1. Introduction
Water contamination from toxic organic chemical waste from pharmaceutical, textile and electrochemical industries and their adverse effects on human and aquatic life has received tremendous attention in recent years. Exposure to above the threshold limits of these chemicals leads to skin discoloration, damage to nervous system and organs, and developmental effects [
1]. The toxic chemicals include nitrophenols, azo dyes and heavy metals, and water containing these needs to be treated before it is safely discharged into waterbodies or land. Various approaches have been employed in the removal of harmful pollutants such as adsorption, ion-exchange, solvent extraction, photochemical reactions, etc. [
2], and among these techniques, adsorption using activated carbon is widely used as the process is economical and effective in the removal of various pollutants. However, this method allows for the adsorption of the pollutants but not their degradation to non-toxic substances.
In recent years, photocatalysis or photodegradation methods using metallic and metal oxide nanoparticles such as silver, gold, platinum, palladium, copper, nickel, cobalt, iron, zinc oxide (ZnO), and titanium dioxide (TiO2), have gained much research attention for complete reduction of toxic chemical waste to non-toxic substances in water [
3,
4,
5,
6,
7]. Nanoparticles on solid polymer supports such as polysulfone, polypropylene, poly(vinylidene fluoride), polyamide, cellulose acetate, and sodium alginate offers improved catalytic properties along with material sustainability, as these materials can be used repeatedly [
7,
8,
9]. Silver nanoparticles supported on various inorganic and organic substrates such as zeolite, silica or fiber glass, carbon materials, natural macro-porous materials, and polymers have been recognized as effective photocatalysts and antimicrobial agents. Nanocomposite beads based on the natural polymer sodium alginate and silver nanoparticles offer extended physical and chemical properties and are currently being considered for point-of-use drinking water disinfection [
8,
9,
10].
Sodium alginate is a linear polysaccharide found in marine brown algae and is composed of irregular blocks of β-D-mannuronic acid (M) and α-L-guluronic residues (G). Due to its non-toxicity, degradability, and bio-compatibility alginate-based gels have attracted numerous biomedical applications such as, drug and protein delivery, wound dressing, 3D bioprinting for tissue engineering, scaffolds for cell growth and organoid morphogenesis, and flexible electronics for health monitoring [
11,
12,
13,
14,
15].
Silver nanoparticles synthesized by green chemistry using the extracts of plants or plant products have been used in the fabrication or engineering of alginate-nanocomposite beads. The phytochemicals present in the extract act both as reducing agent for the reduction of silver salt to silver nanoparticles and stabilizing the resulting nanoparticles against aggregation [
16,
17]. Date palm, commonly known as
Phoenix dactylifera is one of the oldest cultivated varieties of date palm trees with nutritional, economic, and environmental benefits. There are about 5000 varieties of date palm that are grown in different regions of the world, and the nutritional and phytochemical values vary among the dates. Among these ajwa dates are widely cultivated in the Al Madinah and surrounding regions of Saudi Arabia. This type of date has high sugar (34.5% glucose, 25.6% fructose, and 0.5% sucrose) and mineral (3%) content compared to other varieties of dates [
18,
19]. These dates seeds are a rich source of polyphenols, flavonoids, glycosides, phenolic acids, proteins, and carbohydrates, and demonstrates antioxidant, anti-inflammatory, antimicrobial and anti-tumor properties [
19,
20].
In this study, we have developed reusable alginate-based silver nanocomposite beads by in situ chemical reduction and gelation method for a quick reduction of 2-nitrophenol. The silver nanoparticles were first synthesized by green chemistry using the extract of ajwa dates seeds as an efficient source for the reduction and stabilization, and then incorporated into alginate beads by ionotropic crosslinking using calcium ions. To the best of our knowledge this is the first study in the fabrication of alginate-silver nanocomposite beads using the extracts of ajwa dates seed.
2. Experimental
2.1. Materials
Ajwa date (Phoenix dactylifera) seed powder was purchased from a grocery store in Hoora, Bahrain. Silver nitrate (AgNO3), sodium alginate (NaC6H7O6), sodium hydroxide (NaOH), sodium borohydride (NaBH4), 2-nitrophenol (2-NP), Congo red (CR)and calcium chloride (CaCl2) were purchased from Sigma and used as received. Deionized water collected from a Millipore system (Elix Technology, Germany) with a conductivity of 18.2 MΩ cm-1 was used for all aqueous sample preparations. The antibacterial properties of the synthesized nanoparticles, nanocomposite beads and films were evaluated against three different types of bacteria, Staphylococcus aureus (S. aureus), Escherichia coli (E. Coli) and Salmonella typhimurium (S. typhimurium). The bacteria were obtained from the Ministry of Health, Kingdom of Bahrain (MOH, Bahrain, Microbiologiscs, France).
2.2. Preparation of Date Seeds Extract
About 1.00g of the date seeds powder was added to 100ml of water in a beaker and boiled for 30 min under magnetic stirring. The mixture was air cooled and centrifuged for 10 min at an rpm of 4200 to remove any suspended materials. The clear extract was used fresh in the synthesis of silver nanoparticles.
2.3. Green Synthesis of Silver Nanoparticles
Silver nanoparticles were synthesized by reducing the silver nitrate by the freshly prepared date seeds extract as follows. AgNO3 (1 mM, 5ml) was placed in a screw capped glass vial and stirred gently using a magnet. Freshly prepared date seeds extract (1 ml) was added dropwise into the vial with continuous stirring. After about 5 min, NaOH (0.5 M, 50 μl) was added dropwise and the mixture was stirred overnight for the completion of the reaction. Upon addition of NaOH, the color of the solution turned pale yellow and finally to brown (after 24 h). The observed color changes are an indication of formation of nanoparticles. The solution containing the nanoparticle was centrifuged (rpm 10000) for 10 min, and the sedimented nanoparticles was washed repeatedly with water and dried in an oven at 70 °C.
2.4. Synthesis of Alginate-Silver Nanocomposite Beads
Alginate beads containing silver nanoparticles was prepared by sequential chemical reduction and gelation method as described as follows. In this method, silver nanoparticles were first synthesized using the date seeds extract and then incorporated into the alginate beads during the gelation. Silver nitrate solution of concentration 1mM was first prepared by dissolving 0.0175g of the salt in 100 ml of water. To this solution 20 ml of fresh date seeds extract was added under magnetic stirring, followed by the addition of 1 ml of 0.5 M NaOH. Upon addition of sodium hydroxide, the solution turned to pale grey and then to pale brown indicating the onset of formation of silver nanoparticles. The solution was continuously stirred for an additional time of 24h for the completion of the reaction, followed by the addition of 2.20 g of sodium alginate. The solution was heated to 60 °C and continuously stirred for another 24h for the complete dissolution of sodium alginate resulting in a homogenous mixture. The solution was air cooled and then stored in the refrigerator to remove any air bubbles. The prepared alginate-silver nanoparticle solution was injected into 200 ml of 5 wt% calcium chloride solution using a plastic syringe of 20 ml capacity at a rate of 20 drops per minute The resulting black color beads were washed thoroughly with water and dried at 70 °C until constant weight was maintained.
