1. Introduction
Cervical cancer is one of the most common cancers among all women in the world. According to age meta-analysis, the annual world incidence of cervical cancer is 604,127 women in 2020 and varies by geographical area [
1]. Cervical cancer accounts for approximately 70% of all cancer cases in developing countries with 90% of mortality rate [
1,
2]. In most cases, co-administration of cisplatin and paclitaxel is the main procedure treatment for advanced cervical cancer. However, the response rate of the combined drugs is 29.1%-67% in patients who had recurrence after receiving combination treatment [
3]. Despite the improvement following cisplatin combination therapy, the 5-year survival rate of patients with cervical cancer remains low [
4]. Furthermore, tumor recurrence and the development of chemotherapy drug resistance are virtually unavoidable and are a major cause of less effective treatment [
5]. As a result, there is an immediate requirement of aggressive systemic therapies that use a single drug or a combination of drugs [
6]. However, the rising price and adversity of chemotherapy drugs cause a challenge for patients in developing countries. Therefore, alternative herbal supplements for cancer treatment remain an unavoidable option for patients in developing countries [
7].
To overcome cancer resistance, many efforts have been concentrated on natural products for the development of anticancer drugs with a diverse range of anticancer multiple mechanisms. "Polyphenol compounds" are known as one of the potential natural product groups in cancer prevention [
8]. The main anticancer effects of these compounds are associated with their antioxidant and antiinflammatory properties, which involve multiple mechanisms of action [
9]. Recently, peanuts (
Arachis hypogaea L.) are a valuable source of biological function as well as a potential source of natural polyphenol compounds [
10]. Peanut skin procyanidins (PSP) and their derivatives significantly inhibited prostate cancer via apoptotic cell death induction and cell cycle arrest mechanisms [
11]. Peanut skins contain resveratrol, known as the naturally occurring phytoalexin that peanuts produce in response to stress, possessing anticancer properties [
12]. We previously demonstrated that peanut skin extracts of Valencia genotypes (ICG15042 and KK4) possessing histone deacetylase (HDAC) inhibitory activity inhibited the growth of various cancer cell types (liver, colon, cervix, and breast cancer cells) in a dose and time-dependent manner in vitro [
13]. Our findings are in accordance with previous publications that link HDAC inhibitory activity of the compounds to the inhibition of a tumor growth [
14,
15]. Peanut skin extract possessing HDAC inhibitory activity suppressed activity of HDAC enzymes in the cancer cells causing an accumulation of acetylated forms of histone proteins leading to apoptotic cancer cell death [
13]. In addition, the phenolic acids of Valencia KK4-type peanut skin extract analyzed by HPLC include
p-coumaric, vanillic, ferulic,
p-hydroxybenzoic, sinapinic, and syringic acids [
13]. The two phenolic acids,
p-coumaric and vanillic acids, were the most abundant compounds among all identified phenolic acids in KK4-type peanut skin, however, some HPLC peaks in chromatogram of the peanut skin extract have yet to be identified [
16].
Valencia KK4-type peanut skin ethanolic extract (KK4-PSE) exhibited anticancer activity against HeLa cervical cancer cells in vitro [
13], however, its anticancer activity in vivo and its interaction with current anticancer drugs, such as cisplatin and 5-fluorouracil (5-FU), have not yet been explored. Based on the findings that ethanolic extracts of Thai noni juice (TNJ) products and
Tiliacora triandra leaf powder enhance the anticancer effect against human cholangiocarcinoma (CCA) cells and reduce toxicity of 5-FU, cisplatin and gemcitabine in nude mouse xenograft models [
17,
18], we therefore hypothesize that KK4-PSE would enhance anticancer activity of cisplatin or 5-FU against cervical cancer cells both in vitro and in nude mouse xenograft models. In the present study, we explored the anticancer potential of KK4-PSE to inhibit cervical cancer cell growth both in single and combination drug treatments with cisplatin or 5-FU in vitro (HeLa cervical cancer cells) and in vivo (nude mouse xenografts). The mechanisms underlying the synergistic drug interactions were also investigated. The results from this study may help researchers better understand and develop Valencia KK4-type peanut skin extract as a potential chemotherapeutic agent for cervical cancer treatment and/or chemoprevention program.
2. Materials and Methods
2.1. Materials and Reagents
RPMI-1640 medium was obtained from Gibco-BRL (Gaithersburg, MD, USA), whereas fetal bovine serum (FBS) was purchased from Cytiva (Kremplstrasse, Pasching, Austria). Propidium iodide (PI) and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA), whereas the Annexin V-FITC was obtained from Biolegend (San Diego, CA, USA). Cisplatin and 5-fluorouracil (5-FU) were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA) and PanReac Applichem (Castellar del Valles, Spain), respectively. The antibodies against p21 (2946), p53 (2524), Bcl-2 (2870), Bax (2772), pERK1/2 (9107), ERK1/2 (4377), acetyl-histone H3 (9671), and CDK4 (12790) were obtained from Cell Signaling Technology (Beverly, MA, USA). The anti-Cyclin B1 (GNS1) antibody was purchased from Santa Cruz Biotechnology (Dallas, TX, USA).
2.2. Cell Culture and Nude Mouse Xenograft Models
HeLa and Vero cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin (all from Gibco, New York, NY, USA). The cultures were maintained at 37 °C in a humidified atmosphere containing 5% CO2. Only exponentially growing cells were used for all subsequent experiments. Female BALB/CAJcl-Nu/Nu mice (4–6 weeks old, weighing 25–30 g) were procured from Nomura Siam International (Bangkok, Thailand). The mice were housed in individual ventilated cages (IVCs) at 23 ± 2 °C, with humidity maintained at 30–60% and a 12-hour light/dark cycle (350–400 Lux). All animal procedures were conducted at the Northeast Laboratory Animal Center, Khon Kaen University.
2.3. Preparation of KK4-PSE
Valencia KK4-type peanuts were harvested from a field crop grown at Khon Kaen University's Field Crop Research Station, Thailand, during the 2019 season (October 2018 to February 2019). Seed skins were separated, dried in a hot air oven at 60 °C for 6 hours, then powdered and stored under sterile conditions in plastic bags at 4 °C. For extraction, 1 g of peanut skin powder was mixed with absolute ethanol at a 1:40 (w/v) ratio and stirred at room temperature for 48 hours. The resulting supernatant was filtered through a Whatman No. 4 filter paper and concentrated by evaporation. The extract was subsequently stored at 20 °C until use.
2.4. Cell Viability Assay
Cell proliferation assay was performed using the MTT Assay. Cells were seeded into 96-well plates at a density of 8
cells/well and incubated at 37 °C for 24 h. The cells were then treated for various periods of time (24, 48 and 72 h) with varying concentrations of KK4-PSE alone or in combination with a sub-toxic dose of cisplatin or 5-FU drug at IC
20 concentration (the concentrations required to inhibit 20% of cell growth inhibition) and a vehicle control (0.5 % DMSO:EtOH, v/v). At a specified exposure time, the culture media were removed, and each well was filled with a fresh medium containing 1.2 mM MTT solution. After incubation at 37 °C for 2 h, DMSO (100 µL/well) was added and incubated for 15 min at room temperature. The absorbance (A) of dissolved formazan was measured at 570 nm using a Spectramax M5 microplate fluorometer (Molecular Devices Cooperation, Sunnyvale, CA, USA), and the optical density (O.D.) at 655 nm was measured to subtract the optical density of cellular debris at 570 nm. The experiments were performed at least three times.
