1. Introduction
The rapid identification of compound with optimal pharmacokinetic characters plays a pivotal role in drug research. To achieve this, in vitro absorption, distribution, metabolism, and excretion (ADME) screening assays are extensively developed and employed. These assays generate data that proves to be predictive of a compound's pharmacokinetic properties in both preclinical species and humans.[
1,
2] During drug discovery process and lead optimization researchers face the challenge of conducting a multitude of experiments within a limited timeframe. High throughput screening (HTS) systems emerge as indispensable tools, enabling the evaluation of up to 100,000 individual compounds in just a few days or weeks. The extensive utilization of preclinical animals in vivo to execute these experiments raises not only the specter of exorbitant development costs but also the ethical quandary of aligning with the escalating societal and regulatory expectations regarding the minimization of animal testing in pharmaceutical research.[
3] In addition, taking and preparing samples from blood, urine or tissues is time-consuming, the resulting metabolites are in extremely low quantities and the biological matrix present is a confounding factor that can make analysis very difficult. However, information on the enzymatic transformations that the compounds under study undergo in the body is necessary for both the development and the authorization process. For this reason, there is an increasing focus on in vitro systems avoiding biological matrix in the development of pharmaceuticals to test the solubility and solution stability of drugs in simulated gastrointestinal fluid, the absorption of active substances in various epithelial cells membranes[
4] , as well as the metabolism of drugs.[
5]
In vitro biomimetic models have the potential to provide support or even serve as alternatives to the established gold standard in vitro liver microsome based methods. In 1979,
Groves et al. [
6] first applied synthetic metalloporphyrins in biomimetic oxidation reactions. Using a meso-tetraphenylporphyrin iron(III) – iodosylbenzene system, they modeled hydroxylation and epoxidation reactions catalyzed by cytochrome P450 (CyP450) for simple alkanes and olefins in dichloromethane solution. Nowadays the utilization of metalloporphyrins as bioinspired oxidation catalysts is an evolving topic of research. By understanding the CyP450 mechanism of action, chemists have been able to successfully mimic several types of oxidation reactions using metalloporphyrins as catalysts.[
7] Metalloporphyrins have also been employed in investigations of drug metabolism and the synthesis of metabolites, showcasing their versatility in biochemical research.[
8,
9] Recently, as a model system for drug metabolism metalloporphyrin have been utilized as magnetic nanoparticles,[
10] liver-on-a-chip nanoparticles[
11] and as an immobilized catalyst in a continuous flow packed bed reactors[
10]
One crucial aspect influencing the activity of metalloporphyrins is their pH sensitivity.
Wolak et al. examined the catalytic mechanism of meso-tetrakis(2,4,6-trimethyl-3-sulphonyloxyphenyl) porphyrin - iron(III) dihydrate (FeTPPS(2,4,6-Me)) in relation to the chemical properties of the medium. They found that pH significantly affects the selectivity and the rate of catalyst degradation[
12]. FeTPPS(2,4,6-Me) porphyrin complex as a redox partner promotes homolytic O-O bond cleavage of peroxide bond containing oxidants when the pH < 3.5 and pH > 7, which is analogous to the mitochondrial one-electron oxidation within living bodies. Between pH 4 and 7, the heterolytic O-O bond cleavage is the thermodynamically favored mechanism. This process corresponds to the two electron oxidation reaction catalyzed by the microsomal CyP450 enzyme system, which also occurs physiologically.[
13] Also a key features of the application of synthetic metalloporphyrin-based systems is the rapid degradation of the catalyst in homogeneous oxidative media. The rate of degradation is highly dependent on the structure, the reaction medium and the quality of the oxidant used.[
14]
Advancements in label-free, high-throughput mass spectrometry (MS) analysis has significantly advanced the capabilities for screening compound libraries. Notably, ambient ionization techniques like Acoustic Mist Ionization (AMI, 2-3 samples/s),[
15] Desorption Electrospray Ionization (DESI, 2.7 samples/s),[
16] Electrospray Ionization (ESI, 0.4 samples/s), and Acoustic Droplet Ejection (ADE, 0.45 samples/s),[
17] as well as conventional Matrix-Assisted Laser Desorption/Ionization (MALDI, 2.5 samples/s)[
18] and hybrid techniques such as Infrared Matrix-Assisted Laser Desorption Electrospray Ionization (IR-MALDESI 0.5-1.3 samples/s),[
19] have shown promising results in terms of analytical speed. Despite their clear advantages over traditional label-based photometric methods, fast MS-based analysis applications remain relatively underutilized.
Rapid Evaporative Ionization Mass Spectrometry (REIMS) is an ambient ionization technique, initially pioneered for use in clinical environment for the real-time classification of biological samples and human tissue types.[
20] Its ability to translate molecular information of mobilized sample aerosol to mass spectral data points offers desirable solutions for a wider range of applications. With laser powered sample mobilization and (OPO; Optical Parametric Oscillator laser source) easier to automate sampling devices transferred the REIMS technique into an advanced, automated sampling platform. LA-REIMS (laser assisted-REIMS) has been already used in multiple scientific disciplines such as salivary metabolomics,[
21] microbiology,[
22] metabolic biofluid phenotyping,[
22] and food authentication. [
22] LA-REIMS can be used as a metabolomics platform in cervical cancer screening.[
23]
Here, in this study we report the first early-stage drug research related application of automated and laser-assisted-REIMS (LA-REIMS) platform, which allows high-throughput analysis of CyP450-derived metabolic stability of drug substances using synthetic metalloporphyrin-based biomimetic oxidation reactions. LA-REIMS can also allow users to monitor in real time drug consumption and metabolite formation.
2. Materials and Methods
Materials
Analytical grade chemicals were used in the experiments. Methanol, acetonitrile, acetic acid, sodium dihydrogen phosphate, sodium hydrogen phosphate, disodium hydrogen phosphate, tert-butyl hydroperoxide (tBuOOH) as co-oxidizing agent for metalloporphyrin and drug substrates were purchased from Merck Kft (Budapest, Hungary). The catalyst mezotetrakis-(sulfonyloxyphenyl)iron porphyrin (FeTPPS) was purchased from Frontier Scientific Inc (Utah, USA). The formic acid used for analytical measurements was purchased from Alfa Aesar (Ward Hill, Massachusetts, USA). The water used for the measurements was produced using a Milli-Q water purification system (Millipore, Bedford, Massachusetts, USA).
