1. Introduction
The strength and range of motion of the great toe/ first metatarsophalangeal joint (1st-MTJ) are critical for normal walking and balance.[
1,
2,
3,
4] The great toe flexors and extensors play a significant role in sensation/proprioception, generating propulsive forces, weight-bearing, foot clearance, and supporting the foot arch during different phases of gait and other functional activities.[
5,
6,
7,
8,
9] Reduced muscle strength, limited range of motion, and/or loss of sensation in the great toe can affect the individual’s balance, ability to walk, perform activities of daily living (ADLs), and reduce participation in community activities, resulting in a reduction in the individual’s quality of life (QoL).[
10,
11,
12,
13,
14,
15,
16,
17] Furthermore, various pathological conditions (neurological and non-neurological) such as peripheral neuropathy, radiculopathy, Charcot-Marie-Tooth disease, and hallux/toe deformities have also been associated with the atrophy and/or reduction of the strength of great toe muscles.[
18,
19,
20,
21,
22,
23,
24] Multiple studies have also reported an association of great toe strength (GTS) with age and sex, making it a potential clinical biomarker that could be used to detect or evaluate the onset and progression of different health conditions.[
7,
25,
26,
27,
28,
29]
Despite its critical biomechanical and functional roles, GTS, particularly great toe extension strength (GTES), is often overlooked during routine clinical practice due to the lack of a reliable and robust tool for GTES measurement and the subjective nature of existing methods.[
30,
31,
32,
33] To address the clinical need and limitations of existing GTS measurement methods and devices, we recently developed the ToeScale, a novel, portable device.[
34,
35] The preliminary studies using the ToeScale and comparing it with the results from manual muscle test (MMT) indicate an association between the values obtained by both methods and the ToeScale showing stronger discriminative ability than MMT.[
35] In addition, the ToeScale output has a force development curve over time, not just peak force that can be measured using a hand-held dynamometer but also information on how the force was developed at different times. However, the characterization of this Toescale output curve for clinical use is yet to be further examined.
Thus, the primary aim of this study is to characterize the output force development curve of the ToeScale by manually hand-picking features from the force development curve based on its relevance to the muscle’s physiological performance and assess how the different GTES parameters related to demographic variables such as age and sex and grip strength. The secondary objective is to explore the feasibility of employing machine learning (ML) methods to characterize ToeScale output curves by age and sex based on the time series data of the force development curve.
2. Materials and Methods
2.1. Study Design, Inclusion Criteria, and Measurement Protocol
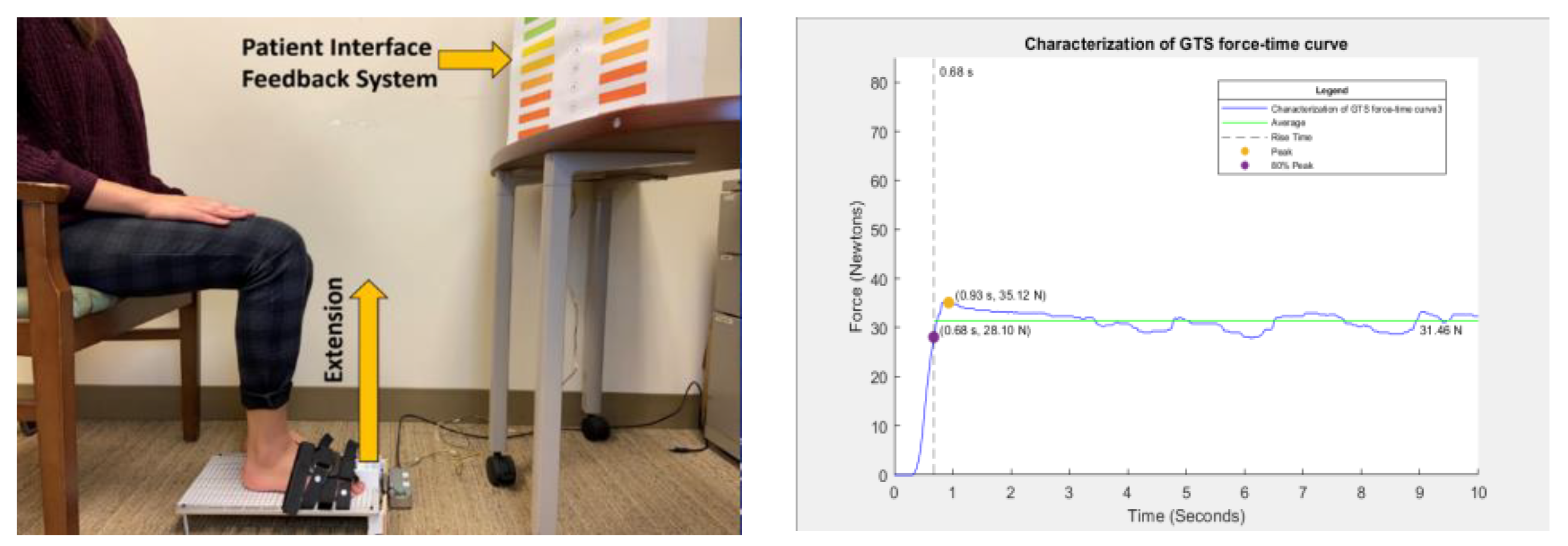
