Submitted:
13 June 2024
Posted:
13 June 2024
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. Kefir Grains Preparation
2.2. Repeated Batch Fermentations of 24 or 48 h
2.3. Microbiological and Chemical Characterization of the Fermented Beverages
2.4. Volatile Characterization
2.5. Odor Activity Value (OAV)
2.6. Aromatic Profile of the Fermented Milks
2.7. Antibacterial Activity Assay
2.8. Statistical Analyses
3. Results and Discussion
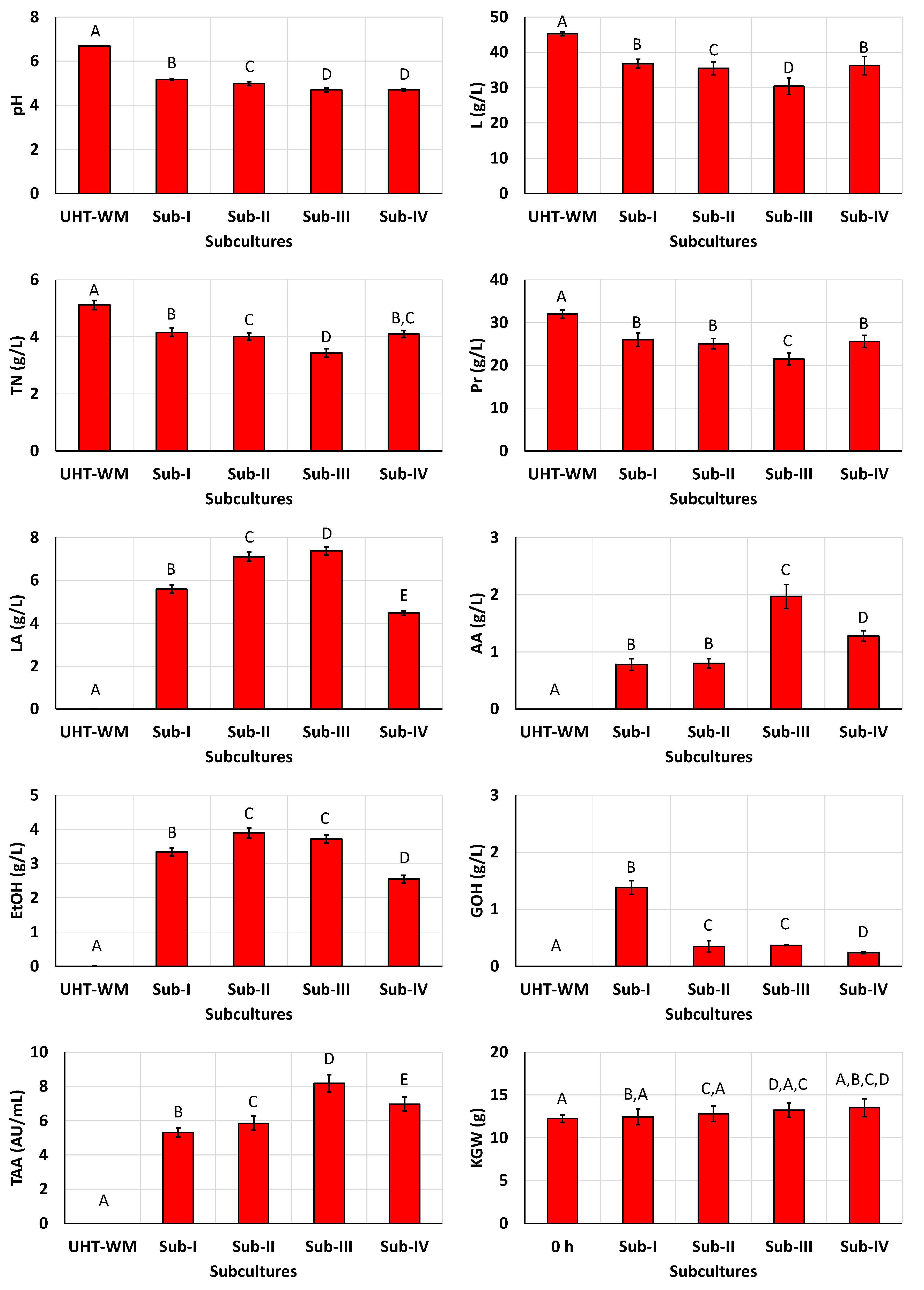
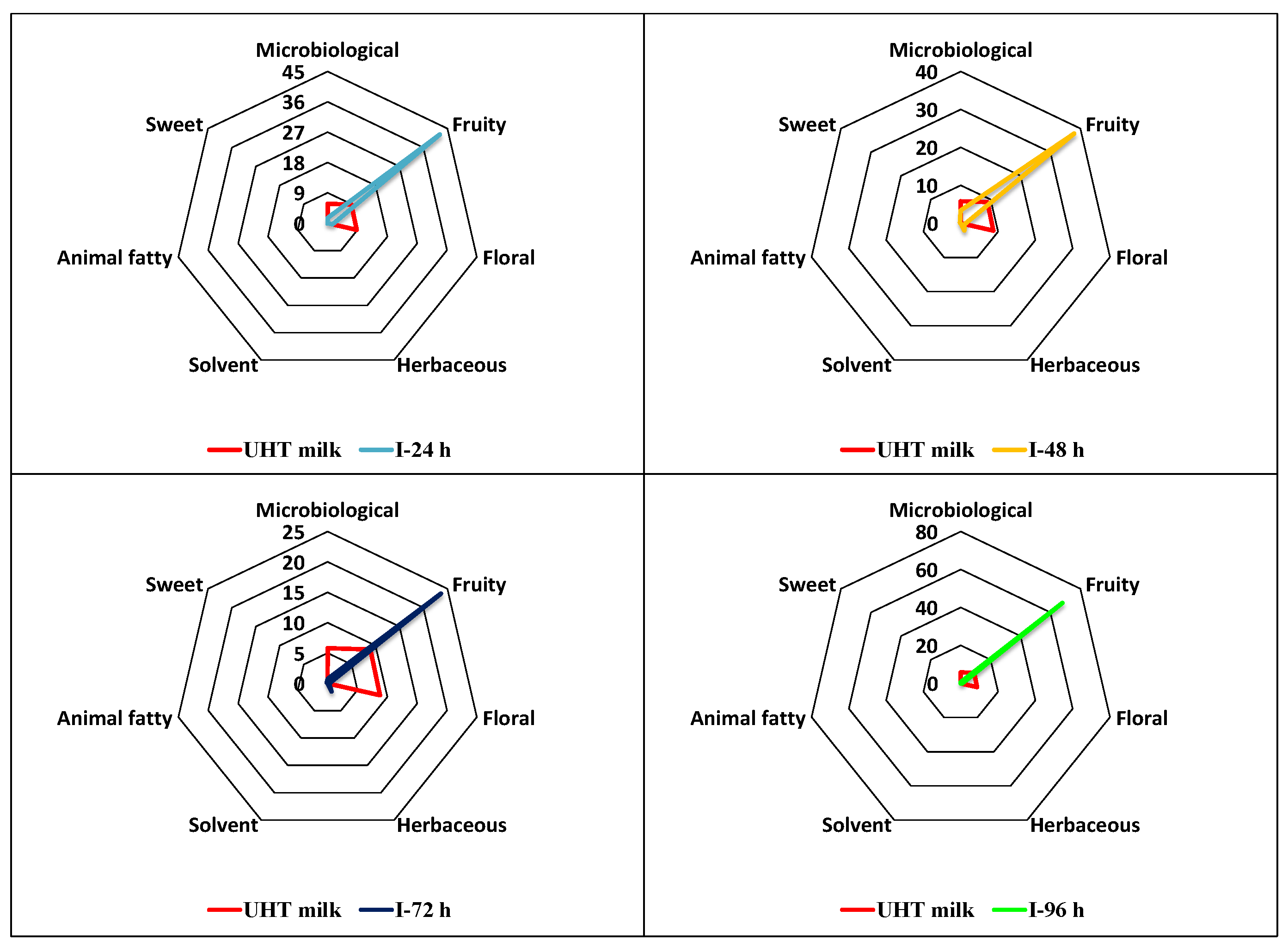
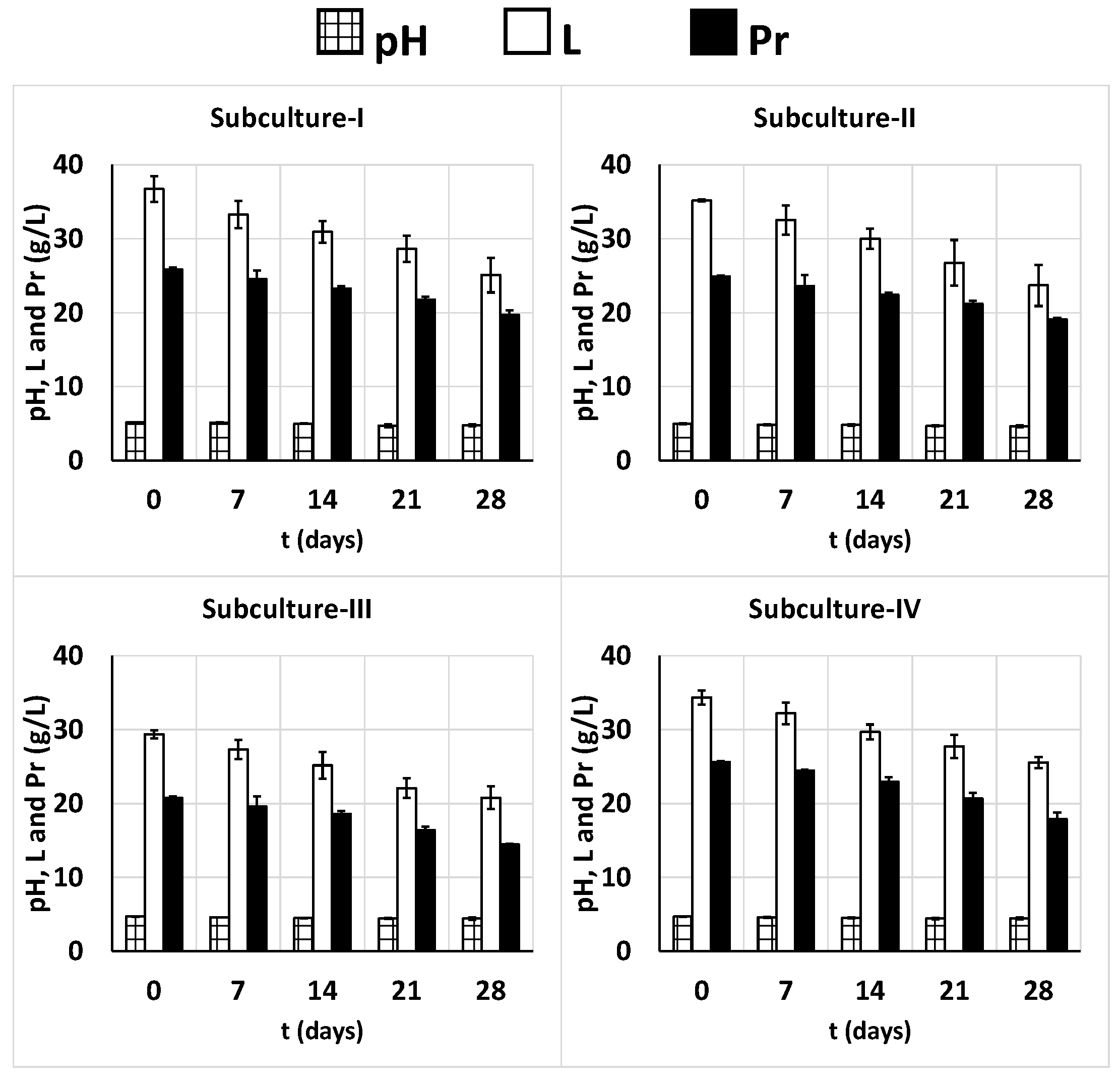
3.1. Static Fermentation of Whole Milk in Consecutive 24-h Batch Subcultures
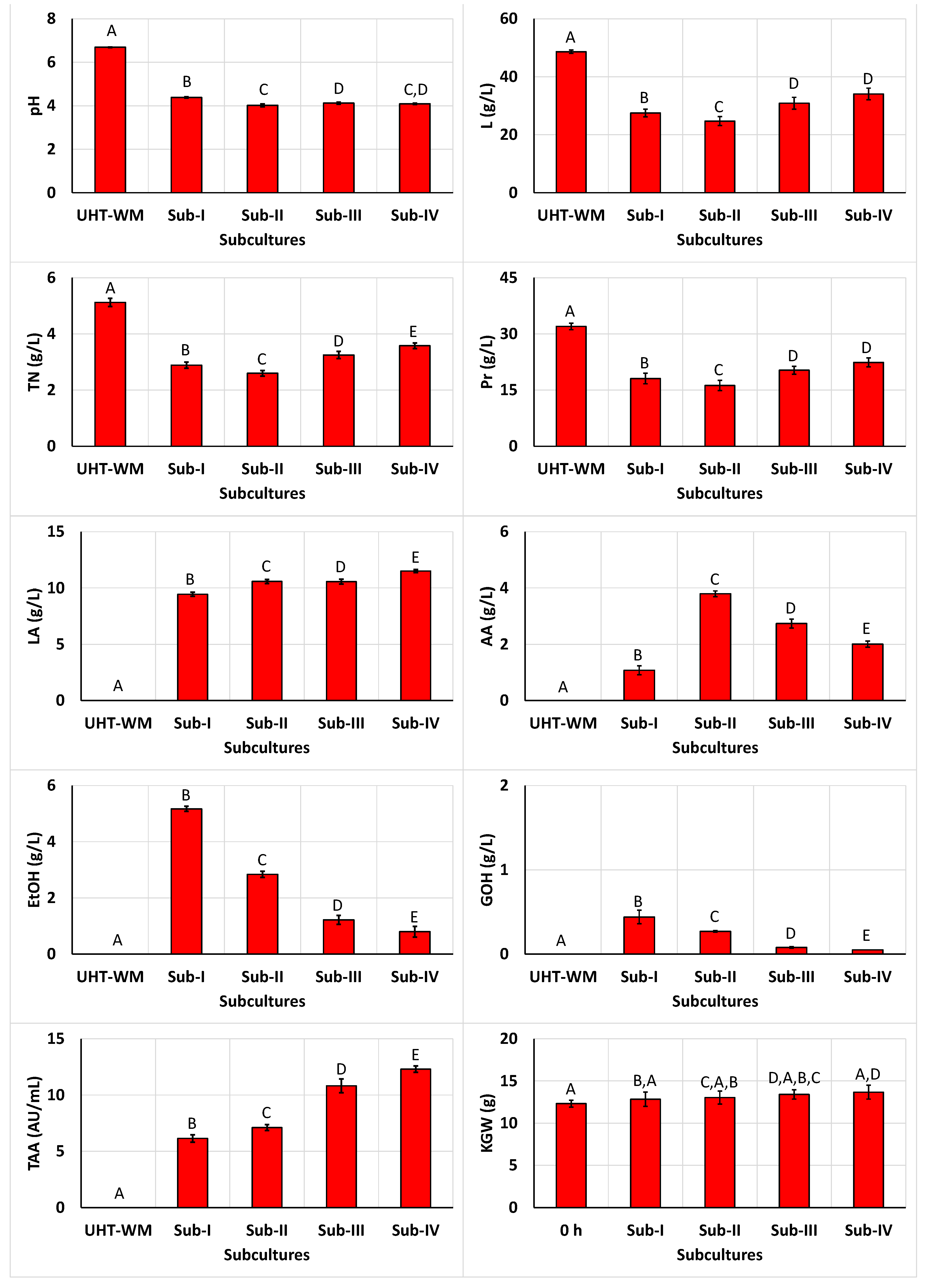
