1. Introduction
Different scientific disciplines are considering the United Nations sustainable development goals for 2030 with its environmental, social and economic pillars in their activities [
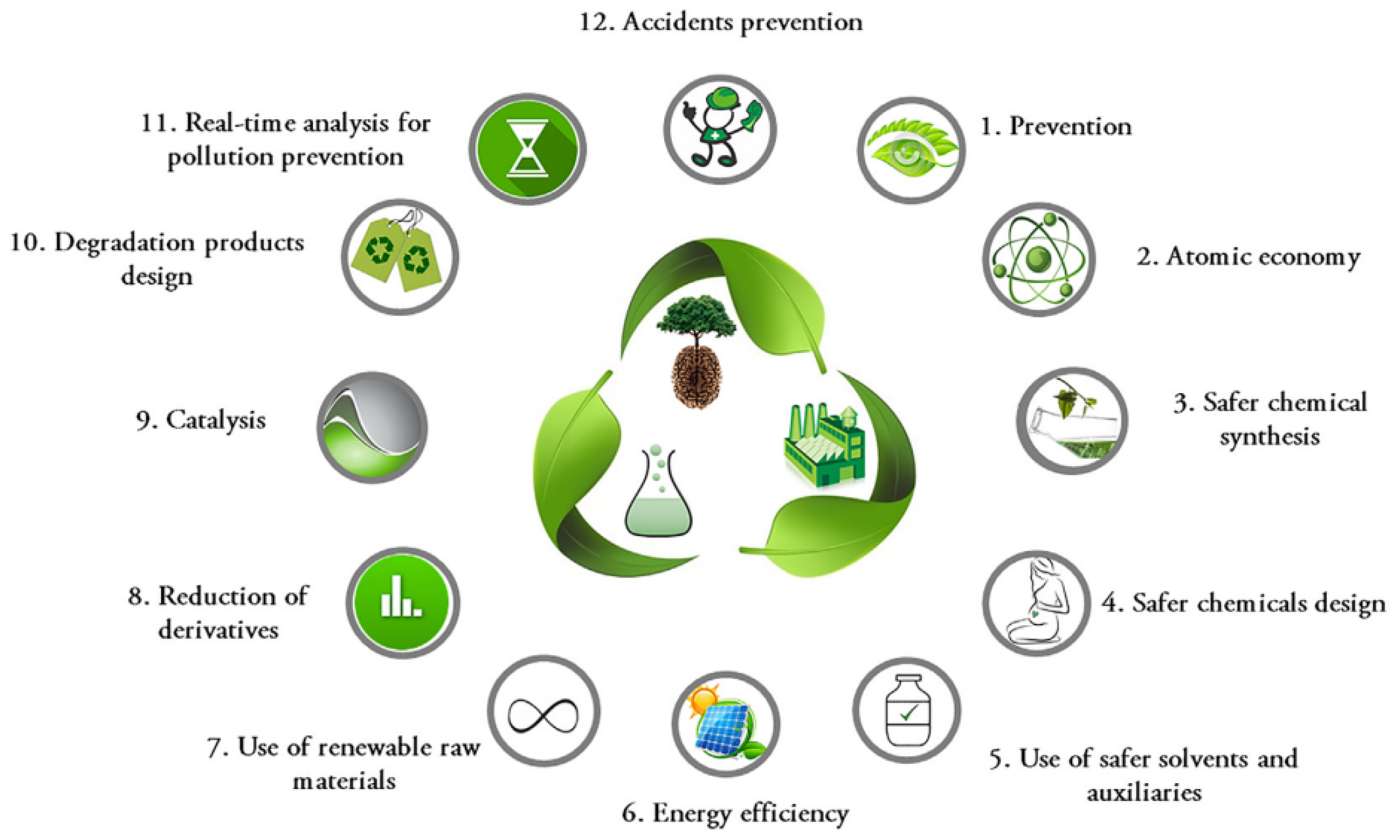
1]. Analysts are raising awareness to move toward more sustainable practice in chemistry. Analytical chemistry is a unique player in environmental and health sustainability. In one-way, analytical chemistry acts as a tool to test the toxicity level in different media and on the other way it utilizes chemicals that can hazard environment and human. At the end of the 20th Century, the concept of green chemistry was introduced by Anastas and Warner [
2] with 12 principles of green chemistry to reduce health and environment footprints as presented in
Figure 1.
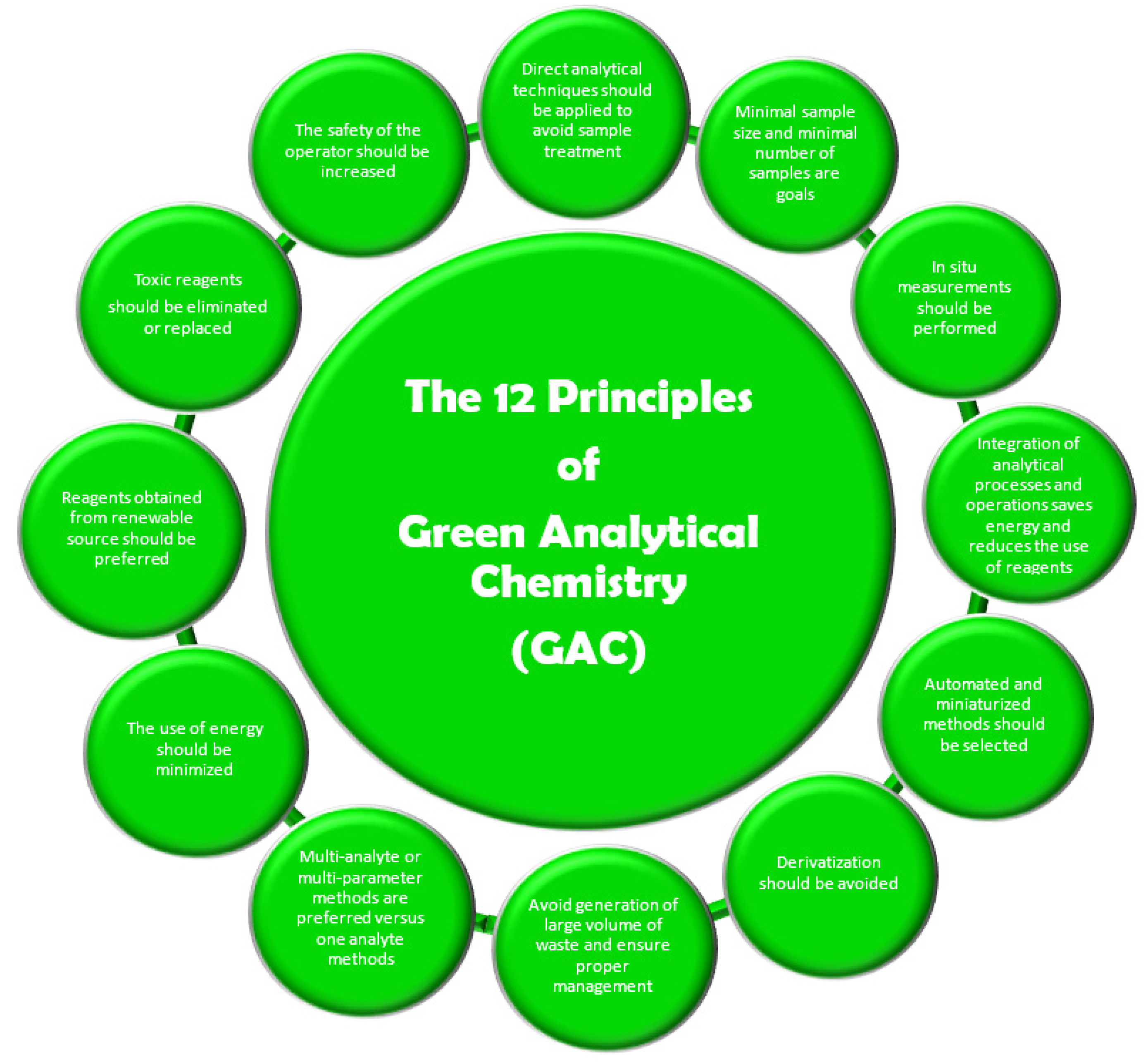
Twelve principles of green analytical chemistry (GAC) were later proposed and published by Galuszka et al. [
3] adapted for analytical chemistry and represented a basic guideline for going green in analysis as shown in
Figure 2.