In this cross-sectional study, data was collected from a convenience sample of younger (aged between 18-24 years) and older adults (> 60 years of age). All participants completed a demographic and physical activity questionnaire and a grip strength assessment using a Jamar handgrip dynamometer. Finally, the great toe extension strength (GTES) was assessed using ToeScale, a novel, portable device. GTES was measured with the participants seated with their knee and ankle at 90° as shown in
Figure 1 below. In this seated position, the participants were instructed to raise their great toe, i.e., extend it against the toe cap of ToeScale as hard as possible and try to aim for higher force for 10 seconds continuously. We selected a 10-second duration for the trials based on our previous work.[
35] The measurement of GTES using ToeScale is shown in
Figure 1.
2.2. Great Toe Extension Strength Characterization and Classification
The device recorded the force in kilograms with a sampling frequency of 50 Hz, resulting in a force-time curve with 500 data points for each participant. For the first objective, the force-time curve was characterized using MATLAB. The different parameters/features of the GTES curve extracted included peak force, time to 80% of the peak, i.e., rise time, average force after reaching the 80% of the peak force, percentage of data points in each trial equal to or above the average value, and the RFD (80% peak force divided by the rise time). The different GTES parameters extracted from the GTES curve are shown in
Figure 1 below. These parameters are then compared across age groups/sex. This study chooses 80% of peak force as the cut-off to calculate rise time. The literature reports that all muscle/muscle group motor units are recruited at 80% of maximum voluntary contraction, emphasizing its significance.[
36] Using 80% of peak force as the cut-off would also help standardize the rise time calculation. The other clinically meaningful measure of GTES is RFD, which has been associated with postural stability and could be a potential biomarker for acute muscle damage and exercise-induced fatigue.[
37] RFD in this study is defined as the rate of rise in toe strength per unit time (Ns-1) to reach 80% of the peak toe strength.
For the second objective, Python[
38] programming language was used to apply machine learning (ML) algorithms to classify the GTES force-time curves based on age and sex. The units of all force data, i.e., GS and GTES force-time curves were converted to newtons before analyses. As this is a preliminary analysis, only supervised ML models were applied to the GTES force-time curves using age and sex as the two target variables for classification. We applied the k-nearest neighbors (k-NN), support vector machine (SVM), and random forest (RF) as those methods were frequently used models for time-series data. Before running any of the ML models, the GTES data with missing data were imputed based on the time-point within the 10-second trial where the data was missing. Once the data was cleaned and imputed, the ML models were applied directly to the raw dataset (500 data points), without using the manually extracted features in the traditional analyses to assess the feasibility of using ML as a method to classify the given GTES force-time curves based on the demographic variables of age and sex.
