Submitted:
11 June 2024
Posted:
12 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. ALK
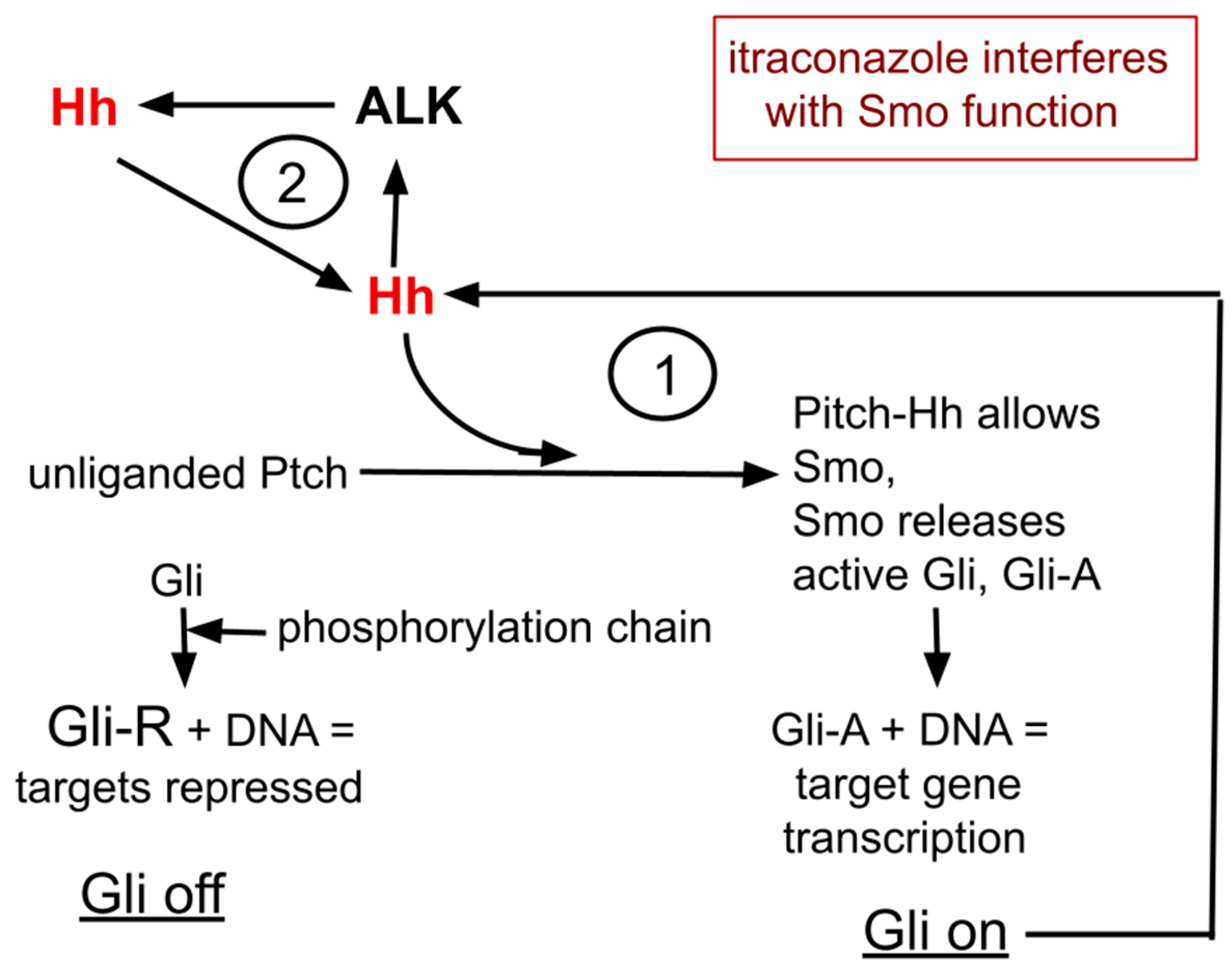
3. Hedgehog
4. ALK and Hh form a Cyclic Amplifying System
5A. Hh and the Repurposed Drug Itraconazole
5B. Itraconazole Caveats
5C. Additional Potential Benefits of Itraconazole
6. Repurposed Drug Cilostazol
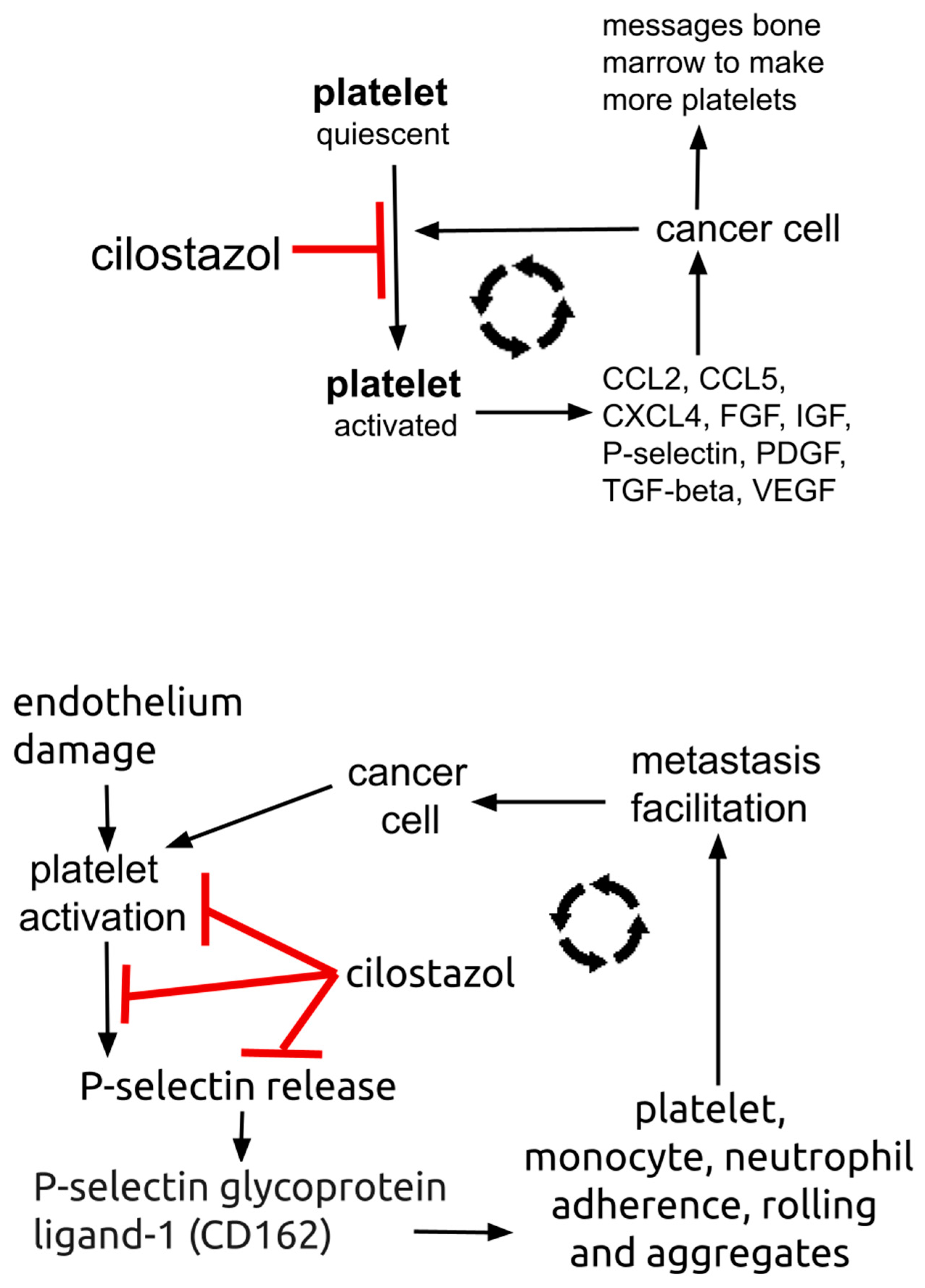
6.1. Cilostazol
6.2. Trophic Function of Platelets in Cancer:
7. Discussion
8. Conclusions
References
- Poei, D.; Ali, S.; Ye, S.; Hsu, R. ALK inhibitors in cancer: mechanisms of resistance and therapeutic management strategies. Cancer Drug Resist. 2024, 7, 20. [Google Scholar] [CrossRef]
- Gemelli, M.; Albini, A.; Catalano, G.; Incarbone, M.; Cannone, M.; Balladore, E.; Ricotta, R.; Pelosi, G. Navigating resistance to ALK inhibitors in the lorlatinib era: a comprehensive perspective on NSCLC. Expert Rev. Anticancer. Ther. 2024, 1–15. [Google Scholar] [CrossRef]
- Fabbri, L.; Di Federico, A.; Astore, M.; Marchiori, V.; Rejtano, A.; Seminerio, R.; Gelsomino, F.; De Giglio, A. From Development to Place in Therapy of Lorlatinib for the Treatment of ALK and ROS1 Rearranged Non-Small Cell Lung Cancer (NSCLC). Diagnostics 2023, 14, 48. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Xia, Q.; Liu, K.; Dong, Z. New perspectives for targeting therapy in ALK-positive human cancers. Oncogene 2023, 42, 1959–1969. [Google Scholar] [CrossRef]
- Schneider, J.L.; Lin, J.J.; Shaw, A.T. ALK-positive lung cancer: a moving target. Nat. Cancer 2023, 4, 330–343. [Google Scholar] [CrossRef]
- Li, W.; Sparidans, R.W.; Wang, Y.; Lebre, M.C.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. P-glycoprotein (MDR1/ABCB1) restricts brain accumulation and cytochrome P450-3A (CYP3A) limits oral availability of the novel ALK/ROS1 inhibitor lorlatinib. Int. J. Cancer 2018, 143, 2029–2038. [Google Scholar] [CrossRef]
- Baba, K.; Goto, Y. Lorlatinib as a treatment for ALK-positive lung cancer. Futur. Oncol. 2022, 18, 2745–2766. [Google Scholar] [CrossRef]
- Bauer, T.M.; Felip, E.; Solomon, B.J.; Thurm, H.; Peltz, G.; Chioda, M.D.; Shaw, A.T. Clinical Management of Adverse Events Associated with Lorlatinib. Oncol. 2019, 24, 1103–1110. [Google Scholar] [CrossRef]
- Kilickap, S.; Ak, S.; Dursun, O.U.; Sendur, M.A.; Karadurmus, N.; Demirci, U. Safety of lorlatinib in ALK-positive non-small-cell lung cancer and management of central nervous system adverse events. Futur. Oncol. 2023, 19, 2003–2012. [Google Scholar] [CrossRef]
- Bergaggio, E.; Tai, W.-T.; Aroldi, A.; Mecca, C.; Landoni, E.; Nüesch, M.; Mota, I.; Metovic, J.; Molinaro, L.; Ma, L.; et al. ALK inhibitors increase ALK expression and sensitize neuroblastoma cells to ALK.CAR-T cells. Cancer Cell 2023, 41, 2100–2116. [Google Scholar] [CrossRef]
- Berger, L.-A.; Janning, M.; Velthaus, J.-L.; Ben-Batalla, I.; Schatz, S.; Falk, M.; Iglauer, P.; Simon, R.; Cao, R.; Forcato, C.; et al. Identification of a High-Level MET Amplification in CTCs and cfTNA of an ALK-Positive NSCLC Patient Developing Evasive Resistance to Crizotinib. J. Thorac. Oncol. 2018, 13, E243–E246. [Google Scholar] [CrossRef]
- Costa, D.B. Resistance to ALK inhibitors: Pharmacokinetics, mutations or bypass signaling? Cell Cycle 2016, 16, 19–20. [Google Scholar] [CrossRef]
- Cooper, A.J.; Sequist, L.V.; Lin, J.J. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat. Rev. Clin. Oncol. 2022, 19, 499–514. [Google Scholar] [CrossRef]
- Kast, R.E.; Alfieri, A.; Assi, H.I.; Burns, T.C.; Elyamany, A.M.; Gonzalez-Cao, M.; Karpel-Massler, G.; Marosi, C.; Salacz, M.E.; Sardi, I.; et al. MDACT: A New Principle of Adjunctive Cancer Treatment Using Combinations of Multiple Repurposed Drugs, with an Example Regimen. Cancers 2022, 14, 2563. [Google Scholar] [CrossRef]
- Halatsch, M.-E.; Kast, R.E.; Karpel-Massler, G.; Mayer, B.; Zolk, O.; Schmitz, B.; Scheuerle, A.; Maier, L.; Bullinger, L.; Mayer-Steinacker, R.; et al. A phase Ib/IIa trial of 9 repurposed drugs combined with temozolomide for the treatment of recurrent glioblastoma: CUSP9v3. Neuro-Oncol. Adv. 2021, 3, vdab075. [Google Scholar] [CrossRef]
- Reshetnyak, A.V.; Rossi, P.; Myasnikov, A.G.; Sowaileh, M.; Mohanty, J.; Nourse, A.; Miller, D.J.; Lax, I.; Schlessinger, J.; Kalodimos, C.G. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature 2021, 600, 153–157. [Google Scholar] [CrossRef]
- Huang, H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int. J. Mol. Sci. 2018, 19, 3448. [Google Scholar] [CrossRef]
- Katic, L.; Priscan, A. Multifaceted Roles of ALK Family Receptors and Augmentor Ligands in Health and Disease: A Comprehensive Review. Biomolecules 2023, 13, 1490. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Anaplastic lymphoma kinase (ALK): Structure, oncogenic activation, and pharmacological inhibition. Pharmacol. Res. 2013, 68, 68–94. [Google Scholar] [CrossRef]
- Choo, J.R.-E.; A Soo, R. Lorlatinib for the treatment of ALK-positive metastatic non-small cell lung cancer. Expert Rev. Anticancer. Ther. 2020, 20, 233–240. [Google Scholar] [CrossRef]
- Shaw, A.T.; Solomon, B.J.; Besse, B.; Bauer, T.M.; Lin, C.-C.; Soo, R.A.; Riely, G.J.; Ou, S.-H.I.; Clancy, J.S.; Li, S.; et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2019, 37, 1370. [Google Scholar] [CrossRef]