Unlike techniques such as capillary electrophoresis [
4,
5], supercritical fluid chromatography [
6] and sensor-based analytical techniques [
7] that are quite green from a sustainable point of view, liquid chromatography utilizes larger amounts of organic solvent in the mobile phase [
8] and generating huge amounts of toxic wastes and emitting relatively large amounts of carbon dioxide that affect global warming through slowing the production of ozone in the lower stratosphere. Moreover, the extensive use of volatile organic compounds (VOC) acts also as ozone-depleting chemical to further contribute to the global warming potential [
9].
In analytical practice, normal phase and HILIC phase chromatography utilize more toxic non-polar organic solvents than reversed phase chromatography and are thus considered less green with more possibilities for toxic solvent accumulation. However, reversed phase chromatography is more commonly applied in routine analysis thus the consideration of all is important. Analytical and preparative liquid chromatographic methods are integral part of analytical separations including chiral separations, identifications, analytical characterizations, and determinations of chemicals. They contribute strongly to pharmaceutical research from drug discovery and development process to routine quality control. The Environmental Protection Agency (EPA) [
10], and the International Organization for Standardization (ISO) promotes transferring classical liquid chromatographic methods to green analytical chemistry [
11].
Organic solvent usage and waste production account for more than half of the greening of a classical method. Therefore, the primary focus when transforming a reference LC method with toxic organic solvent in the mobile phase into the greener method is the replacement of the toxic solvent with a greener one and whenever possible the reduction of solvent consumption and thus also the waste generation. In practice, using a green solvent to replace a toxic one is an important principle among others for easy transfer to green analytical methods. Greening the liquid chromatographic methods can among other aspects be easily enhanced by replacing the classical solvent with a greener alternative. The use of biodegradable solvents can further consolidate the reduction in waste generation. Whenever not already existed in the classical method and if available this could be combined with the replacement of the separation column with a higher performance one. Militarization of chromatographic columns plays also a vital role in method greening. The use of monolithic or core-shell columns with improved performance and thus shorter length and internal diameter significantly reduce the analysis time and thus will save solvent and energy and enhance the greenness of the method. The same is also true for 3 µm and sub-2-µm particle columns where smaller particle size is reflected by larger surface area and better separation performance, when necessary a UHPLC instrument is applied to resist the high backpressure associated with the use of small particles [
12].
The use of shorter columns with faster analysis time would also reduce the instrumental energy consumption per run which would decrease the carbon footprint and further increase the greenness of the method. In principle, less energy consumption related to both solvent and instrument lower the carbon emission and enhance the greenness of the method. This would contribute to total carbon dioxide emission. It has been shown that analytical laboratories emit about 22% of the amount of carbon dioxide emission associated with petrol car per day [
13]. Therefore, HPLC and UHPLC instruments are regarded as energy-intensive instrumental techniques associated with high carbon footprint this can be minimized by depending on renewable energy as solar power and wind energy or by reducing the analysis time to decrease the energy consumption [
14]. The energy consumption of HPLC and UHPLC instruments differ based on vendor and version, instruments with low energy consumption are desired. LC vendor companies should consider further investments to improve their instrumentations in term of reduced energy demand for power saving to contribute to a lower carbon footprint and render their instrument less polluting. On the other hand, companies as well as research and educational laboratories should aim to implement newer more efficient LC instruments with lower energy consumption to reduce the carbon footprint associated with analysis. Scientists suggest the inclusion of energy consumption and carbon footprint to the validation criteria of new analytical methods [
13].
Sustainable analytical chemistry should be globally adapted in the near future. Pharmacopeia should implement newer alternative greener methods and modernize traditional LC analytical methods to elevate the sustainability in analytical chemistry. Therefore, scientists should suggest more alternative green LC separation methods to replace traditional non-green methods. This refinement is mandatory shortly to improve the analytical sustainability of pharmacopeial methods. However, the total switch to sustainable methods should start earlier in the global pharmaceutical industry and research laboratories. Until being officially included as method validation criteria, chemical and pharmaceutical companies should consider method sustainability to their laboratory guidelines.