2.5. Drug Interaction Determination
The combination index (CI) was calculated according to the median-effect principle to estimate the interactions between KK4-PSE with both chemotherapy drugs [
19,
20]. The CI values for 50% growth inhibition were calculated using the following equation:
where D1 is a dose of drug 1 (cisplatin or 5-FU) in a combination treatment with drug 2 (KK4-PSE) to produce 50% cell viability; Dx1 is a dose of drug 1 in a single treatment to produce 50% cell viability; D2 is a dose of drug 2 in a combination treatment with drug 1 to produce 50% cell viability; Dx2 is a dose of drug 2 in a single treatment to produce 50% cell viability; α = 1 for mutually non-exclusive modes of drug action. The dose reduction index (DRI) indicates the extent of dose reduction (fold) of the combined dose tested compared to the dose in a single agent treatment. The DRI was calculated using the following equation:
where D is a dose of a drug combined with the other drug to produce 50% cell viability; Dx is a dose of a drug in a single drug treatment to produce 50% cell viability.
2.6. Cell Cycle Analysis
Human cervical cancer (HeLa) cells (2.5
10
5 cells/mL) were plated in a 5.5 cm culture dish and incubated for 24 h. The cells were then treated with various concentrations of KK4-PSE, either alone or in combination with the IC
20 subtoxic dose of the chemotherapy drug (cisplatin or 5-FU). Propidium Iodide (PI) staining was performed as described previously [
21]. Synergistic concentrations of KK4-PSE and 5-FU were used to treat HeLa cells for 48 h. The analysis of DNA content in HeLa cells was conducted using propidium iodide staining and the BD FACSCanto II flow cytometer (Becton Dickinson, San Jose, CA, USA). Cell cycle distribution in Sub-G1, G0/G1, S, and G2/M phases was determined using the BD FACSDiva software. We gratefully acknowledge the assistance of the team at the Research Instrument Center, Khon Kaen University, Thailand, for facilitating this analysis.
2.7. Apoptosis Detection by Flow Cytometry
Cellular apoptosis was evaluated using Vybrant Apoptosis Assay Kit #2, Molecular Probes, Invitrogen Corporation (Carlsbad, CA, U.S.A.) according to the manufacturer’s instructions. Apoptotic cells were stained with Annexin-V FITC and propidium iodide (PI) as previously described.21 Briefly, HeLa cells were seeded at a density of 1 106 cells/dish in a 5.5 cm culture dish and incubated for 24 h. Synergistic concentrations of KK4-PSE and 5-FU were used to treat HeLa cells for 48 h. Thereafter, cells were washed twice with cold PBS and resuspended in 1X ice-cold Annexin-binding buffer (100 µL/dish). Each tube was then loaded with 400 µL of 1X binding buffer and the cells were incubated with Annexin V-FITC and PI in the dark for 15 minutes at room temperature. To evaluate the effect of treatment on cell death, apoptotic cells were identified by Annexin V/propidium iodide staining and analyzed using the BD FACSDiva software. Specifically, the analysis quantified the percentage of Annexin V-positive and propidium iodide-negative cells, representing early apoptosis.
2.8. Western Blot Analysis
HeLa cells were plated at a density of 1 106 cells/dish in a 5.5 cm culture dish. Synergistic concentrations of KK4-PSE and 5-FU were used to treat HeLa cells for 48 h. Total proteins were extracted using RIPA lysis buffer (Amresco, Solon, OH, USA) with a protease inhibitor cocktail and the protein concentration was determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). The protein bands were resolved by SDS-PAGE (12.5%) and transferred to the polyvinylidene fluoride (PVDF) membrane. After incubation for 1 hour at room temperature in TBST containing 5% skim milk, the blot membrane was then incubated with primary antibodies overnight at 4 °C. The primary antibodies used for western blotting analysis included anti-p21 (1:2000), anti-p53 (1:1,000), anti-Bcl-2 (1:1000), anti-Bax (1:1000), anti-pERK1/2 (1:2,000), anti-ERK1/2 (1:1,000), anti-Cyclin B (1:1,000), anti-CDK4 (1:1000), and anti-acetyl-histone H3 (1:1000). The membrane was washed with TBST before being incubated with the corresponding horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA). The blots were then developed with a chemiluminescence reagent (Bio-Rad, CA, USA) and exposed to the X-ray film.
2.9. Antitumor Activity of KK4-PSE in Nude Mouse Xenograft Models
Female nude mice (BALB/cAJcl-Nu/Nu, 4-6 weeks old) were obtained from Nomura Siam International (Bangkok, Thailand) and maintained at Khon Kaen University's Northeast Laboratory Animal Center. The animal experiments were approved by Khon Kaen University’s Institutional Animal Care and Use Committee (approval No. IACUC-KKU-20/62; date of registration 21 March 2019) and performed in accordance with guidelines established by the National Research Council of Thailand’s Ethical Principles and Guidelines for the Use of Animal in Scientific Purposes (License No. U1-00998-2558). The study was carried out in compliance with the ARRIVE guidelines. To obtain cervical cancer xenografts, HeLa cell suspensions (4
10
6 cells in 100 µL) were subcutaneously injected into the right anterior lateral thoracic wall of each mouse.
22 After cell implantation for 14 days, mice were divided into nine groups of five mice each. Mice in the experimental groups were given intraperitoneal injections of KK4-PSE at doses of 100 and 200 mg/kg body weight twice a day, cisplatin at a dose of 3 mg/kg body weight twice a day, 5-FU at a dose of 10 mg/kg body weight twice a day, and combinations of KK4-PSE and cisplatin or 5-FU. In the control group, mice were given an equal volume of normal saline solution. A digital vernier caliper was used to measure the tumor volume which was calculated every two days as the following formula:
The tumor growth inhibition ratio (TGI, %) was determined using the following formula:
where A was mean tumor weight of the vehicle control group and B was tumor weight of the treated group.
2.10. Toxicological Evaluation in Nude Mouse Xenograft Models
Toxicities of the drugs on xenograft mice during treatments were assessed by monitoring body weight changes, organ weight, and histopathology of organs (liver, kidneys, and spleen). After 14 days, the mice were sacrificed, and the tumors and visceral organs were weighed before being fixed in 10% formalin solution for the further experiments. Additionally, a toxicity analysis was conducted through the assessment of body weight changes (%BWC) following formula:
Following fixation in 10% formalin and subsequent dehydration and clearing in the tissue processor, the tumor, liver, kidney, and spleen tissues were paraffin embedded. Samples were sectioned at 5 μm using a Leica RM2255 Fully Automated Rotary Microtome (Leica Microsystems, Wetzlar, Germany) and mounted onto glass slides. Deparaffinization was achieved using xylene, followed by rehydration through a graded series of ethanol solutions (99%, 95%, and 70%) and rinsing in distilled water. The rehydrated tissue sections were then stained with hematoxylin and eosin (H&E) and examined under a light microscope.
