1. Introduction
In rare cases SARS-CoV-2 mRNA vaccination entails a variety of chronic sequelae summarily addressed as post-acute COVID-19 vaccination syndrome (PACVS) [
1]. PACVS imposes as a distinct etiology [
1,
2]. However, it shares features with complex multisystemic dysautonomia syndromes not related to vaccination, such as myalgic encephalomyelitis/ chronic fatigue syndrome (ME/CFS) [
3,
4], postural tachycardia syndrome (POTS) [
5], fibromyalgia/chronic pain syndrome [
6], small fiber neuropathy (SFN) [
7] or mast cell activation syndrome (MCAS) [
8].
Initial results of our recent study [
2] suggest that the serological vaccination–response of PACVS-affected individuals is significantly altered, allowing objective discrimination of PACVS from the normal post-vaccination state by diagnostic blood markers, most notably increases in interleukins 6 and 8 and alterations of certain receptor-antibodies. While these alterations of blood markers are not disease-specific, they lend support to the notion that PACVS is a somatic syndrome. However, the clinical presentation of that putative syndrome is so far poorly defined.
Here we attempt to more precisely delineate the disease phenotype of PACVS by (i) evaluating a host of clinical data registered in the context of the above study [
2] and (ii) analyzing a broad panel of established blood markers indicative of specific etiologies and organ dysfunctions. Based on these data, we propose a consolidated clinical phenotype of PACVS, which may enable a deeper comprehension of the fundamental pathological mechanism of that novel syndrome and hopefully promote the development of dedicated therapy concepts.
2. Materials and Methods
2.1. Study Participants
Recruitment and selection of study participants has previously been described [
2]. Briefly, N = 159 females and N = 32 males (mean/ median age = 40/ 39 years) suffering from chronic sequelae of SARS-CoV-2 mRNA vaccination for five months or more were included [
2]. Clinical trial protocols were approved by the local ethics board of Heinrich-Heine University Düsseldorf (study number 2022-1948). The investigation conforms to the principles outlined in the World’s Medical Association
Declaration of Helsinki. Before inclusion, all participants have given written informed consent.
2.2. Laboratory Measurements
PACVS-associated features of blood markers were determined in blood samples obtained from the study participants by cubital vein puncture. Blood samples were kept at 4 °C until delivery to the laboratory within 48 h. Serum was prepared immediately upon receipt and stored at −20 °C or −80 °C until analysis. Hemolytic sera were rejected, based on enhanced values of free hemoglobin. Receptor-antibodies were determined with commercially available immuno-assays (CellTrend GmbH, Luckenwalde, Germany) [
2]. SARS-CoV-2 serology was monitored by panIg-reactivity against SARS-CoV-2 spike S1 protein (SAB) and SARS-CoV-2 nucleocapsid protein (NAB) [
9]. Soluble Neurofilament light chains (sNFL) were measured using the SIMOA NF-light V2 Advantage Kit (Quanterix Corporation, Billercia, MA01821, USA). sNFL values were adjusted to body mass index (BMI) and age, and z-scores were calculated according to a published control cohort [
10]. All other laboratory test were performed by accredited routine laboratory diagnostic procedures and related to established routine reference values. Reference values for total IL-8 were derived from reference values of free IL-8 (< 62 pg/ml) by multiplication with five, estimating the erythrocyte-bound fraction at 80% according to [
11].
2.3. Clinical Data
Clinical data were retrieved by online registry of symptoms established for ME/CSF, POTS, MCAS and SFN [
4,
5,
7,
8] and/ or frequently exhibited by subjects suffering from chronic post-vaccination sequelae (
Table 1). The maximal number of queries in the questionnaire was limited by ethical concerns. Therefore, not all guideline criteria of ME/CSF, POTS, MCAS or SFN [
4,
5,
7,
8] could be registered. Incidentally, a very similar selection of symptoms has previously been monitored in a survey of COVID-19 sequelae [
12]. Of note, symptoms registered for the study subjects (i) had been triggered by SARS-CoV-2 mRNA vaccination and (ii) had persisted at time of sample acquisition for five months or more. Symptoms were registered based on self-assessment, which in most cases was corroborated by external physicians. However, medical case histories were not subjected to systematic analysis by the study team, nor were participants subjected to direct physical examination by the study team.
2.3. Statistical Methods
Graph Pad Prism 9 for Windows or Apple Macintosh (released 2020) were used for quantitative data analysis. Non-normally distributed data (Shapiro-Wilk) was descriptively analyzed by mean/median values and interquartile-range. Differences between study subjects and controls were analyzed by the Mann-Whitney-U test (two-tailed). Spearman’s correlations were based on 95% confidence limits and assumed to be good at r ≥ 0.7. Statistical significance was assumed at p < 0.0001. Missing data was handled by listwise deletion. Participants were assigned to ME/CSF, POTS, MCAS or SFN when reporting more than the cohort-average of related symptoms as specified in published guidelines [
4,
5,
7,
8] and/ or when having been explicitly diagnosed with that syndrome by a physician. Overlap of syndrome assignments was analyzed by Chi-square tests. Draw-io V21.6.1 (JGraph Ltd., Northampton, UK, release 2023) was used for extended Venn-diagram analysis.