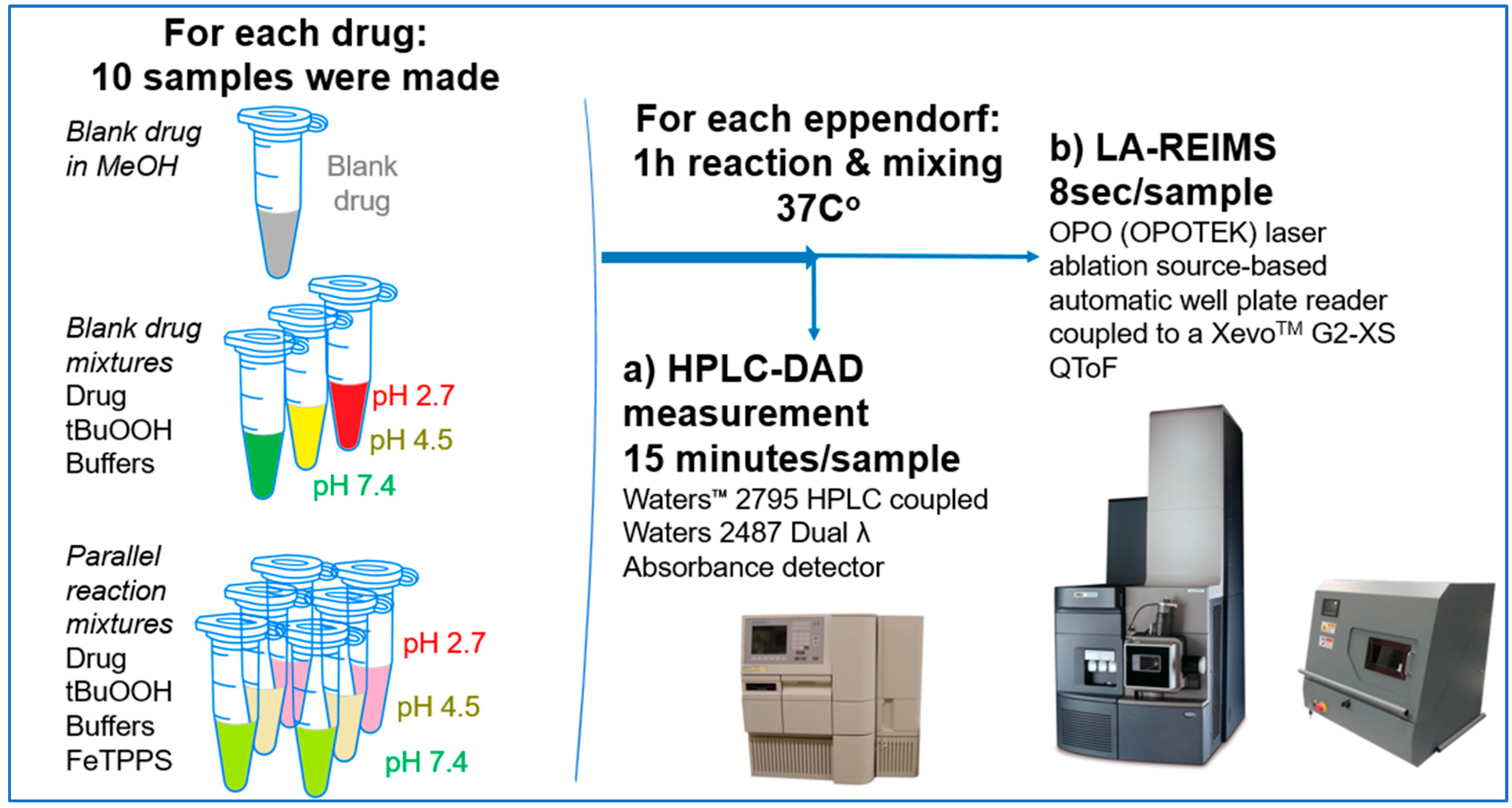
LA-REIMS Well Plate Reader
For this work we utilized a custom-made LA-REIMS platform prototype which is designed for sampling from well plates. This LA-REIMS prototype is for research purpose only. The LA-REIMS technique is based on a direct analysis of the aerosol generated by laser ablation (OPOTEK OPO laser, λ=2940nm, 5mJ/pulse, 20Hz shot frequency, 6ns pulse width). The generated droplets were introduced into the REIMS atmospheric interface where the ion generation occurs via collision with a high temperature impactor surface. For focusing the ablation beam uncoated spheric calcium fluoride lens with 50mm focal length were used. Motorized 3D stages (Thorlabs, Newton, New Jersey, USA) has been used for positioning the well plates in X-Y-Z directions. Raw data was acquired by using a XevoTM G2-XS TOF-MS (Waters Corporation; Milford, MA, USA), the spectra were acquired in positive ion mode over the mass to charge range 50-1200 with 0.5 sec/scans. Leucine enkephalin was used as a lock mass material. Data was processed with Waters MassLynxTM 4.2 software.
HPLC-DAD-MS Measurements
The HPLC-DAD-MS experiments were performed using a Waters MicromassTM Quattro Ultima Pt tandem quadrupole mass spectrometer coupled to a Waters 2795 liquid chromatography system (Milford, MA, USA). Detection was performed with a Waters 2487 Dual λ Absorbance detector. The analysis was performed at 40 °C on a Kinetex XB C18 column (150x4.6 mm, 2.6 µm) (Phenomenex, Torrance, CA, USA) at a mobile phase flow rate of 1 ml/min. The eluent A composition used was 0.1 vol % formic acid dissolved in water and eluent B was a 95:5 mixture of MeCN:H2P to which 0.1 vol % formic acid was added. The gradient elution program: a linear gradient of 5-100% B was applied in the range 0-11 min, and an isocratic phase of 100% B was applied in the range 11-13 min. This was followed by a 2-min equilibration period before the next injection using a 5% B composition. The injection volume was adjusted to 5 µl and the chromatographic profile was recorded at a wavelength of 220 (± 4) nm. The mass spectrometry conditions were as follows: ion source ESI, positive ion mode, scanning ion mode (150 - 600 m/z), drying gas (N2) temperature 350 °C, flow rate 5.5 l/min, nebulizer gas (N2) pressure 6 bar, quadrupole temperature 120 °C, capillary voltage 2500 V, fragmentor voltage 60 V. Data were processed using MassLynxTM 4.0 software.
