Submitted:
11 June 2024
Posted:
12 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Fish Samples and DNA Extraction
2.2. Library Preparation and Sequencing
2.3. Sequence Analysis
2.3.1. Reads Quality Control and Host Removal
2.3.2. Taxonomic Classification
2.3.3. Metagenome Assembly and SDDV Genome Recovery
2.3.4. SDDV Genome Annotation
2.3.5. Comparative Genomics and Phylogenetic Analyses
3. Results
3.1. Taxonomic Profiles of Bacteria and Viruses
3.2. SDDV Genome Recovery
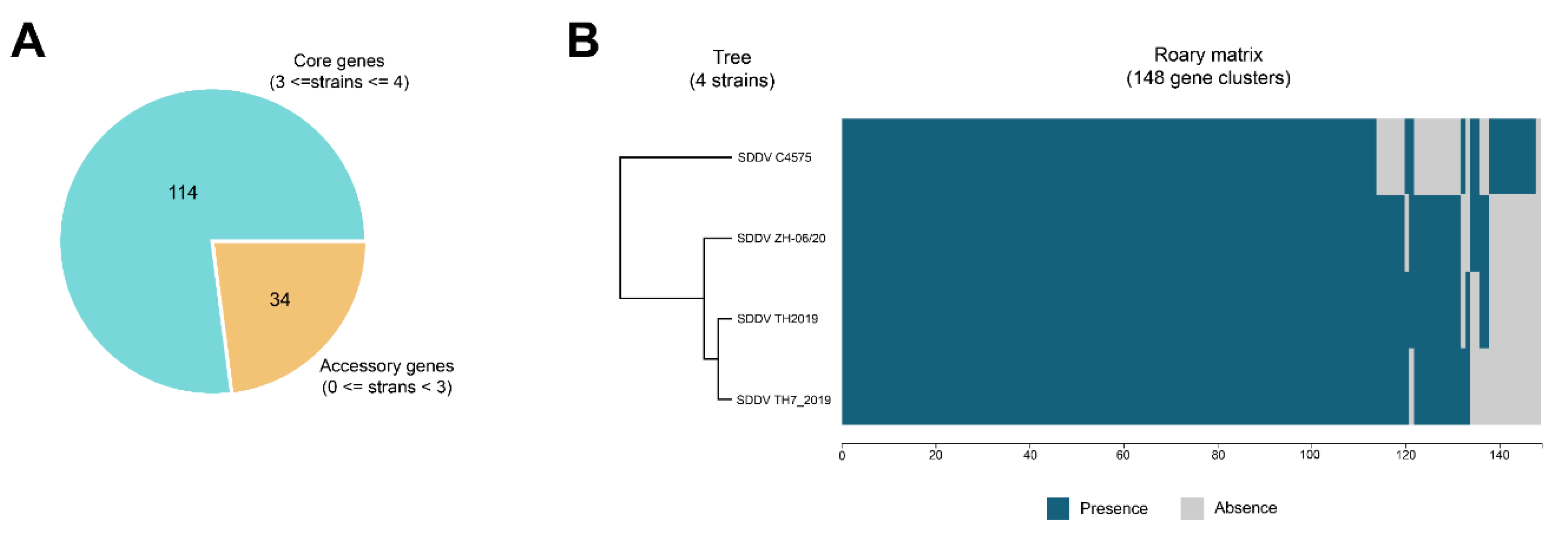
3.3. SDDV Genome Characterization
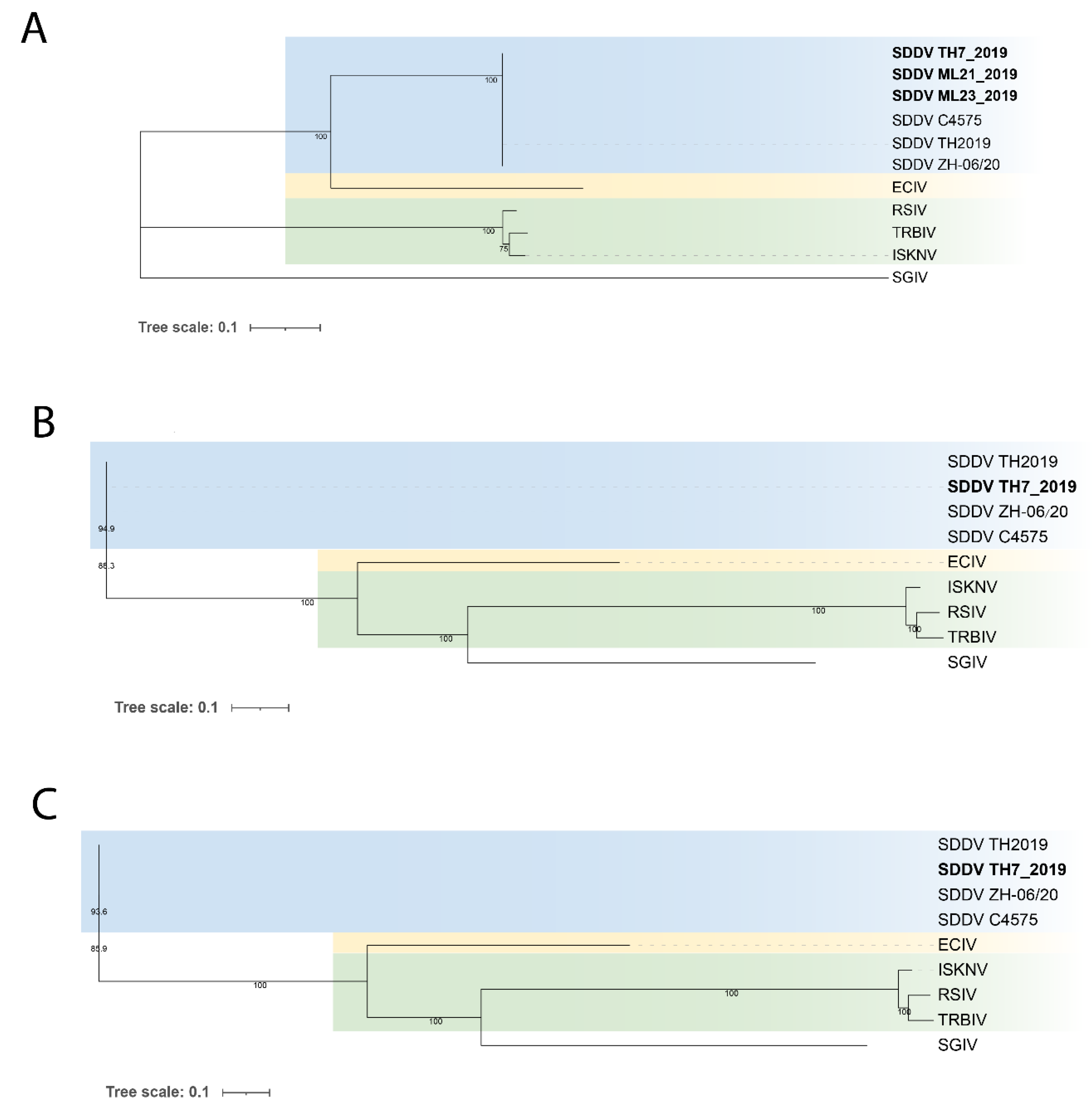
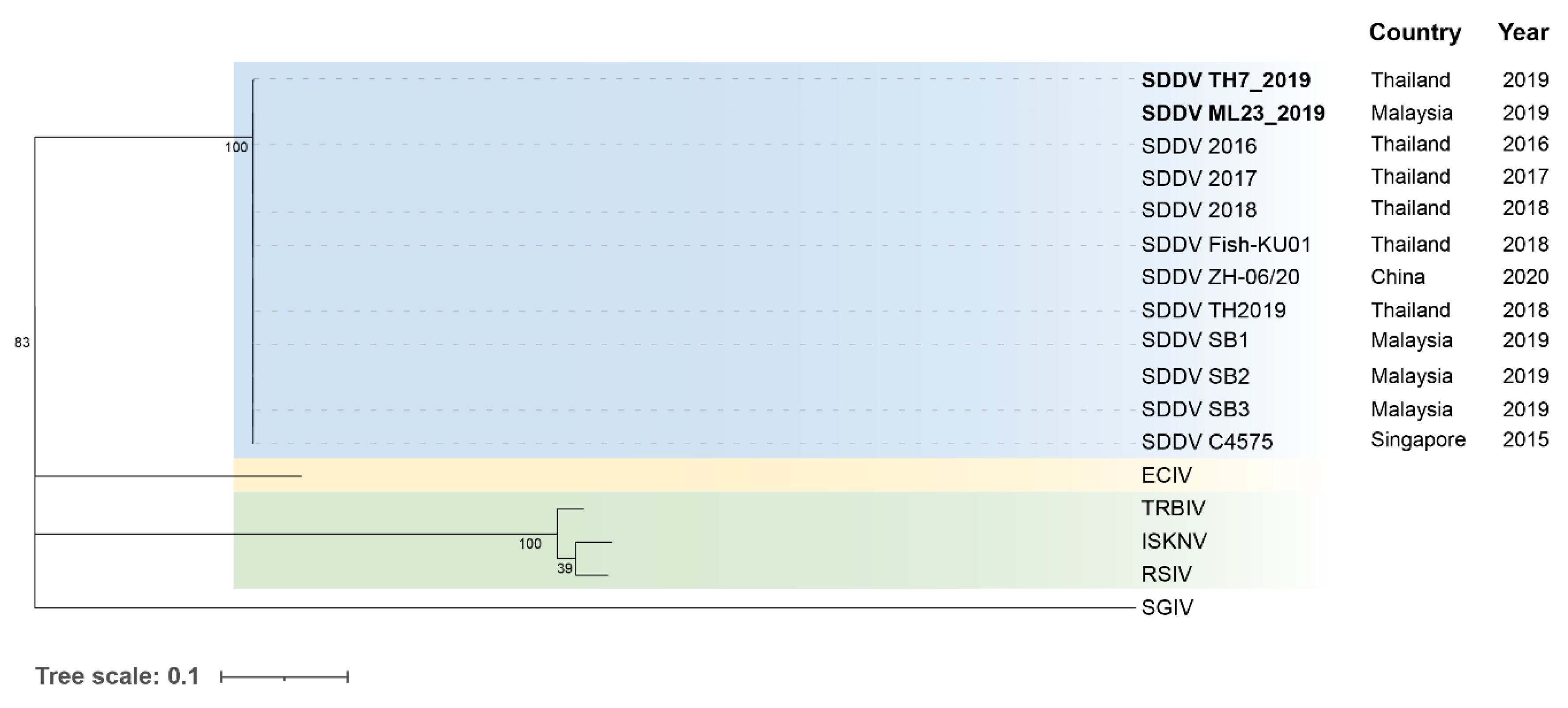
3.4. Comparison Genomics and Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, G.H.; Zhu, Z.Y.; Lo, L.C.; Wang, C.M.; Lin, G.; Feng, F.; Pang, H.Y.; Li, J.; Gong, P.; Liu, H.M.; Tan, J.; Chou, R.; Lim, H.; Orban, L. Genetic variation and population structure of Asian seabass (Lates calcarifer) in the Asia-Pacific region, Aquaculture. 2009, 1, 22-28. https://doi.org/10.1016/j.aquaculture.2009.03.053. [CrossRef]
- Gibson-Kueh, S.; Chee, D.; Chen, J.; Wang, Y.H.; Tay, S.; Leong, L.N.; Ng, M.L.; Jones, J.B.; Nicholls, P.K.; Ferguson, H.W. The pathology of ‘scale drop syndrome’ in Asian seabass, Lates calcarifer Bloch, a first description. J. Fish. Dis. 2012, 35, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Gibson-Kueh; S. Diseases of Asian seabass (or barramundi), Lates calcarifer Bloch, Murdoch University, 2012, pp. 162.
