Submitted:
11 June 2024
Posted:
12 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Characteristics of Mesenchymal Stromal Cells (MSCs)
2.1. The Identification, Distribution and Pathophysiological Role of Mesenchymal Stromal Cells (MSCs)
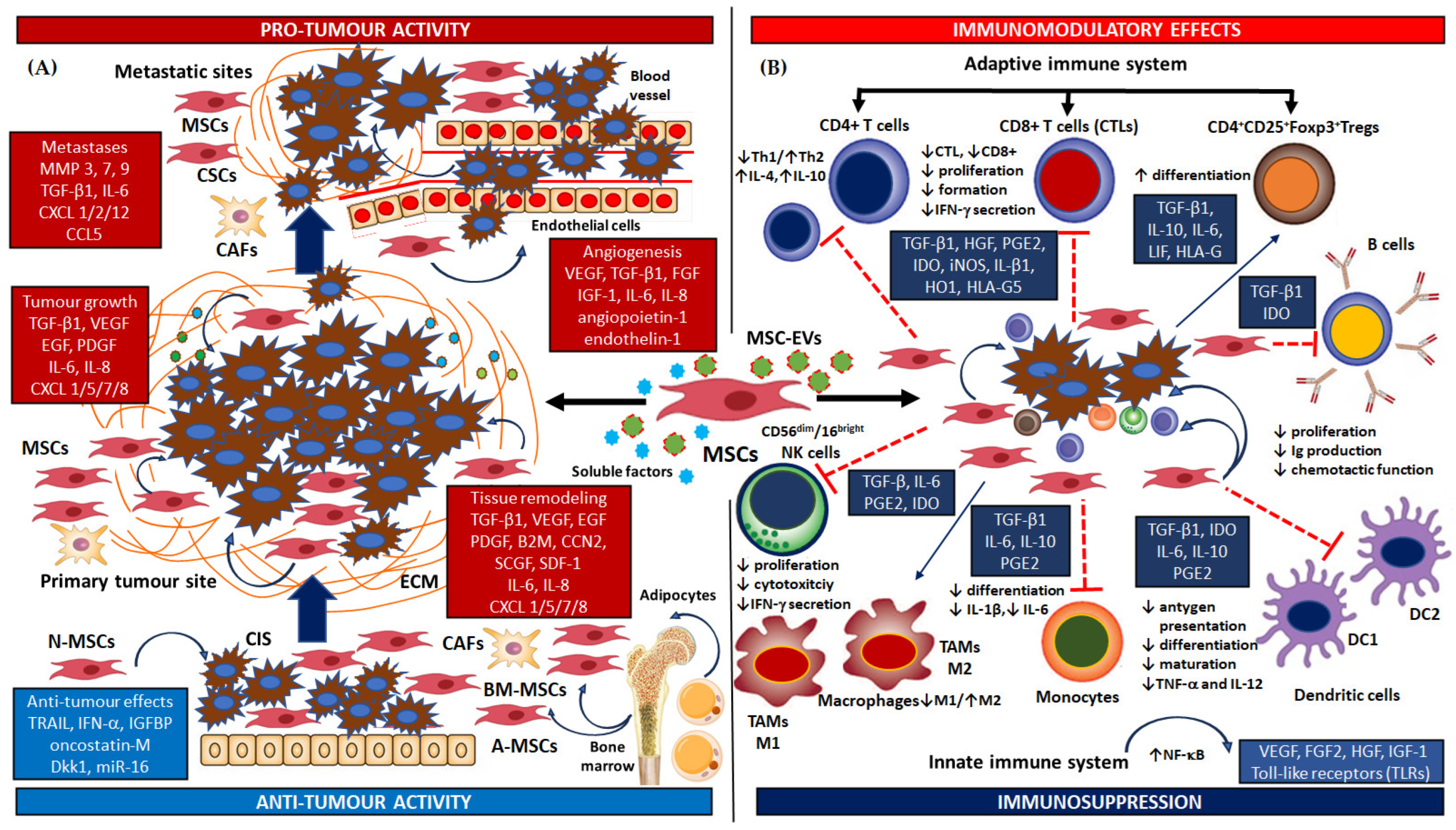
2.2. Dual Roles and the Bidirectional Effect of Mesenchymal Stromal Cells in the Tumour Microenvironment
2.2.1. Pro-Tumour Activity of MSCs
2.2.2. Anti-Tumour Activity of MSCs
2.3. Modulation of Immune and Inflammatory Cells by Mesenchymal Stromal Cells
2.3.1. The Adaptive Immune Response
2.3.2. The Innate Immune Response
3. Studies on the Role of Mesenchymal Stromal Cells (MSCs) in Head and Neck Cancer. Effects of the Secretome on HNC. Therapeutic Potential of MSCs. The Pharmacological Strategies of MSC-Based Therapies in HNC
3.1. The Stemness Phenotype of Mesenchymal Stromal Cells (MSCs) in HNC. The role of MSCs in Tumorigenesis, Progression and Drug-Resistance Mechanisms in HNC
3.1.1. In Vitro Models of HNSCC
3.1.2. In Vivo and Animal Models of HNSCC
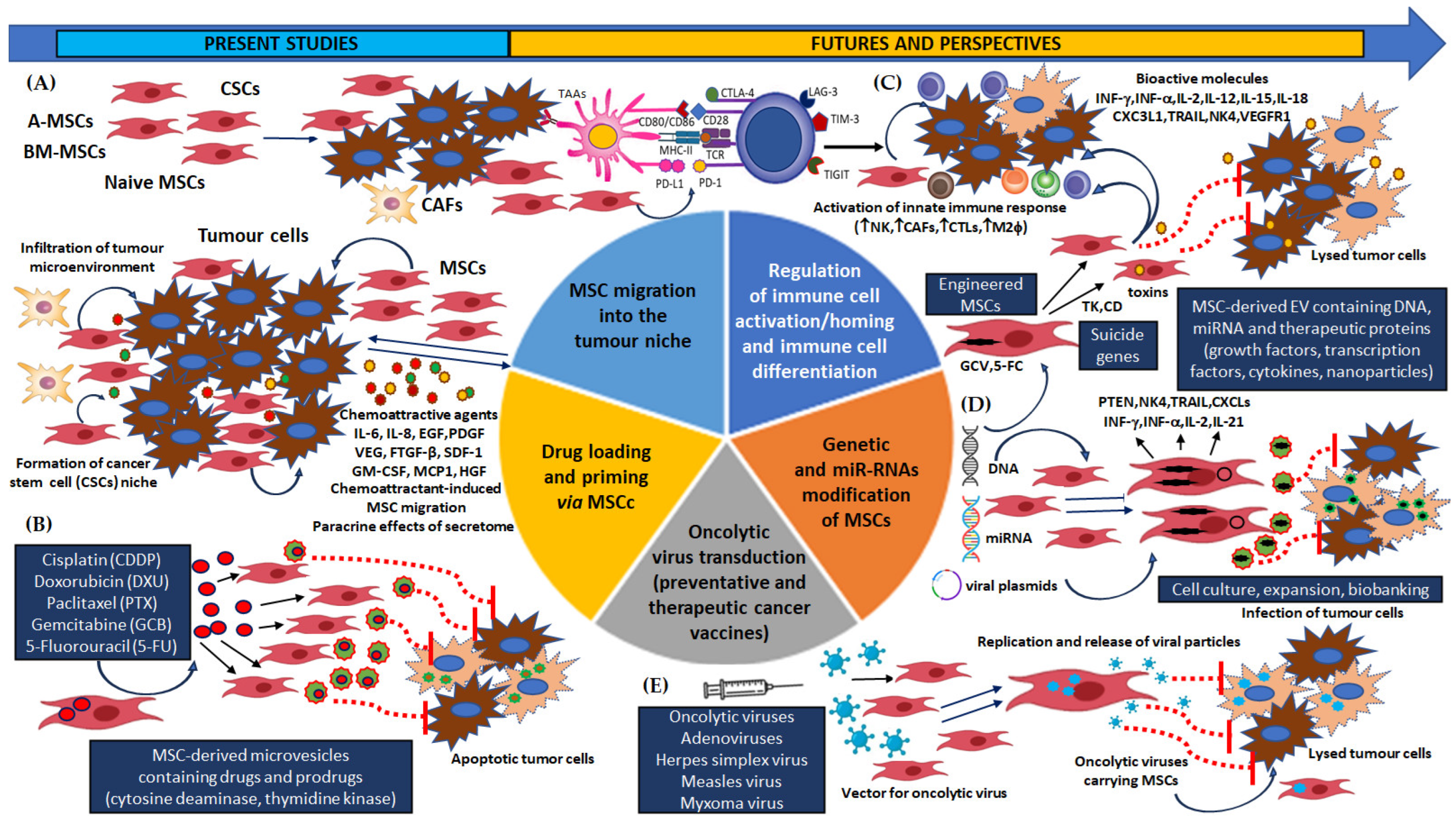
3.2. The Pharmacological Strategies of MSC-Based Treatment for Human Tumours. MSCs as Carriers of Anti-Tumour Therapeutic Biological Compounds and Their Clinical Application for Oncological Therapy
3.3. The Pharmacological Strategies of MSC-Based Treatment for HNC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A-MSCs | Adipose tissue-derived MSCs |
| B2M | Beta 2 microglobulin |
| BM-MSCs | Bone-marrow-derived MSCs |
| BMP | Bone morphogenetic protein |
| CAFs | Cancer-associated fibroblasts |
| CCN2 | Cellular communication network factor 2 |
| CCL | C-C motif chemokine ligand |
| CDDP | Cisplatin |
| CIS | Carcinoma in situ |
| CSCs | Cancer stem cells |
| CTLs | Cytotxic T cells |
| CXCL | C-X-C motif chemokine ligand |
| Dkk | Dickkopf-related protein |
| DXR | Doxorubicin |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| FGF | Fibroblast growth factors |
| 5-FU | 5-Fluorouracyl |
| GTA | Ganciclovir |
| GCB | Gemcitabine |
| HGF | Hepatocyte growth factor |
| IDO | Indoleamine 2,3-dioxygenase |
| iNOS | Nitric oxide synthase |
| INF-α/β/γ | Type-I interferon alpha/betha/gamma |
| HO1 | Heme oxygenase |
| MARSH | Restoration of salivary hypofunction |
| MAPKs | Mitogen-activated protein kinases |
| MMPs (MT-MMPs) | Matrix metalloproteinases, also known as matrix metallopeptidases |
| MSCs | Mesenchymal stromal/stem cells |
| N-MSC | Naïve MSCs |
| NKs | Activated natural killer cells or CD56dim/CD16bright cells |
| SCGF | Stromal cell growth factor-beta |
| SDF-1 | Stromal cell-derived factor 1 |
| PDGF | Platelet-derived growth factor |
| PGE2 | Prostaglandin E2 |
| PTEN | Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase |
| PTX | Paclitaxel |
| RIX | Radiation-induced xerostomia |
| SFR | Saliva Flow Rate |
| TAMs M1/2 | Tumour-associated macrophages M1/2 |
| TGF-β1 | Transforming growth factor beta 1 |
| TLRs | Toll-like receptors |
| TRAIL | TNF-related apoptosis-inducing ligand |
| Treg | Regulatory T cells, known as suppressor T cells or CD4+CD25+Foxp3+ cells |
| UC-MSCs | Umbilical-cord MSCs |
| VEGF | Vascular endothelial growth factor |
References
- Charap, A.J.; Enokida, T.; Brody, R.; Sfakianos, J.; Miles, B.; Bhardwaj, N.; Horowitz, A. Landscape of natural killer cell activity in head and neck squamous cell carcinoma. J. Immunother. Cancer 2020, 8, e001523. [CrossRef]
- El-Naggar, A.K.; Chan, C.J.; Grandis, J.R.; Takata, T.; Slootweg, P.J. WHO Classification of Head and Neck Tumours, 4th ed.; IARC: Lyon, France, 2017; ISBN 9789283224389.