3. Results
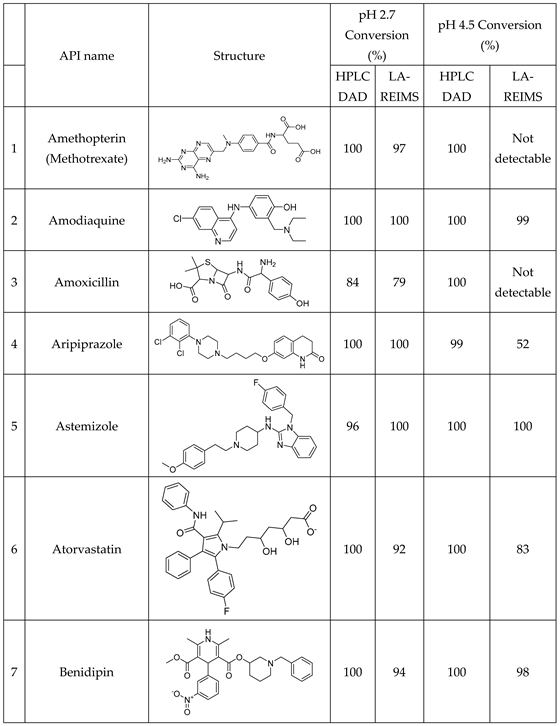
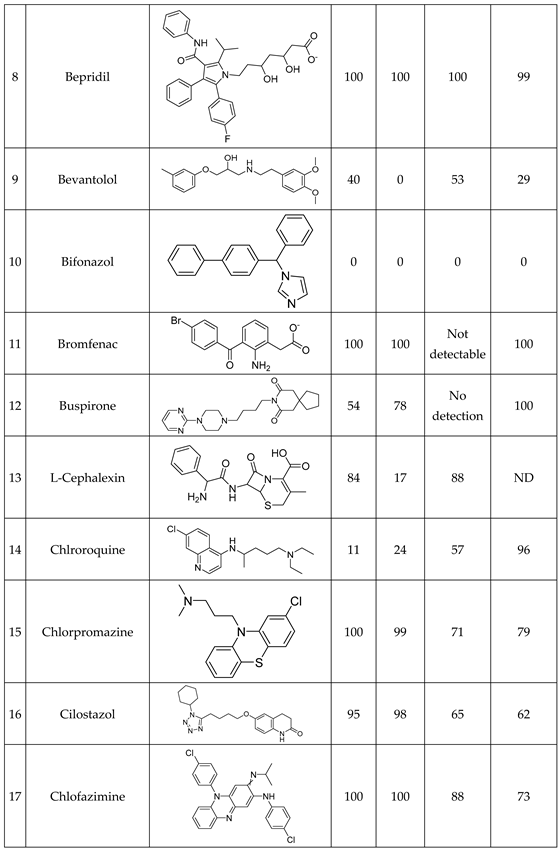
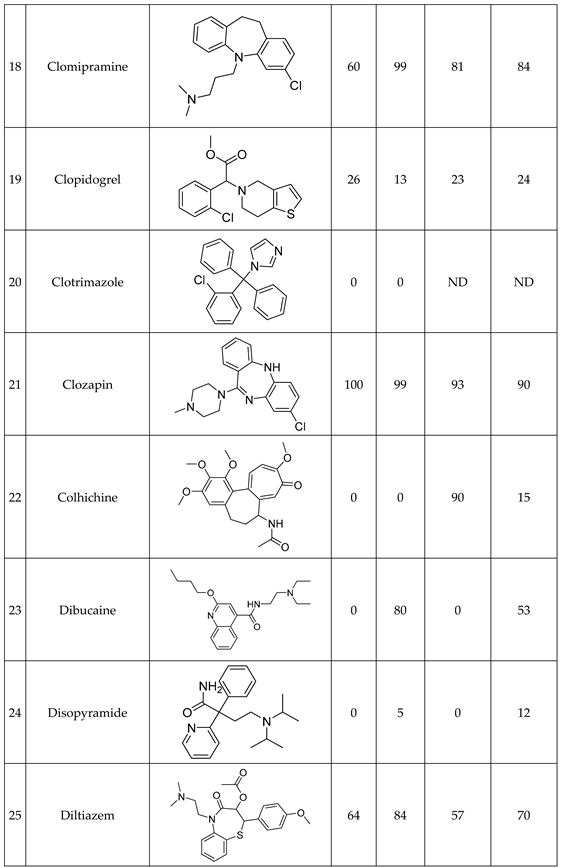
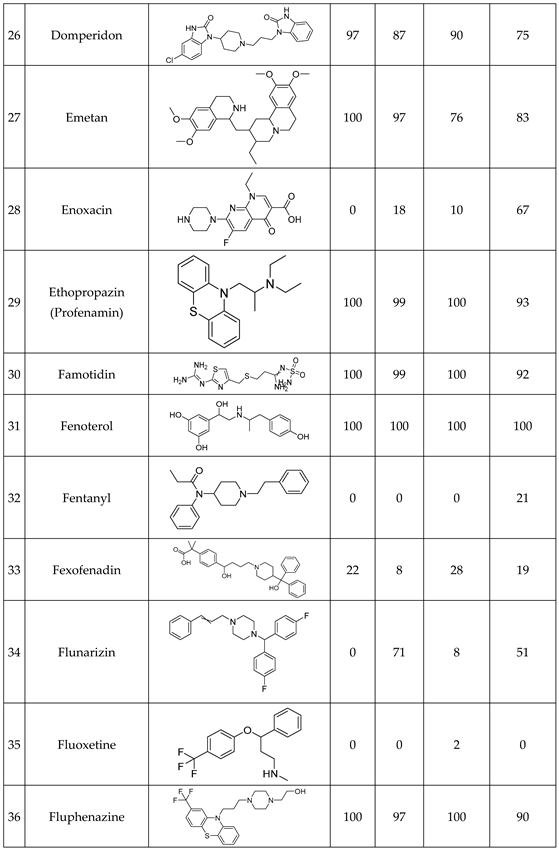
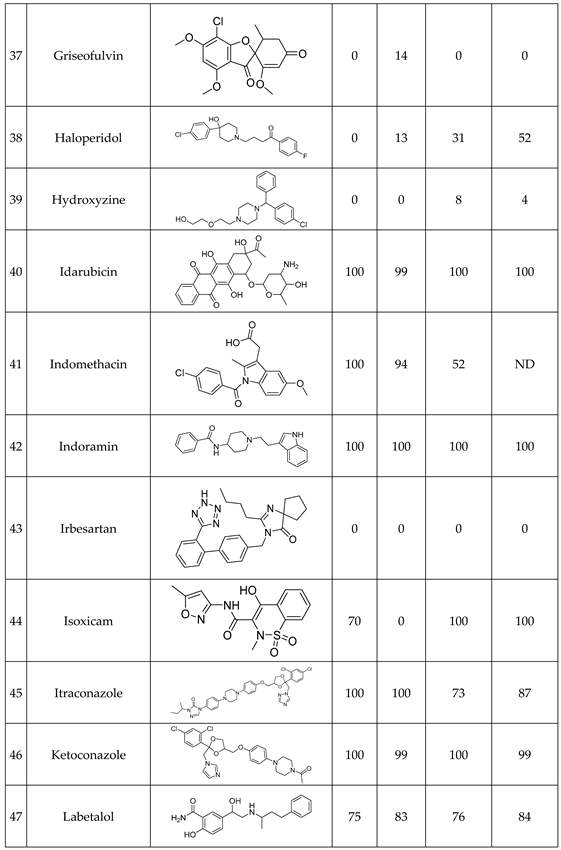
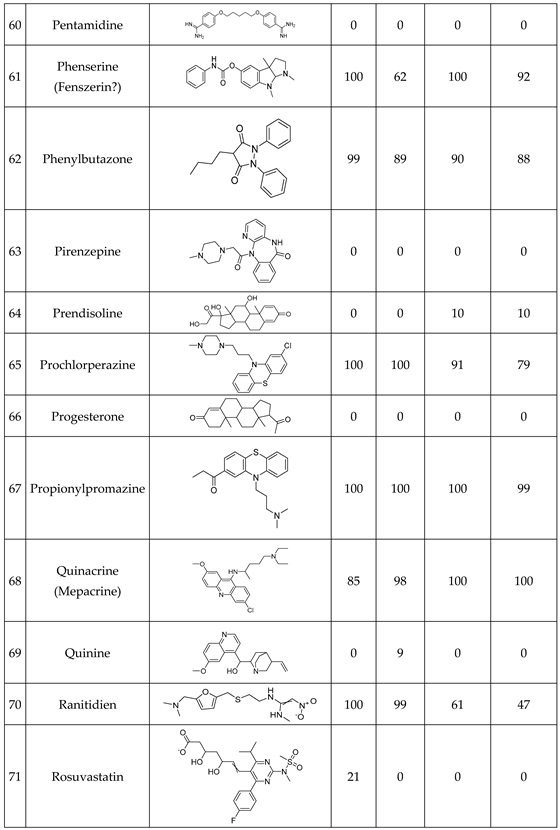
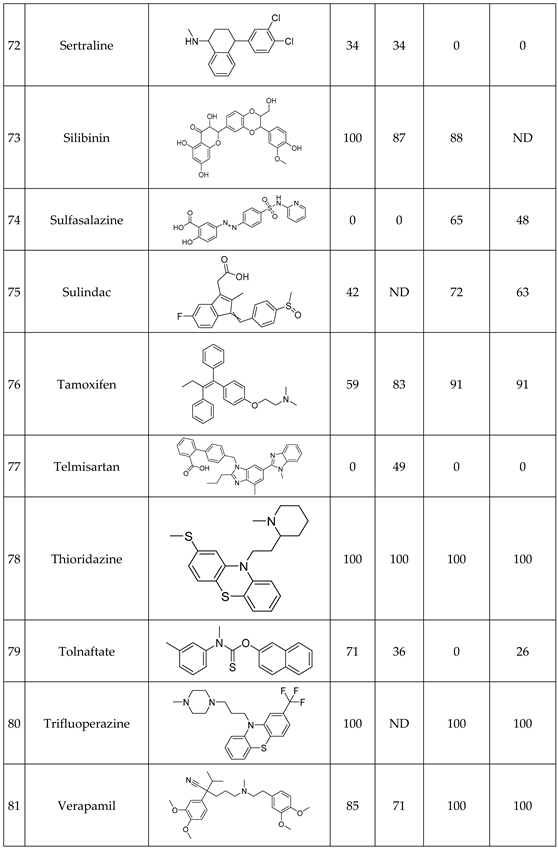
We have studied the biomimetic stability for 77 drugs on 3 pH level. The experiments were performed in 1.5mL microcentrifuge tubes ((Eppendorf® Safe-Lock). The total volume of each reaction mixtures and blanks was 0.5 mL. For the reaction mixtures 370 μl of methanol, 10 μl of 10 mM drug stock solution has been transferred into each tube. To the biomimetic samples it was added 13.6 μl of FeTPPS (0.735 mM, dissolved in methanol) solution, then to the pH-dependent oxidizing agent-only and biomimetic samples were added 100 μl of aqueous buffer solution (64 mM) of appropriate pHs (pH 2.7: formic acid, pH 4.5: acetic acid with sodium acetate; pH 7.4: monosodium- and disodium-phosphate). To the reaction mixtures it was added 6.8μl of
tBuOOH stock solution (147 mM). The concentrations of the stock solutions and porphyrin solution are adjusted so that the proportions of each component in the final reaction mixture were 1:10:100 as the porphyrin:substrate:
tBuOOH. For each drug substrate we prepared 10 samples and the final concentration for each drug were 200 µM in each sample tube. The 10 samples were: 1 sample of standard dissolved in methanol (QC sample), which served as an additional reference for mass accuracy. Blank reaction solutions were also measured at 3 pH levels: pH 2.7, pH 4.5, and pH 7.4. Blank reaction solutions were, contained all components except for the metalloporphyrin catalyst Biomimetic metabolism reactions were also conducted at 3 pH levels: Two parallel reaction solutions were prepared for each biomimetic reaction solution (parallel reaction mixtures). The reaction solutions contained the drug substance, the oxidizing agent (tert-butyl hydroperoxide), the metalloporphyrin catalyst, and the necessary buffers. The reason for conducting reactions at three different pH levels is to explore the pH dependency and optimal conditions of the metalloporphyrin catalyst behavior, which was mentioned in the introduction. Our results reflected that the activity of the applied metalloporphyrin catalyst is pH dependent.
