1. Introduction
The first osseointegrated dental implants were probably made with commercially pure titanium (Ti cp) and used to manufacture parts for aircraft bodies, and they did not have surface treatment. The Ti cp used today in medical devices has a lower percentage of impurities than the old implant. The Harvard Consensus Development Conference on Dental Implants [
1], held in 1979, mentioned that the implants should have a success rate of at least 75% during the first 5 years. Even with the need for knowledge of the mechanisms involved in osseointegration and the interactions of proteins with the surface of the implants, this low success rate was reached. With the increase in scientific knowledge about osseointegration, modifications in surgical instruments and techniques, changes in the shape and dimensions of implants, and better dental implant surface treatments, the success rate of dental implants increased [
2] to 95-98%. The dental implant success rate is higher than hip and knee orthopedic prostheses (75%).
Nowadays, the cp Ti for biomedical applications is specified by the ASTM F67, ISO 5832, and BN 1642 technical standards. The titanium for biomaterials applications has a lower percentage of impurities (Fe, C, N, O) than the cp Ti used in other areas. Pure Ti is classified into grades 1 to 4 (Ti G1 to G4). The most used dental implants are grades 2 and 4, which have adequate biocompatibility, mechanical strength, and corrosion resistance. Ti Grade 4 has higher percentages of alloying elements (C, O, and Fe) and mechanical properties than Ti G1-G4. The addition of alloy elements increases the Ti mechanical strength. Dental implants with small diameters and orthopedic prostheses are made with Ti-6Al-4V alloy according to the ASTM F136 Standard (Ti grade 5) specification [
3]. The disadvantage of Ti G5 is the possibility of delivering harmful ions (Al and V). In each country, the medical Ti manufacturing is controlled by its Health Agency.
The dental implant’s surface treatment changes the roughness, increases the surface area, stimulates interactions with proteins, promotes cell adhesion, and reduces osseointegration time [
4]. After surface treatments, it is possible to stimulate cell proliferation, reduce the time for the formation of bone matrix around the implants, reduce the reopening time for prosthesis installation, and increase the predictability of the treatment [
4].
In clinical practice, the most straightforward procedure to quantify the primary stability of implants is to measure the insertion torque. The disadvantage of torque is the impossibility of following the increase in secondary stability (osseointegration) during tissue healing. The torque measurement can cause damage to the implant-bone interface. The recommended methodology to monitor the rise in secondary stability is to use the resonance frequency to measure the ISQ (implant stability quotient) like the Ostell Mentor
® device. The Ostell Mentor
® device calculates the ISQ and measures primary stability (immediately after insertion) and secondary (after osseointegration) [
5]. The ultrasonic vibration produced by the Ostell Mentor
® device varies with the percentage of the surface of the threads in contact with the bone. It discriminates the fixation of the implant in one or two bone walls. Some studies have shown discrepancies between the ISQ value and implant stability measurements based on insertion torque and bone density.
The objective of the present work was to compare the titanium dental implants’ primary and secondary stability 45 and 60 days after insertion surgery in humans.