2.3. Data Analysis Plan
The descriptives were calculated for all variables, including the different parameters of the GTES force-time curve. ANOVAs, independent sample t-tests, and correlations were conducted to assess and compare the relationship of the different GTES parameters with GS and demographics such as age, sex, and body mass index (BMI). In the machine learning approach, different supervised ML models were applied to the GTES force-time curves, and the models‘ accuracy and area under the receiver operating characteristic curve (AUC) were calculated and compared.
3. Results
3.1. Demographics
Thirty-one participants completed the study, and their demographics are presented below in
Table 1.
3.2. Traditional Analyses
Table 2 below summarizes the different GTES parameters and the GS. The peak GTES, average GTES, and rate of force development (RFD) were lower, and the rise time to 80% of the peak was longer among older adults than younger adults. While there was no statistical significance in the difference in any GTES parameters except rise time between older and younger adults, there was a clinically meaningful difference of over 8N in the peak GTES and over 10N/s in the RFD. The independent samples t-test showed that all the differences in GTES parameters between males and females were statistically significant, with a difference of > 13N in peak GTES, > 14N/s in RFD, and >1.5s rise time. Males had a higher peak GTES and RFD and a quicker rise time than females. A two-way ANOVA of the GTES parameters showed a lack of significance in the interaction effect of age and sex. However,
Table 2 shows that older females have the least peak GTES and RFD and the longest rise time, which warrants further investigation.
Correlation analyses in
Table 3 below show that peak GTES correlates more strongly with BMI than GS, especially among older adults (r=0.594). A significant (p<0.05) moderate positive correlation for the total sample(r=0.545), older adults (r=0.519), and younger adults (r=0.546) was observed in the correlation between peak GTES and GS.
3.3. Machine Learning Analyses
The first step in applying ML to characterize the GTES curve was to check whether machine learning classifiers can support the evidence from the traditional analyses by accurately differentiating between age and sex. The accuracy of all three classifiers was the same (66.67%) when age was the target variable. With sex as the target variable, the SVM classifier had the highest accuracy of 66.67 %, while the k-NN (5 nearest neighbors) and RF classifiers had an accuracy of 55.56% each. By further varying the hyperparameters such as kernel type, number of nearest neighbors, etc., the k-NN (10 nearest neighbors) classifier had the highest accuracy of 77.78% when sex was the target variable. The accuracy scores and the AUC values for the different models are presented in
Table 4 below.
4. Discussion
In this study, we characterized the ToeScale output curve by applying traditional methods based on key features for muscle performance and explored the feasibility of applying ML methods to classify the time series data. To our knowledge, this is the first study to quantify great toe strength beyond just peak force. The key parameters extracted from the GTES force development curve included the peak GTES, average GTES, rise time, and RFD. The results showed statistically significant differences in all GTES parameters between males and females, and the statistically significant differences observed while comparing the GTES parameters by age were in the rise time and RFD. The differences in the peak and average GTES between older and younger adults observed in this study were 7N and 8N, respectively, and this is supported by many studies reporting similar trends and differences in great toe strength (GTS) among healthy older and younger adults.[
28,
31] Existing studies[
39,
40] also support the sex-based differences (~ 7 - 8N), and in this study, males had a higher peak and average GTES than females by 16N and 10N, respectively. Another important component of muscle strength that is often overlooked is RFD. Due to its clinical and physiological significance, there has been an increase in the number of studies reporting RFD and its association with muscle function and performance.[
37,
41,
42,
43,
44] Among the studies reporting RFD, very few specifically reported RFD of the great toe muscles. Kamasaki et. al 2024 and Sarikaya et al 2022 [
45,
46] reported a strong association of RFD in the great toe with better functional mobility and balance outcomes. Additionally, Kamasaki et. al 2024 [
