Introduction
Mammals can be differentiated from other vertebrates by their ability to produce and secrete milk from their mammary glands to feed and provide vital nutrients for the growth of their offspring (Langer, 2008). The function of this organ is overall the same in different mammalian species, however, their development, shape, and structure can vary according to their reproductive and ecological adaptations (Akers, 2016). Literature regarding the morphology and physiology of cetacean mammary glands is limited, with few anatomical and histological descriptions in species such as baleen whales (Mackintosh & Wheeler, 1929; Oftedal, 1997). Moreover, throughout different reproductive stages, the morphology and physiology of the mammary glands can vary, and this has been seen in different mammalian species (Sinha et al., 1970; Cowie et al., 1980; Ji et al., 2006). Nonetheless, in cetaceans, knowledge of the developmental pattern of their mammary gland is scarce, with few morphological and histological examinations in baleen whales (Mackintosh & Wheeler 1929; Oftedal, 1997) and more recently with the use of ultrasound in finless porpoises (Neophocaena asiaeorientalis) (Zeng et al., 2017). This review presents the current diagnostic techniques used to assess the mammary glands in humans and animals, including imaging methods, in an attempt to provide an adequate background on how some of these techniques have been used in marine mammal medicine but mainly to underline what is missing in the literature regarding the assessment of the mammary glands in cetaceans. For example, to our knowledge, marine mammal medicine literature is devoid of an accurate ultrasonographic and volumetric description of the mammary glands of small cetaceans. We addressed the need to develop imaging protocols that could serve for the evaluation of the mammary gland in cetaceans with different demographic parameters and undergoing different reproductive stages. This could allow optimal management not only for populations under human care but also translate to health assessments for free-ranging animals.
Anatomy and Histology of the Mammary Gland
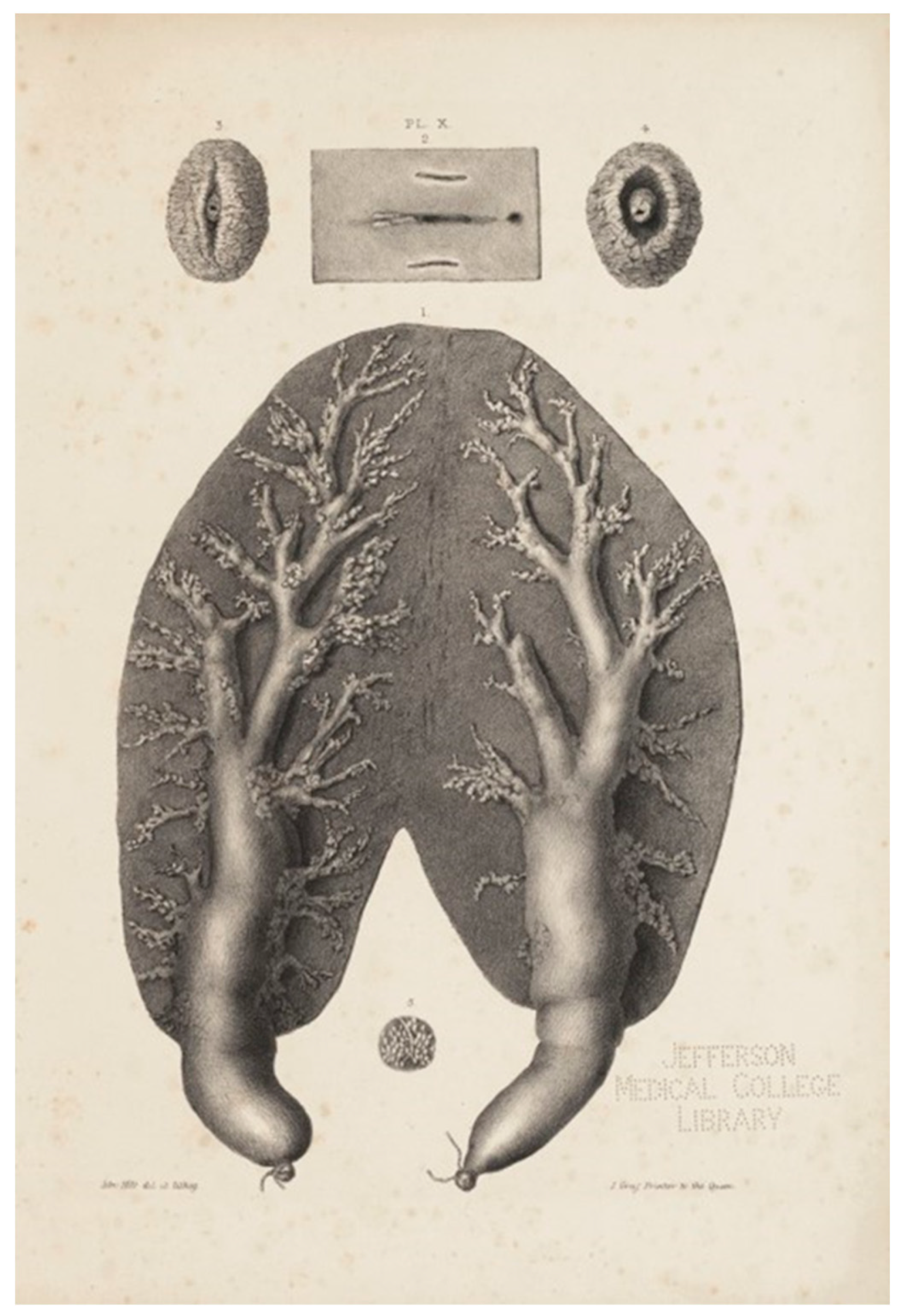
The mammary glands in most marine mammals are located in the mid-caudal abdomen, except in sirenians, whose mammary glands are positioned axillary (Gulland et al., 2018). Cetaceans have paired mammary glands which are long, flattened, and slender located longitudinally and internally along the body on both sides of the genital slit (Slijper, 1962). They have a principal duct which can be measured in lactating females, characterized by fine speckles distributed homogeneously along the parenchyma (Brook et al., 2002; Saviano, 2013). The mammary parenchyma is divided into lobules by connective tissue septa (Ridgway, 1972). The teats can be found at the caudal end of the glands with an external opening on each side of the genital groove (Oftedal, 1997; Muraco, 2015). These openings are called mammary slits, which help to determine the sex of a cetacean, although mammary slits have also been found in male cetaceans (Gulland et al., 2018). In 1840, Sir Astley Paston Cooper described the mammary glands of a porpoise carcass, which were dissected, inflated, and ligated at both extremities of the nipples. He depicted, probably for the first time, how the mammary slits are situated on both sides of the vaginal and anal slit (
Figure 1). He also illustrated how the slits look when they are slightly and completely open, exposing the nipple and its orifice.