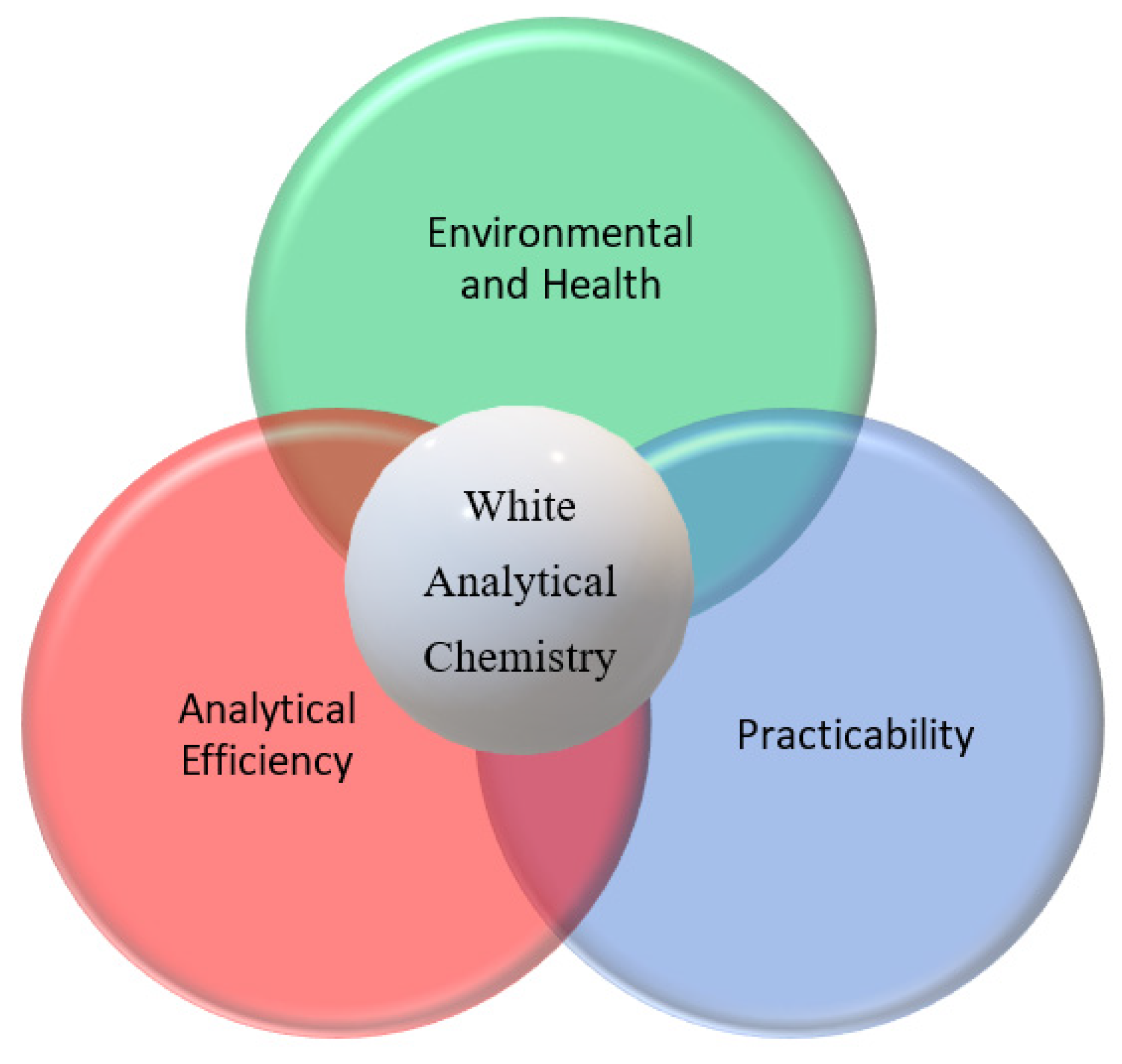
In the few past years, further terms have been popularized extending the consideration beyond green analytical chemistry. The term blue analytical chemistry is concerned with ensuring the practicability of the green analytical method concerning ease of use and cost-effectiveness [
15]. In many cases greening the analytical method would be at the expense of its performance. Thus, the method will become greener while the analytical performance will be compromised and this might affect its intended application. It is necessary to keep a good adequate level of method performance (e.g. precision, sensitivity) when greening it to ensure that the method can fulfil the purpose. Therefore, a further advancement toward better sustainable analytical chemistry has been considered by Nowak et al. In 2021 came up with a new approach beyond green analytical chemistry named white analytical chemistry (WAC) as an extension with red green and blue principles [
16]. WAC considers beyond environmental aspects of the analytical method its analytical and practical aspects. Under the term WAC, the three main components namely method greenness with a green color component, method analytical efficiency with a red color component, and method practicability with a blue color component are included as represented in
Figure 3. The three components are weighted to give an overall white color strength representing the sustainability percentage of the method [
16,
17].
Several reviews have been published on green and beyond analytical chemistry, however did not present a clear solvent selection guide or method transfer guide to shift a traditional LC method based on toxic organic solvent to a more sustainable method [
18,
19,
20,
21,
22,
23,
24,
25].
This review aims to give insight into green solvent selection for chromatographic application while considering environmental, health, and chromatographic suitability and compatibility aspects. This should encourage analysts in industrial companies, research institutes, and the educational sector to rapidly transfer their well-established conventional LC into sustainable LC methods and eliminate the use of toxic organic solvents in the mobile phase that is harmful to the environment and humans. The awareness on solvent and instrument energy consumption should also encourage the use of high separation efficiency columns that can allow fast analysis with reduced energy consumption and lower carbon footprint. The paper also aims to discourage the use of intensively power-consuming old liquid chromatographic instrumentation.
2. Solvent Selection
2.1. Solvent Selection Guidelines
Several solvent selection guidelines like that of Pfizer, GSK, Sanofi, and the combined approach of the three (Pfizer, GSK, Sanofi), AstraZeneca, ETH Zurich approach, Rowan University approach, ACS GCI solvent selection guide, International Council for Harmonization (ICH) Q3C (R8) guidelines, and CHEM21 guide were published for ranking and rating organic solvents according to their environmental, health and safety (EHS) problems considering similar or sometimes different criteria where solvents appeared sometimes with different ranking priorities [
26,
27,
28,
29]. All probably lack the emphasis on sustainable solvents for liquid chromatographic analysis. In fact, solvent selection guidelines to rank solvents based on their greenness are mainly established with orientation to the use of the solvent in synthesis and might be biased when considering their use for chromatographic analysis. For instance, according to CHEM21 solvent selection guideline, the environmental (E) profile for dihydrolevoglucosenone (Cyrene) is scored 7 and assigned as a problematic solvent because of the high boiling point and thus the difficulty of evaporating when used in synthesis. However, this high boiling point is considered advantages when thinking about its suitability for chromatographic analysis because it makes it easy and inexpensive to recycle and allows the possibility to run heated and superheated liquid chromatography. Therefore, the ranking for chromatography can be reversed depending on liquid chromatographic suitability. An ideal disadvantage-free completely sustainable organic solvent for LC analysis is still unavailable. Based on the EHS environmental, health, and safety index a favourable green solvent for chromatographic analysis is the one that can be produced from biomass routes with low energy and low cost compared to petrochemical routes and the one that is also biodegradable. Biobased solvents should whenever possible be integrated in liquid chromatographic analysis to enhance sustainability. For instant Cyrene is an organic solvent that is available as bio-based chemical from renewable feedstock and has shown promising potential for use as organic solvent in chromatography [
30] as represented in
Figure 4.