2.5. Synthesis of Peelable Alginate-Silver Nanocomposite Films
Peelable alginate-silver nanocomposite film was prepared by a mist spray gelation method as described as follows. A 10ml of alginate-silver nanoparticle solution described in the previous section was placed in a clean glass petri dish, and mist sprayed with CaCl2 solution (5 wt%). The petri dish was covered and left overnight at room temperature for the completion of gelation. The dark brown color film was carefully peeled off from the petri dish and washed repeatedly with water and dried at 50 °C until constant weight was maintained.
2.6. UV-Vis Absorption Spectroscopy
The formation of silver nanoparticles was confirmed by measuring the absorbance of the silver nanoparticle solution using a double-beam Shimadzu UV-1800 spectrophotometer. The solution (3 ml) was placed in a quartz cuvette (Helma) of 1 cm path length, and the absorption spectrum was recorded in the wavelength range 250-1000 nm with a resolution of 1mm. Water was used as the blank reference for all measurements.
2.7. Scanning Electron Microscopy (SEM)
The size and morphology of the alginate-silver nanocomposite beads were characterized using a scanning electron microscope operating at a voltage of 10 kV (Inovenso, IEM-11). The samples were sputtered with gold using a Inovenso SPT-20 coater.
2.8. Catalytic Degradation Studies of 2-Nitrophenol
The catalytic degradation of2-nitrophenol (2-NP) by the alginate-silver nanocomposite beads was followed using a UV-Vis spectrophotometer. A solution of 2-nitrophenol (0.13 mM, 2.5 ml) was placed in a quartz cuvette of 1 cm pathlength and the absorbance was recorded. After this, 0.5 ml of freshly prepared NaBH
4 (0.1 M) was added to the solution and the absorbance was recorded again. About 10 nanocomposite beads were then added to the solution and the change in absorbance was recorded in intervals of 3 min for a period of 15 min. The percentage degradation of 2-nitrophenol was calculated using the following equation,
where,
A0 and
At are the absorbance at time zero and absorbance at time
t, respectively.
2.9. Antibacterial Activity
The antibacterial activity of the synthesized silver nanoparticles against different types of Gram-positive and Gram-negative bacterial such as Staphylococcus aureus (S. aureus), Escherichia coli (E. coli) and Salmonella typhimurium (S. typhimurium) was carried out using Kirby-Bauer Disk Diffusion Susceptibility Test method. The bacteria strains were spread on a nutrient agar (LB agar) medium using a sterile spreader in all directions. The filter paper discs were loaded with silver nanoparticles with aseptic precautions and then the agar plate was incubated at 37 °C for 24 h. The zone of inhibition was observed and measured after 24 h of incubation.
3. Results and Discussion
3.1. Ajwa date Seeds Mediated Green Synthesis of Silver Nanoparticles
Plant extracts mediated synthesis of metallic nanoparticles is a desired method as it is environmentally friendly and toxic reagents are not used in the process. The formation of silver nanoparticles using the aqueous extract of Ajwa date seeds was studied by visual observation and spectrophotometrically. Upon the addition of the date seed extract to the silver nitrate solution, a color change to pale brown was observed, which indicates the in situ chemical reduction of silver ions (Ag
+) to silver nanoparticles (Ag
0). The formation of silver nanoparticles was quantified using UV-Vis absorption spectroscopy and a distinct surface plasmon resonance (SPR) peak centered at 421 nm confirmed the presence of silver nanoparticles in the solution, as shown in
Figure 1.
This peak arises due to collective oscillations of conduction electrons in the electromagnetic field of the incident light [
16,
17]. The Ajwa date seeds are rich in phytochemicals such as polyphenols, flavonoids including rutin, catechins, iso-flavonoids and lignans [
18,
19,
20]. These phytochemicals present in the extract of ajwa date seeds are responsible for the chemical reduction and for subsequent stabilization of the resulting silver nanoparticles. The peak at 276 nm for the dates seeds is attributed to the active phytochemicals that are responsible for the chemical reduction. Absence of this peak in the spectrum of the silver nanoparticles corelate to the reaction and reduction in concentration of the active phytochemical. The nanoparticles were stable against aggregation for more than a month, with no obvious change in the position and intensity of the SPR peak observed in the absorption spectrum.
3.2. Formation and Morphology of Alginate Beads and Film
Silver particles encapsulated alginate beads were prepared by ionotropic crosslinking with divalent cations such as calcium ions (Ca
2+). The divalent cations bind to the guluronate blocks of the sodium alginate chains, as the blocks allows a high degree of coordination with the cations. The guluronate blocks of one polymer then forms physical junctions (crosslink points) with the guluronate blocks of adjacent polymer chains. This type of crosslinking and formation of a gel is termed the egg-box model of crosslinking [
21].