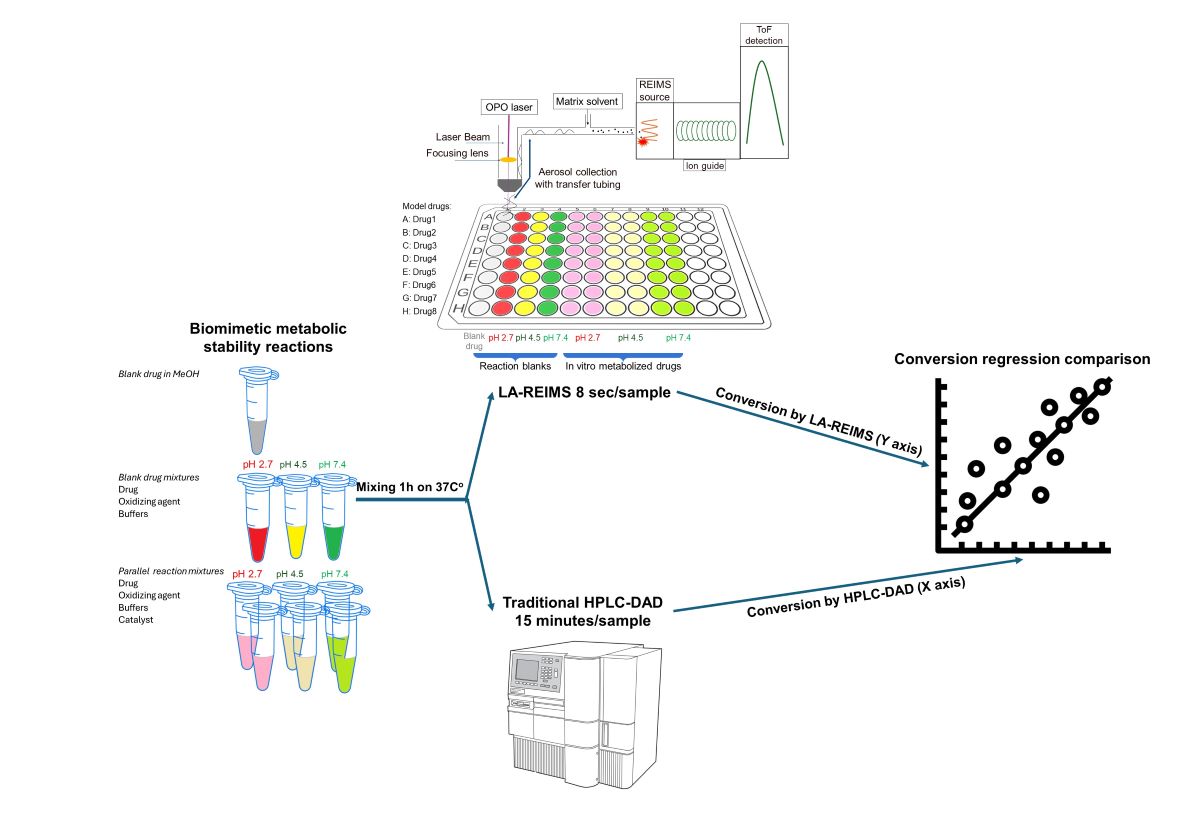
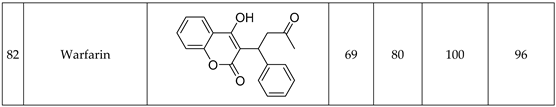
Figure 1.
Experimental workflow of the high-throughput drug stability assessment
Figure 1.
Experimental workflow of the high-throughput drug stability assessment
The prepared solutions were pipetted into the well plate, each row contains all of solutions from each sample (
Figure 2.). Each well contains 100 µl of solution, however during the study it was measured that we could use routinely 30 µl sample volumes too. Data acquisition was started row by row, with one raw file containing all the ten datapoint of a given active pharmaceutical ingredient.