45] reported a higher RFD among younger adults compared to older adults, which is consistent with the findings of this study, where the RFD was ~15N/s higher among younger adults. Although Kamaski et al. did not report any sex-based differences, our study showed that males had 21N/s higher RFD than females.[
46] Finally, our study revealed that older adults had a longer rise time than younger adults. While none of the existing studies reported results on rise time, the prolonged rising time in older adults is expected as muscle responses have been reported to slow down due to aging.[
47] This study shows a decline in all GTES parameters with age (
Table 1), consistent with studies reporting the effects of age on muscle strength.[
28,
31,
45]
The correlation analyses summarized in
Table 2 revealed moderate levels of association between the different GTES parameters and BMI, which varied by age, and a low to moderate correlation between GS and BMI across all age groups. Among all the GTES parameters, peak GTES had the highest correlation with BMI, particularly among older adults (r = 0.594, p=0.012). The rise time nor RFD were statistically significantly correlated with BMI. The analyses between the different GTES parameters with GS show that all GTES parameters were statistically significantly correlated with GS with moderate levels of association (
Table 2). However, when evaluated separately, while the peak GTES was statistically significantly correlated with GS in both younger (r=0.546, p=0.043) and older adults (r=519, p=0.033), the average GTES (r=0.516, p=0.034) and RFD (r=0.473, p=0.055) were more strongly correlated with GS among older adults when compared to younger adults. While BMI and GS are well-established measures commonly used as biomarkers of physical health and mobility and overall muscle strength status.[
48,
49,
50] They are known for their low sensitivity to changes in mobility when compared to measures of lower extremity functions or muscle strength.[
47] Studies have reported foot or great toe strength measures as better predictors of physical health and mobility as they are directly involved in walking.[
51,
52] The moderate correlations between GTES parameters, especially the peak forces between BMI and GS, highlighted the potential of GTES as a clinical marker for health and functional assessment. The differences between older and younger people's correlations between GTES vs. BMI and GTES vs. GS suggested that changes in BMI and GS are different from GTES with aging. In future studies, we should include mobility measures to confirm the better predictability of GTES and include more age ranges to examine how GTES parameters change with age.
Different ML approaches have been used for time series data classification. We applied the k-NN, SVM, and RF ML models as they are simple and widely applied classifiers reported to be accurate and reliable for time series classification.[
53] The three different ML models used to classify the GTES force development curves had moderate accuracies ranging between 55% - 78%, with all models having an accuracy of 66.67% when age was used as the target variable. When sex was used as the target variable, while the SVM had an accuracy of 66.67%, the RF and k-NN (k=5) models had the lowest accuracy of 55.56%. The model with the highest accuracy was the k-NN classifier with k = 10 with sex as the target variable, which had an accuracy of 77.78%. However, the AUC of this model was 0.36, making this model less reliable for sex-based classification despite the high accuracy. While these models aligned with the results of the traditional analyses, the low AUC scores, particularly for sex-based classification, indicated less effectiveness of the machine learning models in distinguishing between the classes (male/female). The low-moderate AUC values could be attributed to the relatively small sample size and imbalance between males and females.[
54] All ML classifiers using age as the target variable had higher AUC scores (0.5 – 0.75) when compared to ML classifiers using sex as the target variable (0.1 – 0.5), which further confirmed the impact of the imbalance of the sex distribution. Despite the low-moderate AUC scores, the results are promising, and the logical next step in the ML analyses would involve using the manually extracted GTES parameters as “features” to classify by age or sex and compare the accuracy and AUC scores with the results reported in this study.