Hierarchical clustering of symptoms was performed using a modified Jaccard index as similarity measure, defined as the mean of the standard Jaccard index and the reversed Jaccard index in which event and non-event are exchanged. The modified Jaccard index was used because some symptoms occurred in the majority of patients. Clusters were selected at the level h = 1.8 using the cut-tree function of the computer language R, based on medical knowledge. The patients were then assigned to the eight clusters previously defined using the modified Jaccard index. This was done in three different ways: Proportion of symptoms in the cluster (prop), Jaccard index with the symptoms to the cluster, and modified Jaccard index (average of Jaccard index and the Jaccard index of the negated values).
Association of blood marker abnormalities (ferritin index, fT3, IgG3, IgG4 and z-score of sNFL) with symptom clusters was tested by plotting the scatter of each blood marker exhibiting abnormalities against corresponding cluster-assignments of the patients based on (i) the proportion of cluster-specific symptoms presented by the patient, (ii) the Jaccard index, or (iii) a modified Jaccard index defined as the mean of the classic Jaccard index and the reversed Jaccard index with respect to the symptoms not indicated by the corresponding patient and not lying within the symptom cluster.
3. Results
3.1. Prevalence of ME/CFS-, POTS-, SFN- and MCAS-Associated Symptoms in PACVS
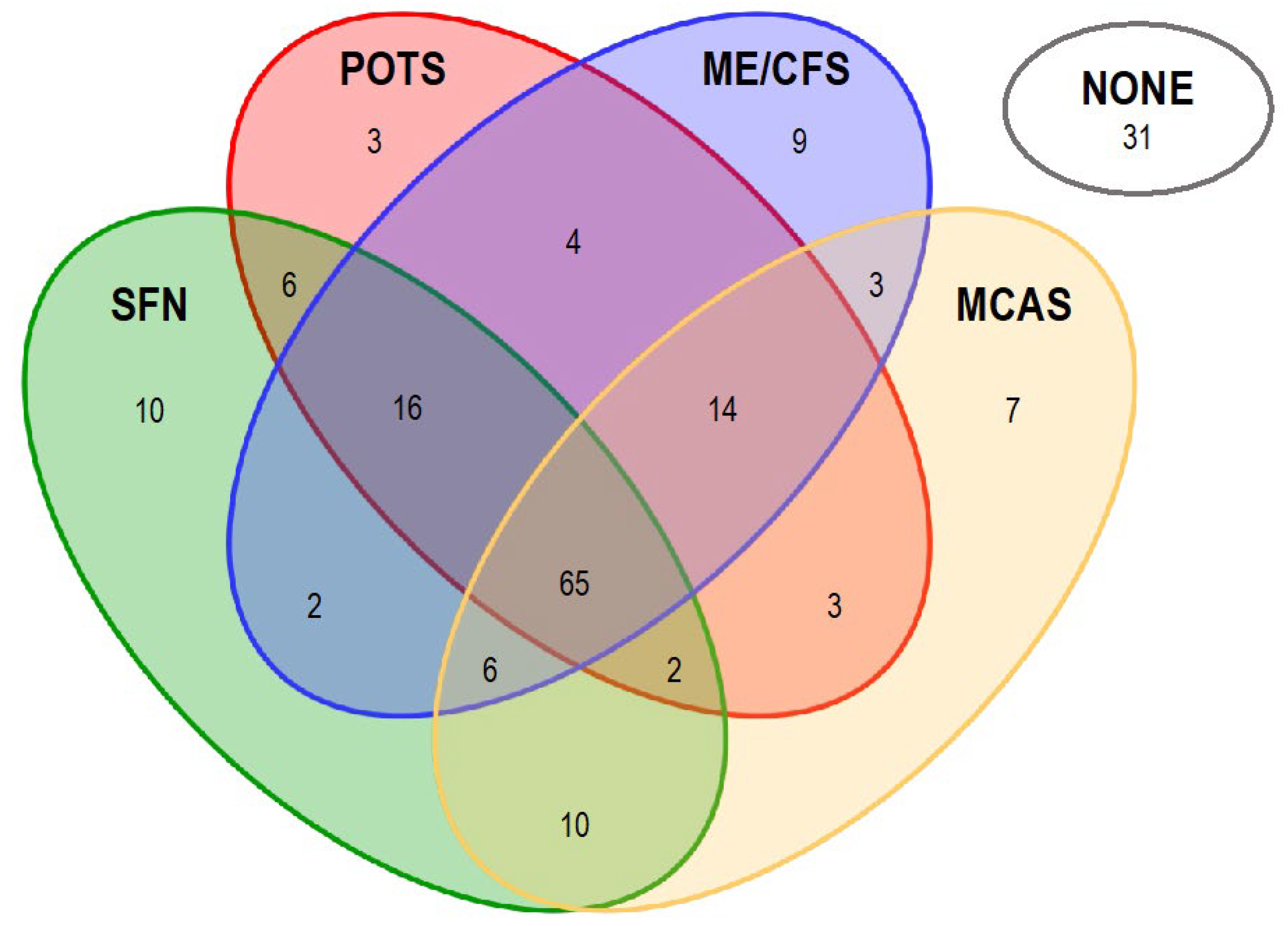
Symptoms registered for PACVS-subjects participating in this study are listed in
Table 1. Symptoms conforming to criteria established for ME/CSF, POTS, MCAS or SFN [
4,
5,
7,
8] were the ones most frequently registered. Based thereon, a majority of PACVS-cases in the study could be assigned to at least one of these syndromes (
Figure 1). However, study participants also reported a variety of disease symptoms not associated with ME/CSF, POTS, MCAS or SFN (
Table 1). Covariance analysis of syndrome assignments showed that only 29/191 PACVS-cases in the study could be unambiguously assigned to either ME/CSF, POTS, MCAS or SFN. The majority (131/191) of cases qualified simultaneously for more than one of the above syndromes. One third (65/191) of cases even qualified simultaneously for all four syndromes. In contrast, 16 % (31/191) of cases could not be assigned to any one of the four syndromes (
Figure 1). These observations support the previous proposition [
1,
2] that PACVS constitutes a disease, or syndrome,