2. Materials and Methods
Twenty-four dental implants were inserted in 24 patients treated at the Specialization Clinic in Implant Dentistry at the State University of Rio de Janeiro (UERJ, Brazil).
Patients were divided into two groups with 12 patients. The first group returned for prosthetic rehabilitation 45 days after implant placement, and the 12 patients from the second group returned 60 days after surgery. The patients were informed about the surgical procedures. All volunteers gave written consent for the scientific use of the data. The study was conducted following the Declaration of Helsinki and approved by the Institutional Review Board (Ethics Committee) of the UERJ implant dentistry specialization (protocol 01-2022 of 05/12/2022).
The inclusion and exclusion criteria and characteristics of the study patients are described in
Table 1. Men were 33.3% (N = 8), women were 66.6% (N = 16), white color was 50%, and brown or black color was 50%.
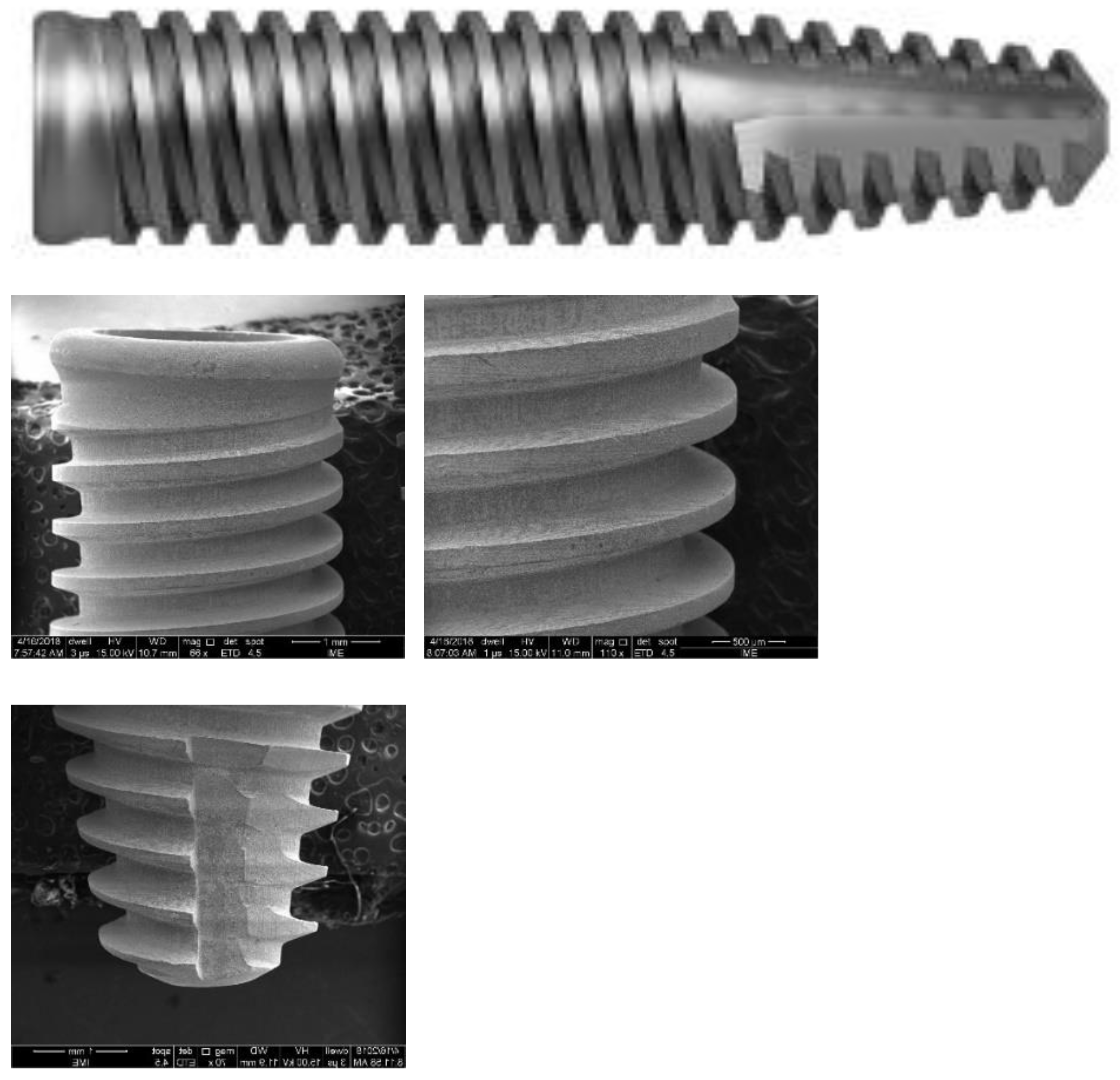
Systhex Company supplied the analyzed cp Ti G4 dental implants (Curitiba, PR, Brazil). The implant Model Avantt was used with a diameter of 3.5 mm and a length of 8.5 mm.
Figure 1 shows the Avantt implant design. It has an internal hexagonal connection and a Morse taper. The coronal region is enlarged to provide better mechanical locking. The implant has a conical apex with three V-shaped threads. The apex is dome-shaped, without a hole, and has grooves that allow for better cutting and anchoring of the implant in the bone. The surface was acid-etched.
Four students from the same specialization class in implantology performed the implant insertion surgeries. The surgeons followed the placement instructions and protocol suggested by the manufacturer. Before the surgery, the surgical team was trained with the Systhex system. Implants that did not anchor with a torque less than 35 N.cm or greater than 60 N.cm were not considered adequate for this research. After the surgeries, the cover screws were placed on the implants, and the implants were kept submerged for 45 and 60 days before receiving the prosthesis. The primary stability was measured at implant installation using a torquemeter and with the Ostell Mentor® device. The secondary stability was measured 45 and 60 days after surgery using the Ostell Mentor® device.
Osseointegration was assessed using periapical or panoramic radiographs according to literature suggestion6. The absence of a radiolucent image indicated bone loss around the implant. The treatment was successful, with the lack of implant mobility at 45 and 60 days at the prosthesis installation.
Early dental implant failure was defined as those that occurred before prosthetic rehabilitation. The implants were separated by insertion area: anterior and posterior maxilla and anterior and posterior mandible. The same examiner assessed primary and secondary stability. This procedure improves the reliability of the findings by eliminating subjective criteria. Clinical evaluation was performed by probing around the implant. The implants with periodontal pockets smaller than 3 mm were considered healthy. Periapical radiographs and computed tomography complemented clinical assessment. Images with a bone loss greater than 3 mm were considered unhealthy and classified as failures. The success rate was calculated based on primary and secondary stability. Implants with mechanical stability assessed by touch and an ISQ greater than 50 were considered successful.
