1. Introduction
Lindernia dubia (L.) Pennell (yellowseed false-pimpernel) is an annual herb of small size, native to North America, where it grows in borders of ponds, river banks and other moist to wet habitats. According to Lewis [
1], this species shows great morphological plasticity, especially in vegetative characters; stems can be erect, ascending or prostrate, and they can root at the lower nodes; leaves are simple, opposite and sessile, and their shape is variable, from lanceolate to ovate or suborbicular. Flowers are gamopetalous, zygomorphic, chasmogamous or cleistogamous [
2]; the fruit is a septicidal capsule with ellipsoid or globose shape, containing numerous seeds, ellipsoid or rectangular [
1].
Nowadays, the distribution of this species has expanded from America to various other regions of the World, as distant as Europe, Korea or Taiwan [
3,
4]. The Global Biodiversity Information database [
4] mentions that there is evidence of impact by this species in Italy, Romania and Japan. Specifically, in Europe
L. dubia (hereafter referred as LIDDU) has been appointed as an invasive species in natural aquatic habitats of the Netherlands [
5], Romania [
6], and Italy [
7].
In crop environments, LIDDU has been acknowledged as a weed in rice paddies, where its occurrence seems to be expanding [
8,
9,
10]. Despite the fact that rice is a staple food crop, there is little information on the traits possessed by LIDDU that promote its spread and on its performance as a weed, probably because other weeds of rice, such as
Echinochloa spp. and
Cyperus spp., are by far much more harmful [
11]. In the case of Europe, the cultivation of rice is concentrated in two countries, Italy (60.3 %) and Spain (15.5 %) (rice cropped in Russian Federation not included) [
12], and in both countries LIDDU has been reported as an alien weed of rice [
13]. Additionally, LIDDU may also occur in other productive moist habitats, such as meadows, plant nurseries or treatment wetlands.
Treatment wetlands are constructed aquatic habitats intended for the restoration of ecosystems or the management of wastewater; they are regarded as nature-based solutions in various contexts [
14]; among the most commonly plants used in constructed wetlands (CW) are
Typha spp. [
15].
Typha spp. can be established in constructed wetlands from rhizomes, seeds or seedlings; while the supply of rhizomes requires much resources (rhizome collection, logistics expenses), seeds are easy to collect and use. However, direct sowing is less successful than the establishment from seedlings, due to seed drift by water movement. This fact, along with the need for large number of plants (5 to 50 individuals·m
-2 depending on the type of wetland) [
16], has placed the focus on nursery-propagated plants for CW establishment [
17]. In this respect, proper sanitation is essential to produce marketable plants and to prevent the spread of pests; some authors highlight that weed growth in container-grown nursery stock is a serious problem not only in terms of economic losses but also in terms of the risk of spread of troublesome plant species, such as alien or invasive plant species [
18]. In this regard, we hypothesized that helophyte nurseries may represent an environment prone to weed infestation, due to high availability of growth resources such as water and nutrients. To the best of our knowledge, the occurrence of weeds in helophyte nurseries has not been addressed in the published literature.
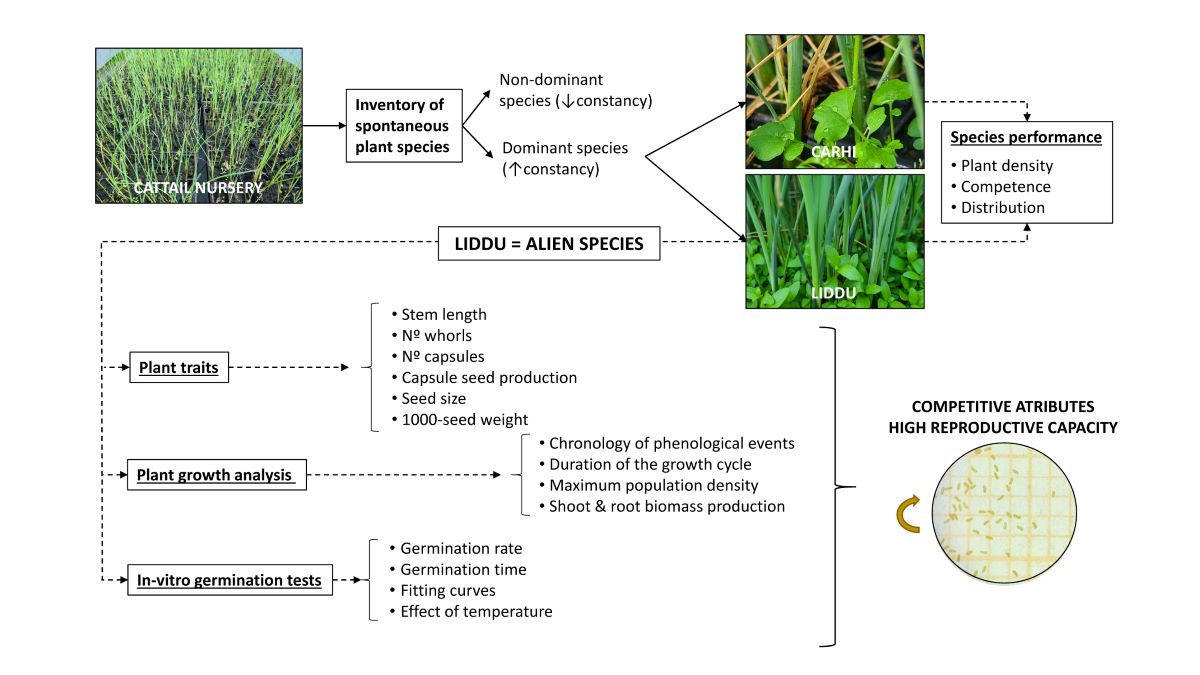
In order to shed some light on spontaneous flora occurring in helophyte nurseries and the interspecific competition between dominant species, we set up a mesocosm experiment with the helophyte Typha domingensis Pers. (commonly known as cattail) intentionally grown from seed in a nursery of Spain for constructed wetlands. The primary objectives were to develop an inventory of potential weeds in cattail nurseries and to assess the occurrence of the prevailing taxa and their introduction pathways in order to understand the source and species that should be controlled. In the course of species identification works, the alien species Lindernia dubia (L.) Pennel was recorded; this finding encouraged the research on the performance and characterization of this species. Thus, in this paper, i) a case study of weeds in cattail seedlings is presented, ii) a new record of the alien species L.dubia is reported, iii) the distribution of dominant weeds is assessed, and iv) traits of L.dubia, a dominant alien species, are determined in order to build a dataset for the estimation of its weedy potential.