sui generis, which shares certain features with, but is distinct from, ME/CSF, POTS, MCAS or SFN. Therefore, we readdressed the data by an unbiased approach.
3.2. Unbiased Clustering of PACVS-Associated Symptoms
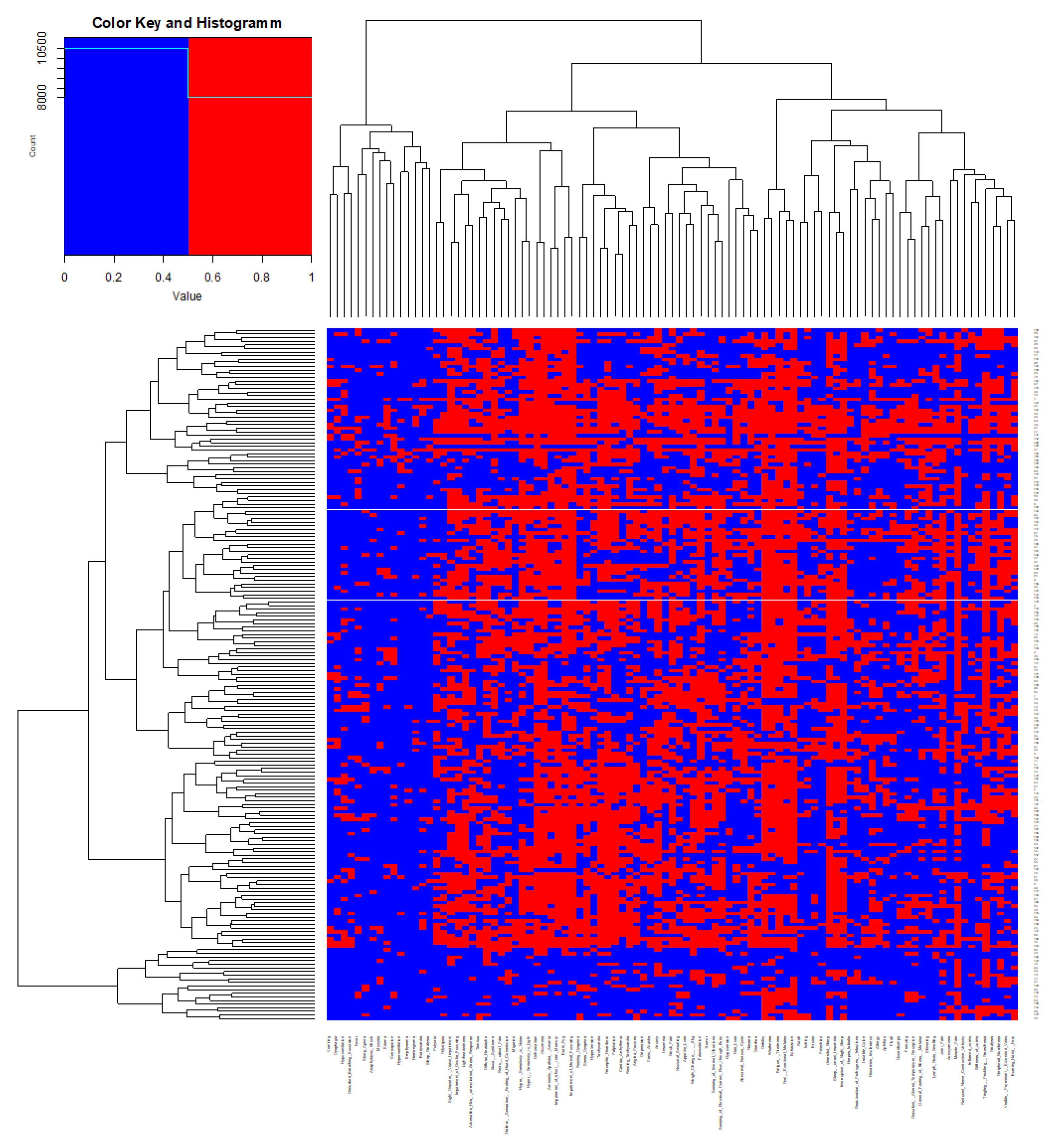
To delineate the clinical characteristics of
PACVS in a hypothesis-free manner, we performed cluster analyses of the symptoms and diagnoses listed in
Table 1. Clearest results were obtained by analyzing Jaccard distances of similarities and dissimilarities (
Figure 2). Eight major clusters of symptoms could thus be identified (
Figure 2, bottom). These clusters appeared plausible and meaningful in medical terms since they exhibited a reasonable degree of coherence and were consistent with established etiologies: Cluster 1 comprised symptoms of generally reduced well-being commonly associated with serious consuming diseases (B-symptoms); cluster 2 encompassed symptoms of peripheral nervous dysfunction; cluster 3 contained the core symptoms of chronic fatigue and malaise; cluster 4 contained key symptoms of cardiovascular dysfunction; cluster 5 comprised common migraine-like symptoms (cognitive impairment, headache, visual and acoustic dysfunction); cluster 6 encompassed symptoms of psycho-motoric disturbances and anxiety disorder; cluster 7 combined sleep disturbances and cutaneous symptoms; the symptoms assembled in cluster 8 were consistent with disturbed autonomous regulation of breathing and eating.
Table 2 provides a complete listing of the symptoms in each of the above symptom clusters.
3.3. Prevalence of Major PACVS-Associated Symptoms
Study participants were assigned to symptom clusters according to (i) proportion of symptoms in the cluster, (ii) the Jaccard index, or (iii) the modified Jaccard index (i. e. the average of the Jaccard index and the Jaccard index of the negated values) (
Figure 3). The outcome of these three approaches was comparable: Almost all participants fitted into cluster 3 (chronic fatigue, malaise), which apparently comprises a cross-cohort symptomatology characteristic of PACVS. Cluster 2 (peripheral neuropathy, dysesthesia, motor weakness, pain, vasomotor dysfunction), cluster 4 (cardiovascular impairment), and cluster 5 (cognitive impairment, headache, optic, oculo-motoric and acoustic dysfunctions) were also frequently represented in the study cohort, mostly overlapping with the symptoms of cluster 3. The set of symptoms represented by these clusters apparently characterize subgroups of PACVS symptomatology. Of note, by the Jaccard index alone more patients were assigned to cluster 5 than to cluster 3. In contrast, almost no participants fitted into cluster 1 (general unwellness) and cluster 8 (dysphagia, breathing impairment), which appear to comprise unspecific symptoms not characteristic of PACVS.