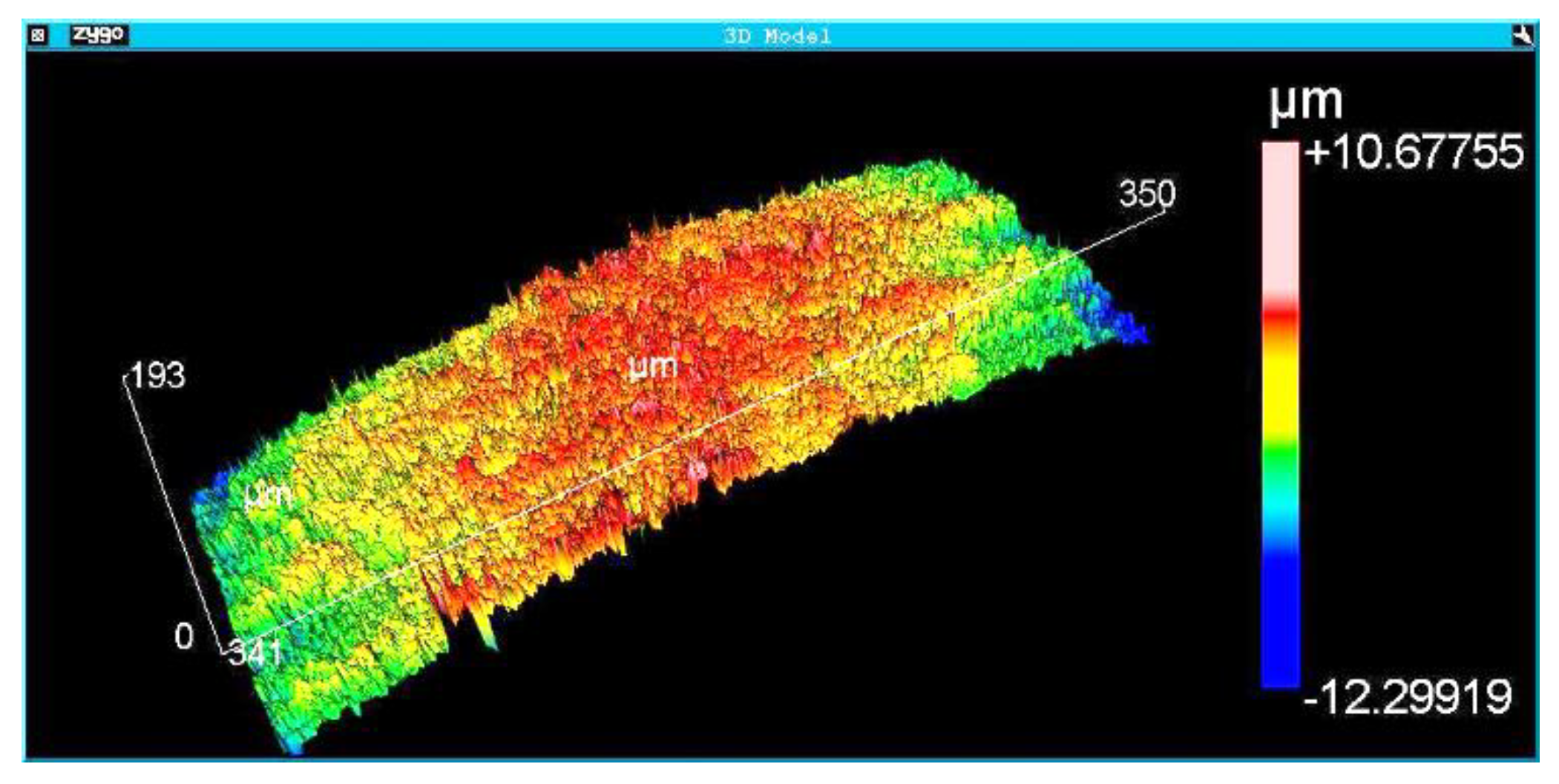
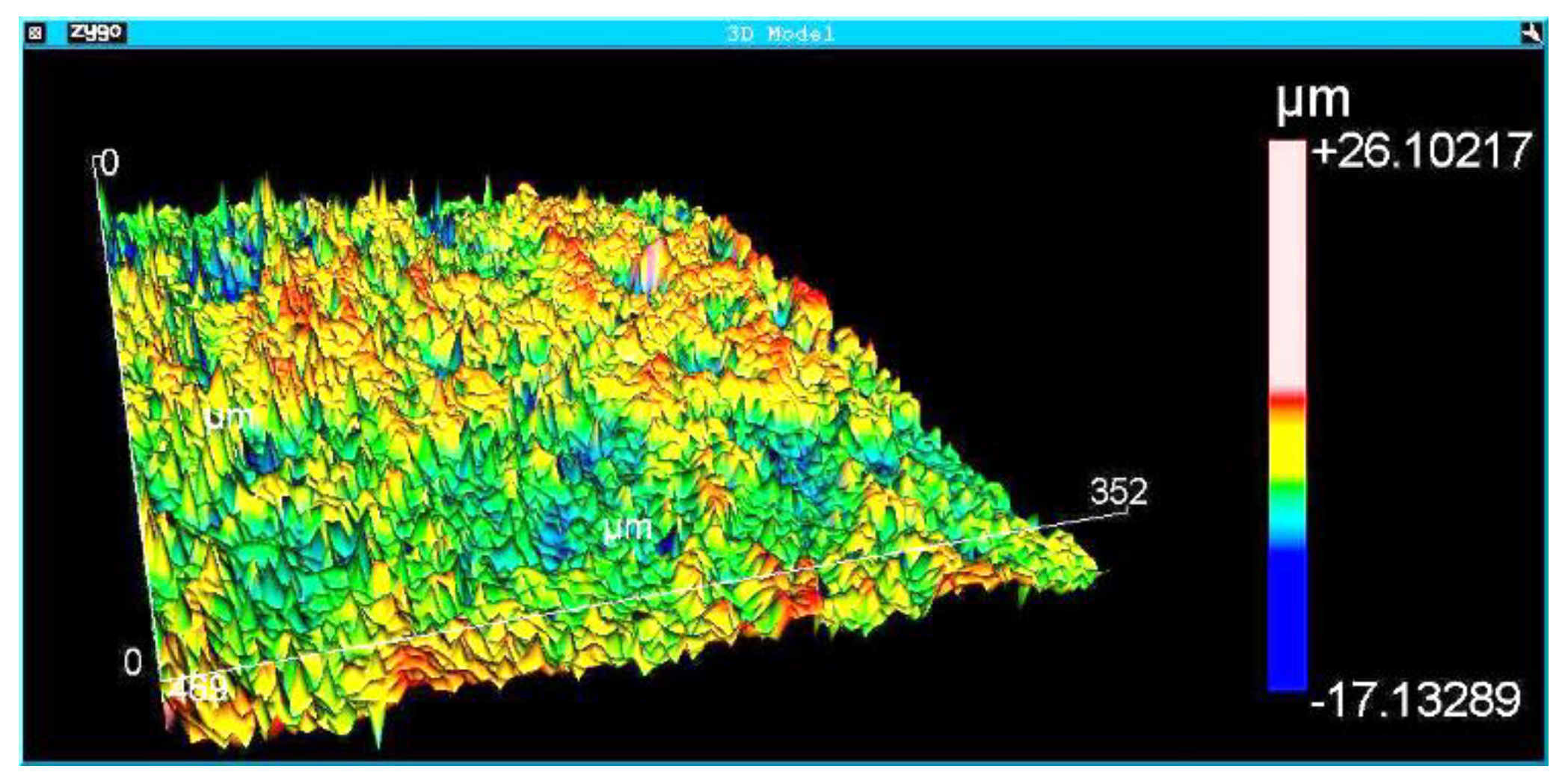
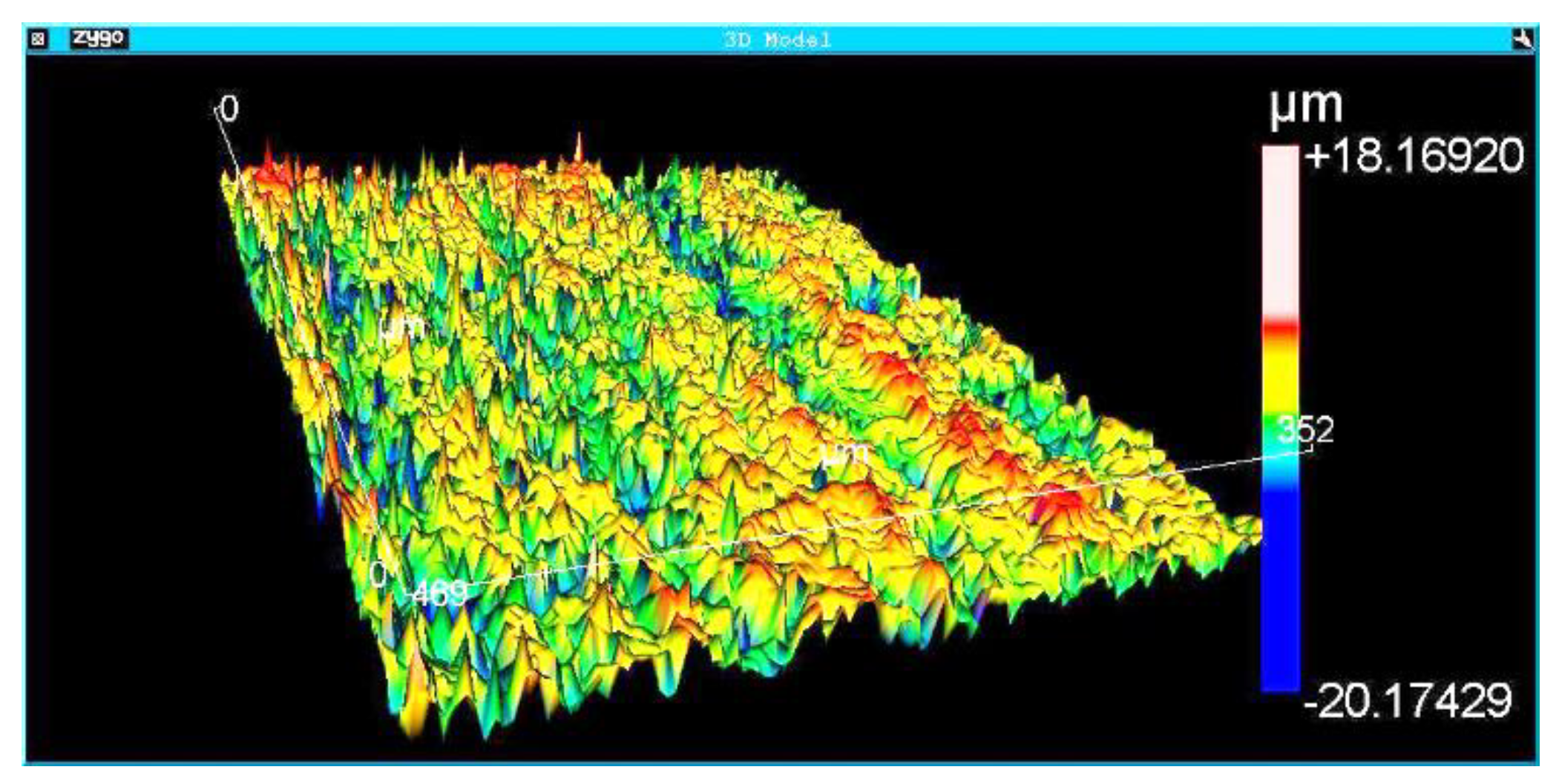
The surface morphology of the Implants was analyzed using a scanning electron microscope (FEI QUANTA FEG 250) at different magnifications. The results were complemented with roughness measurements. Roughness analysis was performed by interferometry with the Zygo NewView 7100 optical roughness meter (Zygo Corporation, Connecticut, United States). The implants’ roughness parameters Ra, Rsk, Rms, Rku, PV, Rpk, Rk, and R3z were obtained. The definitions of the roughness parameters are available in the Technical Standard ISO 4287 (ISO 4287:1997 Geometrical Product Specifications (GPS)–- Surface texture: Profile method - Terms, definitions, and surface texture parameters). Although the roughness parameter Ra is the most analyzed in the literature, previous results of the authors of the present work showed that other roughness parameters, such as Rz and PV, have the most significant influence on the osseointegration of dental implants. Three areas of each implant were measured. The interferometry technique produces a 3D image of the measurement region. Roughness measurement with a non-destructive optical interferometer provides a better understanding of surface morphology and roughness than measurements with a contact profilometer.