- de Groof, A.; Guelen, L.; Deijs, M.; van der Wal, Y.; Miyata, M.; Ng, K.S.; van Grinsven, L.; Simmelink, B.; Biermann, Y.; Grisez, L.; van Lent, J.; de Ronde, A.; Chang, S.F.; Schrier, C.; van der Hoek, L. A Novel Virus Causes Scale Drop Disease in Lates calcarifer. PLoS Pathog. 2015, 11, e1005074. [Google Scholar] [CrossRef] [PubMed]
- Kayansamruaj, P.; Dong, H.T.; Hirono, I.; Kondo, H.; Senapin, S.; Rodkhum, C. Genome characterization of piscine ‘Scale drop and Muscle Necrosis syndrome’-associated strain of Vibrio harveyi focusing on bacterial virulence determinants. J. Appl. Microbiol. 2018, 124, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Mabrok, M.; Algammal, A.M.; Sivaramasamy, E.; Hetta, H.F.; Atwah, B.; Alghamdi, S.; Fawzy, A.; Avendano-Herrera, R.; Rodkhum, C. Tenacibaculosis caused by Tenacibaculum maritimum: Updated knowledge of this marine bacterial fish pathogen. Front. Cell Infect. Microbiol. 2022, 12, 1068000. [Google Scholar] [CrossRef] [PubMed]
- Nurliyana, M.; Lukman, B.; Ina-Salwany, M.Y.; Zamri-Saad, M.; Annas, S.; Dong, H.T.; Rodkhum, C.; Amal, M.N.A. First evidence of scale drop disease virus in farmed Asian seabass (Lates calcarifer) in Malaysia. Aquaculture. 2020, 528, 735600. [Google Scholar] [CrossRef]
- Senapin, S.; Dong, H.; Meemetta, W.; Gangnonngiw, W.; Sangsuriya, P.; Vanichviriyakit, R.; Sonthi, M.; Nuangsaeng, B. Mortality from scale drop disease in farmed Lates calcarifer in Southeast Asia. J. Fish. Dis. 2019, 42, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, Y.; Fu, W.; Su, H.; Zhang, L.; Huang, C.; Weng, S.; Yu, F.; He, J.; Dong, C. Scale Drop Disease Virus Associated Yellowfin Seabream (Acanthopagrus latus) Ascites Diseases, Zhuhai, Guangdong, Southern China: The First Description. Viruses. 2021, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, N.; Liu, L.; Lin, Q.; Wang, F.; Lai, Y.; Jiang, H.; Pan, H.; Shi, C.; Wu, S. Genotype and host range analysis of infectious spleen and kidney necrosis virus (ISKNV). Virus Genes. 2011, 42, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Fusianto, C.K.; Becker, J.A.; Subramaniam, K.; Whittington, R.J.; Koda, S.A.; Waltzek, T.B.; Murwantoko; Hick, P. M.; Zheng, C. Genotypic Characterization of Infectious Spleen and Kidney Necrosis Virus (ISKNV) in Southeast Asian Aquaculture. Transb. Emerg. Dis. 2023, 2023, 1–16. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Soontara, C.; Dong, H.T.; Phiwsaiya, K.; Senapin, S. Draft genome sequence of scale drop disease virus (SDDV) retrieved from metagenomic investigation of infected barramundi, Lates calcarifer (Bloch, 1790). J. Fish. Dis. 2020, 43, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Mokili, J.L.; Rohwer, F.; Dutilh, B.E. Metagenomics and future perspectives in virus discovery. Curr. Opin. Virol. 2012, 2, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Munang'andu, H.M. Environmental Viral Metagenomics Analyses in Aquaculture: Applications in Epidemiology and Disease Control. Front. Microbiol. 2016, 7, 1986. [Google Scholar] [CrossRef]
- Rigou, S.; Santini, S.; Abergel, C.; Claverie, J.M.; Legendre, M. Past and present giant viruses diversity explored through permafrost metagenomics. Nat. Commun. 2022, 13, 5853. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, F.; Ren, L.; Xiong, Z.; Wu, Z.; Dong, J.; Sun, L.; Zhang, T.; Hu, Y.; Du, J.; Wang, J.; Jin, Q. Unbiased parallel detection of viral pathogens in clinical samples by use of a metagenomic approach. J. Clin. Microbiol. 2011, 49, 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Charoenwai, O.; Senapin, S.; Dong, H.T.; Sonthi, M. Detection of scale drop disease virus from non-destructive samples and ectoparasites of Asian sea bass, Lates calcarifer. J. Fish. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Charoenwai, O.; Meemetta, W.; Sonthi, M.; Dong, H.T.; Senapin, S. A validated semi-nested PCR for rapid detection of scale drop disease virus (SDDV) in Asian sea bass (Lates calcarifer). J. Virol. Methods. 2019, 268, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014, 30, 15, 2114–2120. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome. Biol. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Breitwieser, F.P.; Thielen, P.; Salzberg, S.L. Bracken: estimating species abundance in metagenomics data. PeerJ Comput. Sci. 2017, 3, e104. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. 2009.
- Rstudio Team. RStudio: Integrated Development Environment for R. 2015. http://www.rstudio.com/.
- Li, D.; Luo, R.; Liu, C.M.; Leung, C.M.; Ting, H.F.; Sadakane, K.; Yamashita, H.; Lam, T.W. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods. 2016, 102, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: architecture and applications. BMC Bioinformatics. 2009, 10, 421. [Google Scholar] [CrossRef]
- Manni, M.; Berkeley, M.R.; Seppey, M.; Simão, F.A.; Zdobnov, E.M. BUSCO Update: Novel and Streamlined Workflows along with Broader and Deeper Phylogenetic Coverage for Scoring of Eukaryotic, Prokaryotic, and Viral Genomes. Mol. Biol. Evol. 2021, 38, 4647–4654. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.; Chen, G.L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.C.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome. Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, Y. pyGenomeViz: A genome visualization python package for comparative genomics. 2022. https://github.com/moshi4/pyGenomeViz.