- Schönherr, C.; Ruuth, K.; Kamaraj, S.; Wang, C.-L.; Yang, H.-L.; Combaret, V.; Djos, A.; Martinsson, T.; Christensen, J.G.; Palmer, R.H.; et al. Anaplastic Lymphoma Kinase (ALK) regulates initiation of transcription of MYCN in neuroblastoma cells. Oncogene 2012, 31, 5193–5200. [Google Scholar] [CrossRef]
- Hasan, K.; Nafady, A.; Takatori, A.; Kishida, S.; Ohira, M.; Suenaga, Y.; Hossain, S.; Akter, J.; Ogura, A.; Nakamura, Y.; et al. ALK is a MYCN target gene and regulates cell migration and invasion in neuroblastoma. Sci. Rep. 2013, 3, 3450. [Google Scholar] [CrossRef]
- Neal, J.W.; Sequist, L.V. Exciting New Targets in Lung Cancer Therapy: ALK, IGF-1R, HDAC, and Hh. Curr. Treat. Options Oncol. 2010, 11, 36–44. [Google Scholar] [CrossRef]
- Brechbiel, J.; Miller-Moslin, K.; Adjei, A.A. Crosstalk between hedgehog and other signaling pathways as a basis for combination therapies in cancer. Cancer Treat. Rev. 2014, 40, 750–759. [Google Scholar] [CrossRef]
- Piteša, N.; Kurtović, M.; Bartoniček, N.; Gkotsi, D.S.; Čonkaš, J.; Petrić, T.; Musani, V.; Ozretić, P.; Galdo, N.A.R.-D.; Sabol, M. Signaling Switching from Hedgehog-GLI to MAPK Signaling Potentially Serves as a Compensatory Mechanism in Melanoma Cell Lines Resistant to GANT-61. Biomedicines 2023, 11, 1353. [Google Scholar] [CrossRef]
- Rovida, E.; Stecca, B. Mitogen-activated protein kinases and Hedgehog-GLI signaling in cancer: A crosstalk providing therapeutic opportunities? Semin. Cancer Biol. 2015, 35, 154–167. [Google Scholar] [CrossRef]
- Ok, C.Y.; Singh, R.R.; Vega, F. Aberrant Activation of the Hedgehog Signaling Pathway in Malignant Hematological Neoplasms. Am. J. Pathol. 2011, 180, 2–11. [Google Scholar] [CrossRef]
- Aberger F, Kern D, Greil R, Hartmann TN. Canonical and noncanonical Hedgehog/GLI signaling in hematological malignancies. Vitam Horm. 2012;88:25-54. [CrossRef]
- Skoda, A.M.; Simovic, D.; Karin, V.; Kardum, V.; Vranic, S.; Serman, L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn. J. Basic Med. Sci. 2018, 18, 8–20. [Google Scholar] [CrossRef]
- Stecca, B.; Mas, C.; Clement, V.; Zbinden, M.; Correa, R.; Piguet, V.; Beermann, F.; Ruiz, I.; Altaba, A. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc. Natl. Acad. Sci. USA 2007, 104, 5895–5900. [Google Scholar] [CrossRef]
- Hwang, S.-H.; White, K.A.; Somatilaka, B.N.; Wang, B.; Mukhopadhyay, S. Context-dependent ciliary regulation of hedgehog pathway repression in tissue morphogenesis. PLOS Genet. 2023, 19, e1011028. [Google Scholar] [CrossRef]
- Singh, R.R.; Cho-Vega, J.H.; Davuluri, Y.; Ma, S.; Kasbidi, F.; Milito, C.; Lennon, P.A.; Drakos, E.; Medeiros, L.J.; Luthra, R.; et al. Sonic Hedgehog Signaling Pathway Is Activated in ALK-Positive Anaplastic Large Cell Lymphoma. Cancer Res. 2009, 69, 2550–2558. [Google Scholar] [CrossRef]
- Chen, S.; Wang, B.; Fu, X.; Liang, Y.; Chai, X.; Ye, Z.; Li, R.; He, Y.; Kong, G.; Lian, J.; et al. ALKAL1 gene silencing prevents colorectal cancer progression via suppressing Sonic Hedgehog (SHH) signaling pathway. J. Cancer 2021, 12, 150–162. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Zhuang, R.; Guo, X.; Feng, Y.; Shen, F.; Liu, W.; Zhang, Y.; Tong, H.; Sun, W.; et al. Efficacy and Resistance of ALK Inhibitors in Two Inflammatory Myofibroblastic Tumor Patients with ALK Fusions Assessed by Whole Exome and RNA Sequencing. OncoTargets Ther. 2020, 13, 10335–10342. [Google Scholar] [CrossRef]
- Chen, C.; Breslin, M.B.; Lan, M.S. Sonic hedgehog signaling pathway promotes INSM1 transcription factor in neuroendocrine lung cancer. Cell. Signal. 2018, 46, 83–91. [Google Scholar] [CrossRef]
- Watkins, D.N.; Berman, D.M.; Burkholder, S.G.; Wang, B.; Beachy, P.A.; Baylin, S.B. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 2003, 422, 313–317. [Google Scholar] [CrossRef]
- Park, K.-S.; Martelotto, L.G.; Peifer, M.; Sos, M.L.; Karnezis, A.N.; Mahjoub, M.R.; Bernard, K.; Conklin, J.F.; Szczepny, A.; Yuan, J.; et al. A crucial requirement for Hedgehog signaling in small cell lung cancer. Nat. Med. 2011, 17, 1504–1508. [Google Scholar] [CrossRef]
- Ishiwata, T.; Iwasawa, S.; Ebata, T.; Fan, M.; Tada, Y.; Tatsumi, K.; Takiguchi, Y. Inhibition of Gli leads to antitumor growth and enhancement of cisplatin-induced cytotoxicity in large cell neuroendocrine carcinoma of the lung. Oncol. Rep. 2018, 39, 1148–1154. [Google Scholar] [CrossRef]
- Kim, J.; Aftab, B.T.; Tang, J.Y.; Kim, D.; Lee, A.H.; Rezaee, M.; Kim, J.; Chen, B.; King, E.M.; Borodovsky, A.; et al. Itraconazole and Arsenic Trioxide Inhibit Hedgehog Pathway Activation and Tumor Growth Associated with Acquired Resistance to Smoothened Antagonists. Cancer Cell 2013, 23, 23–34. [Google Scholar] [CrossRef]
- Wang, X.; Wei, S.; Zhao, Y.; Shi, C.; Liu, P.; Zhang, C.; Lei, Y.; Zhang, B.; Bai, B.; Huang, Y.; et al. Anti-proliferation of breast cancer cells with itraconazole: Hedgehog pathway inhibition induces apoptosis and autophagic cell death. Cancer Lett. 2017, 385, 128–136. [Google Scholar] [CrossRef]