- The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517(7536), 576–582. [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71(3), 209–249. [CrossRef]
- Gormley, M.; Creaney, G.; Schache, A; Ingarfield, K.; Conway D.I.. Reviewing the epidemiology of head and neck cancer: Definitions, trends and risk factors. Br. Dent. J. 2022, 233, 780–786. [CrossRef]
- Johnson, D. E.; Burtness, B.; Leemans, C. R.; Lui, V. W. Y.; Bauman, J. E.; Grandis, J. R. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2023, 9(1), 4. [CrossRef]
- 7. Goyal, N.; Day, A.; Epstein, J.; Goodman, J.; Graboyes, E.; Jalisi, S.; Kiess, A. P.; Ku, J. A.; Miller, M. C.; Panwar, A; et al. Head and neck cancer survivorship consensus statement from the American Head and Neck Society. Laryngoscope Investig. Otolaryn. 2021, 7(1), 70–92. [CrossRef]
- Atashi, F.; Vahed, N.; Emamverdizadeh, P.: Fattahi, S.: Paya L. Drug resistance against 5-fluorouracil and cisplatin in the treatment of head and neck squamous cell carcinoma: A systematic review. J Dental Res Dental Clin Dental Prospect. 2021, 15(3), 219–225. [CrossRef]
- Sola, A.M.; Johnson, D.E.; Grandis, J.R. Investigational multitargeted kinase inhibitors in development for head and neck neoplasms. Expert Opin. Investig. Drugs. 2019, 28(4), 351–363. [CrossRef]
- National Comprehensive Cancer Network. Head and neck cancer. 2020. https://www.nccn.org/professionals/ physician_gls/ pdf/head-and-neck.pdf.
- Cancer, I.A.f.R.o. List of Classifications by cancer sites with sufficient or limited evidence in humans, 2019, 1-127. IARC Monographs On The Identification Of Carcinogenic Hazards To Humans. https://monographs.iarc.fr/wp-content/uploads/2019/07/Classification_by_cancer_site_127.pdf.
- Gupta, B.; Johnson N.W.; Kumar, N. Global Epidemiology of Head and Neck Cancers: A Continuing Challenge. Oncol., 2016, 91, 13–23. [CrossRef]
- Ng, J.H.; Iyer, N.G.; Tan M-H.; Edgren, G. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study, Head Neck. 2017, 39, 297–304. [CrossRef]
- Du, E.; Mazul, A.L.; Farquhar, D.; Brennan, P.; Anantharaman, D.; Abedi-Ardekani, B.; Weissler, M.C.; Hayes, D.N.; Olshan, A.F.; Zevallos, J.P. Long-term Survival in Head and Neck Cancer: Impact of Site, Stage, Smoking, and Human Papillomavirus Status. Laryngoscope 2019, 129(11), 2506–2513. [CrossRef]
- Sun, Z.; Sun, X.; Chen, Z.; Du, J.; Wu, Y. Head and Neck Squamous Cell Carcinoma: Risk Factors, Molecular Alterations, Immunology and Peptide Vaccines. Int. J. Pept. Res. Ther. 2022, 28(1), 19. [CrossRef]
- Miranda-Galvis, M.; Loveless, R.; Kowalski, L.P.; Teng, Y. Impacts of Environmental Factors on Head and Neck Cancer Pathogenesis and Progression. Cells 2021, 10, 389. [CrossRef]
- Saada-Bouzid, E.; Peyrade, F.; Guigay, J. Molecular genetics of head and neck squamous cell carcinoma. Curr. Opin. Oncol. 2019, 31 (3), 131–137. [CrossRef]
- Zhang, Y. X.; Koneva, L. A.; Virani, S.; Arthur, A. E.; Virani, A.; Hall, P. B.; Warden, C. D.; Carey, T. E.; Chepeha, D. B.; Prince, M. E.; et al. Subtypes of HPV-Positive Head and Neck Cancers Are Associated with HPV Characteristics, Copy Number Alterations, PIK3CA Mutation, and Pathway Signatures. Clin. Cancer Res. 2016, 22 (18), 4735–4745. [CrossRef]
- Leemans, C. R.; Snijders, P. J. F.; Brakenhoff, R. H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer. 2018, 18(10), 662. [CrossRef]
- Chow, .LQ.M.; Head and neck cancer. N. Engl. J. Med. 2020, 382, 60–72. [CrossRef]
- Dong, H.; Shu, X.; Xu, Q.; Zhu, C.; Kaufmann, A. M.; Zheng, Z. M.; Albers, A. E.; Qian, X. Current Status of Human Papillomavirus-Related Head and Neck Cancer: From Viral Genome to Patient Care. Virologica Sinica 2021, 36(6), 1284–1302. [CrossRef]
- Wittekindt, C.; Wagner, S.; Bushnak, A.; Prigge, E. S.; von Knebel Doeberitz, M.; Würdemann, N.; Bernhardt, K.; Pons-Kühnemann, J.; Maulbecker-Armstrong, C.; Klussmann, J. P. Increasing Incidence rates of Oropharyngeal Squamous Cell Carcinoma in Germany and Significance of Disease Burden Attributed to Human Papillomavirus. Cancer Prev. Res. (Phila.) 2019, 12(6), 375–382. [CrossRef]
- Zamani, M.; Grønhøj, C.; Jensen, D. H.; Carlander, A. F.; Agander, T.; Kiss, K.; Olsen, C.; Baandrup, L.; Nielsen, F. C.; Andersen, E.; Friborg, J.; von Buchwald, C. The current epidemic of HPV-associated oropharyngeal cancer: An 18-year Danish population-based study with 2,169 patients. Eur J Cancer (Oxford, England: 1990) 2020, 134, 52–59. [CrossRef]
- de Freitas, A. C.; de Oliveira, T. H. A.; Barros, M. R.; Venuti, A. hrHPV E5 oncoprotein: Immune evasion and related immunotherapies. J. Exp. Clin. Cancer Res. 2017, 36. [CrossRef]
- Pal, A.; Kundu, R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front. Microbiol. 2020, 10. [CrossRef]
- Canning, M.; Guo, G.; Yu, M.; Myint, C.; Groves, M. W.; Byrd, J. K.; Cui, Y. Heterogeneity of the Head and Neck Squamous Cell Carcinoma Immune Landscape and Its Impact on Immunotherapy. Front. Cell Dev. Biol. 2019, 7. [CrossRef]
- Lawrence, M. S.; Sougnez, C.; Lichtenstein, L.; Cibulskis, K.; Lander, E.; Gabriel, S. B.; et al. Comprehensive genomic characterization of head and neck squamous cell carcinomas. The Cancer Genome Atlas Network. Nature 2015, 517, 576–582. [CrossRef]
- Seiwert, T. Y.; Zuo, Z. X.; Keck, M. K.; Khattri, A.; Pedamallu, C. S.; Stricker, T.; Brown, C.; Pugh, T. J.; Stojanov, P.; Cho, J.; et al. Integrative and Comparative Genomic Analysis of HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinomas. Clin. Cancer Res. 2015, 21 (3), 632-641. [CrossRef]
- Rühle, A.; Grosu, A. L.; Nicolay, N. H. De-Escalation Strategies of (Chemo)Radiation for Head-and-Neck Squamous Cell Cancers-HPV and Beyond. Cancers 2021, 13(9), 2204. [CrossRef]
- Ventz, S.; Trippa, L.; Schoenfeld, J. D. Lessons Learned from Deescalation Trials in Favorable Risk HPV-Associated Squamous Cell Head and Neck Cancer-A Perspective on Future Trial Designs. Clin. Cancer Res. 2019, 25(24), 7281–7286. [CrossRef]
- Amin, M. B.; Greene, F. L.; Edge, S. B.; Compton, C. C.; Gershenwald, J. E.; Brookland, R. K.; Meyer, L.; Gress, D. M.; Byrd, D. R.; Winchester, D. P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA: Cancer J. Clin. 2017, 67(2), 93–99. [CrossRef]
- Gillison, M. L.; Trotti, A. M.; Harris, J.; Eisbruch, A.; Harari, P. M.; Adelstein, D. J.; Jordan, R. C. K.; Zhao, W.; Sturgis, E. M.; Burtness; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet (London, England) 2019, 393(10166), 40–50. [CrossRef]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. De-ESCALaTE HPV Trial Group. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet (London, England) 2019, 393(10166), 51–60. [CrossRef]
- Strober, W.; Shishido, S.; Wood, B.; Lewis, J. S.; Jr, Kuhs, K.; Ferris, R. L.; Faden, D. L. Two for the price of one: Prevalence, demographics and treatment implications of multiple HPV mediated Head and Neck Cancers. Oral Oncol. 2020, 100, 104475. [CrossRef]
- Mondino, A.; Vella, G.; Icardi, L. Targeting the tumor and its associated stroma: One and one can make three in adoptive T cell therapy of solid tumors, Cytokine Growth Factor Rev. 2017, 36, 57–65. [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–37. [CrossRef]
- Dogan, V.; Rieckmann, T.; Münscher, A.; Busch, C.J. Current studies of immunotherapy in head and neck cancer. Clin. Otolaryngol. 2018, 43, 13–21. [CrossRef]
- Cuiffo, B.G.; Karnoub, E. Mesenchymal stem cells in tumor development: Emerging roles and concepts. Cell Adh. Migr. 2012, 6, 220–230. [CrossRef]
- Böhrnsen, F.; Fricke, M.; Sander, C.; Leha, A.; Schliephake, H.; Kramer, F.J. Interactions of human MSC with head and neck squamous cell carcinoma cell line PCI-13 reduce markers of epithelia-mesenchymal transition. Clin Oral Investig. 2015, 19(5), 1121–1128. [CrossRef]
- Wei, S.; Li, M.; Wang, Q.; Zhao, Y.; Du, F.; Chen, Y.; Deng, S.; Shen, J.; Wu, K.; Yang, J.; Sun, Y.; Gu, L.; Li, X.; Li, W.; Chen, M.; Ling, X.; Yu, L.; Xiao, Z.; Dong, L.; Wu, X. Mesenchymal Stromal Cells: New Generation Treatment of Inflammatory Bowel Disease. J. Inflamm. Res. 2024, 17, 3307–3334. [CrossRef]
- Bianco, P. Mesenchymal Stem Cells. Annu. Rev. Cell Dev. Biol. 2014, 30, 677–704. [CrossRef]
- Kfoury, Y.; Scadden, D.T. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 2015, 16, 239–253. [CrossRef]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7. [CrossRef]
- Shi, L.; Chen, L.; Gao, X.; Sun, X.; Jin, G.; Yang, Y.; Shao, Y.; Zhu, F.; Zhou, G. Comparison of different sources of mesenchymal stem cells: Focus on inflammatory bowel disease. Inflammopharmacology 2024, 32(3), 1721–1742. [CrossRef]
