1. Introduction
Phosphorus (P) play an indispensable role in global agriculture, underpinning the sector’s ability to meet the world’s increasing food demands (Sultenfuss and Doyle. 1999). As a vital component of plants, P is essential for energy transfer, photosynthesis, and cell division, making it a cornerstone of crop productivity and plant health. Agricultural intensification, aimed at achieving higher yields to feed a burgeoning population, has further entrenched the reliance on P-based fertilizers and increasing mining activities that disrupt the natural landscape and ecosystems where the quarry is located due to land violations, air emissions, water pollution, and noise (Cordell et al. 2009; Cordell and White 2011). This reliance has been pivotal in boosting food production, but it has also highlighted the nutrient’s finite availability to meet increasing demand and the challenges of sustainable management (Cordell and White 2013; FAO 2022). The past years have seen a heightened recognition of these challenges, spurring research and investment into more efficient fertilization methods and P recovery from waste streams.
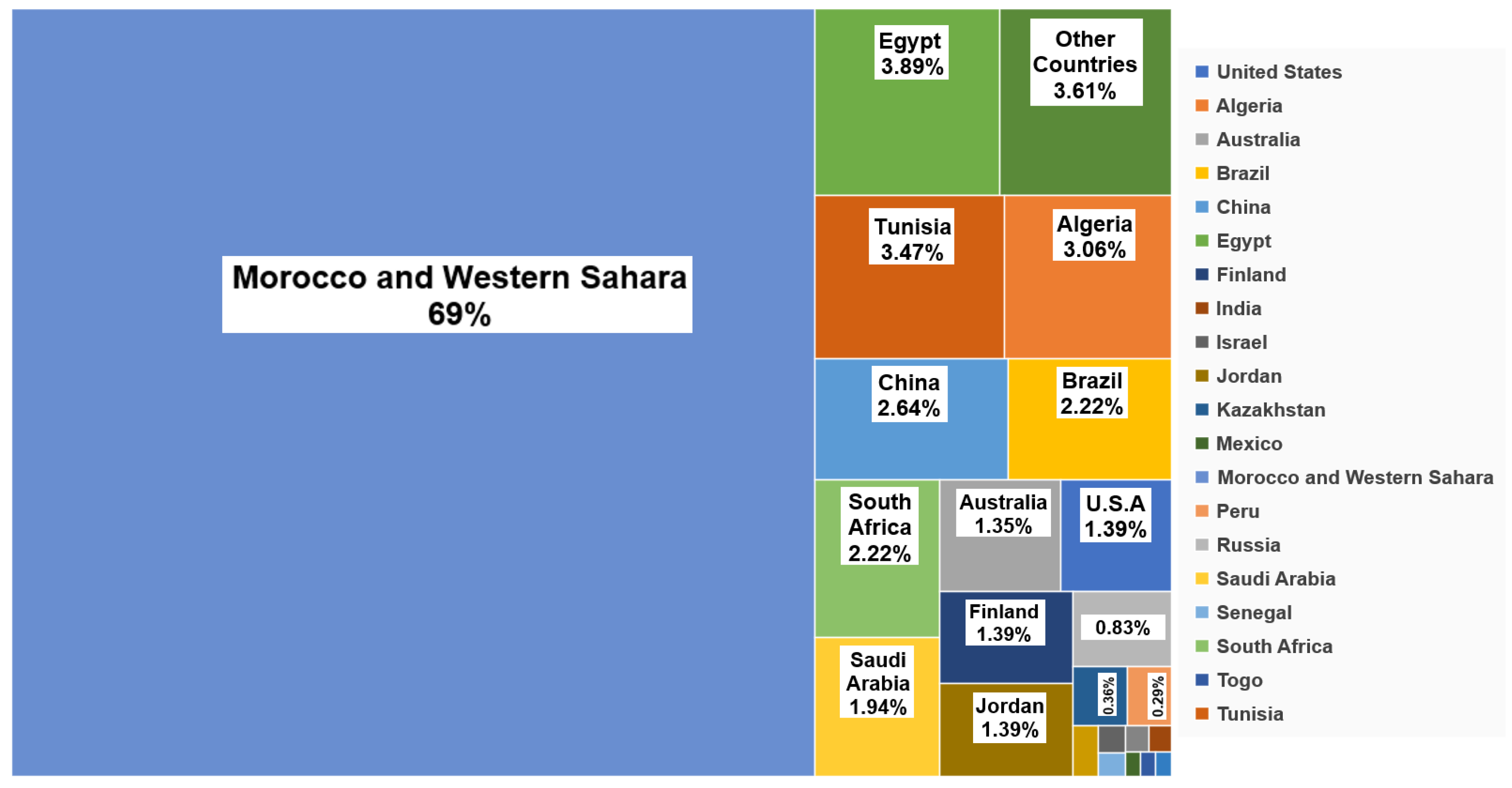
The global processing and transportation of P fertilizers costs is reliant on inexpensive fossil fuels, and energy costs. Phosphorite rocks are one of the most widely traded commodities in the international market, with approximately 220 million tons transported globally annually (Cordell and White 2011; Stephen M. Jasinski 2023). This situation poses a challenge, as P fertilizer prices are contingent on the trade and the geopolitical stability of a limited number of countries. Phosphorus stocks are primarily located in Morocco (69.44%), Egypt (3.89%), Tunisia (3.47%), Algeria (3.09%), China (2.64%), Brazil (2.2%), South Africa (2.2%), Saudi Arabia (1.94%), Australia (1.53%), and the United States (1.39%) (Cordell and White 2011; Stephen M. Jasinski 2023) (
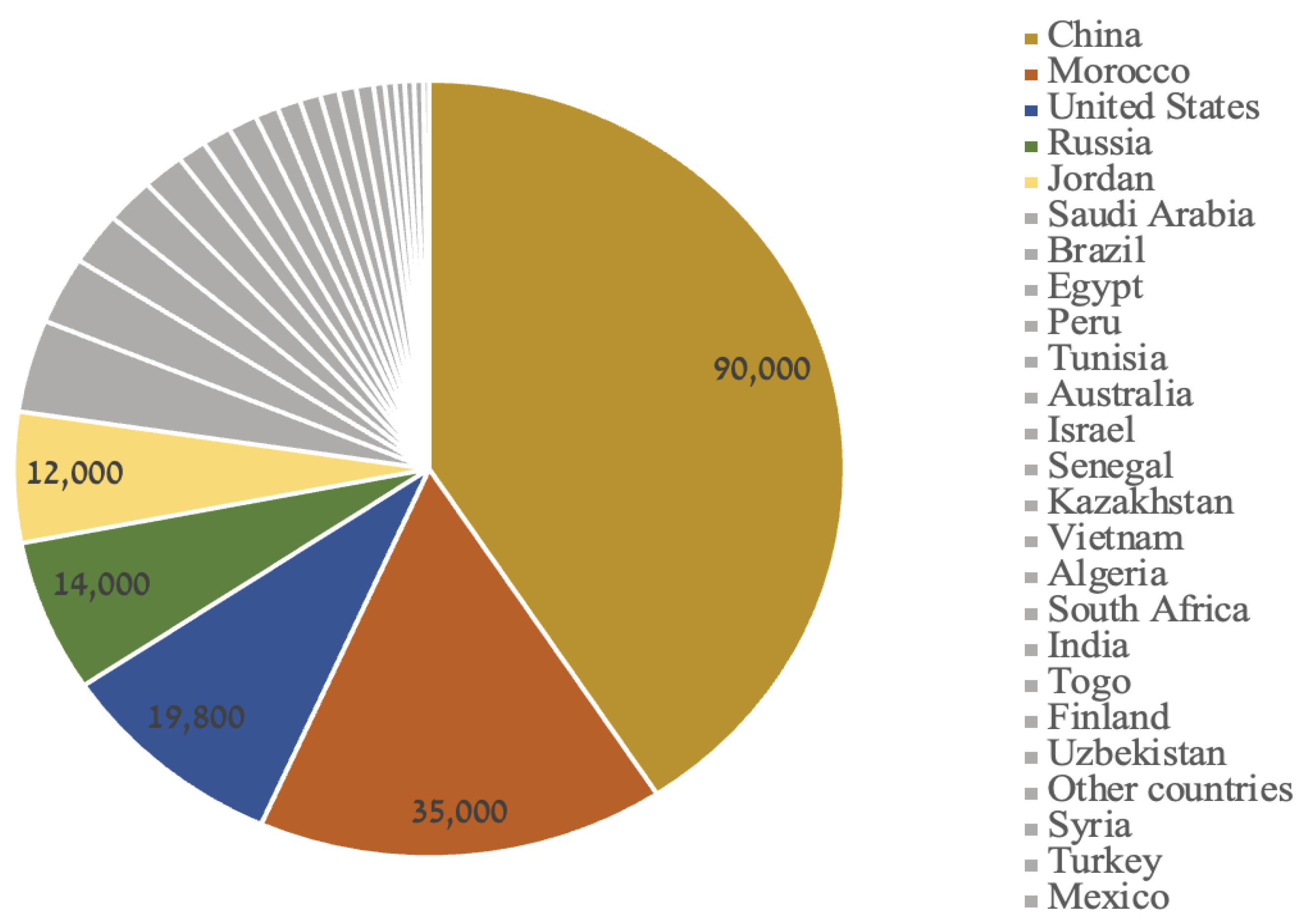
Figure 1). However, global phosphorus production (for all uses) is currently enhanced by China (41%), Morocco (16%), the USA (9%), and Russia (6%) with a total of 220.75 million metric tons in 2023 (US National Minerals Information Center 2023) (
Figure 2).