In lactating dams, mammary glands appear swollen and can be observed anterior to the genital groove (Muraco, 2015).
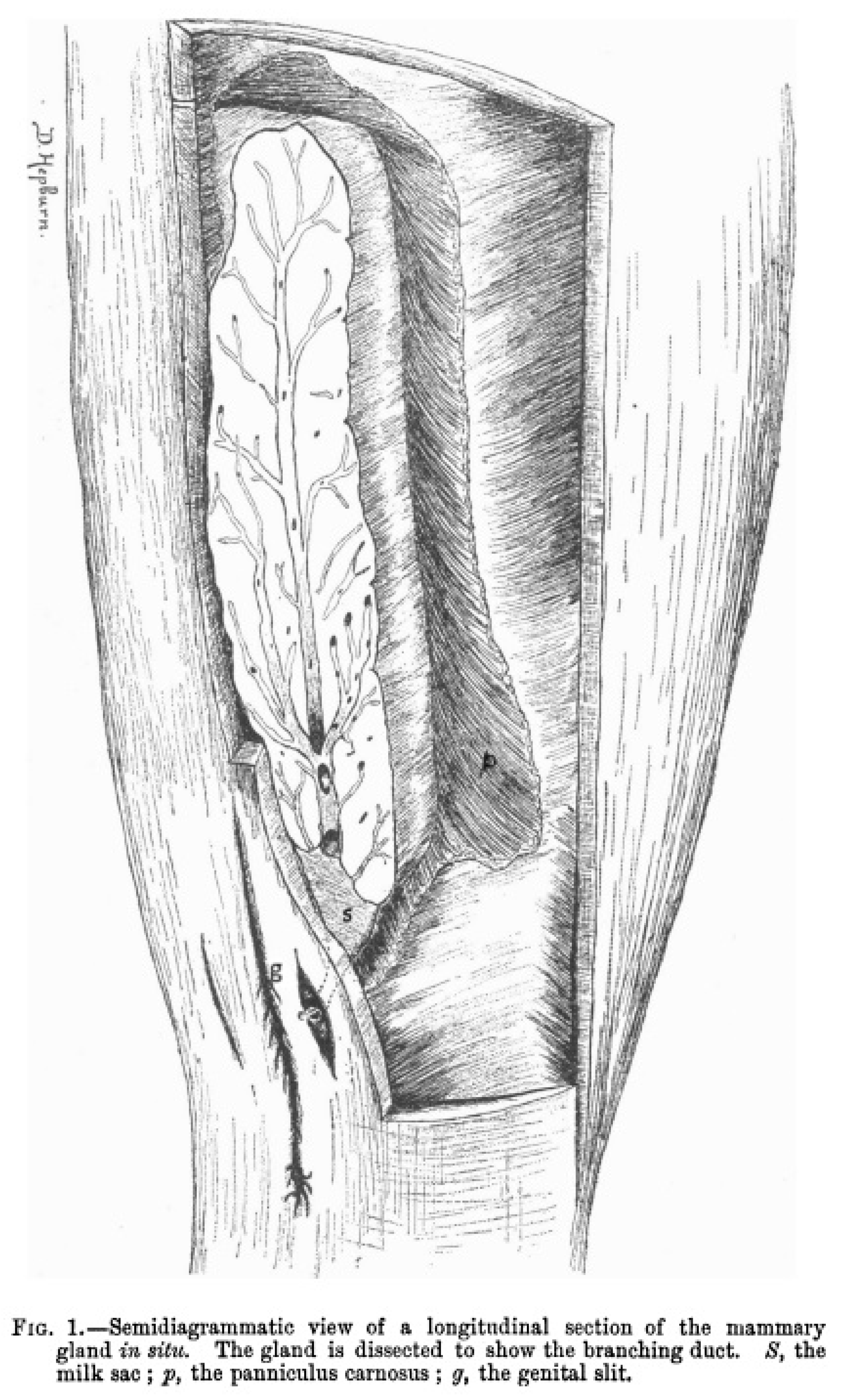
Figure 2 shows an illustration of the anatomic location of a dissected mammary gland in a gravid porpoise (
Phocoena Communis) where the branching duct is clearly visible, and a milk reservoir can be found at the posterior end of the mammary gland. This reservoir follows the long axis of the gland, projecting itself to the exterior by a single nipple (Hepburn, 1893).
Even though there is no clear division between cetacean immature and mature mammary glands, recognition of immature glands can be easily achieved (Mackintosh & Wheeler, 1929). Cetacean immature mammary glands are mainly formed of connective tissue, with a small number of blood vessels and ducts which are bordered by groups of cells creating clusters of alveoli in lobules of small size. Regarding mature mammary glands, they develop in stages correlated with lactation (Plön, 2007).