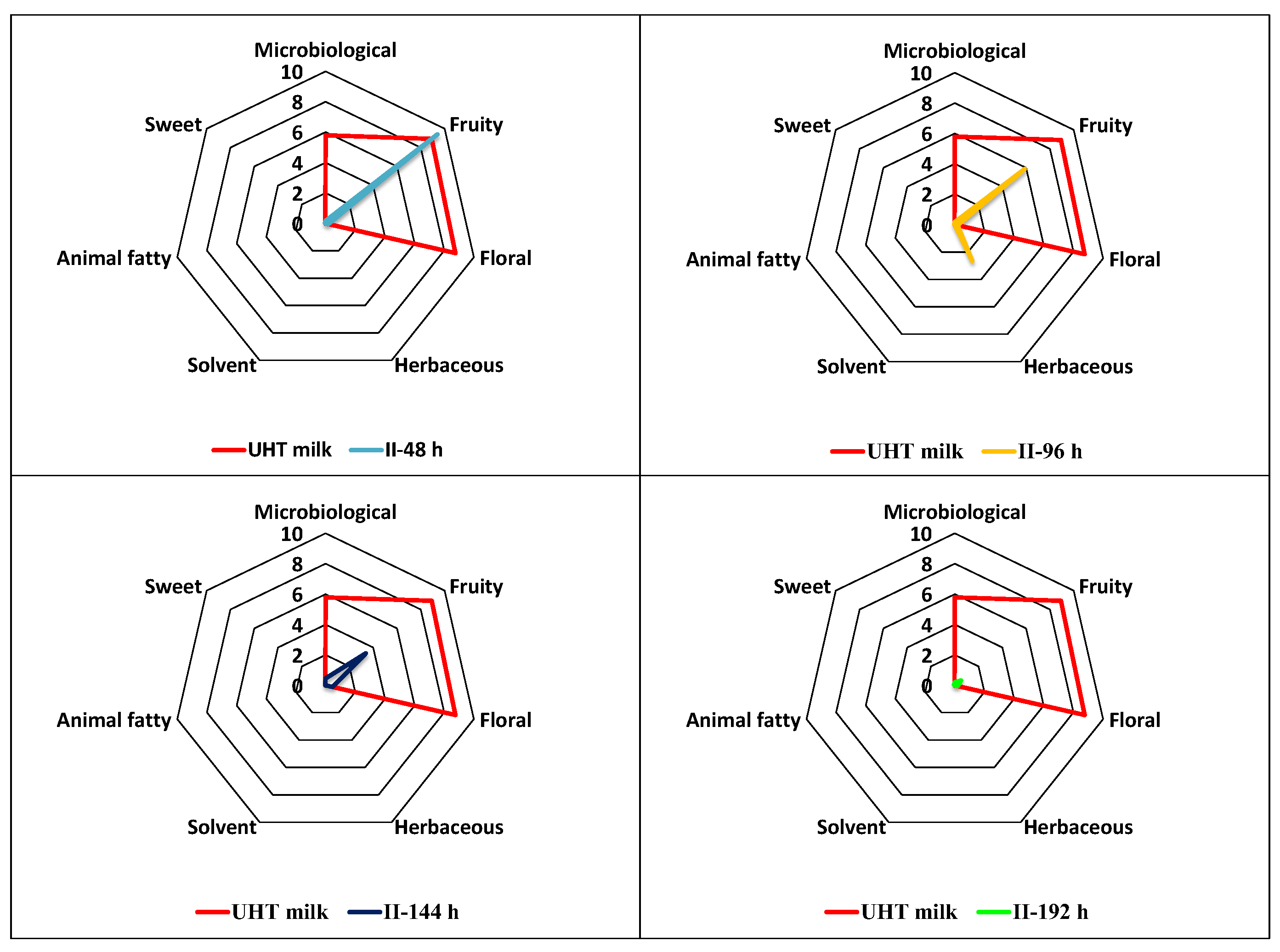
3.2. Static Fermentation of Whole Milk in Consecutive 48-h Batch Subcultures
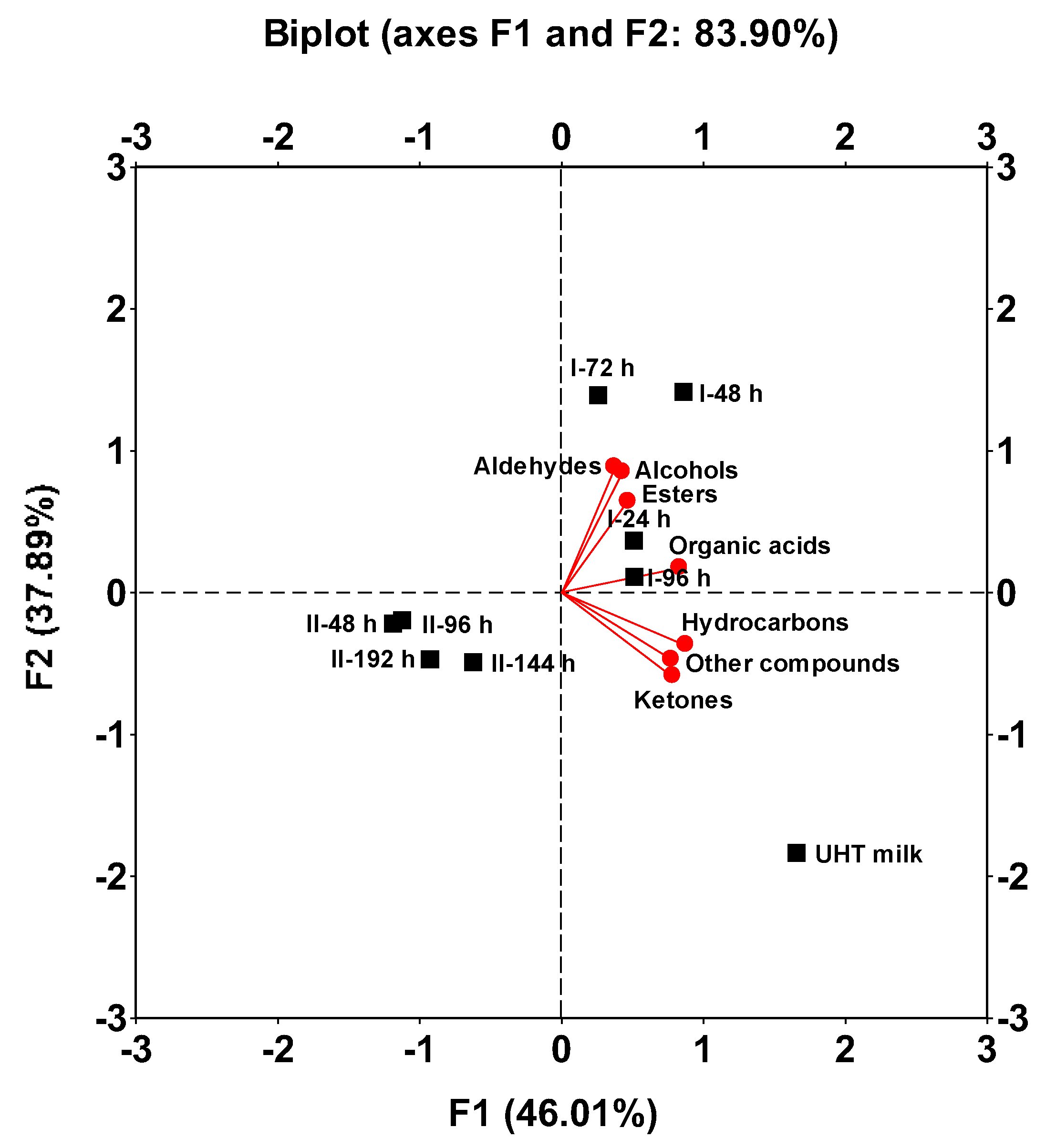
3.3. Volatile Composition of Beverages Obtained through Static Discontinuous Fermentations I and II
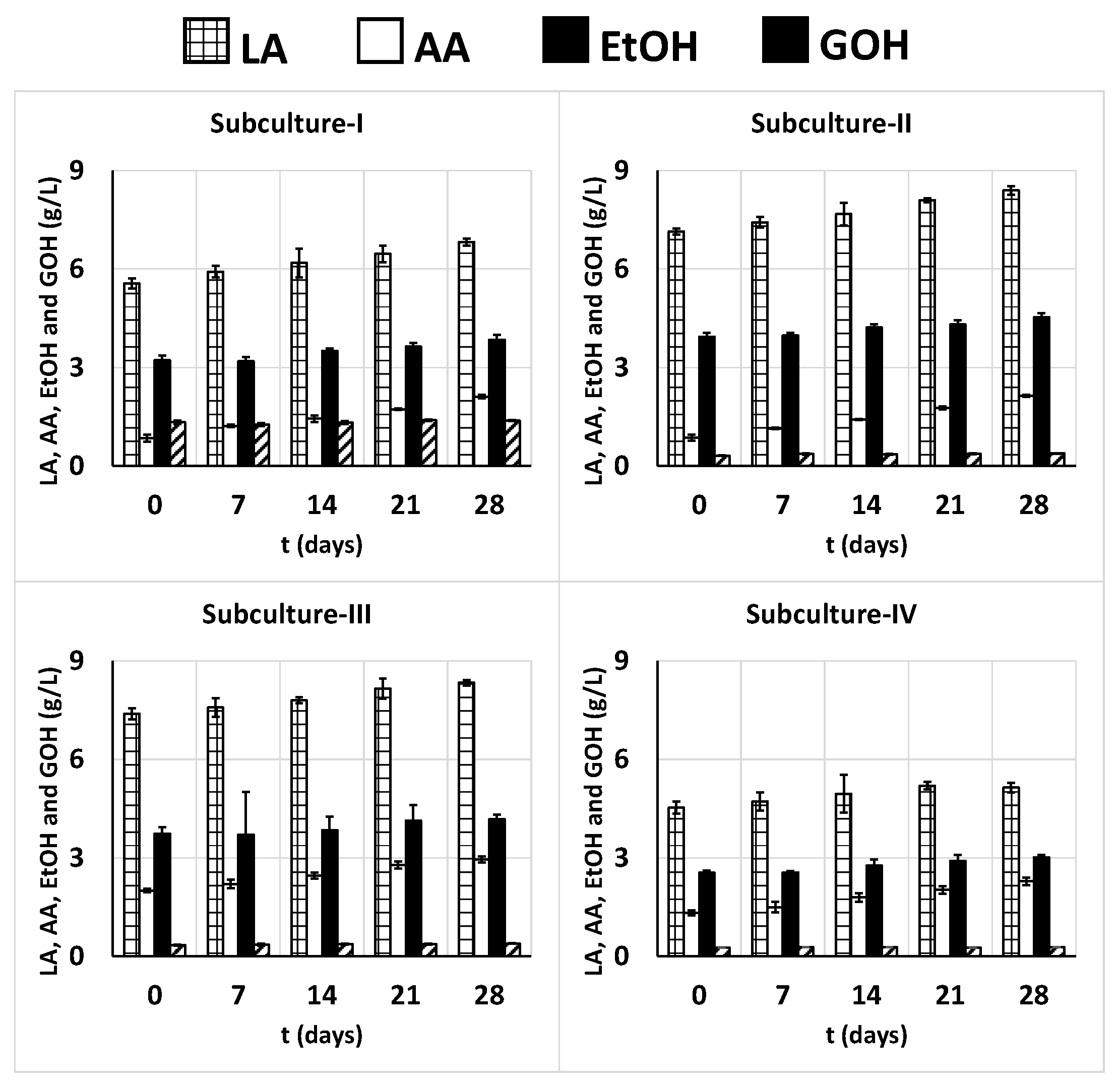
3.3.1. Organic Acids
3.3.2. Alcohols
3.3.3. Aldehydes