The current study has several limitations. Firstly, this study has a small sample size and an uneven distribution of males and females included in the study. In addition, we collected only a single trial of GTES and GS measurements, resulting in a smaller dataset for the machining learning methods. Secondly, while the results of this study are comparable to the trends in great toe strength reported in existing studies, the extension strength results were difficult to compare with literature as most existing studies looking at age and sex-based differences primarily report on the great toe flexion strength.
5. Conclusion
The results of this pilot study are promising as the differences in the GTES parameters are consistent with the current evidence on the age and sex-based differences of great toe strength and provide more information than just the peak strength and rate of force development. The peak GTES is more strongly correlated with BMI than GS with BMI. Thus, these findings indicate that different GTES parameters could potentially provide insights, other than GS or BMI, into different physical health and mobility-related outcomes and how they are affected by aging and other health conditions. Additionally, the ML classifiers with moderate accuracies and low to moderate AUC scores are consistent with the results of the traditional analyses. However, future studies with a larger sample size, more methodological rigor, and measurement of both flexion and extension strength of the great toe with balance and functional mobility measures to better understand the impact of GTS on mobility and balance are warranted.
Author Contributions
Conceptualization, H.W; methodology, H.W.; software, R.C., L.P.; validation, H.W., R.C.; formal analysis, R.C., L.P.; investigation, H.W., R.C., L.P.; resources, H.W.; data curation, R.C., L.P.; writing—original draft preparation, R.C.; writing—review and editing, H.W., R.C., L.P.; visualization, L.P., R.C.; supervision, H.W.; project administration, H.W.; funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding and it was all funded by the Technology for Occupational Performance (TOP) Lab at the University of Florida
Institutional Review Board Statement
Ethical review and approval were waived for this study as the data was collected during device demonstration at different workshops conducted within the community
Informed Consent Statement
Patient consent was waived because this study did not involve collecting any of the names or even the exact ages of the participants. None of the identifiable information was obtained for this study.
Data Availability Statement
The data presented in this study may be requested from the authors. For deidentified human participant data, contact the corresponding author (H.W.). Institutional approvals and data use agreements may be required. The deidentified data are not yet publicly available because the study is ongoing.
Acknowledgments
We would like to thank the residents and staff at Oak Hammock at the University of Florida for their time and allowing us to conduct our workshop. We would also like to thank the undergraduate research assistants from the Active Learning Program (ALP) course offered at the University of Florida. We would also like to thank the Department of Occupational Therapy for the resources and support.
Conflicts of Interest
H.W., is an inventor on The University of Oklahoma Health Sciences Center patent on ToeScale used in this work, and R.C. will receive royalties from any future commercialization of the device. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Mann, R.A.; Hagy, J.L. The Function of the Toes in Walking, Jogging and Running. Clin. Orthop. Relat. Res. 1979, 24–29. [Google Scholar] [CrossRef]