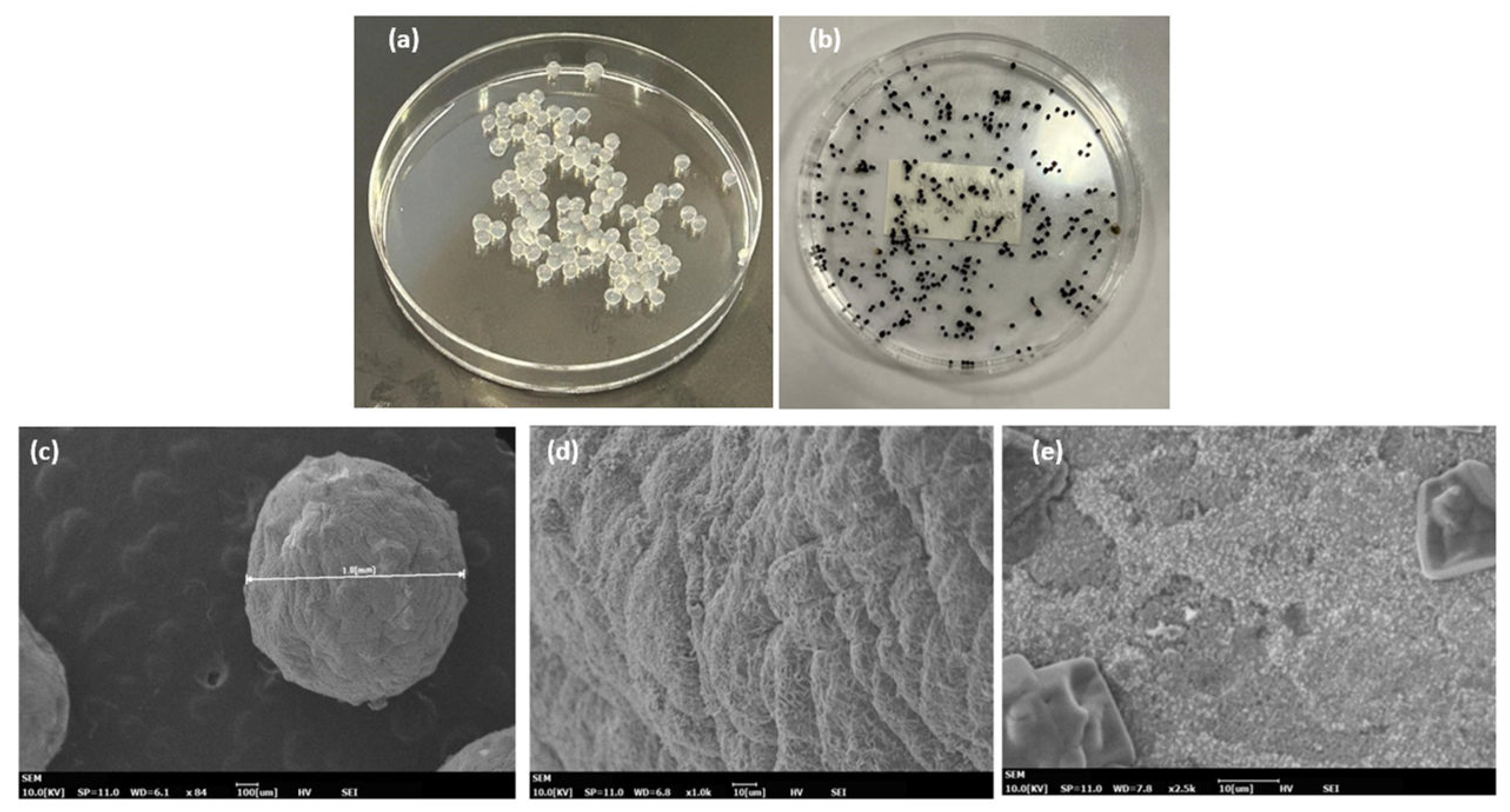
The alginate beads containing silver particles prepared in this study were pale brown in color in their hydrated state and black when completely dry as shown in
Figure 2(b). The dry beads were close to spherical shape with an average size of 1.2 μm as shown by the SEM micrograph in
Figure 2(c). The surface of the nanocomposite beads were compact with dense particulate clusters of silver in the form of plates (
Figure 2(d)). To verify the presence of silver nanoparticles, the alginate beads were soaked in phosphate buffer solution and dissociated.
The dissociation causes chelation of Ca2+ by PO43- and HPO42- ligands, any releasing the alginate and silver nanoparticles in solution. The resulting viscous solution was analyzed by UV-Vis absorption spectroscopy. A strong SPR peak around 420 nm confirmed the presence of silver nanoparticles in the alginate-nanocomposite beads. The nanocomposite film does not show any significant morphology and the silver nanoparticles are evenly distributed on the surface of the film.
3.3. Catalytic Degradation of 2-Nitrophenol (2-NP)
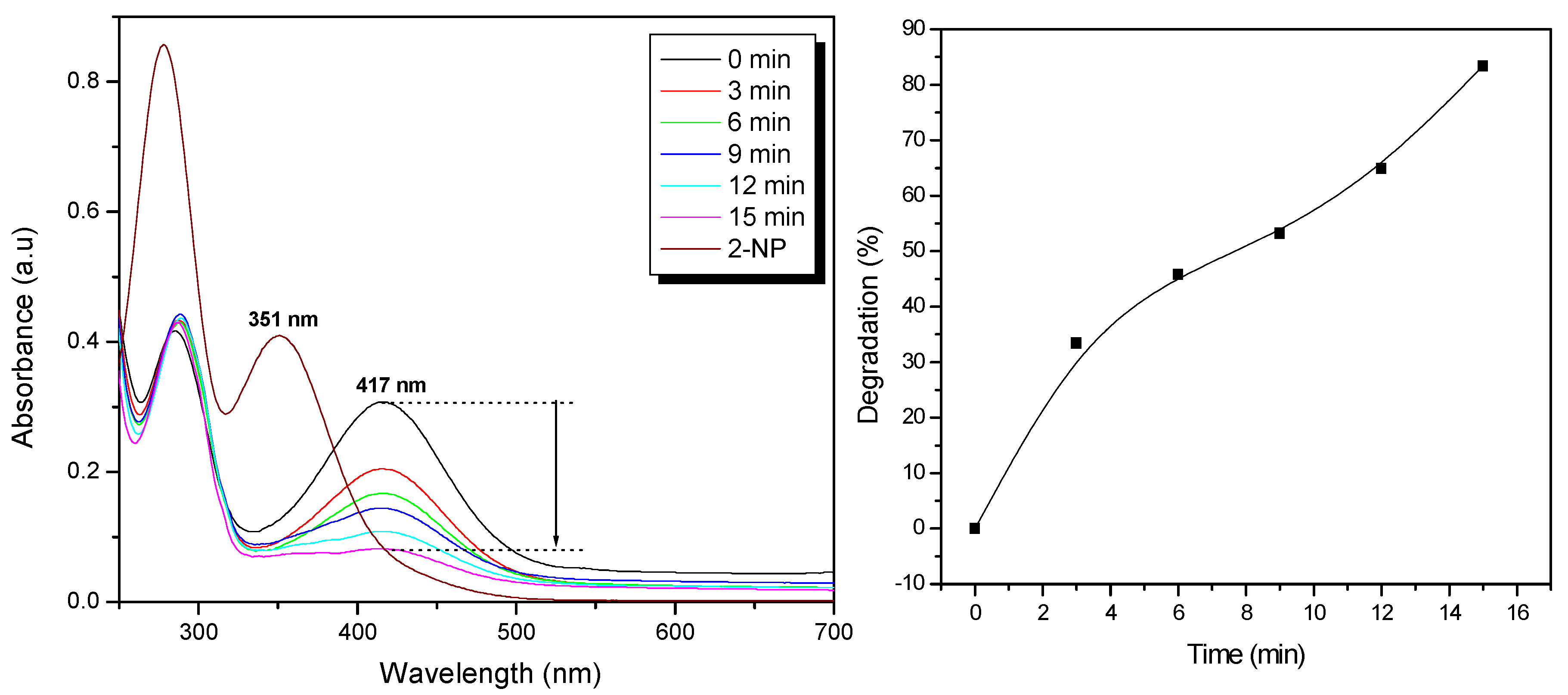
Effluents of dye and pesticide industries contain 2-NP which is an environmental hazard and is known to cause methemoglobinemia. The reduced product of 2-NP is 2-amino phenol (2-AP) which is a non-toxic product. The catalytic activity of the alginate-silver nanocomposite beads in the degradation of 2-NP in the presence of NaBH
4 was studied using UV-Vis absorption spectroscopy. The time-dependent change in absorbance during the degradation of 2-NP is shown in
Figure 3(a). The absorption peak at 281 nm corresponds to 2-NP and this shifts to 416 nm due to the formation of 2-nitrophenolate ion (due to deprotonation of the -OH group) upon the addition of NaBH
4. The reduction in the absorption of 2-nitrophenolaote ion corresponds to the formation of 2-AP, however this reaction has a large kinetic barrier due to the large potential difference between the reducing agent (NaBH4) and 2-NP [
22]. As a result, a catalyst is required to overcome the large energy barrier associated with this reduction process at room temperature.