- Hu, Q.; Hou, Y.-C.; Huang, J.; Fang, J.-Y.; Xiong, H. Itraconazole induces apoptosis and cell cycle arrest via inhibiting Hedgehog signaling in gastric cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 1–11. [Google Scholar] [CrossRef]
- Liang, G.; Liu, M.; Wang, Q.; Shen, Y.; Mei, H.; Li, D.; Liu, W. Itraconazole exerts its anti-melanoma effect by suppressing Hedgehog, Wnt, and PI3K/mTOR signaling pathways. Oncotarget 2017, 8, 28510–28525. [Google Scholar] [CrossRef]
- Liu, M.; Liang, G.; Zheng, H.; Zheng, N.; Ge, H.; Liu, W. Triazoles bind the C-terminal domain of SMO: Illustration by docking and molecular dynamics simulations the binding between SMO and triazoles. Life Sci. 2018, 217, 222–228. [Google Scholar] [CrossRef]
- Gutzmer, R.; Solomon, J.A. Hedgehog Pathway Inhibition for the Treatment of Basal Cell Carcinoma. Target. Oncol. 2019, 14, 253–267. [Google Scholar] [CrossRef]
- Patel, S.; Armbruster, H.; Pardo, G.; Archambeau, B.; Kim, N.H.; Jeter, J.; Wu, R.; Kendra, K.; Contreras, C.M.; Spaccarelli, N.; et al. Hedgehog pathway inhibitors for locally advanced and metastatic basal cell carcinoma: A real-world single-center retrospective review. PLOS ONE 2024, 19, e0297531. [Google Scholar] [CrossRef]
- Ning, H.; Mitsui, H.; Wang, C.Q.; Suárez-Fariñas, M.; Gonzalez, J.; Shah, K.R.; Chen, J.; Coats, I.; Felsen, D.; Carucci, J.A.; et al. Identification of anaplastic lymphoma kinase as a potential therapeutic target in Basal Cell Carcinoma. Oncotarget 2013, 4, 2237–2248. [Google Scholar] [CrossRef]
- Singh, R.R.; Kunkalla, K.; Qu, C.; Schlette, E.; Neelapu, S.S.; Samaniego, F.; Vega, F. ABCG2 is a direct transcriptional target of hedgehog signaling and involved in stroma-induced drug tolerance in diffuse large B-cell lymphoma. Oncogene 2011, 30, 4874–4886. [Google Scholar] [CrossRef]
- Prabavathy, D; Swarnalatha, Y; Ramadoss, N. Lung cancer stem cells—origin, characteristics and therapy. Stem Cell Investig. 2018, 5, 6–6. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, L.; Wu, D. Clinical significance of cancer stem cell markers in lung carcinoma. Acta Biochim. Pol. 2021, 68, 187–191. [Google Scholar] [CrossRef]
- Li, W.; Sparidans, R.W.; Wang, Y.; Lebre, M.C.; Beijnen, J.H.; Schinkel, A.H. Oral coadministration of elacridar and ritonavir enhances brain accumulation and oral availability of the novel ALK/ROS1 inhibitor lorlatinib. Eur. J. Pharm. Biopharm. 2019, 136, 120–130. [Google Scholar] [CrossRef]
- Li, W.; Sparidans, R.W.; Wang, Y.; Lebre, M.C.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. P-glycoprotein (MDR1/ABCB1) restricts brain accumulation and cytochrome P450-3A (CYP3A) limits oral availability of the novel ALK/ROS1 inhibitor lorlatinib. Int. J. Cancer 2018, 143, 2029–2038. [Google Scholar] [CrossRef]
- Ghadi, M.; Hosseinimehr, S.J.; Amiri, F.T.; Mardanshahi, A.; Noaparast, Z. Itraconazole synergistically increases therapeutic effect of paclitaxel and 99mTc-MIBI accumulation, as a probe of P-gp activity, in HT-29 tumor-bearing nude mice. Eur. J. Pharmacol. 2021, 895, 173892. [Google Scholar] [CrossRef]
- Lam, A.; Hoang, J.D.; Singleton, A.; Han, X.; Bleier, B.S. Itraconazole and clarithromycin inhibit P-glycoprotein activity in primary human sinonasal epithelial cells. Int. Forum Allergy Rhinol. 2015, 5, 477–480. [Google Scholar] [CrossRef]
- Lempers, V.J.C.; Heuvel, J.J.M.W.v.D.; Russel, F.G.M.; Aarnoutse, R.E.; Burger, D.M.; Brüggemann, R.J.; Koenderink, J.B. Inhibitory Potential of Antifungal Drugs on ATP-Binding Cassette Transporters P-Glycoprotein, MRP1 to MRP5, BCRP, and BSEP. Antimicrob. Agents Chemother. 2016, 60, 3372–3379. [Google Scholar] [CrossRef]
- Piérard, G.; Arrese, J.; Piérard-Franchimont, C. Itraconazole. Expert Opin. Pharmacother. 2000, 1, 287–304. [Google Scholar] [CrossRef]
- Gupta, A.; Unadkat, J.D.; Mao, Q. Interactions of azole antifungal agents with the human breast cancer resistance protein (BCRP). J. Pharm. Sci. 2007, 96, 3226–3235. [Google Scholar] [CrossRef]
- Inderbinen, S.G.; Zogg, M.; Kley, M.; Smieško, M.; Odermatt, A. Species-specific differences in the inhibition of 11β-hydroxysteroid dehydrogenase 2 by itraconazole and posaconazole. Toxicol. Appl. Pharmacol. 2021, 412, 115387. [Google Scholar] [CrossRef]
- Head, S.A.; Shi, W.; Zhao, L.; Gorshkov, K.; Pasunooti, K.; Chen, Y.; Deng, Z.; Li, R.-J.; Shim, J.S.; Tan, W.; et al. Antifungal drug itraconazole targets VDAC1 to modulate the AMPK/mTOR signaling axis in endothelial cells. Proc. Natl. Acad. Sci. 2015, 112, 201512867–85. [Google Scholar] [CrossRef]
- Head, S.A.; Shi, W.Q.; Yang, E.J.; Nacev, B.A.; Hong, S.Y.; Pasunooti, K.K.; Li, R.-J.; Shim, J.S.; Liu, J.O. Simultaneous Targeting of NPC1 and VDAC1 by Itraconazole Leads to Synergistic Inhibition of mTOR Signaling and Angiogenesis. ACS Chem. Biol. 2016, 12, 174–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Chu, F.; Wu, H.; Xiao, X.; Ye, J.; Li, K. Itraconazole inhibits tumor growth via CEBPB-mediated glycolysis in colorectal cancer. Cancer Sci. 2024, 115, 1154–1169. [Google Scholar] [CrossRef]
- Steel, H.C.; Tintinger, G.R.; Theron, A.J.; Anderson, R. Itraconazole-mediated inhibition of calcium entry into platelet-activating factor-stimulated human neutrophils is due to interference with production of leukotriene B4. Clin. Exp. Immunol. 2007, 150, 144–150. [Google Scholar] [CrossRef]