To our knowledge, literature is scarce on marine mammal mammary gland dimensions and masses. What is known is mainly related to mysticetes such as the blue (Balaenoptera musculus) and fin (Balaenoptera physalus) whales, which have flat glands measuring around 65 cm of width, 1.5 to 2 m long and with a thickness between 20 and 30 cm (Oftedal, 1997). Humpback whale (Megaptera novaeangliae) mammary glands have also been measured (Lillie, 1915), with a length of 1.7m and 45cm wide. Lactating mammary gland depth of other mysticetes such as fin, sei (Balaenoptera borealis), right (Eubalaena), and minke (Balaenoptera) whales, have also been recorded. Measurements vary from 10 cm deep in the minke whale, to 25 cm deep in a right whale (Oftedal, 1997). In relation to mass, Oftedal (1997) recorded the mammary gland mass of a lactating blue whale, measuring 1.5 m x 65 x 20 cm; the total mass of the two mammary glands weighed around 225 kg.
Information regarding measurements of odontocete mammary glands is also scarce. Lactating spotted dolphins (Stenella frontalis) and spinner dolphins (Stenella longirostris) have mammary glands measuring on average around 27 cm in length and 2.8 of depth (Pilson & Waller, 1970). Another odontocete species, the long-finned pilot whale (Globicephala melas), was recorded to have lactating mammary glands measuring 15 cm wide, 45 cm long, and 7.5 cm deep; while a stranded dwarf sperm whale (Kogia sima) with a calf, was documented to have a left mammary gland weighting 351 gr with the following dimensions: 48.4 cm long, 4.4 cm wide, and with a thickness of 3 cm. The total mass of both mammary glands of this whale was around 700g (Oftedal, 1997). Mammary gland masses of other species have also been recorded, varying between 570 g in a common dolphin (Delphinus delphis) whose mammary glands measured 32cm by 7cm by 2cm from the left side, and 31cm by 7cm by 2cm from the right side; to 597 g in a lactating harbor porpoise (Phocoena phocoena) whose right mammary gland measured 30.5cm by 7.5cm by 2.3 cm (Oftedal, 1997). It has been suggested that there is an approximate correlation between mammary gland mass and linear gland proportions in cetaceans such as the common dolphin, harbor porpoise, blue and dwarf sperm whales (Oftedal, 1997).
Functions of Prolactin on the Mammary Gland
Regarding mammary gland development, prolactin plays a major role in lactation, mammogenesis, galactopoiesis, and female reproduction (Horseman, 1999; Muraco et al., 2020) and has been widely studied in humans and rodents (Horseman, 1999). Nonetheless, information regarding prolactin in marine mammals, and especially dolphins, is scarce, focusing on the study of its physiological effects in several cetacean species, which is to regulate lactation and the rate of milk production (Cozzi et al., 2016).
Despite prolactin having multiple roles in different mammalian species, birds, and even fish, its main target organ, and the most sensitive, is the mammary gland. Prolactin can promote mammary gland growth, initiate the production of milk, and sustain lactation with the help of other metabolic, local, and reproductive processes (Trott et al., 2012). For mammary growth promotion, it has been observed in mice that prolactin can influence terminal end bud regression and ductal side branching (Brisken et al., 1999). It is known that basal concentrations of prolactin fluctuate depending on the reproductive stage of the female during and after gestation, increasing at the time of late gestation, peaking when parturition is about to initiate and decreasing during lactation (Neville et al., 2002). Concentrations of prolactin can also increase during proestrus, and decrease during diestrus, but compared to progesterone, it declines during estrous (BenJonathan et al., 2008). Synergy between these hormones can result in the development of mammary alveoli and branching morphogenesis (Trott et al., 2012).
In marine mammals, prolactin is known to be of the utmost importance for the development of mammary gland secretory cells, increasing in otariids usually one or two days before parturition and showing highest levels around 0-3 days postpartum (Gulland et al., 2018). In captive bottlenose dolphins, it was reported that prolactin concentrations began to rise with the progression of pregnancy and reached its highest concentrations in the last month before parturition (Muraco et al., 2020), which has also been detected in humans (Kletzky et al., 1985). Moreover, they also found that during the dolphins’ first trimester of pregnancy, prolactin concentrations were higher in normal pregnancies than abnormal ones, and higher during the luteal phase.
Common Methods for the Assessment of the Mammary Gland Morphology and Physiology
Laboratory Investigations
Blood samples are the most common biological samples to measure prolactin levels in both humans and production animals such as dairy cows (Saleem et al., 2018; García-Ispierto et al., 2009). These levels might be influenced by different factors in healthy individuals, hence, to accurately interpret prolactin value results, ample reference data must be obtained. Factors that need to be considered include age, sex, stress, circadian rhythm, and even drug ingestion (Cowden et al., 1976). To address the need to obtain reference ranges of prolactin concentrations in healthy subjects and individuals with diverse diseases, Cowden et al. (1976) used serum samples to study the effect on prolactin levels of different demographic parameters such as age and sex, as well as of other factors such as circadian rhythm, stress, and pregnancy, in individuals with no illnesses. Their research also included measurements of basal prolactin levels in individuals with diverse pathological conditions such as prolactinoma and growth hormone deficiency. With the information obtained from this study, Cowden and colleagues developed a standardized approach to routinely assess prolactin levels in the laboratory. Their results showed that there was no statistically significant difference between basal prolactin levels between men and women in the study, although literature states that it is common for female subjects to have higher mean basal prolactin levels than in men (Friesen and Hwang, 1973). Nonetheless, a significant fall in prolactin levels has been found in postmenopausal women, which could be influenced by estrogen (Cowden et al., 1979). Age was also found to influence basal prolactin levels, especially in women. Cowden and colleagues propose that the hyperprolactinemia found in neonates could be either of fetoplacental origin or estrogen related.