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232-235. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Kaas, R.S.; Leekitcharoenphon, P.; Aarestrup, F.M.; Lund, O. Solving the Problem of Comparing Whole Bacterial Genomes across Different Sequencing Platforms. PLOS ONE. 2014, 9, e104984. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Benson, G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015, 31, 3691–3693. [Google Scholar] [CrossRef] [PubMed]
- Kerddee, P.; Dong, H.T.; Chokmangmeepisarn, P.; Rodkhum, C.; Srisapoome, P.; Areechon, N.; Del-Pozo, J.; Kayansamruaj, P. Simultaneous detection of scale drop disease virus and Flavobacterium columnare from diseased freshwater-reared barramundi Lates calcarifer. Dis. Aquat. Organ. 2020, 140, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.F.; Ng, K.S.; Grisez, L.; De Groof, A.; Vogels, W.; Van der Hoek, L.; Deijs, M. Novel Fish Pathogenic Virus, in: W. I.P.O.I. Bureau (Ed.) 2018.
- Dang, M.; Dien, T.D.; Van, U.P.; Ha, V.T.; Dung, V.V.; Hieu, N.T.D.; Hua, V.C.; Hue, N.T.K.; Giang, N.T.T.; Truong, V.H.; Dong, H.T. The first description of histopathology of Lates calcarifer herpesvirus (LCHV) infection in barramundi (Lates calcarifer). Aquaculture. 2023, 565, 739091. [Google Scholar] [CrossRef]
- Girisha, S.K.; Puneeth, T.G.; Nithin, M.S.; Naveen Kumar, B.T.; Ajay, S.K.; Vinay, T.N.; Suresh, T.; Venugopal, M.N.; Ramesh, K.S. Red sea bream iridovirus disease (RSIVD) outbreak in Asian seabass (Lates calcarifer) cultured in open estuarine cages along the west coast of India: First report. Aquaculture. 2020, 520, 734712. [Google Scholar] [CrossRef]
- Sumithra, T.G.; Krupesha Sharma, S.R.; Neelima, L.; Dhanutha, N.R.; Joshy, A.; Anusree, V.N.; Gayathri, S.; Raghu, R.K.; Praveen, N.D.; Thomas, S.; Rajesh, K.M. Red sea bream iridovirus infection in cage farmed Asian sea bass (Lates calcarifer): Insights into the pathology, epizootiology, and genetic diversity. Aquaculture. 2022, 548, 737571. [Google Scholar] [CrossRef]
- Domingos, J.A.; Shen, X.; Terence, C.; Senapin, S.; Dong, H.T.; Tan, M.R.; Gibson-Kueh, S.; Jerry, D.R. Scale Drop Disease Virus (SDDV) and Lates calcarifer Herpes Virus (LCHV) Coinfection Downregulate Immune-Relevant Pathways and Cause Splenic and Kidney Necrosis in Barramundi Under Commercial Farming Conditions. Front. Genet. 2021, 12, 666897. [Google Scholar] [CrossRef] [PubMed]
- Bass, D.; Stentiford, G.D.; Wang, H.C.; Koskella, B.; Tyler, C.R. The Pathobiome in Animal and Plant Diseases. Trends Ecol. Evol. 2019, 34, 996–1008. [Google Scholar] [CrossRef]
- Kim, W.S.; Oh, M.J.; Kim, J.O.; Kim, D.; Jeon, C.H.; Kim, J.H. Detection of megalocytivirus from imported tropical ornamental fish, paradise fish Macropodus opercularis. Dis. Aquat. Organ. 2010, 90, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Kurita, J.; Nakajima, K. Megalocytiviruses Viruses 2012, 4, 521-538. [CrossRef] [PubMed]
- Fu, Y.; Li, Y.; Zhang, W.; Fu, W.; Li, W.; Zhu, Z.; Weng, S.; He, J.; Dong, C. Effectively protecting Asian seabass Lates calcarifer from ISKNV-I, ISKNV-II, RSIV-II and SDDV by an inactivated ISKNV-I and SDDV bivalent vaccine. Aquaculture. 2023, 566, 739218. [CrossRef]
- Halaly, M.A.; Subramaniam, K.; Koda, S.A.; Popov, V.L.; Stone, D.; Way, K.; Waltzek, T.B. Characterization of a Novel Megalocytivirus Isolated from European Chub (Squalius cephalus). Viruses. 2019, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.; Deijs, M.; Canuti, M.; van Schaik, B.D.; Faria, N.R.; van de Garde, M.D.; Jachimowski, L.C.; Jebbink, M.F.; Jakobs, M.; Luyf, A.C.; Coenjaerts, F.E.; Claas, E.C.; Molenkamp, R.; Koekkoek, S.M.; Lammens, C.; Leus, F.; Goossens, H.; Ieven, M.; Baas, F.; van der Hoek, L. A sensitive assay for virus discovery in respiratory clinical samples. PLoS One. 2011, 6, e16118. [Google Scholar] [CrossRef] [PubMed]
- De Vries, M.; Oude Munnink, B.B.; Deijs, M.; Canuti, M.; Koekkoek, S.M.; Molenkamp, R.; Bakker, M.; Jurriaans, S.; Van Schaik, B.D.C.; Luyf, A.C.; Olabarriaga, S.D.; Van Kampen, A.H.C.; Van der Hoek, L. Performance of VIDISCA-454 in feces-suspensions and serum. Viruses. 2012, 4, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- He, J.G.; Deng, M.; Weng, S.P.; Li, Z.; Zhou, S.Y.; Long, Q.X.; Wang, X.Z.; Chan, S.-M. Complete Genome Analysis of the Mandarin Fish Infectious Spleen and Kidney Necrosis Iridovirus. Virology. 2001, 291, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.J.; Chinchar, V.G. Ranaviruses. Springer Cham. 2015.
