Submitted:
13 June 2024
Posted:
13 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
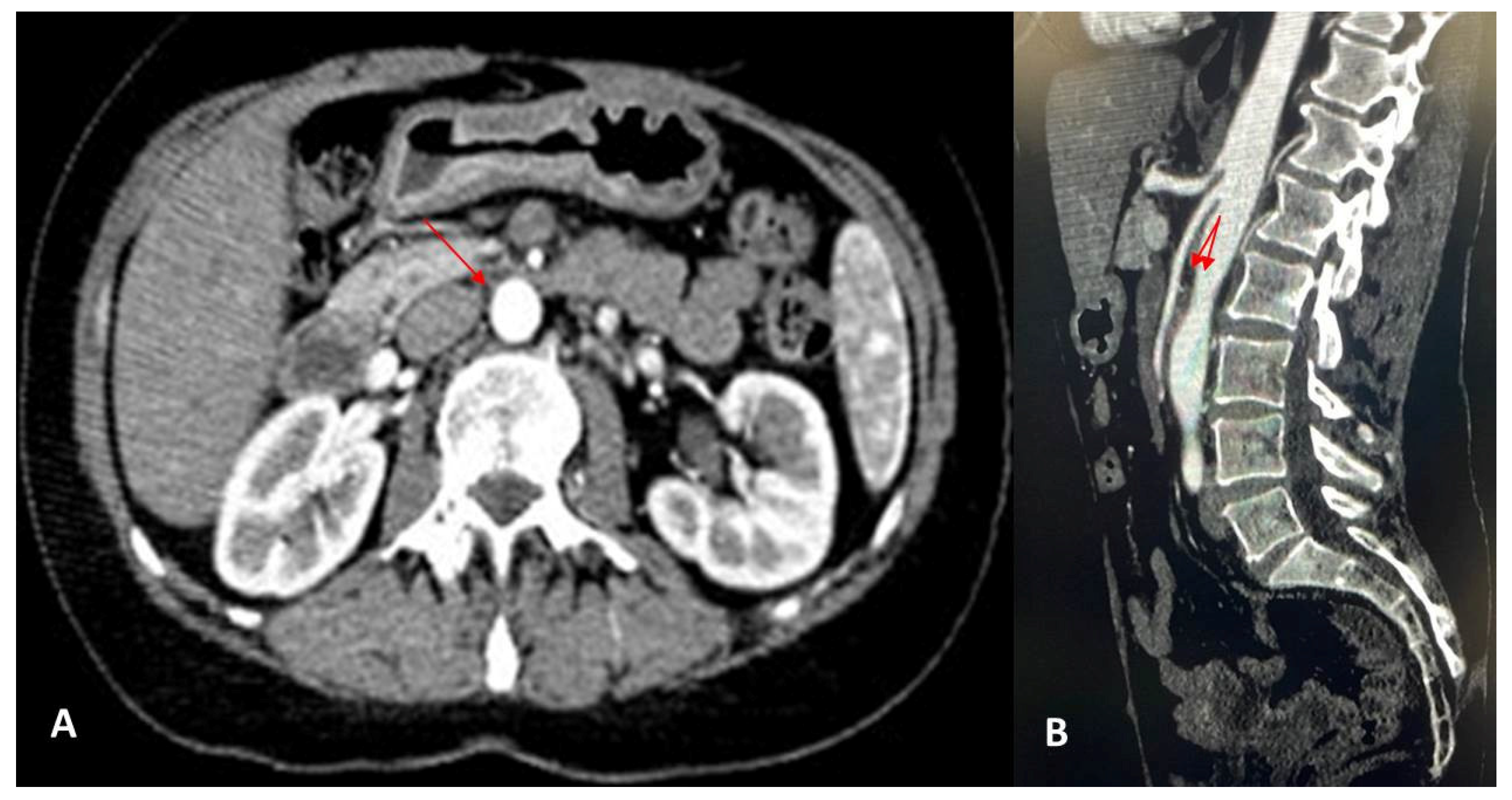
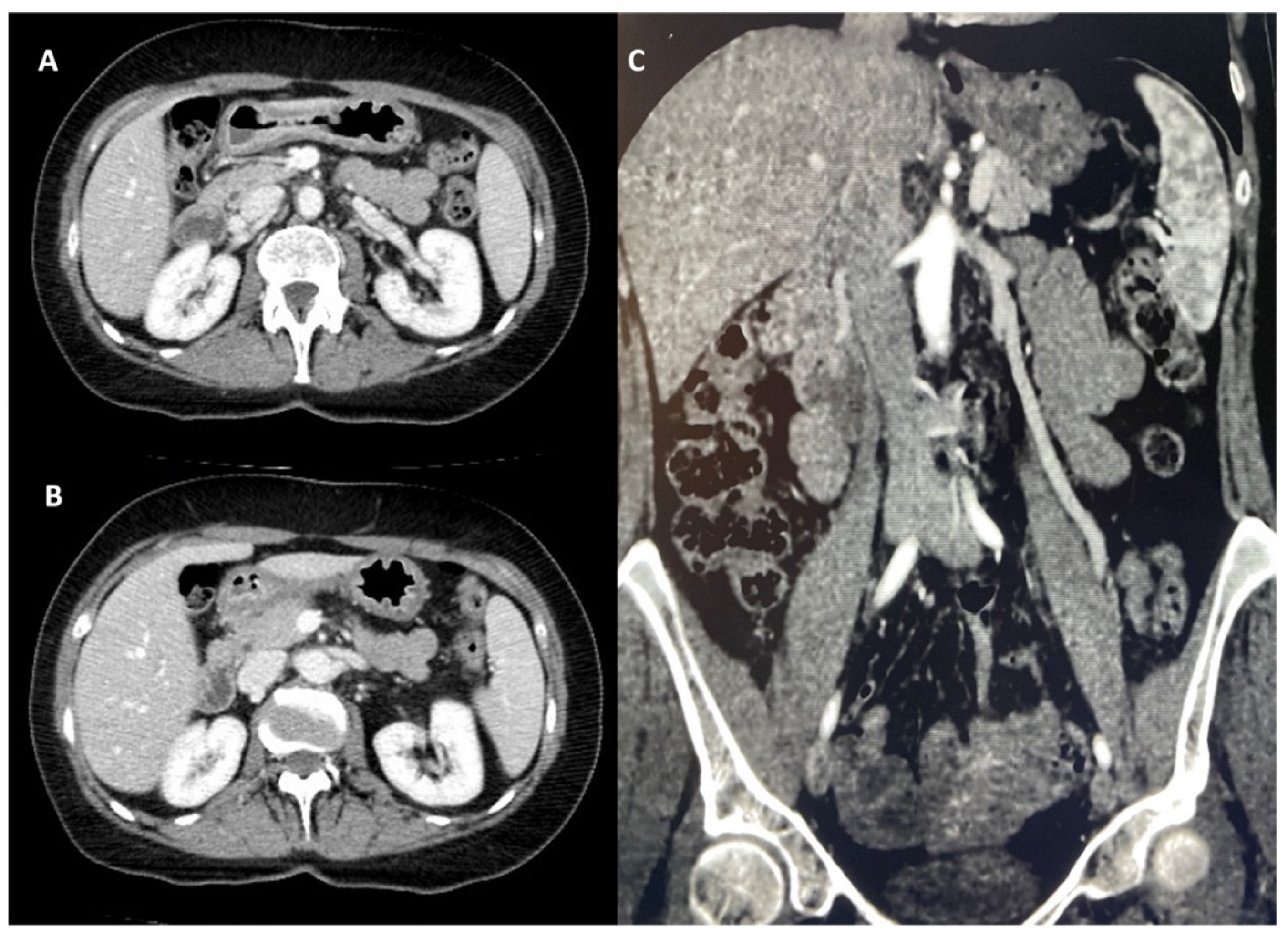
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Claro, M.; Sousa, D.; Abreu da Silva, A.; Grilo, J.; Martins, JA. Wilkie’s Syndrome: An Unexpected Finding. Cureus 2021, 14, 13–e20413. [Google Scholar] [CrossRef]
- Oka, A.; Awoniyi, M.; Hasegawa, N.; Yoshida, Y.; Tobita, H. ; Ishimura, N., Ishihara, S. Superior mesenteric artery syndrome: Diagnosis and management. World J Clin Cases 2023, 11, 3369–3384. [Google Scholar] [CrossRef]
- Unal, B.; Aktaş, A.; Kemal, G.; Bilgili, Y.; Güliter, S.; Daphan, C.; Aydinuraz, K. Superior mesenteric artery syndrome: CT and ultrasonography findings. Diagn Interv Radiol 2005, 11, 90–5. [Google Scholar]
- Oka, A.; Awoniyi, M.; Hasegawa, N.; Yoshida, Y.; Tobita, H.; Ishimura, N.; Ishihara, S. Superior mesenteric artery syndrome: Diagnosis and management. World J Clin Cases 2023, 11, 3369–3384. [Google Scholar] [CrossRef]
- Kaur, R.; Airey, D. Nutcracker syndrome: A case report and review of the literature. Front Surg 2022, 9, 984500. [Google Scholar] [CrossRef]
- Ananthan, K.; Onida, S.; Davies, A.H. Nutcracker Syndrome: An Update on Current Diagnostic Criteria and Management Guidelines. Eur J Vasc Endovasc Surg 2017, 53, 886–894. [Google Scholar] [CrossRef]
- Kurklinsky, A.K.; Rooke, T.W. Nutcracker phenomenon and Nutcracker syndrome. Mayo Clin Proc 2010, 85, 552–9. [Google Scholar] [CrossRef]
- Ananthan, K.; Onida, S.; Davies, A.H. Nutcracker Syndrome: An Update on Current Diagnostic Criteria and Management Guidelines. Eur J Vasc Endovasc Surg 2017, 53, 886–894. [Google Scholar] [CrossRef]
- Gulleroglu, K.; Gulleroglu, B.; Baskin, E. Nutcracker syndrome. World J Nephrol 2014, 3, 277–81. [Google Scholar] [CrossRef]
- de Macedo, G.L. , Dos Santos, M.A., Sarris, A.B., Gomes, R.Z. Diagnosis and treatment of the Nutcracker syndrome: a review of the last 10 years. J Vasc Bras 2018, 17, 220–228. [Google Scholar]