3.4. Abnormalities of Blood Markers in PACVS
We have recently reported that PACVS can be discriminated from the normal vaccination response by alterations in certain receptor antibodies (increases in angiotensin II type 1 receptor antibodies and decreases alpha-2B adrenergic receptor antibodies) and increases in IL-6 and IL-8 [
2]. In addition, here, we investigated in the sera of the study participants an extended panel of established blood markers for organ-specific dysfunctions. Value distribution in the study-cohort was compared to z-scores (sNFL) or age-adjusted normal ranges of these parameters in healthy individuals.
As summarized in
Table 3, the following abnormalities were observed: 17% of the cases exhibited abnormally high eGFR (> 120 ml/min) combined with abnormally low serum urea (< 18 mg/dl). fT3 values were in all cases either below the 95% confidence limit (34% of cases) or in the lower half of the normal range (66% of cases). 61% of cases exhibited a low ferritin-index and subnormal levels of transferrin, soluble transferrin receptor and transferrin saturation. 27% of cases exhibited serum levels of sNFL above the 90th percentile [
10]. Almost half of the study participants exhibited IgG-subclass imbalances, most notably decreased IgG-2 (44% of cases) or increased IgG-3 (11% of cases). IL-6 was increased in 60% of cases. IL-8 was increased in 90% of cases assuming the free fraction was determined [
2] and in 83 % of cases assuming determinations included the erythrocyte-bound fraction (estimated at 80 % according to [
11]) possibility released during transport of uncentrifuged serum samples (i. e. total IL-8). One third of cases even presented IL-8 values above 30,000 pg/ml corresponding to > 100-fold increases in total IL-8. Total cholesterol and triglycerides were increased in 30 and 20 %, respectively of the cases. However, LDL-levels and LDL/HDL-ratios were not increased accordingly and fasting before serum sampling was not assured. Cardiac markers and parameters of liver function exhibited a normal distribution in the study cohort. Associations of abnormally distributed blood markers with symptom clusters (
Table 2) were undetectable or insignificant (p > 0.0001).
