1. Introduction
Several agricultural methods, practices or technologies are currently being used by farmers for maximal yields and plant growth, these include crop rotation, use of fertilizer (both organic and inorganic) for improved soil health, using genetic modified seeds, and the monitoring plants to detect pathogens, pests and stress (Mahlein 2016; Roper et al., 2021). Plant’s growths and developments requires the study of the controls processes the coordination processes that occurs within the organs, cells and in the entire plant. This studies usually involves changes in gene expression which occur as responses to the changes occurring within the environment especially climatic changes such as drought, heat, rainfall, e.t.c. Several imaging methodologies are currently available for use in collecting data for quantitative studies of plants their growths and developments. Multi-level data, from macroscopic (Jones, 1992; Freschet et al, 2021; Roper et al., 2021).
In future decades, increased productivity in agriculture is a necessity. To meet the food demands of the rising global populations which is grossly being hampered by drought, poor soil quality, flooding, new emerging plant diseases and high temperatures with resulting negative impacts on plant yields. To assist in overcoming these challenges, in situ studies of rhizosphere interactions, using specialized plant growth houses/chambers or systems are developed which are able to mimic the natural growth environments for disease detection, stress detection, etc to determine how best to tackle these problems (Mahlein et al., 2016; Farber et al., 2019; Mohammed et al., 2019)
There are also many diagnostic technologies, methods and tools that are being employed to study plants and their development and growth creating more awareness about plants and how to promote their health. Also, several hand-held and at-the-point-of-use technologies are available , including devices with a lateral flows or portable-devices for easy use on the field (Roper et al., 2021). However, the recent lab-based methods and techniques available for plant’s diagnostics for at-the-point-of-use plant monitorings are not sufficient or are limited in certain capacities as these devices usually requiring harvested and or processed plant tissue, thus un-conducive for continued monitorings. This review provides information on several methodologies being used in monitoring plants and how they improve awareness about plants.