- Barsoum, M.K. , Shepherd, R.F., Welch, T.J. Patient with both Wilkie syndrome and Nutcracker syndrome. Vasc Med 2008, 13, 247–50. [Google Scholar] [CrossRef]
- Vulliamy, P. , Hariharan, V., Gutmann, J., Mukherjee, D. Superior mesenteric artery syndrome and the ‘Nutcracker phenomenon’. BMJ Case Rep 2013, 2013, bcr2013008734. [Google Scholar] [CrossRef]
- Inal, M. , Unal Daphan, B., Karadeniz Bilgili, MY. Superior mesenteric artery syndrome accompanying with Nutcracker syndrome: a case report. Iran Red Crescent Med J 2014, 16, e14755. [Google Scholar] [CrossRef]
- Alenezy, A. , Obaid, AD., Qattan, A.A., Hamad, A. Superior mesenteric artery syndrome and Nutcracker phenomenon. Saudi J Med Med Sci 2014, 2, 223–5. [Google Scholar]
- Nunn, R. , Henry, J., Slesser, A.A.P., Fernando, R., Behar, N. A model example: coexisting superior mesenteric artery syndrome and the Nutcracker phenomenon. Case Rep Surg 2015, 2015, 649469. [Google Scholar]
- Iqbal, S. , Siddique, K., Saeed, U., Khan, Z., Ahmad, S. Nutcracker Phenomenon With Wilkie’s Syndrome in a Patient With Rectal Cancer. J Med Cases, 2016; 7, 282–285. [Google Scholar]
- Heidbreder, R. Co-occurring superior mesenteric artery syndrome and Nutcracker syndrome requiring Roux-en-Y duodenojejunostomy and left renal vein transposition: a case report and review of the literature. J Med Case Rep 2018, 12, 214. [Google Scholar] [CrossRef]
- Al-Zoubi, N.A. Nutcracker Syndrome Accompanying With Superior Mesenteric Artery Syndrome: A Case Report. Clin Med Insights Case Rep 2019, 12, 1179547619855383. [Google Scholar] [CrossRef]
- Shi, Y. , Shi, G., Li, Z., Chen, Y., Tang, S., Huang, W. Superior mesenteric artery syndrome coexists with Nutcracker syndrome in a female: a case report. BMC Gastroenterol 2019, 19, 15. [Google Scholar] [CrossRef]