The increasing need to find less invasive methods to measure prolactin has made both human and animal researchers assess if it is feasible to measure prolactin in a non-invasive way with samples such as urine (Keely et al., 1994; Leaños-Miranda et al., 2008; Muraco et al., 2020) and saliva from humans, dogs, and rhesus macaques (Macaca mulatta) (Steinfeld et al., 2000; Gutiérrez et al., 2019; Lindell et al., 1999). In regards to marine mammals, to our knowledge, the only non-invasive methods described in the current literature to assess prolactin levels in female bottlenose dolphins belongs to the studies performed by Muraco et al. (2020), where they used dolphin urine to measure prolactin levels throughout several reproductive stages; and from Steinman et al. (2012) where they validated urinary immunoassays including several hormones such as prolactin for their use in managed belugas (Delphinapterus leucas). However, the literature is devoid of basal ranges of prolactin in healthy female cetaceans of various ages and reproductive stages, which could be useful to predict an optimal development of the mammary gland for lactation and calf survival.
Physical Assessment
The most common way to evaluate the health of the mammary glands in both humans and animals is through a physical examination. As part of an integrative assessment of the human breasts, a clinical breast exam (CBE) provides vital information for the diagnosis of an array of breast diseases and abnormalities, for which clinicians need to have the necessary skills to perform an adequate examination (Henderson et al., 2022). Moreover, this exam can aid in the early detection of breast cancer as a complement to mammography (McDonald et al., 2004). Physical alterations that might be noticed by the patient include changes in the skin around the breasts, pain, nipple discharge, among other abnormalities (Henderson et al., 2022). Although literature has various ways to describe the CBE technique, the basic components of this examination, which include visual inspection and palpation of the breasts, are always included in these descriptions (McDonald et al., 2004). The first step of the exam is the visual inspection of the breasts, assessing different parameters such as symmetry, shape, temperature, texture, venous patterns, and size of the breasts, as well as shape, texture, color, and size of the nipples (Henderson et al., 2022; McDonald et al., 2004). The next step after the visual inspection, is palpation of the superficial and deep breast tissue, usually performed with the clinician’s finger pads to examine the consistency of the breast in order to detect lesions or masses, as well as to evaluate the nipple areolar complex (Henderson et al., 2022). The MammaCare technique is one of the most common methods used for palpation of the breast tissues, which describes how the finger pads should move and how much pressure to apply during the assessment (Pennypacker & Pilgrim, 1993). Palpation also includes assessment of the lymph nodes while the patient is sitting down, extending to the axillary nodes (Coleman et al., 2001). In the literature, to document the results of a breast examination, terms such as symmetrical or asymmetrical, ptotic or pendulous shape, soft, dense, and nodular texture are used; as well as pink, everted, or presence of discharge to describe the nipple-areolar complex (Henderson et al., 2022). To reference the skin, terms like dry, edema, warm, open sores, and presence or absence or erythema are used (Henderson et al., 2022). Important factors to consider for an effective CBE include how the breast tissue is positioned, how much breast area is assessed, finger motion, how many fingers and how much pressure to use, and how much time is spent in the examination (McDonald et al., 2004).
In recent years, the use of CBE has been under scrutiny due to the more modern diagnostic tools available for clinicians, particularly for the detection of breast cancer (Zafar, 2014), even though years back CBE alone could detect breast cancer without the need of other type of examinations (McDonald et al., 2004). Moreover, this modality is usually not performed in a standardized way by examiners, so for these reasons, a study was conducted to assess the sensitivity and specificity of a CBE alone compared to performing it along additional diagnostic techniques (Zafar, 2014). The detected lumps were assessed by ultrasound or mammogram along with the CBE, with fine needle aspiration cytology for confirmation of the diagnosis. Their results showed that a structured CBE is sensible enough to diagnose even a small lump; although they found that inspection can be better for detecting some malignancy indicators such as slight skin dimpling and nipple defacement. Studies also report that additional factors that can have a significant impact on the sensitivity and specificity of a CBE include type of tumor, examiner, and patient (McDonald et al., 2004).
Regarding veterinary medicine, physical assessment of the mammary gland has been described mainly for production animals such as lactating cattle and to a lesser extent for small ruminants (Blagitz et al., 2013; Klaas et al., 2004; dos Santos et al., 2016). Advantages of performing this type of clinical examination in the udder and teat of production animals include being a low-cost technique, relatively fast, and able to provide important clinical information for the early detection of pathologies such as mastitis or intramammary infections (Blagitz et al., 2013). As in human medicine, physical examination of the udder and teats in veterinary medicine include a visual inspection followed by palpation. Blagitz and colleagues (2013) created a palpation score of the teats of meat-producing ewes, which included descriptions of the texture, volume, symmetry, and thickness of the orifice, as well as presence or absence of thelitis (inflammation or swelling of the teat) and cisternitis (inflammation of the teat cistern). For udder assessment, they focused on its texture and presence or absence of nodules. They also recorded signs of inflammation by examining if the supramammary lymph nodes presented any abnormality. They concluded that to create an effective mastitis control program, veterinarians need to include microbiological tests, a thorough clinical examination, and milk somatic cell counts (MSCCs).