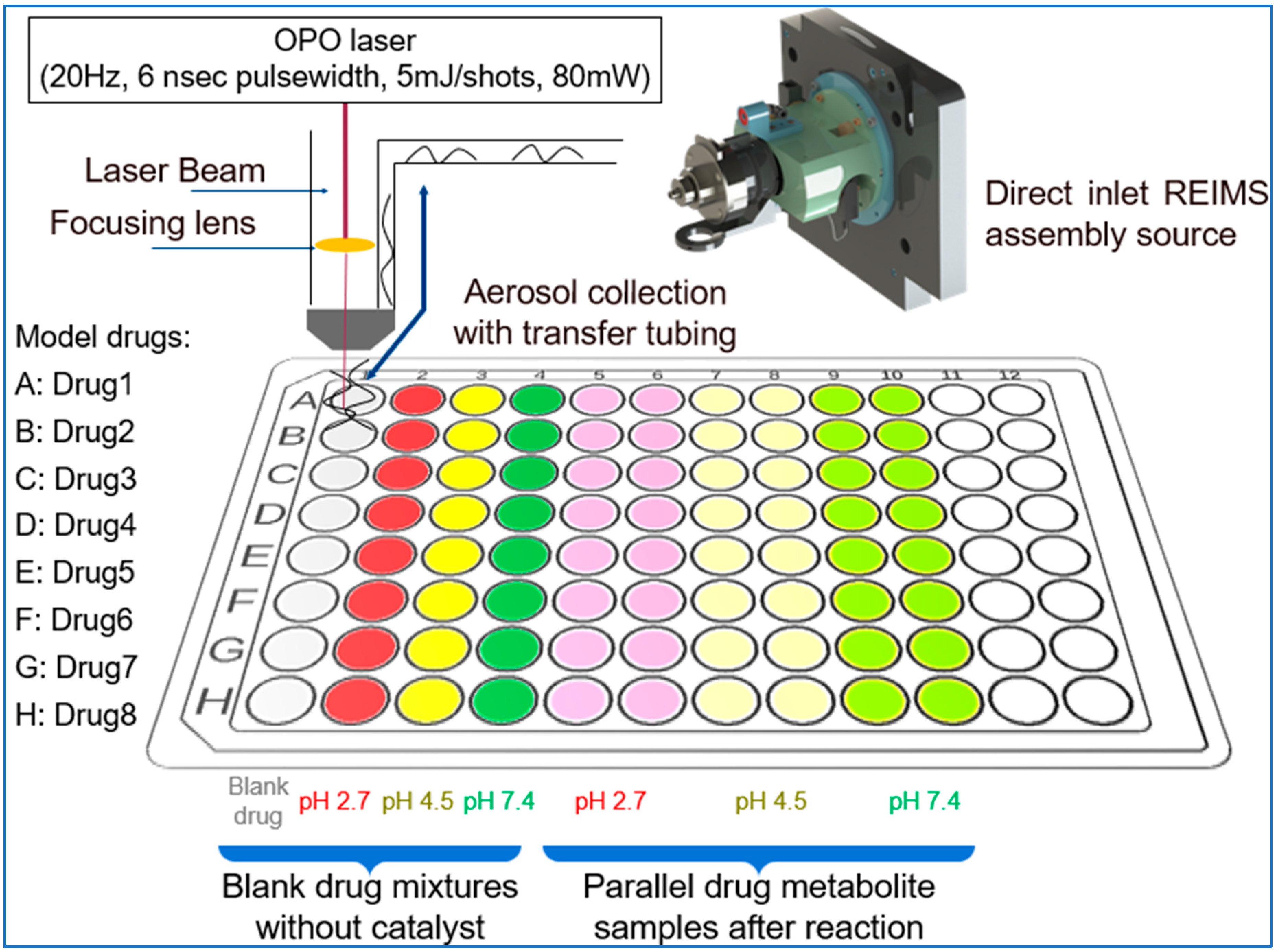
Here bellow, on
Figure 3 one can see the results obtained on the example of aripiprazole. The chromatogram below shows that the acquisition of the ten independent datapoint required only 1.5minutes. It is clearly visible from the ion chromatogram of aripiprazole (second trace from the top) that the degradation is close to complete in pH2.7, while in pH4.5 and pH 7.4 the degradation is not significant. The appearance of the major degradation products can also be followed as it is illustrated on the bottom three traces in
Figure 3. To obtain these results for this compound would have taken 2.5 hours with a conventional HPLC-UV-MS.
3.2. Regression Comparison of Results Obtained Using LA-REIMS and LC-UV-MS
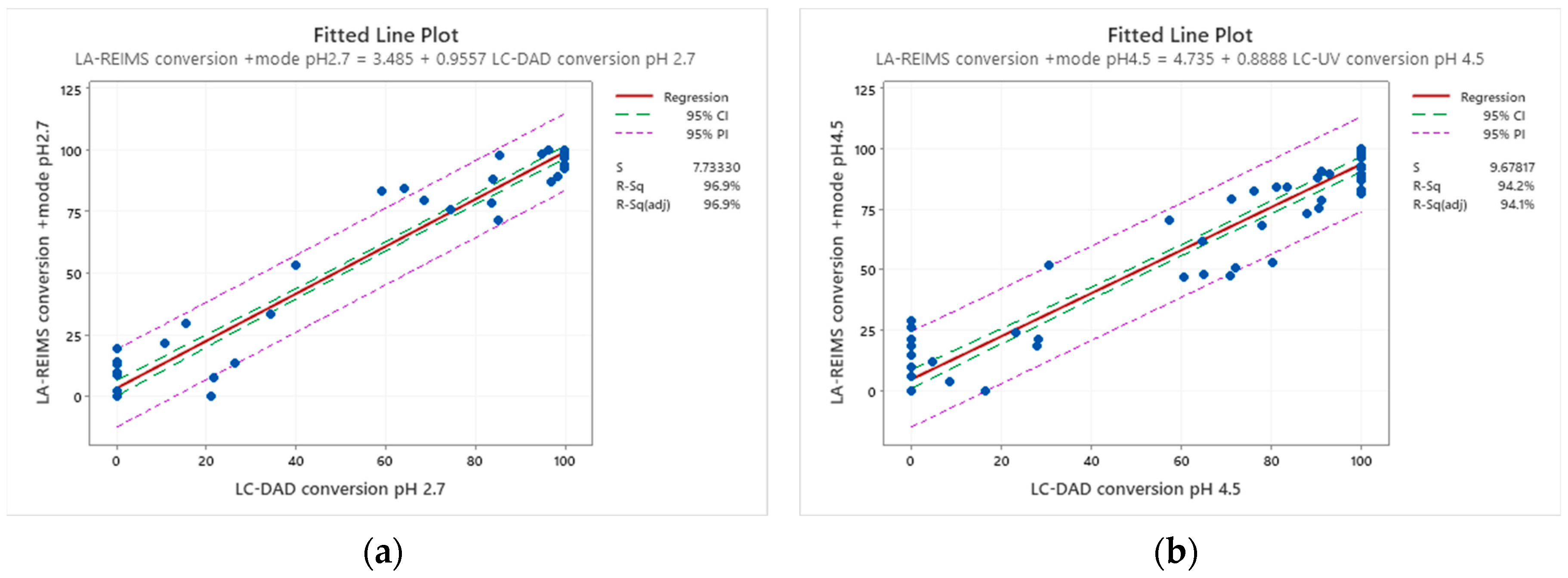
Conversion rates are calculated by the loss percent of the peak integral of the blank drug’s M+ compared to the drug’s M+ post-reaction value at their corresponding pHs. Good agreement is visible at the endpoints of drug conversion in the LC-DAD vs LA-REIMS plot on pH2.7 and pH4.5 after we omitted 10 and 3 outliers because of sample quality and instrument settings (
Figure 5). Due to the matrix effect of buffers caused ion suppression at the detection, the sensitivity of LA-REIMS is reduced by orders of magnitude, especially on pH7.4. However, this occurrence is acceptable as beyond pH 7, the formation of the artificial oxo-dimer takes place, resulting in the deactivation of the metalloporphyrin catalyst.