- Diab, S.; Hayek, F. Combined Superior Mesenteric Artery Syndrome and Nutcracker Syndrome in a Young Patient: A Case Report and Review of the Literature. Am J Case Rep 2020, 21, e922619. [Google Scholar] [CrossRef]
- Lin, T.H. , Lin, C.C., Tsai, J.D. Superior mesenteric artery syndrome and Nutcracker syndrome. Pediatr Neonatol 2020, 61, 351–352. [Google Scholar] [CrossRef]
- Farina, R. , Iannace, F.A., Foti, P.V., Conti, A., Inì, C., Libra, F., Fanzone, L., Coronella, M.E., Santonocito, S., Basile, A. A Case of Nutcracker Syndrome Combined with Wilkie Syndrome with Unusual Clinical Presentation. Am J Case Rep 2020, 21, e922715. [Google Scholar] [CrossRef]
- Wang, C. , Wang, F., Zhao, B., Xu, L., Liu, B., Guo, Q., Yang, X., Wang, R. Coexisting Nutcracker phenomenon and superior mesenteric artery syndrome in a patient with IgA nephropathy: A case report. Medicine (Baltimore) 2021, 100, e26611. [Google Scholar] [CrossRef]
- Suárez-Correa, J. , Rivera-Martínez, W.A., González-Solarte, K.D., Guzmán-Valencia, C.F., Zuluaga-Zuluaga, M.V.S., Juan, C. Nutcracker Syndrome Combined with Wilkie Syndrome: Case Report. Rev. colomb. Gastroenterol 2022, 37, 306–310. [Google Scholar]
- Laskowski, T. , Tihonov, N., Richard, M., Katz, D., d’Audiffret, A., Lim, S. Concurrent Nutcracker syndrome and superior mesenteric artery syndrome requiring duodenojejunal bypass and left renal vein transposition. Ann Vasc Surg 2022, 2, 100099. [Google Scholar]
- Khan, H. , Al-Jabbari, E., Shroff, N., Barghash, M., Shestopalov, A., Bhargava, P. Coexistence of superior mesenteric artery syndrome and Nutcracker phenomenon. Radiol Case Rep 2022, 17, 1927–1930. [Google Scholar] [CrossRef]
- Ober, M.C. , Lazăr, F.L., Achim, A., Tirinescu, D.C., Leibundgut, G., Homorodean, C., Olinic, M., Onea, H.L., Spînu, M., Tătaru, D., et al. Interventional Management of a Rare Combination of Nutcracker and Wilkie Syndromes. J Pers Med 2022, 12, 1461. [Google Scholar] [CrossRef]
- Güngörer, V. , Öztürk, M., Arslan, Ş. A rare cause of recurrent abdominal pain; the coexistence of Wilkie’s syndrome and Nutcracker syndrome. Arch Argent Pediatr 2023, 121, e202102373. [Google Scholar]