3.3.4. Ketones
3.3.5. Esters
3.3.6. Aromatic Hydrocarbons
3.3.7. Other Compounds
3.4. Analysis of the Effect of Incubation Time on the Volatile Composition of the Fermented Beverages
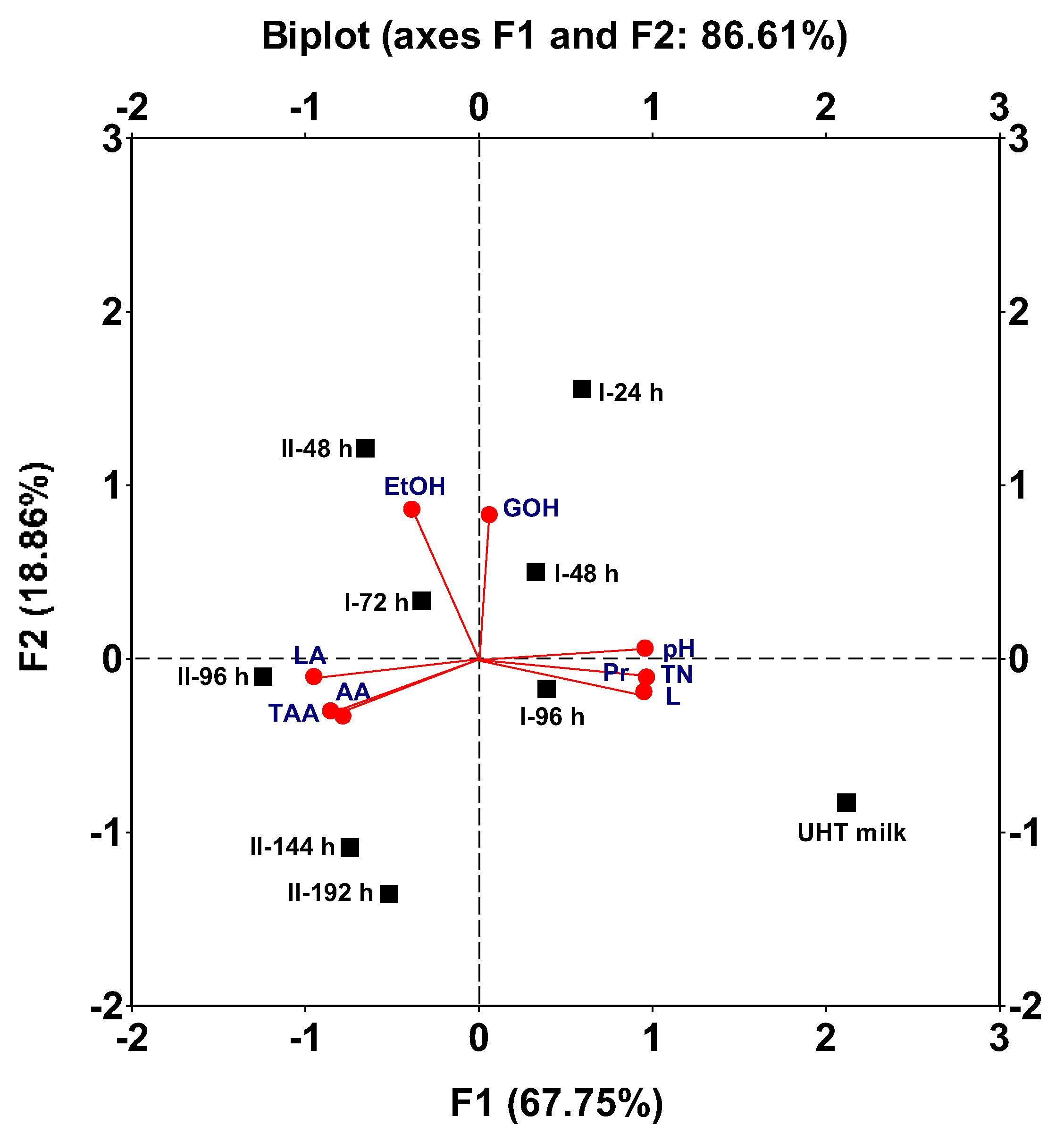
3.5. Analysis of the Aromatic Profile of the Fermented Milks
3.6. Comparison of the Chemical Composition of Unfermented Milk