3. Discussion
Nowadays, the spread of alien species is a matter of concern, especially when they present invasive traits. In an agricultural or horticultural environment, the record of an alien plant species may represent a potential weed infestation and/or a risk for nearby ecosystems. One of the main goals of this work was to gain insight into the spontaneous flora occurring in cattail nurseries. Certainly, the use of potted cattail seedlings infested with weeds for constructed wetlands may result in the spread of undesirable plant species, including potentially invaders.
In the present work, the inventory of species accompanying cattail seedlings -grown in a substrate free of plant propagules- revealed low diversity; it also showed that such species have preference for moist habitats [
21]. Thus, CYPER was reported as the most frequent alien species on river banks of the Cantabric watershed of Spain [
22]. AGSST is widely distributed in Europe and Spain, where it appears in humid meadows [
21]. CYPRO and ECHCG have been acknowledged as major weeds of rice fields globally [
11], and DIGSA has been reported as a weed in rice fields of India and USA [
11]. In Spain, CYPRO, ECHCG and SONOL were reported as main weeds in sprinkler irrigated rice [
23]. In a study of the success of alien weeds in irrigated Mediterranean orchards, CYPRO and ECHCG were categorized as ‘high successful’ invaders [
24]. Notwithstanding the above, the constancy values of the above-mentioned species were low in our case-study; the dominant species were others, LIDDU (first in the rank) and CARHI.
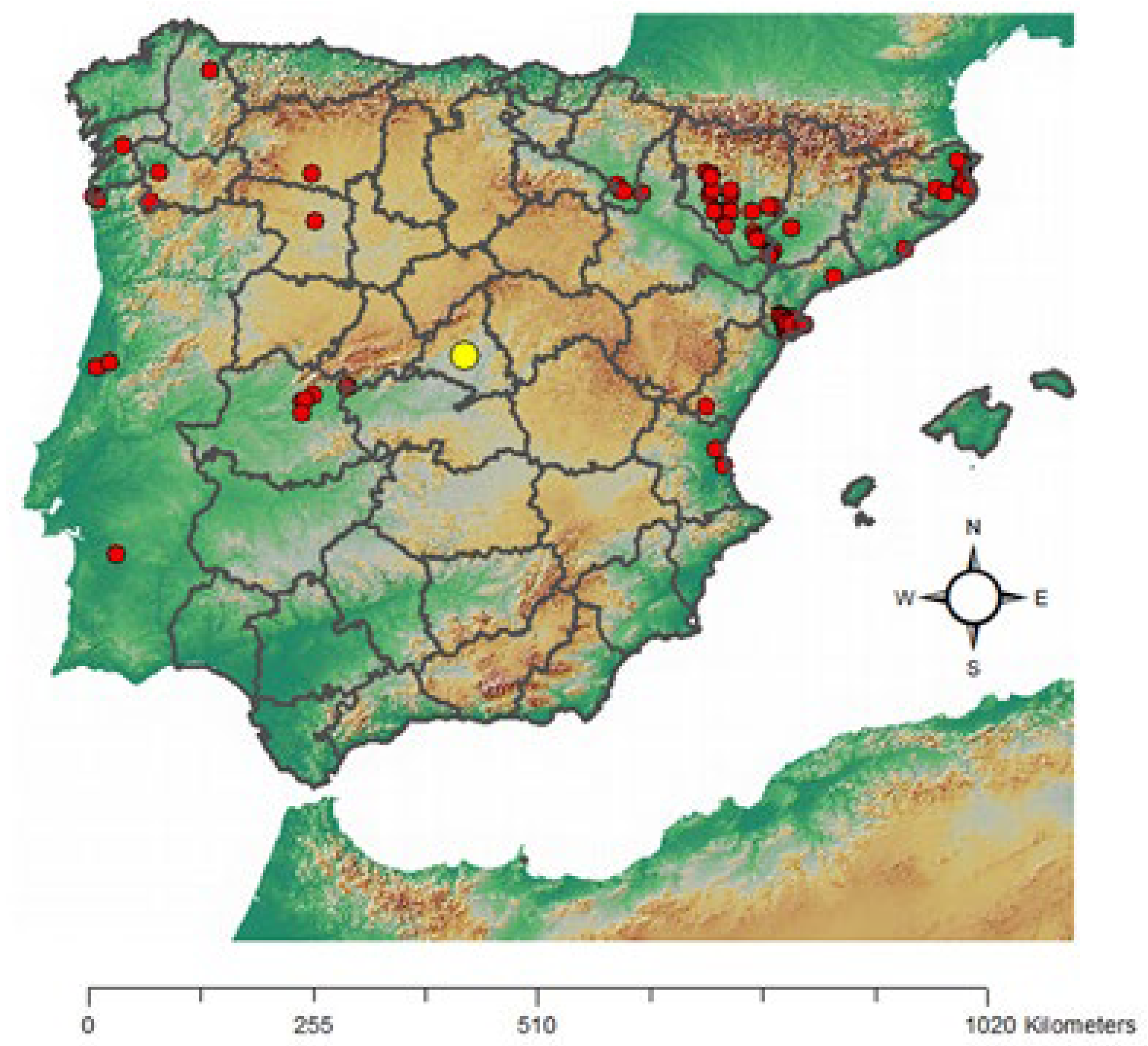
To the best of our knowledge, the occurrence of the alien species LIDDU in our area of study (Madrid, Spain) has not been reported so far [
20,
25,
26]. According to the Information System of the Plants of Spain [
20] the closest record of the occurrence of LIDDU was located in the proximities of the Rosarito reservoir, i.e. 138 km from our location. It is worth noting that this species was cited as a global plant invader by Laginhas & Bradley [