1
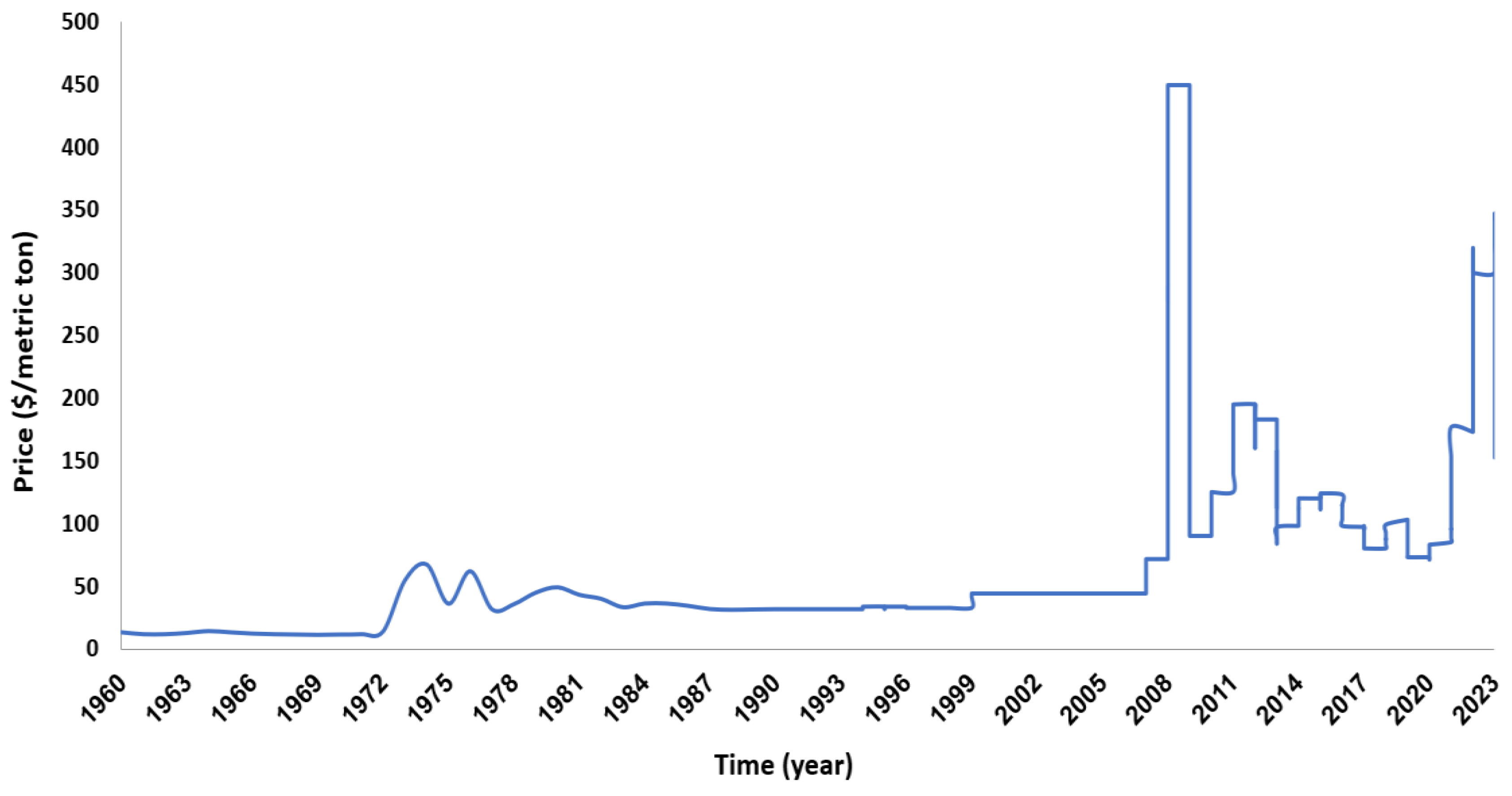
The price of P fertilizer skyrocketed by up to 800% during the 2008 economic crisis partially due to increasing costs of extraction, processing, and shipping (Cordell and White 2011; The World Bank 2017), compounding concerns for economic uncertainty in P markets (
Figure 2). Moreover, P price is forecast to increase, as long as demand continues to rise, and no new production substitutes are available. Al Rawashdeh, (2023) have estimated the short-run and the long-run price elasticities of demand for P, forecasting the elasticities of price at –0.003 and –0.061, respectively. This implies that 1% increase in quantities of P demanded will lead to 3.3% increase in price in the short run, and 1.6% increase in price in the long run. These recent results are consistent with previous results, including Denbaly and Vroomen, (1993) who found that price elasticities for phosphate were historically inelastic.
The extraction and refinement of Phosphorite rock, a primary source of P for agricultural fertilizers, is a process fraught with environmental and ecological implications. In producing one ton of P, the process yields approximately five tons of phospho-gypsum, a by-product laden with carbon, radioactive elements, and heavy metals like cadmium and uranium that are toxic to soils and humans and hence (Cordell et al. 2009; Carpenter and Bennett 2011; Cordell and White 2011). Such by-products represent a significant environmental hazard, contributing to the growing concern over industrial carbon footprints and the contamination of natural ecosystems. Furthermore, the application of industrial P fertilizers is encumbered by inherently low utilization efficiency. Soil P-fixation processes immobilize the P, severely restricting its availability to plants and thereby undermining the efficacy of fertilization efforts. To compensate for the resultant P deficiency, agricultural practices often resort to the overuse of fertilizers. This overuse initiates a cascade of environmental degradation, including the leaching of excess nutrients into groundwater systems, the pollution of water bodies, the eutrophication of aquatic ecosystems, and the creation of anoxic dead zones. Such zones inflict harm not only on water reservoirs but also on human economic activities like fishing and tourism (De-Bashan and Bashan 2004; Haygarth et al. 2013; Litaor et al. 2013, 2016; Ayele and Atlabachew 2021). The nutrient-rich effluents emanating from municipal and agricultural sources, characterized by high P concentrations, exacerbate the problem, significantly contributing to the P load in waterways and furthering the eutrophication process. That underscores the critical need for a paradigm shift in P management, that aligns with the principles of a circular economy and mitigates the adverse environmental impacts associated with current practices.
Figure 3.
Phosphorus rock prices 1960-2023 (Database: World Bank, 2023).
Figure 3.
Phosphorus rock prices 1960-2023 (Database: World Bank, 2023).