- Fujita, M. Role of the metatarsophalangeal (MTP) joints of the foot in level walking. Nihon Seikeigeka Gakkai Zasshi 1985, 59, 985–97. [Google Scholar] [PubMed]
- Miyazaki, M. Role and Movement of the Toes During Walking. Nihon Seikeigeka Gakkai Zasshi 1993, 67, 606–616. [Google Scholar] [PubMed]
- Fanous, J.; Rice, C. How Important is the Big Toe?: Functional Anatomy of Hallux Flexion. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Nawoczenski, D.A.; Baumhauer, J.F.; Umberger, B.R. Relationship Between Clinical Measurements and Motion of the First Metatarsophalangeal Joint During Gait*. J. Bone Jt. Surg. 1999, 81, 370–6. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Peterson, C.; Kautz, S.; Neptune, R. Relationships between muscle contributions to walking subtasks and functional walking status in persons with post-stroke hemiparesis. Clin. Biomech. 2011, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, J.; Brüggemann, G. The potential of human toe flexor muscles to produce force. J. Anat. 2012, 221, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, J.-P.; Sanno, M.; Willwacher, S.; Heinrich, K.; Brüggemann, G.-P. The potential of toe flexor muscles to enhance performance. J. Sports Sci. 2013, 31, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, J.; Koyama, K. Force-generating capacity of the toe flexor muscles and dynamic function of the foot arch in upright standing. J. Anat. 2019, 234, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Mickle, K.J.; Munro, B.J.; Lord, S.R.; Menz, H.B.; Steele, J.R. ISB Clinical Biomechanics Award 2009. Clin. Biomech. 2009, 24, 787–791. [Google Scholar] [CrossRef]
- Nix, S.E.; Vicenzino, B.T.; Collins, N.J.; Smith, M.D. Gait parameters associated with hallux valgus: A systematic review. J. Foot Ankle Res. 2013, 6, 9. [Google Scholar] [CrossRef]
- Kamonseki, D.H.; Gonçalves, G.A.; Yi, L.C.; Júnior, I.L. Effect of stretching with and without muscle strengthening exercises for the foot and hip in patients with plantar fasciitis: A randomized controlled single-blind clinical trial. Man. Ther. 2016, 23, 76–82. [Google Scholar] [CrossRef]
- Yokozuka, M.; Okazaki, K.; Sakamoto, Y.; Takahashi, K. Correlation between functional ability, toe flexor strength, and plantar pressure of hallux valgus in young female adults: a cross-sectional study. J. Foot Ankle Res. 2020, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, S.; Fong Yan, A.; Sinclair, P.; Hunt, A. The evidence for improving balance by strengthening the toe flexor muscles: A systematic review. Gait Posture 2020, 81, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Futrell, E.E.; Roberts, D.; Toole, E. The effects of intrinsic foot muscle strengthening on functional mobility in older adults: A systematic review. J. Am. Geriatr. Soc. 2022, 70, 531–540. [Google Scholar] [CrossRef]
- Jaffri, A.H.; Koldenhoven, R.; Saliba, S.; Hertel, J. Evidence for Intrinsic Foot Muscle Training in Improving Foot Function: A Systematic Review and Meta-Analysis. J. Athl. Train. 2022, 58, 941–951. [Google Scholar] [CrossRef]
- de Souza, T.M.M.; Coutinho, V.G.d.O.; Tessutti, V.D.; de Oliveira, N.R.C.; Yi, L.C. Effects of intrinsic foot muscle strengthening on the medial longitudinal arch mobility and function: A systematic review. J. Bodyw. Mov. Ther. 2023, 36, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Myerson, M.S.; Shereff, M.J. The pathological anatomy of claw and hammer toes. J. Bone Jt. Surg. 1989, 71, 45–49. [Google Scholar] [CrossRef]
- Bus, S.A.; Yang, Q.X.; Wang, J.H.; Smith, M.B.; Wunderlich, R.; Cavanagh, P.R. Intrinsic Muscle Atrophy and Toe Deformity in the Diabetic Neuropathic Foot: A magnetic resonance imaging study. Diabetes Care 2002, 25, 1444–1450. [Google Scholar] [CrossRef]