2.11. In Situ Apoptosis Detection
The level of apoptosis within tissue sections was assessed using the terminal deoxyuridine nick-end labeling (TUNEL) assay. Following the protocol of the In Situ Cell Death Detection kit (Roche Applied Science, Mannheim, Germany), all specimens were sequentially fixed with 10% formaldehyde, dehydrated, paraffin-embedded, and sectioned into 5 µm-thick histological sections. After TUNEL staining, slides were counterstained with 10% hematoxylin, dehydrated, mounted, and visualized under a light microscope (Olympus CX31, Tokyo, Japan). TUNEL-positive cells were identified and quantified by counting the number of brown-stained (DAB-positive) cells per field image using the particle analysis tool in ImageJ Fiji software, adapted from the method as described previously [
23]. The data were then expressed as the average percentage of TUNEL-positive cells.
2.12. Statistical Analysis
Statistical analyses were performed using SPSS 22.0 (IBM, Manassas, VA, USA). The data are presented as mean ± standard deviation (SD) and mean ± SEM (for independent experiments). Graphical representations were generated with GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA). One-way analysis of variance (ANOVA) was employed to assess statistically significant differences between the control and experimental groups, followed by Duncan's post hoc test for pairwise comparisons. The p values < 0.05 were considered statistically significant. All experiments were performed in triplicate.
4. Discussion
Chemotherapy drug resistance in cervical cancer remains a critical obstacle to effective practical therapy. It is important to identify pharmacologically safe agents capable of enhancing conventional therapy while reducing side effects from chemotherapy drugs. Polyphenol agents appear to have the potential to solve this problem, as several studies have reported that polyphenol agents possess antitumor activity against various types of cancer [
24]. Peanut (
Arachis hypogaea L.) seed skin (testa) has long been considered as an important source of polyphenols. Recently, polyphenol compounds from peanut testa, particularly procyanidins, demonstrated remarkable activity against a variety of cancer cell lines [
25]. According to our previous study, Valencia-type peanut skin extracts exhibited antitumor activity against several human carcinoma cell lines (breast, cervical, colon, and liver) in a dose- and time-dependent manner [
13]. The antitumor activity of Valencia-type peanut skin extract is associated with a variety of mechanisms, including the activation of caspases (cysteinyl aspartate-specific proteases), the production of reactive oxygen species (ROS), and the inactivation of histone deacetylases [
26,
27]. KK4-PSE inhibited the growth of human cervical adenocarcinoma (HeLa) cell line by inhibiting the HDAC enzyme and inducing cell apoptosis [
13]. In this study, we further employed
in vitro models to investigate the drug-interaction effects against HeLa cells and
in vivo experiments to assess the antitumor efficacy and organ toxicity profiles in mouse models. The KK4-PSE exhibited potent antiproliferative activity against HeLa cells (IC
50 = 41.32 ± 0.74 µg/mL) at 72 h exposure. This effect was significantly less pronounced in Vero cells (IC
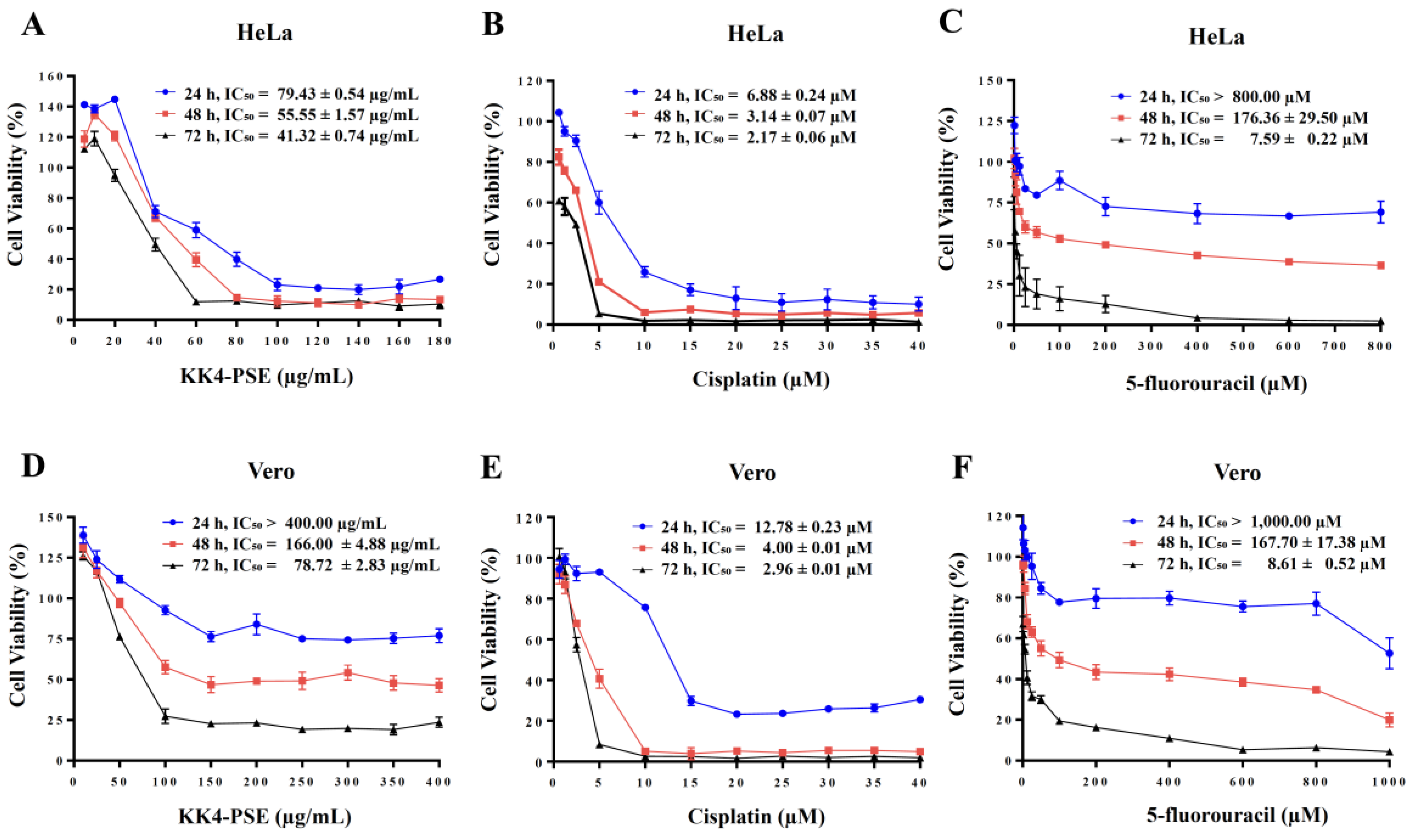
50 = 78.72 ± 2.83 µg/mL), suggesting a selective cytotoxicity towards cancer cells (
Figure 1).