To address the dual challenges of P management, namely, the reliance on non-renewable rock phosphate and the associated water pollution, there is a pressing need to embrace alternative approaches that encapsulate the principles of a circular economy. Recycling P from wastewater and reintegrating it into agricultural systems emerges as a sustainable solution with the potential to significantly reduce our dependence on mined rock phosphate (Steen 1998; Cordell et al. 2009; Barnea et al. 2012; Wendling et al. 2013). This strategy involves reprocessing wastewater to recover P, thereby transforming a waste product into a valuable resource. Such an approach not only helps conserve finite P reserves but also mitigates the environmental impacts of P run-off. By reintroducing recycled P into the agricultural cycle, we can close the loop, minimizing the leaching and eutrophication that result from the excessive use of conventional P fertilizers. Moreover, recycling P aligns with the broader objectives of resource conservation, waste reduction, and economic efficiency, which are at the heart of a circular economy. This paradigm shift presents an opportunity to reassess and redesign the entire P life cycle, from extraction to use, recovery, and reuse thereby creating a more resilient agricultural system that can withstand the challenges of resource scarcity and environmental degradation.
Water treatment residuals (WTR), a by-product of clarification pretreatment of surface water, e.g., in the seawater desalination industry, offer a significant yet underutilized potential for P recovery. These residuals result from the addition of coagulants like ferric chloride (FeCl3) or Al2(SO4)3 to the treated water, which, under neutral pH conditions, precipitate into a heterogeneous sludge rich in surface area, possessing a pronounced affinity for anion adsorption, including phosphate ions (Ippolito et al. 2011; Zohar et al. 2020; Zohar and Forano 2021). Conventionally, the desalination sector has leaned towards the environmentally unsustainable practice of landfilling massive quantities of iron-rich WTR (Fe-WTR), presenting a missed opportunity for resource recovery and a source of environmental degradation (Netanyahu 2017).
The prospect of repurposing Fe-WTR for P removal from dairy wastewater presents a dual benefit: environmentally, it diverts a substantial waste stream away from landfills, and economically, it generates a valuable alternative to traditional fertilizers. The Recycling of P from wastewater can be achieved through various methods, including co-precipitation or use of adsorbents. Co-precipitation can be accomplished using chemicals such as ferric chloride (FeCl3) and aluminum sulfate (Al2(SO4)3), in the form of minerals like struvite (NH4MgPO4·6H2O), strengite (FePO4·2H2O), vivianite (Fe2+Fe2+2(PO4)2·8H2O), hydroxyapatite (Ca5(PO4)3(OH)), fluorapatite (Ca5(PO4)3F), chlorapatite (Ca5(PO4)3Cl) (Roldán et al. 2002; Wilfert et al. 2015; Fink et al. 2016; Hughes and Rakovan 2019). Adsorption can be achieved using adsorbing materials like layered double hydroxides (LDH), oxides and oxyhydroxides, porous nano silicates, and polymer ligand exchangers (Wendling et al. 2013; Zohar et al. 2017; Bacelo et al. 2020; Zohar and Forano 2021). These absorbent materials that efficiently capture P show very limited release capability, posing a challenge to reusability (Cordell et al. 2011; Wendling et al. 2013). Moreover, this approach does not align with circular economy principles of reducing waste materials through reuse and may not be cost-effective (Loganathan et al. 2014). However, using wastewater as a P source not only adheres to sustainability but also renders/improves P lability and phyto-availability since organic compounds in wastewater weaken phosphorus binding to the adsorbents surfaces (Yang. et al. 2006; Song et al. 2011; Banet et al. 2020). We demonstrated this innovative approach with dairy wastewater and Al- and Fe-based WTRs in Litaor et al., 2019; Zohar et al., 2020, 2018, 2017; Zohar and Forano, 2021.
The primary objective of this paper is to present a detailed cost-benefit analysis of adopting a circular economy framework in the production of P. This analysis will delve into the economic and environmental ramifications of transitioning from traditional, linear models of P fertilizer production to a circular approach that incorporates the recycling of iron waste and dairy wastewater. By repurposing these waste streams into an effective P fertilizer, this paper aims to demonstrate a viable strategy for reducing the depletion of finite global P reserves (Smol 2019). In contrast to linear processes, which are characterized by high input and waste, the circular model embodies the efficient reuse of resources, thereby fostering their long-term sustainability, lessening the burden of environmental and economic waste, and consequently contributing to global economic growth and ecological health (Kirchherr et al. 2017; Berg et al. 2018). This shift not only decreases the dependency on resource-rich nations but also resonates with the ethos of the United Nations’ Sustainable Development Goals, seeking harmony between economic viability, environmental integrity, and social equity (Van Kauwenbergh 2010; Linderholm et al. 2012; Smol 2019). Ultimately, this research will quantify the tangible benefits and highlight potential challenges of implementing a circular economy in P production, thus providing a comprehensive blueprint for stakeholders to make informed decisions about sustainable agricultural practices.
2. Circular P Production
The subsequent section details the P recovery process, from the production and utilization of adsorbents to the recovery and application of P in agricultural settings, forming the basis of this comprehensive cost-benefit evaluation.
Zohar and Forano, (2021), investigated two types of adsorbents for their efficacy in P recovery from wastewater. The first, a synthetic adsorbent, was a commercial calcined oxide material known as layered double hydroxide (LDH), with the formula Mg0.7Al0.3O1.15, provided by KISUMA-Chemicals. The LDH’s layered oxides structure contributes to high sorption capacity. The second was a recycled material derived from the residues of water treatment processes in desalination facilities, known as Fe-WTR, which is composed of a heterogeneous mix of calcite, Fe hydroxides, clays, and organic matter from seawater. Both adsorbents were subjected to P loading using dairy wastewater after clarification with nano-composites (Rytwo 2012), resulting in P and organic compounds enriched adsorbents, designated P-LDH and P-Fe -WTR. Total P concentration in the P-LDH was 7752 mg kg-1, while the P-Fe-WTR had 6100 mg kg-1.