- Li, C.Y.; Wu, X.Y.; Tong, J.B.; Yang, X.X.; Zhao, J.L.; Zheng, Q.F.; Zhao, G.B.; Ma, Z.J. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015, 6(1), 55. [CrossRef]
- Takano, T.; Taira, Y.; Suzuki, R.; Matsumoto. H. Immortalized Canine Adipose-Derived Mesenchymal Stem Cells Maintain the Immunomodulatory Capacity of the Original Primary Cells. Int. J. Mol. Sci. 2023, 24(24), 17484. [CrossRef]
- Jin, Q.H.; Kim, H.K.; Na, J.Y.; Jin C.; Seon, J.K. Anti-inflammatory effects of mesenchymal stem cell-conditioned media inhibited macrophages activation in vitro. Sci. Rep. 2022, 12, 4754. [CrossRef]
- Fernández Vallone, V.B.; Romaniuk, M.A.; Choi, H.; Labovsky, V.; Otaegui, J.; Chasseing, N.A. Mesenchymal stem cells and their use in therapy: What has been achieved? Differentiation 2013, 85(1-2), 1-10. [CrossRef]
- Linard, C,; Brachet, M.; Strup-Perrot, C.; Busson, E.; Bonneau, M.; Lataillade, J-J.; Bey, E.; Benderitter, M. Long-term effectiveness of local BMMSCs for skeletal muscle regeneration: A proof of concept obtained on a pig model of severe radiation burn. Stem Cell Res. Ther. 2018; 9, 299. [CrossRef]
- Felker, S.; Shrestha, A.; Bailey, J.; Pillis, D.M.; Siniard, D.; Malik P. Differential CXCR4 expression on hematopoietic progenitor cells versus stem cells directs homing and engraftment. JCI Insight 2022, 7(9), e151847. [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [CrossRef]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8. [CrossRef]
- Gervois, P.; Struys, T,; Hilkens, P.; Bronckaers, A.; Ratajczak, J.; Politis, C.; Brône, B.; Lambrichts, I.; Martens, W. Neurogenic maturation of human dental pulp stem cells following neurosphere generation induces morphological and electrophysiological characteristics of functional neurons. Stem Cells Dev. 2015, 24(3), 296–311. [CrossRef]
- Bourin, P.; Bunnell, B.; Casteilla, L.; Dominici, M.; Katz, A.; March, K.; Redl, H.; Rubin, J.; Yoshimura, K.; Gimble, J. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International So, Cytotherapy 2013, 15, 641–648. [CrossRef]
- Dingal, P.C.; Bradshaw, A.M.; Cho, S.; Raab, M.; Buxboim, A.; Swift, J.; Discher, D.E. Fractal heterogeneity in minimal matrix models of scars modulates stiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nat. Mater. 2015, 14(9), 951–960. [CrossRef]
- Worthley, D.L.; Churchill, M.; Compton, J.T.; Tailor, Y.; Rao, M.; Si, Y.; Levin, D.; Schwartz, M.G.; Uygur, A.; Hayakawa, Y.; Gross, S.; Renz, B.W.; Setlik, W,; Martinez, A.N.; Chen, X.; Nizami, S.; Lee, H.G.; Kang, H.P.; Caldwell, J.M.; Asfaha, S.; Westphalen, C.B.; Graham, T.; Jin, G.; Nagar, K.; Wang, H.; Kheirbek, M.A.; Kolhe, A.; Carpenter, J.; Glaire, M.; Nair, A.; Renders, S.; Manieri, N.; Muthupalani, S.; Fox, J.G.; Reichert, M.; Giraud, A.S.; Schwabe, R.F.; Pradere, J.P.; Walton, K.; Prakash, A.; Gumucio, D.; Rustgi, A.K.; Stappenbeck, T.S.; Friedman, R.A.; Gershon, M.D.; Sims, P.; Grikscheit, T.; Lee, F.Y.; Karsenty, G.; Mukherjee, S.; Wang, T.C. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 2015, 160, 269–284. [CrossRef]
- Smolinska, A.; Chodkowska, M.; Kominek, A.; Janiec, J.; Piwocka, K.; Sulejczak, D.; Sarnowska, A. Stemness properties of SSEA-4+ subpopulation isolated from heterogenous Wharton’s jelly mesenchymal stem/stromal cells. Front Cell Dev Biol. 2024, 12, 1227034. [CrossRef]
- Maeda, K.; Enomoto, A.; Hara, A.; Asai, N.; Kobayashi, T.; Horinouchi, A.; Maruyama, S.; Ishikawa, Y.; Nishiyama, T.; Kiyoi, H;. Kato, T.; Ando, K.; Weng, L.; Mii, S.; Asai, M.; Mizutani, Y.; Watanabe, O.; Hirooka, Y.; Goto, H.; Takahashi, M. Identification of Meflin as a Potential Marker for Mesenchymal Stromal Cells. Sci. Rep. 2016, 6. [CrossRef]
- Aggoune, D.; Sorel, N.; Bonnet, M.L.; Goujon, J.M.; Tarte, K.; Hérault, O.; Domenech, J.; Réa, D.; Legros, L.; Johnson-Ansa, H.; Rousselot, P.; Cayssials, E.; Guerci-Bresler, A.; Bennaceur-Griscelli, A.; Chomel, J.C.; Turhan, A.G. Bone marrow mesenchymal stromal cell (MSC) gene profiling in chronic myeloid leukemia (CML) patients at diagnosis and in deep molecular response induced by tyrosine kinase inhibitors (TKIs). Leuk. Res. 2017, 60, 94–102. [CrossRef]
- Montelatici, E,; Baluce, B.; Ragni, E.; Lavazza, C.; Parazzi, V.; Mazzola, R.; Cantarella, G.; Brambilla, M.; Giordano, R.; Lazzari, L. Defining the identity of human adipose-derived mesenchymal stem cells. Biochem. Cell Biol. 2015, 82, 74–82. [CrossRef]
- Lv, F.J.; Tuan, R.S.; Cheung, K.M.C.; Leung, V.Y.L. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014, 32, 1408–1419. [CrossRef]
- Lootens, T.; Roman, B.I.; Stevens, C.V.; De Wever, O.; Raedt, R. Glioblastoma-Associated Mesenchymal Stem/Stromal Cells and Cancer-Associated Fibroblasts: Partners in Crime? Int J Mol Sci. 2024, 25(4), 2285. [CrossRef]
- Mishra, P.J.; Banerjee, D. Activation and differentiation of mesenchymal stem cells. Methods Mol. Biol., Humana Press Inc. 2017, 201–209. [CrossRef]
- Tang, H.; Chu, Y.; Huang, Z.; Cai, J.; Wang, Z. The metastatic phenotype shift toward myofibroblast of adipose-derived mesenchymal stem cells promotes ovarian cancer progression. Carcinogenesis 2019, 41(2), 182-193. [CrossRef]
- Matsumoto, K.; Xavier, S.; Chen, J.; Kida, Y.; Lipphardt, M.; Ikeda, R.; Gevertz, A.; Caviris, M.; Hatzopoulos, A.K.; Kalajzic, I,; Dutton, J.; Ratliff, B.B.; Zhaom, H.; Darzynkiewicz, Z.; Rose-John, S.; Goligorsky, M.S. Instructive Role of the Microenvironment in Preventing Renal Fibrosis. Stem Cells Transl. Med. 2017, 6, 992–1005. [CrossRef]
- Chen, S.; Liang, B.; Xu, J. Unveiling heterogeneity in MSCs: Exploring marker-based strategies for defining MSC subpopulations. J. Transl. Med. 2024, 22(1), 459. [CrossRef]
- Soundararajan, M.; Kannan, S. Fibroblasts and mesenchymal stem cells: Two sides of the same coin?. J Cell Physiol. 2018, 233(12), 9099–9109. [CrossRef]
- Denu, R.A.; Nemcek, S.; Bloom, D.D.; Goodrich, A.D.; Kim, J.; Mosher, D.F.; Hematti, P. Fibroblasts and Mesenchymal Stromal/Stem Cells Are Phenotypically Indistinguishable. Acta Haematol. 2016, 136, 85–97. [CrossRef]
- Kahounová, Z.; Kurfürstová, D.; Bouchal, J.; Kharaishvili, G.; Navrátil, J.; Remšík, J.; Šimečková, Š.; Študent, V.; Kozubík, A.; Souček, K. The fibroblast surface markers FAP, anti-fibroblast, and FSP are expressed by cells of epithelial origin and may be altered during epithelial-to-mesenchymal transition. Cytom. Part A., 2018, 93, 941–951. [CrossRef]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; Deschler, D.G.; Varvares, M.A.; Mylvaganam, R.; Rozenblatt-Rosen, O.; Rocco, J.W.; Faquin, W.C.; Lin, D.T.; Regev, A.; Bernstein, B.E. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624.e24. [CrossRef]
- Janeczek-Portalska, K., Leferink, A.; Groen, N.; Fernandes, H.; Moroni, L.; van Blitterswijk, C.; de Boer, J. Endothelial differentiation of mesenchymal stromal cells. PLoS ONE 2012, 7(10), e46842. [CrossRef]
- Suila, H.; Hirvonen, T.; Kotovuori, A.; Ritamo, I.; Kerkelä, E.; Anderson, H.; Natunen, S.; Tuimala, J.; Laitinen, S.; Nystedt, J.; Räbinä, J.; Valmu, L. Human umbilical cord blood-derived mesenchymal stromal cells display a novel interaction between P-selectin and galectin-1. Scand. J. Immunol. 2014, 80, 12–21. [CrossRef]
- L Ramos, T.; Sánchez-Abarca, L.I.; Muntión, S.; Preciado, S.; Puig, N.; López-Ruano, G.; Hernández-Hernández, Á.; Redondo, A.; Ortega, R.; Rodríguez, C.; Sánchez-Guijo, F.; del Cañizo, C. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. 2016, 14, 2. [CrossRef]
- Moravcikova, E.; Meyer, E.M.; Corselli, M.; Donnenberg, V.S.; Donnenberg, A.D. Proteomic Profiling of Native Unpassaged and Culture-Expanded Mesenchymal Stromal Cells (MSC). Cytom. Part A., 2018, 93, 894–904. [CrossRef]
- Chen, Q.H.; Wu, F.; Liu, L.; Chen, H.; Zheng R.Q.; Wang, H.L.; Yu, L.N. Mesenchymal stem cells regulate the Th17/Treg cell balance partly through hepatocyte growth factor in vitro. Stem Cell Res Ther 2020,11, 91. [CrossRef]
- Ciuculescu, F.; Giesen, M.; Deak, E.; Lang, V.; Seifried, E.; Henschler, R. Variability in chemokine-induced adhesion of human mesenchymal stromal cells. Cytotherapy 2011, 13, 1–8. [CrossRef]
- Guan, X.; Ma, X.; Zhang, L.; Feng, H.; Ma, Z. Evaluation of CD24 as a marker to rapidly define the mesenchymal stem cell phenotype and its differentiation in human nucleus pulposus. Chin. Med. J (Engl) 2014, 127(8), 1474–1481. PMID: 24762592.