27], and that it was reported as an invasive plant species in some regions of Europe [
5]. In eastern Spain (provinces of Tarragona and Valencia) it was reported within the list of emergent rice field flora (flooded rice crops), although with little presence [
28]. Results of seed weight in this work and literature data [
29,
30] showed that LIDDU seeds are lightweight so, the accidental introduction of this alien species into our region might have occurred by a wide range of pathways. It could have even occurred by waterfowl, a pathway suggested by Lovas-Kiss et al. [
31] for LIDDU dispersal in Hungary, and by Soons et al. [
32] for small seeds in general. In this respect, it is worth noting that our facilities are about 500 m from the Manzanares river (closest river).
CARHI (hairy bittercress) is a well-known weed species, widely studied in the literature. Vaughn et al. [
33] stated that CARHI was a major pest in nurseries in USA and highlighted that CARHI occurred ‘
in patches in nursery crops and can solidly fill nursery pots if left uncontrolled’ (sic). Warnings about CARHI as a weed of plant nurseries have also been launched in Spain at least since 1997 [
34]. These authors highlighted as competitive attributes of CARHI, large seed production (up to 5000 seeds per plant), quick generation times (30-60 days per generation, in favorable climates), and enhanced mechanisms of seed dispersal (ballistic seed dispersal).
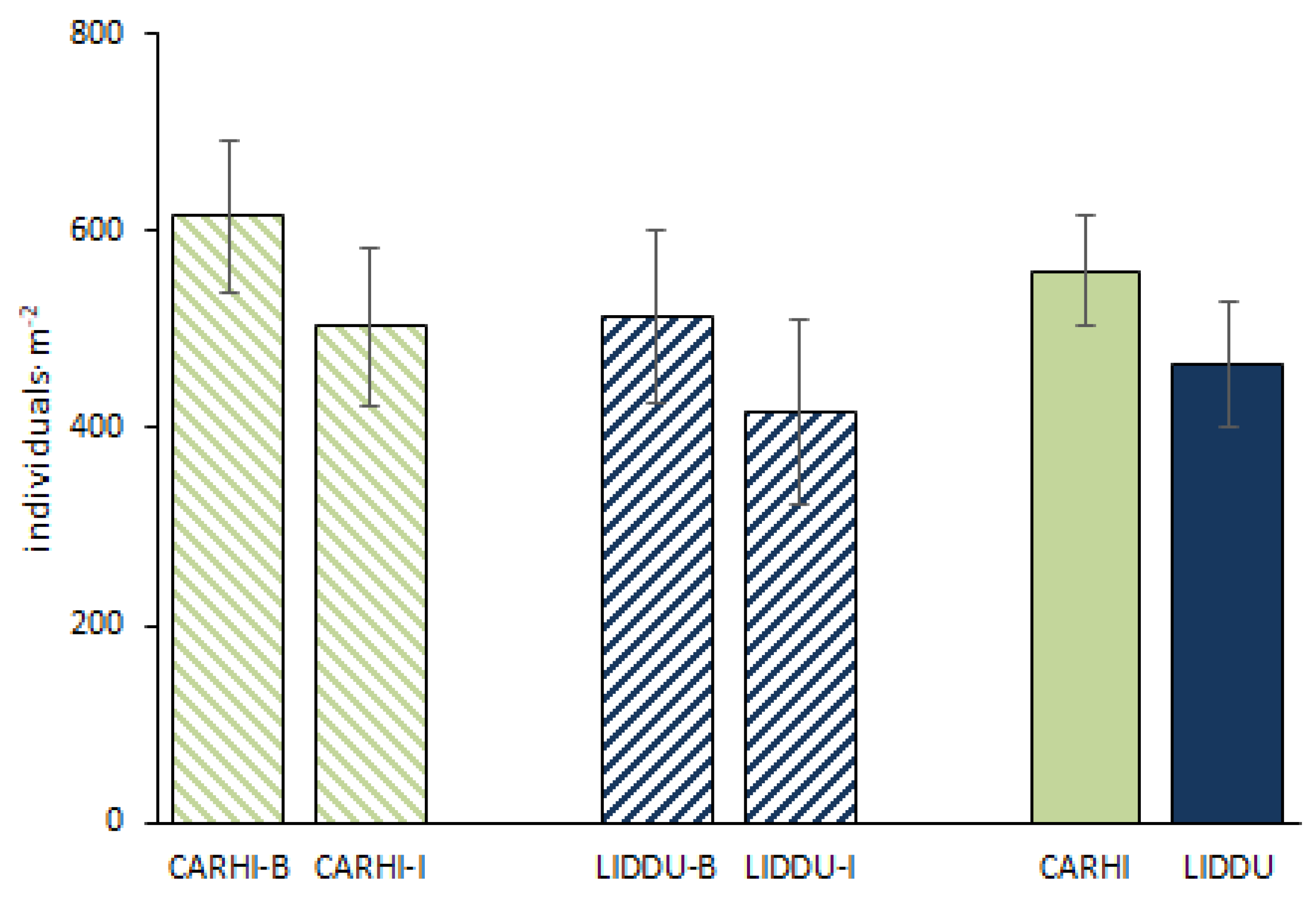
The occurrence of LIDDU and CARHI across seedling trays seemed to be influenced by the proximity to the corridors, although no statistically significant differences were found due to high variability in the counts; e.g. LIDDU presented 63 % coefficient of variation for plant counts in the inner trays. Despite variability, it was noticed that LIDDU performed a little worse in cells all around surrounded by cattails (