Upon addition of the nanocomposite beads (10 beads) into the solution, the initial absorption (0.30) at 416 nm decreased significantly reaching 0.05 in 15 min. This decrease corresponds to about 83% degradation of 2-NP. At the same time, the intensity of the absorption peak at 300 nm increased which indicates the formation of 2-AP, the reaction being accelerated by the silver nanoparticles present in the nanocomposite. The degradation kinetics is shown in
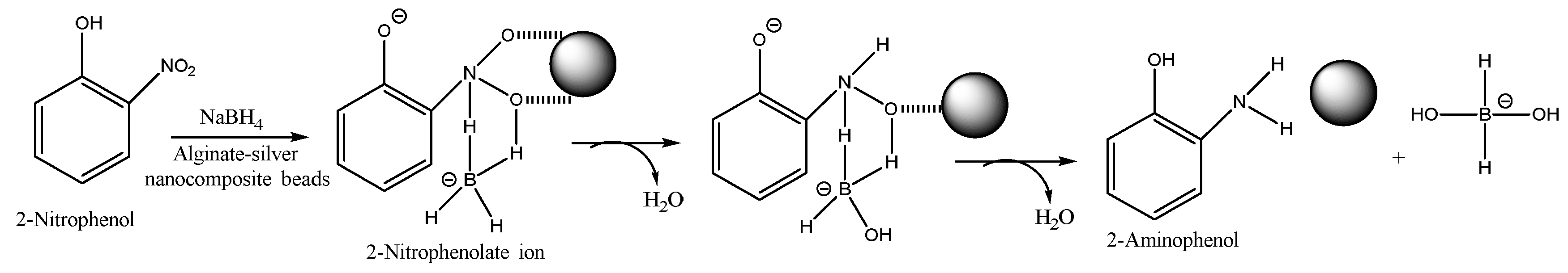
Figure 3(b) and a degradation of 83% is observed in just 15 min of the reaction. The rate of reaction could be shortened by improving the surface morphology of the nanocomposite beads by making them more porous which would allow higher diffusion of the 2-nitrophenolate ion into the beads for faster reaction with the active proton species, or by increasing the number of beads. In the absence of the nanocomposite beads the degradation reaction was extremely slow (more than 3 days) confirming the major catalytic role of the silver nanoparticles. The degradation reaction mechanism [
21,
22,
23] in the presence of the nanocomposite takes place in four steps such as: (i) adsorption of 2-NP on to the nanocomposite bead, (ii) diffusion of 2-NP to the active site, (iii) reaction of 2-NP to form the adsorbed product, and (iv) desorption of the product from the nanocomposite. A schematic representing the degradation of 2-NP in the presence of the strong reducing agent, NaBH
4, is shown in
Figure 4.
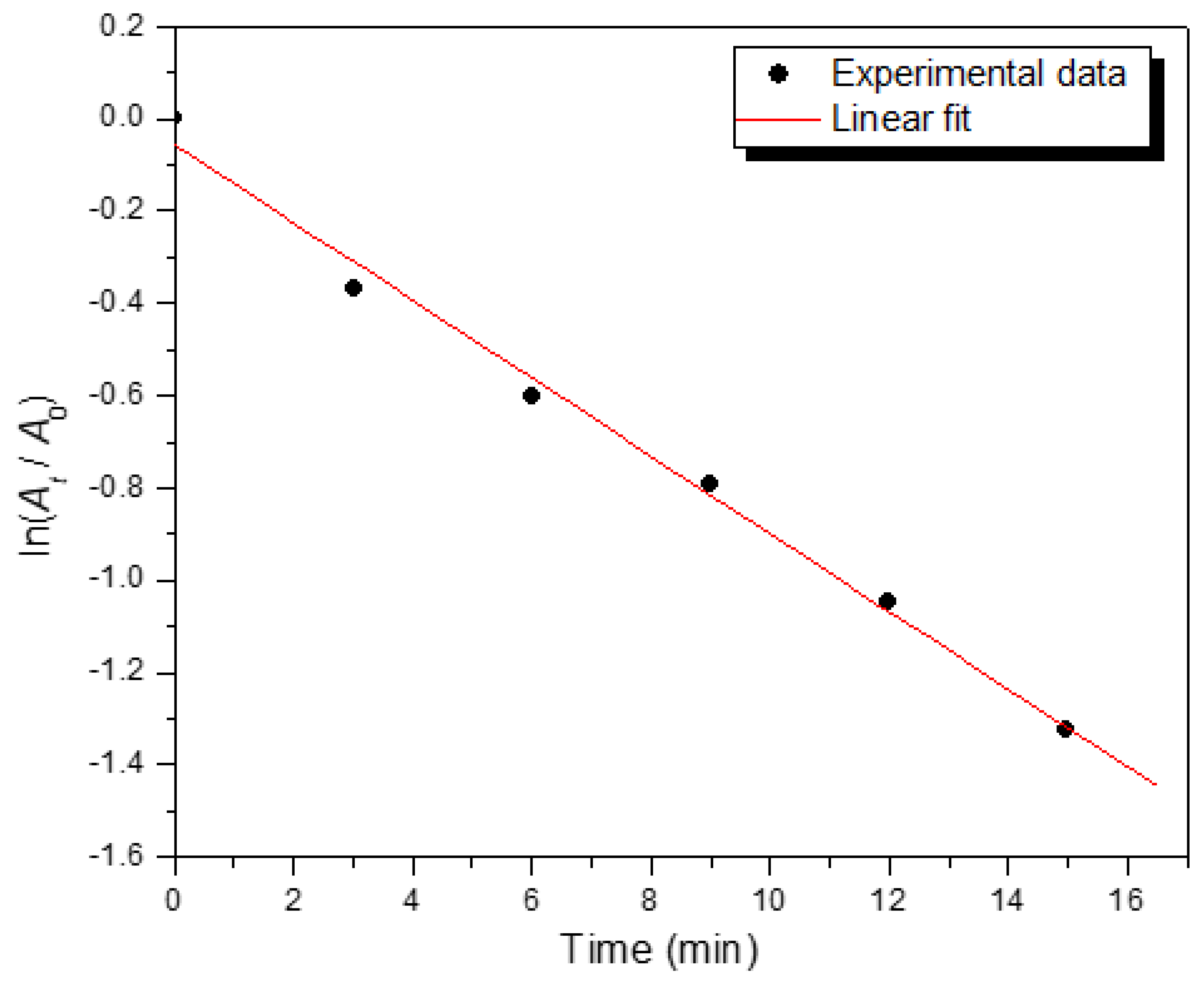
The rate constant (
k) of the degradation was determined from the linear plot of ln(
At/
A0) versus reaction time (
t) in min (
Figure 5) according to the following linear equation,
where
Ct and
C0 are the concentration of 2-NP, and
At and
A0 are the absorbances at time
t, and
t = 0, respectively,
k (min
-1) is the rate constant of the reaction.