- Teo, G.S.; Ankrum, J.A.; Martinelli, R.; Boetto, S.E.; Simms, K.; Sciuto, T.E.; Dvorak, A.M.; Karp, M.; Carman, C.V. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-α-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cells 2012, 30(11), 2472–2486. [CrossRef]
- Steingen, C.; Brenig, F.; Baumgartner, L.; Schmidt, J.; Schmidt, A.; Bloch, W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J Mol Cell Cardiol. 2008, 44(6), 1072–1084. [CrossRef]
- Hou, Y.; Ryu, C.H.; Jun, J.A.; Kim, S.M.; Jeong, C.H.; Jeun, S.S. IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol. Int. 2014, 38, 1050–1059. [CrossRef]
- Dreyer, C.H. Jørgensen, .R.; Overgaard, S.; Qin, L.; Ding, M. Vascular Endothelial Growth Factor and Mesenchymal Stem Cells Revealed Similar Bone Formation to Allograft in a Sheep Model. Biomed Res Int. 2021, 2021, 6676609. [CrossRef]
- Scherzed, A.; Hackenberg, S.; Froelich, K.; Rak, K.; Schendzielorz, P.; Gehrke, T.; Hagen, R.; Kleinsasser, N.; The differentiation of hMSCs counteracts their migration capability and pro-angiogenic effects in vitro. Oncol. Rep. 2016, 35, 219–226. [CrossRef]
- Mao, Y.; Keller, E.T.; Garfield, D.H.; Shen, K.; Wang, J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013, 32, 303–315. [CrossRef]
- Li, Y.; Zhong, X.; Zhang, Y.; Lu, X. Mesenchymal stem cells in gastric cancer: Vicious but hopeful. Front. Oncol. 2021, 11, 617677. [CrossRef]
- Sanchez-Vega, F.; Mina, M.; Armenia, J.; Chatila, W.K.; Luna, A.; La, K.C, Dimitriadoy, S.; Liu, D.L.; Kantheti, H.S.; Saghafinia, S.; Chakravarty, D.; Daian, F.; Gao, Q.; Bailey, M.H.; Liang, W.W.; Foltz, S.M.; Shmulevich, I.; Ding, L.; Heins, Z.; Ochoa, A.; Gross, B.; Gao, J.; Zhang, H.; Kundra, R.; Kandoth, C.; Bahceci, I.; Dervishi, L.; Dogrusoz, U.; Zhou, W.; Shen, H.; Laird, P.W.; Way, G.P.; Greene, C.S.; Liang, H.; Xiao, Y.; Wang, C.; Iavarone, A.; Berger, A.H.; Bivona, T.G.; Lazar, A.J.; Hammer, G.D.; Giordano, T.; Kwong, L.N.; McArthur, G.; Huang, C.; Tward, A.D.; Frederick, M.J.; McCormick, F.; Meyerson, M. Cancer Genome Atlas Research Network; Van Allen, E.M.; Cherniack, A.D.; Ciriello, G.; Sander, C.; Schultz, N. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 2018, 173(2), 321–337.e10. [CrossRef]
- Torsvik, A.; Bjerkvig, R. Mesenchymal stem cell signaling in cancer progression. Cancer Treat. Rev. 2013, 39(2), 180–188. [CrossRef]
- Cuiffo, B.G.; Campagne, A.; Bell, G.W.; Lembo, A.; Orso, F.; Lien, E.C.; Bhasin, M.K.; Raimo, M.; Hanson, S.E., Marusyk, A.; El-Ashry, D.; Hematti, P.; Polyak, K.; Mechta-Grigoriou, F.; Mariani, O.; Volinia, S.; Vincent-Salomon, A.; Taverna, D.; Karnoub, A.E. MSC-regulated microRNAs converge on the transcription factor FOXP2 and promote breast cancer metastasis. Cell Stem Cell. 2014, 15, 762–774. [CrossRef]
- Ohkouchi, S.; Block, G.J.; Katsha, A.M.; Kanehira, M.; Ebina, M.; Kikuchi, T.; Saijo, Y.; Nukiwa, T.; Prockop, D.J. Mesenchymal Stromal Cells Protect Cancer Cells From ROS-induced Apoptosis and Enhance the Warburg Effect by Secreting STC1. Mol. Ther. 2012, 20, 417–423. [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017, 35(4), 851–8. [CrossRef]
- Kupcova Skalnikova, H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie 2013, 95, 2196–2211. [CrossRef]
- Tachida ,Y.; Sakurai, H.; Okutsu, J.; Proteomic Comparison of the Secreted Factors of Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue and Dental Pulp. J. Proteomics Bioinform. 2015, 89(12). [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer, 2016, 16, 582–598. [CrossRef]
- de Miranda, M.C.; Melo, M.I.A.; Cunha, P.D.S.; Gentilini, J Júnior.; Faria, J.A.Q.A.; Rodrigues, M.A.; Gomes, D.A. Roles of mesenchymal stromal cells in the head and neck cancer microenvironment. Biomed. Pharmacother. 2021, 144, 112269. [CrossRef]
- Lourenco, S.; Teixeira, V.H.; Kalber, T.; Jose, R.J.; Floto, R.A.; Janes, S.M. Macrophage migration inhibitory factor-CXCR4 is the dominant chemotactic axis in human mesenchymal stem cell recruitment to tumors. J. Immunol. 2015, 194(7), 3463–3474. [CrossRef]
- Wobus, M.; List, C.; Dittrich, T.; Dhawan, A.; Duryagina, R.; Arabanian, L. S.; Kast, K.; Wimberger, P.; Stiehler, M.; Hofbauer, L. C.; Jakob, F.; Ehninger, G.; Anastassiadis, K.; Bornhäuser, M. Breast carcinoma cells modulate the chemoattractive activity of human bone marrow-derived mesenchymal stromal cells by interfering with CXCL12. Int. J. Cancer 2015, 136(1), 44–54. [CrossRef]
- Kalimuthu, S.; Oh, J. M.; Gangadaran, P.; Zhu, L.; Lee, H. W.; Rajendran, R. L., Baek, SH.; Jeon, Y.H.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. In Vivo Tracking of Chemokine Receptor CXCR4-Engineered Mesenchymal Stem Cell Migration by Optical Molecular Imaging. Stem Cells Int. 2017, 8085637. [CrossRef]
- Sheta, M.; Taha, E. A.; Lu, Y.; Eguchi, T. Extracellular Vesicles: New Classification and Tumor Immunosuppression. Biology 2023, 12(1), 110. [CrossRef]
- Norozi, F.; Ahmadzadeh, A.; Shahrabi, S.; Vosoughi, T.; Saki, N. Mesenchymal stem cells as a double-edged sword in suppression or progression of solid tumor cells. Tumour Biol. 2016, 37(9), 11679–11689. [CrossRef]
- Zhang, R.; Qi, F.; Zhao, F.; Li, G.; Shao, S.; Zhang, X.; Yuan, L.; Feng, Y. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis. 2019, 10(4), 273. [CrossRef]
- Barcellos-de-Souza, P.; Comito, G.; Pons-Segura, C.; Taddei, M.L.; Gori, V.; Becherucci, V.; Bambi, F.; Margheri, F.; Laurenzana, A.; Del Rosso, M.; Chiarugi, P. Mesenchymal Stem Cells are Recruited and Activated into Carcinoma-Associated Fibroblasts by Prostate Cancer Microenvironment-Derived TGF-β1. Stem Cells. 2016, 34(10), 2536–2547. [CrossRef]
- Aoto, K.; Ito, K.; Aoki, S. Complex formation between platelet-derived growth factor receptor beta and transforming growth factor beta receptor regulates the differentiation of mesenchymal stem cells into cancer-associated fibroblasts. Oncotarget 2018, 9, 34090–34102. [CrossRef]
- Li, G. C.; Zhang, H. W.; Zhao, Q. C.; Sun, L. I.; Yang, J. J.; Hong, L.; Feng, F.; Cai, Lei. Mesenchymal stem cells promote tumor angiogenesis via the action of transforming growth factor beta1. Oncol. Lett. 2016, 11, 1089–1094. [CrossRef]
- Li, W.; Zhou, Y.; Yang, J.; Zhang, X.; Zhang, H.; Zhang, T.; Zhao, S.; Zheng, P.; Huo, J.; Wu, H. Gastric cancer-derived mesenchymal stem cells prompt gastric cancer progression through secretion of interleukin-8. J. Exp. Clin. Cancer Res. 2015, 34, 52. [CrossRef]
- Guo, X.; Zhao, Y.; Yan, H.; Yang, Y.; Shen, S.; Dai, X.; Ji, X.; Ji, F.; Gong, X.G.; Li, L.; Bai, X.; Feng, X.H.; Liang, T.; Ji, J.; Chen, L.; Wang, H.; Zhao, B. Single tumor-initiating cells evade immune clearance by recruiting type II macrophages. Genes Dev. 2017, 31, 247–259. [CrossRef]
- Powell, D.; Lou, M.; Barros Becker, F.; Huttenlocher, A. Cxcr1 mediates recruitment of neutrophils and supports proliferation of tumor-initiating astrocytes in vivo. Sci. Rep. 2018, 8, 13285. [CrossRef]
- Lan, T.; Luo, M.; Wei, X. Mesenchymal stem/stromal cells in cancer therapy. J. Hematol. Oncol. 2021, 14, 195. [CrossRef]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Mol. Biol. Rep. 2020, 47(6), 4587–4629. [CrossRef]
- Li, L.; Li, J.C.; Yang, H.; Zhang, X.; Liu, L.L.; Li Y, Zeng, T.T.; Zhu, Y.H.; Li, X.D.; Li, Y.; Xie, D.; Fu, L.; Guan, X.Y. Expansion of cancer stem cell pool initiates lung cancer recurrence before angiogenesis. Proc. Natl. Acad. Sci. U S A. 2018, 115(38), E8948-E8957. [CrossRef]
- Liongue, C.; Sertori, R.; Ward, A.C. Evolution of cytokine receptor signaling. J. Immunol. 2016, 197(1), 11–8. [CrossRef]
- Zhang, X.; Hu, F.; Li, G.; Li, G.; Yang, X.; Liu, L.; Zhang, R.; Zhang, B.; Feng, Y. Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling. Cell Death Dis. 2018, 9(2), 25. [CrossRef]
- Liu, C.; Feng, X.; Wang, B.; Wang, X.; Wang, C.; Yu, M.; Cao, G.; Wang, H. Bone marrow mesenchymal stem cells promote head and neck cancer progression through Periostin-mediated phosphoinositide 3-kinase/Akt/mammalian target of rapamycin. Cancer Sci. 2018, 109(3), 688-698. [CrossRef]
- McGuire, J.J.; Frieling, J.S.; Lo, C.H.; Li, T.; Muhammad, A.; Lawrence, H.R.; Lawrence, N.J.; Cook, L.M.; Lynch, C.C. Mesenchymal stem cell-derived interleukin-28 drives the selection of apoptosis resistant bone metastatic prostate cancer. Nat. Commun. 2021, 12(1), 723. [CrossRef]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene, 2017, 36(11), 1461–1473. [CrossRef]
- Wang, W.; Zhong, W.; Yuan, J.; Yan, C.; Hu, S.; Tong, Y.; Mao, Y.; Hu, T.; Zhang, B.; Song G. Involvement of Wnt/β-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget 2015, 6(39), 42276–42789. [CrossRef]
- Chen, Y. C.; Gonzalez, M. E.; Burman, B.; Zhao, X.; Anwar, T.; Tran, M.; Medhora, N.; Hiziroglu, A.B.; Lee, W.; Cheng, Y.H.; Choi, Y.; Yoon, E.; Kleer, C.G. Mesenchymal Stem/Stromal cell engulfment reveals metastatic advantage in Breast Cancer. Cell Rep. 2019, 27(13), 3916–3926.e5. [CrossRef]
- Lu, L.; Chen, G.; Yang, J.; Ma, Z.; Yang, Y.; Hu, Y.; Lu, Y.; Cao, Z.; Wang, Y.; Wang, X. Bone marrow mesenchymal stem cells suppress growth and promote the apoptosis of glioma U251 cells through downregulation of the PI3K/AKT signaling pathway. Biomed. Pharmacother. 2019, 112, 108625. [CrossRef]
- Akimoto, K.; Kimura, K.; Nagano, M.; Takano, S.; To’a Salazar, G.; Yamashita, T.; Ohneda O. Umbilical cord blood-derived mesenchymal stem cells inhibit, but adipose tissue-derived mesenchymal stem cells promote, glioblastoma multiforme proliferation. Stem Cells Dev. 2013, 22, 1370–1386. [CrossRef]
- Xuan, X.; Tian, C.; Zhao, M.; Sun, Y.; Huang C. Mesenchymal stem cells in cancer progression and anticancer therapeutic resistance. Cancer Cell Int. 2021, 21(1), 595. [CrossRef]
- Ho, I. A., Toh, H. C., Ng, W. H., Teo, Y. L., Guo, C. M., Hui, K. M., Lam, P.Y.P. Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells 2013, 31, 146–155. [CrossRef]
