnostic process for tremors, potentially enhancing the accuracy of distinguishing between ET and FT [
23,
24,
25]. By integrating detailed psychometric evaluations into the routine assessment of patients with tremors, clinicians could better understand the patient’s condition, which is crucial for effective treatment planning. In addition, although the psychometric data scores of patients with ET were lower than those of patients with FT, there was also a disorder in the psychometric profiles of patients with ET. This may be related to the neurodegenerative and cognitive disorders accompanying ET [
26,
27,
28].
This aspect of our study correlates with the insights provided by Hopfner et al. (2016), who explored the implications of symptomatic management outcomes in patients with ET. They highlighted the variability in patient responses to treatment, and the importance of tailoring management strategies based on individual symptoms and disease characteristics [
29]. This is particularly relevant to our findings, which suggests that the underlying psychometric differences between ET and FT could influence how patients respond to standard treatment protocols. The increased BMI of patients with FT indicates that not only psychiatry and neurology should be involved in the treatment method, but also dietitians. Moreover, the detailed demographic analysis in our study revealed that certain therapies might be more effective in one group than in another and influenced by factors such as age and sex.
Our contribution emphasises the need for further research to explore how demographic and psychometric distinctions between tremor types can be effectively translated into personalised treatment plans. Future studies should consider these factors when assessing the efficacy of different therapeutic interventions to enhance the overall management of tremor disorders and improve patient outcomes in clinical practice.
Our study raises important questions concerning the biological underpinnings that might differentiate ET from FT, particularly in progression and response to therapy. The distinct psychometric and demographic profile identification suggests that personalised treatment strategies could be more efficient than the current one-size-fits-all approaches. Furthermore, the significant rate of misdiagnosis indicates the need for improved diagnostic protocols, possibly by incorporating advanced imaging techniques or revised clinical guidelines that consider our findings.
Future studies should focus on longitudinal patient tracking to observe ET and FT progression under various treatment modalities. In addition, exploring the genetic markers or environmental factors that could contribute to the phenotypic expression of these tremors would be invaluable. These efforts would not only refine the diagnostic accuracy, but also enhance therapeutic outcomes by tailoring interventions to specific tremor types and individual patient characteristics.
Despite the wide range of data obtained in our study, it still has some limitations. First, the sample size, although adequate for detecting significant differences, might not have captured the full spectrum of variability within the tremor population. Second, the cross-sectional nature of our study design limited our ability to infer causality or track tremor progression over time. In addition, while we used a robust statistical framework to analyse our data, reliance on self-reported measures for some psychometric evaluations might have introduced bias or variability in the data accuracy. Incorporating objective biomarkers or neuroimaging data could enhance the reliability of distinguishing between ET and FT.
Addressing these limitations in future studies would refine our understanding and contribute to more definitive conclusions, thereby not only solidifying the foundations laid by our current study but also expanding the scope of tremor disorder management methods.
5. Conclusions
Our discoveries related to the clinical and psychometric distinctions between ET and FT underscore significant differences that are not only statistically relevant but also clinically actionable. Our analysis highlighted a higher ET prevalence in males and an association of FT with more pronounced psychometric impairments, including higher anxiety and depression scores. These observations align with our initial hypothesis that the two tremor types would manifest distinct clinical profiles that could inform diagnostic and therapeutic strategies.
Moreover, the substantial misdiagnosis rates in our study highlight the need for increased clinical awareness and diagnostic precision. Our study suggests that integrating more rigorous psychometric assessments into routine clinical evaluations could reduce these rates and ultimately enhance patient care.
By demonstrating clear distinctions in the clinical presentation and psychometric parameters, we pave the way for future research exploring tailored therapeutic interventions that address the specific needs of each tremor type, potentially improving outcomes and patient satisfaction.
Author Contributions
Conceptualization, M.B. and S.Ç.; methodology, M.B., SÇ. and MD; software, M.E.; validation, MB., M.E. and M.D.; formal analysis, M.D.; investigation, M.D.; resources, M.E.; data curation, M.B. and M.E.; writing—original draft preparation, M.B.; writing—review and ediing, M.B., M.E. and M.D; visualization, M.B.; supervision, M.D.; project administration, M.D.; Not funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Çukurova University School of Medicine (04/05/2020-039).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data of the study can be accessed by contacting the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lidstone, S.C.; Costa-Parke, M.; Robinson, E.J.; Ercoli, T.; Stone, J.; FMD GAP Study Group. Functional movement disorder gender, age and phenotype study: A systematic review and individual patient meta-analysis of 4905 cases. J. Neurol. Neurosurg. Psychiatry 2022, 93, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, G.S.; Nielsen, G.; Teodoro, T.; Yogarajah, M.; Coebergh, J.A.; Dilley, M.D.; Martino, D.; Edwards, M.J. Management of functional neurological disorder. J. Neurol. 2020, 267, 2164–2172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lidstone, S.C.; Lang, A.E. How do i examine patients with functional tremor? Mov. Disord. Clin. Pract. 2020, 7, 587. [Google Scholar] [CrossRef] [PubMed]