Klaas et al. (2004) evaluated the feasibility of applying systematic clinical examinations to the udders of Danish dairy herds, in order to enhance udder health assessments on dairy farms. For this study, 19 variables were used to characterize udder and teats, adding parity and lactation stage as variables, as well as somatic cell count (SCC) and milk yield. Research technicians performed the udder clinical examinations according to the methodologies by Rosenberger (1979) and Houe et al., (2002), assessing all quarters of the cow. Some of the clinical and morphological variables used for this study included asymmetry between quarters, udder and teat shape, clinical mastitis, udder edema, knotty tissue, teat wound, among others. These variables were detected with the previously described techniques of visual inspection and/or palpation. This study demonstrated that the stability in the type of udder patterns can function as evidence that performing clinical examinations of the udder can be a valuable tool not only for veterinary practice but also for research purposes. Udder health in small ruminants also relies on visual assessments, with literature determining that healthy udders should be properly attached to the animal’s abdomen and have a large volume and rounded shape, which are ideal for milk production (Vrdoljak et al., 2020). Palpation is also a crucial part of the mammary gland examination in ruminants such as goats, which can aid to identify if there is an inadequate use of milking equipment in dairy goat farms (Ramos et al., 2020).
To the best of our knowledge, there are only a few descriptions of physical assessment in the mammary glands of marine mammals, mainly in species such as polar bears under human care, using palpation (Gulland et al., 2018). In cetaceans, assessment of the mammary glands is usually performed when there are visual changes detected that could indicate the presence of inflammation. Mauroo et al. (2008) reported that a female Indo-Pacific bottlenose dolphin (Tursiops aduncus) was diagnosed with parasitic mastitis after detecting signs of inflammation. Ultrasonography proved to be essential for a definitive diagnosis, as what Brook et al., (2002) performed in three Indonesian bottlenose dolphins, although on the latter case there were no physical changes noted in their mammary glands; incidental findings were reported during their routine ultrasound exam. In one routine ultrasound examination, they found enlargement of the mammary glands in one individual, with dilation of the lactiferous ducts, and presence of a lesion in the abdominal muscle. Enlargement of the mammary glands was also detected with ultrasound in two specimens as an incidental finding, until a year later they visualized a parasite in two of these animals with ultrasonography. These cases show that the physical evaluation of the mammary glands in cetaceans needs to be complemented with other diagnostic techniques such as ultrasound and laboratory exams for the definite diagnosis of mastitis.
Diagnostic Imaging Techniques
In this section, the main imaging techniques used for the assessment of the mammary glands in human and veterinary medicine will be discussed. It is important to note that mammary glands can change structurally due to different factors such as age. In the early life of humans, this organ is mainly composed of adipose tissue, while fibroglandular tissue appears at puberty extending until adulthood until it decreases again with time. Regarding glandularity, there are also variations according to age which can affect the density and radiographic appearance during a diagnostic imaging procedure (Lemoigne et al., 2007). Age, hormones, and the use of prescription drugs can hamper the diagnosis of breast abnormalities and diseases, making imaging a complementary diagnostic tool for the breast investigation (Maciejewski et al., 2003).
X-ray and Magnetic Resonance (MR) Mammography
One of the most common imaging techniques used in human medicine to assess women's breast tissues is called X-ray mammography. This method is comprised of an X-ray shadowgram which irradiates the breast from different directions, with a support plate for the breast with an X-ray tube that can rotate to create specific radiographic projections; the X-rays transmitted are then documented by an image receptor (Analoui et al., 2012; Lemoigne et al., 2007). For this image modality, quality of the images is of the utmost importance, as to detect abnormalities such as micro-calcifications and masses, high resolution and low contrast are needed, with a low dose of radiation to minimize the risk of exposure in the patient (Noel, 2007). Moreover, for the system to be capable of demarcating the borders of the breasts’ fine structures, it needs enough special resolution (Analoui et al., 2012). An important characteristic of X-ray mammography is the compression of the breast during the examination which helps to lower the dose of radiation, to enhance contrast, avoid loss of sharpness due to movement, as well as allowing an improved assessment of the breast tissues by avoiding their overlap (Lemoigne et al., 2007). X-ray mammography can detect soft tissue abnormalities and calcifications of different shapes and sizes, as well as disruptions in breast architecture (Lemoigne et al., 2007). For this imaging modality, to achieve the best visualization of breast tissues and to accurately detect any sign of abnormality, the use of the right equipment with precise and accurate specifications by trained experts, is needed (Williams et al., 2021). Limitations of this method include limited image latitude and gradient of the film which can hamper the detection of tumors if they are positioned in a highly opaque or lucent area of breasts with particularly high amounts of fibroglandular tissue (Analoui et al., 2012). Nowadays, the risk of radiation exposure in this imaging technique has dropped significantly due to full-field digital mammography (Noel, 2007). In this modality, an electronic detector is used to absorb the radiation applied to the breast tissues, and to enhance image processing as well as storage, these processes are performed independently (Analoui et al., 2012; Pisano & Yaffe, 2005). After image acquisition is completed, digital image-processing methods are applied to modify both brightness and contrast without the need to expose the patient multiple times (Pisano & Yaffe, 2005).
Magnetic resonance imaging (MRI) is a conventional imaging technique which has greatly contributed to diagnosing different pathological conditions and monitoring health in the general population (Pagani et al, 2008). This diagnostic method shares features with other techniques such as ultrasound, computed tomography (CT), and radiography but differs in image formation and how it generates signal (Bolas et al, 2010). Assessment of the breast through MRI is a functional method that has become one of the most sensitive imaging techniques to detect breast cancer in women, mainly using T1-weighted contrast-enhanced imaging as well as T2- and diffusion-weighted imaging (Mann et al., 2019). This method is known for its high accuracy in discriminating between different types of breast abnormalities and whether they are of benign or malignant nature (Mann et al., 2019). By applying contrast material such as gadolinium chelate, MRI can assess how permeable blood vessels are, achieving a better signal on T1-weighted images by shortening the local T1 time (Knopp et al., 1999). An important consideration of breast MRI analyses is the need for interpretation by radiologists with vast experience in breast imaging, including the above-mentioned techniques. Moreover, to obtain images with an excellent diagnostic quality, a breast coil should be used for all examinations (Mann et al., 2019). By using a breast coil, the breast tissue spreads in a way that enhances the diagnostic precision to detect abnormalities and avoids respiration to produce motion artifacts (Yeh et al., 2014).