- Hanks, B.A.; Holtzhausen, A.; Evans, K. S.; Jamieson, R.; Gimpel, P.; Campbell, O.M.; Hector-Greene, M.; Sun, L.; Tewari, A.; George, A.; Starr, M.; Nixon, A.B.; Augustine, C.; Beasley, G.; Tyler, D.S.; Osada, T.; Morse, M.A.; Ling, L.; Lyerly, H. K.; Blobe, G. C. J. Clin. Invest. 2020, 123(9), 3925–3940. [CrossRef]
- Bortolotti, F.; Ukovich, L.; Razban, V.; Martinelli, V.; Ruozi, G.; Pelos, B.; Dore, F.; Giacca, M.; Zacchigna, S. In vivo therapeutic potential of mesenchymal stromal cells depends on the source and the isolation procedure. Stem Cell Rep. 2015, 4, 332–339. [CrossRef]
- Bajetto, A.; Pattarozzi, A.; Corsaro, A.; Barbieri, F.; Daga, A.; Bosio, A.; Gatti, M.; Pisaturo, V.; Sirito, R.; Florio T. Different effects of human umbilical cord mesenchymal stem cells on glioblastoma stem cells by direct cell interaction or via released soluble factors. Front. Cell. Neurosci. 2017, 11, 312. [CrossRef]
- Capilla-Gonzalez, V., Lopez-Beas, J., Escacena, N., Aguilera, Y., de la Cuesta, A., Ruiz-Salmeron, R.; Martín, F.; Hmadcha, A.; Soria, B. PDGF restores the defective phenotype of adipose-derived mesenchymal stromal cells from diabetic patients. Mol. Ther. 2018, 26, 2696–2709. [CrossRef]
- Perez, L.M.; de Lucas, B.; Galvez, B.G. Unhealthy stem cells: When health conditions upset stem cell properties. Cell. Physiol. Biochem. 2018, 46, 1999–2016. [CrossRef]
- Rivera, FJ; de la Fuente, A.G.; Zhao C, Silva ME, Gonzalez GA, Wodnar R, Feichtner M, Lange S, Errea O, Priglinger E, O’Sullivan A, Romanelli P, Jadasz, J.J.; Brachtl. G.; Greil, R.; Tempfer. H.; Traweger, A.; Bátiz, L.F, Küry, P.; Couillard-Despres, S.; Franklin, R.J.M.; Aigner, L. Aging restricts the ability of mesenchymal stem cells to promote the generation of oligodendrocytes during remyelination. Glia. 2019, 67(8), 1510–1525. [CrossRef]
- Liang, W.; Chen, X.; Zhang, S; Fang, J.; Chen, M.; Xu, Y.; Chen, X. Mesenchymal stem cells as a double-edged sword in tumor growth: Focusing on MSC-derived cytokines. Cell Mol. Biol. Lett, 2021, 26(1), 3. [CrossRef]
- Timaner, M.; Tsai, K.K.,; Shaked, Y. The multifaceted role of mesenchymal stem cells in cancer. Semin Cancer Biol. 2020, 60, 225–237. [CrossRef]
- Le Naour, A.; Prat, M.; Thibault, B.; Mével, R.; Lemaitre, L.; Leray, H.; Joubert, M.V.; Coulson, K.; Golzio, M.; Lefevre, L.; Mery, E.; Martinez, A.; Ferron, G.; Delord, J.P.; Coste, A.; Couderc, B. Tumor cells educate mesenchymal stromal cells to release chemoprotective and immunomodulatory factors. J Mol Cell Biol. 2020, 12(3), 202–215. [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. (Weinh). 2021, 8(15), e2004433. [CrossRef]
- Panchalingam, K.M..; Jung, S.; Rosenberg, L.; Behie, L.A. Bioprocessing strategies for the large-scale production of human mesenchymal stem cells: A review. Stem Cell Res. Ther. 2015, 6, 225. [CrossRef]
- Sharma, R.R.; Pollock, K.; Hubel, A.; McKenna, D. Mesenchymal stem or stromal cells: A review of clinical applications and manufacturing practices. Transfusion 2014, 54(5), 1418–1437. [CrossRef]
- Haddad, R.; Saldanha-Araujo, F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: What do we know so far? Biomed. Res. Int. 2014, 2014, 216806. [CrossRef]
- Lawson, D. A.; Bhakta, N. R.; Kessenbrock, K.; Prummel, K. D.; Yu, Y.; Takai, K., Zhou A.; Eyob, H.; Balakrishnan, S.; Wang, C.Y.; Yaswen, P.; Goga, A.; Werb, Z. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 2015, 526(7571), 131–135. [CrossRef]
- Bhonde, R.R.; Sheshadri, P, Sharma S.; Kumar, A. Making surrogate β-cells from mesenchymal stromal cells: Perspectives and future endeavors. Int. J. Biochem. Cell Biol. 2014, 46, 90–102. [CrossRef]
- López de Padilla, C. M., Niewold, T. B. The type I interferons: Basic concepts and clinical relevance in immune-mediated inflammatory diseases. Gene 2016, 576(1 Pt 1), 14–21. [CrossRef]
- Barrat, F.J.; Crow, M.K.; Ivashkiv, L.B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat, Immunol. 2019, 20(12), 1574–1583. [CrossRef]
- Domenis, R.; Cifù, A.; Quaglia, S.; Pistis, C.; Moretti, M.; Vicario, A.; Parodi, P.C.; Fabris, M.; Niazi, K.R.; Soon-Shiong, P.; Curcio, F. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells derived exosomes. Sci. Rep. 2018, 8, 1–11. [CrossRef]
- Dunn, C.M.; Kameishi, S.; Cho, Y.K.; Song, S.U.; Grainger, D.W.; Okano, T. (2022). Interferon-Gamma Primed Human Clonal Mesenchymal Stromal Cell Sheets Exhibit Enhanced Immunosuppressive Function. Cells 2022, 11(23), 3738. [CrossRef]
- Ungerer, C.; Quade-Lyssy, P.; Radeke, H.H.; Henschler, R.; Königs, C.; Köhl, U.; Seifried, E.; Schüttrumpf, J. Galectin-9 is a suppressor of T and B cells and predicts the immune modulatory potential of mesenchymal stromal cell preparations. Stem Cells Dev. 2014, 23(7), 755–766. [CrossRef]
- Hori, A.; Takahashi, A.; Miharu, Y.; Yamaguchi, S.; Sugita, M.; Mukai, T.; Nagamura, F.; Nagamura-Inoue, T. Superior migration ability of umbilical cord-derived mesenchymal stromal cells (MSCs) toward activated lymphocytes in comparison with those of bone marrow and adipose-derived MSCs. Front Cell Dev Biol. 2024, 12, 1329218. [CrossRef]
- Chen, H.W.; Chen, H.Y.; Wang, L.T.; Wang, F.H.; Fang, L.W.; Lai, H.Y.; Chen, H.H.; Lu, J.; Hung, M.S.; Cheng, Y.; Chen, M.Y.; Liu, S.J.; Chong, P.; Lee, O.K.; Hsu, S.C. Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines. J. Immunol. 2013, 190(10), 5065–5077. [CrossRef]
- Yen, B.L.; Yen, M.L.; Hsu, P.J,; Liu, K.J.; Wang, C.J.; Bai, C,H.; Sytwu, H.K. Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Reports. 2013, 1(2), 139–151. [CrossRef]
- Cao, W.; Cao, K.; Cao, J.; Wang, Y.; Shi, Y. Mesenchymal stem cells and adaptive immune responses. Immunol. Lett. 2015, 168(2), 147–153. [CrossRef]
- Le Blanc, K.; Davies, L.C. Mesenchymal stromal cells and the innate immune response. Immunol. Lett. 2015, 68(2), 140–146. [CrossRef]
- Lee, H.J.; Ko, J.H.; Jeong, H.J.; Ko, A.Y.; Kim, M.K.; Wee, W.R,.; Yoon, S.O.; Oh, J.Y. Mesenchymal stem/stromal cells protect against autoimmunity via CCL2-dependent recruitment of myeloid-derived suppressor cells. J. Immunol. 2015, 194(8), 3634–3645. [CrossRef]
- Davies, L.C.; Heldring, N.; Kadri, N.; Le Blanc, K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells. 2017, 35(3), 766–776. [CrossRef]
- Gaber, T.; Schönbeck, K.; Hoff, H.; Tran, C.L.; Strehl, C.; Lang, A.; Ohrndorf, S.; Pfeiffenberger, M.; Röhner, E.; Matziolis, G.; Burmester, G.R.; Buttgereit, F.; Hoff, P. CTLA-4 Mediates Inhibitory Function of Mesenchymal Stem/Stromal Cells. Int. J. Mol Sci. 2018, 19(8), 2312. [CrossRef]
- Zhu, Q.; Zhang, X.; Zhang, L.; Li, W.; Wu, H.; Yuan, X.; Mao, F.; Wang, M.; Zhu, W.; Qian, H.; Xu. W. The IL-6-STAT3 axis mediates a reciprocal crosstalk between cancer-derived mesenchymal stem cells and neutrophils to synergistically prompt gastric cancer progression. Cell Death Dis. 2014, 5(6), e1295. [CrossRef]
- Ma, O.K.; Chan, K.H. Immunomodulation by mesenchymal stem cells: Interplay between mesenchymal stem cells and regulatory lymphocytes. World J. Stem Cells 2016, 8(9), 268–278. PMID: 27679683; PMCID: PMC5031888. [CrossRef]
- Wang, M.Y.; Zhou, T.Y.; Zhang, Z.D.; Liu, H.Y.; Zheng, Z.Y.; Xie, H.Q. Current therapeutic strategies for respiratory diseases using mesenchymal stem cells. Med. Comm. 2020, 2(3), 351–380. [CrossRef]
- Lee, H,J.; Oh, J.Y. Mesenchymal Stem/Stromal Cells Induce Myeloid-Derived Suppressor Cells in the Bone Marrow via the Activation of the c-Jun N-Terminal Kinase Signaling Pathway. Int. J. Mol. Sci. 2024, 25(2), 1119. [CrossRef]
- Korneev, K. V.; Atretkhany, K. N.; Drutskaya, M. S.; Grivennikov, S. I.; Kuprash, D. V.; Nedospasov, S. A. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine 2017, 89, 127–135. [CrossRef]
- Deguine, J.; Barton, G. M. MyD88: A central player in innate immune signaling. F1000 Med. Rep. 2014, 6, 97. [CrossRef]
- Ye, W.; Hu, M. M.; Lei, C. Q.; Zhou, Q.; Lin, H.; Sun, M. S.; Shu, H. B. TRIM8 Negatively Regulates TLR3/4-Mediated Innate Immune Response by Blocking TRIF-TBK1 Interaction. J. Immunol. (Baltimore, Md.:1950) 2017, 199(5), 1856–1864. [CrossRef]
- Shah, P.; Omoluabi, O.; Moorthy, B.; Ghose, R. Role of Adaptor Protein Toll-Like Interleukin Domain Containing Adaptor Inducing Interferon β in Toll-Like Receptor 3- and 4-Mediated Regulation of Hepatic Drug Metabolizing Enzyme and Transporter Genes. Drug Metab. Dispos. 2016, 44(1), 61–67. [CrossRef]
- Affolter, A.; Lammert, A.; Kern, J.; Scherl, C,.; Rotter, N. Precision Medicine Gains Momentum: Novel 3D Models and Stem Cell-Based Approaches in Head and Neck Cancer. Front. Cell Dev. Biol. 2021, 9, 666515. [CrossRef]
- Naaldijk, Y.; Johnson, A.A.; Ishak, S.; Meisel, H.J.; Hohaus, C.; Stolzing, A. Migrational changes of mesenchymal stem cells in response to cytokines, growth factors, hypoxia, and aging. Exp. Cell Res. 2015, 338(1), 97–104. [CrossRef]
- Qiu, X.; Zhang, Y.; Zhao, X.; Zhang, S.; Wu, J.; Guo, H.; Hu, Y. Enhancement of endothelial differentiation of adipose derived mesenchymal stem cells by a three-dimensional culture system of microwell. Biomaterials 2015, 53, 600–608. [CrossRef]
- Kwon, Y.W.; Heo, S.C.; Jeong, G.O.; Yoon, J.W.; Mo, W.M.; Lee, M.J.; Jang, I.H.; Kwon, S.M.; Lee, J.S.; Kim, J.H. Tumor necrosis factor-α-activated mesenchymal stem cells promote endothelial progenitor cell homing and angiogenesis. Biochim. Biophys. Acta. 2013, 1832(12), 2136–2144. [CrossRef]
- Liu, X.; Zhou, Z.; Zeng, W.N.; Zeng, Q.; Zhang, X. The role of toll-like receptors in orchestrating osteogenic differentiation of mesenchymal stromal cells and osteoimmunology. Front. Cell Dev. Biol. 2023, 11, 1277686. [CrossRef]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [CrossRef]