2. Methods for Studying and Monitoring Plants
2.1. Phynotypic and Physiological Studies
These includes the study of traits and physical characteristics (phenological and morpho-physiological traits) such as growth rate, green-canopy-cover, total plant’s biomass, the plant’s height, the total number of seeds in a plant, total pod number, flowering, vigour, pod formation, maturity of seedling, leaf loss, wilting, growth rates, time to reach initiation of flowering, the time duration for pod to set, time to reach full maturity, the length of the vegetative stage, duration of reproduction stages, duration of time for complete seed fillings, seed lengths. They also include plant morphologies such as leaf size, the growth of stem, the plant’s height, and plant root’s development (Tang et al., 2022; Yang et al., 2018; Mohammed et al., 2024).
2.2. Genotypic and Molecular Studies
This includes the study of the genetic characteristics and proteins and gene expression in plants.
2.2.1. Genomics
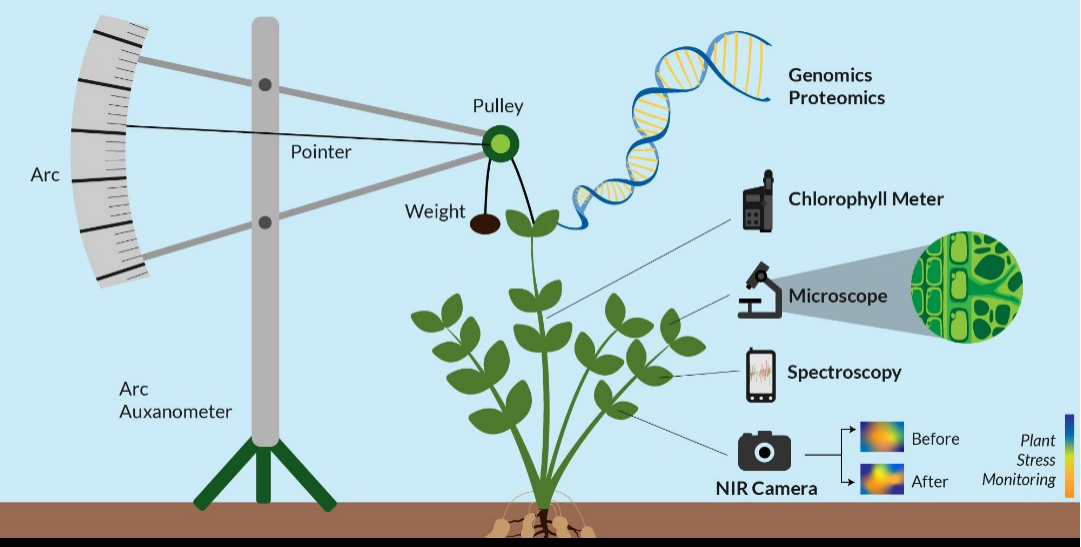
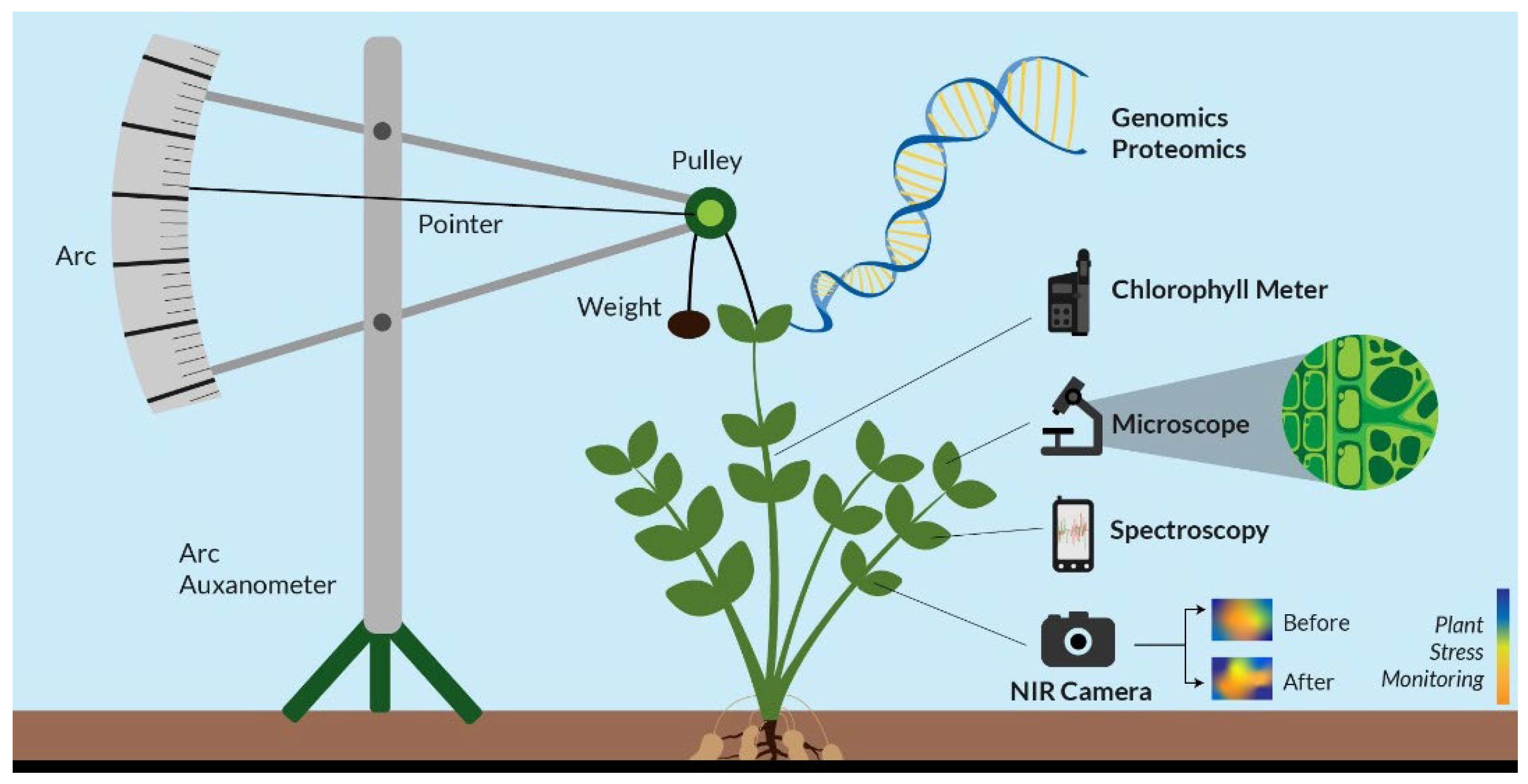
Genomics is an inter-disciplinary aspect of biology that is focused on the structures, evolutions, functions, mappings, and editings of genome.The genome is the organism's total DNA set, which includes all its genes their hierarchies, three-dimensional structures and configurations (
Figure 1). These genes are highly important as they direct protein productions which is assisted by enzymes and the messenger molecules. Genomics differs from genetics, an involves the studies of the individual gene(s) and what role(s) they play in genetic inheritances. Rather, genomics is aimed at collective characterizations and quantifications of gene(s).
Functional genomics is the approach that makes use of a vast wealth of data which are produced through genomic projects (these include genome sequencing(s)) describing how they function and interaction especially genes and proteins.
Structural genomics works on describing the three-dimensional structures of all proteins which are encoded by specific given genomes.
Epigenomics studies the complete/total set of epigenetics which studies how and the way genes are expressed and change while remaining constant.