i.e. in the inner trays). This observation is considered in line with [
5], who inferred from their results that LIDDU would not easily out-compete fast-growing erect plant species; however, these authors suggested that the impact of LIDDU on small or minute plant communities could be adverse. Competition could explain the differences observed between tray positions in the present study. Another explanation could be the unintentionally human contribution to seed dispersal (workers walked through the corridors) and wind dispersal; open corridors may result in preferential air flow paths, entailing higher seed dispersal towards the closest trays.
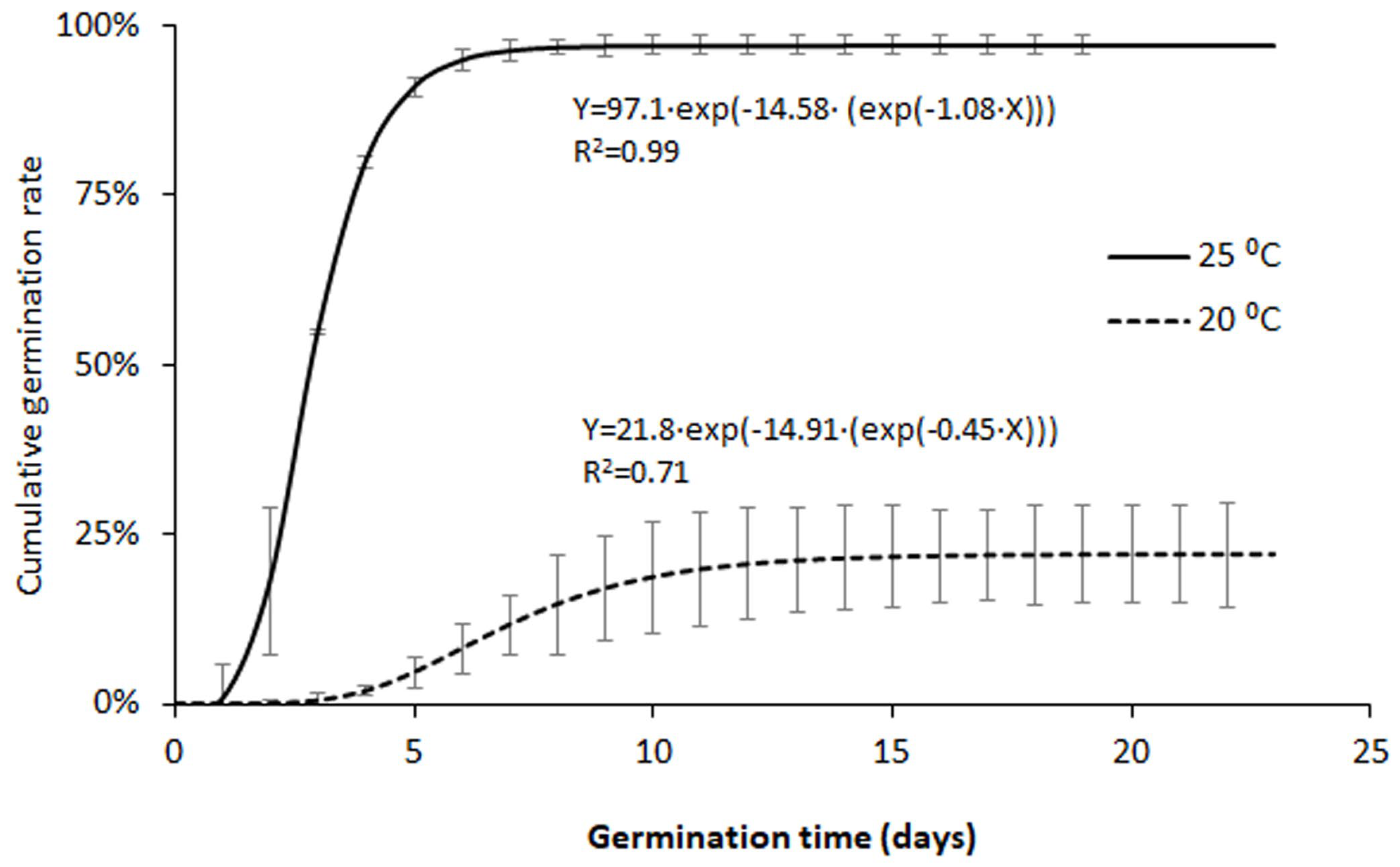
In-vitro germination tests showed that the seeds of LIDDU have the potential to germinate very quickly at 25 ⁰C, and that the germination rate is good, especially for naturally-dehisced seeds. Interestingly, growth of hypocotyl roots was observed at an early plantlet stage (see
supplementary material S1), an observation that has not been previously reported in literature. The only datum on LIDDU germination found in relevant literature was the 63 % germination rate reported for the control treatment in the study of waterfowl seed dispersal by Lovas-Kiss et al. [
31]; their test was conducted at room temperature (20-25 ⁰C), and 16 h light/8 h dark photoperiod. Consistent with those temperature conditions, the value reported was intermediate between the rates we found at 25 ⁰C and 20 ⁰C in this work. Furthermore, the trends observed in our work seem to be consistent with previous studies of freshwater wetlands and other aquatic habitats, that highlighted the capacity of LIDDU to germinate over other species present in seed banks [
35] [