- Ulivi, V.; Tasso, R.; Cancedda, R.; Descalzi, F. Mesenchymal stem cell paracrine activity is modulated by platelet lysate: Induction of an inflammatory response and secretion of factors maintaining macrophages in a proinflammatory phenotype. Stem Cells Dev. 2014, 23(16), 1858–1869. [CrossRef]
- Watts, T.L.; Cui, R.; Szaniszlo, P.; Resto, V.A.; Powell, D.W.; Pinchuk, I.V.; PDGF-AA mediates mesenchymal stromal cell chemotaxis to the head and neck squamous cell carcinoma tumor microenvironment, J. Transl. Med. 2016, 14(1), 337. [CrossRef]
- Kansy, B.A.; Dißmann, P.A.; Hemeda, H.; Bruderek, K.; Westerkamp, A.M.; Jagalski, V.; Schuler, P.; Kansy, K.; Lang, S.; Dumitru, C.A.; Brandau, S. The bidirectional tumor - mesenchymal stromal cell interaction promotes the progression of head and neck cancer. Stem Cell Res. Ther. 2014, 5, 95. [CrossRef]
- Ji, X.; Zhang, Z.; Han, Y,; Song, J.; Xu, X.; Jin, J.; Su, S.; Mu, D.; Liu, X.; Xu, S.; Cui, H.; Zhao, Z,; Wang, Y.; Liu, H.; Mesenchymal stem cells derived from normal gingival tissue inhibit the proliferation of oral cancer cells in vitro and in vivo. Int. J. Oncol. 2016, 49, 2011–2022. [CrossRef]
- Wang, J.; Yang, W.; Wang, T.; Chen, X.; Wang, J.; Zhang, X.; Cai, C.; Zhong, B.; Wu, J.; Chen, Z.; Xiang, A.P.; Huang, W. Mesenchymal Stromal Cells-Derived β2-Microglobulin Promotes Epithelial-Mesenchymal Transition of Esophageal Squamous Cell Carcinoma Cells. Sci. Rep. 2018, 8(1), 5422. [CrossRef]
- Scherzed, A.; Hackenberg, S.; Radeloff, A. Froelich, K.; Rak, K.; Hagen, R.; Kleinsasser, N. Human mesenchymal stem cells promote cancer motility and cytokine secretion in vitro. Cells Tissues Organs. 2014, 198, 327–337. [CrossRef]
- Wu, Y.L.; Li, H.Y.; Zhao, X.P.; Jiao, J.Y.; Tang, D.X.; Yan, L.J.; Wan, Q.; Bin Pan, C. Mesenchymal stem cell-derived CCN2 promotes the proliferation, migration and invasion of human tongue squamous cell carcinoma cells. Cancer Sci. 2017, 108, 897–909. [CrossRef]
- Coccè, V.; Farronato, D.; Brini, A.T.; Masia, C.; Giannì, A.B.; Piovani, G.; Sisto, F.; Alessandri, G.; Angiero, F.; Pessina, A. Drug Loaded Gingival Mesenchymal Stromal Cells (GinPa-MSCs) Inhibit In Vitro Proliferation of Oral Squamous Cell Carcinoma. Sci. Rep. 2017, 7(1), 9376. [CrossRef]
- Wang, J.; Cui, R.; Clement, C.G.; Nawgiri. R.; Powell. D.W.; Pinchuk, I.V.; Watts, T.L. Activation PDGFR-α/AKT Mediated Signaling Pathways in Oral Squamous Cell Carcinoma by Mesenchymal Stem/Stromal Cells Promotes Anti-apoptosis and Decreased Sensitivity to Cisplatin. Front. Oncol. 2020, 10, 552. [CrossRef]
- Liu, C.; Billet, S.; Choudhury, D.; Cheng, R.; Haldar, S.; Fernandez, A.; Biondi, S.; Liu, Z.; Zhou, H.; Bhowmick, N.A, Bone marrow mesenchymal stem cells interact with head and neck squamous cell carcinoma cells to promote cancer progression and drug resistance. Neoplasia 2021, 23, 118–128. [CrossRef]
- Liu, C.; Feng, X.; Wang, B.; Wang, X.; Wang. C.; Yu, M.; Cao, G.; Wang, H. Bone marrow mesenchymal stem cells promote head and neck cancer progression through Periostin-mediated phosphoinositide 3-kinase/Akt/mammalian target of rapamycin. Cancer Sci. 2018, 109, 688–698. [CrossRef]
- Jiang, C.; Zhang, Q.; Shanti, R.M.; Shi, S.; Chang, T.H.; Carrasco, L.; Alawi, F.; Le, A.D. Mesenchymal Stromal Cell-Derived Interleukin-6 Promotes Epithelial–Mesenchymal Transition and Acquisition of Epithelial Stem-Like Cell Properties in Ameloblastoma Epithelial Cells. Stem Cells. 2017, 35, 2083–2094. [CrossRef]
- Li, W.; Han, Y.; Zhao, Z.; Ji, X.; Wang, X.; Jin, J.; Wang, Q.; Guo, X.; Cheng, Z,; Lu, M.; Wang, G.; Wang, Y.; Liu, H. Oral mucosal mesenchymal stem cell-derived exosomes: A potential therapeutic target in oral premalignant lesions. Int. J. Oncol. 2019, 54, 1567–1578. [CrossRef]
- Almeida, R.D.A.C.; Andrade, E.S.D.S.; Barbalho, J.; Vajgel, A.; Vasconcelos BCDE, Recurrence rate following treatment for primary multicystic ameloblastoma: Systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 359–367. [CrossRef]
- Hendra, F.N.; Van Cann, E.M.; Helder, M.N.; Ruslin, M.; de Visscher, J.G.; Forouzanfar, T.; de Vet, H.C.W. Global incidence and profile of ameloblastoma: A systematic review and meta-analysis. Oral Dis. 2020, 26, 12–21. [CrossRef]
- Shi, S.; Zhang, Q.; Xia, Y.; You, B.; Shan, Y.; Bao, L.; Li, L.; You, Y.; Gu, Z. Mesenchymal stem cell-derived exosomes facilitate nasopharyngeal carcinoma progression. Am. J. Cancer Res. 2016, 6, 459–472. PMID: 27186416, PMCID: PMC4859673.
- Hong, D.; Liu, T.; Huang, W.; Liao, Y.; Wang, L.; Zhang, Z.; Chen, H.; Zhang, X.; Xiang, Q. Gremlin1 Delivered by Mesenchymal Stromal Cells Promoted Epithelial-Mesenchymal Transition in Human Esophageal Squamous Cell Carcinoma. Cell Physiol. Biochem. 2018, 47(5), 1785–1799. [CrossRef]
- Nakayama, A.; Aoki, S.; Uchihashi, K.; Nishijima-Matsunobu, A.; Yamamoto, M.; Kakihara, N.; Iwakiri, R.; Fujimoto, K.; Toda, S. Interaction between Esophageal Squamous Cell Carcinoma and Adipose Tissue in Vitro. Am. J. Pathol. 2016, 186, 1180–1194. [CrossRef]
- Wang, Y.; Fan, H.; Zhou, B.; Ju, Z.; Yu, L.; Guo, L.; Han, J.; Lu, S. Fusion of human umbilical cord mesenchymal stem cells with esophageal carcinoma cells inhibits the tumorigenicity of esophageal carcinoma cells. Int. J. Oncol. 2012, 40, 370–377. [CrossRef]
- Li, L.; Tian, H.; Yue, W.; Zhu, F.; Li, S,; Li, W. Human mesenchymal stem cells play a dual role on tumor cell growth in vitro and in vivo. J. Cell. Physiol. 2011, 226, 1860–1867. [CrossRef]
- Liotta, F.; Querci, V.; Mannelli, G.; Santarlasci, V.; Maggi, L.; Capone, M.; Rossi, M.C.; Mazzoni, A.; Cosmi, L.; Romagnani, S.; Magg, E.; Gallo, O. Annunziato F, Mesenchymal stem cells are enriched in head neck squamous cell carcinoma, correlates with tumour size and inhibit T-cell proliferation. Br. J. Cancer. 2015, 112, 745–754. [CrossRef]
- Mazzoni, A.; Capone, M.; Ramazzotti, M.; Vanni, A.; Locatello, L.G.; Gallo, O.; De Palma, R.; Cosmi, L.; Liotta, F.; Annunziato, F.; Maggi, L. IL4I1 Is Expressed by Head–Neck Cancer-Derived Mesenchymal Stromal Cells and Contributes to Suppress T Cell Proliferation. J. Clin. Med. 2021, 10, 2111. [CrossRef]
- Schuler, P.J.; Westerkamp, A.M.; Kansy, B.; Bruderek, K.; Dissmann, P.A.; Dumitru, C.A.; Lang, S.; Jackson, E.K.; Brandau, S. Adenosine metabolism of human mesenchymal stromal cells isolated from patients with head and neck squamous cell carcinoma. Immunobiology 2017, 222, 66–74. [CrossRef]
- Ping, Q.; Yan, R.; Cheng, X.; Wang, W.; Zhong, Y.; Hou, Z.; Shi, Y.; Wang, C.; Li, R. Cancer-associated fibroblasts: Overview, progress, challenges, and directions. Cancer Gene Ther. 2021, 28 (9), 984–999. [CrossRef]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 2017, 276, 121–144. [CrossRef]
- Rowan, B.G.; Lacayo, E.A.; Sheng, M.; Anbalagan, M.; Gimble, J.M.; Jones, R.K.; Joseph, W.J.; Friedlander, P.L.; Chiu, E.S.; Human Adipose Tissue-Derived Stromal/Stem Cells Promote Migration and Early Metastasis of Head and Neck Cancer Xenografts. Aesthetic Surg. J. 2015, 36, 93–104. [CrossRef]
- Salo, S.; Bitu, C.; Merkku, K.; Nyberg, P.; Bello, I.O.; Vuoristo, J.; Sutinen, M.; Vähänikkilä, H.; Costea, D.E.; Kauppila, J.; Lehenkari, P.; Dayan, D.; Vered, M.; Risteli, J.; Salo, T. Human Bone Marrow Mesenchymal Stem Cells Induce Collagen Production and Tongue Cancer Invasion. PLoS ONE. 2013, 8(10), e77692. [CrossRef]
- Hong, D.; Liu, T.; Huang, W.; Liao, Y.; Wang, L.; Zhang, Z.; Chen, H.; Zhang, X.; Xiang, Q. Gremlin1 delivered by mesenchymal stromal cells promoted epithelial-mesenchymal transition in human esophageal squamous cell carcinoma. Cell. Physiol. Biochem. 2018, 47, 1785–1799. [CrossRef]
- Castellone, M.D.; Laatikainen, L.E.; Laurila, J.P.; Langella, A.; Hematti, P.; Soricelli, A.; Salvatore, M.; Laukkanen, M.O. Brief Report: Mesenchymal Stromal Cell Atrophy in Coculture Increases Aggressiveness of Transformed Cells. Stem Cells. 2013, 31, 1218–1223. [CrossRef]
- Mazzoni, A.; Maggi, L.; Montaini, G.; Ramazzotti, M.; Capone, M.; Vanni, A.; Locatello, L.G.; Barra, G.; De Palma, R.; Gallo, O.; Cosmi, L.; Liotta, F.; Annunziato, F. Human T cells interacting with HNSCC-derived mesenchymal stromal cells acquire tissue-resident memory like properties. Eur. J. Immunol. 2020, 50(10), 1571–1579. [CrossRef]
- Chen, Y.; Wang, X.; Fang, J.; Song, J.; Ma, D.; Luo, L.; He, B.; Xia, J.; Lui, V.W.Y.; Cheng, B.; Wang, Z. Mesenchymal stem cells participate in oral mucosa carcinogenesis by regulating T cell proliferation. Clin. Immunol. 2019, 198, 46–53. [CrossRef]
- Ziebart, A.; Huber, U.; Jeske, S.S.; Laban, S.; Doescher, J.; Hoffmann, T.K.; Brunner, C.; Jackson, E.K.; Schuler, P.J. The influence of chemotherapy on adenosine-producing B cells in patients with head and neck squamous cell carcinoma. Oncotarget 2017, 9, 5834–5847. [CrossRef]
- Bruna, F.; Arango-Rodríguez, M.; Plaza, A.; Espinoza, I.; Conget, P. The administration of multipotent stromal cells at precancerous stage precludes tumor growth and epithelial dedifferentiation of oral squamous cell carcinoma. Stem Cell Res. 2017, 18, 5–13. [CrossRef]
- Bruna, F.; Plaza, A.; Arango, M.; Espinoza, I.; Conget, P. Systemically administered allogeneic mesenchymal stem cells do not aggravate the progression of precancerous lesions: A new biosafety insight. Stem Cell Res. Ther. 2018, 9, 137. [CrossRef]
- Meng, W.; Wu, Y.; He, X.; Liu, C.; Gao, Q.; Ge, L.; Wu, L.; Liu, Y.; Guo, Y.; Li, X.; Liu, Y.; Chen, S.; Kong, X.; Liang, Z.; Zhou, H. A systems biology approach identifies effective tumor-stroma common targets for oral squamous cell carcinoma. Cancer Res. 2014, 74, 2306–2315. [CrossRef]
- Kumar, J.D.; Holmberg, C.; Kandola, S.; Steele, I.; Hegyi, P.;p Tiszlavicz, L.; Jenkins, R.; Beynon, R.J.; Peeney, D.; Giger, O.T.; Alqahtani, A.; Wang, T.C.; Charvat, T.T.; Penfold, M.; Dockray, G.J.; Varro, A. Increased expression of chemerin in squamous esophageal cancer myofibroblasts and role in recruitment of mesenchymal stromal cells. PLoS ONE 2014, 9(7), e104877. [CrossRef]