- Tsubamoto, H.; Ueda, T.; Inoue, K.; Sakata, K.; Shibahara, H.; Sonoda, T. Repurposing itraconazole as an anticancer agent. Oncol. Lett. 2017, 14, 1240–1246. [Google Scholar] [CrossRef]
- Ueda, T.; Tsubamoto, H.; Inoue, K.; Sakata, K.; Shibahara, H.; Sonoda, T. Itraconazole Modulates Hedgehog, WNT/β-catenin, as well as Akt Signalling, and Inhibits Proliferation of Cervical Cancer Cells. Anticancer. Res. 2017, 37, 3521–3526. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, W. Itraconazole Alters the Stem Cell Characteristics of A549 and NCI-H460 Human Lung Cancer Cells by Suppressing Wnt Signaling. Med Sci. Monit. 2019, 25, 9509–9516. [Google Scholar] [CrossRef]
- Wang, W.; Dong, X.; Liu, Y.; Ni, B.; Sai, N.; You, L.; Sun, M.; Yao, Y.; Qu, C.; Yin, X.; et al. Itraconazole exerts anti-liver cancer potential through the Wnt, PI3K/AKT/mTOR, and ROS pathways. Biomed. Pharmacother. 2020, 131, 110661. [Google Scholar] [CrossRef]
- Mazieres, J.; He, B.; You, L.; Xu, Z.; Jablons, D.M. Wnt signaling in lung cancer. Cancer Lett. 2005, 222, 1–10. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; He, J.; Li, J.; Shi, J.; Cho, W.C.; Liu, X. Wnt signaling as potential therapeutic target in lung cancer. Expert Opin. Ther. Targets 2016, 20, 999–1015. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Li, M.-Y.; Ng, C.S.H.; Yang, S.-L.; Wang, S.; Zou, C.; Dong, Y.; Du, J.; Long, X.; et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol. Cancer 2017, 16, 1–12. [Google Scholar] [CrossRef]
- He, Y.; Jiang, X.; Duan, L.; Xiong, Q.; Yuan, Y.; Liu, P.; Jiang, L.; Shen, Q.; Zhao, S.; Yang, C.; et al. LncRNA PKMYT1AR promotes cancer stem cell maintenance in non-small cell lung cancer via activating Wnt signaling pathway. Mol. Cancer 2021, 20, 1–21. [Google Scholar] [CrossRef]
- Mohamed, A.W.; Elbassiouny, M.; Elkhodary, D.A.; Shawki, M.A.; Saad, A.S. The effect of itraconazole on the clinical outcomes of patients with advanced non-small cell lung cancer receiving platinum-based chemotherapy: a randomized controlled study. Med Oncol. 2021, 38, 1–9. [Google Scholar] [CrossRef]
- Gerber, D.E.; Putnam, W.C.; Fattah, F.J.; Kernstine, K.H.; Brekken, R.A.; Pedrosa, I.; Skelton, R.; Saltarski, J.M.; Lenkinski, R.E.; Leff, R.D.; et al. Concentration-dependent Early Antivascular and Antitumor Effects of Itraconazole in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 6017–6027. [Google Scholar] [CrossRef]
- Halatsch, M.-E.; Dwucet, A.; Schmidt, C.J.; Mühlnickel, J.; Heiland, T.; Zeiler, K.; Siegelin, M.D.; Kast, R.E.; Karpel-Massler, G. In Vitro and Clinical Compassionate Use Experiences with the Drug-Repurposing Approach CUSP9v3 in Glioblastoma. Pharmaceuticals 2021, 14, 1241. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Heath, E.I.; Smith, D.C.; Rathkopf, D.; Blackford, A.L.; Danila, D.C.; King, S.; Frost, A.; Ajiboye, A.S.; Zhao, M.; et al. Repurposing Itraconazole as a Treatment for Advanced Prostate Cancer: A Noncomparative Randomized Phase II Trial in Men With Metastatic Castration-Resistant Prostate Cancer. Oncol. 2013, 18, 163–173. [Google Scholar] [CrossRef]
- Lee, M.; Hong, H.; Kim, W.; Zhang, L.; Friedlander, T.W.; Fong, L.; Lin, A.M.; Small, E.J.; Wei, X.X.; Rodvelt, T.J.; et al. Itraconazole as a Noncastrating Treatment for Biochemically Recurrent Prostate Cancer: A Phase 2 Study. Clin. Genitourin. Cancer 2018, 17, e92–e96. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brahmer, J.R.; Juergens, R.A.; Hann, C.L.; Ettinger, D.S.; Sebree, R.; Smith, R.; Aftab, B.T.; Huang, P.; Liu, J.O. Phase 2 Study of Pemetrexed and Itraconazole as Second-Line Therapy for Metastatic Nonsquamous Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2013, 8, 619–623. [Google Scholar] [CrossRef]
- Tsubamoto, H.; Sonoda, T.; Yamasaki, M.; Inoue, K. Impact of combination chemotherapy with itraconazole on survival of patients with refractory ovarian cancer. . 2014, 34, 2481–7. [Google Scholar]
- Tsubamoto, H.; Sonoda, T.; Yamasaki, M.; Inoue, K. Impact of combination chemotherapy with itraconazole on survival for patients with recurrent or persistent ovarian clear cell carcinoma. . 2014, 34, 2007–14. [Google Scholar]
- Zheng, H.; Yang, H.; Gong, D.; Mai, L.; Qiu, X.; Chen, L.; Su, X.; Wei, R.; Zeng, Z. Progress in the Mechanism and Clinical Application of Cilostazol. Curr. Top. Med. Chem. 2020, 19, 2919–2936. [Google Scholar] [CrossRef]
- Kherallah, R.Y.; Khawaja, M.; Olson, M.; Angiolillo, D.; Birnbaum, Y. Cilostazol: a Review of Basic Mechanisms and Clinical Uses. Cardiovasc. Drugs Ther. 2021, 36, 777–792. [Google Scholar] [CrossRef]
- Pratt, C.M. Analysis of the cilostazol safety database. Am. J. Cardiol. 2001, 87, 28–33. [Google Scholar] [CrossRef]
- Galyfos, G.; Sianou, A. Cilostazol for Secondary Prevention of Stroke: Should the Guidelines Perhaps Be Extended? Vasc. Spéc. Int. 2017, 33, 89–92. [Google Scholar] [CrossRef]
- Sorkin, E.M.; Markham, A. Cilostazol. Drugs Aging 1999, 14, 63–71. [Google Scholar] [CrossRef]