4. Discussion
4.1. The Clinical Phenotype of PACVS
The vast majority of cases included in this study [
2] exhibited in varying proportion a symptomatic triplet of (i) malaise/ chronic fatigue, (ii) cognitive impairment and (iii) peripheral neuropathic symptoms. We assume that these symptom clusters represent facets of a single complex syndrome, since it seems unlikely and counter-intuitive, that a single and unique cause (i. e. SARS-CoV-2 vaccination) should trigger several distinct etiologies. The clinical phenotype of that putative syndrome differs markedly from long-termed sequelae following SARS-CoV-2 vaccination in published case reports, which encompass dysfunctions of the eyes (scintillating scotoma, double vision), malaise (fever, myalgia, fatigue), renal dysfunction (nephrotic syndrome, IgA-nephropathy, lupus nephritis), and a variety of other auto-immune manifestations [
13,
14,
15]. Apart from malaise, none of these sporadic sequelae of SARS-CoV-2 vaccination figured prominently among the clinical features reported by the majority of the participants of this study.
Most notably, Guillain-Barré syndrome (GBS) observed in association with SARS-CoV-2 vaccination in more than 100 cases worldwide [
16,
17] was not presented by a single one of the 191 cases studied here, even though GBS and associated symptoms were explicitly addressed in the recruitment questionnaire of our study. Interestingly, the association of GBS with SARS-CoV-2 vaccination has been attributed to the reactivation or exacerbation of pre-existing GBS [
18]. In analogy, many other sequelae occasionally reported in the context of SARS-CoV-2 vaccination may similarly be based on the exacerbation or reactivation of preexisting or dormant diseases. In contrast, the panoply of symptoms reported by the participants of this study seems to occur de novo and to share similarities with endogenous complex dysautonomia syndromes such as ME/CFS, MCAS, POTS and SFN.
4.2. PACVS-Associated Alterations of Diagnostic Markers
We have recently demonstrated [
2] that PACVS can be discriminated from the normal post-vaccination state by increases in interleukins 6 and 8 and alterations of certain receptor-antibodies. Here we demonstrate a variety of further blood markers, alterations of which are associated with PACVS. These alterations may add to the general characterization of PACVS as a disease entity but they were not significantly associated with specific subsets of symptoms presented by the study participants. The following notable alterations of blood markers were observed in the participants of our study:
4.2.1. Increased eGFR
Abnormally high eGFR combined with abnormally low serum urea as presented by 17% of the study cases could possibly indicate insipid diabetes/ hypopituitarism, which has occasionally been observed in conjunction with SARS-CoV-2 mRNA vaccination [
19,
20,
21]. In keeping with that notion, a third of the study participants reported clinical symptoms fitting hypopituitarism e. g. diabetes insipidus (i. e. polyuria, polydipsia and hypotonia, see:
Table 1). However, these symptoms were not correlated with abnormally high eGFR or abnormally low serum urea. Regarding the thyroid axis, we didn’t observe abnormally low TSH levels. However, we neither measured copeptin nor performed any functional endocrinological testing.
4.2.2. Low fT3
More than 90 % of the study participants presented unimpaired thyroid function based on reference values of TSH and fT4. Abnormalities of TSH and fT4 exhibited by < 10 % of the cases could mostly be related to pre-existing auto-immune thyroid diseases reported in the case history and/ or indicated by increases in TPO antibodies (18 cases) or TSH-R antibodies (1 case). All study participants exhibited low fT3 values, which in conjunction with normal fT4 and normal TSH is thought to reflect oblique or incipient hypothyroidism as an unspecific epiphenomenon of critical illness also addressed as “low-T3-syndrome” [
22]. In keeping with that notion, fT3 levels did not exhibit a different distribution in the eight symptom clusters, which were indistinguishable from each other based on fT3 values alone.
4.2.3. Disturbance of Iron Storage
61 % of PACVS-subjects exhibited a low ferritin-index and subnormal levels of transferrin, soluble transferrin receptor and transferrin saturation. These abnormalities were not correlated with age and gender, rendering enhanced iron-loss due to menstrual bleeding an unlikely cause. Imbalances in iron-metabolism and iron-storage as suggested by the above markers are a possible cause of chronic fatigue reported by a majority of study participants. However, the above abnormalities were not significantly correlated to chronic fatigue reported as a single symptom, nor was the distribution of these values significantly different between the eight symptom clusters (p > 0.0001). Of note, the indications of altered iron-metabolism and decreased iron-storage observed here in the context of PACVS did not encompass hyper-ferritinemia, which is frequently observed in severe courses [
23] or chronic sequelae [
24] of COVID-19.
4.2.4. Increase in Soluble Neurofilament Light Chains (sNFL)
27% of the PACVS-cohort exhibited serum levels of the neuroaxonal damage marker sNFL above the 90th percentile [
10]. However, increased sNFL was not significantly associated with symptoms or symptom clusters of central or peripheral neurological dysfunction. It must be taken into account, however, that possible neuronal dysfunctions reported by the study participants were not corroborated by a standardized neurological examination nor by electrophysiological measurements of nerve conductivity or nuclear magnetic resonance imaging of the central nervous system.