2.2.2. Transcriptomics
Transcriptomic analysis of bacteria associated with plant is done using two approaches either RNA sequencings (RNA-seq) or the gene(s) expression micro-arrays, this is able to reveal the gene(s) which are expressed differently under certain environmental conditions e.g for detection of genes that give responses when certain plant extract are present (Coutinho et al., 2015; Levy et al., 2018). In most cases, the bacteria are usually cultured first from host plants before RNA-seq is used. Metatranscriptomic on the other hand, sequences transcripts from the from the sample of the entire community present, providing more informations about simultaneous transcriptional states for an arrays of micro-organisms which maybe present in the samples (Coutinho et al., 2015; Levy et al., 2018).
2.2.3. Proteomics
The use of Proteomics or metaproteomics methods are majorly based on the use of the liquid-chromatography, the mass spectrometry techniques and with one obtaining semi quantitative informations about the majority of bacteria protein types which are produced in specific environmental samples (
Figure 1). They also use proteins to enable the understanding of how microbes are able to contribute to changes in the soil ecosystem, providing more information about the secreted enzymes present in the soil, the microbial types and their ability to produce these metabolites. After protein extraction, isolation and fractionation is done after which mass spectroscopy is carried out, and the final results are compared with the data base proteome (Levy
et al., 2018).
2.3. Metabolomics
Metabolomics as a tool for analysis is becoming more recognized in the industrial and scientific sectors it intergrates both statistics and analytical chemistry to provide an understanding of the level of metabolites and how they change within a chemical or biological system (Levy et al., 2018; Shen et al., 2023). Plant metabolomics is a useful study area in plant sciences where quantitative analysis for metabolites is done for plant systems. It is also used as a tool for phenotypic and diagonistic research in plants (Hong et al., 2016) these include Plant products characterization and evaluation (Favilla et al., 2013), Plant pharmacology and nutrition (Schmidt et al., 2008), Plant diseases and resistance (Hantao et al., 2013), Plant genetic mutant and phenotyping (Hong et al., 2016). Chromatographic metabolomics on the other hand has widely for performing plant’s genetic mutants or phenotyping study. It is a powerful tool, that uses tensor method(s) and plays a highly important role to aid in understanding how important metabolite(s) traits and the associated targeted genetic factor(s) help in plant development (Khakimov et al., 2016; 2017). The fast developments of metabolomics as a technology is profoundly useful in the field of plant sciences. Anther method is the use of Mass spectrometry based metabolomic or lipidomic measurement(s) which requires specified extraction procedures for different molecule to be MS-compatible (Stearns et al., 2012, enabling the study of smaller volumes of metabolites and lipids contents (Nakayasu et al., 2016). Another method is the use of nuclear magnetic resonance (NMR) metabolomics, to study metabolites that are present in soil study the types of responses given by certain organism to the different surrounding habitat stimuli thus revealing pre-symptomatic signs and signals of inherent plant stress(es) and plant disease(s) (Rochfort et al., 2005).
Tools, Devices and Machines Used for Monitoring Plant
Crescograph: is the device used for measurement plant growth, and it was first designed by an Indian scientist known as Sir Jagdish Chandra Bose in the 20
th century. It used batches of clocks gears alongside a dark glass-plate for recording the tiny movement(s) of the top most tip of the plant. It provides real-time informations about the plant’s growth, for studying it’s water status in the soil-plant atmosphere and also measures and records the plant growth under certain environmental and climatic factors such as temperature, chemical reactions, electric supply, the effect of gases a modern electronic device was designed by Randall Fontes which has a range of 1:1000 to 1:10,000 of an inch (Bose, 1920) (
Figure 1).
3.2 Auxanometer: is a instrument used for determining the rate of growth and development in plants and it usually consists of a flexible wire which runs over a pulley that is connected to an indicator that shows the value of plant growth on a curved shaped scale (Bovie and Bovie, 1915; Gallagher et al., 1976 ).
3.3 Potometer: is a H-shaped device with a capillary tube instrument used for measuring the rate of transpiration in green leafy plant shoot. It tells the rate of uptake and evaporation of water. It also measures the loss of water from the leaves (
Figure 1). It is known as the Ganong’s potometer or a transpirometer. Water uptake is in plant is usually caused by photosynthesis and transpiration (Bohdan 1974).
3.4 Non-Contact Device- These devices are able to measure the growths of plant by the use of a single sensor or set of sensors. They study the movements in plants without making physical contact with them and be use at a minimal distance or close to them the sensor is able to sense the movements in the plant and also keeps records for other further measurements an example is the sensor metre.
3.5 MRI (magnetic resonance imaging): uses analytical techniques to for studies of water, its properties and is done without ionizing or breaking down the water sample. The use of portable magnetic resonance imaging (MRI) a a tool for field experiments is expanding rapidly and provides substantial advantage(s) in plant science(s) (
Figure 1). The MRI allows for a non-invasive studying of the content of plant water, its structure, it’s fow, it’s stress responses, and any other physiological process(es), as such a large array of informations maybe obtained together while the method is being used, and can be measured in-situ within the natural enviroment of the plant (such as greenhouses, climate chambers, and in natural environments), as they are handy portable devices. (Blystone
et al., 2024).