Computed Tomography (CT) Scanning
CT scanning is one of the most useful methods to provide complete cross-sectional images of internal structures, commonly applied in human medicine to assess normal and abnormal organs (González-Romano et al., 2000). CT has been used in less extent to assess the breast tissue in women and living animals mainly due to the high radiation exposure and because mammography, which is the preferred screening test to detect breast abnormalities (Boone et al., 2006; Løberg et al., 2015) and ultrasonography, can provide superior information than CT (González-Romano et al., 2000). Nonetheless, a CT system has been developed to scan the breast, showing that the subjective assessment of the resulting CT images provide superior anatomical detail, can detect microcalcifications, and even soft tissue elements of a tumor (Boone et al., 2006).
Animal studies aimed to assess the mammary gland with this imaging technique are also scarce, focusing mainly on dairy animals such as the goat. González-Romano et al. (2000), assessed the caprine mammary gland by CT, X-rays, and histology. With CT it was possible to visualize the different udder structures and the surrounding fascia and muscles. Moreover, visualization of the mammary vessels after the injection of contrast medium was also possible and different areas of the lactiferous sinus were identified, with the possibility of obtaining its volume. The study concluded that with the use of CT, X-rays, and histology, it is possible to assess the normal anatomy of the mammary gland in goats. To our knowledge, this imaging technique has not been applied yet to assess the mammary gland of live marine mammals, although it is commonly used to detect pathologies that may be hard to identify in conventional radiographs or ultrasonography (Gulland et al., 2018). However, the application of postmortem imagining modalities, including postmortem CT scanning, on stranded cetaceans has grown over the years as marine mammal veterinarians and personnel working in stranding response programs have become more aware of the strengths of virtopsy (Tsui & Kot, 2015). At the same time, it could be useful to evaluate the morphology and characteristics of mammary glands, and other organs and systems of deceased stranded cetaceans.
Ultrasonography of the Mammary Gland
With the use of ultrasound, it has been possible to observe that glandular tissue increases throughout pregnancy and by the 6th to12th week, the predominant tissue is composed of adipose origin (Morozova et al., 1997, Geddes, 2009). Furthermore, another study made lactating women undergo semi-quantitative ultrasound to measure their adipose and glandular tissue, observing that there was twice as much glandular tissue as of adipose origin (Ramsey et al., 2005). Nevertheless, these proportions varied among women, comprising up to 80% of glandular tissue in some of them.
The anatomy of the lactating breast has unique features compared to other phases of breast development which need to be considered when trying to diagnose lactation pathologies through scanning techniques and ultrasonography (Geddes, 2009). When performing a mammogram of the lactating breast, limitations such as an increase in glandular tissue and milk secretions have to be considered, as this can enhance radiodensity and hamper radiograph interpretation (Chersevani et al., 1995). Imaging modalities such as magnetic resonance imaging (MRI) and computed tomography (CT) have many diagnostic advantages over other techniques such as radiography, nonetheless, their role in detecting pathologies in the lactating breast are scarce (Geddes, 2009). In comparison, ultrasound has detected small structures located in the breast, due to its improved image resolution in recent years and being a non-invasive technique (Geddes, 2009). Even though ultrasonography has several advantages over other imaging modalities in the assessment of the lactating breast, it is important to have a proper knowledge of anatomy and of its changes during lactation (Jokich et al., 1992). Regarding the gross anatomy of lactating breasts, Ramsey et al. (2005) used high-resolution ultrasound for its assessment, finding less ducts than what has previously described. Moreover, instead of lactiferous sinuses, they found that ductal branches were draining into glandular tissue merging into the principal collecting duct a few inches close to the nipple. Later, it was discovered that the lactating breast has milk ducts that only increase during milk ejection, transporting milk to the nipple instead of storing it for later removal (Ramsey et al., 2004).
B-Mode Ultrasound
When using ultrasonography for breast assessment, it is important to note that this technique needs a higher resolution than other imaging procedures, especially for its subcutaneous tissue (Geddes, 2009). A linear array transducer with the characteristics of being electronically focused and having a frequency of 7-12 MHz should be used for this assessment, adding several focal zones to have a better resolution in the area of interest (Smith, 2001). For larger lactating breasts, using a 5MHz probe might the best choice for increasing the penetration of the breast tissue and enhance focus at depth, and when adjusting the time compensation curve, it should range from a steep slope for dense breasts to a gentle slope for fattier breasts (Geddes, 2009). It has also been found that when assessing the non-lactating breast, moderate compression gives better results for improved image quality and to observe small masses situated in a profound section of the breast (Stavros, 2004). Nonetheless, when scanning the lactating breast, it is not recommended to apply compression as it might alter the shape of milk ducts, affecting the visualization of these structures (Geddes, 2009). When assessing the normal breast through ultrasound, the subcutaneous fat should look like a layer beneath the skin surface with a hypoechoic echogenicity (Geddes, 2009). In women, Cooper’s ligaments can be found between the two breast’s fascia, appearing as echogenic lines starting from the posterior aspect of the breast to the skin; these ligaments can cause posterior shadowing due to their fibrous and curved texture which can be avoided by changing the angle and pressure induced through the probe (Baker et al., 2002).