The measured data of implant stability were expressed in frequencies (ISQ) and percentages. The percentages of successes and failures corresponding to the different times were compared and calculated using the’ Agostino and Pearson omnibus normality test for independent samples. Statistical significance was fixed at (P < 0.05). Origin 7.0 software was used for the calculations.
3. Results
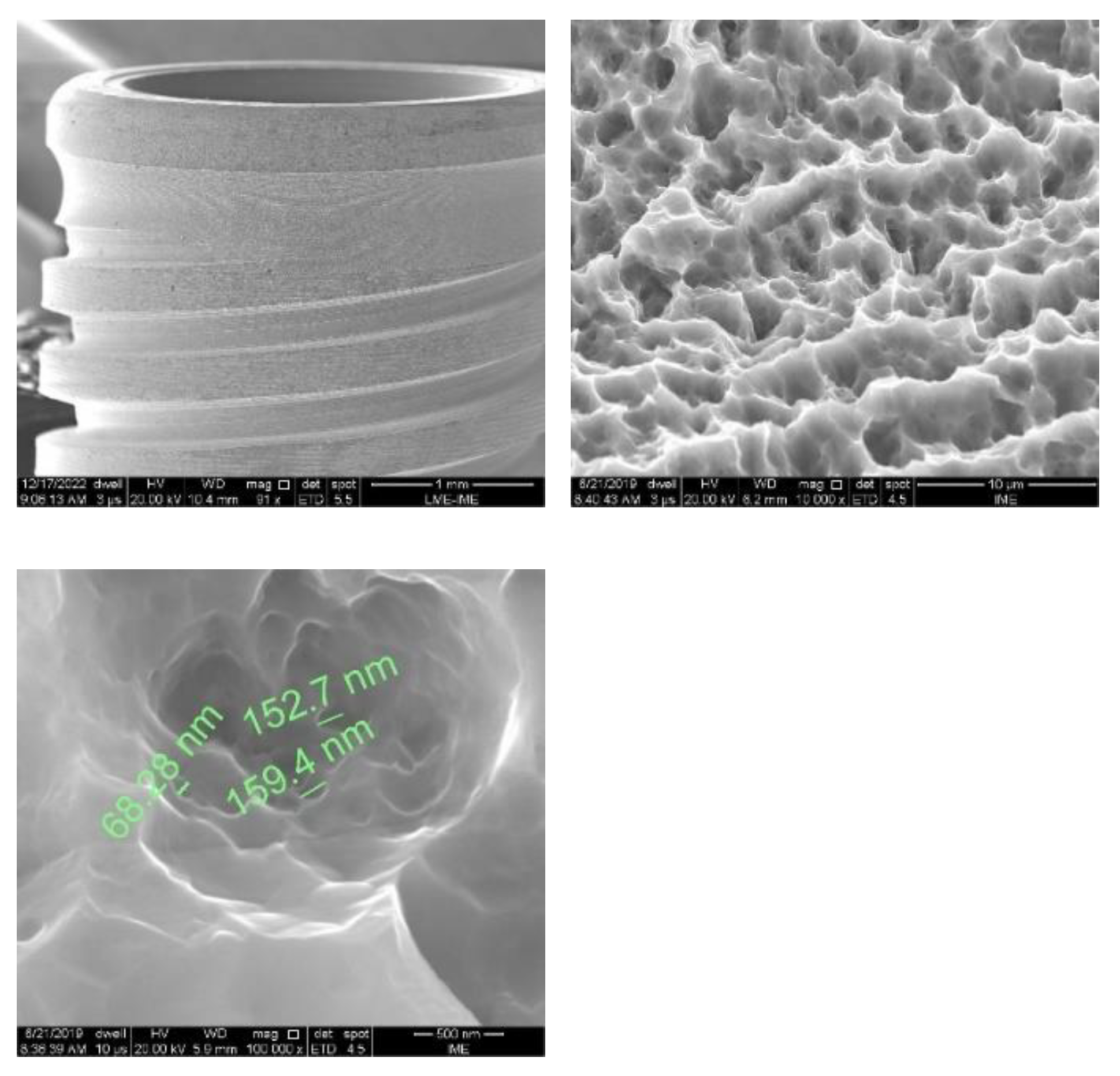
Figure 2,
Figure 3 and
Figure 4 show the surfaces of Avantt dental implants. A typical surface characteristic of acid etching treatment was observed in all analyzed regions. The surface is homogeneous and has micro and nano-roughness. The surface of the implants did not show contaminants from the manufacturing process.