- McHutchison, C.; Blair, G.W.; Appleton, J.P.; Chappell, F.M.; Doubal, F.; Bath, P.M.; Wardlaw, J.M. Cilostazol for Secondary Prevention of Stroke and Cognitive Decline. Stroke 2020, 51, 2374–2385. [Google Scholar] [CrossRef]
- Goto, S. Cilostazol: Potential mechanism of action for antithrombotic effects accompanied by a low rate of bleeding. Atheroscler. Suppl. 2005, 6, 3–11. [Google Scholar] [CrossRef]
- Schrör, K. The pharmacology of cilostazol. Diabetes, Obes. Metab. 2002, 4, S14–S19. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Kimura, K.; Otsuka, T.; Toyoda, K.; Uchiyama, S.; Hoshino, H.; Sakai, N.; Okada, Y.; Origasa, H.; Naritomi, H.; et al. Dual Antiplatelet Therapy With Cilostazol for Secondary Prevention in Lacunar Stroke: Subanalysis of the CSPS.com Trial. Stroke 2023, 54, 697–705. [Google Scholar] [CrossRef]
- S. A, U.; Y.I, S.; Y.D, K.; T.K, Y.; S.G, H.; K.F, M.; Y.E, O.; Nj, T.; H.N, Y.; C.O, G.; et al. Benefit of Cilostazol in Patients with High Risk of Bleeding: Subanalysis of Cilostazol Stroke Prevention Study 2. Cerebrovasc. Dis. 2014, 37, 296–303. [Google Scholar] [CrossRef]
- Chai, E.; Chen, J.; Li, C.; Zhang, X.; Fan, Z.; Yang, S.; Zhao, K.; Li, W.; Xiao, Z.; Zhang, Y.; et al. The Efficacy and Safety of Cilostazol vs. Aspirin for Secondary Stroke Prevention: A Systematic Review and Meta-Analysis. Front. Neurol. 2022, 13, 814654. [Google Scholar] [CrossRef]
- Naka, H.; Nomura, E.; Kitamura, J.; Imamura, E.; Wakabayashi, S.; Matsumoto, M. Antiplatelet Therapy as a Risk Factor for Microbleeds in Intracerebral Hemorrhage Patients: Analysis Using Specific Antiplatelet Agents. J. Stroke Cerebrovasc. Dis. 2013, 22, 834–840. [Google Scholar] [CrossRef]
- Lin, M.P.; Meschia, J.F.; Gopal, N.; Barrett, K.M.; Ross, O.A.; Ertekin-Taner, N.; Brott, T.G. Cilostazol Versus Aspirin for Secondary Stroke Prevention: Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2020, 30, 105581. [Google Scholar] [CrossRef]
- Tamai, Y.; Takami, H.; Nakahata, R.; Ono, F.; Munakata, A. Comparison of the Effects of Acetylsalicylic Acid, Ticlopidine and Cilostazol on Primary Hemostasis Using a Quantitative Bleeding Time Test Apparatus. Pathophysiol. Haemost. Thromb. 1999, 29, 269–276. [Google Scholar] [CrossRef]
- Kambayashi, J.; Liu, Y.; Sun, B.; Shakur, Y.; Yoshitake, M.; Czerwiec, F. Cilostazol as a Unique Antithrombotic Agent. Curr. Pharm. Des. 2003, 9, 2289–2302. [Google Scholar] [CrossRef]
- Kherallah, R.Y.; Khawaja, M.; Olson, M.; Angiolillo, D.; Birnbaum, Y. Cilostazol: a Review of Basic Mechanisms and Clinical Uses. Cardiovasc. Drugs Ther. 2021, 36, 777–792. [Google Scholar] [CrossRef]
- Liu, Y.; Shakur, Y.; Yoshitake, M.; Kambayashi, J. Cilostazol (Pletal®): A Dual Inhibitor of Cyclic Nucleotide Phosphodiesterase Type 3 and Adenosine Uptake. Cardiovasc. Drug Rev. 2001, 19, 369–386. [Google Scholar] [CrossRef]
- Murata, K.; Kameyama, M.; Fukui, F.; Ohigashi, H.; Hiratsuka, M.; Sasaki, Y.; Kabuto, T.; Mukai, M.; Mammoto, T.; Akedo, H.; et al. Phosphodiesterase type III inhibitor, cilostazol, inhibits colon cancer cell motility. Clin. Exp. Metastasis 1999, 17, 525–530. [Google Scholar] [CrossRef]
- Gremmel, T.; Frelinger, A.L., 3rd; Michelson, A.D. Platelet Physiology. Semin. Thromb. Hemost. 2016, 42, 191–204. [Google Scholar] [CrossRef]
- Beitia, M.; Delgado, D.; Mercader, J.; Sánchez, P.; de Dicastillo, L.L.; Sánchez, M. Action of Platelet-Rich Plasma on In Vitro Cellular Bioactivity: More than Platelets. Int. J. Mol. Sci. 2023, 24, 5367. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Goszka, M.; Serwin, N.; Roszak, M.; Grygorcewicz, B.; Heryć, R.; Dołęgowska, B. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2021, 64, 84–94. [Google Scholar] [CrossRef]
- Svendsen, M.N.; Lykke, J.; Werther, K.; Christensen, I.J.; Nielsen, H.J. Concentrations of VEGF and VEGFR1 in Paired Tumor Arteries and Veins in Patients With Rectal Cancer. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2004, 14, 611–615. [Google Scholar] [CrossRef]
- Werther, K.; Christensen, I.J.; Nielsen, H.J. Determination of vascular endothelial growth factor (VEGF) in circulating blood: significance of VEGF in various leucocytes and platelets. Scand. J. Clin. Lab. Investig. 2002, 62, 343–350. [Google Scholar] [CrossRef]
- Werther, K.; Bülow, S.; Hesselfeldt, P.; Jespersen, N.F.K.; Svendsen, M.N.; Nielsen, H.J. VEGF concentrations in tumour arteries and veins from patients with rectal cancer. APMIS 2002, 110, 646–650. [Google Scholar] [CrossRef]
- Morris, K.; Schnoor, B.; Papa, A.-L. Platelet cancer cell interplay as a new therapeutic target. Biochim. et Biophys. Acta (BBA) - Rev. Cancer 2022, 1877, 188770. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Yoda, S.; Lennerz, J.K.; Langenbucher, A.; Lin, J.J.; Rooney, M.M.; Prutisto-Chang, K.; Oh, A.; Adams, N.A.; Yeap, B.Y.; et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clin. Cancer Res. 2020, 26, 2535–2545. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Z.; Tian, Y.; Li, Z.; Liu, Z.; Zhu, S. The critical role of platelet in cancer progression and metastasis. Eur. J. Med Res. 2023, 28, 1–12. [Google Scholar] [CrossRef]