According to Zohar and Forano, (2021), who conducted research using the same adsorbents, the concentrations of TDP released from the adsorbents indicate that P was significantly more labile in P-Fe/O-WTR than in P-LDH in 0.01M KCl solution. Upon mixing with dairy wastewater, P-Fe/O-WTRs demonstrated superiority, yielding approximately 90 mg TDP kg-1, compared to the 1.3–6.4 mg TDP kg-1 produced from P-LDH. Thus, despite P-Fe/O-WTR having a lower P content than P-LDH, it retained much higher P solubility. The practical implication of these findings is that while synthetic materials prove more effective in removing excess P (e.g., treating polluted streams), recycled materials may serve as a valuable resource in P recovery, especially when combined with additional recycling efforts, offering an alternative P fertilizer.
Furthermore, Zohar et al., (2024), evaluated the potential for P-LDH and P-Fe-WTR to perform as P sources, i.e., allow for P release and its subsequent assimilation in plant biomass. The efficiency of the recycled P was tested using lettuce (Lactuca sativa) as a bioindicator in pot experiments, being a plant requiring high P, with quick life cycle, high immunity to crop diseases, and rapid responses to drip irrigation and fertilization (Hoque et al. 2010). In this study, the performance of a conventional slow-release fertilizer (positive control (Super phosphate)) and non-treated soil (negative control) was compared with the P-loaded adsorbents. The difference in performance was taken into consideration in our cost-benefit analysis.
Applying P-Fe-WTR was comparable to the positive control, as it has released this essential nutrient effectively in P-deficient soil more than P-LDH. After the lettuce growth experiment, the length of lettuce leaves in the P-Fe-WTR treatment measured 21.63±2.3 cm, closely resembling the positive control treatment (approximately 22.10±1.2 cm). In contrast, the P-LDH treatment resulted in a length of 14.63±3 cm, more akin to the negative control treatment (12.08±1.6 cm), the presented values exhibit statistically significant differences (P<0.05). Regarding fresh-weight biomass, the results corresponded to the plant height, with the P-Fe-WTR treatment producing 50.8±10.7 g, surpassing the positive control treatment (38.5±6.5 g). Conversely, the P-LDH treatment yielded 12.8±6.7 g, exceeding the biomass of the negative control treatment (5.5±1.5 g), the presented values exhibit statistically significant differences (P<0.05). This promotes crop growth with efficiency comparable to that achieved with commercial fertilizers. The organic compounds abundant in dairy effluents impacted organic and inorganic P binding in P-Fe-WTR, rendering a weaker binding than in P-LDH; due to the LDH’s high sorption capacity and strength was less affected by the organic compounds’ presence, resulting in reduced P availability to the plant. Adding P-Fe-WTR to soils provides a more readily available P source for plants than P-LDH, which is evident in biomass, nutrient concentration in lettuce plants, leaf length, and overall plant development.
Thus, despite P-Fe-WTR having a lower P content than P-LDH, it retained much higher P solubility and phyto-availability. The practical implication of these findings is that while synthetic materials prove more effective in removing excess P (e.g., treating polluted streams), recycled materials may serve as a valuable resource in P recycling, especially when combined with additional recycling efforts, offering an alternative P fertilizer.
3. Cost Analysis of Alternative P Fertilizer Production
In the context of this study, a cost-benefit analysis is conducted to evaluate the economic viability and environmental impact of the circular reproduction of P as detailed above. This analysis takes-into-account the initial investment in the recovery process, operational costs, and the market value of the P-loaded adsorbents as alternative fertilizers. It also considers the environmental benefits derived from diverting waste from landfills, reducing the need for traditional mining, and mitigating water pollution through P removal. The analysis is critical in assessing the overall sustainability of the proposed circular economy approach, highlighting the trade-offs and synergies between economic efficiency and environmental stewardship.
The production costs associated with creating alternative P fertilizers, such as P-Fe-WTR and P-LDH, are critical to determining the economic feasibility of the circular P production approach. Considering the most efficient and cost-effective practices known today, the associated production costs of both adsorbents depend on dairy wastewater used as P source, including the volume processed and the chemicals in use.
2 Clarification pretreatment of the dairy wastewater (solid-liquid separation), used in the presented case, were 3% nano-composite (N/C 24) and 40% ZETAG 9088 (Rytwo 2012), adding a cost of 204 Euros per 111 L treated wastewater; While these chemicals are effective, as they efficiently remove organic solids but leave the high P content in solution, exploring other cost-effective alternatives for clarification methods is advisable.
Additionally, the cost considers the energy required for production, measured in kilowatt-hours, and the total volume of wastewater treated, reported in cubic meters.
Table 1 details the expenses incurred in manufacturing 1 ton of P-Fe-WTR fertilizer, which has a P concentration of 6100 mg kg
-1. Given that Fe-WTR is a recycled waste material and thus incurs no raw material cost, the overall production expense for 1 ton of this fertilizer alternative is estimated at 329 Euros, the highest portion of the cost is attributed to the clarification pretreatment. Conversely, the LDH adsorbent represents a significant cost factor due to its synthetic nature. The cost implication of using LDH, which is not only more expensive but also carries environmental concerns, is substantial. The financial outlay for creating 1 ton of P-LDH, with a 7752 mg kg
-1, is considerably higher, totaling 3,329 Euros (
Table 1).