To quantitatively evaluate the drug interaction effects, the Chou-Talalay method was employed to calculate combination index (CI) and dose reduction index (DRI) values [
19,
20]. These parameters serve as critical indicators of the interaction type, revealing whether the combination exhibits synergism, antagonism, or additivity as described previously [
19,
28]. These parameters determine whether the combined effect on cancer cells is additive (CI = 0.90–1.1), synergistic (CI < 0.90), or antagonistic (CI > 1.3) [
19,
28]. After 48 hours of exposure, the combination of KK4-PSE and cisplatin exhibited an additive effect on HeLa cells (CI = 1.18 ± 0.06). Conversely, the KK4-PSE and 5-FU combination demonstrated a slight synergism at the same time point (CI = 0.49 ± 0.02) (
Table 1). This synergism translated to a 3.19-fold and 21.78-fold reduction in the required doses of Cis and 5-FU, respectively, in HeLa cells after 48 hours exposure. Notably, the 5-FU combination further facilitated dose reduction by 21.78-fold and 5.09-fold at 48 and 72 hours, respectively. These findings suggest that KK4-PSE in combination with 5-FU exhibits greater potential for enhanced HeLa cell inhibition compared to the combination with cisplatin.
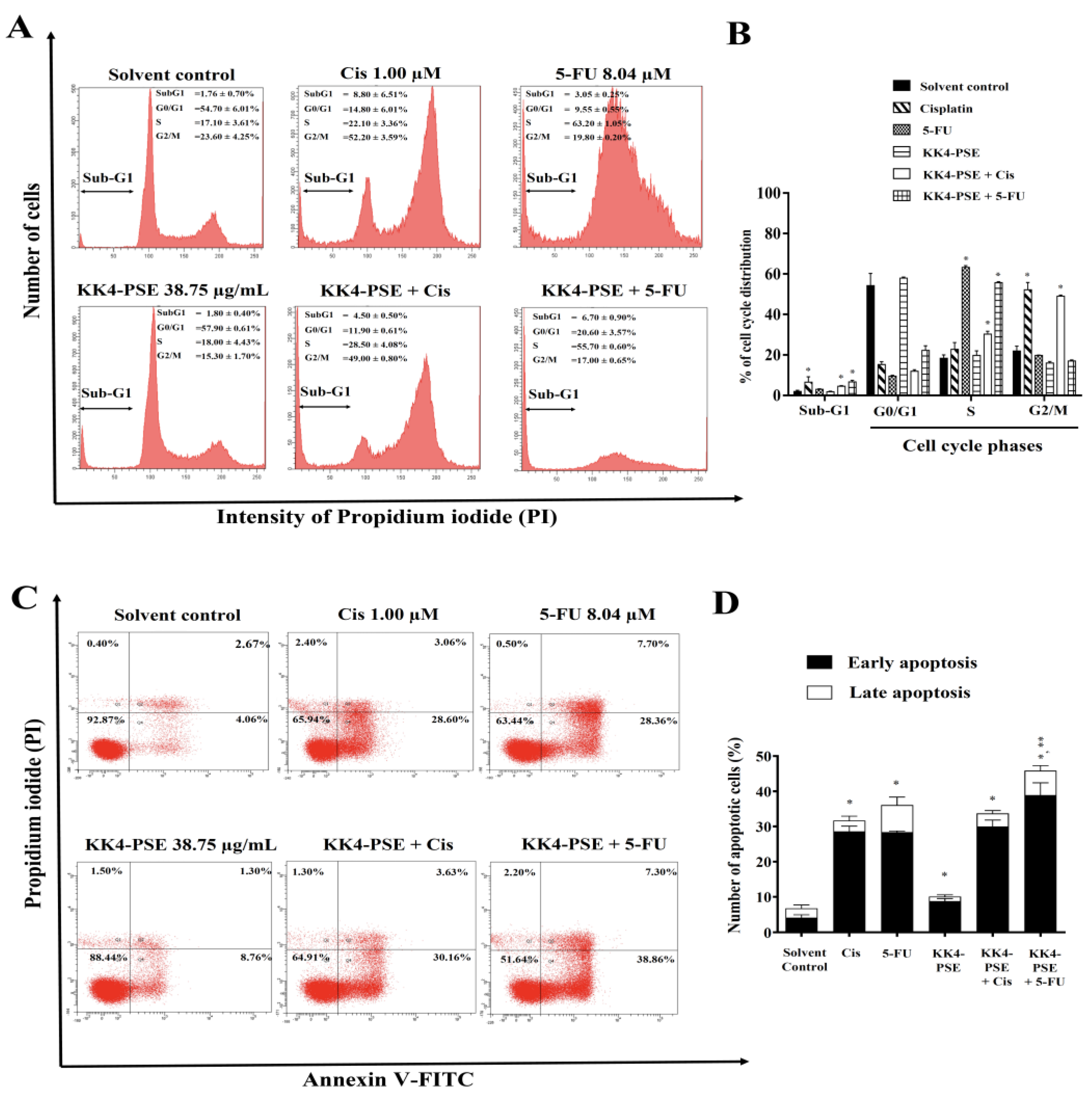
Combined treatment of KK4-PSE with either cisplatin or 5-FU potently suppressed HeLa cell proliferation through a dual mechanism: cell cycle arrest and apoptosis inductions. Treatment-specific cell cycle arrest was observed, with Cis inducing G2/M arrest and 5-FU targeting the S phase (
Figure 2A and 2B). In consistent with our findings, 5-FU triggered S-phase arrest in drug-resistant neck & head carcinoma cell lines (UM-SCC-23 and UM-SCC-2/WR) and colon cancer cells [
29,
30]. Interestingly, 5-FU combinations can activate the thymidylate synthase (TS) gene [
31]. This activation, however, appears to provide cancer cells with additional time to repair DNA damage induced by 5-FU, ultimately contributing to drug resistance [
32,
33]. Combinations of KK4-PSE with cisplatin/5-FU led to a significant increase in the sub-G1 population, indicating apoptosis induction (
Figure 2A and 2B). Indeed, apoptosis analysis further confirmed and visualized this enhanced apoptosis (
Figure 2C and 2D).
Several cancer cells were defeated by the synergistic effect of 5-FU and herb extract, which switched the manner of action from cell cycle disruption to increasing reactive oxygen species-mediated cell death [
34,
35]. In this study, our findings revealed that a combination treatment of cisplatin and KK4-PSE promoted cancer cell growth inhibition through up-regulating p21 and Cyclin B1 (
Figure 3). Cyclin B1 protein is suppressed by p21 protein through preventing the activation of Cyclin B1-cdc2 complexes, resulting in G2/M phase cell cycle disruption at the end [
36]. Notably, the combination treatment of 5-FU and KK4-PSE caused the up-regulation of pERK1/2 (
Figure 3). Like this finding, low-intensity DNA damage induced ERK activation causing cell cycle arrest by phosphorylation of p53 [
37]. Similarly, activating ERK1/2 can cause histone H3 phosphorylation by activating downstream histone kinases, resulting in oncotic cell death [
38]. Furthermore, ERK activation is linked to cell death caused by ROS through signaling pathways downstream of p53 activation [
39].