3.6 High quality 3D informations for microscopic morphology of plant tissue: this method studies the cell spatial organization and tissue intercellular spaces providing a better understanding of the physiological processes occuring within the plant or tissue cell. An example of such tool is the X-ray micro-CT which provides 3D micro-structural informations about the inter-cellular pore spaces, sizes and tissue shapes. It’s limitation is its inability to properly segement the cells because of the density difference(s) at cell-to-cell interface(s). Also, deep learning models have been trained and also tested allowing for segmention of cells individually using an X-ray micro-CT scan images for the parenchyma tissues or samples with different cell and porosity characteristics (Blystone et al., 2024; Leen et al., 2024).
3.7 X-ray micro-computed tomography (micro-CT): this device acquires complex 3D images of plants structure and the great advancement in developing high-resolution X-ray micro-CT lab instrument(s) making it much easier for plant scientist and researchers to carry out meaningful studies with out the use of synchrotron facilities (Piovesan et al., 2021; Duncan et al., 2022; Leen et al., 2024).
3.8 Contrast-enhanced micro-CT: this device scans and provides images by highlighting the plant cell wall structure (Wang et al., 2017; Xiao et al., 2021; Leen et al., 2024).
3.9 Disease detection: Precise, accurate and quick early disease detection has great significance in ensure precise spraying to avoid extensive spread of disease(s) and improved crop yields and quality (Zhang et al., 2023). Never the less, the signs and symptoms between the healthy leaves, and early leaf disease infection are quite similar, which makes detecting the early characteristics of the diseases still a challenge. The BPNN (also known as the back propagation neural network), SVM (also known as the support vector machine) and RF (also known as the radio frequency) are model machine learning which have been developed and tested for the detection of gray-mold and anthracnose in strawberry early, respectively, with the use of spectral fingerprints, VIs used alone or together provide rapid, precise, and also non-destructive identification of the strawberry’s types of gray-mold and anthracnose disease in early stage (Wang et al., 2015; 2018) which showed much success with accuracies of 97.78%, 94.44% and 93.33% in the three different machine learning model types (BPNN, SVM and RF), respectively (Cortis et al., 2017; Wu et al., 2019). This machine learning models may also be considered for detection of several other diseases in other plants.
3.10 Drought monitoring: The thermography device is a useful tool for monitoring changes that occur in plant water-use behaviour and responses to water. The quantity of the emission of thermal infrared (TIR) radiations coming from a plant is closely or approximately related to the plant’s body temperature, which indirectly depends on received radiation and plant transpiration rate as the rate at which latent heat vaporizes at the leaf’s surface (Jones, 1992). Combinations of the thermal infrared (TIR) and the hyper-spectral imaging used in monitoring how drought affects a plant’s transpiration rate (E) was quantified, with the TIR indices being used in monitoring plant’s drought stress and in developing prediction models, providing more accurate values of plant temperatures, ambient air temperature and relative humidity (Mertens et al., 2023).
3.11. Imaging Techniques and Approaches
Imaging techniques include thermography, fluorescent imaging, RGB imaging, and hyper-spectral imaging (Farber et al., 2019). The RGB imaging works by utilizing digital cameras which are able to measure the change(s) in transmittance. Also simple short digital videos and images maybe used to monitor a different sets of plants in the field. These devices may be used on plants in conjunction with smart-phone sensors, and drones for monitoring larger fields. Also, several machine learning algorithms are presently being designed for detection of patterns to show disease infections. In the study by Mahlein, the several use for RGB imaging are described (Roper et al., 2021; Mahlein et al., 2019).
3.11.1 Fluorescent imaging: it is a non-invasive imaging technique which helps with visualizing biological processes that occurs within a living organism. This method is highly similar to the RGB imaging; it however differs with the use of a laser along side the camera, and allows for the occurrence of fluorescent excitations. One the most regular uses of the fluorescent imaging technique is the chlorophyll SPAD meter, it reads/ detects the fluorescence in the plant and compares it with standard baseline values (
Figure 1). when chlorophyll is excited by specific light type it fluorescences naturally allowing the amount of chlorophyll to be read (Roper
et al., 2021; Bolhar-Nordenkampf
et al., 1989). They are also used for photographing fluorescent-dyes and fluorescent-proteins helping in marking molecular mechanisms and structures. This technique has low cost, is highly sensitive and relatively safe.
3.11.2 Chlorophyll fluorescence: this portable hand held device helps to provide information about photosynthetic activities of a plant. The technique is based on the Since chlorophyll is fluorescent nature of chlorophyll under intensive sunlight, it works as a simple fluorimeter that takes measurements in the field. It is non-destructive, non-invasive and very handy for field use, although it is unable to carry out the diagnose of specified types of abiotic or biotic stress factors its response may fluctuate depending on the type of factors (either biotic or abiotic) (Roper et al., 2021; Mohammed et al., 2019).