The amount of fat deposited in the glandular tissue can affect the ultrasonographic appearance of the breast. For example, fatty tissue can look hypoechoic with respect to the glandular tissue which has echogenic appearance, but in other cases it can appear isoechoic (Geddes, 2009). Regarding the mammary ducts, ultrasound depicts them as hypoechoic linear structures of small size, changing their size depending on their proximity to the areola. These structures have a larger size under the areola and decrease their diameter when they get closer to the periphery of the breast (Teboul & Halliwell, 1995). Mammary ducts from a non-lactating breast do not change their shape when compression is applied to them, unless they have liquid content in their lumen such as blood. In this regard, color doppler imaging can make the distinction between a duct and a blood vessel vessel or detect lesions within a duct (Ballesio et al., 2007; Geddes, 2009). Difference in echogenicity between the lactating and non-lactating breast is minor, although there are variations regarding milk ducts, as ducts from the lactating breast easily compress under a small amount of pressure (Geddes, 2009). The ultrasonographic appearance of milk flow can be observed as echogenic flecks generated from the reflection of the milk lipid components (Ramsey et al., 2004). With the production and accumulation of milk in breast tissue, the echogenicity of glandular tissue increases (Geddes, 2009).
B-mode ultrasound has also been used in veterinary medicine to explore the structure and functional parameters of the mammary gland in different dairy animals such as ewes and heifers (Barbagianni et al., 2016; Nishimura et al., 2010). This imaging modality can be used to obtain information about the internal structure of the udder, to assess the nipple, mammary parenchyma, and gland cistern, as well as to examine blood flow anomalies, all in real time (Barbagianni et al., 2016). Research has shown that the assessment of the grey-scale intensity of the mammary parenchyma can aid to understand the quantity of fluids and glandular tissue present in the parenchyma (Barbagianni et al., 2016). If this assessment is performed, sections of the parenchyma containing ducts or blood vessels, should be avoided (Barbagianni et al., 2015; Petridis et al., 2014). The normal sonographic appearance of the ewe’s mammary parenchyma appears homogeneous with medium echogenicity and lactiferous ducts and vessels with an anechoic appearance (Barbagianni et al., 2016). Ultrasonography in these species has shown changes in the parenchyma depending on the reproductive status of the ewes such as pregnancy and termination of lactation (Petridis et al., 2014). Moreover, it is possible to detect mastitis, characterized by non-homogenous areas in the mammary gland parenchyma, with alternating hypo- and hyperechoic regions (Franz et al., 2003). However, while mild inflammation shows normal echogenicity in some individuals (Barbagianni et al., 2016), in severe mastitis the mammary parenchyma loses its normal ultrasonographic appearance, showing a non-homogeneous texture with regions of varied echogenicity, indicating a severely damaged parenchyma (Barbagianni et al., 2016). Ultrasonographic characteristics of this mammary pathology also include enhanced echogenicity of the mammary secretion due to its increased number of cells (Floeck & Winter, 2006). B-mode ultrasound has also been used in ewes to detect small mammary abscesses when these structures are still difficult to palpate, as well as to differentiate them from cysts or haematomas (Barbagianni et al., 2016). A mammary abscess can be observed in ultrasound as a structure with circular shape, a capsule and content of hypoechoic nature (Floeck & Winter, 2006).
B-mode ultrasound has also shown changes depending on the stage of development in the mammary glands of Holstein Freisian heifers (Nishimura et al., 2010). The organs of two-month-old animals were characterized by presenting a homogeneous and hypoechoic appearance, while in the rest of the groups there were two distinct sections within the mammary tissue, one in the superficial section with a not well-defined heterogeneous and hypoechoic appearance and the other section looking homogeneous with medium echogenicity. In pregnant heifers, their mammary glands presented irregular and distinctive anechoic or hypoechoic areas like lactiferous sinuses. It was suggested that the different hypoechoic areas found in the mammary glands might represent ducts and lactiferous sinuses, concluding that B-mode ultrasound can be an important imaging technique to distinguish and evaluate the internal structures of heifer udders.
Most of the studies related to the morphology and physiology of the mammary gland in cetaceans have relied mainly on freshly deceased animals or by using biopsy on live individuals. With the use of imaging techniques such as B-mode ultrasound, non-invasive studies can be performed to assess these anatomical structures as what has been done to characterize the morphology of other organs in cetaceans, such as the reproductive tract of Indo-Pacific bottlenose dolphins under human care (Brook, 2001; Wu et al., 2010b). B-mode ultrasound has not been used in cetaceans as for other animals or humans. The existing information focuses on how the mammary gland changes during different reproductive stages in two species, the finless porpoise (Neophocaena asiaeorientalis) (Zeng et al, 2017) and the bottlenose dolphin (Tursiops truncatus) (Muraco et al, 2020). Zeng et al. (2017) found that mammary glands of immature finless porpoises had less defined borders than mature animals, and that compared with the homogeneous appearance of the parenchyma with medium echogenicity, the mammary duct system was characterized for being anechoic or hypoechoic. The mammary glands' appearance when viewed in the transverse section was characterized by an oblong shape with its ducts collapsed or rounded. In the longitudinal assessment, the large sinus could be visualized in the posterior end of the mammary gland, with the connecting canal traveling through the center of the mammary tissue, branching out to the anterior end of the gland. Moreover, body length affected significantly both mammary gland depth and width among reproductive stages. The most notorious difference was found in the mammary gland depth in late -lactating animals compared to mid-lactating individuals, having a greater depth in the late-lactating porpoises. Regarding immature animals and animals in mid-and late-pregnancy, gland depth was less than in mid-lactating animals. Width measurements of the gland showed that late-lactating animals had a greater width than animals in mid- and late- pregnancy. These findings can be of importance to depict the developmental pattern of the cetacean mammary gland.