- Stegner, D.; Dütting, S.; Nieswandt, B. Mechanistic explanation for platelet contribution to cancer metastasis. Thromb. Res. 2014, 133, S149–S157. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Xu, R. Human Cancer and Platelet Interaction, a Potential Therapeutic Target. Int. J. Mol. Sci. 2018, 19, 1246. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Gil-Pulido, J.; Sarukhanyan, E.; Burkard, P.; Shityakov, S.; Schonhart, C.; Stegner, D.; Remer, K.; Nurden, P.; Nurden, A.T.; et al. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived Galectin-3. Blood 2020, 135, 1146–1160. [Google Scholar] [CrossRef]
- Xue, J.; Deng, J.; Qin, H.; Yan, S.; Zhao, Z.; Qin, L.; Liu, J.; Wang, H. The interaction of platelet-related factors with tumor cells promotes tumor metastasis. J. Transl. Med. 2024, 22, 1–13. [Google Scholar] [CrossRef]
- Foss, A.; Muñoz-Sagredo, L.; Sleeman, J.; Thiele, W. The contribution of platelets to intravascular arrest, extravasation, and outgrowth of disseminated tumor cells. Clin. Exp. Metastasis 2020, 37, 47–67. [Google Scholar] [CrossRef]
- Roweth, H.G. Platelet Contributions to the (Pre)metastatic Tumor Microenvironment. Semin. Thromb. Hemost. 2023, 50, 455–461. [Google Scholar] [CrossRef]
- Schlesinger, M. Role of platelets and platelet receptors in cancer metastasis. J. Hematol. Oncol. 2018, 11, 1–15. [Google Scholar] [CrossRef]
- Goubran, H.A.; Burnouf, T.; Radosevic, M.; El-Ekiaby, M. The platelet–cancer loop. Eur. J. Intern. Med. 2013, 24, 393–400. [Google Scholar] [CrossRef]
- Shi, M.; Zhao, W.; Zhou, F.; Chen, H.; Tang, L.; Su, B.; Zhang, J. Neutrophil or platelet-to-lymphocyte ratios in blood are associated with poor prognosis of pulmonary large cell neuroendocrine carcinoma. Transl. Lung Cancer Res. 2020, 9, 45–54. [Google Scholar] [CrossRef]
- Akdag, G.; Alan. ; Dogan, A.; Yildirim, S.; Kinikoglu, O.; Batu, A.; Kudu, E.; Geçmen, G.G.; Isik, D.; Sever, O.N.; et al. Prognostic scores in pulmonary large cell neuroendocrine carcinoma: A retrospective cohort study. Heliyon 2024, 10, e25029. [Google Scholar] [CrossRef]
- Han, Y.; Wang, J.; Hong, L.; Sun, L.; Zhuang, H.; Sun, B.; Wang, H.; Zhang, X.; Ren, X. Platelet–lymphocyte ratio is an independent prognostic factor in patients with ALK-positive non-small-cell lung cancer. Futur. Oncol. 2017, 13, 51–61. [Google Scholar] [CrossRef]
- Andersen, B.L.; Myers, J.; Blevins, T.; Park, K.R.; Smith, R.M.; Reisinger, S.; Carbone, D.P.; Presley, C.J.; Shields, P.G.; Carson, W.E. Depression in association with neutrophil-to-lymphocyte, platelet-to-lymphocyte, and advanced lung cancer inflammation index biomarkers predicting lung cancer survival. PLOS ONE 2023, 18, e0282206. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, L.; Zhang, B.; Zhang, L.; Wang, C. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: a systematic review and meta-analysis. Sci. Rep. 2016, 6, 22618. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, S.; Liu, Y.; Ma, L.; Zhu, J.; Xin, Y.; Wang, Y.; Yang, C.; Cheng, Y. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J. Clin. Lab. Anal. 2019, 33, e22964. [Google Scholar] [CrossRef]
- Liu, N.; Mao, J.; Tao, P.; Chi, H.; Jia, W.; Dong, C. The relationship between NLR/PLR/LMR levels and survival prognosis in patients with non-small cell lung carcinoma treated with immune checkpoint inhibitors. Medicine 2022, 101, e28617. [Google Scholar] [CrossRef]
- Stakiw, J.; Radosevic, M.; Burnouf, T.; Goubran, H.A. Platelet–Cancer Interactions. Semin. Thromb. Hemost. 2014, 40, 296–305. [Google Scholar] [CrossRef]
- Garofano, K.; Rashid, K.; Smith, M.; Brantner, C.; Suwunnakorn, S.; Diemert, D.; Gordon, O.; Horvath, A.; Khan, S.; Popratiloff, A.; et al. Prostate cancer cell-platelet bidirectional signaling promotes calcium mobilization, invasion and apoptotic resistance via distinct receptor-ligand pairs. Sci. Rep. 2023, 13, 1–16. [Google Scholar] [CrossRef]
- Najafi, S.; Asemani, Y.; Majidpoor, J.; Mahmoudi, R.; Aghaei-Zarch, S.M.; Mortezaee, K. Tumor-educated platelets. Clin. Chim. Acta 2024, 552, 117690. [Google Scholar] [CrossRef]
- Coenen, D.M.; Heinzmann, A.C.A.; Oggero, S.; Albers, H.J.; Nagy, M.; Hagué, P.; Kuijpers, M.J.E.; Vanderwinden, J.-M.; van der Meer, A.D.; Perretti, M.; et al. Inhibition of Phosphodiesterase 3A by Cilostazol Dampens Proinflammatory Platelet Functions. Cells 2021, 10, 1998. [Google Scholar] [CrossRef]
- Melese, E.S.; Franks, E.; Cederberg, R.A.; Harbourne, B.T.; Shi, R.; Wadsworth, B.J.; Collier, J.L.; Halvorsen, E.C.; Johnson, F.; Luu, J.; et al. CCL5 production in lung cancer cells leads to an altered immune microenvironment and promotes tumor development. OncoImmunology 2021, 11, 2010905. [Google Scholar] [CrossRef]
- Kong, P.; Yang, X.; Zhang, Y.; Dong, H.; Liu, X.; Xu, X.; Zhang, X.; Shi, Y.; Hou, M.; Song, B. Palbociclib Enhances Migration and Invasion of Cancer Cells via Senescence-Associated Secretory Phenotype-Related CCL5 in Non-Small-Cell Lung Cancer. J. Oncol. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Xia, L.; Zhu, X.; Zhang, L.; Xu, Y.; Chen, G.; Luo, J. EZH2 enhances expression of CCL5 to promote recruitment of macrophages and invasion in lung cancer. Biotechnol. Appl. Biochem. 2019, 67, 1011–1019. [Google Scholar] [CrossRef]