36]. In our tests the germination rate sharply decreased at 20 ⁰C, a fact that pointed to thermophilic preferences of this species. Although our findings refer to seed lots from an only location, the response of seed germination to temperature obtained in this work suggests that infestation by LIDDU is not likely to happen in the cold season of temperate climates, at least in a short period of time since the introduction of this species. Nevertheless, Simons & Jansen [
5] categorized LIDDU as a naturalized invasive in the Netherlands (temperate Atlantic north-western European climate), but estimated that the transition from being a casual alien plant to that status could have taken about ten years. Additionally, there is evidence of the environmental impact by LIDDU in warmer countries of Europe such as Italy and Romania [
4].
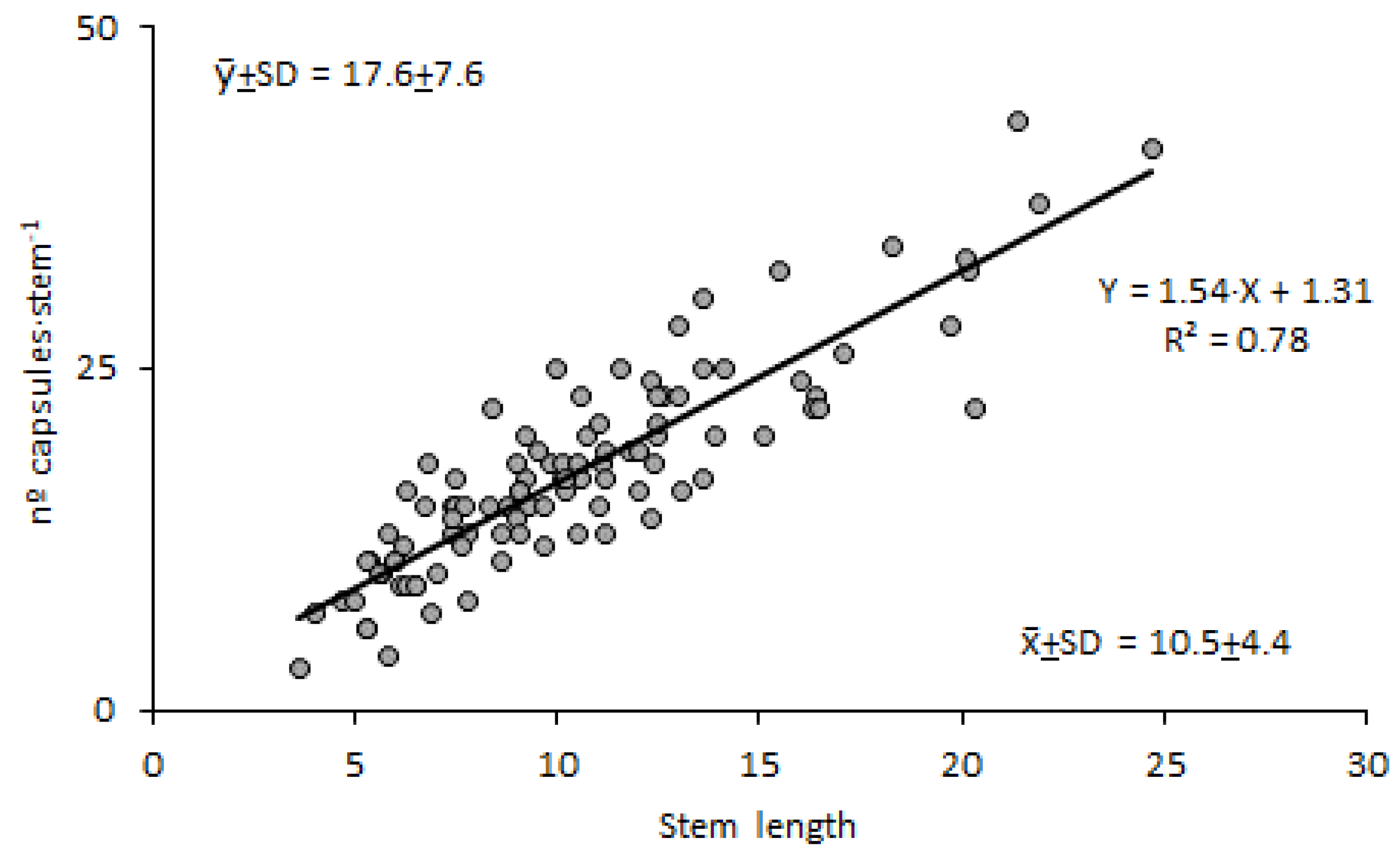
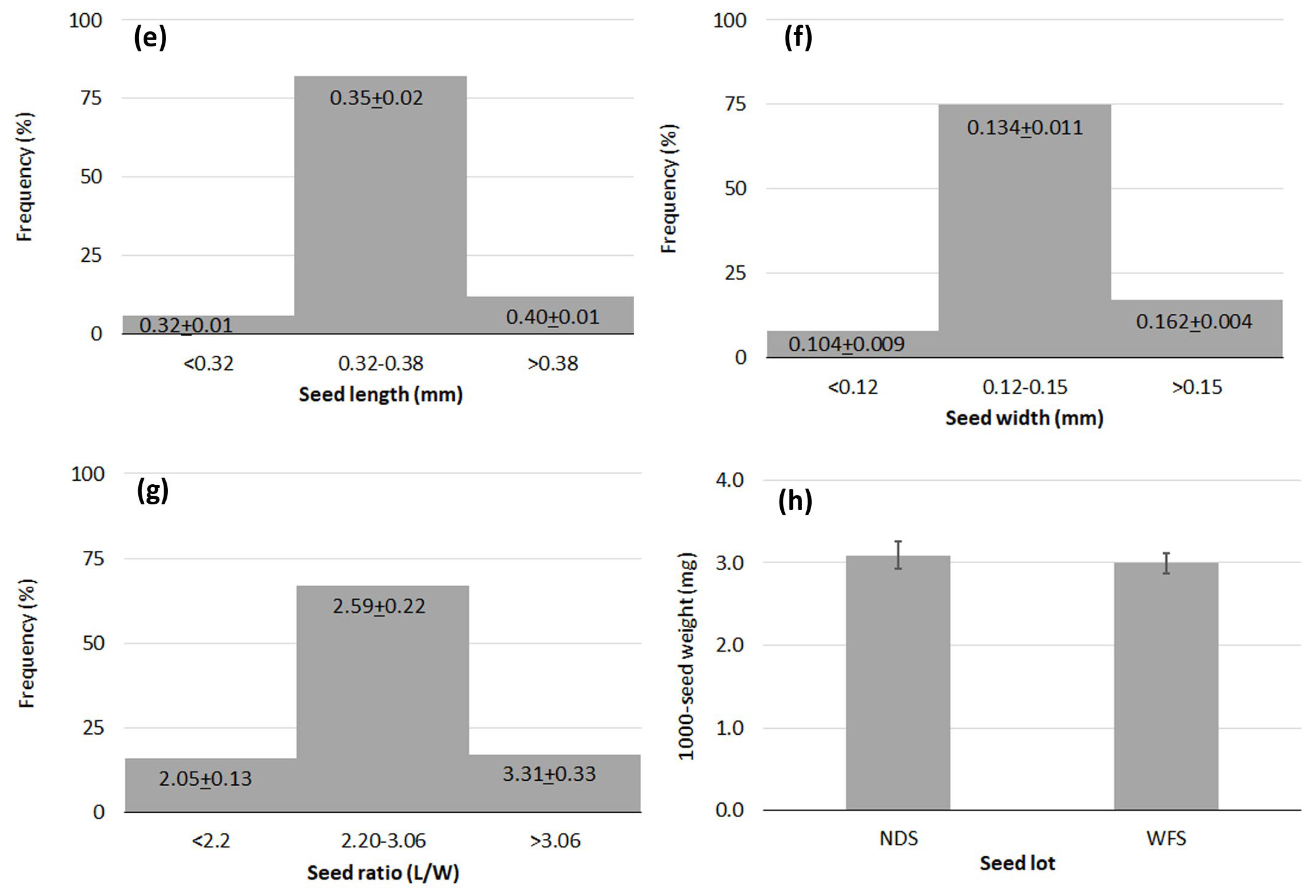
Chronology of phenological events and plant traits data presented in this work suggest that LIDDU possess competitive attributes such as morphological variability, early flowering, long seeding time, short growth cycle, small and light seeds, and high seed production. Peralta and Royuela [
37] cited that LIDDU had a summer phenology in North Spain, flowering from July to September, like we observed in central Spain. Data accompanying the botanical description of LIDDU in North America by [
38] reads ‘
flowering year-round’; Baker [
30] observed that LIDDU flowering ended in autumn (September) in California. As regards plant size, values in this work were in line with the botanical description of LIDDU by Lewis [
1]. Results of seed length and width were consistent with the taxonomical study of Linderniaceae conducted in Korea by Bazarragchaa et al [
39], and also with data provided in the study of endozoochorous seed dispersal in Hungary [
31]. Bazarragchaa et al.[
39] described the inflorescence of LIDDU as in axillary flower pairs, solitary axillary or branching in subtending leaf. Despite the fact that the number of capsules in a single stem was not reported by those authors, it can be inferred that the finding of nearly 2 fruits (capsules) per leaf whorl in our work is in line with their inflorescence description. Data on capsule seed number or plant seed number were not found in the consulted literature. Some data on 1000-seed weight were found but appeared controversial to a certain extend. Thus, the Seed Information Database [