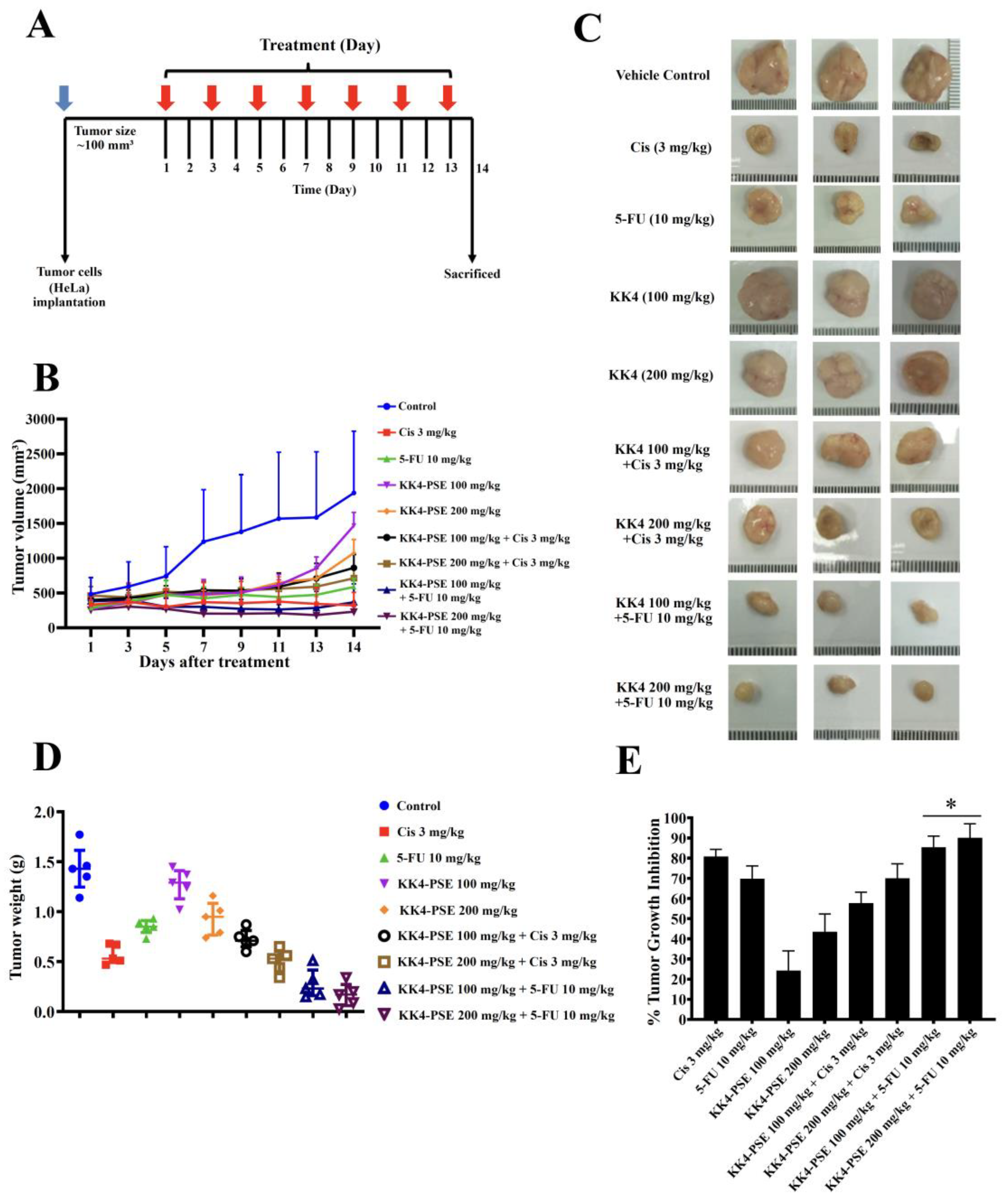
The BALB/cAJcl-Nu/Nu nude mouse HeLa xenograft model was used to investigate the empirical basis for the preclinical administration of 5-FU in combination with KK4-PSE. In this study, KK4-PSE enhanced tumor suppression activity of 5-FU greater than that of cisplatin (
Figure 4B–4E). This is in accordance with the
in vitro findings, in which KK4-PSE synergistically enhances the antiproliferative activity of 5-FU while the interaction of KK4-PSE and cisplatin is additive or antagonistic (
Table 1). There were no significant differences in tumor volumes between the combination treatments (5-FU + KK4-PSE) and 5-FU single drug treatment, however, the mean values of tumor volumes of the combination treatments (5-FU + KK4-PSE) were less than that of 5-FU single drug treatment (
Figure 4B). Nevertheless, significant differences in tumor weights and tumor growth inhibition ratios between the combination treatments (5-FU + KK4-PSE) and the 5-FU single drug treatment were observed (
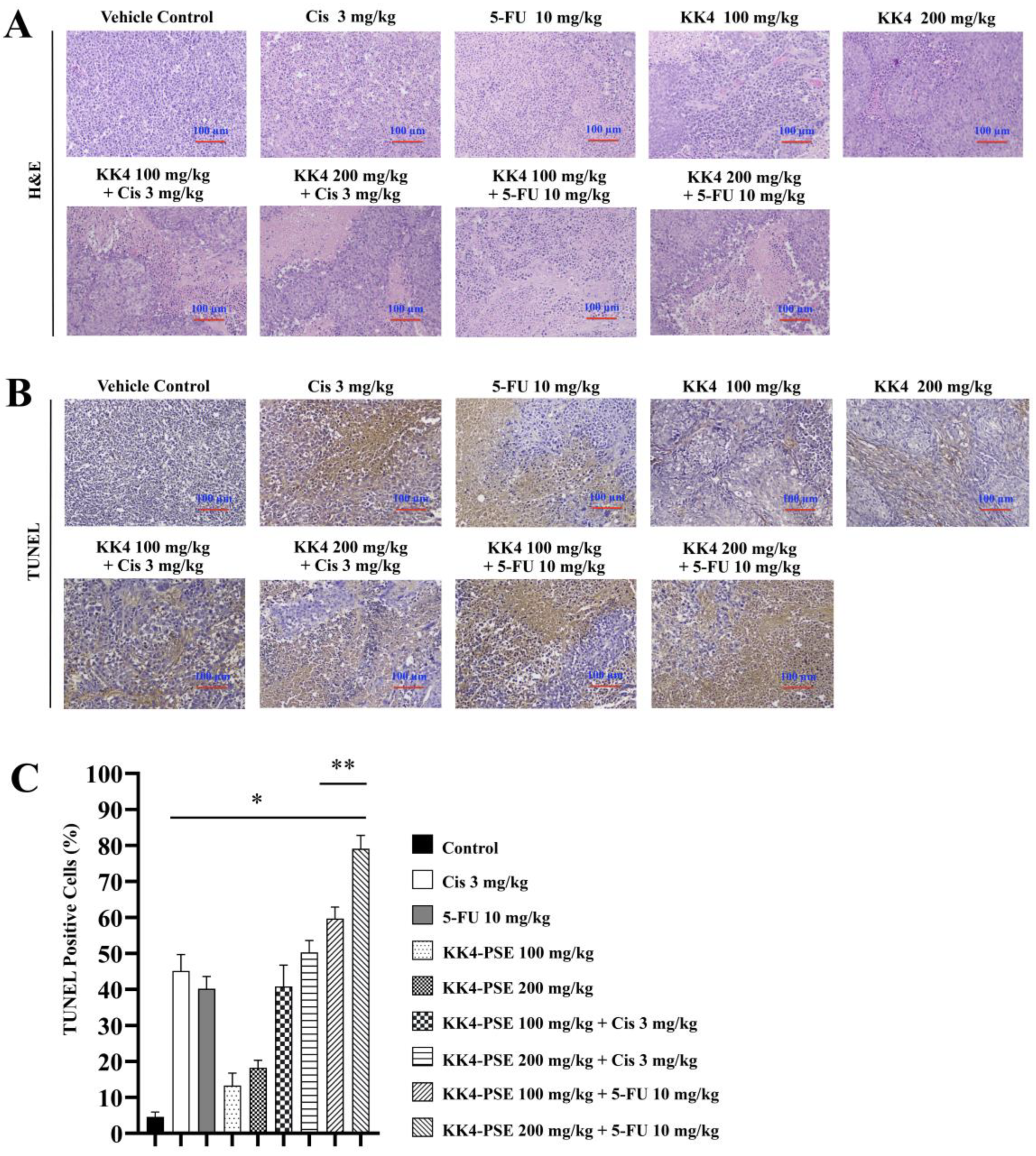
Figure 4D and 4E). In consistent with this findings, significant differences in the percentages of TUNEL-positive cells representing apoptotic-positive cells in the mouse tumors between the combination treatments (5-FU + KK4-PSE and the 5-FU single drug treatment were observed (
Figure 5B and 5C).
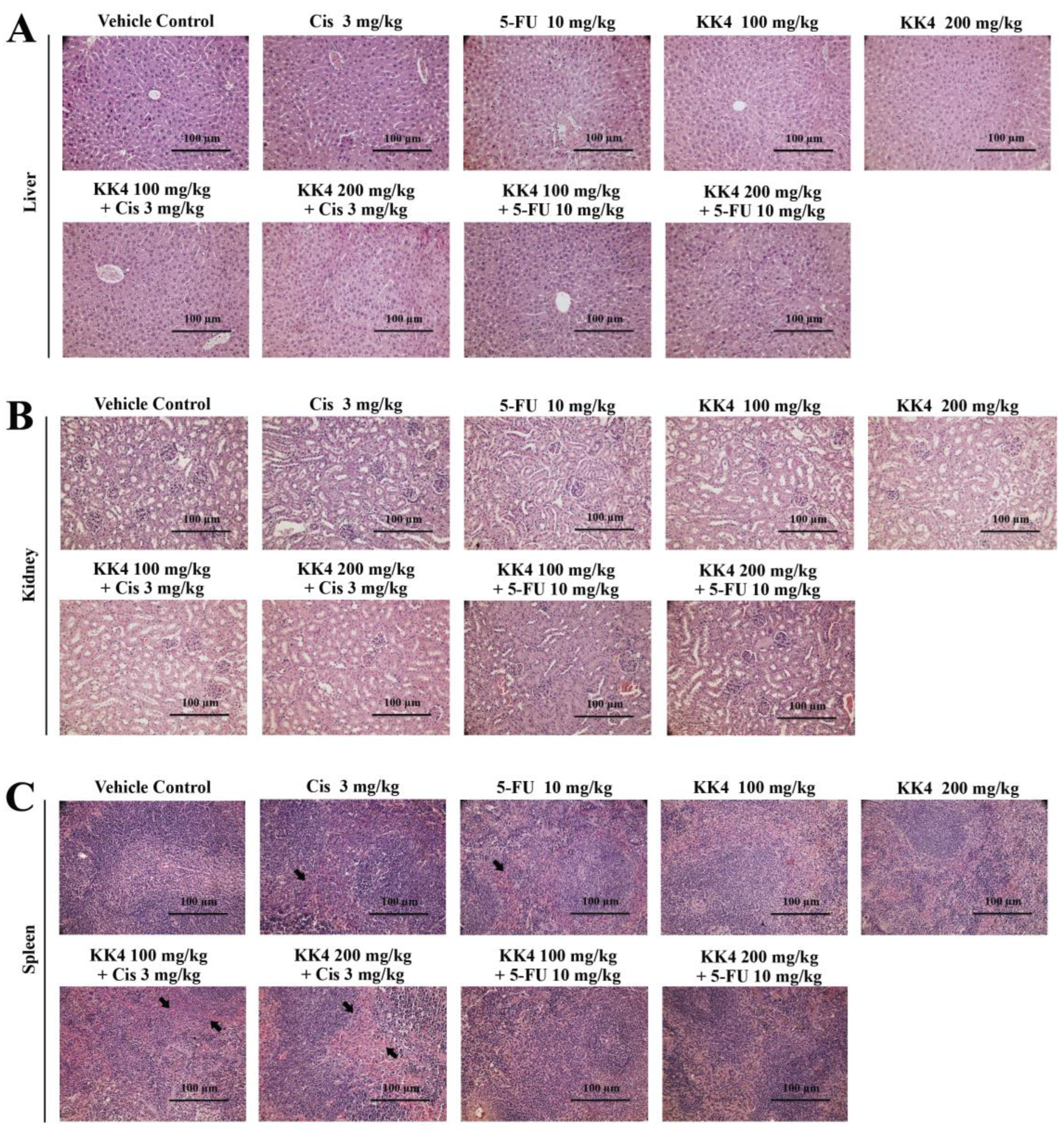
Toxicological evaluation revealed that nude mice treated with 5-FU alone and in combination with KK4-PSE had slightly negative impacts on body weight gain following the intervention sessions (