- Tan, T.T.; Lai, R.C.; Padmanabhan, J.; Sim, W.K.; Choo, A.B.H.; Lim, S.K. Assessment of Tumorigenic Potential in Mesenchymal-Stem/Stromal-Cell-Derived Small Extracellular Vesicles (MSC-sEV). Pharm. 2021, 14, 345. [CrossRef]
- Zielske, S.P.; Livant, D.L.; Lawrence, T.S. Radiation Increases Invasion of Gene-Modified Mesenchymal Stem Cells into Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 843–853. [CrossRef]
- Escacena, N.; Quesada-Hernandez, E.; Capilla-Gonzalez, V.; Soria, B.; Hmadcha, A. Bottlenecks in the efficient use of advanced therapy medicinal products based on mesenchymal stromal cells. Stem Cells Int. 2015, 2015, 895714. [CrossRef]
- Melen, G. J.; Franco-Luzon, L.; Ruano, D.; Gonzalez-Murillo, A.; Alfranca, A.; Casco, F., Lassaletta, Á.; Alonso, M.; Madero, L.; Alemany, R.; García-Castro, J.; Ramírez, M. Influence of carrier cells on the clinical outcome of children with neuroblastoma treated with high dose of oncolytic adenovirus delivered in mesenchymal stem cells. Cancer Lett. 2016, 371(2), 161–170. [CrossRef]
- Carlander, A.F.; Gundestrup, A.K.; Jansson, P.M.; Follin, B.; Hoeeg, C.; Kousholt, B.S.; Larsen, R.T.; Jakobsen, K.K.; Rimborg, S.; Fischer-Nielsen, A.; Grønhøj, C.; Buchwald, C.V.; Lynggaard, C.D. Mesenchymal Stromal/Stem Cell Therapy Improves Salivary Flow Rate in Radiation-Induced Salivary Gland Hypofunction in Preclinical in vivo Models: A Systematic Review and Meta-Analysis. Stem Cell Rev. Rep. 2024, 20(4), 1078–1092. [CrossRef]
- Jansson, P.M.; Lynggaard, C.D.; Carlander, A.F.; Jensen, S.B.; Follin, B.; Hoeeg, C.; Kousholt, B.S.; Larsen, R.T.; Grønhøj, C.; Jakobsen, K.K.; Rimborg, S.; Fischer-Nielsen, A.; Menon, J.M.L.; on Buchwald, C. Mesenchymal stromal/stem cell therapy for radiation-induced salivary gland hypofunction in animal models: A protocol for a systematic review and meta-analysis. Systematic reviews 2022, 11(1), 72. [CrossRef]
- Jakobsen, K.K.; Carlander, A.F.; Todsen, T.; Melchiors, J.; Paaske, N.; Østergaard Madsen, A.K.; Kloch Bendtsen, S.; Mordhorst, C.; Stampe, H.; Kastrup, J.; Ekblond, A.; Haack-Sørensen, M.; Farhadi, M.; Maare, C.; Friborg, J.; Lynggaard, C.D.; Werner Hauge, A.; Christensen, R.; Grønhøj, C.; von Buchwald, C. Mesenchymal Stem/Stromal Cell Therapy for Radiation-Induced Xerostomia in Previous Head and Neck Cancer Patients: A Phase II Randomized, Placebo-Controlled Trial. Clin. Cancer Res: An official journal of the American Association for Cancer Research 2024, 30(10), 2078–2084. [CrossRef]
- Strojan, P.; Plavc, G.; Kokalj, M.; Mitrovic, G.; Blatnik, O.; Lezaic, L.; Socan, A.; Bavec, A.; Tesic, N.; Hartman, K.; Svajger, U. Post-radiation xerostomia therapy with allogeneic mesenchymal stromal stem cells in patients with head and neck cancer: Study protocol for phase I clinical trial. Radiol Oncol. 2023, 57(4), 538–549. [CrossRef]
- Jakobsen, K.K.; Carlander, A.F.; Grønhøj, C.; Todsen, T.; Melchiors, J.; Paaske, N.; Madsen, A K.Ø.; Kastrup, J.; Ekblond, A.; Haack-Sørensen, M.; Farhadi, M.; Maare, C.; Friborg, J.; Lynggard, C. D.; von Buchwald, C. Effectiveness and safety of mesenchymal stem/stromal cell for radiation-induced hyposalivation and xerostomia in previous head and neck cancer patients (MESRIX-III): A study protocol for a single-centre, double-blinded, randomised, placebo-controlled, phase II study. Trials 2023, 24(1), 567. [CrossRef]
- Lynggaard, C. D., Grønhøj, C., Christensen, R., Fischer-Nielsen, A., Melchiors, J., Specht, L., Andersen, E., Mortensen, J., Oturai, P., Barfod, G. H., Haastrup, E. K., Møller-Hansen, M., Haack-Sørensen, M., Ekblond, A., Kastrup, J., Jensen, S. B., & von Buchwald, C. Intraglandular Off-the-Shelf Allogeneic Mesenchymal Stem Cell Treatment in Patients with Radiation-Induced Xerostomia: A Safety Study (MESRIX-II). Stem Cells Transl Med. 2022, 11(5), 478–489. [CrossRef]
- Lynggaard, C.D.; Grønhøj, C.; Jensen, S.B.; Christensen, R.; Specht, L.; Andersen, E.; Andersen, T.T.; Ciochon, U.M.; Rathje, G.S.; Hansen, A.E.; Stampe, H.; Fischer-Nielsen, A.; von Buchwald, C. Long-term Safety of Treatment with Autologous Mesenchymal Stem Cells in Patients with Radiation-Induced Xerostomia: Primary Results of the MESRIX Phase I/II Randomized Trial. Clin Cancer Res. 2022, 28(13), 2890–2897. [CrossRef]
- Blitzer, G. C., Paz, C., Glassey, A., Ganz, O. R., Giri, J., Pennati, A., Meyers, R. O., Bates, A. M., Nickel, K. P., Weiss, M., Morris, Z. S., Mattison, R. J., McDowell, K. A., Croxford, E., Chappell, R. J., Glazer, T. A., Rogus-Pulia, N. M., Galipeau, J., & Kimple, R. J. Functionality of bone marrow mesenchymal stromal cells derived from head and neck cancer patients - A FDA-IND enabling study regarding MSC-based treatments for radiation-induced xerostomia. Radiother Oncol. 2024, 192, 110093. [CrossRef]
- Grønhøj, C.; Jensen, D.H.; Vester-Glowinski, P.; Jensen, S.B.; Bardow, A.; Oliveri, R.S.; Fog, L.M.; Specht, L.; Thomsen, C.; Darkner, S.; Jensen, M.; Müller, V.; Kiss, K.; Agander, T.; Andersen, E.; Fischer-Nielsen, A.; von Buchwald, C. Safety and Efficacy of Mesenchymal Stem Cells for Radiation-Induced Xerostomia: A Randomized, Placebo-Controlled Phase 1/2 Trial (MESRIX). Int. J. Radiat. Oncol. Biol. Phys. 2018, 101(3), 581–592. [CrossRef]
- Blitzer, G.C.; Rogus-Pulia, N. M.; Mattison, R.J.;Varghese, T.; Ganz, O.; Chappell, R.; Galipeau, J.; McDowell, K.A.; Meyers, R.O.; Glazer, T.A.; Kimple, R.J. Marrow-Derived Autologous Stromal Cells for the Restoration of Salivary Hypofunction (MARSH): A pilot, first-in-human study of interferon gamma-stimulated marrow mesenchymal stromal cells for treatment of radiation-induced xerostomia. Cytotherapy 2023, 25(11), 1139–1144. [CrossRef]
- Blitzer, G.C.; Rogus-Pulia, N. M.; Mattison, R.J.;Varghese, T.; Ganz, O.; Chappell, R.; Galipeau, J.; McDowell, K.A.; Meyers, R.O.; Glazer, T.A.; Kimple, R.J. Marrow-Derived Autologous Stromal Cells for the Restoration of Salivary Hypofunction (MARSH): Study protocol for a phase 1 dose-escalation trial of patients with xerostomia after radiation therapy for head and neck cancer: MARSH: Marrow-Derived Autologous Stromal Cells for the Restoration of Salivary Hypofunction. Cytotherapy 2022, 24(5), 534–543. [CrossRef]
- Lynggaard, C.D.; Jersie-Christensen, R.; Juhl, M.; Jensen, S.B.; Grønhøj, C.; Melchiors, J.; Jacobsen, S.; Møller-Hansen, M.; Herly, M.; Ekblond, A.; Kastrup, J.; Fischer-Nielsen, A.; Belstrøm, D.; von Buchwald, C. Intraglandular mesenchymal stem cell treatment induces changes in the salivary proteome of irradiated patients. Commun. Med. (Lond.) 2022, 2(1), 160. [CrossRef]
- Chulpanova, D.S.; Kitaeva, K.V.; Tazetdinova, L.G.; James, V.; Rizvanov, A.A.; Solovyeva, V.V. Application of Mesenchymal Stem Cells for Therapeutic Agent Delivery in Anti-tumor Treatment. Front. Pharmacol. 2018, 9, 259. [CrossRef]
- Shen, C.J.; Chan, T.F.; Chen, C.C.; Hsu, Y.C.; Long, C.Y.; Lai, C.S. Human umbilical cord matrix-derived stem cells expressing interferon-beta gene inhibit breast cancer cells via apoptosis. Oncotarget 2018, 7, 34172–34179. [CrossRef]
- Liu, X., Hu, J., Li, Y., Cao, W., Wang, Y., Ma, Z., Li, F. Mesenchymal stem cells expressing interleukin-18 inhibit breast cancer in a mouse model. Onco. Lett. 2018, 15(5), 6265–6274. [CrossRef]
- Ciavarella, S.; Grisendi, G.; Dominici, M.; Tucci, M.; Brunetti, O.; Dammacco, F., Silvestris, F. In vitro anti-myeloma activity of TRAIL-expressing adipose-derived mesenchymal stem cells. Br. J. Haematol. 2012, 157, 586–598. [CrossRef]
- Fakiruddin, K.S.; Baharuddin, P.; Lim, M.N.; Fakharuzi, N.A.; Yusof, N.A.; Zakaria, Z. Nucleofection optimization and in vitro anti-tumourigenic effect of TRAIL-expressing human adipose-derived mesenchymal stromal cells. Cancer Cell Int. 2014, 14, 122. [CrossRef]
- Guo, X.R.; Yang, Z.S.; Tang, X.J.; Zou, D.D.; Gui, H.; Wang, X.L., Ma, S.N.; Yuan, Y.H.; Fang, J.; Wang, B.; Zhang, L.; Sun, X.Y.; Warnock, G.L.; Dai, L.J.; Tu, H.J. The application of mRNA-based gene transfer in mesenchymal stem cell-mediated cytotoxicity of glioma cells. Oncotarget 2016, 7, 55529–55542. [CrossRef]
- Jiang, X.; Fitch, S.; Wang, C.; Wilson, C.; Li, J.; Grant, G.A., Yang, F. Nanoparticle engineered TRAIL-overexpressing adipose-derived stem cells target and eradicate glioblastoma via intracranial delivery. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, 13857–13862. [CrossRef]
- Li, G.C.; Zhang, H.W.; Zhao, Q.C.; Sun, L.I.; Yang, J.J.; Hong, L., Feng F.; Cai, L. Mesenchymal stem cells promote tumor angiogenesis via the action of transforming growth factor beta1. Oncol. Lett. 2016, 11, 1089–1094. [CrossRef]
- Mangraviti, A.; Tzeng, S.Y.; Gullotti, D.; Kozielski, K.L.; Kim, J.E.; Seng, M.; Abbadi, S.; Schiapparelli, P.; Sarabia-Estrada, R.; Vescovi, A.; Brem, H.; Olivi, A.; Tyler, B.; Green, J.J.; Quinones-Hinojosa, A. Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials 2016, 100, 53–66. [CrossRef]
- Rincon, E.; Cejalvo, T.; Kanojia, D.; Alfranca, A.; Rodriguez-Milla, M.A.; Gil Hoyos, R.A.; Han, Y.; Zhang, L.; Alemany, R.; Lesniak, M. S.; García-Castro, J. Mesenchymal stem cell carriers enhance antitumor efficacy of oncolytic adenoviruses in an immunocompetent mouse model. Oncotarget 2017, 8(28), 45415–45431. [CrossRef]
- Guo, Y.; Zhang, Z.; Xu, X.; Xu, Z.; Wang, S.; Huang, D.; Li, Y.; Mou, X.; Liu, F.; Xiang, C. Menstrual blood-derived stem cells as delivery vehicles for oncolytic adenovirus virotherapy for Colorectal Cancer. Stem Cells Dev. 2019, 28, 882–896. [CrossRef]
- Du, W.; Seah, I.; Bougazzoul, O.; Choi, G.; Meeth, K.; Bosenberg, M.W.; et al. Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, E6157–E6165. [CrossRef]