4.2.5. IgG-Subclass Imbalances
Almost half of the study cohort exhibited IgG-subclass imbalance, most notably decreases of IgG-2 and/ or increases in IgG-3. Similar imbalances have been observed in ME/CFS unrelated to vaccination [
25], in influenza [
26] and in severe or prolonged COVID-19 [
27]. The latter phenomenon is thought to reflect alterations in spike S1-protein specific IgG subclass utilization [
27,
28], which may also be the case with the IgG-subclass imbalances observed here in PACVS. Levels of IgG3 and IgG4 did not vary across the individual symptom clusters, which were indistinguishable based on these parameters.
4.2.6. Systemic Inflammation
A diagnostic feature presented by almost all of the study participants was a significant increase in IL-6 and/ or IL-8 in combination with normal CRP. This constellation distinguishes PACVS-cases from the normal healthy post-vaccinations state [
2]. Increased blood levels of IL-6 and IL-8 are considered early and sensitive albeit unspecific markers of increased and persistent systemic inflammation. Most notably, increases in total IL-8 observed in ME/CFS occurring after COVID-19 are thought to support the notion that a state of persistent systemic hyperinflammation is one cause for prolonged or more severe courses of that disease [
29]. It is conceivable that chronically enhanced systemic inflammation may also be the cause of low fT3 levels and alterations of iron metabolism observed in a significant fraction of the participants of this study. Moreover, persistent systemic inflammation would be a plausible cause of malaise, chronic fatigue and other symptoms frequently reported by the study participants (see
Table 2, clusters 2, 3 and 5).
4.2.7. Lipids
In view of the age distribution of the study participants, the significant increases in cholesterol and triglycerides observed in one third of the cohort would be noteworthy. However, these increases were not matched by corresponding increases in LDL or the LDL/HDL-ratio. Therefore, they probably don’t reflect a genuine disorder of lipid metabolism. More probably they are due to a non-fasting state during sample acquisition.
5. Limitations
Any conclusions to be drawn from our study must take into account that the proposed disease phenotype of PACVS is solely based on symptoms, afflictions and ailments reported by the study participants or their local doctors. These reports were not corroborated by neurological examinations, objective measurements of nerve function, imaging of nerve structures or other clinical investigations performed in the context of the study in a stratified and standardized manner. Moreover, the pre-analytic conditions of laboratory analyses performed on blood samples of the study participants were insufficiently controlled. In particular the time lapse between blood drawing and separation of serum was comparatively long (up to 48 h at 4 °C) and we cannot exclude that some of the laboratory results were thereby affected. Most notably, we are unable to ascertain whether free or total IL-8 was determined. However, this distinction does not alter the overall result regarding the prevalence of systemic hyperinflammation in the study cohort.
6. Conclusions
The data presented here and in our previous publication [
2] outline a chronic syndrome probably triggered by SARS-CoV-2 mRNA-vaccination. This putative syndrome commonly addressed as PACVS [
1] is probably not the only chronic health impairment associated with SARS-CoV-2 vaccination. It must be distinguished from the (re-)activation of GBS [
16,
17,
18] and other immunologic diseases [
13,
14,
15], which has also been observed in the context of SARS-CoV-2 vaccination. The clinical presentation of PACVS based on the registry of this study comprises a symptomatic triplet of (i) malaise/ chronic fatigue (symptom cluster 3), (ii) migraine-like symptoms/ cognitive impairment (symptom cluster 5), and (iii) peripheral nerve dysfunction (symptom cluster 2). In addition, PACVS may be characterized (and distinguished from the normal post-vaccination state) by a characteristic set of blood markers: Almost all of PACVS cases studied here exhibited increases in interleukins 6 and 8, alterations of certain receptor-antibodies [
2], and low fT3 levels [
22]. More than half of the cases exhibited imbalances in IgG-subclass distribution and serological indications of impaired iron-storage. A smaller subset of cases presented markers of renal hyper-perfusion (17 %) or significant increases in circulating sNFL (30%). However, none of these laboratory findings were limited to clinical subsets of the cases. Nor were they linked to any specific symptom constellation of PACVS as derived from Jaccard clustering.