29] reports 0.01 g as mean value for 1000-seed weight, whereas the Baker Seed Collection database, 0.003 g for the same seed amount [
30]. However, a note on the former value says that minor covering structures could have been included in the weights. Therefore, our results of 1000-seed weight are consistent with Baker’s, and support the hypothesis that minor covering structures were included in the weights reported for LIDDU by the Seed Information Database [
29].
In the literature there has been much discussion on what plant traits can be associated with invasiveness, as well as on the dependence of plant traits on the environmental context; however, it is widely accepted that traits related to fecundity and species dispersal are essential to assess the potential success of an invader [
40]. Concerning weed fecundity, Norris [
41] underlined the risks of extrapolating data from controlled conditions to field conditions, but at the same time acknowledged that many papers report experiments carried out under artificial conditions; besides, this author encouraged for data on more weed species. In this sense, our estimates of plant seed production and maximum plant reproductive capacity should be taken with caution, however they provide a basis along with other results from this work for management decisions of LIDDU, a potentially invasive species little investigated in Europe.
4. Materials and Methods
4.1. Site description
This study was developed at the nursery of aquatic plants of the Technical University of Madrid (UPM, Spain) (40 26’ 36” N, 3º 44’ 18” W, 650 m a.s.l.). The climate at the site is temperate with dry and hot summers; normal values of temperature are: 14.6 ⁰C annual mean temperature, -7.4 ⁰C absolute minimum temperature (January) and 40 ⁰C absolute maximum temperature (August) [