- Song, W.J.; Qin, Q.W.; Qiu, J.; Huang, C.H.; Wang, F.; Hew, C.L. Functional genomics analysis of Singapore grouper iridovirus: complete sequence determination and proteomic analysis. J. Virol. 2004, 78, 12576-90. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, Y.; Cai, J.; Wei, S.; Gao, R.; Qin, Q. Identification and characterization of a tumor necrosis factor receptor-like protein encoded by Singapore grouper iridovirus. Virus Res. 2013, 178, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Gammon, D.B.; Gowrishankar, B.; Duraffour, S.; Andrei, G.; Upton, C.; Evans, D.H. Vaccinia virus-encoded ribonucleotide reductase subunits are differentially required for replication and pathogenesis. PLoS Pathog. 2010, 6, e1000984. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, X.; Liu, H.; Gong, J.; Ouyang, Z.; Cui, H.; Cao, J.; Zhao, Y.; Wang, X.; Jiang, Y.; Qin, Q. Complete sequence determination of a novel reptile iridovirus isolated from soft-shelled turtle and evolutionary analysis of Iridoviridae. BMC Genomics. 2009, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Kurita, J.; Nakajima, K. Megalocytiviruses. Viruses. 2012, 4, 521-539. [CrossRef] [PubMed]
- Eaton, H.E.; Ring, B.A.; Brunetti, C.R. The genomic diversity and phylogenetic relationship in the family Iridoviridae. Viruses. 2010, 2, 1458-75. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Kitamura, S.; Jung, S.J.; Miyadai, T.; Tanaka, S.; Fukuda, Y.; Kim, S.R.; Oh, M.J. Genetic variation and geographic distribution of megalocytiviruses. J. Microbiol. 2008, 46, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Syed Musthaq, S.; Sudhakaran, R.; Ishaq Ahmed, V.P.; Balasubramanian, G.; Sahul Hameed, A.S. Variability in the tandem repetitive DNA sequences of white spot syndrome virus (WSSV) genome and suitability of VP28 gene to detect different isolates of WSSV from India. Aquaculture. 2006, 256, 34–41. [Google Scholar] [CrossRef]