2.2. G-Score
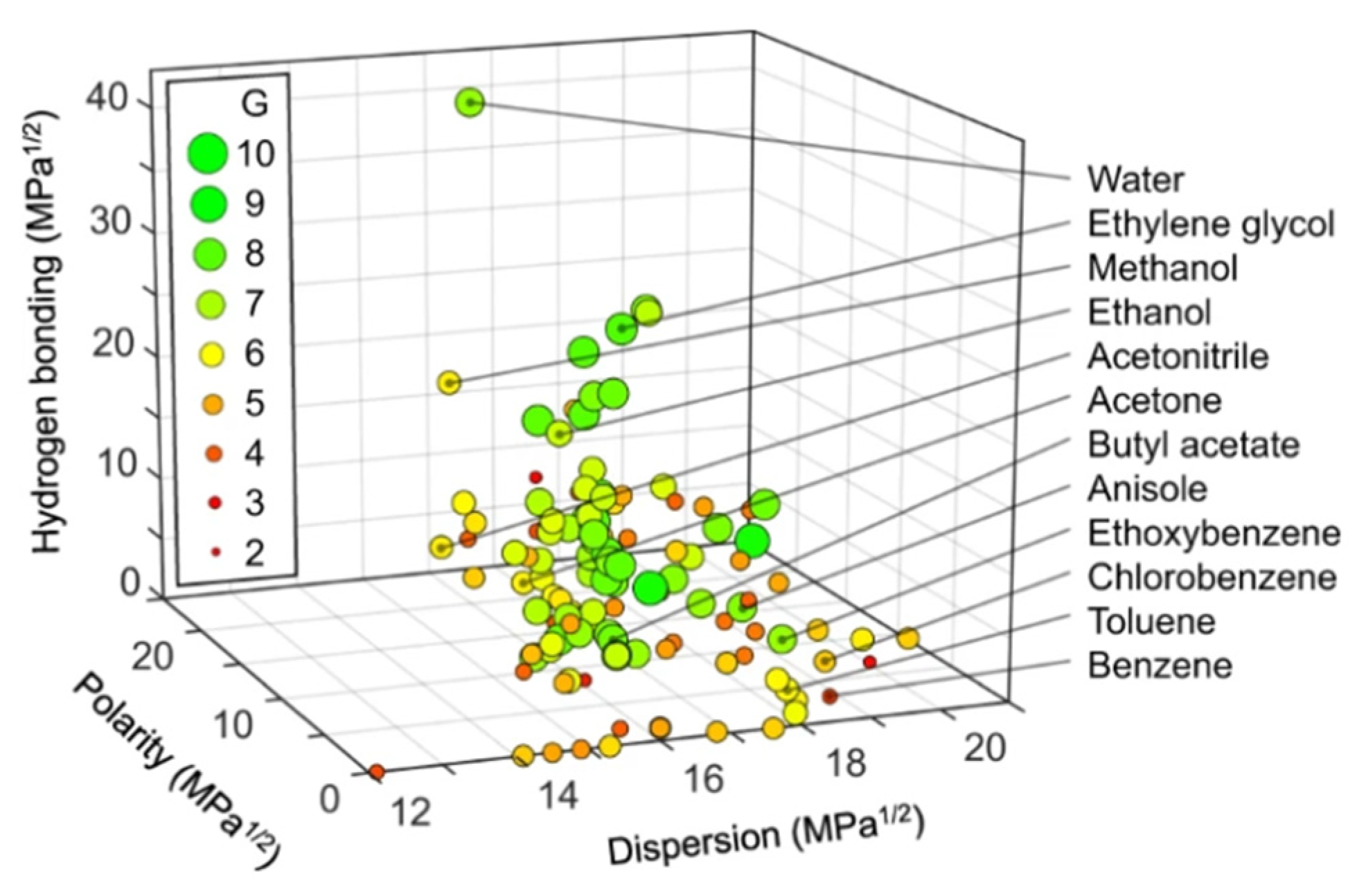
Hansen space for solvent selection evaluates solvent greening based on Hansen Solubility Parameters (HSP) [
31]. The score of solvent systems with their GSK greenness (G) Score is available freely as a web tool in the Hansen Space with a G score graph under
http://green-solvent-tool.herokuapp.com as shown in
Figure 5.
The highest G score value is 10 to indicate a fully eco-friendly solvent. In practice, most common liquid chromatography green solvents have a G score between 6 and 8. Solvents with a G score below 6 are not preferred in green chromatography. Propylene carbonate still has the highest G score as an LC green organic solvent with a value of 8.8. However, propylene carbonate suffers among other disadvantages like pressure fluctuation and high viscosity, from low water solubility and thus miscibility with aqueous mobile phase portion. This could be improved by the mixed solvent concept through adding another more soluble green co-eluent as ethanol (in a tertiary mobile phase system) to improve the solubility [
32]. The author of this manuscript calculated the G score value of Cyrene which has been recently proposed as green organic solvent for chromatographic application by El Deeb et al. [
33].
The G score of Cyrene is not readily available in the free web of Hansen space but has been calculated according to the following equations [
35] considering Health (H), Safety (S), Environment (E) and Waste Disposal (W) categories of the GSK's Solvent Sustainability Guide shown in
Table 1 [
36]:
Where H category includes the subcategories Health Hazard (HH) and Exposure Potential (EP) and can be calculated using the solvent values in the GSK solvent guide (in
Table 1) according to the flowing equation
and S category represents the safety category that includes the subcategories Flammability & Explosion (F&E) and Reactivity & Stability (R&S) and can be calculated using the solvent values in the GSK solvent guide (in
Table 1) according to the following equation.
and E category to represent the Environmental category with subcategories Air impact (Air) and Aqueous impact (Aqua) can be calculated using the solvent values in the GSK solvent guide (in
Table 1) according to the following equation.
and W category to represent waste Disposal with the subcategories Incineration (I), Recycling (R), Bio Treatment (BT) and Volatile organic compounds (VOC) can be calculated using the solvent values in the GSK solvent guide (in
Table 1) according to the following equation
If unavailable one can calculate the G-score of any organic solvent based on information mentioned in the GSK solvent sustainability guide according to the above equation.
2.3. Relative Hazard
The relative hazard indicates the chemical hazard of the substance (in this case the organic solvent) relative to the chemical hazard of chloroform (CH
sub/CH
CHCl3). Relative hazard could be used to indicate the degree of chemical risk associated with the use of solvent thus a smaller value indicates a greener solvent. The chemical hazard of chloroform (CH
CHCl3) equals 5.75. A simple model called weight hazard number (WHN) can be used to calculate the chemical hazard of the substance. According to WHN model, the chemical hazard of a substance is calculated based on the following equation.
where
Ncat is the number of hazards of a given category according to safety data sheet (SDS) of the substance (solvent).