Author Contributions
Conceptualization, Anna Mundorf, Matthias Schott, Marc Pawlitzki, Sven Guenther Meuth, Frank Klawonn, Jana Ruhrländer and Fritz Boege; Data curation, Anna Mundorf, Matthias Schott, Karl J. Lackner, Karin Schulze-Bosse, Marc Pawlitzki, Sven Guenther Meuth, Frank Klawonn, Jana Ruhrländer and Fritz Boege; Formal analysis, Anna Mundorf, Matthias Schott, Karl J. Lackner, Marc Pawlitzki, Frank Klawonn and Fritz Boege; Funding acquisition, Harald Heidecke; Investigation, Anna Mundorf, Amelie Semmler, Harald Heidecke, Falk Steffen, Stefan Bittner, Karl J. Lackner, Marc Pawlitzki, Frank Klawonn, Jana Ruhrländer and Fritz Boege; Methodology, Anna Mundorf, Amelie Semmler, Harald Heidecke, Falk Steffen, Stefan Bittner, Karl J. Lackner, Sven Guenther Meuth, Jana Ruhrländer and Fritz Boege; Project administration, Anna Mundorf, Amelie Semmler, Harald Heidecke, Karin Schulze-Bosse and Fritz Boege; Resources, Harald Heidecke, Falk Steffen, Stefan Bittner, Karl J. Lackner, Karin Schulze-Bosse, Frank Klawonn, Jana Ruhrländer and Fritz Boege; Software, Frank Klawonn and Jana Ruhrländer; Supervision, Matthias Schott, Karin Schulze-Bosse, Marc Pawlitzki, Sven Guenther Meuth, Jana Ruhrländer and Fritz Boege; Validation, Anna Mundorf, Amelie Semmler, Matthias Schott, Karl J. Lackner, Sven Guenther Meuth, Frank Klawonn, Jana Ruhrländer and Fritz Boege; Visualization, Anna Mundorf, Amelie Semmler and Frank Klawonn; Writing – original draft, Amelie Semmler, Marc Pawlitzki, Jana Ruhrländer and Fritz Boege; Writing – review & editing, Anna Mundorf, Matthias Schott, Falk Steffen, Stefan Bittner, Karl J. Lackner, Karin Schulze-Bosse, Marc Pawlitzki, Sven Guenther Meuth, Frank Klawonn, Jana Ruhrländer and Fritz Boege. All authors have read and agreed to the published version of the manuscript.
Funding
Laboratory analyses were funded by the Medical Faculty of the Heinrich Heine University Düsseldorf and the University Hospital Düsseldorf. Online data registry was funded by Selbsthilfegruppe Post-Vac-Syndrom Deutschland e. V.. Receptor-antibody measurements were provided free of charge by CellTrend GmbH, Luckenwalde, Germany.
Institutional Review Board Statement
Clinical trial protocols were approved by the local ethics board of Medical Faculty of Heinrich-Heine University Düsseldorf (study number 2022-1948). The investigation conforms to the principles outlined in the World´s Medical Association Declaration of Helsinki. Before inclusion, all participants have given written informed consent.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy concerns by the study participants.
Acknowledgments
Technical assistance is gratefully acknowledged to Alexander Weinstein, Susanne Herrmann, Petra Meirowski.
Conflicts of Interest
Harald Heidecke and Kai Schulze-Forster is co-owner and chief executive officer of CellTrend GmbH, Luckenwalde, Germany. The other authors have no conflict of interest to declare.
References
- Scholkmann, F.; May, C.-A. COVID-19, post-acute COVID-19 syndrome (PACS, “long COVID”) and post-COVID-19 vaccination syndrome (PCVS, “post-COVIDvacsyndrome”):Similarities and differences. Pathology - Research and Practice 2023. [CrossRef]
- Semmler, A.; Mundorf, A.K.; Kuechler, A.S.; Schulze-Bosse, K.; Heidecke, H.; Schulze-Forster, K.; Schott, M.; Uhrberg, M.; Weinhold, S.; Lackner, K.J.; et al. Chronic Fatigue and Dysautonomia following COVID-19 Vaccination Is Distinguished from Normal Vaccination Response by Altered Blood Markers. Vaccines (Basel) 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.J.; Son, C.G. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med 2020, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med 2011, 270, 327–338. [Google Scholar] [CrossRef]
- Fedorowski, A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med 2019, 285, 352–366. [Google Scholar] [CrossRef]
- Bair, M.J.; Krebs, E.E. Fibromyalgia. Ann Intern Med 2020, 172, ITC33–ITC48. [Google Scholar] [CrossRef] [PubMed]
- Tavee, J.; Zhou, L. Small fiber neuropathy: A burning problem. Cleve Clin J Med 2009, 76, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Hartmann, K.; Bonadonna, P.; Niedoszytko, M.; Triggiani, M.; Arock, M.; Brockow, K. Mast Cell Activation Syndromes: Collegium Internationale Allergologicum Update 2022. Int Arch Allergy Immunol 2022, 183, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Kuechler, A.S.; Weinhold, S.; Boege, F.; Adams, O.; Muller, L.; Babor, F.; Bennstein, S.B.; Pham, T.U.; Hejazi, M.; Reusing, S.B.; et al. A Diagnostic Strategy for Gauging Individual Humoral Ex Vivo Immune Responsiveness Following COVID-19 Vaccination. Vaccines (Basel) 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Benkert, P.; Meier, S.; Schaedelin, S.; Manouchehrinia, A.; Yaldizli, O.; Maceski, A.; Oechtering, J.; Achtnichts, L.; Conen, D.; Derfuss, T.; et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol 2022, 21, 246–257. [Google Scholar] [CrossRef]