42].
The aquatic plant facilities included experimental wetlands, a greenhouse for plant propagation and laboratories for plant characterization. These facilities were intended to produce cattail plants for the establishment of external constructed wetlands, as well as for research on phytodepuration. The greenhouse was endowed with an automated system for ventilation (20 ⁰C set point); in addition, doors remained open during summertime. In the interior of the greenhouse, benches were conventionally arranged; two adjacent rows of benches in the middle (central rows) plus two separate rows at each side of the central rows with two aisle spaces of 1.10 m width in between. Benches were watertight by means of a polyethylene (PE) pond liner. They were periodically filled up with tap water to simulate shadow ponds. Every year in the period from 2017 to 2023, cattails were raised from seed in 96-cell plastic trays (52.5 x 33 x 8 cm) placed in the simulated water ponds; before seeding, trays were filled with a commercial substrate (Pindstrup Mosebrug S.A.E. code 51212). After emergence, seedlings were thinned to one per cell and let to grow until planting in constructed wetlands. The alien LIDDU was noticed for the first time in the summer of 2022.
4.2. Weed inventory and assessment of the dominant species
The occurrence of spontaneous plant species in cattail seedlings was studied in autumn 2022. At that time, cattails were about 50-65 cm tall and exhibited 5-7 green leaves. Identification of spontaneous plant species growing in the trays was carried out through botanical keys and monographs [
21] [
43]. Species constancy was assessed as the ratio of the number of seedling trays with a determined species to the total number of seedling trays (n=174). The indexes of diversity (H) [
44] and dominance (D) [
45] were calculated from tray counts.