- Wongteerasupaya, C.; Pungchai, P.; Withyachumnarnkul, B.; Boonsaeng, V.; Panyim, S.; Flegel, T.W.; Walker, P.J. High variation in repetitive DNA fragment length for white spot syndrome virus (WSSV) isolates in Thailand. Dis. Aquat. Organ. 2003, 54, 253-7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xiao, C.T.; Fan, Y.; Cai, Z.; Lu, C.; Zhang, G.; Jiang, T.; Tan, Y.; Peng, Y. Homologous recombination shapes the genetic diversity of African swine fever viruses. Vet. Microbiol. 2019, 236, 108380. [Google Scholar] [CrossRef] [PubMed]
- Goller, K.V.; Malogolovkin, A.S.; Katorkin, S.; Kolbasov, D.; Titov, I.; Höper, D.; Beer, M.; Keil, G.M.; Portugal, R.; Blome, S. Tandem repeat insertion in African swine fever virus, Russia, 2012. Emerg. Infect. Dis. 2015, 21, 731-2. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Iversen, E.S., Jr.; Parmigiani, G. Classification of Missense Mutations of Disease Genes. J. Am. Stat. Assoc. 2005, 100, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Balasco, N.; Damaggio, G.; Esposito, L.; Colonna, V.; Vitagliano, L. A comprehensive analysis of SARS-CoV-2 missense mutations indicates that all possible amino acid replacements in the viral proteins occurred within the first two-and-a-half years of the pandemic. Int. J. Biol. Macromol. 2024, 266, 131054. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.Y.; Jia, K.T.; Yang, B.; Huang, J. Complete genome sequence of a Megalocytivirus (family Iridoviridae) associated with turbot mortality in China. Virol J. 2010, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yoshiura, Y.; Kamaishi, T.; Yoshida, K.; Nakajima, K. Prevalence of red sea bream iridovirus among organs of Japanese amberjack (Seriola quinqueradiata) exposed to cultured red sea bream iridovirus. J. Gen. Virol. 