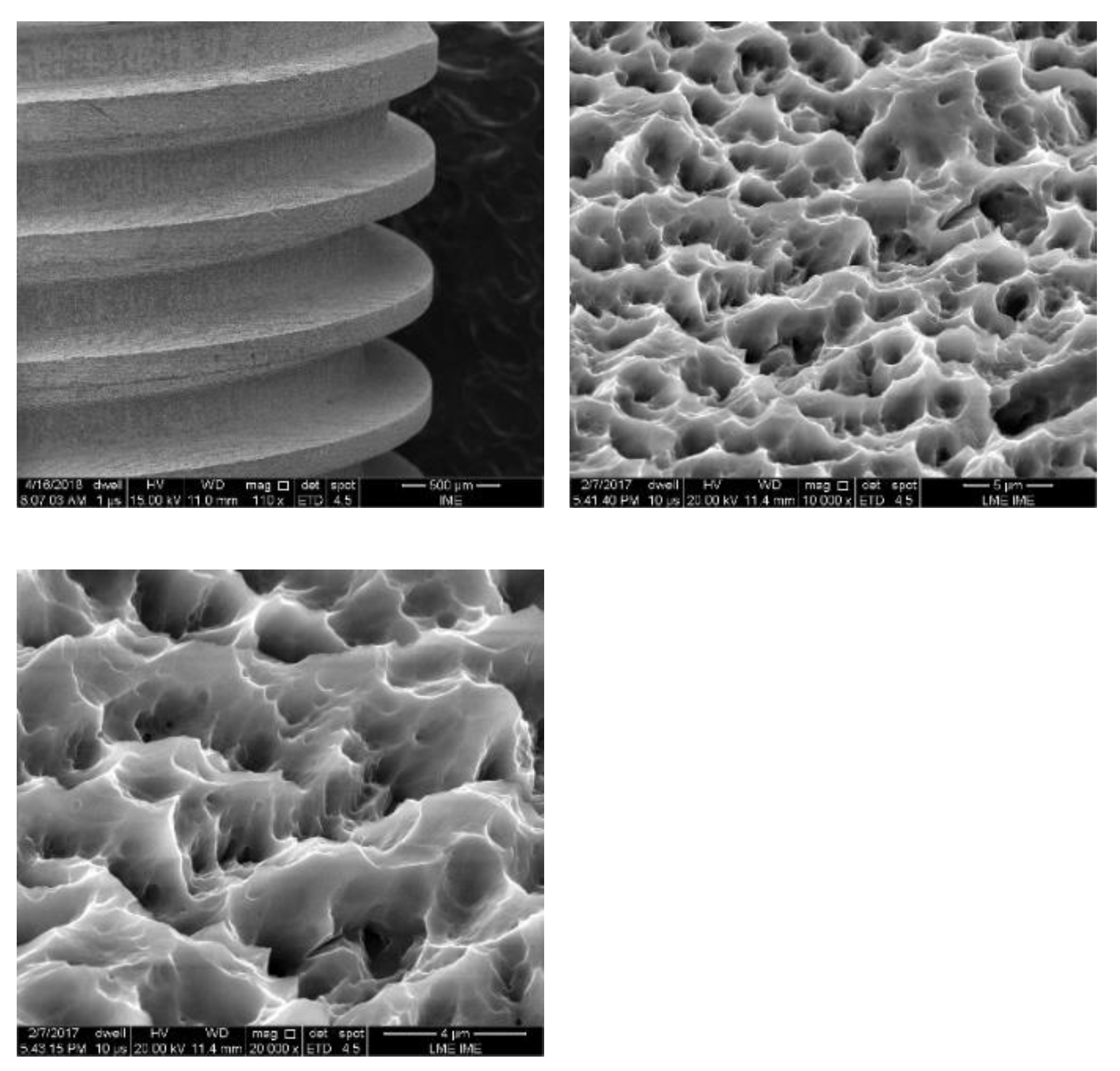
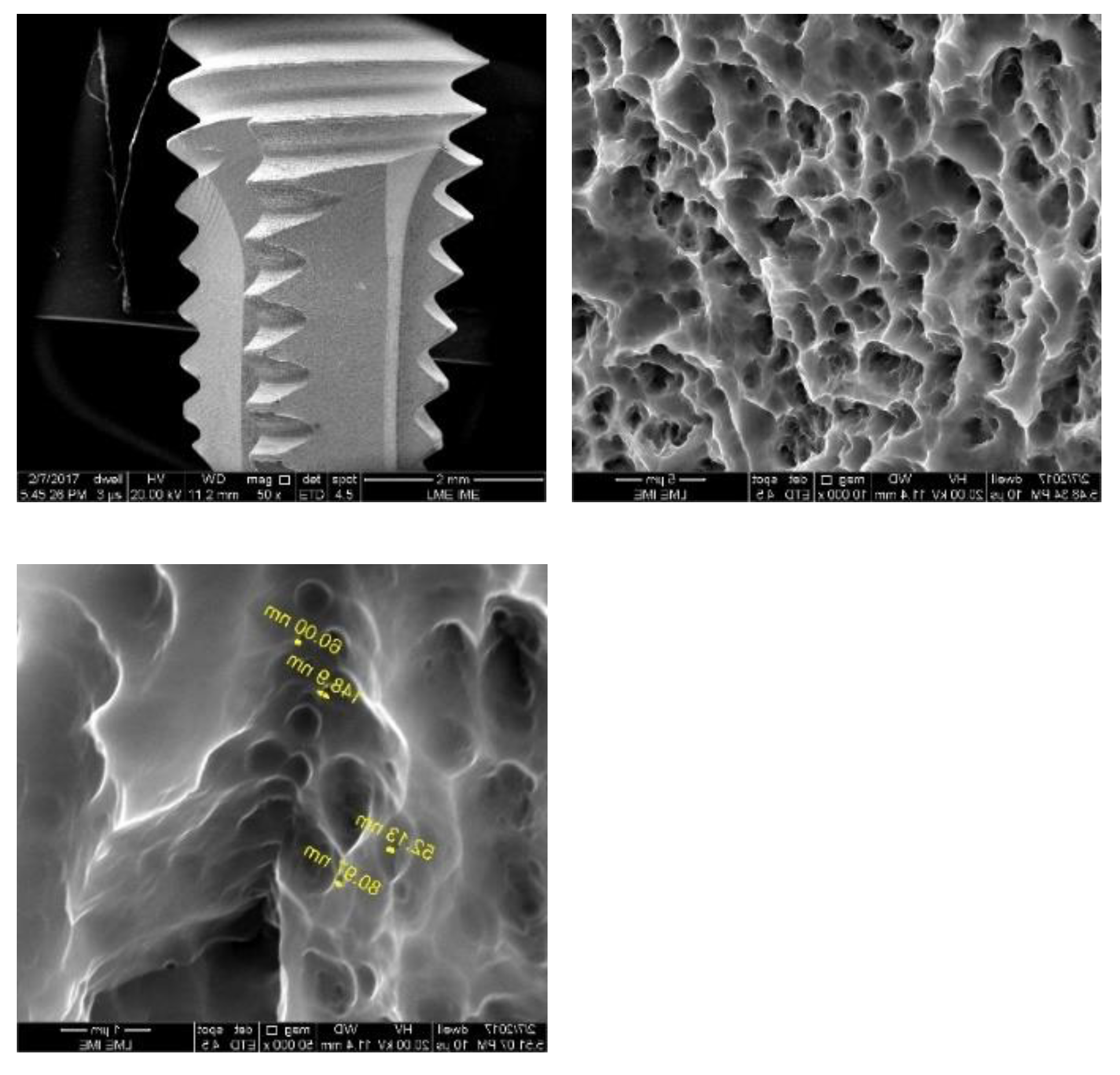
Figure 5,
Figure 6 and
Figure 7 show the surface morphologies of the implants obtained by interferometry during roughness measurements. The results showed that the Avantt implant has a high level of mechanical locking, good primary stability, and a good ISQ index. The acid treatment increased the contact area between the implant and the native bone, contributing to the primary stability.
Table 2 shows the descriptive statistics data of ISQ after dental implant immediately after insertion and after 45 and 60 days after surgery. The ANOVA test at the 0.05 level indicates that the ISQ means are significantly different. The ISQ value 45 days or 65 days after the surgery is lower than the measured after insertion.
In the statistical analysis of the stability values measured with ISQ, it was considered that the null hypothesis is that the means of all ISQ immediately after implant insertion and 45 days after surgery or 60 days after surgery are equal. The alternative hypothesis is that the means ISQ are different.
The ANOVA test shows that at the 0.05 level, the data means are significantly different (P = 0.0 and F = 124.7).
Table 3 shows the statistical analysis of the comparison of the mean using the Bonferroni Test. The same result was obtained using the Scheffe Test, the Scheffe Test, and the Tukey Test, which show that the ISQ values after 45 days and 60 days are significantly different from the start value.
Roughness measurements were taken at the implant threads’ vertices, flanks, and bottom.
Table 4 shows the average and standard deviation of different roughness parameters (StDev). The analyzed regions presented homogeneous roughness with similar values for all areas.
The statistical analysis showed that the ISQ 45 days after surgery was not different from 60 days after surgery (
Table 3). The secondary stabilities are similar 45 and 60 days after surgery. Loading the dental prostheses on the Avantt implant platform 45 days after insertion in natural bone is possible. The natural tissues in the implant installation region are entirely healed and follow the inclusion and exclusion criteria shown in
Table 1.