The occurrence of LIDDU was assessed in terms of population density and distribution across the nursery, as compared to CARHI, the other dominant weed species in this study. A randomized block design with four blocks and two replications per block was used. To this end, two categories: border (trays placed next to the aisle) and inner (trays placed on the inner area of the bench table) were made. Population density (individuals·m-2) was calculated from the number of individuals counted in each seedling tray, considering the tray surface area (1536 cm2·tray-1).
4.3. Growth chamber experiments
Two types of experiments were developed in a plant growth chamber (300 L capacity, forced air circulation, 6 x F30W/Gro-Lux T8) to gain insight into LIDDU growth cycle and seed germination.
The first one was a pot experiment, where the pot dimensions were: top diameter, 10.5 cm; bottom diameter, 8.5 cm; and height, 9.5 cm. Pots (n=4) were filled up with the same substrate as the one used in the cattail nursery (Pindstrup Mosebrug S.A.E. code 51212). A control (no sown treatment) was used to check the absence of plant propagules in the substrate. Mature fruits (light-brown capsules, starting to break) were taken from senescent LIDDU plants in the seedling trays; seeds were spread on the pot surface, using 50 capsules·pot
-1; capsules were left on the ground. Then, the just-sown pots were placed in a water bath inside the growth chamber at 25 ⁰C constant temperature and 14 h day/10 h dark cycle. Water level in the bath was kept at pot surface level (daily water replenishment). Dates of the start of the phenological stages: visible cotyledons, flowering, fruiting, seed dehiscence and plant senescence (
supplementary material S1), were recorded and expressed as DAS (Days After Sowing). Once the seed dehiscence was observed, naturally-dehisced seeds from the potted plants were collected on a daily basis; after seed cleaning (debris separation under stereomicroscope) they were gathered in a seed lot named NDS (=Naturally-Dehisced Seeds). When plants were fully dry (brown-colored shoots), they were uprooted in order to determine the following parameters: population density (individuals·m
-2), shoots dry matter content (%), average growth rate (dry mass accumulated per week and ground area, g·week
-1·m
-2) and ratio shoots/roots (dry weight basis). Dry weights were determined by oven-drying at 105 ⁰C.
The other type of experiments were in-vitro seed germination tests. Germination of NDS was performed in covered Petri-dishes filled with filter paper moistened with distilled water; 2 thermal regimes (20 and 25 ⁰C constant temperature) were tested using three replicates of about 100 seeds each (n = 3 x 100). The photoperiodic regime was 14 h light/10 h dark. Observations were made on a daily basis under a Leica EZ4 stereomicroscope 35 x (transmitted illumination). A seed was considered to have germinated when the radicle emerged (see supplementary material S1). The germination rate was calculated as the number of germinated seeds divided by the total number of seeds in the Petri dish. Dates of the first observation of visible cotyledons and lateral root growth were annotated as well.
Another seed lot was prepared and tested for in-vitro germination. All seeds (naturally-dehisced plus not-dehisced seeds) inside 50 mature LIDDU capsules were extracted by shaking and by hand with the help of a thin brush and a spatula; after seed cleaning under stereomicroscope, seeds were gathered in a seed lot named WFS (Whole Fruit Seeds). WFS germination was tested at 25 ⁰C constant thermal regime, following the same procedure as for NDS tests. Final germination rate of WFS was compared to that of NDS in order to assess the seed dispersal rate of LIDDU capsules.
4.4. LIDDU traits
Plant traits studied were the following: stem length (SL), number of whorls per stem (NW), number of capsules per stem (NC), seed production per capsule (NS), seed size, 1000-seed weight, and maximum reproductive capacity in absence of competence. One hundred shoots at senescence stage (brown shoots) were randomly sampled from cattail seedling trays in the nursery for SL, NW and NC; from these plants, fifty capsules -dry capsules starting to open- were taken at random for NS. Seed extraction and seed cleaning (separation from debris) were performed by hand with the help of laboratory equipment (thin brushes, tweezers, spatulas, stereomicroscope); afterwards, seeds from each capsule were counted under a stereomicroscope to determine NS. Seed size measurements included seed length (L) and width (W) and ratio L/W [
39]; the sample size for seed measurements was one hundred seeds. Thousand-seed weight (air-dry seeds) was determined in triplicate using a five decimal precision balance. This trait was studied for the two lots of seeds, NDS and WFS (n=3 x 2 x 1000).
The maximum reproductive capacity (individuals per ground area, pl·m-2) was calculated as the product of mean NS, mean NC, seed dispersal rate and optimum germination rate. It was assumed that the optimum germination rate was the highest asymptote value found for the germination curves in the germination tests.
4.5. Statistical analysis
Descriptive statistics (mean, standard deviation, minimum, maximum, coefficients of variation) were calculated. Data of LIDDU traits were graphically presented in terms of frequency (percentage in a category). Population densities of dominant species were analyzed by one-way ANOVA with the location of plants in the nursery as between-subject factor and two weed dominant species (factor with two levels) as a within-subject factor. For the study of seed germination, data were fitted to the S-shaped Gompertz function: Y=a*exp(-b*exp(-c*T), using the cumulative germination rate as dependent variable and the germination time (days) as independent variable; parameters a, b and c in the Gompertz function were calculated by successive iterations. Data analyses were conducted using the statistics software Statgraphics 19®.