Table 2). Prolonged dosing of 5-FU has been reported to cause a significant hepatotoxicity as measured by biochemical and histopathological markers, in which total protein content (TPC) and overall liver weight were reduced in particular [
40]. In this study, mice administered with 5-FU combined with high dose-KK4-PSE exhibited a significant increase in liver weight when compared with other treatment groups, suggesting that hepatotoxicity in mice may be relieved by the 5-FU combination treatment to minimize 5-FU toxicity. These results are in accordance with previous studies demonstrating that some herb extracts and phenolic compounds can reduce the hepatotoxicity of 5-FU by scavenging free radicals and lowering damage caused by the reactive oxygen species (ROS) [
41,
42]. Furthermore, our data showed that KK4-PSE treatments, both alone and in combination with 5-FU, significantly increased the mouse kidney as well as liver weights (
Table 2). The treatments with 5-FU alone or in combination with KK4-PSE significantly reduced spleen weight. However, the spleen hyaloserositis was significantly reduced in the 5-FU combination treatment with KK4-PSE when compared to the 5-FU alone treatment (
Figure 6C), suggesting that KK4-PSE may also reduce spleen toxicity caused by 5-FU. Taken together, the KK4-PSE appeared to reduce the toxicities of 5-FU chemotherapy. However, clinical studies on the anticancer activity and the toxicity of the combined 5-FU and KK4-PSE are required.