- Castro, B.N. , Ferreira, A.R., Graça, S., Oliveira, M. Combined superior mesenteric artery syndrome and nutcraker syndrome presenting as acute pancreatitis: a case report. J Vasc Bras 2023, 22, e20220161. [Google Scholar] [CrossRef]
- Pacheco, T.B.S. , Chacon, A.C.M., Brite, J., Sohail, A.H., Gangwani, M.K., Malgor, R.D., Levine, J., do Amaral Gurgel, G. Nutcracker phenomenon secondary to superior mesenteric artery syndrome. J Surg Case Rep 2023, 2023, rjac622. [Google Scholar] [CrossRef]
- Alonso-Canal, L. , Santos-Rodríguez, A., Gil-Fournier-Esquerra, N., García-Centeno, P. Wilkie’s and Nutcracker’s syndromes overlapping a case of functional dyspepsia. Rev Gastroenterol Peru 2023, 43, 74–6. [Google Scholar] [CrossRef]
- Brogna, B. , La Rocca, A., Giovanetti, V., Ventola, M., Bignardi, E., Musto, L.A. An interesting presentation of a rare association of the Wilkie and Nutcracker syndromes. Radiol Case Rep 2023, 18, 2677–2680. [Google Scholar] [CrossRef]
- Forte, A.; Santarpia, L.; Venetucci, P.; Barbato, A. Aorto-mesenteric compass syndrome (Wilkie’s syndrome) in the differential diagnosis of chronic abdominal pain. BMJ Case Rep 2023, 16, e254157. [Google Scholar] [CrossRef]
- Kim, S.H. Doppler US and CT Diagnosis of Nutcracker Syndrome. Korean J Radiol 2019, 20, 1627–1637. [Google Scholar] [CrossRef]
- Makam, R.; Chamany, T.; Potluri, V.K.; Varadaraju, P.J.; Murthy, R. Laparoscopic management of superior mesentric artery syndrome: A case report and review of literature. J Minim Access Surg 2008, 4, 80–2. [Google Scholar] [CrossRef]
- Fromm, S.; Cash, J.M. Superior mesenteric artery syndrome: an approach to the diagnosis and management of upper gastrointestinal obstruction of unclear etiology. South Dakota Journal of Medicine 1990, 43, 5–10. [Google Scholar]
- Muheilan, M.; Walsh, A.; O’Brien, F.; Tuite, D. Nutcracker syndrome, conservative approach: a case report. J Surg Case Rep 2022, 2022, rjac423. [Google Scholar]