- Zheng, Z.; Jia, S.; Shao, C.; Shi, Y. Irradiation induces cancer lung metastasis through activation of the cGAS–STING–CCL5 pathway in mesenchymal stromal cells. Cell Death Dis. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Fong, Y.-C.; Lee, C.-Y.; Chen, M.-Y.; Tsai, H.-C.; Hsu, H.-C.; Tang, C.-H. CCL5 increases lung cancer migration via PI3K, Akt and NF-κB pathways. Biochem. Pharmacol. 2009, 77, 794–803. [Google Scholar] [CrossRef]
- Spaks, A.; Svirina, D.; Spaka, I.; Jaunalksne, I.; Breiva, D.; Tracums, I.; Krievins, D. CXC chemokine ligand 4 (CXCL4) is predictor of tumour angiogenic activity and prognostic biomarker in non-small cell lung cancer (NSCLC) patients undergoing surgical treatment. Biomarkers 2016, 21, 474–478. [Google Scholar] [CrossRef]
- Gerrits, A.J.; Frelinger, A.L.; Michelson, A.D. Whole Blood Analysis of Leukocyte-Platelet Aggregates. Curr. Protoc. Cytom. 2016, 78, 6–15. [Google Scholar] [CrossRef]
- Lecot, P.; Ardin, M.; Dussurgey, S.; Alcazer, V.; Moudombi, L.; Abrantes, M.P.; Hubert, M.; Swalduz, A.; Hernandez-Vargas, H.; Viari, A.; et al. Gene signature of circulating platelet-bound neutrophils is associated with poor prognosis in cancer patients. Int. J. Cancer 2022, 151, 138–152. [Google Scholar] [CrossRef]
- Chen, M.; Geng, J.-G. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch. Immunol. et Ther. Exp. 2006, 54, 75–84. [Google Scholar] [CrossRef]
- Coupland, L.A.; Parish, C.R. Platelets, Selectins, and the Control of Tumor Metastasis. Semin. Oncol. 2014, 41, 422–434. [Google Scholar] [CrossRef]
- Nomura, S.; Shouzu, A.; Omoto, S.; Hayakawa, T.; Kagawa, H.; Nishikawa, M.; Inada, M.; Fujimura, Y.; Ikeda, Y.; Fukuhara, S. Effect of cilostazol on soluble adhesion molecules and platelet-derived microparticles in patients with diabetes. . 1998, 80, 388–92. [Google Scholar]
- O'Donnell, M.; Badger, S.; Sharif, M.; Makar, R.; McEneny, J.; Young, I.; Lee, B.; Soong, C. The Effects of Cilostazol on Exercise-induced Ischaemia–reperfusion Injury in Patients with Peripheral Arterial Disease. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 326–335. [Google Scholar] [CrossRef]
- Kariyazono, H.; Nakamura, K.; Shinkawa, T.; Yamaguchi, T.; Sakata, R.; Yamada, K. Inhibition of Platelet Aggregation and the Release of P-Selectin from Platelets by Cilostazol. Thromb. Res. 2001, 101, 445–453. [Google Scholar] [CrossRef]
- Rubio, V.E.C.; Segura, P.P.; Muñoz, A.; Farré, A.L.; Ruiz, L.C.; Lorente, J.A. High plasma levels of soluble P-Selectin and Factor VIII predict venous thromboembolism in non-small cell lung cancer patients: The Thrombo-Nsclc risk score. Thromb. Res. 2020, 196, 349–354. [Google Scholar] [CrossRef]
- Klinkhardt, U.; Bauersachs, R.; Adams, J.; Graff, J.; Lindhoff-Last, E.; Harder, S. Clopidogrel but not aspirin reduces P-selectin expression and formation of platelet-leukocyte aggregates in patients with atherosclerotic vascular disease*. Clin. Pharmacol. Ther. 2003, 73, 232–241. [Google Scholar] [CrossRef]
- Rao, A.K.; Vaidyula, V.R.; Bagga, S.; Jalagadugula, G.; Gaughan, J.; Wilhite, D.B.; Comerota, A.J. Effect of antiplatelet agents clopidogrel, aspirin, and cilostazol on circulating tissue factor procoagulant activity in patients with peripheral arterial disease. . 2006, 96, 738–43. [Google Scholar]
- Berger, G.; Hartwell, D.W.; Wagner, D.D. P-Selectin and platelet clearance. . 1998, 92, 4446–52. [Google Scholar]
- Goto, S.; Ichikawa, N.; Lee, M.; Goto, M.; Sakai, H.; Kim, J.J.; Yoshida, M.; Handa, M.; Ikeda, Y.; Handa, S. Platelet surface P-selectin molecules increased after exposing platelet to a high shear flow. . 2000, 19, 147–51. [Google Scholar]
- Michelson, A.D.; Barnard, M.R.; Hechtman, H.B.; MacGregor, H.; Connolly, R.J.; Loscalzo, J.; Valeri, C.R. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc. Natl. Acad. Sci. 1996, 93, 11877–11882. [Google Scholar] [CrossRef]
- Fabricius, H.; Starzonek, S.; Lange, T. The Role of Platelet Cell Surface P-Selectin for the Direct Platelet-Tumor Cell Contact During Metastasis Formation in Human Tumors. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Geng, J.-G.; Chen, M.; Chou, K.-C. P-selectin Cell Adhesion Molecule in Inflammation, Thrombosis, Cancer Growth and Metastasis. Curr. Med. Chem. 2004, 11, 2153–2160. [Google Scholar] [CrossRef]
- Chen, M.; Geng, J.-G. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch. Immunol. et Ther. Exp. 2006, 54, 75–84. [Google Scholar] [CrossRef]
- Omi, H.; Okayama, N.; Shimizu, M.; Fukutomi, T.; Nakamura, A.; Imaeda, K.; Okouchi, M.; Itoh, M. Cilostazol inhibits high glucose-mediated endothelial-neutrophil adhesion by decreasing adhesion molecule expression via NO production. Microvasc. Res. 2004, 68, 119–125. [Google Scholar] [CrossRef]
- Weintraub, W.S. The vascular effects of cilostazol. Can. J. Cardiol. 2006, 22, 56B–60B. [Google Scholar] [CrossRef]
- Sohn, M.; Lim, S. The Role of Cilostazol, a Phosphodiesterase-3 Inhibitor, in the Development of Atherosclerosis and Vascular Biology: A Review with Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 2593. [Google Scholar] [CrossRef]