The phenolic acid contents of KK4-PSE were partially identified in our previous study based on availability of phenolic acid standards [
13]. The study indicated that
p-hydroxybenzoic and
p-coumaric acids were the most prevalent components in KK4-PSE. Furthermore, these phenolic components possessed histone deacetylase inhibitory activity against breast and cervical cancer cell lines [
43]. Recent report revealed that peanut skin includes a variety of bioactive substances that may be divided into three categories: stilbenes, phenolic acids, and flavonoids, in which phenolic compounds are considered key active molecules with a wide variety of biological activity [
44]. Peanut skin extracts have considerable amounts of phenolic compounds, including
p-coumaric, cinnamic acids, ferulic, caffeic, chlorogenic, and quinic acids [
45,
46]. Gaafar et al. (2015) also reported that a crude extract from peanut skin exhibited cytotoxic effects on HCT116 (colon cancer), HepG2 (liver cancer), and MCF-7 (breast cancer) cells [
25]. Furthermore, polyphenolic extract of peanut skin showed no cytotoxicity on normal epithelial cells and human peripheral blood cells [
26]. Resveratrol found in peanut skin exhibits anticancer activity through a mechanism that involves disrupting the cellular signaling pathway and triggering apoptosis [
47]. Nonetheless, other promising active compounds in KK4-PSE will be studied further before starting the clinical trial.
Author Contributions
Conceptualization, T.S.; methodology, T.S., G.S., and J.J.; formal analysis, J.J.; investigation, J.J., J.P., S.U., K.W., and A.S.; resources, T.S., G.S., S.J., B.S.; data curation, J.J., T.S., and G.S.; writing-original draft preparation, J.J., A.S., T.S.; writing-review and editing, T.S., G.S., S.J., B.S.; visualization, J.J., T.S.; supervision, T.S.; funding acquisition, T.S., S.J. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Antiproliferative effects of the single agent treatments of 5-FU, cisplatin, and KK4-PSE. Human cervical cancer HeLa cells (A, B, C), and non-cancer Vero cells (D, E, F) were treated with various concentrations of KK4-PSE or chemotherapeutic drugs (5-FU, cisplatin) for 24, 48 and 72 h. The percentages of cell viability were calculated relative to the solvent control treatment (0.50% ethanol + 0.50% DMSO). Data shown were mean ± SEM from three independent experiments.
Figure 1.
Antiproliferative effects of the single agent treatments of 5-FU, cisplatin, and KK4-PSE. Human cervical cancer HeLa cells (A, B, C), and non-cancer Vero cells (D, E, F) were treated with various concentrations of KK4-PSE or chemotherapeutic drugs (5-FU, cisplatin) for 24, 48 and 72 h. The percentages of cell viability were calculated relative to the solvent control treatment (0.50% ethanol + 0.50% DMSO). Data shown were mean ± SEM from three independent experiments.
Figure 2.
Effects of the combination treatments under the synergistic conditions on inductions of cell cycle arrest and apoptosis. (A) Representative DNA histograms of HeLa cells treated with IC20 sub-toxic doses (48 h-exposure) of KK4-PSE, 5-FU, and cisplatin either alone or combination treatments are shown. (B) The percentages of cells at different cell cycle phases are shown as bar graphs of the mean from three independent experiments. (C) The representative dot plots represent the flow cytometry analysis of apoptosis induction in HeLa cells. (D) The bar graph shows the mean of the percentage of apoptotic cells from three independent experiments. HeLa cells were treated with solvent control (0.25% ethanol + 0.25% DMSO), cisplatin (Cis) (1.00 µM), 5-FU (8.04 µM), KK4-PSE (38.75 µg/mL), and the respective combination treatments for 48 hours. *p < 0.05 and **p < 0.05 indicate a significant difference compared with the solvent control and single agent treatments, respectively.
Figure 2.
Effects of the combination treatments under the synergistic conditions on inductions of cell cycle arrest and apoptosis. (A) Representative DNA histograms of HeLa cells treated with IC20 sub-toxic doses (48 h-exposure) of KK4-PSE, 5-FU, and cisplatin either alone or combination treatments are shown. (B) The percentages of cells at different cell cycle phases are shown as bar graphs of the mean from three independent experiments. (C) The representative dot plots represent the flow cytometry analysis of apoptosis induction in HeLa cells. (D) The bar graph shows the mean of the percentage of apoptotic cells from three independent experiments. HeLa cells were treated with solvent control (0.25% ethanol + 0.25% DMSO), cisplatin (Cis) (1.00 µM), 5-FU (8.04 µM), KK4-PSE (38.75 µg/mL), and the respective combination treatments for 48 hours. *p < 0.05 and **p < 0.05 indicate a significant difference compared with the solvent control and single agent treatments, respectively.
Figure 3.
Effect of KK4-PSE in combination with cisplatin (Cis) or 5-FU on the levels of proteins involved in apoptosis and ERK signaling. HeLa cells were treated with the solvent control (0.25% ethanol + 0.25% DMSO), KK4-PSE (38.75 µg/mL), and Cis (1.00 µM) or 5-FU (8.04 µM) for single and combined agent treatments under the synergistic conditions (48 h exposure). (A) The representative protein bands from western blot analysis were shown. Total ERK1/2 and β-actin were used as loading controls. (B) Bar graph represents mean ± SD of relative fold of protein expression from three independent experiments. “*” and “**” denotes a statistically significant difference (p < 0.05) compared with the solvent control treatment and single agent treatments, respectively.
Figure 3.
Effect of KK4-PSE in combination with cisplatin (Cis) or 5-FU on the levels of proteins involved in apoptosis and ERK signaling. HeLa cells were treated with the solvent control (0.25% ethanol + 0.25% DMSO), KK4-PSE (38.75 µg/mL), and Cis (1.00 µM) or 5-FU (8.04 µM) for single and combined agent treatments under the synergistic conditions (48 h exposure). (A) The representative protein bands from western blot analysis were shown. Total ERK1/2 and β-actin were used as loading controls. (B) Bar graph represents mean ± SD of relative fold of protein expression from three independent experiments. “*” and “**” denotes a statistically significant difference (p < 0.05) compared with the solvent control treatment and single agent treatments, respectively.
Figure 4.