The degradation reaction follows a pseudo-first order reaction kinetics with respect to the alginate-silver nanocomposite beads because the concentration of NaBH
4 (10 mM) was much higher than that of 2-NP (1 mM). The rate constant for the degradation reaction was determined to be 1.40 × 10
-3 s
-1. The reaction kinetics and the rate constant obtained agree with reported values for catalytic reduction of nitrophenol compounds by green synthesized silver and gold nanoparticles and polymer nanocomposites [
21,
23,
24,
25].
3.4. Catalytic Performance of Alginate-Silver Nanocomposite Beads
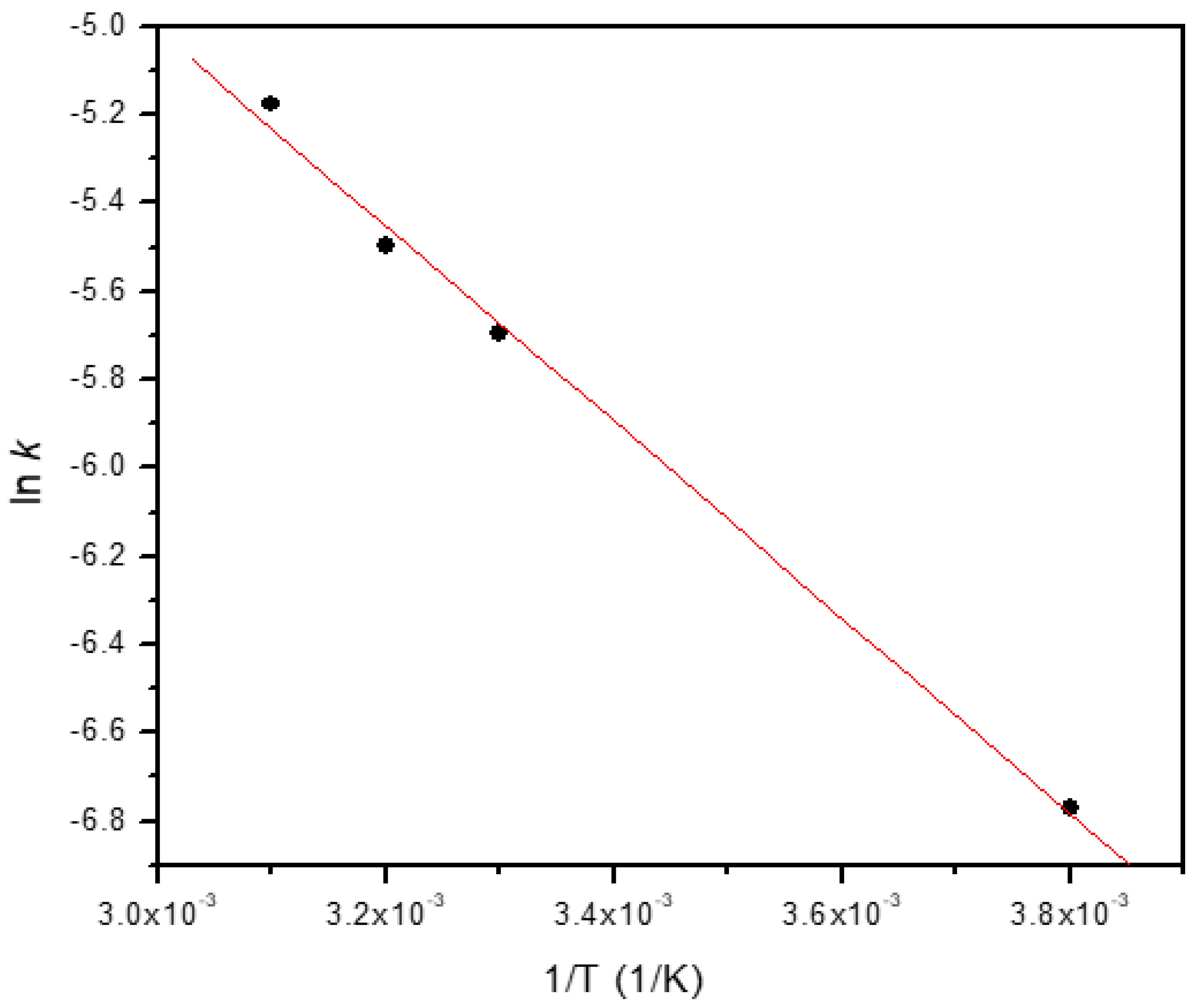
The activation energy (
Ea) for the degradation process was determined from the gradient of a plot of ln
(k) versus 1/
T according to Arrhenius equation as [
26],
where
A = frequency factor or Arrhenius constant,
R = 8.314 J K
-1 mol
-1,
T = absolute temperature in Kelvin, and
k = rate constant.
From a linear plot of ln(
k) versus 1/
T, the
Ea and
A were determined from the gradient and intercept as 18.45 kJ mol
-1 and 5.19 s
-1, respectively (
Figure 6).