3.11.3 Hyperspectral imaging: this technique uses an electromagnetic spectrum to analyse light and evaluates the changes which aren't visible in the RGB images formed. It is able to detect the more significant changes more effectively than the visual and the fluorescence images counterparts, but is limited in that only general changes can be detected on plant surfaces. In future with more significant studies, the use of hyper-spectral patterns may be more precise and specific in detecting changes. The work of Zhang et al. 2012 for example, where analysis of the hyper-spectral features for yellow rust disease was done and followed by statistical analysis, it was possible to differentiate the yellow rust disease from nutritional leaf/ plant deficiencies (Roper et al., 2021; Zhang et al., 2012).
3.11.4 Polydiacetylene polymer (PDA) and functional DNA single-walled carbon nanotubes: this methodology was carried out in a laboratory setting where (SWCNTs) were incorporated inside the plant leaves before imaging to study the leave molecular contents it may also have promising potentials to be used in the field.
3.11.5 Root imaging: Instead of using the traditional method where there is a destructive sampling of soil, root washing, and advancement is the imaging technology, which allows for the significant advance(s) in phenotyping of roots making root studies much easier (Farber et al., 2019). it allows for observation of roots in the soil, and measurements can be taken repeatedly, using the X-ray tomography (Mooney et al., 2012) to the use of the electrical resistant tomography method (Amato et al., 2008; Baykalov et al., 2023). 2D images of the growing roots can taken using the non-destructive techniques such as rhizo-boxes, or (mini-) rhizotrons (MR). The Rhizotron systems uses a fat, transparent (plexi-)glass window to take larger but images with similar structures better than tube installed MR cameras, although the infrastructure costs are more, with greater or more significant disturbance within the soil and may have reduced flexibility for experimental uses. A better method is the use of MR camera systems that are able to observe the timings (phenology) of the plant’s root emergence, it’s growth and it’s decay rate in the field providing the window for an un-disturbed rhizosphere which may not be possible with other methods (Freschet et al., 2021; Baykalov et al., 2023).
3.12. Spectroscopy techniques and approaches
One major method used for rapid diagnosis is the use of spectroscopy, it is used in molecular methods which includes PCR (real-time) method, and the ELISA (which are commonly used in plant disease(s) diagnosis and can be very invasive sometimes). Some common methods of spectroscopy include X-ray spectroscopy, Raman spectroscopy, and mass spectrometry. The Raman spectroscopy works by detecting vibrational frequencies of molecules present in the sample; and can also determine the type(s) of chemical footprints of the structure(s) for identification of molecules by using the illumination from a monochromatic laser. The interaction between the and the sample results in a shift in energy helping to tell the type of molecules present. Altangerel et al. 2017 worked on developing a hand-held Raman spectroscopy instrument which uses the coleus lime as it’s model organisms and carotenoids and anthocyanins (photosynthetic pigments) as target molecules (Roper et al., 2021; Altangerel et al., 2017).
3.13. Electrical-Based Approaches
Many several studies have used electrical components to monitor plants in-vivo. They require external materials and equipments and also use nanotechnology for integration into plants examples of such are:
3.13.1 Microneedle Electrodes: In the study carried out by Jeon et al. Salinity was measured by using real-time monitoring systems which were developed to detect the salinity (a very important factor for plant growth and health) in tomato plants in a no-destructive manner, which is a highly important factor in the plant health and crop yield (Roper et al., 2021; Jeon et al., 2017).
3.13.2 Organic Electrochemical Transistor-Based Sensors (OECT): these devices have been explored for it’s use as biosensing tools. It based on the use of conductive polymers (poly (3,4- ethylene-dioxythiophene) which are doped with diverse attached side groups) and films or channels were put in close contacts with electrodes and electrolytes with the electrode being connected with a channel along gate electrodes for electrical connections. Coppede et al. were able to develop an OECT sensor which can be used for continuously monitoring plant health based by studying the changing sap solutes (Roper et al., 2021; Coppede et al., 2017).
4. Fostering of More Methodologies and Techniques
The current available lab methods and techniques for plant analysis and devices are not sufficient for plant monitoring at point-use. These techniques and devices can allow for precision in agriculture, minimizing usage of expensive resources and maximizing crop yield. In addition, the availability of the affordable technologies with ready accessible for large and small farming is highly vital necessity for increased agricultural productions. Some of the pressing challenges of some these technologies includes their implementation under field conditions. Some of the major factors to put into considerations for the successful use of in-vivo sensors may include, yet not limited to, it’s accuracy, sensitivity, specificity, ease of use, cost, durability, and ultimately the environmental after impacts. More researches should be done for developing deep learning frame-works for replacing traditional machine learning methods for detection of early plant disease infection, detection of stress, and plant monitoring to foster better understanding of plants under different environmental conditions.