- Kramer, G.; Dominguez-Vega, Z.T.; Laarhoven, H.S.; Brandsma, R.; Smit, M.; van der Stouwe, A.M.; Elting, J.W.J.; Maurits, N.M.; Rosmalen, J.G.; Tijssen, M.A. Similar association between objective and subjective symptoms in functional and organic tremor. Park. Relat Disord. 2019, 64, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.D.; McCreary, M. How Common is Essential Tremor? Update on the Worldwide Prevalence of Essential Tremor. Tremor Other Hyperkinet. Mov. 2021, 11, 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Louis, E.D.; Ferreira, J.J. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov. Disord. 2010, 25, 534–541. [Google Scholar] [CrossRef]
- Shanker, V. Essential tremor: Diagnosis and management. BMJ 2019, 366, l4485. [Google Scholar] [CrossRef] [PubMed]
- Welton, T.; Cardoso, F.; Carr, J.A.; Chan, L.L.; Deuschl, G.; Jankovic, J.; Tan, E.K. Essential tremor. Nat. Rev. Dis. Primers 2021, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, F.; Deuschl, G. Is essential tremor a single entity? Eur. J. Neurol. 2018, 25, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, N.; Li, J.; Duan, L.; Chen, C.; Zeng, Y.; Xi, J.; Jiang, Y.; Peng, R. Reclassification of patients with tremor syndrome and comparisons of essential tremor and essential tremor-plus patients. J. Neurol. 2022, 269, 3653–3662. [Google Scholar] [CrossRef] [PubMed]
- Govorova, T.G.; Popova, T.E.; Tappakhov, A.A.; Andreev, M.E. Clinical Features of Essential Tremor in the Two Ethnic Groups. Pers. Psychiatry Neurol. 2023, 3, 54–60. [Google Scholar] [CrossRef]
- Pan, M.K.; Kuo, S.H. Essential tremor: Clinical perspectives and pathophysiology. J. Neurol. Sci. 2022, 435, 120198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Q.; He, R.; Huang, H.; Cao, H.; Wang, X.; Liu, H.; Wang, C.; Lei, L.; Wang, P.; Cui, G.; et al. Age and Sex Affect Essential Tremor (ET) Plus: Clinical Heterogeneity in ET Based on the National Survey in China. Aging Dis. 2023, 14, 1360–1373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A.G.; Jiménez-Jiménez, F.J. Current and Future Neuropharmacological Options for the Treatment of Essential Tremor. Curr. Neuropharmacol. 2020, 18, 518–537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pick, S.; Goldstein, L.H.; Perez, D.L.; Nicholson, T.R. Emotional processing in functional neurological disorder: A review, biopsychosocial model and research agenda. J. Neurol. Neurosurg. Psychiatry. 2019, 90, 704–711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sasikumar, S.; Strafella, A.P. The neuroimaging evidence of brain abnormalities in functional movement disorders. Brain 2021, 144, 2278–2283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van den Berg, K.R.E.; Helmich, R.C. The Role of the Cerebellum in Tremor—Evidence from Neuroimaging. Tremor Other Hyperkinet. Mov. 2021, 11, 49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dominguez-Vega, Z.T.; Kramer, G.; Elting, J.W.J.; Tijssen, M.A.J.; Maurits, N.M. Three Days of Measurement Provide Reliable Estimates of Daily Tremor Characteristics: A Pilot Study in Organic and Functional Tremor Patients. Tremor Other Hyperkinet. Mov. 2021, 11, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mavroudis, I.; Kazis, D.; Kamal, F.Z.; Gurzu, I.L.; Ciobica, A.; Pădurariu, M.; Novac, B.; Iordache, A. Understanding Functional Neurological Disorder: Recent Insights and Diagnostic Challenges. Int. J. Mol. Sci. 2024, 25, 4470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macchi, Z.A.; Kletenik, I.; Olvera, C.; Holden, S.K. Psychiatric Comorbidities in Functional Movement Disorders: A Retrospective Cohort Study. Mov. Disord. Clin. Pract. 2021, 8, 725–732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spagnolo, P.A.; Garvey, M.; Hallett, M. A dimensional approach to functional movement disorders: Heresy or opportunity. Neurosci. Biobehav. Rev. 2021, 127, 25–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pavletic, A.J. Insights into Chronic Functional Movement Disorders: The Value of Qualitative Psychiatric Interviews. Psychosomatics 2017, 58, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Yasar, H.; Balibey, H.; Tekeli, H.; Alay, S.; Senol, M.G.; Türker, T.; Bayar, N. The levels of anxiety and depression in young male patients with essential tremor. Psychiatry Behav. Sci. 2014, 4, 66. [Google Scholar] [CrossRef]
- Miller, K.M.; Okun, M.S.; Fernandez, H.F.; Jacobson CE 4th Rodriguez, R.L.; Bowers, D. Depression symptoms in movement disorders: Comparing Parkinson’s disease, dystonia, and essential tremor. Mov. Disord. 2007, 22, 666–672. [Google Scholar] [CrossRef] [PubMed]
- O’Suilleabhain, P.; Berry, D.S.; Lundervold, D.A.; Turner, T.H.; Tovar, M.; Louis, E.D. Stigma and Social Avoidance in Adults with Essential Tremor. Mov. Disord. Clin. Pract. 2023, 10, 1317–1323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Louis, E.D.; Faust, P.L. Essential Tremor Within the Broader Context of Other Forms of Cerebellar Degeneration. Cerebellum 2020, 19, 879–896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faust, P.L. Is essential tremor a degenerative disorder or an electric disorder? Degenerative disorder. Int. Rev. Neurobiol. 2022, 163, 65–101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Louis, E.D. Essential tremor as a neuropsychiatric disorder. J. Neurol. Sci. 2010, 289, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, F.; Deuschl, G. Managing Essential Tremor. Neurotherapeutics 2020, 17, 1603–1621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).