These contrasting cost profiles underscore the economic advantage of utilizing waste-derived adsorbents over synthetic alternatives. Moreover, the environmental impact, not directly accounted for in the cost but significant in a broader sustainability context, favors the use of recycled materials like Fe-WTR, which align with the goals of a circular economy by minimizing waste and reducing the reliance on non-renewable resources.
4. Economic Benefits of P Recovery
The direct economic benefits derived from P recovery, as investigated in this study, are considerable.
Table 2 presents a bifurcated view of these advantages: the first category (Section A) delineates cost savings in agricultural applications of P, and the second (Section B) enumerates the environmental benefits stemming from reduced industrial P misuse.
In Section A, we calculate substantial cost reductions from reusing Fe-WTR. In Israel, where the detailed process of P recovery is currently being experimented with, diverting Fe-WTR from landfills can save approximately 40 Euros per ton, while in Europe, this figure is markedly higher at 150 Euros per ton (European Environment Agency 2023). Avoidance of penalties for discharging P-laden wastewater, a central contributor to eutrophication and aquatic pollution, could represent a mitigation cost of around 500 Euros per incident (Israeli Ministry of Environmental Protection 2023). The reuse of P also diminishes reliance on quarry mining, with an attendant cost saving of 345 Euros per ton and reduces the need for costlier commercial P fertilizers. Recycling P from organic sources, such as dairy wastewater, saves significant costs. This cyclical use of P not only economizes resources but also plays a pivotal role in reducing environmental damage, making it a dual boon of sustainability and economic sensibility.
Section B focuses on related environmental benefits quantified in related research, particularly the cost implications of soil and water pollution by P. The ecological damage from P pollution in aquatic ecosystems is profound, threatening the survival of unique species and the ecological equilibrium. Assessing the monetary costs of reversing eutrophication - an outcome of P pollution - is complex due to fluctuating factors like the intensity of contamination and the size of the affected water bodies. According to studies by Dodds et al., (2009) and Martín-Hernándaez et al., (2021), the restoration expenses to return an aquatic ecosystem to its baseline state in the U.S. have been significant. Combined restoration costs range from
$0.3 to
$2.8 billion, with the loss in recreational value estimated between
$0.37 and
$1.16 billion annually. Notably, these figures do not consider the extensive time frames required for ecosystems to fully recover, often spanning years, nor do they account for the potential loss of biodiversity that may never be recuperated.
3
While
Table 1 and
Table 2 indicate that the immediate financial returns from the use of the alternative P fertilizer investigated in this study are modest, the long-term economic outlook is promising. The methodology outlined herein paves the way for enduring profitability, particularly considering projected trends in the P market, as well as possible returns to scale in the recycling P-process. Anticipated price escalations, fueled by dwindling natural P reserves and the burgeoning global demand linked to population growth, suggest a favorable shift in the cost-benefit equilibrium. Current and future profitability is also influenced by transportation expenditures; as the costs of fuel climb globally, as depicted in
Figure 1, the expense of transporting P has followed suit. Thus, the adoption of the proposed circular approach to P fertilizer production not only presents an environmentally responsible practice but also a strategic economic advantage as market dynamics evolves.
5. Conclusions
The cost-benefit analysis conducted to produce 1 ton of alternative P fertilizer P-Fe-WTR, with a 1.397% P2O5 composition, underscores a persuasive argument for its adoption. The production incurs variable costs, predominantly from wastewater treatment and energy usage, amounting to approximately 329 Euros per ton. However, the broader fiscal benefits, substantially tip the scales in favor of this alternative approach. Notably, the diversion of iron sludge from landfills affords tangible cost savings 40 Euros per ton in Israel and as much as 150 Euros per ton in Europe validated by figures from the Israeli Ministry of Environmental Protection and the European Environment Agency.
The financial implications extend beyond mere savings. The hefty fines imposed for the release of P-load wastewater, which attract penalties of up to 500 Euros per ton due to the potential for eutrophication and pollution, serve as a significant deterrent against conventional disposal methods. When juxtaposed with the costs of commercial fertilizers and quarry-extracted P, the P-Fe-WTR alternative emerges as a distinctly economical solution.
The analysis presented goes beyond cost savings, highlighting the profound environmental merits of reducing eutrophication and mitigating pollution through the reuse of P-Fe-WTR. This sustainable pathway not only aligns with environmental priorities but also demonstrates clear economic incentives. In contrast to synthetic adsorbents, which are economically and environmentally costly, the study reveals that recycled adsorbents offer an enhanced proposition, circumventing the challenges of limited availability and suboptimal P release observed with synthetic options in plant-soil systems (Zohar and Forano 2021; Zohar et al. 2024). The results presented could even improve over tile with upscaling volume of production using recycled P-Fe WTR processes.
By leveraging two distinct waste streams to create a valuable new resource, this study not only reaffirms the viability of a circular economy in P fertilizer production but also sets a precedent for integrating environmental stewardship with economic rationality. Projections indicate a sustained increase in the cost of commercial fertilizers, driven by heightened demand stemming from population growth, urbanization, and changing dietary habits. Geopolitical tensions and supply chain disruptions amplify this trend, potentially exacerbating price volatility. To navigate this landscape effectively, we embrace strategies focused on resource efficiency, innovation in agricultural practices, and sustainable P management to mitigate the impact of rising prices and ensure food security for future generations. The adoption of P-Fe-WTR as an alternative P fertilizer stands as a compelling cost-benefit prospect, advocating for a shift towards more sustainable and economically viable agricultural practices.