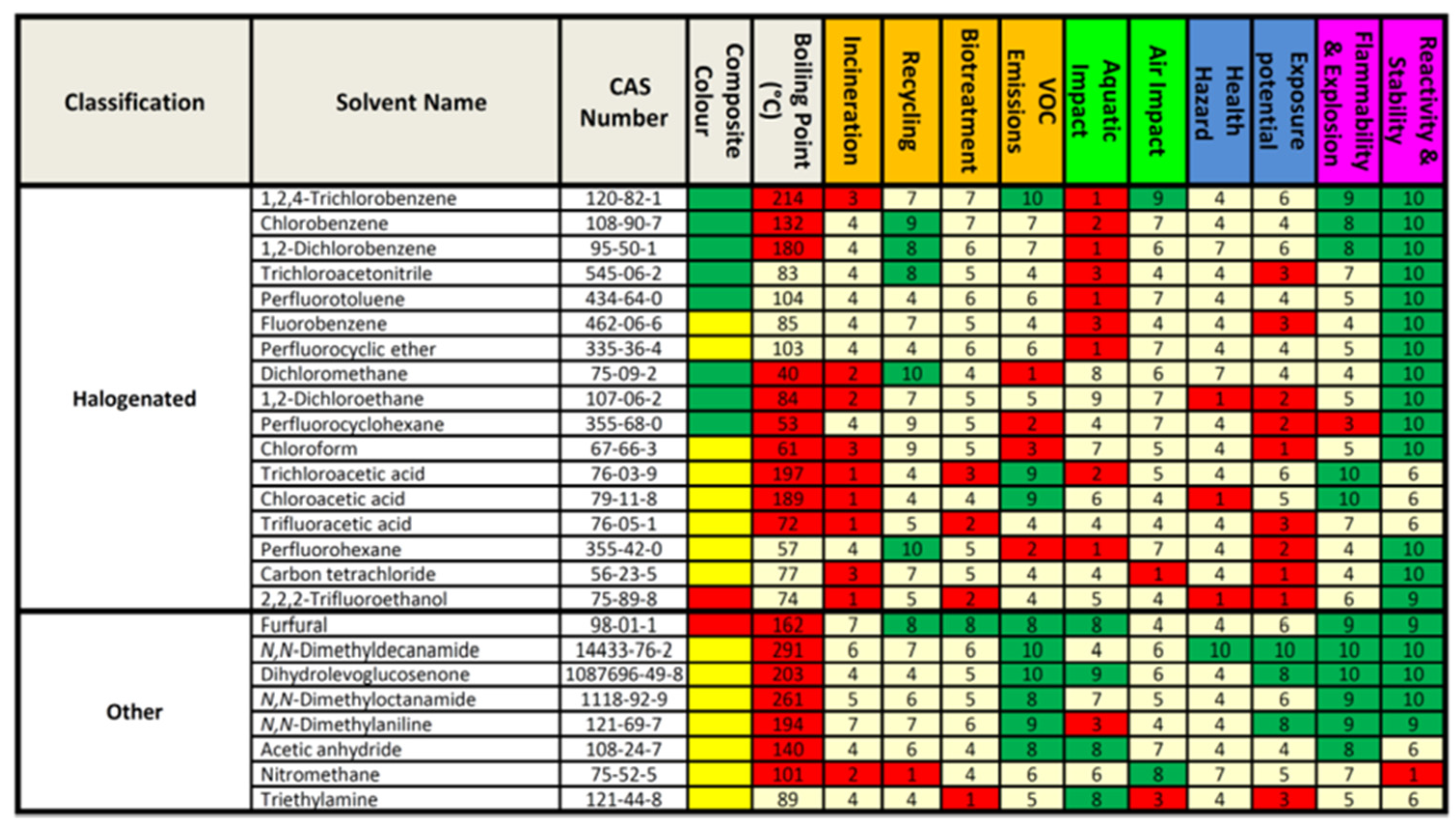
Values of Chemical Hazards according to WHN for each of the common solvents are provided in
Table 2 either obtained from reference [
37] or calculated by the author based on recent SDS category values of each solvent. It is worth noting that the relative hazard can be multiplied by the mass of the substance to give what is referred to as Chloroform-oriented Toxicity Estimation Scale (ChlorTox Scale) based on the following equation to act as indicator for chemical risk [
37].
2.4. Consideration of Chromatographic Suitability
When choosing an organic solvent for use as a component in the mobile phase of liquid chromatographic analysis more information is required about the solvent than its greenness score to judge its suitability for the analysis to substitute the traditional hazardous organic solvent in the reference method. Some factors are not considered in either G-score or Relative hazards but are LC-relevant and can play a good role in selecting a green organic solvent for chromatographic applications. These factors include compatibility with the detector, miscibility of the organic solvent with the aqueous phase of the mobile phase, elution power, density, boiling point, and purity.
The primary detection method in HPLC and UHPLC is UV/Vis spectrometry thus the transparency of the solvent in this region accounts for its advantages as a green solvent otherwise its applicability will be limited to substances that can absorb beyond the ultraviolet (UV)/visible cut-off value of the solvent. Compatibility with other common detection techniques like mass spectrometry and fluorescence detection is of advantage. Therefore, the compatibility with the used detectors should be known. Actually, UV transparency is an important limiting factor to the implementation of a new green organic solvent in various chromatographic application. The narrower the transparency range the less the possibility to apply for a wide range of substances that absorb only out of this transparency range or have very weak absorbance within the transparency range that did not fulfil the required sensitivity for the intended application.
It is important to consider the miscibility of the green organic solvent with the aqueous component of the mobile phase when it substitutes the old organic solvent. In case of very low solubility, a co-eluent may be added in small amounts to improve the solubility. Otherwise, an alternative green solvent should be tried. It is worth noting that solubility is somewhat involved in greenness consideration. In general, low water solubility and high Log P value indicates high bioaccumulation and aquatic toxicity. It is important also to consider the solving power of the solvent to solubilize analytes depending on their polarity. The Elution Power of a newly implemented organic solvent in LC should also be considered as well as its compatibility with different stationary phases for normal and reversed phase chromatography. Showing a similar selectivity and retention behavior to the toxic organic solvent in replacement would make method transfer easier. A primary impression about the elution power of the new green solvent compared to the old toxic solvent can be expected by comparing the polarity parameter Kamlet-Taft (π*) values [
38]. The high density of the organic solvent should be considered in view of the developed back pressure. As mentioned before the boiling point of the organic solvent for chromatographic application is preferably to be high to facilitate waste treatment and offer the possibility for high-temperature separation [
39].
Green solvents assigned and ranked for synthesis or purification require less purity than solvents for chromatographic analysis where the presence of impurities as contaminant elements might hinder its application through reactivity with analytes, non-transparency in detection, and fluctuation with non-smooth baseline. This should be an issue to consider when trying to implement a new green solvent for the application in chromatographic analysis. The comprehensive testing of new potential green organic solvents for chromatographic analysis is essential to advance the field of sustainable analytical chemistry.
In
Table 2 the author of this manuscript listed properties and parameters of 17 solvents for normal, HILIC and reversed phase liquid chromatography taking in account suitability parameters for liquid chromatographic analysis in addition to greenness. The suitability requirements should be balanced against greening requirements to choose the best solvent for the intended application. The values for each solvent are based on the solvent data sheet SDS, G- Score, and relative hazards resources. Subjected to a future update with more solvents,
Table 2 should act as a current collated solvent selection guide for liquid chromatography.
Certain parameters have been particularly mentioned in
Table 2 to give a rapid indication of the health and safety of the solvent. For instance, a value lower than 2000 mg/kg of the health measure rat oral LED50 can indicate a harmful solvent according to European Parliament and Council Regulation (EC) No 1272/2008 [
40]. The vapor pressure of the solvent can reflect its volatility and thus its ozone-depleting potential. Substances with high vapor pressure will vaporize more readily as stated by the United States Environmental Protection Agency. A vapor pressure of 10 hPa at 20 ◦C or more (0.01 kPa at 293.15 K or more) represents a VOC ozone-affecting solvent [
41]. The WHO classified inorganic pollutants as very volatile, volatile, and semi-volatile organic compounds also depending on the boiling point, a low boiling point indicates a more volatile organic compound [
42]. The flash point as a critical measure of flammability shows the lowest temperature at which the substance can vaporize to form an ignitable mixture also gives a rapid indication of the solvent safety and should be above 60°C [
43]. A high Partition coefficient n-octanol/water (log P value) value of more than 4 indicates high lipophilicity and bio-accumulation potential [
44].