In general, the results obtained with the LA REIMS workstation are nicely correlates with the conventional HPLC results, while required significantly less analytical runtime.
3.2. Solvent Requirements and Measurement Time
The high throughput screening capable LA-REIMS technology can be a greener alternative compared to traditional LC-based techniques due to its lower solvent requirement. While conventional liquid chromatography (LC) methods often demand significant amounts of solvents for analytical procedures, LA-REIMS technology consumes less solvent. This reduction in solvent usage not only minimizes environmental impact but also contributes to cost-effectiveness and sustainability. By requiring lower volume of solvents, LA-REIMS facilitates an eco-friendlier approach in analytical chemistry, aligning with the growing emphasis on green chemistry practices. Consequently, its integration into high throughput screening processes not only enhances efficiency but also underscores its role in advancing environmentally conscious analytical methodologies.
Table 1.
Comparison of solvent requirements and measurement times of measurement techniques(* UHPLC results are from the literature, presented for comparability[
24])
Table 1.
Comparison of solvent requirements and measurement times of measurement techniques(* UHPLC results are from the literature, presented for comparability[
24])
| Technique |
One sample |
10 sample ( to characterize a drug) |
770 sample (whole dataset) |
| HPLC based |
15 ml
15 min |
150 ml
150 min |
11,550 ml
212.5 h |
| UHPLC based* |
0.3 ml
1 min |
3 ml
10 min |
231 ml
~13 h |
| HTS LA-REIMS |
0.02 ml
8 sec |
0.2 ml
80 sec |
15.4 ml
< 1 h 45 min |
4. Discussion
In conclusion, LA-REIMS emerges as a promising technique, demonstrating potential suitability for semi-quantitative high-throughput metabolic stability in an optimized solvent environment. Notably, LA-REIMS possesses a distinct speed advantage over conventional HPLC measurements, without compromising sensitivity or incurring significant information loss. Reduced measurement time and the use of the LA-REIMS technique also reduces solvent requirements by orders of magnitude. LA-REIMS is thus a greener, more environmentally friendly measurement technique that is ideally suited to the modern pharmaceutical industry. The LA-REIMS can also facilitate the real-time recording of pharmacokinetic curves directly from a well plates, eliminating the need for sample preparation, which can also support the traditional ADME microsomal metabolic stability research. Our future endeavors aim comprehensive exploration of microsomal systems, leveraging upon this methodology.
Author Contributions
Conceptualization, Tamás Karancsi and György Tibor Balogh; Data curation, András Marton, Zsombor Mohácsi, Balázs Csillag, Júlia Balogh, Richárd Schäffer and Tamás Karancsi; Formal analysis, Zsombor Mohácsi, Balázs Decsi and Balázs Csillag; Funding acquisition, György Tibor Balogh; Investigation, András Marton, Zsombor Mohácsi, Balázs Decsi, Balázs Csillag and Richárd Schäffer; Methodology, András Marton, Zsombor Mohácsi, Balázs Decsi, Júlia Balogh and Richárd Schäffer; Project administration, Tamás Karancsi and György Tibor Balogh; Resources, Tamás Karancsi and György Tibor Balogh; Software, Tamás Karancsi; Supervision, Tamás Karancsi and György Tibor Balogh; Validation, András Marton; Writing – original draft, András Marton; Writing – review & editing, Júlia Balogh, Richárd Schäffer, Tamás Karancsi and György Tibor Balogh.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Acknowledgments
"The scientific work/research and/or results publicised in this article was reached with the sponsorship of Gedeon Richter Talentum Foundation in framework of Gedeon Richter Excellence PhD Scholarship of Gedeon Richter.
References
- Williamson, B.; Wilson, C.; Dagnell, G.; Riley, R.J. Harmonised High Throughput Microsomal Stability Assay. J. Pharmacol. Toxicol. Methods 2017, 84, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Obach, R.S.; Baxter, J.G.; Liston, T.E.; Silber, B.M.; Jones, B.C.; MacIntyre, F.; Rance, D.J.; Wastall, P. The Prediction of Human Pharmacokinetic Parameters from Preclinical and in Vitro Metabolism Data. J. Pharmacol. Exp. Ther. 1997, 283, 46–58. [Google Scholar] [PubMed]
- Kiani, A.K.; Pheby, D.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; Falsini, B.; Capodicasa, N.; Miertus, S.; et al. Ethical Considerations Regarding Animal Experimentation. J. Prev. Med. Hyg. 2022; 63, No. 2S3, E255 Pages. [Google Scholar] [CrossRef]
-
The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham (CH), 2015; ISBN 978-3-319-15791-7. [Google Scholar]
- Asha, S.; Vidyavathi, M. Role of Human Liver Microsomes in in Vitro Metabolism of Drugs-a Review. Appl. Biochem. Biotechnol. 2010, 160, 1699–1722. [Google Scholar] [CrossRef] [PubMed]
- Groves, J.T.; Nemo, T.E.; Myers, R.S. Hydroxylation and Epoxidation Catalyzed by Iron-Porphine Complexes. Oxygen Transfer from Iodosylbenzene. J. Am. Chem. Soc. 1979, 101, 1032–1033. [Google Scholar] [CrossRef]