Table 5 shows data from the Agostino-Pearson test. This statistic test aims to verify if the shape of the distribution is similar to the shape of the normal distribution. It is a combination of the skewness test and the kurtosis test. According to Table 6, there was no statistical difference in the results obtained in the ISQ indices between the groups studied in the periods of 45 and 60 days of insertion (p > 0.05).
4. Discussion
Planning for implant-supported prosthetic rehabilitation involves assuring the primary stability of the implant [
7]. The prosthesis loading can be immediate, mediate, or delayed. The results of the present study showed that it is safe to load the definitive prosthesis 45 days after surgery, which is a delayed procedure.
The surface treatment changes the morphology, increases the contact area of the implant with the native bone, and improves interactions between proteins and the implant surface [
8]. Literature shows good clinical results of dental implants installed with 25 to 45 N.cm in the mandibles [
9] tested applying a counter torque of 25 N/cm. The success rate was 97.7 % after 60 days of healing.
The clinical results of the present study showed that when implants are installed with torques above 35 N.cm, initial stability is obtained to allow events associated with the mechanisms of osseointegration. In addition, the surgeon must avoid high torques not to deform and damage the implant surface and induce cracks nucleation in the bone [
10]. Literature showed no relationship between insertion torque and resonance frequency [
11,
12].
There are divergences in the literature regarding the influence of the diameter and shape of the dental implant on primary stability. Researchers evaluated the primary stability of 60 implants divided into 3 groups with different geometries, and it was observed that there was no statistical difference between cylindrical implants and conical implants [
13,
14]. These data are relevant to the results of the present work. The results of the present work showed that regardless of implant surface area and diameter, there is no significant difference in the ISQ index between both groups. Primary stability must be higher than 35 N.cm [
12,
15]. Other researchers found that the stability increases as the implant length and diameter increase, and the mechanical stability of a conical implant is greater than that of a cylindrical one.