- Velasquez, C.A.; Saeyeldin, A.; Zafar, M.A.; Brownstein, A.J.; Erben, Y. A systematic review on management of Nutcracker syndrome. J Vasc Surg Venous Lymphat Disord 2018, 6, 271–278. [Google Scholar] [CrossRef]

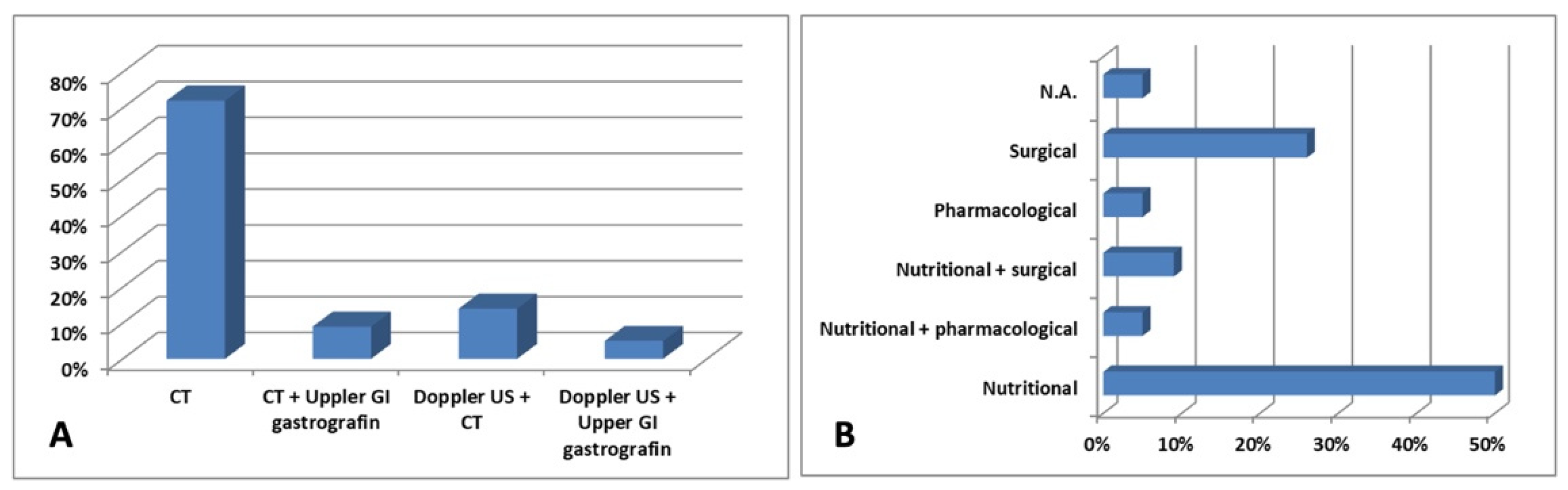
| Reference | Patient | Clinical manifestations | Diagnosis | Treatment |
|---|---|---|---|---|
| Barsoum et al., 2008 [11] | 29-year-old female | Early satiety and post-prandial epigastric abdominal pain |
CT Upper GI gastrografin |
Enteral nutrition |
| Vulliamy et al., 2013 [12] | 55-year-old male | Vomiting, epigastric pain and bloating | CT | N.A. |
| Inal et al., 2014 [13] | 28-year-old male | Cachexia and intermittent abdominal pain | CT | Enteral nutrition |
| Alenezy et al., 2014 [14] | 17-year-old male | Abdominal pain and intermittent vomiting | CT | Fluid and electrolytes replacement and nasogastric tube decompression |
| Nunn et al., 2015 [15] | 19-year-old female | Severe epigastric pain associated with emesis and anorexia | CT | Enteral nutrition |
| Iqbal et al., 2016 [16] | 62-year-old male | Cachexia | CT | Enteral nutrition |
| Heidbreder; 2018 [17] | 20-year-old female | Severe left flank and lower left quadrant pain, abdominal pain, nausea, and vomiting | CT | Roux-en-Y duodenojejunostomy and LRV transposition |
| Al-Zoubi; 2019 [18] | 38-year-old female | Intermittent left-sided loin pain | CT | LRV transposition |
| Shi et al., 2019 [19] | 32-year-old female | Severe bloating, epigastric pain, left flank ache, nausea and occasional vomiting | CT | Fluid resuscitation with parenteral and enteral nutritional support, plus mosapride citrate dispersible tablets 5 mg thrice a day |
| Diab et al., 2020 [20] | 18-year-old male | Crampy post-prandial abdominal pain associated with bilious vomiting, and signs of varicocele | CT | Regular assumption of a liquid diet |