5. Conclusions
Several different sets of needs are required for better studies of plant diagnostics technologies and the most suitable technologies for farmers will mostly be dependent on land size they cultivate, the crop types and their specific needs, natural, economical, and social environments. The current technologies available for measurement of plant’s health and/or diagnosis of disease have high costs, are invasive, and need to be sent as samples to laboratory facilities for processing, therefore, there is urgent need for developing more simple, cheaper, accurate and precise point of use technologies methodologies and devices for better understanding of plant growth and health monitoring. Development arrays of sensors and new innovative techniques and technologies are a necessity to meet agricultural food demands for the increasing large world populations.
Author Contributions
All enlisted authors have contributed to this review study by editing, providing information and graphical presentation for this research.
Funding
No funding were received and as such are not reported for this work.
Data Availability Statement
No datasets were generated or analysed for this study).
Conflicts of Interest
The authors declare that there are no conflict of interest.
References
- Altangerel N., Ariunbold G. O., Gorman C., Alkahtani M. H., Borrego E. J., Bohlmeyer D., Hemmer P., Kolomiets M. V., Yuan J. S., Scully M. O. (2017) In vivo diagnostics of early abiotic plant stress response via Raman spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 114 (13), 3393−3396.
- Amato M., Basso B., Celano G., Bitella G., Morelli G., Rossi R. (2008). In situ detection of tree root distribution and biomass by multi-electrode resistivity imaging. Tree Physiol. 28(10):1441–8.
- Atkinson J. A., Pound M. P., Bennett M. J., Wells D. M. (2019). Uncovering the hidden half of plants using new advances in root phenotyping. Curr Opin Biotechnol. 55:1–8.
- Blystone Shannan, Magali Nuixe, Amidou Sissou Traoré, Hervé Cochard, Catherine Picon-Cochard and Guilhem Pagés. (2024).Towards portable MRI in the plant sciences Plant Methods (2024) 20:31. [CrossRef]
- Bolhar-Nordenkampf H., Long S., Baker N., Oquist G., Schreiber U., Lechner E. (1989). Chlorophyll fluorescence as a probe of the photosynthetic competence of leaves in the field: A review of current instrumentation. Funct. Ecol. 3, 497−514.
- Bose, Jagadis Chandra (1920). Researches on growth of plants. Nature. 105 (2646): 615-617. [CrossRef]
- Bovie W. T., and Bovie W. T. (1915). A simplified Precision Auxanometer. American journal of Botany, 2(2): 95-99. [CrossRef]
- Colnago L. A., Wiesman Z., Pages G., Musse M., Monaretto T., Windt C. W., et al. (2021). Low feld, time domain NMR in the agriculture and agrifood sectors: an overview of applications in plants, foods and biofuels. J Magn Reson.. 323:106899. [CrossRef]
- Coppede N., Janni M., Bettelli M., Maida C. L., Gentile F., Villani M., Ruotolo R., Iannotta S., Marmiroli N., Marmiroli M., Zappettini A. (2017). An in vivo biosensing, biomimetic electrochemical transistor with applications in plant science and precision farming. Sci. Rep. 7 (1), 16195.
- Cortés V., Rodriguez A., Blasco J., Rey B., Besada C., Cubero S., Aleixos N. (2017). Prediction of the level of astringency in persimmon using visible and near-infrared spectroscopy. J Food Eng. 204:27–37.
- Coutinho B.G., Licastro D., Mendonca-Previato L., Camara M., Venturi V. (2015). Plant-influenced gene expression in the rice endophyte Burkholderia kururiensis M130. Mol. Plant Microbe Interact. 28, 10–21. [Google Scholar] [CrossRef] [Green Version].
- Duncan K. E., Czymmek K. J., Jiang N., Thies A. C., Topp C. N. (2022). X-ray microscopy enables multiscale high-resolution 3D imaging of plant cells, tissues, and organs. Plant Physiol. 188:831–45.
- Farber C.; Mahnke M., Sanchez L., Kurouski D. (2019). Advanced spectroscopic techniques for plant disease diagnostics. A review. TrAC, Trends Anal. Chem. 118, 43−49.
- Favilla S., Durante C., Vigni M. L., Cocchi M. Assessing feature relevance in NPLS models by VIP. Chemometr Intell Lab Syst. 2013;129:76–86.
- Freschet G. T., Pagès L., Iversen C. M., Comas L. H., Rewald B., Roumet C., et al. (2021). A starting guide to root ecology: strengthening ecological concepts and standardising root classifcation, sampling, processing and trait measurements. New Phytol. 232(3): 973–1122.
- Gallagher J. N, Biscoe P. V., Saffell R. A. (1976).A sensitive Auxanometer for field use. Journal of experimental Botany. 27(4): 704. [CrossRef]
- Hantao L. W., Toledo B. R., de Lima Ribeiro F. A., Pizetta M., Pierozzi C. G., Furtado E. L., Augusto F. (2013) Comprehensive two-dimensional gas chromatography combined to multivariate data analysis for detection of disease-resistant clones of Eucalyptus. Talanta. 116:1079–84.