Muraco et al. (2020) assessed the mammary gland of lactating and non-lactating bottlenose dolphins under human care with B-mode ultrasound, finding that the parenchyma of non-lactating females was difficult to observe, presenting a hyperechoic border with homogeneous echogenicity. When assessed through a transverse view, hyperechoic ligaments could be observed, which were used as landmarks to locate the gland in non-lactating dolphins. On the other hand, the mammary gland parenchyma of lactating females could be easily found with ultrasound, presenting a homogeneous and hypoechoic echogenicity, containing lactiferous ducts with an anechoic appearance and a well-defined hyperechoic border and ligaments. Therefore, the use of B-mode ultrasound can be a potentially valuable tool to evaluate the morphological and physiological characteristics of the mammary gland throughout the different reproductive stages of small cetaceans (Muraco et al., 2020) but more studies are needed to find a correlation between mammary gland morphology and demographic parameters such as age, body size, and weight.
Color Doppler Ultrasound
Ultrasound allows the assessment of an organ’s blood flow by using color Doppler sonography, which can be used to detect mammary blood vessels (Barbagianni, 2016). When color Doppler ultrasound is performed, blood vessels appear either blue or red depending if the red cells are moving towards or away from the probe and according to the settings established at the beginning of the examination (Petridis et al., 2017). This modality has been used in human medicine to evaluate the vessel arrangement, blood flow, and vascularization extent of women’s breasts (Giuseppetti et al., 1998); as well as to assess vascularization of mammary lesions and to demonstrate inflammatory hyperemia in abscesses and mastitis (Busilacchi et al., 2012). In veterinary medicine, color Doppler sonography has been used in different dairy animals to document changes during lactogenesis in healthy and diseased udders of ewes (Barbagianni et al., 2015), to evaluate blood flow disorders in goats and sheep (Petridis et al., 2014; Barbagianni, 2016), and to measure the variability of mammary blood flow in lactating cows (Götze et al, 2010). However, to our knowledge, the literature is devoid on the use of color Doppler sonography to assess the mammary blood flow of cetacean mammary glands. Knowledge gained from this assessment could be useful to assess if mammary blood flow changes not only during lactation but also during different reproductive states and mammary gland pathologies such as mastitis.
3-D Ultrasound
With the development of 3-D ultrasound, the limited two-dimensional assessment of three-dimensional anatomy has been addressed, overcoming the difficulties of mentally integrating, quantifying, and visualizing different organs (Fenster et al., 2011). Moreover, research studies are incorporating the use of 3-D ultrasound into different procedures such as biopsy and therapeutic techniques (Carson & Fenster, 2009). To generate 3-D ultrasound images, there are different methodologies that can be applied, including free-hand scan with or without position sensing, mechanical scan, and 2-D array scan for dynamic 3-D ultrasound (Fenster et al., 2011). For the sonographic assessment of breast tissues, 2-D ultrasound scanning requires a skillful operator that can envision the organ as a 3-D structure. By using 3-D ultrasound techniques, the mammary gland tissues can be better analyzed by having a clearer depiction of the borders and morphology (Shipley et al., 2005). 3-D ultrasound has also the advantages to provide complementary information compared to 2-D for the general breast tissue assessment (Rotten et al., 1999) such as better interpretation of infiltration, different breast diseases, and intracystic structures (Blohmer et al., 1996). To improve the sonographic evaluation of the mammary gland in women, a system was developed to obtain images acquired from ultrasonographic examinations of the breast, diminish attenuation artifacts and generate a 3-D volume model suitable for clinical purposes (Shipley et al., 2005). Compared to human medicine, in veterinary medicine the use of this technique is much less frequent. Reports describe the use of this modality to determine the reproductive tract, pregnancy diagnosis, and other uses in zoos, small animal, and equine medicine (Franz et al., 2004). In cows and goats, 3-D ultrasound has been used to evaluate their mammary glands providing good-quality, 3-D images of the organ and its different structures, including the parenchyma and lactiferous ducts (Franz et al., 2004; Fasulkov et al., 2018). To our knowledge, in marine mammal medicine, 3-D ultrasound has not been used to study and measure the mammary gland volume.
Shear-Wave Elastography
Shear wave elastography is a newer ultrasonographic technique which induces the movement of lateral shear waves with acoustic pulses created by the transducer to analyze the stiffness of a tissue in a quantitative way (Barr & Zhang, 2015; Jung et al., 2020). Evaluating elasticity by this imaging modality can provide additional information to B-mode ultrasonography by including stiffness as a property that can be measured with ultrasound (Kamaya et al., 2013). In human medicine, elastography has been applied as a non-invasive technique mainly to evaluate liver fibrosis, and more recently to study tissue stiffness in organs such as prostate, kidney, thyroid, lymph nodes, muscles, as well as the breast (Sigrist et al., 2017). In veterinary medicine, shear wave elastography has been used to assess normal soft tissue organs in dogs, to distinguish between benign and malignant conditions of lymph nodes and mammary tumors, and to assess changes in liver stiffness after radiofrequency ablation in dogs (Feliciano et al., 2015; Glińska-Suchocka et al., 2013; Jung et al., 2020). It has also been applied in cats, showing that it can characterize the normal appearance of their kidneys, liver, and spleen in both an objective and subjective way (White et al., 2014). However, to our knowledge, shear-wave elastography has not been applied in marine mammal medicine, which could function as a complementary imaging modality to assess the normal and abnormal stiffness of the mammary glands in cetaceans, which could aid in the diagnosis of pathologies such as mastitis.