An activation energy of 17 kJ mol
-1 has been reported for the degradation of 2-NP using calcium alginate beads containing iron-silver bimetallic nanoparticles [
5]. Our results agree with this confirming the excellent catalytic property of the nanocomposite beads. The results indicate that the catalytic reduction have a low potential barrier and the catalytic reduction reactions occur via surface catalysis. In comparison to 4-NP (
Ea = 10.51 kJ mol
-1), the obtained activation energy for 2-NP is higher by a factor of about 1.5, and this increase is attributed to the steric hinderance of 2-NP. The thermodynamic parameters of degradation reaction such as enthalpy change, (Δ
H), and entropy change (Δ
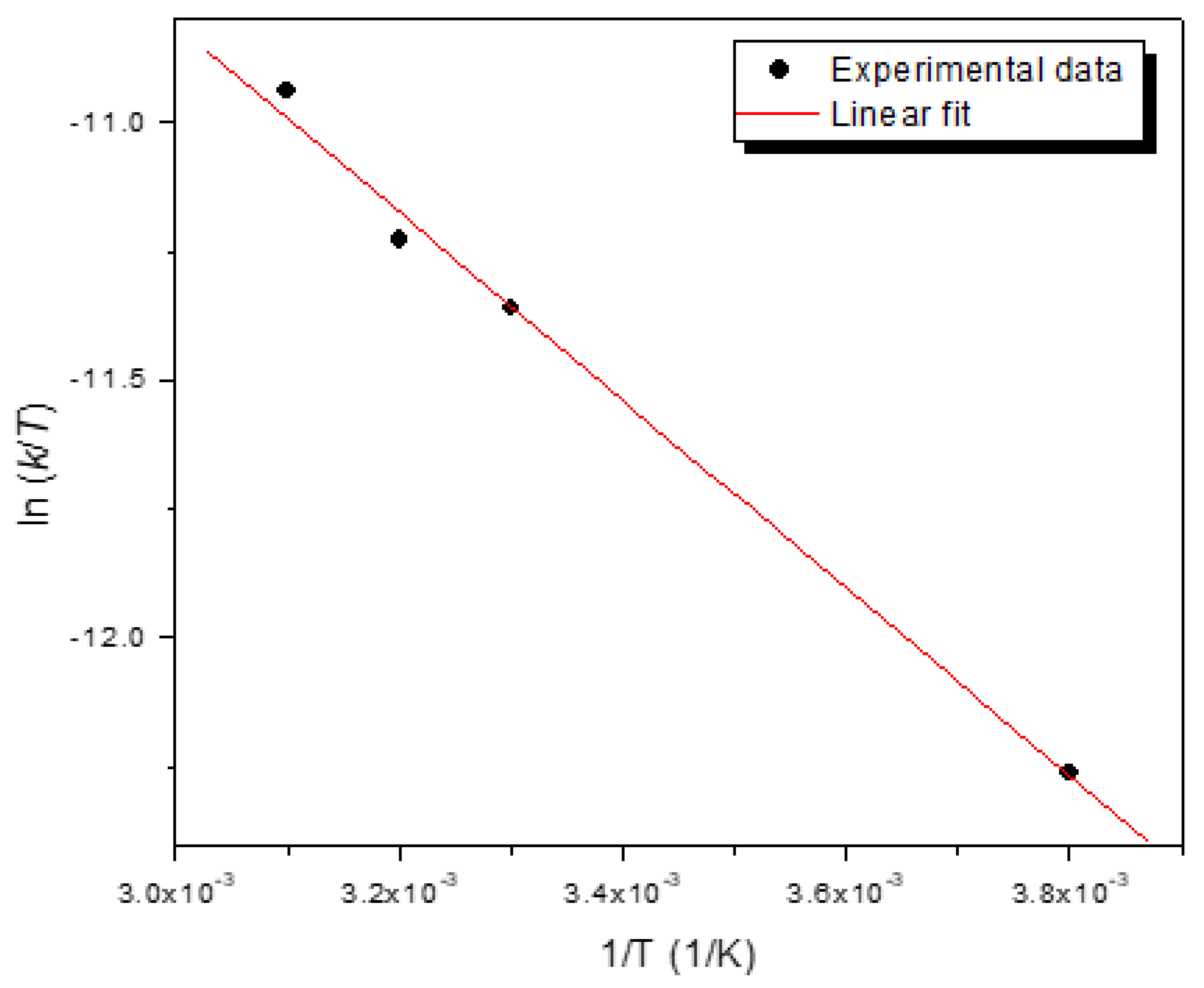
S) were determined using Eyring equation as [
5,
27],
where
kB is the Boltzmann constant (1.381 × 10
-23 JK
-1),
h is the Planck’s constant (6.626 × 10
-34 J.s),
R is the ideal gas constant, and
T is the absolute temperature in Kelvin.
From a linear plot of ln(
k/
T) versus 1/
T, the enthalpy (ΔH), and entropy(ΔS) change for the degradation of 2-NP were determined to be 15.22 kJ mol
-1 and −197.50 J mol
-1 K
-1, respectively. These values agree with reported values of ΔH = 12.77 kJ mol
-1 and ΔS = −198.42 J mol
-1 K
-1 for the degradation of 2-NP using alginate nanocomposite beads containing iron and silver nanoparticles [
5]. The negative entropy values indicate that the randomness on the interface between the nanocomposite bead and 2-NP decrease during the degradation process.