- Hong J., Yang L., Zhang D., Shi J. (2018). Plant metabolomics: an indispensable system biology tool for plant science. Int J Mol Sci. 17(6):767.
- Jeon E., Choi S., Yeo K.-H., Park K. S., Rathod M. L., Lee J. (2017) Development of electrical conductivity measurement technology for key plant physiological information using microneedle sensor. J. Micromech. Microeng. 27 (8), 085009.
- Jones H. G. (1992). Plants and microclimate: a quantitative approach to environmental plant physiology. Cambridge: Cambridge University Press. p. 460.
- Kamal T., Cheng S., Khan I. A., Nawab K., Zhang T., Song Y., et al. (2021) Potential uses of LF-NMR and MRI in the study of water dynamics and quality measurement of fruits and vegetables. J Food Process Preserv. 43(11):e14202. [CrossRef]
- Khakimov B., Mongi R. J., Sørensen K. M., Ndabikunze B. K., Chove B. E., Engelsen S. B. (2016). A comprehensive and comparative GC–MS metabolomics study of non-volatiles in Tanzanian grown mango, pineapple, jackfruit, baobab and tamarind fruits. Food Chem. 213:691–9.
- Khakimov B., Rasmussen M. A., Kannangara R. M., Jespersen B. M., Munck L., Engelsen S. B. (2017) From metabolome to phenotype: GC-MS metabolomics of developing mutant barley seeds reveals effects of growth, temperature and genotype. Sci Rep. 7(1):8195.
- Leen Van Doorselaer, Pieter Verboven and Bart Nicolai (2024). Automatic 3D cell segmentation of fruit parenchyma tissue from X-ray micro CT images using deep learning Van Doorselaer et al. Plant Methods (2024) . [CrossRef]
- Levy A., Salas Gonzalez I., Mittelviefhaus M., Clingenpeel S., Herrera Paredes S., Miao J., Wang K., Devescovi G., Stillman K., Monteiro F., et al. (2018). Genomic features of bacterial adaptation to plants. Nat. Genet. 50, 138–150. [Google Scholar] [CrossRef] [Green Version].
- Mahlein, A. K. (2016) Plant Disease Detection by Imaging Sensors Parallels and Specific Demands for Precision Agriculture and Plant Phenotyping. Plant Dis. 100 (2), 241−251.
- Mertens Stien, Lennart Verbraeken, Heike Sprenger, Sam De Meyer, Kirin Demuynck, Bernard Cannoot, Julie Merchie, Jolien De Block, Jonathan T. Vogel, Wesley Bruce6, Hilde Nelissen, Steven Maere Dirk Inzé and Nathalie Wuyts (2023). Monitoring of drought stress and transpiration rate using proximal thermal and hyperspectral imaging in an indoor automated plant phenotyping platform Plant Methods 19:132. [CrossRef]
- Mohammed Mitache, Aziz Baidani, Bouchaib Bencharki and Omar Idrissi. (2024). Exploring the impact of light intensity under speed breeding conditions on the development and growth of lentil and chickpea. Plant Methods 20:30 . [CrossRef]
- Mohammed G. H., Colombo R., Middleton E. M.; Rascher U.; van der Tol C., Nedbal, L., Goulas, Y., Perez-Priego O., Damm A., Meroni M., Joiner J., Cogliati S., Verhoef W., Malenovsky Z., Gastellu-Etchegorry J.-P., Miller J. R., Guanter L., Moreno J., Moya I., Berry J. A., Frankenberg C., Zarco-Tejada P. J. (2019). Remote sensing of solar-induced chlorophyll fluorescence (SIF) in vegetation: 50 years of progress. Remote Sens. Environ. 231, 111177.
- Mooney S. J., Pridmore T. P., Helliwell J., Bennett M. J. (2012). Developing X-ray computed tomography to non-invasively image 3-D root systems architecture in soil. Plant Soil. 352(1):1–22.
- Nakayasu, E. S.; Nicora, C. D.; Sims, A. C.; Burnum-Johnson, K. E.; Kim, Y. M.; Kyle, J. E.; Matzke, M. M.; Shukla, A. K.; Chu, R. K.; Schepmoes, A. A.; et al. (2016). MPLEx: A robust and universal protocol for single-sample integrative proteomic, metabolomic, and lipidomic analyses. mSystems 2016, 1, e00043-16. [Google Scholar] [CrossRef] [PubMed] [Green Version].