2.5. Liquid Chromatography Sustainability Guideline
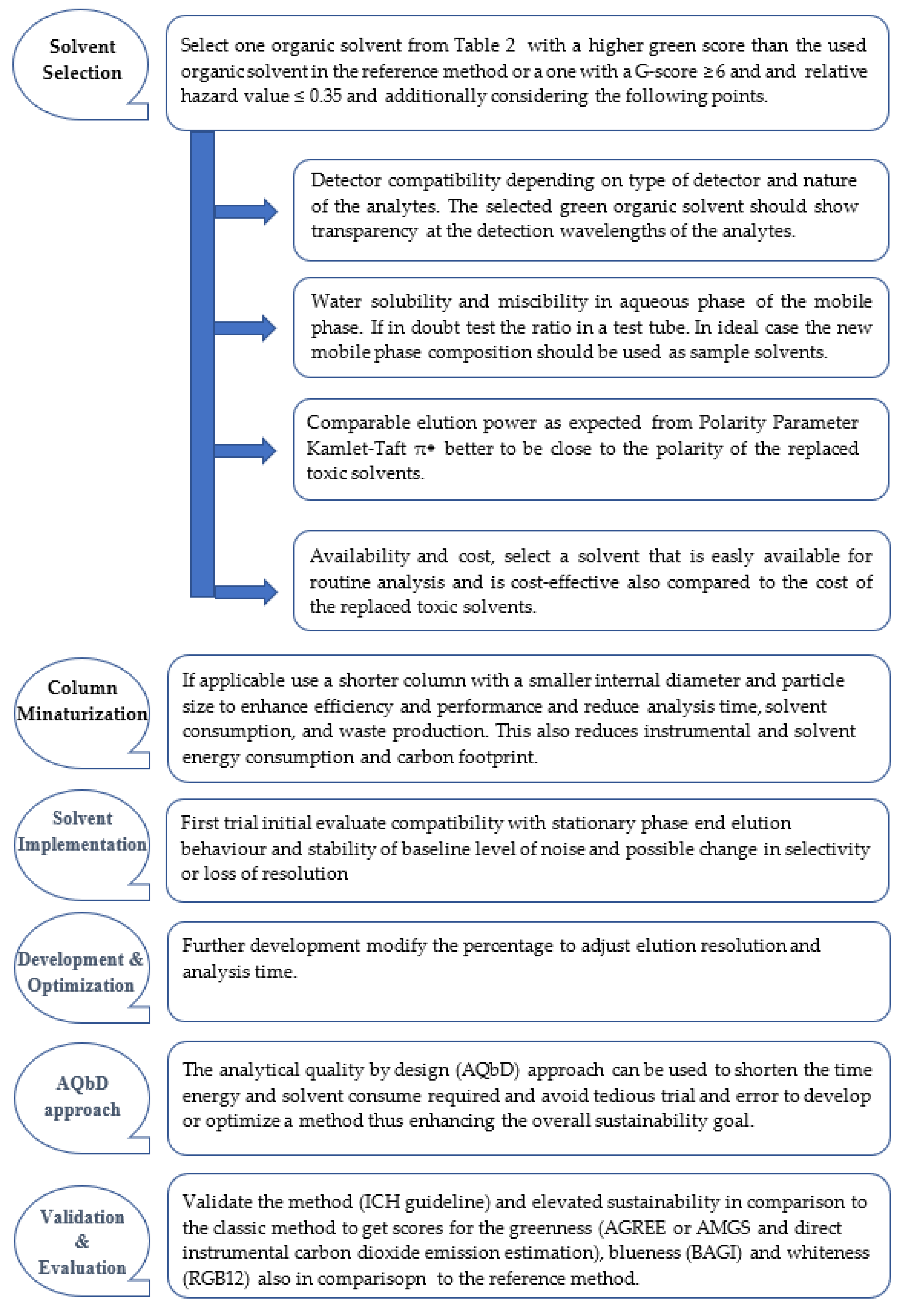
The following chart represents a simple guide to transfer traditional classical non-green LC method into a greener more sustainable LC method by organic solvent replacement and whenever applicable by changing to a higher separation efficiency column (chart 1). It is worth noting that greening the sample preparation method if applicable using the same green liquid as the sample solvent would further enhance the overall method green score. The suggested transformation just concentrates on eliminating the toxic organic solvent by green alternative solvent replacement and possibly smaller columns to reduce solvent consumption, waste production, and analysis time. There is an economic and environmental benefit associated with organic solvent waste reduction in analysis. In ideal cases, organic solvent should be eliminated whenever possible like in the case of transferring to heated or superheated water chromatography [
45]. This could also be applied to high boiling point liquids like Cyrene as suggested by El Deeb et al. [
33].
It is worth mentioning that the greener replacement solvent should not be of significant larger volume than the replaced toxic solvent to avoid increasing the overall use of organic solvent in the method which negatively impacts the greenness profile. In some cases, green solvents could be worse because of the significantly larger volume required to replace the toxic solvent. The strategy in
Chart 1 acts as a greening guideline in help with
Table 2 that would help to subjectively choose a proper green solvent and implement it in the LC method to elevate its analysis sustainability.
3. Post-Greening Method Evaluation
It is advisable to assess the greening profile of the developed or transformed green method. The evaluation tools would demonstrate the superiority of a proposed green method over a traditional reported method.
3.1. Greening Evaluation
Many recently developed analytical chromatographic methods fail to meet green analytical criteria. Several evaluation tools have gained significant recognition and acceptance within the analytical chemical society mostly using a friendly shareware software that generates a colored pictogram in some of them with a quantitative numerical value in percentage where 100 % reflects full alignment [
46,
47].
Here are two recommended tools to use in evaluating the greenness of your method. The first is AGREE, which stands for Analytical GREEnness Metric Approach and Software considering the 12 principles of green analytical chemistry and presented as a pictogram with a score in the middle and green yellow, and red colors for each segment to indicate the agreement level with the greenness principle. It is an easy-to-use, user-friendly software with a simple illustrative colored pictogram. It represents a comprehensive well-recognized tool commonly used to evaluate greenness and compare methods after transformation from classical to green [
48,
49].
The second is referred to as AMGS and stands for Analytical Method Greenness Score. Is a tool to compare method greenness considering three main issues namely instrument energy, solvent energy (energy demand associated with solvent production and incineration for disposal), and solvent EHS aspects [
50]. It is an open-source spreadsheet calculator is available online at
https://www.acsgcipr.org/amgs. The lower the overall score of AMGS the greener the method. The detailed scores of greenness percentage will be given for each of the three components instrument energy, solvent energy, and EHS.
It is worth noting that, the carbon footprint associated with the use of HPLC or UHPLC instrument can be directly calculated according to the following equation to get a value for kilogram carbon dioxide equivalent (carbon footprint) per analysis.
The reference constant value for the emission factor is 0.247 kg CO2/kWh. Instrument power differs depending on the analytical instrument [
51].
3.2. Blueness Evaluation
The Blue Applicability Grade Index (BAGI) is a free available software available under
https://Bagi-Index.Anvil.App. It involves involving 10 questions with variable choices of each to evaluate the practicability of the method. The software evaluates practicability aspects and ease of application including the type of analysis, number of elements, the analytical technique, the sample preparation, number of samples analyzed per hour, reagents, pre-concentration, degree of automation, and amount of sample [
52].