- Chung, K.; Suh, B.; Shy, M.; Cho, S.; Yoo, J.; Park, S.; Moon, H.; Park, K.; Choi, K.; Shim, D.; et al. Different clinical and magnetic resonance imaging features between Charcot–Marie–Tooth disease type 1A and 2A. Neuromuscul. Disord. 2008, 18, 610–618. [Google Scholar] [CrossRef]
- Chang, R.; Kent-Braun, J.A.; Hamill, J. Use of MRI for volume estimation of tibialis posterior and plantar intrinsic foot muscles in healthy and chronic plantar fasciitis limbs. Clin. Biomech. 2012, 27, 500–505. [Google Scholar] [CrossRef]
- Soysa, A.; Hiller, C.; Refshauge, K.; Burns, J. Importance and challenges of measuring intrinsic foot muscle strength. J. Foot Ankle Res. 2012, 5, 1–14. [Google Scholar] [CrossRef]
- Stewart, S.; Ellis, R.; Heath, M.; Rome, K. Ultrasonic evaluation of the abductor hallucis muscle in hallux valgus: a cross-sectional observational study. BMC Musculoskelet. Disord. 2013, 14, 45–45. [Google Scholar] [CrossRef] [PubMed]
- Jastifer, J.R. Intrinsic muscles of the foot: Anatomy, function, rehabilitation. Phys. Ther. Sport 2023, 61, 27–36. [Google Scholar] [CrossRef]
- Bryant, A.; Tinley, P.; Singer, K. Plantar pressure distribution in normal, hallux valgus and hallux limitus feet. Foot 1999, 9, 115–119. [Google Scholar] [CrossRef]
- Hara, Y.; Hara, N.; Matsudaira, K.; Oka, H. A comparison of muscle strength testing for great toe extension. J. Orthop. Sci. 2011, 16, 765–767. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L. The differential effects of foot sole sensory on plantar pressure distribution between balance and gait. Gait Posture 2013, 37, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Mickle, K.J.; Angin, S.; Crofts, G.; Nester, C.J. Effects of Age on Strength and Morphology of Toe Flexor Muscles. J. Orthop. Sports Phys. Ther. 2016, 46, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-Y.; Tsai, Y.-J.; Liao, Y.-T.; Yang, Y.-C.; Lu, F.-H.; Lin, S.-I. Reactive balance control in older adults with diabetes. Gait Posture 2018, 61, 67–72. [Google Scholar] [CrossRef]
- De Win, M.M.L.; Theuvenet, W.J.; Roche, P.W.; A De Bie, R.; Van Mameren, H. The paper grip test for screening on intrinsic muscle paralysis in the foot of leprosy patients. . 2002, 70, 16–24. [Google Scholar]
- Spink, M.; Fotoohabadi, M.R.; Menz, H. Foot and Ankle Strength Assessment Using Hand-Held Dynamometry: Reliability and Age-Related Differences. Gerontology 2010, 56, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Ciesla, N.; Dinglas, V.; Fan, E.; Kho, M.; Kuramoto, J.; Needham, D. Manual Muscle Testing: A Method of Measuring Extremity Muscle Strength Applied to Critically Ill Patients. J. Vis. Exp. 2011, e2632–e2632. [Google Scholar] [CrossRef] [PubMed]
- Bruening, D.A.; Ridge, S.T.; Jacobs, J.L.; Olsen, M.T.; Griffin, D.W.; Ferguson, D.H.; Bassett, K.E.; Johnson, A.W. Functional assessments of foot strength: a comparative and repeatability study. BMC Musculoskelet. Disord. 2019, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang H, Hile E, Ghazi M. Apparatus and method for measuring toe flexion and extension. US1140 2284B2, 2022.
- Hile, E.S.; Ghazi, M.; Chandrashekhar, R.; Rippetoe, J.; Fox, A.; Wang, H. Development and Earliest Validation of a Portable Device for Quantification of Hallux Extension Strength (QuHalEx). Sensors 2023, 23, 4654. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.X. Motor-Unit Recruitment Plays an Important Role in Determining the Relationship Between Muscle Force and Force Variability. Biomed. J. Sci. Tech. Res. 2018, 8. [Google Scholar] [CrossRef]
- Rodríguez-Rosell, D.; Pareja-Blanco, F.; Aagaard, P.; González-Badillo, J.J. Physiological and methodological aspects of rate of force development assessment in human skeletal muscle. Clin. Physiol. Funct. Imaging 2018, 38, 743–762. [Google Scholar] [CrossRef]
- Python 3.12.3 n.d.