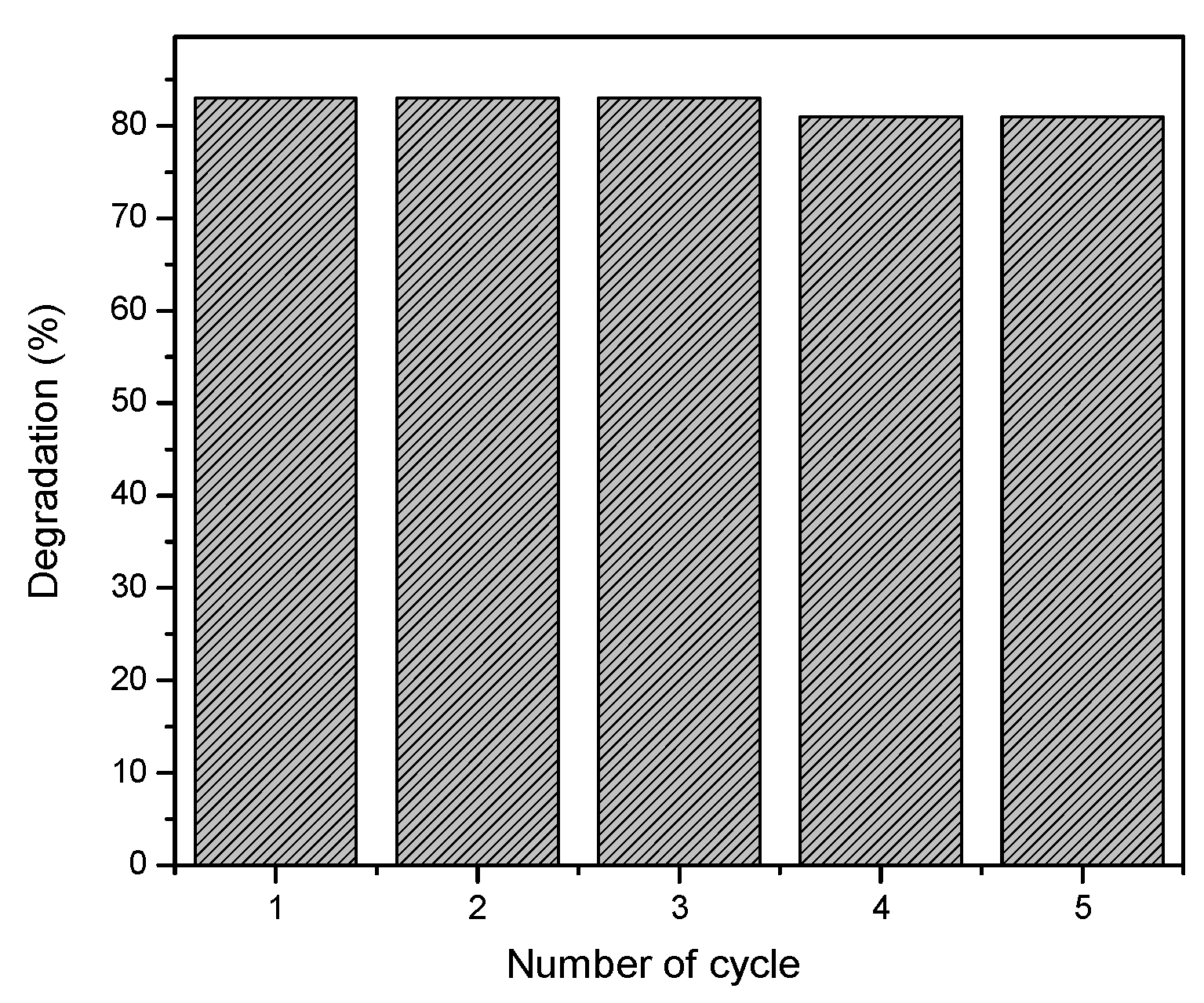
The conversion efficiency of the alginate-silver nanocomposite beads was also evaluated for 5 successive cycles and the results are shown in
Figure 8.
After the first cycle of 2-NP degradation, the beads were removed from the solution and washed repeatedly with water and then placed into a fresh solution containing 2-NP and NaBH4. The conversion efficiency of the nanocomposite beads was constant within the range of 83-80% up to 5 successive cycles which indicates good catalytic efficiency on repeated usage. During this process, no leaching of silver nanoparticles from the beads was observed.
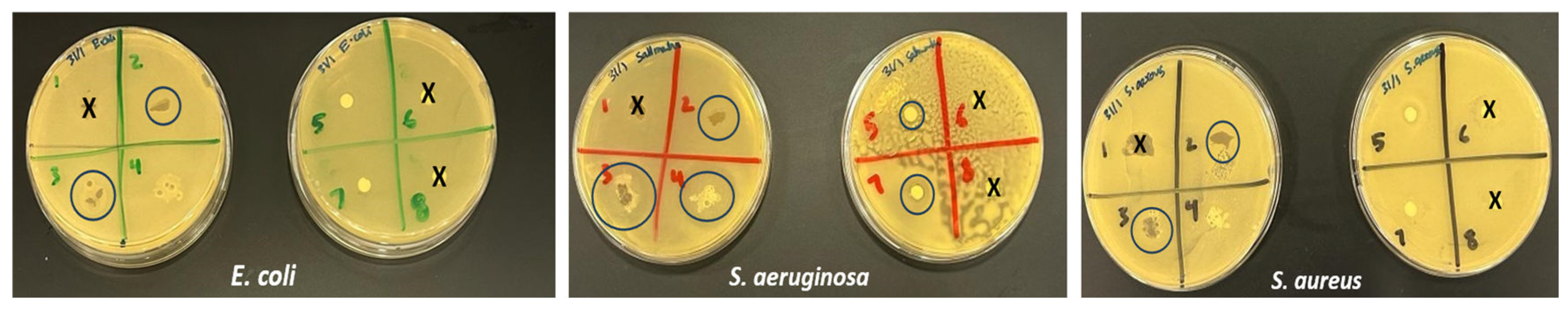
3.5. Antibacterial Properties
The silver nanoparticles (No. 7), alginate-silver nanocomposite beads (No. 3), and alginate-silver nanocomposite film (No. 2) exhibited weak to good antibacterial properties against
Escherichia coli,
Staphylococcus aureus and
Pseudomonas aeruginosa. The bacterial agar plates with the zone of inhibition for a concentration of 10 mM AgNO3 is shown in
Figure 9. The ajwa seeds extract (No. 5) showed a very weak antibacterial effect against all the three types of bacteria, and interestingly the neat alginate beads (No. 4) showed a comparable effect. The silver nanoparticles (No. 2) were effective against all the three types of bacteria, and antibacterial effect of silver nanoparticles is well known. The alginate-silver nanocomposite beads exhibited a larger zone of inhibition relative to the neat alginate beads which indicates the synergistic antibacterial effect of the nanocomposites. Similar synergistic effect has been observed for many green synthesized silver nanocomposites [
28].
The mechanism of interaction of silver nanoparticles with the bacteria is mainly ionic. The silver nanoparticles and silver ions (released from the nanoparticles) can accumulate in the pits of the cell wall and leads to denaturation of the cell membrane [
29]. In addition, the silver nanoparticles could penetrate the cell membrane leading to denaturation and rupture of organelles resulting in lysis. Further, the silver nanoparticles can disrupt bacterial signal transduction leading to cell apoptosis and termination of the bacterial cell multiplication.
4. Conclusions
Alginate-silver nanocomposites in the form of spherical beads and thin films were successfully fabricated using silver nanoparticles synthesized using the extract of ajwa dates seed. The nanocomposite beads were effective in the catalytic degradation of 2-nitrophenol and 80% degradation was achieved in 15 min. The beads showed good reusability with no appreciable decrease in the degradation capacity even up to 5 successive cycles of operation. The degradation followed a pseudo-first order reaction kinetics. The nanocomposite exhibited antibacterial effect against three clinically important pathogens. Overall, this study has laid the foundation for a new effective strategy as an alternative to high-cost commercial catalysis for the detoxification of organic pollutants. The new material developed through the eco-friendly green approach in addition to the catalytic properties has the potential to treat hospital wastewater in the future.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Niaz A, Fischer J, Barek B, et al. A novel voltametric method for the determination of maleic acid using silver amalgam paste electrode. Electroanalysis 2009; 21(15): 1719-1722.