- Pereira, M.M.; Dias, L.D.; Calvete, M.J.F. Metalloporphyrins: Bioinspired Oxidation Catalysts. ACS Catal. 2018, 8, 10784–10808. [Google Scholar] [CrossRef]
- Balogh, G.T.; Keserû, G.M. Metalloporphyrin Mediated Biomimetic Oxidations. A Useful Tool for the Investigation of Cytochrome P450 Catalyzed Oxidative Metabolism. Arkivoc 2004, 2004, 124–139. [Google Scholar] [CrossRef]
- Bernadou, J.; Meunier, B. Biomimetic Chemical Catalysts in the Oxidative Activation of Drugs. Adv. Synth. Catal. 2004, 346, 171–184. [Google Scholar] [CrossRef]
- Balogh, G.T.; Decsi, B.; Krammer, R.; Kenéz, B.; Ender, F.; Hergert, T.; Balogh-Weiser, D. Effect of Binding Linkers on the Efficiency and Metabolite Profile of Biomimetic Reactions Catalyzed by Immobilized Metalloporphyrin. Metabolites 2022, 12, 1269. [Google Scholar] [CrossRef]
- Decsi, B.; Krammer, R.; Hegedűs, K.; Ender, F.; Gyarmati, B.; Szilágyi, A.; Tőtős, R.; Katona, G.; Paizs, C.; Balogh, G.T.; et al. Liver-on-a-Chip‒Magnetic Nanoparticle Bound Synthetic Metalloporphyrin-Catalyzed Biomimetic Oxidation of a Drug in a Magnechip Reactor. Micromachines 2019, 10, 668. [Google Scholar] [CrossRef]
- Wolak, M.; van Eldik, R. Mechanistic Studies on Peroxide Activation by a Water-Soluble Iron(III)–Porphyrin: Implications for OO Bond Activation in Aqueous and Nonaqueous Solvents. Chem. – Eur. J. 2007, 13, 4873–4883. [Google Scholar] [CrossRef]
- Nam, W.; Han, H.J.; Oh, S.-Y.; Lee, Y.J.; Choi, M.-H.; Han, S.-Y.; Kim, C.; Woo, S.K.; Shin, W. New Insights into the Mechanisms of O−O Bond Cleavage of Hydrogen Peroxide and Tert-Alkyl Hydroperoxides by Iron(III) Porphyrin Complexes. J. Am. Chem. Soc. 2000, 122, 8677–8684. [Google Scholar] [CrossRef]
- Cunningham, I.D.; Danks, T.N.; Hay, J.N.; Hamerton, I.; Gunathilagan, S.; Janczak, C. Stability of Various Metalloporphyrin Catalysts during Hydrogen Peroxide Epoxidation of Alkene. J. Mol. Catal. Chem. 2002, 185, 25–31. [Google Scholar] [CrossRef]
- Smith, M.J.; Ivanov, D.P.; Weber, R.J.M.; Wingfield, J.; Viant, M.R. Acoustic Mist Ionization Mass Spectrometry for Ultrahigh-Throughput Metabolomics Screening. Anal. Chem. 2021, 93, 9258–9266. [Google Scholar] [CrossRef] [PubMed]
- Wleklinski, M.; Loren, B.P.; Ferreira, C.R.; Jaman, Z.; Avramova, L.; Sobreira, T.J.P.; Thompson, D.H.; Cooks, R.G. High Throughput Reaction Screening Using Desorption Electrospray Ionization Mass Spectrometry. Chem. Sci. 2018, 9, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Zhang, J.; Liu, C.; Covey, T.R.; Olah, T.V.; Weller, H. (Bud) N.; Shou, W.Z. Ultrahigh-Throughput and Chromatography-Free Bioanalysis of Polar Analytes with Acoustic Ejection Mass Spectrometry. Anal. Chem. 2020, 92, 13525–13531. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Ries, R.; Kleiner, C.; Bischoff, D.; Luippold, A.H.; Bretschneider, T.; Büttner, F.H. Automated MALDI Target Preparation Concept: Providing Ultra-High-Throughput Mass Spectrometry–Based Screening for Drug Discovery. SLAS Technol. 2019, 24, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.; Radosevich, A.J.; Sawicki, J.W.; Chang-Yen, D.; Talaty, N.N.; Gopalakrishnan, S.M.; Williams, J.D.; Elsen, N.L. High-Throughput Label-Free Biochemical Assays Using Infrared Matrix-Assisted Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2021, 93, 6792–6800. [Google Scholar] [CrossRef] [PubMed]
- Balog, J.; Szaniszlo, T.; Schaefer, K.-C.; Denes, J.; Lopata, A.; Godorhazy, L.; Szalay, D.; Balogh, L.; Sasi-Szabo, L.; Toth, M.; et al. Identification of Biological Tissues by Rapid Evaporative Ionization Mass Spectrometry. Anal. Chem. 2010, 82, 7343–7350. [Google Scholar] [CrossRef] [PubMed]
- Wijnant, K.; Van Meulebroek, L.; Pomian, B.; De Windt, K.; De Henauw, S.; Michels, N.; Vanhaecke, L. Validated Ultra-High-Performance Liquid Chromatography Hybrid High-Resolution Mass Spectrometry and Laser-Assisted Rapid Evaporative Ionization Mass Spectrometry for Salivary Metabolomics. Anal. Chem. 2020, 92, 5116–5124. [Google Scholar] [CrossRef]
- Cameron, S.J.S.; Perdones-Montero, A.; Van Meulebroek, L.; Burke, A.; Alexander-Hardiman, K.; Simon, D.; Schaffer, R.; Balog, J.; Karancsi, T.; Rickards, T.; et al. Sample Preparation Free Mass Spectrometry Using Laser-Assisted Rapid Evaporative Ionization Mass Spectrometry: Applications to Microbiology, Metabolic Biofluid Phenotyping, and Food Authenticity. J. Am. Soc. Mass Spectrom. 2021, 32, 1393–1401. [Google Scholar] [CrossRef]
- Paraskevaidi, M.; Cameron, S.J.S.; Whelan, E.; Bowden, S.; Tzafetas, M.; Mitra, A.; Semertzidou, A.; Athanasiou, A.; Bennett, P.R.; MacIntyre, D.A.; et al. Laser-Assisted Rapid Evaporative Ionisation Mass Spectrometry (LA-REIMS) as a Metabolomics Platform in Cervical Cancer Screening. EBioMedicine 2020, 60, 103017. [Google Scholar] [CrossRef] [PubMed]
- Attwa, M.W.; Bakheit, A.H.; Abdelhameed, A.S.; Kadi, A.A. An Ultrafast UPLC–MS/MS Method for Characterizing the In Vitro Metabolic Stability of Acalabrutinib. Molecules 2023, 28, 7220. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).