2013, 94, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
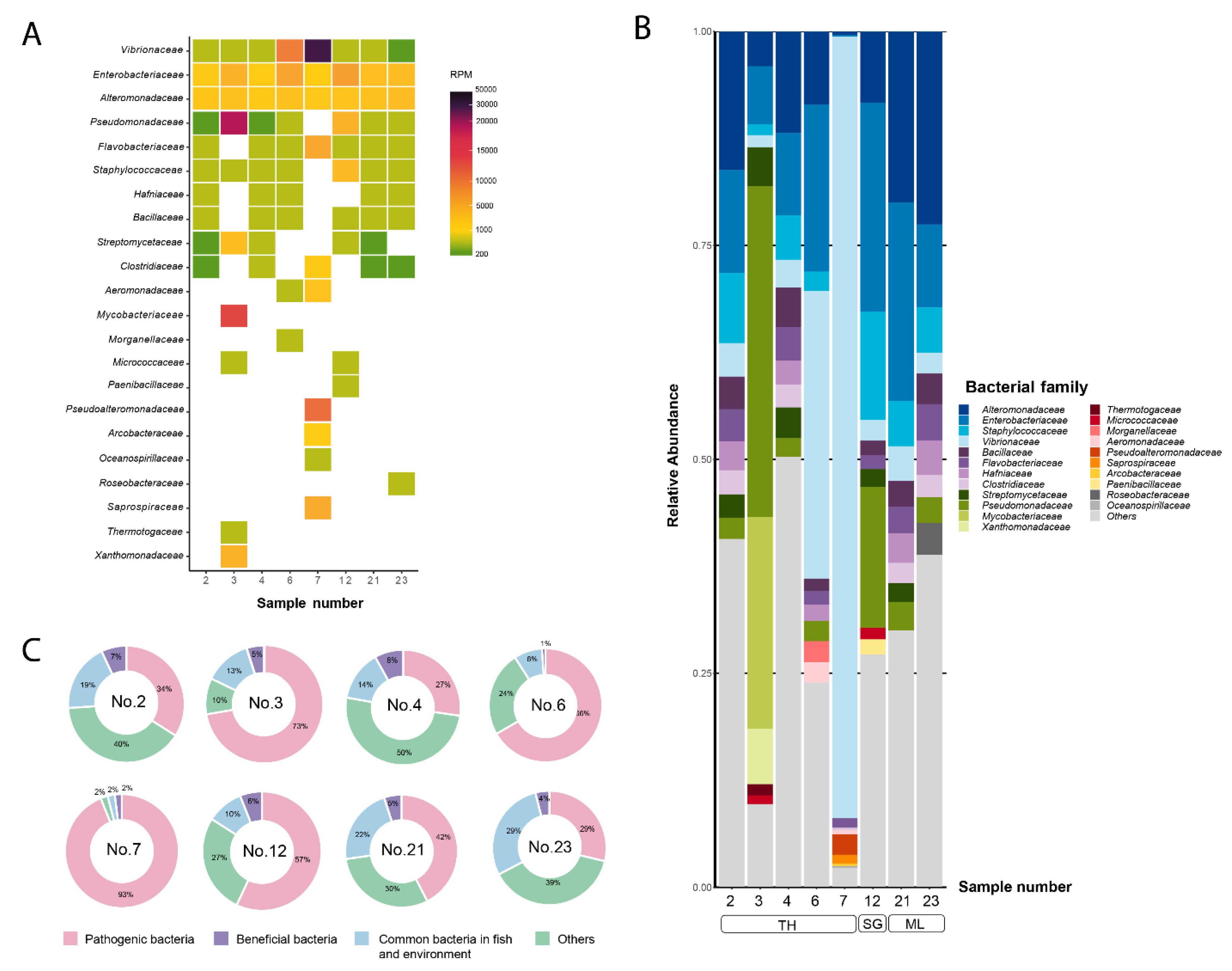
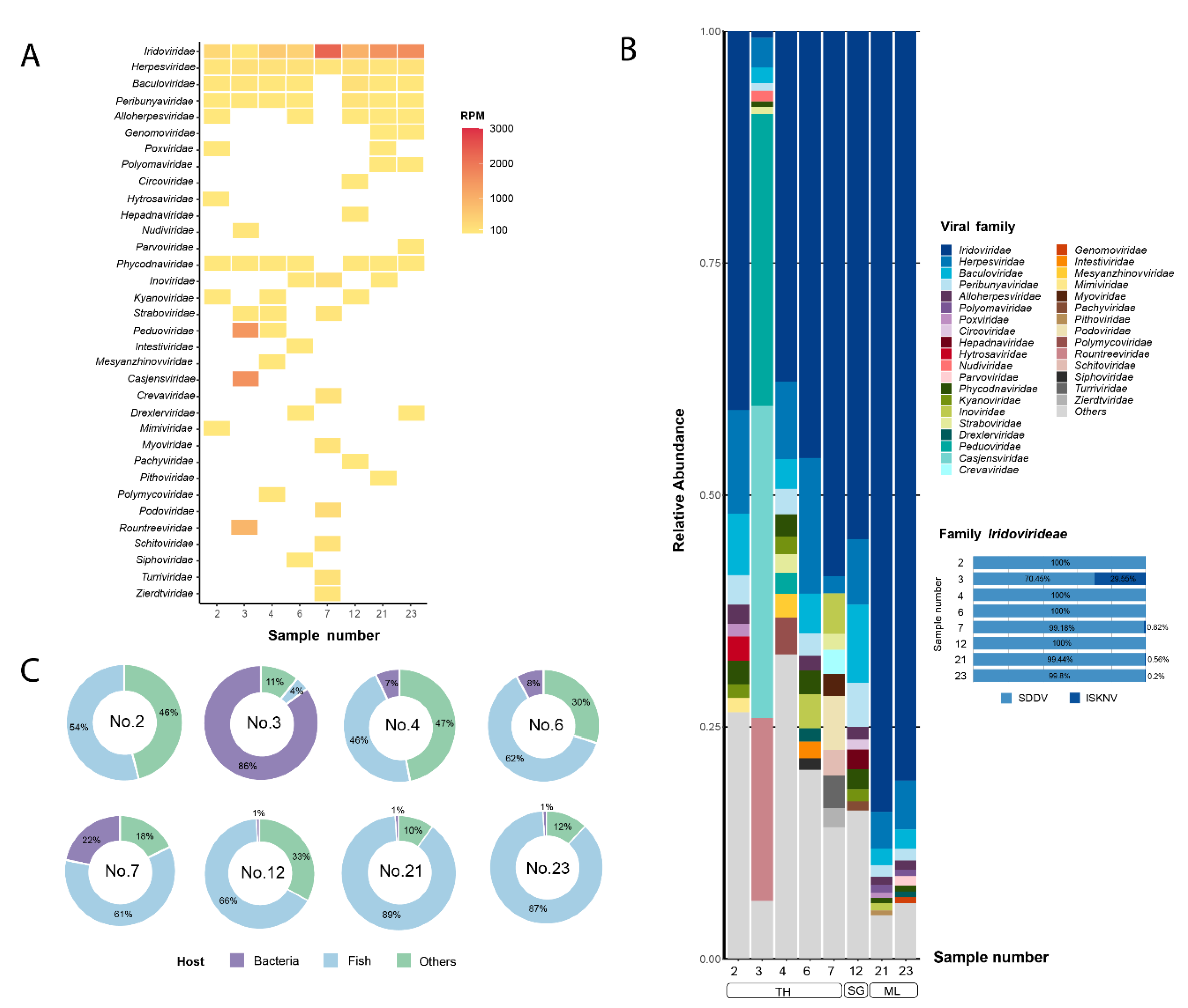
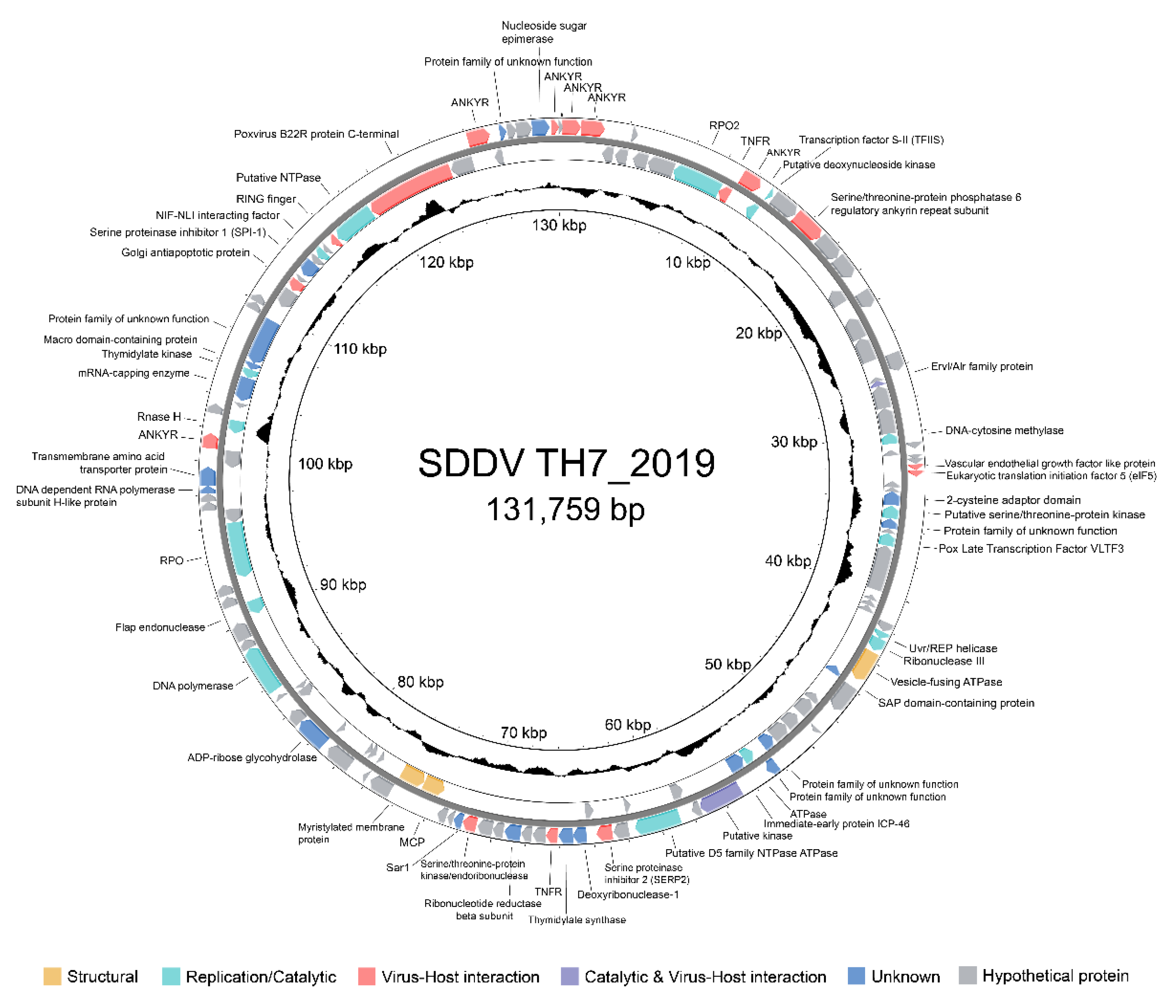
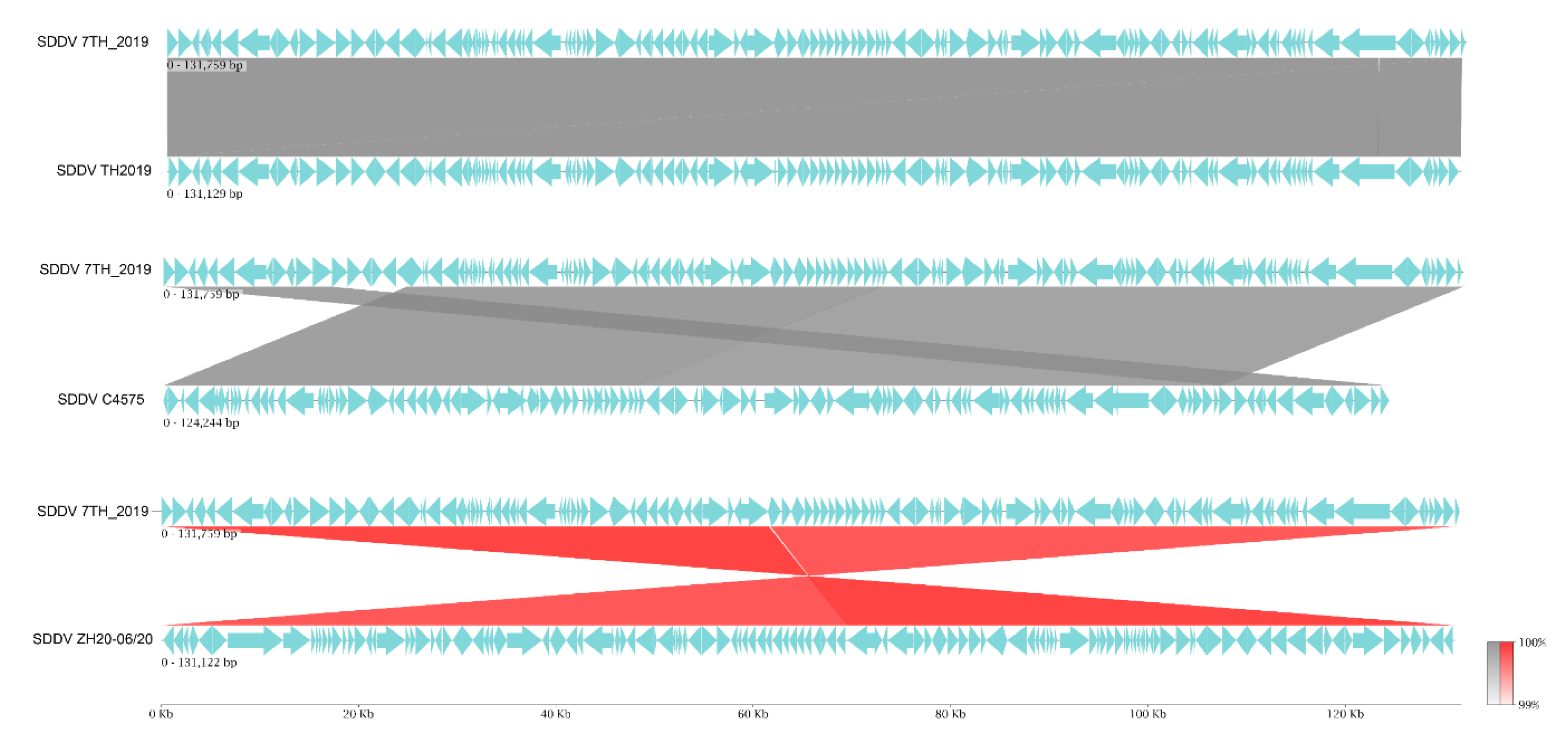
| Metagenomic Sample no. |
Geographical Origin | Year | Organ |
| 2 | Chanthaburi, Thailand | 2016 | Liver |
| 3 | Chanthaburi, Thailand | 2016 | Liver |
| 4 | Chanthaburi, Thailand | 2017 | Liver |
| 6 | Chanthaburi, Thailand | 2018 | Liver |
| 7 | Chanthaburi, Thailand | 2019 | Fin |
| 12 | Singapore | 2019 | Kidney |
| 21 | Selangor, Malaysia | 2019 | Pool internal organs (liver, spleen, and kidney) |
| 23 | Selangor, Malaysia | 2019 | Pool internal organ (liver, spleen, and kidney) |
| Metagenomic sample no. |
Number of Raw Reads | Unclassified reads (%) |
Classified Reads (%) |
Bacterial Reads (%) |
Viral Reads (%) |
| 2 | 1,577,409 | 98.90 | 1.10 | 93.13 | 6.87 |
| 3 | 3,012,060 | 94.16 | 5.84 | 92.04 | 7.96 |
| 4 | 3,144,439 | 98.70 | 1.30 | 88.75 | 11.25 |
| 6 | 1,422,958 | 97.30 | 2.70 | 96.62 | 3.38 |
| 7 | 2,844,173 | 57.19 | 42.81 | 99.09 | 0.91 |
| 12 | 2,017,399 | 97.55 | 2.45 | 92.54 | 7.46 |
| 21 | 1,515,485 | 98.77 | 1.23 | 85.10 | 14.90 |
| 23 | 2,096,503 | 98.69 | 1.31 | 84.42 | 15.58 |
| Metagenomic Sample No. |
Number of SDDV Contigs |
SDDV Strain Name |
Length (bp) | Percent Covered1 |
BUSCO Search | ||
| Complete (C) |
Fragmented (F) | Missing (M) |
|||||
| 2 | 36 | TH2_2016 | 16,035 | 12.3 | 1 | 0 | 9 |
| 4 | 126 | TH4_2017 | 90,045 | 80.0 | 1 | 6 | 3 |