- Reinsberg, J.; Dembinski, J.; Dorn, C.; Behrendt, D.; Bartmann, P.; van Der Ven, H. Determination of total interleukin-8 in whole blood after cell lysis. Clin Chem 2000, 46, 1387–1394. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Cancarevic, I.; Nassar, M.; Medina, L.; Sanchez, A.; Parikh, A.; Hosna, A.; Devanabanda, B.; Vest, M.; Ayotunde, F.; Ghallab, M.; et al. Nephrotic Syndrome in Adult Patients With COVID-19 Infection or Post COVID-19 Vaccine: A Systematic Review. Cureus 2022, 14, e29613. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.M.; Shuai, Z.W.; Ye, D.Q.; Pan, H.F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Finterer, J.; Scorza, F.A. A retrospective analysis of clinically confirmed long post-COVID vaccination syndrome. J Clin Transl Res 2022, 8, 506–508. [Google Scholar] [PubMed]
- Finsterer, J.; Scorza, F.A.; Scorza, C.A. Post SARS-CoV-2 vaccination Guillain-Barre syndrome in 19 patients. Clinics (Sao Paulo) 2021, 76, e3286. [Google Scholar] [CrossRef] [PubMed]
- Abolmaali, M.; Rezania, F.; Behnagh, A.K.; Hamidabad, N.M.; Gorji, A.; Mirzaasgari, Z. Guillain-Barré syndrome in association with COVID-19 vaccination: a systematic review. Immunologic Research 2022, 70, 752–764. [Google Scholar] [CrossRef]
- Finsterer, J. Exacerbating Guillain-Barre Syndrome Eight Days after Vector-Based COVID-19 Vaccination. Case Rep Infect Dis 2021, 2021, 3619131. [Google Scholar] [CrossRef]
- Murvelashvili, N.; Tessnow, A. A Case of Hypophysitis Following Immunization With the mRNA-1273 SARS-CoV-2 Vaccine. J Investig Med High Impact Case Rep 2021, 9, 23247096211043386. [Google Scholar] [CrossRef] [PubMed]
- Ankireddypalli, A.R.; Chow, L.S.; Radulescu, A.; Kawakami, Y.; Araki, T. A Case of Hypophysitis Associated With SARS-CoV-2 Vaccination. AACE Clin Case Rep 2022, 8, 204–209. [Google Scholar] [CrossRef]
- Bouca, B.; Roldao, M.; Bogalho, P.; Cerqueira, L.; Silva-Nunes, J. Central Diabetes Insipidus Following Immunization With BNT162b2 mRNA COVID-19 Vaccine: A Case Report. Front Endocrinol (Lausanne) 2022, 13, 889074. [Google Scholar] [CrossRef]
- Moura Neto, A.; Zantut-Wittmann, D.E. Abnormalities of Thyroid Hormone Metabolism during Systemic Illness: The Low T3 Syndrome in Different Clinical Settings. International Journal of Endocrinology 2016, 2016, 2157583. [Google Scholar] [CrossRef]
- Suriawinata, E.; Mehta, K.J. Iron and iron-related proteins in COVID-19. Clin Exp Med 2022, 1–23. [Google Scholar] [CrossRef]
- Legler, F.; Meyer-Arndt, L.; Mödl, L.; Kedor, C.; Freitag, H.; Stein, E.; Hoppmann, U.; Rust, R.; Wittke, K.; Siebert, N.; et al. Long-term symptom severity and clinical biomarkers in post-COVID-19/chronic fatigue syndrome: results from a prospective observational cohort. eClinicalMedicine 2023, 63. [Google Scholar] [CrossRef]
- Lutz, L.; Rohrhofer, J.; Zehetmayer, S.; Stingl, M.; Untersmayr, E. Evaluation of Immune Dysregulation in an Austrian Patient Cohort Suffering from Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomolecules 2021, 11. [Google Scholar] [CrossRef]
- Gordon, C.L.; Johnson, P.D.; Permezel, M.; Holmes, N.E.; Gutteridge, G.; McDonald, C.F.; Eisen, D.P.; Stewardson, A.J.; Edington, J.; Charles, P.G.; et al. Association between severe pandemic 2009 influenza A (H1N1) virus infection and immunoglobulin G(2) subclass deficiency. Clin Infect Dis 2010, 50, 672–678. [Google Scholar] [CrossRef]
- Yates, J.L.; Ehrbar, D.J.; Hunt, D.T.; Girardin, R.C.; Dupuis, A.P., 2nd; Payne, A.F.; Sowizral, M.; Varney, S.; Kulas, K.E.; Demarest, V.L.; et al. Serological analysis reveals an imbalanced IgG subclass composition associated with COVID-19 disease severity. Cell Rep Med 2021, 2, 100329. [Google Scholar] [CrossRef]
- Schultheiss, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; Girndt, M.; et al. Liquid biomarkers of macrophage dysregulation and circulating spike protein illustrate the biological heterogeneity in patients with post-acute sequelae of COVID-19. J Med Virol 2023, 95, e28364. [Google Scholar] [CrossRef]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Hanitsch, L.G.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun 2022, 13, 5104. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).