Acknowledgments
This work was supported by the Ministry of Science and Technology of Israel (Iris Zohar PI) [grant No. #3-17523].
References
- Al Rawashdeh R (2023) Estimating short-run (SR) and long-run (LR) demand elasticities of phosphate. Miner Econ 36:239–253. [CrossRef]
- Ayele HS, Atlabachew M (2021) Review of characterization, factors, impacts, and solutions of Lake eutrophication: lesson for lake Tana, Ethiopia. Environ Sci Pollut Res 28:14233–14252. [CrossRef]
- Bacelo H, Pintor AMA, Santos SCR, et al. (2020) Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem Eng J 381:122566. [CrossRef]
- Banet T, Massey MS, Zohar I, et al. (2020) Phosphorus removal from swine wastewater using aluminum-based water treatment residuals. Resour Conserv Recycl X 6:100039. [CrossRef]
- Barnea I, Litaor MI, Shenker M (2012) Evaluation of phosphorus management practices in East Mediterranean altered wetland soils. Soil Use Manag 28:35–44. [CrossRef]
- Berg A, Antikainen R, Hartikainen E, et al. (2018) Reports of the Finnish Environment Institute -Circular Economy for Sustainable Development. Reports Finnish Environ Inst 26:24.
- Carpenter SR, Bennett EM (2011) Reconsideration of the planetary boundary for phosphorus. Environ Res Lett 6:. [CrossRef]
- Cordell D, Drangert JO, White S (2009) The story of phosphorus: Global food security and food for thought. Glob Environ Chang 19:292–305. [CrossRef]
- Cordell D, Rosemarin A, Schröder JJ, Smit AL (2011) Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 84:747–758. [CrossRef]
- Cordell D, White S (2011) Peak phosphorus: Clarifying the key issues of a vigorous debate about long-term phosphorus security. Sustainability 3:2027–2049. [CrossRef]
- Cordell D, White S (2013) Sustainable phosphorus measures: Strategies and technologies for achieving phosphorus security. Agronomy 3:86–116. [CrossRef]
- De-Bashan LE, Bashan Y (2004) Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997-2003). Water Res 38:4222–4246. [CrossRef]
- Denbaly M, Vroomen H (1993) Dynamic Fertilizer Nutrient Demands for Corn: A Cointegrated and Error-Correcting System. Am J Agric Econ 75:203–209. [CrossRef]
- Dodds WK, Bouska WW, Eitzmann JL, et al. (2009) Eutrophication of U. S. freshwaters: Analysis of potential economic damages. Environ Sci Technol 43:12–19. [CrossRef]
- European Environment Agency (2023) Landfill Levy.
- FAO (2022) World fertilizer outlook and trends to 2022.
- Fink JR, Inda AV, Tiecher T, Barrón V (2016) Iron oxides and organic matter on soil phosphorus availability. Cienc e Agrotecnologia 40:369–379. [CrossRef]
- Haygarth PM, Delgado A, Chardon WJ, et al. (2013) Phosphorus in soils and its transfer to water: From fine-scale soil processes to models and solutions in landscapes and catchments. Soil Use Manag 29:1–5. [CrossRef]
- Hoque MM, Ajwa H, Othman M, et al. (2010) Yield and postharvest quality of lettuce in response to nitrogen, phosphorus, and potassium fertilizers. HortScience 45:1539–1544. [CrossRef]
- Hughes JM, Rakovan J (2019) The crystal structure of apatite, Ca5(PO4)3(F,OH,Cl). Phosphates Geochemical, Geobiol Mater Importance 48:1–12. [CrossRef]
- Ippolito JA, Barbarick KA, Elliott HA (2011) Drinking Water Treatment Residuals: A Review of Recent Uses. J Environ Qual 40:1–12. [CrossRef]
- Israeli Ministry of Environmental Protection (2023) Landfill Levy.
- Kirchherr J, Reike D, Hekkert M (2017) Conceptualizing the circular economy: An analysis of 114 definitions. Resour Conserv Recycl 127:221–232. [CrossRef]
- Linderholm K, Tillman AM, Mattsson JE (2012) Life cycle assessment of phosphorus alternatives for Swedish agriculture. Resour Conserv Recycl 66:27–39. [CrossRef]
- Litaor MI, Barnea I, Reichmann O, Zohar I (2016) Evaluation of the ornithogenic influence on the trophic state of East Mediterranean wetland ecosystem using trend analysis. Sci Total Environ 539:. [CrossRef]
- Litaor MI, Chashmonai I, Barnea I, et al. (2013) Assessment of phosphorus fertilizer practices in altered wetland soils using uncertainty analysis. Soil Use Manag 29:55–63. [CrossRef]
- Litaor MI, Schechter S, Zohar I, et al. (2019) Making Phosphorus Fertilizer from Dairy Wastewater with Aluminum Water Treatment Residuals. Soil Sci Soc Am J 83:649–657. [CrossRef]
- Loganathan P, Vigneswaran S, Kandasamy J, Bolan NS (2014) Removal and Recovery of Phosphate From Water Using Sorption. Crit Rev Environ Sci Technol 44:847–907. [CrossRef]
- Martín-Hernándaez E, Martín M, Ruiz-Mercado GJ (2021) A geospatial environmental and techno-economic framework for sustainable phosphorus management at livestock facilities. Resour Conserv Recycl 175:. [CrossRef]
- Netanyahu S (2017) Desalination of sea water - resilience, challenges and risks. Minist Environ Prot Isr 4:38–47.