3.3. Whiteness Evaluation
The whiteness of the method can best be evaluated using the RGB12 tool with the freely available Excel sheet to evaluate the three components each with 4 columns aspects. The red component with its 4 aspects covering the scope of application, LOD and LOQ, precision, and accuracy. The green component the 4 aspects namely toxicity of reagent, amount of reagent and waste, consumption of energy and other media, and direct impact. The blue component with its 4 aspects mainly costs-efficiency, time-efficiency, sample consumption, and need for advanced instruments and operational simplicity. By filling the required data in each component, a graphical presentation of red, green, and blue columns with a white column saturation depending on the relative fill of each component will be presented to indicate the percentage of method whiteness [
53].
4. Conclusion
Green and white analytical chemistry are currently gaining significant attention to support the general global move toward sustainability. Analysts aim to move toward a more sustainable future in analytical chemistry that can be implemented in routine analytical work. For instant routine quality control of pharmaceuticals should in future be conducted as sustainable quality control with energy efficient practice and with a minimal environmental burden. Analysis of real pharmaceutical mixtures and bio-analytical application in drug monitoring and forensic investigations should also be conducted with energy efficient cost-effective methods. Currently applied analytical methods still depend on the use of hazardous organic solvents that contraverse method greenness. The use of easily available inexpensive reagents and the simplicity of the method with the possible elimination of laborious steps such as preconcentration derivatization or complex gradient program should also be considered to support the practicability of the method. A handful of alternative organic solvents for chromatographic elutions are demonstrating superiority over routinely used hazardous organic solvents in term of greenness. It is worth noting that any sustainable analytical method could undergo further optimization to elevate its sustainability profiling without sacrificing practicability and method performance. The article should increase the analytical method sustainability awareness. This should enhance the sustainable practice in analytical chemistry using HPLC and UHPLC instruments which are dominant in analysis with cost-effective energy-efficient, eco-friendly methods that reduce carbon dioxide emission and minimize waste production. Reducing carbon footprint and VOC can positively contribute to reducing global warming. The author expects that this paper will provide good insight into the implementation of sustainable analytical chromatography in industrial, research, and educational fields. Sustainable analytical chemistry is currently in increasing practice and will have a crucial role in the near future to maintain sustainability and to contribute more to sustainable development.
Funding
This research received no external funding.
Acknowledgments
Merck Life Science KGaA, Darmstadt, Germany, for kindly supporting research activities on sustainable analytical chemistry.
Conflicts of Interest
The authors declare no conflicts of interest.”.
References
- Wennersten, R.; Qie, S. United Nations Sustainable Development Goals for 2030 and Resource Use; 2018; pp. 317–339. [Google Scholar]
- Green Chemistry By Paul, T. Anastas and John C. Warner. Oxford University Press: Oxford. 2000. Paperback. 135 Pp. £14.99. ISBN 0-19-850698-9. Org Process Res Dev 2000, 4. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC Trends in Analytical Chemistry 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Cizmarova, I.; Parrak, V.; Secnik jr, P.; Secnik, P.; Sopko, L.; Nemergutova, K.; Kovac, A.; Mikus, P.; Piestansky, J. A Simple and Green Capillary Electrophoresis-Mass Spectrometry Method for Therapeutic Drug Monitoring of Colistin in Clinical Plasma Samples. Heliyon 2023, 9, e23111. [Google Scholar] [CrossRef]
- Deeb, S. El; Wätzig, H.; El-Hady, D.A. Capillary Electrophoresis to Investigate Biopharmaceuticals and Pharmaceutically-Relevant Binding Properties. TrAC Trends in Analytical Chemistry 2013, 48, 112–131. [Google Scholar] [CrossRef]
- Datta, S.; Ghosh Auddy, R.; De, A. Supercritical Fluid Chromatography: A Green Approach for Separation and Purification of Organic and Inorganic Analytes. In Green Chromatographic Techniques; Springer Netherlands: Dordrecht, 2014; pp. 55–80. [Google Scholar]
- Ali, M.F.B.; Saraya, R.E.; El Deeb, S.; Ibrahim, A.E.; Salman, B.I. An Innovative Polymer-Based Electrochemical Sensor Encrusted with Tb Nanoparticles for the Detection of Favipiravir: A Potential Antiviral Drug for the Treatment of COVID-19. Biosensors (Basel) 2023, 13, 243. [Google Scholar] [CrossRef]
- Yabré, M.; Ferey, L.; Somé, I.; Gaudin, K. Greening Reversed-Phase Liquid Chromatography Methods Using Alternative Solvents for Pharmaceutical Analysis. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Solomon, K.R.; Tang, X.; Wilson, S.R.; Zanis, P.; Bais, A.F. Changes in Tropospheric Composition and Air Quality Due to Stratospheric Ozone Depletion. Photochemical & Photobiological Sciences 2003, 2, 62–67. [Google Scholar] [CrossRef]
- Taylor, D.A. Principles into Practice Setting the Bar for Green Chemistry. Environ Health Perspect 2010, 118. [Google Scholar] [CrossRef]
- Loste, N.; Roldán, E.; Lomba, L.; Giner, B. Green Chemistry and Environmental Management Systems: Relationships, Synergies, Advantages and Barriers of Joint Implementation at Universities. Environ Manage 2019, 64, 783–793. [Google Scholar] [CrossRef]
- Brice, R.W.; Zhang, X.; Colón, L.A. Fused-core, Sub-2 Μm Packings, and Monolithic HPLC Columns: A Comparative Evaluation. J Sep Sci 2009, 32, 2723–2731. [Google Scholar] [CrossRef]
- Nowak, P.M.; Bis, A.; Rusin, M.; Woźniakiewicz, M. Carbon Footprint of the Analytical Laboratory and the Three-Dimensional Approach to Its Reduction. Green Analytical Chemistry 2023, 4, 100051. [Google Scholar] [CrossRef]
- Ibrahim, A.E.; Abd Elmonem, H.M.; Al-Harrasi, A.; El Deeb, S. Comparative Evaluation of Reversed Stationary Phase Geometries and Greener Systems on HPLC and UHPLC Using Five Recent Hepatitis-C Antivirals. J AOAC Int 2023, 106, 580–587. [Google Scholar] [CrossRef]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue Applicability Grade Index (BAGI) and Software: A New Tool for the Evaluation of Method Practicality. Green Chemistry 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An Approach to Reconcile the Principles of Green Analytical Chemistry and Functionality. TrAC Trends in Analytical Chemistry 2021, 138. [Google Scholar] [CrossRef]
- Hussain, C.M.; Hussain, C.G.; Keçili, R. White Analytical Chemistry Approaches for Analytical and Bioanalytical Techniques: Applications and Challenges. TrAC Trends in Analytical Chemistry 2023, 159. [Google Scholar] [CrossRef]
- de la Guardia, M.; Garrigues, S. The Concept of Green Analytical Chemistry. In Handbook of Green Analytical Chemistry; Wiley, 2012; pp. 1–16. [Google Scholar]
- Sajid, M.; Płotka-Wasylka, J. Green Analytical Chemistry Metrics: A Review. Talanta 2022, 238, 123046. [Google Scholar] [CrossRef]
- Al-Shatti, B.J.; Alsairafi, Z.; Al-Tannak, N.F. Green Chemistry and Its Implementation in Pharmaceutical Analysis. Rev Anal Chem 2023, 42. [Google Scholar] [CrossRef]
- Turner, C. Sustainable Analytical Chemistry—More than Just Being Green. Pure and Applied Chemistry 2013, 85, 2217–2229. [Google Scholar] [CrossRef]
- Hussain, C.M.; Hussain, C.G.; Keçili, R. White Analytical Chemistry Approaches for Analytical and Bioanalytical Techniques: Applications and Challenges. TrAC Trends in Analytical Chemistry 2023, 159, 116905. [Google Scholar] [CrossRef]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An Approach to Reconcile the Principles of Green Analytical Chemistry and Functionality. TrAC Trends in Analytical Chemistry 2021, 138, 116223. [Google Scholar] [CrossRef]
- Psillakis, E.; Pena-Pereira, F. The Twelve Goals of Circular Analytical Chemistry. TrAC Trends in Analytical Chemistry 2024, 175, 117686. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC Trends in Analytical Chemistry 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and Techniques for Solvent Selection: Green Solvent Selection Guides. Sustainable Chemical Processes 2016, 4, 7. [Google Scholar] [CrossRef]
- Diorazio, L.J.; Hose, D.R.J.; Adlington, N.K. Toward a More Holistic Framework for Solvent Selection. Org Process Res Dev 2016, 20, 760–773. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 Selection Guide of Classical- and Less Classical-Solvents. Green Chemistry 2016, 18, 288–296. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Impurities: Guideline for Residual Solvents Q3C(R8).