- Brini, A.T.; Coccè, V.; Ferreira, L.M.; Giannasi, C.; Cossellu, G.; Giannì, A.B.; Angiero, F.; Bonomi, A.; Pascucci, L.; Falchetti, M.L.; Ciusani, E.; Bondiolotti, G.; Sisto, F.; Alessandri, G.; Pessina, A., Farronato, G. Cell-mediated drug delivery by gingival interdental papilla mesenchymal stromal cells (GinPa-MSCs) loaded with paclitaxel. Expert Opin Drug Deliv. 2016, 13(6), 789–798. [CrossRef]
- Layek, B.; Sadhukha, T.; Panyam, J.; Prabha, S. Nano-engineered mesenchymal stem cells increase therapeutic efficacy of anticancer drug through true active tumor targeting. Mol. Cancer Ther. 2018, 17, 1196–1206. [CrossRef]
- Wang, X.; Gao, J.; Ouyang, X.; Wang, J.; Sun, X.; and Lv, Y; Mesenchymal stem cells loaded with paclitaxel-poly(lactic-co-glycolic acid) nanoparticles for glioma-targeting therapy. Int. J. Nanomed. 2018, 13, 5231–5248. [CrossRef]
- Moku, G.; Layek, B.; Trautman, L.; Putnam, S.; Panyam, J.; and Prabha, S. Improving payload capacity and anti-tumor efficacy of mesenchymal stem cells using TAT peptide functionalized polymeric nanoparticles. Cancers 2019, 1, E491. [CrossRef]
- Collino, F.; Bruno, S.; Lindoso; R. S.; Camussi, G. miRNA expression in mesenchymal stem cells. Curr. Pathobiol. Rep. 2014, 2, 101–107. [CrossRef]
- Lang, F.M.; Hossain, A.; Gumin, J.; Momin, E.N.; Shimizu, Y.; Ledbetter, D.; Shahar, T.; Yamashita, S.; Parker Kerrigan, B.; Fueyo, J.; Sawaya, R., Lang, F.F. Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro. Oncol. 2018, 20(3), 380–390. [CrossRef]
- Sharif, S.; Ghahremani, M.H.; Soleimani, M. Delivery of exogenous miR-124 to glioblastoma multiform cells by wharton’s jelly mesenchymal stem cells decreases cell proliferation and migration, and confers chemosensitivity. Stem Cell Rev. 2018, 14, 236–246. [CrossRef]
- Sharif, S.; Ghahremani, M.H.; Soleimani, M. Delivery of exogenous miR-124 to glioblastoma multiform cells by wharton’s jelly mesenchymal stem cells decreases cell proliferation and migration, and confers chemosensitivity. Stem Cell Rev. 2018, 14, 236–246. [CrossRef]
- Bregy, A.; Shah, A.H.; Diaz, M.V.; Pierce, H.E.; Ames, P.L.; Diaz, D.; Komotar, R.J. The role of Gliadel wafers in the treatment of high-grade gliomas. Expert Rev. Anticancer Ther. 2013, 13(12), 1453–1461. [CrossRef]
- Smith, S.J.; Tyler, B.M.; Gould, T.; Veal, G.J.; Gorelick, N.; Rowlinson, J.; Serra, R.; Ritchie, A.; Berry, P.; Otto, A.; Choi, J.; Skuli, N.; Estevez-Cebrero, M.; Shakesheff, K.M.; Brem, H.; Grundy, R.G.; Rahman, R. Overall survival in malignant glioma is significantly prolonged by neurosurgical delivery of etoposide and temozolomide from a thermo-responsive biodegradable paste. Clin. Cancer Res. 2019, 25(16), 5094–5106. [CrossRef]
- Zeng, X.; Qiu, X.C.; Ma, Y.H.; Duan, J.J.; Chen, Y.F.; Gu, H.Y., Wang, J.M.; Ling, E.A.; Wu, J.L.; Wu, W.; Zeng, Y.S. Integration of donor mesenchymal stem cell-derived neuron-like cells into host neural network after rat spinal cord transection. Biomaterials 2015, 53, 184–201. [CrossRef]
- Wang, Q.L.; Wang, H.J.; Li, Z.H.; Wang, Y.L.; Wu, X.P.; Tan, Y.Z. Mesenchymal stem cell-loaded cardiac patch promotes epicardial activation and repair of the infarcted myocardium. J. Cell Mol. Med. 2017, 21, 1751–1766. [CrossRef]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A.; Caputi, S.; Fontana, A.; Mazzon, E.; Trubiani, O. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: A new tool for bone defect repair. Stem Cell Res. Ther. 2018, 9(1), 104. [CrossRef]
- Sheets, K.T.; Bago, J.R.; Hingtgen, S.D. Delivery of Cytotoxic Mesenchymal Stem Cells with Biodegradable Scaffolds for Treatment of Postoperative Brain Cancer. Methods Mol. Biol. 2018, 1831, 49–58. [CrossRef]
- Aliperta, R.; Welzel, P.B.; Bergmann, R.; Freudenberg, U.; Berndt, N.; Feldmann, A.; Arndt, C.; Koristka, S.; Stanzione, M.; Cartellieri, M.; Ehninger, A.; Ehninger, G.; Werner, C.; Pietzsch, J.; Steinbach, J.; Bornhäuser, M.; Bachmann, M.P. Cryogel-supported stem cell factory for customized sustained release of bispecific antibodies for cancer immunotherapy. Sci Rep. 2017, 7, 42855. [CrossRef]
- Johansson, M.; Oudin, A.; Tiemann, K.; Bernard, A.; Golebiewska, A.; Keunen, O.; Fack, F.; Stieber, D.; Wang, B.; Hedman, H.; Niclou, S.P. The soluble form of the tumor suppressor Lrig1 potently inhibits in vivo glioma growth irrespective of EGF receptor status. Neuro. Oncol. 2013, 15(9), 1200–1211. [CrossRef]
- Galland, S.; Stamenkovic, I. Mesenchymal stromal cells in cancer: A review of their immunomodulatory functions and dual effects on tumor progression. J. Pathol. 2020, 250(5), 555–572. [CrossRef]
- Avnet, S.; Lemma, S.; Cortini, M.; Di Pompo, G.; Perut, F., Baldini N.. Pre-clinical models for studying the interaction between mesenchymal stromal cells and cancer cells and the induction of stemness. Front. Oncol. 2019, 9. 305. [CrossRef]
- Li, C.; Zhao, H;.; Cheng, L.; Wang, B. Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci. 2021, 11(1), 187. [CrossRef]
- Wang, J.; Duan, X.; Yang, L.; Liu, X.; Hao, C.; Dong, H.; Gu, H.; Tang, H.; Dong, B.; Zhang, T.; Gao, G.; Liang, R. Comparison of Survival Between Autologous and Allogeneic Stem Cell Transplantation in Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma: A Meta-Analysis. Cell Transplant. 2020;29, 963689720975397. [CrossRef]
- Durand, N.; Zubair, A.C. Autologous versus allogeneic mesenchymal stem cell therapy: The pros and cons. Surgery 2022, 171(5), 1440–1442. [CrossRef]
- Jensen, S.B.; Vissink, A.; Limesand, K.H.; Reyland, M.E. Salivary gland hypofunction and Xerostomia in Head and Neck Radiation patients. J. Natl. Cancer Inst. Monogr. 2019, 2019(53), lgz016. [CrossRef]
- Vissink, A.; van Luijk, P.; Langendijk, J.A.; Coppes, R.P. Current ideas to reduce or salvage radiation damage to salivary glands. Oral Diseases. 2015;21, e1–10. [CrossRef]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.; Adab, F.; Jefferies, S.J.; Scrase, C.; Yap, B.K.; A’Hern, R.P.; Sydenham, M.A.; Emson, M.; Hall, E.; & PARSPORT trial management group. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. The Lancet Oncology 2011, 12, 127–136. [CrossRef]
- Jeong, J.; Baek, H.; Kim, Y.J.; Choi, Y.; Lee, H.; Lee, E.; Kim, E.S.; Hah, J.H.; Kwon, T.K.; Choi, I.J.; Kwon, H. Human salivary gland stem cells ameliorate hyposalivation of radiation-damaged rat salivary glands. Exp. Mol. Med. 2013, 45(11), e58. [CrossRef]
- Lim JY, Yi T, Choi JS, Jang YH, Lee S, Kim HJ; et al. Intraglandular transplantation of bone marrow-derived clonal mesenchymal stem cells for amelioration of post-irradiation salivary gland damage. Oral Oncology 2013, 49, 136–143. [CrossRef]
- Lim, J.Y.; Ra, J.C.; Shin, I.S.; Jang, Y.H.; An, H.Y.; Choi, J.S.; Kim, W.C.; Kim, Y.M. Systemic transplantation of human adipose tissue-derived mesenchymal stem cells for the regeneration of irradiation-induced salivary gland damage. PLoS ONE 2013, 8(8), e71167. [CrossRef]
- Wang, Z.; Xing, H.; Hu, H.; Dai, T.; Wang, Y.; Li, Z.; An, R.; Xu, H.; Liu, Y.; Liu, B. Intraglandular transplantation of adipose-derived stem cells combined with platelet-rich fibrin extract for the treatment of irradiation-induced salivary gland damage. Exp. Ther. Med. 2018, 15(1), 795–805. [CrossRef]
- Li, Z.; Wang, Y.; Xing, H.; Wang, Z.; Hu, H.; An, R.; Xu, H.; Liu, Y.; Liu, B. Protective efficacy of intravenous transplantation of adipose-derived stem cells for the prevention of radiation-induced salivary gland damage. Arch. Oral Biol. 2015, 60(10), 1488–1496. [CrossRef]
- Shin, H.S.; Lee, S.; Hong, H.J.; Lim, Y.C.; Koh, W.G.; Lim, J.Y. Stem cell properties of human clonal salivary gland stem cells are enhanced by three-dimensional priming culture in nanofibrous microwells. Stem. Cell Res. Ther. 2018, 9(1), 74. [CrossRef]
- Choi, J.S.; An, H.Y.; Shin, H.S.; Kim, Y.M.; Lim, JY. Enhanced tissue remodelling efficacy of adipose-derived mesenchymal stem cells using injectable matrices in radiation-damaged salivary gland model. J. Tissue Eng. Regen. Med. 2018, 12(2), e695–e706. [CrossRef]
- Shin, H.S.; Lee, S.; Kim, Y.M.; Lim, J.Y. Hypoxia-activated adipose mesenchymal stem cells prevents Irradiation-Induced Salivary Hypofunction by enhanced paracrine effect through fibroblast growth factor 10. Stem Cells. 2018, 36, 1020–1032. [CrossRef]
- Mulyani, S.W.M.; Astuti, E.R.; Wahyuni, O.R.; Ernawati, D.S.; Ramadhani, N.F. Xerostomia Therapy due to Ionized Radiation using preconditioned bone marrow-derived mesenchymal stem cells. Eur. J. Dent. 2019, 13, 238–242. [CrossRef]
| Author | MSC in in vitro models of HNSCC | |
| Study design | Mechanisms/ Underlying signalling pathway/ Results | |
| Wats et al. [163] |
|
|
| Liu et al. [172] |
|
|
| Salo et al. [188] |
|
|
| Kansy et al. [164] |
|
|
| Ji et al. [165] |
|
|
| Wang et al. [166] |
|
|
| Wu et al. [168] |
|
|
| Coccè et al. [169] |
|
|
| Wang et al. [170] |
|
|
| Liu et al. [171] |
|
|
| Li et al. [174] |
|
|
| Shi et al. [177] |
|
|
| Tian et al. [181] |
|
|
| Hong et al. [189] |
|
|
| Nakayama et al. [179] |
|
|
| Castellone et al. [190] |
|
|
| Wang et al. [180] |
|
|
| Liotta et al. [182] |
|
|
| Mazzoini et al. [183] |
|
|
| Mazzoini et al. [191] |
|
|
| Rowan et al. [187] |
|
|
| Author | MSCs in animal and in vivo models of HNSCC | |
| Study design | Mechanisms/ Underlying signalling pathway/Results | |
| Liu et al. [172] |
|
|
| Kansy et al. [164] |
|
|
| Salo et al. [188] |
|
|
| Ji et al. [165] |
|
|
| Wang et al. [166] |
|
|
| Wu et al. [168] |
|
|
| Liu et al. [171] |
|
|
| Shi et al. [177] |
|
|
| Tian et al. [181] |
|
|
| Hong et al. [189] |
|
|
| Castellone et al. [190] |
|
|
| Wang et al. [180] |
|
|
| Liotta et al. [182] |
|
|
| Chen et al.[192] |
|
|
| Rowan et al. [187] |
|
|
| Zielske et al. [199] |
|
|
| Tan et al. [198] |
|
|
| Bruna et al. [194] |
|
|
| Bruna et al. [195] |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