- Roldán R, Barrón V, Torrent J. (2002) Experimental alteration of vivianite to lepidocrocite in a calcareous medium. Clay Miner 37:709–718. [CrossRef]
- Rytwo G (2012) The use of clay-polymer nanocomposites in wastewater pretreatment. Sci World J 2012:. [CrossRef]
- Smol M (2019) The importance of sustainable phosphorus management in the circular economy (CE) model: the Polish case study. J Mater Cycles Waste Manag 21:227–238. [CrossRef]
- Song X, Pan Y, Wu Q, et al. (2011) Phosphate removal from aqueous solutions by adsorption using ferric sludge. DES 280:384–390. [CrossRef]
- Steen I (1998) Phosphorus availability in the 21st century : Management of a non-renewable resource. Phosphorus Potassium 217:25–31.
- Stephen M. Jasinski (2023) Phosphate Rock, Mineral Commodity Summaries; US Geological Survey: Reston, VA, USA, 2023. Nat Resour US-canadian Relations, Vol 2 Patterns Trends Resour Supplies Policies 122–123.
- Sultenfuss JH, Doyle. WJ (1999) Better Crops With Plant Food - Phosphorus for Agriculture.
- The World Bank (2017) Global Economic Monitor Commodities Phosphate Rock.
- The World Bank (2023) Global Economic Monitor Commodities Phosphate Rock.
- Ukiwe L, Iwu IC, Okere M C (2013) The Role of Inorganic Metal Salts in Wastewater Clarification. J Adv Chem 8:318–322. [CrossRef]
- US National Minerals Information Center (2023) World Mine Production and Reserves, Reserves for China, India, Russia, and Turkey were revised based on Government reports.
- Van Kauwenbergh SJ (2010) World Phosphate Rock Reserves and Resources Technical Bulletin IFDC. Ifdc-T-75 48.
- Wendling LA, Blomberg P, Sarlin T, et al. (2013) Phosphorus sorption and recovery using mineral-based materials: Sorption mechanisms and potential phytoavailability. Appl Geochemistry 37:157–169. [CrossRef]
- Wilfert P, Kumar PS, Korving L, et al. (2015) The Relevance of Phosphorus and Iron Chemistry to the Recovery of Phosphorus from Wastewater: A Review.
- Yang., Zhao YQ, Babatunde AO, et al. (2006) Characteristics and mechanisms of phosphate adsorption on dewatered alum sludge. Sep Purif Technol 51:193–200. [CrossRef]
- Zohar I, Forano C (2021) Phosphorus recycling potential by synthetic and waste materials enriched with dairy wastewater: A comparative physicochemical study. J Environ Chem Eng 9:106107. [CrossRef]
- Zohar I, Ganem HE, DiSegni DM, Jonas-Levi A (2024) The impact of alternative recycled and synthetic phosphorus sources on plant growth and responses, soil interactions and sustainable agriculture - lettuce (Lactuca sativa) as a case model, (Under review).
- Zohar I, Ippolito JA, Bernstein Rose N, Litaor MI (2020) Phosphorus pools in Al and Fe-based water treatment residuals (WTRs) following mixing with agro-wastewater — A sequential extraction study. Environ Technol Innov 18:. [CrossRef]
- Zohar I, Ippolito JA, Massey MS, Litaor IM (2017) Innovative approach for recycling phosphorous from agro-wastewaters using water treatment residuals (WTR). Chemosphere 168:234–243. [CrossRef]
- Zohar I, Massey MS, Ippolito JA, Litaor MI (2018) Phosphorus sorption characteristics in aluminum-based water treatment residuals reacted with dairy wastewater: 1. Isotherms, XRD, and SEM-EDS analysis. J Environ Qual 47:538–545. [CrossRef]
| 1 |
Capacity expansions to phosphate rock production that were expected to be completed by 2026 were ongoing in Brazil, Kazakhstan, Mexico, Morocco, and Russia. Significant new mining projects planned to be completed after 2027 are under development in Australia, Canada, Congo (Brazzaville), Guinea-Bissau, and Senegal. The new mines in Australia and Canada were planned to be primarily used to supply the manufacturing of lithium-iron-phosphate battery cathode active material (US National Minerals Information Center 2023). |
| 2 |
Various chemicals like iron trichloride, iron sulfate, aluminum sulfate, and calcium chloride can clarify wastewater but may unintentionally remove P, which is needed for adsorption (Ukiwe et al. 2013). However, the nanocomposite material 3% N/C 24 (an industrial material manufactured at the GES Your water treatment expert factory, Israel), used in this study, exclusively removes total suspended solids (TSS) while preserving nutrients like P. Physical methods like centrifugation are also available but yield low TSS removal, impacting P adsorption quality. Zohar and Forano, (2021), demonstrated almost twice the P adsorption on Fe-WTR and LDH adsorbents when clarified by N/C 24 compared to centrifugation. |
| 3 |
Using the recycled P material from sludges is not expected to increase soil salinity, as it is often the case that their sludges are washed with tap water in the desalination plant for avoiding soil salinity. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).