- Abe, T.; Tayashiki, K.; Nakatani, M.; Watanabe, H. Relationships of ultrasound measures of intrinsic foot muscle cross-sectional area and muscle volume with maximum toe flexor muscle strength and physical performance in young adults. J. Phys. Ther. Sci. 2016, 28, 14–19. [Google Scholar] [CrossRef]
- Nagano, K.; Okuyama, R.; Taniguchi, N.; Yoshida, T. Gender difference in factors affecting the medial longitudinal arch height of the foot in healthy young adults. J. Phys. Ther. Sci. 2018, 30, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Kawamori N, Rossi SJ, Justice BD, Haff EE, Pistilli EE, O’bryant HS, et al. Peak force and rate of force development during isometric and dynamic mid-thigh clean pulls performed at various intensities. The Journal of Strength & Conditioning Research 2006;20:483.
- Andersen, L.L.; Aagaard, P. Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur. J. Appl. Physiol. 2006, 96, 46–52. [Google Scholar] [CrossRef]
- Peñailillo, L.; Blazevich, A.; Numazawa, H.; Nosaka, K. Rate of force development as a measure of muscle damage. Scand. J. Med. Sci. Sports 2015, 25, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Farup, J.; Rahbek, S.K.; Bjerre, J.; de Paoli, F.; Vissing, K. Associated decrements in rate of force development and neural drive after maximal eccentric exercise. Scand. J. Med. Sci. Sports 2015, 26, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Kamasaki, T.; Otao, H.; Tanaka, S.; Hachiya, M.; Kubo, A.; Okawa, H.; Sakamoto, A.; Fujiwara, K.; Suenaga, T.; Kichize, Y.; et al. Age-specific comparisons in the rate of force development of toe pressure strength and its association with the timed up and go test. Eur. Geriatr. Med. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, F.; Sahin, M. The Effect of Big Toe Strength Development on Some Athletic Performance Parameter in Young Male Footballers. Pakistan Journal of Medical & Health Sciences 2022, 16, 997–1001. [Google Scholar] [CrossRef]
- Arnold, P.; Vantieghem, S.; Gorus, E.; Lauwers, E.; Fierens, Y.; Pool-Goudzwaard, A.; Bautmans, I. Age-related differences in muscle recruitment and reaction-time performance. Exp. Gerontol. 2015, 70, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Manual muscle testing: does it meet the standards of an adequate screening test? Clin. Rehabilitation 2005, 19, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, J.; Stenholm, S.; Rantanen, T.; Heliövaara, M.; Sainio, P.; Koskinen, S. Hand-Grip Strength Cut Points to Screen Older Persons at Risk for Mobility Limitation. J. Am. Geriatr. Soc. 2010, 58, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- A A A Siqueira, V.; Sebastião, E.; Camic, C.L.; Machado, D.R.L. Higher Body Mass Index Values Do Not Impact Physical Function and Lower-Extremity Muscle Strength Performance in Active Older Individuals. . 2022, 15, 330–340. [Google Scholar]
- Kim, M.-J.; Seino, S.; Kim, M.-K.; Yabushita, N.; Okura, T.; Okuno, J.; Tanaka, K. Validation of lower extremity performance tests for determining the mobility limitation levels in community-dwelling older women. Aging Clin. Exp. Res. 2009, 21, 437–444. [Google Scholar] [CrossRef]
- Kusagawa, Y.; Kurihara, T.; Imai, A.; Maeo, S.; Sugiyama, T.; Kanehisa, H.; Isaka, T. Toe flexor strength is associated with mobility in older adults with pronated and supinated feet but not with neutral feet. J. Foot Ankle Res. 2020, 13, 1–8. [Google Scholar] [CrossRef]
- Bagnall, A.; Lines, J.; Bostrom, A.; Large, J.; Keogh, E. The great time series classification bake off: a review and experimental evaluation of recent algorithmic advances. Data Min. Knowl. Discov. 2017, 31, 606–660. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ling, C. Using AUC and accuracy in evaluating learning algorithms. IEEE Trans. Knowl. Data Eng. 2005, 17, 299–310. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).