- Pavel Baykalov, Bart Bussmann, Richard Nair, Abraham George Smith, Gernot Bodner, Ofer Hadar, Naftali Lazarovitch and Boris Rewald. (2023). Semantic segmentation of plant roots from RGB (mini-) rhizotron images— generalisation potential and false positives of established methods and advanced deep-learning models. Plant Methods 19:122. [CrossRef]
- Piovesan A., Vancauwenberghe V., Van De Looverbosch T., Verboven P., Nicolaï B. (2021). X-ray computed tomography for 3D plant imaging. Trends Plant Sci. 26:1171–85.
- Prakash I., Gangashetty Shruthi H., Belliappa, Naresh Bomma, Vinutha Kanuganahalli, Sobhan Babu Sajja, Sunita Choudhary, Ramanagouda Gaviyappanavar, Deekshitha Bomireddy, V. Anil Kumar, Jwala Pranati, Mamta Sharma and Manish K. Pandey (2024). Optimizing speed breeding and seed/ pod chip based genotyping techniques in pigeonpea: A way forward for high throughput line development.Gangashetty et al. Plant Methods. 20:27. [CrossRef]
- Rochfort S. (2005). Metabolomics reviewed: A new “omics” platform technology for systems biology and implications for natural products research. J. Nat. Prod. 68, 1813–1820. [Google Scholar] [CrossRef].
- Roper J. M., Jose F. G., and Hideaki T. (2021). Emerging Technologies for Monitoring Plant Health in Vivo. ACS Omega 2021, 6, 5101−5107. http://pubs.acs.org/journal/acsodf.
- Schmidt B., Jaroszewski J.W., Bro R., Witt M., Stærk D. (2008) Combining PARAFAC analysis of HPLC-PDA profiles and structural characterization using HPLCPDA-SPE-NMR-MS experiments: commercial preparations of St. John’s wort. Anal Chem. 80(6):1978–87.
- Schulze E. D., Beck E., Buchmann N., Clemens S., Müller-Hohenstein K., Scherer-Lorenzen M. Water defciency (Drought). In: Schulze E. D., Beck E., Buchmann N., Clemens S., Müller-Hohenstein K., Scherer-Lorenzen M., editors (2019). Plant ecology. Berlin: Springer, Berlin Heidelberg;. p. 165–202. [CrossRef]
- Shen S., Zhan C., Yang C., Fernie A. R., Luo J. (2023). Metabolomics-centered mining of plant metabolic diversity and function: past decade and future perspectives. Mol Plant. 16(1):43–63.
- Slavik Bohdan (1974). Methods of studying plant water relations. Acad. Publishing House of the Czechoslovak Acad. Of Sciences.
- Stearns J. C., Woody, O. Z., McConkey B. J., Glick B. R. (2012). Effects of bacterial ACC deaminase on Brassica napus gene expression. Mol. Plant-Microbe Interact. 25, 668–676. [Google Scholar] [CrossRef] [Green Version].
- Tang W., Guo H., Baskin C. C, Xiong W., Yang C., Li Z., et al. (2022). Effect of light intensity on morphology, photosynthesis and carbon metabolism of alfalfa (Medicago sativa) seedlings. Plants. 11(13):1688.
- Wang H., Peng J., Xie C., Bao Y., He Y. (2015). Fruit quality evaluation using spectroscopy technology: a review. Sensors. 15(5):11889–927.
- Wang Z., Verboven P., Nicolai B. (2017) Contrast-enhanced 3D micro-CT of plant tissues using diferent impregnation techniques. Plant Methods. 13:1–16.
- Weng S., Qiu M., Dong R., Wang F., Huang L., Zhang D., Zhao J. Fast detection of fenthion on fruit and vegetable peel using dynamic surface-enhanced Raman spectroscopy and random forests with variable selection. Spectrochim Acta Part A Mol Biomol Spectrosc. 2018;200:20–5.
- Wu Y., Li L., Liu L., Liu Y. (2019). Nondestructive measurement of internal quality attributes of apple fruit by using NIR spectroscopy. Multimed Tools Appl. 78(4):4179–95.
- Xiao H., Piovesan A., Pols S., Verboven P., Nicolaï B. (2021) Microstructural changes enhance oxygen transport in tomato (Solanum lycopersicum) fruit during maturation and ripening. New Phytol. 232:2043–56.
- Yang F., Feng L., Liu Q., Wu X., Fan Y., Raza M. A., et al. (2018). Effect of interactions between light intensity and red-to-far-red ratio on the photosynthesis of soybean leaves under shade condition. Environ Exp Bot. 150:79–87.
- Zhang Baohua Yunmeng Ou, Shuwan Yu, Yuchen Liu, Ying Liu and Wei Qiu. (2023). Gray mold and anthracnose disease detection on strawberry leaves using hyperspectral imaging. Zhang et al. Plant Methods 19:148. [CrossRef]
- Zhang J., Pu R., Huang W., Yuan L.; Luo J., Wang J. Using in-situ hyperspectral data for detecting and discriminating yellow rust disease from nutrient stresses. Field Crops Res. 2012, 134, 165−174.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).