- El Deeb, S.; Abdelsamad, K.; Parr, M.K. Greener and Whiter Analytical Chemistry Using Cyrene as a More Sustainable and Eco-Friendlier Mobile Phase Constituent in Chromatography. Pharmaceuticals 2023, 16, 1488. [Google Scholar] [CrossRef]
- Benazzouz, A.; Moity, L.; Pierlot, C.; Sergent, M.; Molinier, V.; Aubry, J.-M. Selection of a Greener Set of Solvents Evenly Spread in the Hansen Space by Space-Filling Design. Ind Eng Chem Res 2013, 52, 16585–16597. [Google Scholar] [CrossRef]
- Fayaz, T.K.S.; Chanduluru, H.K.; Obaydo, R.H.; Sanphui, P. Propylene Carbonate as an Ecofriendly Solvent: Stability Studies of Ripretinib in RPHPLC and Sustainable Evaluation Using Advanced Tools. Sustain Chem Pharm 2024, 37, 101355. [Google Scholar] [CrossRef]
- El Deeb, S.; Abdelsamad, K.; Parr, M.K. Greener and Whiter Analytical Chemistry Using Cyrene as a More Sustainable and Eco-Friendlier Mobile Phase Constituent in Chromatography. Pharmaceuticals 2023, 16, 1488. [Google Scholar] [CrossRef]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and Further Expanding GSK’s Solvent Sustainability Guide. Green Chemistry 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Larsen, C.; Lundberg, P.; Tang, S.; Ràfols-Ribé, J.; Sandström, A.; Mattias Lindh, E.; Wang, J.; Edman, L. A Tool for Identifying Green Solvents for Printed Electronics. Nat Commun 2021, 12, 4510. [Google Scholar] [CrossRef]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and Further Expanding GSK’s Solvent Sustainability Guide. Green Chemistry 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Nowak, P.M.; Bis, A.; Zima, A. ChlorTox Base – a Useful Source of Information on Popular Reagents in Terms of Chemical Hazards and Greenness Assessment. Green Analytical Chemistry 2023, 6, 100065. [Google Scholar] [CrossRef]
- Islam, T.; Islam Sarker, Md.Z.; Uddin, A.H.; Yunus, K. Bin; Prasad, R.; Mia, Md.A.R.; Ferdosh, S. Kamlet Taft Parameters: A Tool to Alternate the Usage of Hazardous Solvent in Pharmaceutical and Chemical Manufacturing/Synthesis - A Gateway towards Green Technology. Analytical Chemistry Letters 2020, 10, 550–561. [Google Scholar] [CrossRef]
- Guillarme, D.; Heinisch, S.; Rocca, J.L. Effect of Temperature in Reversed Phase Liquid Chromatography. J Chromatogr A 2004, 1052, 39–51. [Google Scholar] [CrossRef]
- EUR-Lex Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006.
- United States Environmental Protection Agency Technical Overview of Volatile Organic Compounds.
- World Health Organization Indoor Air Quality: Organic Pollutants. Environmental Technology Letters 1989, 10, 855–858. [CrossRef]
- Flinders Shire Council Classification Of Flammable And Combustible Liquids.
- Lombardo, A.; Roncaglioni, A.; Boriani, E.; Milan, C.; Benfenati, E. Assessment and Validation of the CAESAR Predictive Model for Bioconcentration Factor (BCF) in Fish. Chem Cent J 2010, 4, S1. [Google Scholar] [CrossRef]
- Dembek, M.; Bocian, S. Pure Water as a Mobile Phase in Liquid Chromatography Techniques. TrAC Trends in Analytical Chemistry 2020, 123, 115793. [Google Scholar] [CrossRef]
- Kowtharapu, L.P.; Katari, N.K.; Muchakayala, S.K.; Marisetti, V.M. Green Metric Tools for Analytical Methods Assessment Critical Review, Case Studies and Crucify. TrAC Trends in Analytical Chemistry 2023, 166, 117196. [Google Scholar] [CrossRef]
- Sinzervinch, A.; Torres, I.M.S.; Kogawa, A.C. Tools to Evaluate the Eco-Efficiency of Analytical Methods in the Context of Green and White Analytical Chemistry: A Review. Curr Pharm Des 2023, 29, 2442–2449. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal Chem 2020, 92. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Tobiszewski, M.; Wojnowski, W.; Psillakis, E. A Tutorial on AGREEprep an Analytical Greenness Metric for Sample Preparation. Advances in Sample Preparation 2022, 3, 100025. [Google Scholar] [CrossRef]
- Hicks, M.B.; Farrell, W.; Aurigemma, C.; Lehmann, L.; Weisel, L.; Nadeau, K.; Lee, H.; Moraff, C.; Wong, M.; Huang, Y.; et al. Making the Move towards Modernized Greener Separations: Introduction of the Analytical Method Greenness Score (AMGS) Calculator. Green Chemistry 2019, 21. [Google Scholar] [CrossRef]
- Ballester-Caudet, A.; Campíns-Falcó, P.; Pérez, B.; Sancho, R.; Lorente, M.; Sastre, G.; González, C. A New Tool for Evaluating and/or Selecting Analytical Methods: Summarizing the Information in a Hexagon. TrAC Trends in Analytical Chemistry 2019, 118. [Google Scholar] [CrossRef]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue Applicability Grade Index (BAGI) and Software: A New Tool for the Evaluation of Method Practicality. Green Chemistry 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Hussain, C.M.; Hussain, C.G.; Keçili, R. White Analytical Chemistry Approaches for Analytical and Bioanalytical Techniques: Applications and Challenges. TrAC Trends in Analytical Chemistry 2023, 159, 116905. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).