- Geng, D.-F.; Deng, J.; Jin, D.-M.; Wu, W.; Wang, J.-F. Effect of cilostazol on the progression of carotid intima-media thickness: A meta-analysis of randomized controlled trials. Atherosclerosis 2011, 220, 177–183. [Google Scholar] [CrossRef]
- Wan, H.; Huang, T.; Yang, P.; Wu, T.; Zhang, H.; Wu, Q. Efficacy and Safety of Cilostazol for Atherosclerosis: A Meta-analysis of Randomized Controlled Trials. J. Cardiovasc. Pharmacol. 2021, 79, 390–390. [Google Scholar] [CrossRef]
- Suzuki, K.; Aiura, K.; Ueda, M.; Kitajima, M. The Influence of Platelets on the Promotion of Invasion by Tumor Cells and Inhibition by Antiplatelet Agents. Pancreas 2004, 29, 132–140. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, H.; Li, H.; Mou, H.; Yinwang, E.; Xue, Y.; Wang, S.; Zhang, Y.; Wang, Z.; Chen, T.; et al. Cancer cells reprogram to metastatic state through the acquisition of platelet mitochondria. Cell Rep. 2023, 42, 113147. [Google Scholar] [CrossRef]
- Cereceda, L.; Cardenas, J.C.; Khoury, M.; Silva-Pavez, E.; Hidalgo, Y. Impact of platelet-derived mitochondria transfer in the metabolic profiling and progression of metastatic MDA-MB-231 human triple-negative breast cancer cells. Front. Cell Dev. Biol. 2024, 11, 1324158. [Google Scholar] [CrossRef]
- Veilleux, V.; Pichaud, N.; Boudreau, L.H.; Robichaud, G.A. Mitochondria Transfer by Platelet-Derived Microparticles Regulates Breast Cancer Bioenergetic States and Malignant Features. Mol. Cancer Res. 2023, 22, 268–281. [Google Scholar] [CrossRef]
- Guan, F.; Wu, X.; Zhou, J.; Lin, Y.; He, Y.; Fan, C.; Zeng, Z.; Xiong, W. Mitochondrial transfer in tunneling nanotubes—a new target for cancer therapy. J. Exp. Clin. Cancer Res. 2024, 43, 1–18. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Yu, S.-H.; Lee, S.-E.; Park, J.H.; Cho, G.; Choi, C.; Han, K.; Kim, C.-H.; Kang, Y.C. Platelet-derived mitochondria transfer facilitates wound-closure by modulating ROS levels in dermal fibroblasts. Platelets 2022, 34, 2151996. [Google Scholar] [CrossRef]
- Chen, E.; Chen, Z.; Chen, L.; Hu, X. Platelet-derived respiratory-competent mitochondria transfer to mesenchymal stem cells to promote wound healing via metabolic reprogramming. Platelets 2022, 33, 171–173. [Google Scholar] [CrossRef]
- Levoux, J.; Prola, A.; Lafuste, P.; Gervais, M.; Chevallier, N.; Koumaiha, Z.; Kefi, K.; Braud, L.; Schmitt, A.; Yacia, A. Platelets Facilitate the Wound-Healing Capability of Mesenchymal Stem Cells by Mitochondrial Transfer and Metabolic Reprogramming. Cell Metab. 2021; 99. [Google Scholar] [CrossRef]
- Ross, J.S.; Ali, S.M.; Fasan, O.; Block, J.; Pal, S.; Elvin, J.A.; Schrock, A.B.; Suh, J.; Nozad, S.; Kim, S.; et al. ALK Fusions in a Wide Variety of Tumor Types Respond to Anti-ALK Targeted Therapy. Oncol. 2017, 22, 1444–1450. [Google Scholar] [CrossRef]
- Shreenivas, A.; Janku, F.; Gouda, M.A.; Chen, H.-Z.; George, B.; Kato, S.; Kurzrock, R. ALK fusions in the pan-cancer setting: another tumor-agnostic target? npj Precis. Oncol. 2023, 7, 1–20. [Google Scholar] [CrossRef]
- Inufusa, H.; Adachi, T.; Nakamura, M.; Shindo, K.; Yasutomi, M.; Kimura, Y. Inhibition of experimental metastasis of human adenocarcinoma by cilostazol, a platelet phosphodiesterase inhibitor. Oncol. Rep. 1995, 2, 1079–1083. [Google Scholar] [CrossRef]
- Liu CC, Wu CL, Yeh IC, Wu SN, Sze CI, Gean PW. Cilostazol eliminates radiation-resistant glioblastoma by re-evoking big conductance calcium-activated potassium channel activity. Am J Cancer Res. 2021;11(4):1148-1169.
- Naderbar, L.; Pazhang, Y.; Rezaie, J. Inhibiting AKT signaling pathway with cilostazol and meloxicam synergism for suppressing K562 cells in vitro. J. Biochem. Mol. Toxicol. 2022, 36, e23185. [Google Scholar] [CrossRef]
- Hao, N.; Shen, W.; Du, R.; Jiang, S.; Zhu, J.; Chen, Y.; Huang, C.; Shi, Y.; Xiang, R.; Luo, Y. Phosphodiesterase 3A Represents a Therapeutic Target that Drives Stem Cell–like Property and Metastasis in Breast Cancer. Mol. Cancer Ther. 2020, 19, 868–881. [Google Scholar] [CrossRef]
- Vandenberghe, P.; Delvaux, M.; Hagué, P.; Erneux, C.; Vanderwinden, J.-M. Potentiation of imatinib by cilostazol in sensitive and resistant gastrointestinal stromal tumor cell lines involves YAP inhibition. Oncotarget 2019, 10, 1798–1811. [Google Scholar] [CrossRef]
- Uzawa, K.; Kasamatsu, A.; Baba, T.; Usukura, K.; Saito, Y.; Sakuma, K.; Iyoda, M.; Sakamoto, Y.; Ogawara, K.; Shiiba, M.; et al. Targeting phosphodiesterase 3B enhances cisplatin sensitivity in human cancer cells. Cancer Med. 2013, 2, 40–49. [Google Scholar] [CrossRef]
- Wenzel, J.; Zeisig, R.; Fichtner, I. Inhibition of metastasis in a murine 4T1 breast cancer model by liposomes preventing tumor cell-platelet interactions. Clin. Exp. Metastasis 2009, 27, 25–34. [Google Scholar] [CrossRef]
| drug | general medical use | with lorlatinib |
|---|---|---|
| itraconazole | anti-fungal | Hh, p-gp, 3A4 inhibition |
| cilostazol | thrombosis prevention | growth factor deprivation |
| drug | T1/2 | metabolism by |
inhibition of | side effects |
|---|---|---|---|---|
| itraconazole | 1 d | 3A4 | 3A4, p-gp | ⇧LFT |
| cilostazol | 12 h | 3A4, 2C19 | none | headache, diarrhea |
| lorlatinib | 1 d | 3A4, glucuronidation | none | hyperlipidemia, neuro-psyche |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