| 6 | 71 | TH6_2018 | 30,800 | 23.1 | 1 | 2 | 7 |
| 7 | 1 | TH7_2019 | 131,759 | 100 | 10 | 0 | 0 |
| 12 | 113 | SG12_2019 | 108,163 | 81.9 | 3 | 7 | 0 |
| 21 | 79 | ML21_2019 | 127,278 | 92.9 | 7 | 3 | 0 |
| 23 | 28 | ML23_2019 | 130,742 | 98.9 | 8 | 2 | 0 |
| Species | Isolate Name | Host | Year | Geographical Origin | GC (%) | Length (bp) | ORFs | GenBank accession no. |
Reference |
| Scale drop disease virus (SDDV) | TH7_2019 | Asian seabass (Lates calcarifer) |
2019 | Thailand | 36.6 | 131,759 | 134 | PP660347 | This study |
| Scale drop disease virus (SDDV) | TH2019 | Asian seabass (Lates calcarifer) |
2018 | Thailand | 36.6 | 131,192 | 135 | MN562489 | [12] |
| Scale drop disease virus (SDDV) | C4575 | Asian seabass (Lates calcarifer) |
2015 | Singapore | 37.0 | 124,244 | 129 | KR139659 | [4] |
| Scale drop disease virus (SDDV) | ZH-06/20 | Yellow seabream (Acanthopagrus latus) |
2020 | China | 36.56 | 131,122 | 135 | OM037668 | [9] |
| Infectious spleen and kidney necrosis virus (ISKNV) | Mandarin fish (Siniperca chuatsi) |
2001 | China | 54.78 | 111,362 | 124 | AF371960 | [52] | |
| European chub iridovirus (ECIV) | LEC15001 | European chub (Squalius cephalus) |
2005 | United Kingdom | 38.5 | 128,216 | 108 | MK637631 | [49] |
| Turbot reddish body iridovirus (TRBIV) | Turbot (Scophthalmus maximus) |
2006 | China | 55.0 | 110,104 | 114 | GQ273492 | [67] | |
| Red seabream iridovirus (RSIV) | KagYT-96 | Japanese amberjack (Seriola quinqueradiata) |
1996 | Japan | 53.0 | 112,719 | 117 | MK689686 | [68] |
| Singapore grouper iridovirus (SGIV) | Brown-spotted grouper (Epinephelus tauvina) |
2004 | Singapore | 48.5 | 140.131 | 162 | AY521625 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