| Lin et al., 2020 [21] | 15-year-old male | Postprandial discomfort, nausea, and vomiting | CT | Enteral and parenteral nutrition |
| Farina et al., 2020 [22] | 27-year-old male | Painful post-prandial crises at the sub-acute onset, located at the epigastrium | Doppler US CT |
Endovascular stent grafting |
| Wang et al., 2021[23] | 15-year-old male | Hematuria, fatigue, anorexia, nausea, and recurrent abdominal distension | Doppler US Upper GI gastrografin |
Pulse dose of methylprednisolone 500 mg daily for 3 days, followed by 1 mg/kg orally and mycophenolate mofetil 0.75 g twice a day |
| Suarez-Correa et al., 2022 [24] | 25-year-old male | Postprandial abdominal pain and distension, nausea, vomiting, and distension | CT Upper GI gastrografin |
Enteral nutrition and surgery |
| Laskowski et al., 2022 [25] | 40-year-old female | Nausea, early satiety and diffuse abdominal pain | CT | LRV transposition |
| Khan et al., 2022 [26] | 25-year-old female | Abdominal pain associated with nausea, bilious emesis, and diarrhea | CT | Surgery and conservative therapy |
| Ober et al., 2022 [27] | 45-year-old female | Macroscopic hematuria, intermittent pain in the left flank and hypogastric region, postprandial nausea, and cachexia | Doppler US CT |
Stent implantation in the LRV |
| Gungorer et al., 2022 [28] | 17-year-old male | Abdominal pain, nausea, and vomiting | Doppler US CT |
Surgery |
| Castro et al., 2023 [29] | 18-year-old female | Epigastric pain and emesis | CT | Dietary changes |
| Pacheco et al., 2023 [30] | 26-year-old male | GI obstructive symptoms | CT | Enteral nutrition |
| Alonso-Canal et al., 2023 [31] | 24-year-old male | Functional dyspepsia | CT | Dietary changes |
| Brogna et al., 2023 [32] | 37-year-old female | Abdominal pain with sub-occlusive episodes, nausea, and vomiting | CT | Periodic insertion of a nasogastric tube to decompress the stomach, along with a high-protein diet and parenteral nutritional supplements |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