The implant shape has become an excellent choice for better locking at the time of its installation. Many researchers have been studying this relationship between the locking potential of conical and cylindrical implants. The results showed that the percentage of locking in conical implants in both the maxilla and the mandible is higher than in cylindrical ones [
10,
11]. In the present study, we observed that when installing the conical implant (containing a homogeneous surface with micro and nano-roughness) using a torque between 35 N.cm and 60 N.cm, there was no statistical difference in the percentage of implant loss after 45 and 60 days, showing that its macrogeometry can be one of the main success points to reduce the reopening time.
A limitation of the present work is that only conical implants were used, with equal diameters (3.5/8.5), lengths, and thread shapes. New investigations need to be carried out to analyze the influence of the abovementioned variables.
5. Conclusions
Based on the results obtained in this work and considering the limitations of the methodology used, it can be concluded that:
- (a)
Dental implants installed with adequate primary stability do not show a statistical difference between secondary stability (osseointegration) 45 and 60 days after surgery.
- (b)
For dental implants with micro- and nano-rough surfaces inserted with a torque between 35 and 60 N.cm, it is possible to perform prosthetic rehabilitation six weeks (45 days) after surgery.
Author Contributions
Igor da Silva Brum: Conceptualization, surgery, and co-wrote the manuscript. Carlos Nelson Elias: characterization of samples, surface roughness measurements, analysis of results, writing review, and editing. João Carlos Amorim Lopes: surgery. Lucio Frigo: Analyze the experimental results of the materials. Paulo Gonçalo Pinto dos Santos: co-wrote the manuscript. Jorge José de Carvalho: Analyze the experimental results of the materials. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted following the Declaration of Helsinki and approved by the Institutional Review Board (Ethics Committee) of the UERJ implant dentistry specialization (protocol 01-2022 of 05/12/2022).
Informed Consent Statement
The author’s informed consent has been obtained from the patient(s) to publish this paper.
Acknowledgments
The authors are grateful to the Brazilian Agencies CAPES, CNPq, FAPERJ, and FINEP.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schnitman, P.A.; Shulman, L.B. Recommendations of the consensus development conference on dental implants. J. Am. Dent. Assoc. 1979, 98, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000 2016, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M.A. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Elias, C.N.; Coelho, P.G. Dental implants. Int J Biomater. 2013, 2013, 838565. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Moreno, F.J.; Herrera-Briones, F.J.; Bassam, T.; et al. Factors Affecting Dental Implant Stability Measured Using the Ostell Mentor Device: A Systematic Review. Implant Dent. 2015, 24, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Cosola, S.; Toti, P.; Peñarrocha-Diago, M.; Covani, U.; Brevi, B.C.; Peñarrocha-Oltra, D. Standardization of three-dimensional pose of cylindrical implants from intraoral radiographs: A preliminary study. BMC Oral Heal. 2021, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bayarchimeg, D.; Namgoong, H.; Kim, B.K.; Kim, M.D.; Kim, S.; Kim, T.-I.; Seol, Y.J.; Lee, Y.M.; Ku, Y.; Rhyu, I.-C.; et al. Evaluation of the correlation between insertion torque and primary stability of dental implants using a block bone test. J. Periodontal Implant. Sci. 2013, 43, 30–36. [Google Scholar] [CrossRef] [PubMed]
- da Silva Brum, I.; De Carvalaho, M.A.A.; Dos Santos, P.G.P. Ultrastructural Characterization of the Titanium Surface Degree IV in Dental Implant Aluminum Free (Acid Attack). Journal of Biomaterials and Nanobiotechnology 2020, 11, 151–160. [Google Scholar] [CrossRef]
- Rafael, M.; Marcelo, C.B.; Vinicius, F.; et al. Clinical Evaluation of Anodized Surface Implants Submitted to a Counter Torque of 25 Ncm After 60 Days of Osseointegration: Study in Humans. J. Maxillofac. Oral Surg. 2015, 14, 1–6. [Google Scholar] [CrossRef]
- Mints, D.; Elias, C.; Funkenbusch, P.; Meirelles, L. Integrity of implant surface modifications after insertion. Int. J. Oral Maxillofac. Implant. 2014, 29, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.-J.; Leesungbok, R.; Lee, S.-W.; Heo, Y.-K.; Kang, K.L. Differences in implant stability associated with various methods of preparation of the implant bed: An in vitro study. J. Prosthet. Dent. 2012, 107, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Comuzzi, L.; Tumedei, M.; Pontes, A.E.; Piattelli, A.; Iezzi, G. Primary Stability of Dental Implants in Low-Density (10 and 20 pcf) Polyurethane Foam Blocks: Conical vs Cylindrical Implants. Int. J. Environ. Res. Public Heal. 2020, 17, 2617. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.V.; Elias, C.N.; Cavalcanti, J.H. The effects of surface roughness and design on the primary stability of dental implants. Clin Implant Dent Relat Res. 2011, 13, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Carnovale, F.; Patini, R.; Penarrocha-Oltra, D.; et al. Measurement of the gap between abutment and fixture in dental conical connection implants. A focused ion beam SEM observation. Med Oral Patol Oral Cir Bucal. 2020, 25, e449–e454. [Google Scholar] [CrossRef] [PubMed]
- Svezia, L.; Caotto, F. Short Dental Implants (6 mm) Versus Standard Dental Implants (10 mm) Supporting Single Crowns in the Posterior Maxilla and/or Mandible: 2-Year Results from a Prospective Cohort Comparative Trial. J Oral Maxillofac Res. 2018, 9, e4. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).