- Ganapuram BR, Alle M, Dadigala R, et al. Catalytic reduction of methylene blue and Congo red dyes using green synthesized gold nanoparticles capped by Salmalia malabarica gum. International Nano Letters 2015; 5(1): 215-222.
- Gola D, Kriti A, Bhatt N, et al. Silver nanoparticles for enhanced dye degradation. Current Research in Green and Sustainable Chemistry 2021, 4: 100132-100139.
- Choudhary MK, Kataria J, Sharma S. Evaluation of the kinetic and catalytic properties of biogenically synthesized silver nanoparticles. Journal of Cleaner Production 2018; 198: 882-890.
- Gupta VK, Yola ML, Eren T, Kartal F, et al. Catalytic activity of Fe@Ag nanoparticle involved calcium alginate beads for the reduction of nitrophenols. Journal of Molecular Liquids 2014; 190 (1): 133-138.
- Augustine R, Kalarikkal N, Thomas S. A facile and rapid method for the black pepper leaf mediated green synthesis of silver nanoparticles and the antimicrobial study. Applied Nanoscience 2014, 4:809-818.
- Kastner C, Thunemann F. Catalytic reduction of 4-nitrophenol using silver nanoparticles with adjustable activity. Langmuir 2016, 32: 7383-7391.
- Lin S, Huang R, Cheng W. et al. Silver nanoparticle-alginate composite beads for point-of-use drinking water disinfection. Water Research 2013, 47: 3959-3965.
- Mthombeni NH, Mpenyana-Monytasi I, Onyango, MS. et al. Breakthrough analysis for water disinfection using silver nanoparticles coated resin beads in fixed-bed column. Journal of Hazardous Materials 2012, 217-218: 133-140.
- Lv Y, Lou H, Wang Z. et al. Silver nanoparticle-decorated porous ceramic composite for water treatment. Journal of Membrane Science 2009, 331 (1-2): 50-56.
- Saha S, Pal A, Kundu S. et al. Photochemical green synthesis of calcium-alginate-stabilised Ag and Au nanoparticles and their catalytic application to 4-nitrophenol reduction. Langmuir 2010, 26(4): 2885-2893.
- Martinez-Gómez F, Guerrero J, Matsuhiro B. et al. In vitro release of metformin hydrochloride from sodium alginate/polyvinyl alcohol hydrogels. Carbohydrate Polymers 2017, 155: 182-191.
- Albalwi H, El Fadl FIA, Ibrahim MM. et al. Catalytic activity of silver nanocomposite beads for degradation of basic dye: kinetic and isothermal study. Applied Organometallic Chemistry 2021, 1, 1-11. [CrossRef]
- Fagieh TM, Bakhsh EM, Khan SB. et al. Alginate/banana waste beads supported metal nanoparticles for efficient water remediation. Polymers 2021, 13: 4054-4071.
- Khan SB, Ahmad S, Karnal T. et al. Metal nanoparticles decorated sodium alginate-carbon nitride composite beads as effective catalyst for the reduction of organic pollutants. International Journal of Biological Macromolecules 2020, 164: 1087-1098.
- Alomar A, Qassim T, AlNajjar Y. et al. Green nanotechnology and phytosynthesis of metallic nanoparticles: The green approach, mechanism, biomedical applications and challenges. World Scientific Annual Review of Functional Materials 2024, 1:2430001-2430023. [CrossRef]
- Deen GR, Alhannan F, Henari F, et al. Effects of different parts of the okra plant (Abelmoschus esculentus) on the phytosynthesis of silver nanoparticles: Evaluation of synthesis conditions, nonlinear optical and antibacterial properties. Nanomaterials 2022, 12:4174-4185.
- Mostafa H, Airouyuwa JO, Maqsood S. A novel strategy for producing nanoparticles from date seeds and enhancing their phenolic content and antioxidant properties using ultrasound-assisted extraction: A multivariate based optimization study. Ultrasonics Sonochemistry 2022, 87: 106017-106033.
- Khalid S, Khalid N, Khan RS. Et al. A review on chemistry and pharmacology of ajwa dates fruit and pit. Trends in Food Science and Technology 2017, 63:60-69.
- Eid N, Osmanova H, Natchez C. et al. Impact of palm date consumption on microbiota growth and large intestinal health: A randomized, controlled, cross-over human intervention study. British Journal of Nutrition 2015, 114:1226-1236.
- Cao PL, Lu W, Mata A. et al. Egg-box model-based gelation of alginate and pectin: A review. Carbohydrate Polymers 2020, 242: 116389-116399.
- Gangula A, Podila R, Karanam L. et al. Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived from Breynia rhamnoides. Langmuir 2011, 27:15268-15274.
- Jiang ZJ, Liu CY, Sun LW. Catalytic properties of silver nanoparticles supported on silica spheres. Journal of Physical Chemistry B 2005, 109:1730-1735.
- Kumar I, Gangwar C, Yaseen B. et al. Kinetic and mechanistic studies of the formation of silver nanoparticles by nicotinamide as a reducing agent. ACS Omega 2022, 7:13778-13788.
- Khan SB, Ahmad S, Kamal T. et al. Metal nanoparticles decorated sodium alginate-carbon nitride composite beads as effective catalyst for the reduction of organic pollutants. International Journal of Biological Macromolecules 2020, 164:1087-1098.
- Shimoga G, Palem RR, Lee S-H. et al. Catalytic degradability of p-nitrophenol using ecofriendly silver nanoparticles. Metal 2020, 10 (12): 1661-1672. [CrossRef]
- Meija YR, Bogireddy NKR. Reduction of 4-nitrophenol using green-fabricated metal nanoparticles. RSC Advances 2022, 12: 18661-18675. [CrossRef]
- Farazin A, Mohammadimehr M, Ghasemi AM. et al. Design, preparation and characterization of CS/PVA/SA hydrogels modified with mesoporous Ag2O/SiO2 and curcumin nanoparticles for green, biocompatible, and antibacterial biopolymer film. RSC Advances 2021, 11:32775-32791.
- Cittrarasu V, Kaliannan D, Dharman K. et al. Green synthesis of selenium nanoparticles mediated from Ceropegia bulbosa Roxb extract and its cytotoxicity, antimicrobial, mosquitocidal and photocatalytic activities. Scientific Reports 2021, 11:1032-1046.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).