Effects of KK4-PSE (100 or 200 mg/kg), Cis (3 mg/kg), and 5-FU (10 mg/kg) alone and in combination on the HeLa mouse xenografts. (A) Experimental design of the administration of KK4-PSE, Cis, and 5-FU alone or in combination is shown. (B) Tumor volumes of HeLa inoculated mice after treatments with KK4-PSE, Cis, and 5-FU alone or in combination are shown. (C) Representative photographs of the tumors and (D) tumor weights after surgical excision are shown. (E) The percentages of tumor growth inhibition (%TGI) compared with the control treatments are shown. “*” indicates a significant increase when compared with the single drug treatment (p < 0.05).
Figure 4.
Effects of KK4-PSE (100 or 200 mg/kg), Cis (3 mg/kg), and 5-FU (10 mg/kg) alone and in combination on the HeLa mouse xenografts. (A) Experimental design of the administration of KK4-PSE, Cis, and 5-FU alone or in combination is shown. (B) Tumor volumes of HeLa inoculated mice after treatments with KK4-PSE, Cis, and 5-FU alone or in combination are shown. (C) Representative photographs of the tumors and (D) tumor weights after surgical excision are shown. (E) The percentages of tumor growth inhibition (%TGI) compared with the control treatments are shown. “*” indicates a significant increase when compared with the single drug treatment (p < 0.05).
Figure 5.
Effects of KK4-PSE, cisplatin, and 5-FU in single and combined agent treatments on HeLa cells-inoculated nude mice. (A) Hematoxylin and eosin staining (H&E) was used to analyze the histopathology of mouse tumor slices under a light microscope. (B) Representative tumor sections representing in situ apoptosis were analyzed by TUNEL staining. (C) Bar graph shows the mean percentage of TUNEL-positive cells as representing the level of apoptosis. “*” and “**” denote a statistically significant difference (p < 0.05) compared with the solvent control and single agent treatments, respectively. Scale bar = 100 μm.
Figure 5.
Effects of KK4-PSE, cisplatin, and 5-FU in single and combined agent treatments on HeLa cells-inoculated nude mice. (A) Hematoxylin and eosin staining (H&E) was used to analyze the histopathology of mouse tumor slices under a light microscope. (B) Representative tumor sections representing in situ apoptosis were analyzed by TUNEL staining. (C) Bar graph shows the mean percentage of TUNEL-positive cells as representing the level of apoptosis. “*” and “**” denote a statistically significant difference (p < 0.05) compared with the solvent control and single agent treatments, respectively. Scale bar = 100 μm.
Figure 6.
Histopathology of mouse organs. The tissue sections of (A) liver, (B) kidney and (C) spleen, were stained with hematoxylin and eosin and examined under a light microscope. The black arrow indicates spleen hyaloserositis that is often described as inflammation of the serous membranes (serositis) that cover tissues or organs. Scale bar = 100 μm.
Figure 6.
Histopathology of mouse organs. The tissue sections of (A) liver, (B) kidney and (C) spleen, were stained with hematoxylin and eosin and examined under a light microscope. The black arrow indicates spleen hyaloserositis that is often described as inflammation of the serous membranes (serositis) that cover tissues or organs. Scale bar = 100 μm.
Table 1.
CI and DRI of the combination treatments between KK4-PSE, Cisplatin, and 5-FU against HeLa cells.
Table 1.
CI and DRI of the combination treatments between KK4-PSE, Cisplatin, and 5-FU against HeLa cells.
| Exposure times |
IC50 of KK4-PSE (µg/mL) |
IC20 of Cis (µM) |
IC20 of 5-FU (µM) |
CI |
DRI |
| Alone |
Combination |
Cis |
5-FU |
KK4-PSE |
| 24 h |
79.43 ± 0.54 |
43.37 ± 1.66 |
3.30 ± 0.14 |
- |
1.12 ± 0.03 |
2.09 |
- |
1.56 |
| 48 h |
55.55 ± 1.57 |
45.48 ± 0.29 |
1.00 ± 0.17 |
- |
1.18 ± 0.06 |
3.19 |
- |
1.16 |
| 72 h |
41.32 ± 0.74 |
35.83 ± 1.18 |
0.31 ± 0.07 |
- |
1.02 ± 0.03 |
7.30 |
- |
1.14 |
| 24 h |
79.43 ± 0.54 |
31.79 ± 1.64 |
- |
45.09 ± 3.64 |
N/D |
- |
N/D |
2.13 |
| 48 h |
55.55 ± 1.57 |
23.36 ± 0.23 |
- |
8.04 ± 1.48 |
0.49 ± 0.02 |
- |
21.78 |
2.26 |
| 72 h |
41.32 ± 0.74 |
16.57 ± 1.34 |
- |
1.50 ± 0.11 |
0.60 ± 0.03 |
- |
5.09 |
2.49 |
Table 2.
Body weight, % body weight change (%BWC), and relative organ weight of mice in the vehicle control and treated groups.
Table 2.
Body weight, % body weight change (%BWC), and relative organ weight of mice in the vehicle control and treated groups.
| Groups |
Initial body weight (g) |
Final body weight (g) |
%BWC |
Organ Index (g/100 g Body weight) |
| Liver |
Kidney |
Spleen |
| Vehicle Control |
23.52 ± 1.96 |
23.53 ± 1.45 |
0.04 |
6.27 ± 0.60 |
0.97 ± 0.09 |
1.60 ± 1.68 |
| Cisplatin 3 mg/kg |
23.61 ± 1.03 |
22.97 ± 1.23 |
-2.71 |
5.54 ± 1.88 |
0.80 ± 0.21a
|
0.35 ± 0.18a
|
| 5-FU 10 mg/kg |
21.85 ± 1.12a
|
15.99 ± 1.48a
|
-26.82 |
7.37 ± 0.48 |
1.18 ± 0.10a
|
0.28 ± 0.03a
|
| KK4-PSE 100 mg/kg |
19.87 ± 1.36a
|
20.11 ± 1.65a
|
1.21 |
6.93 ± 0.58 |
1.04 ± 0.07 |
0.68 ± 0.17a
|
| KK4-PSE 200 mg/kg |
19.80 ± 1.01a
|
20.06 ± 0.78a
|
1.31 |
7.13 ± 0.91 |
1.07 ± 0.08 |
0.76 ± 0.27a
|
| KK4-PSE 100 mg/kg + Cis |
22.17 ± 1.05 |
21.20 ± 1.44 |
-4.38 |
6.98 ± 0.06 |
0.93 ± 0.05 |
0.71 ± 0.23a
|
| KK4-PSE 200 mg/kg + Cis |
22.74± 1.55 |
19.73 ± 2.41a,b
|
-13.24 |
7.02 ± 0.94 |
0.87 ± 0.06 |
0.70 ± 0.32a
|
| KK4-PSE 100 mg/kg + 5-FU |
21.35± 1.10a
|
17.82 ± 3.88a
|
-16.53 |
7.17± 1.01 |
1.12 ± 0.10a
|
0.32 ± 0.16a
|
| KK4-PSE 200 mg/kg + 5-FU |
22.97 ± 0.30 |
17.61± 1.63a
|
-23.33 |
7.98 ± 0.46a
|
1.19 ± 0.07a
|
0.21 ± 0.08a
|