and the Beverages Obtained in the Repeated 24- and 48-h Subcultures
3.7. Comparison of the Volatile Composition of Unfermented Milk and the Beverages Obtained in the Repeated 24- and 48-h Subcultures
3.8. Microbiological Stability of the Beverages Obtained in the Repeated 24-h Subcultures
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teijeiro, M.; Pérez, P.F.; De Antoni, G.L.; Golowczyc, M.A. Suitability of Kefir Powder Production using Spray Drying. Food Res. Int. 2018, 112, 169–174. [Google Scholar] [CrossRef]
- Farag, M.A., Jomaa, S.A.; El-Wahed, A.A.; El-seedi, H.R. The Many Faces of Kefir Fermented Dairy Products: Quality Characteristics, Flavor Chemistry, Nutritional Value, Health Benefits, and Safety. Nutrients, 2020, 12, 1–21. [CrossRef]
- Guzel-Seydim, Z.B.; Çağlar, G.; Greene, A.K. A Comparison of Milk Kefir and Water Kefir: Physical, Chemical, Microbiological and Functional Properties. Trends Food Sci. Technol. 2021, 113, 42–53. [Google Scholar] [CrossRef]
- Ganatsios, V.; Nigam, P.; Plessas, S.; Terpou, A. Kefir as a Functional Beverage Gaining Momentum towards Its Health Promoting Attributes. Beverages 2021, 7, 48. [Google Scholar] [CrossRef]
- Bourrie, B.C.T.; Willing, B.P.; Cotter, P.D. The Microbiota and Health Promoting Characteristics of the Fermented Beverage Kefir. Frontiers Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef]
- Pawlos, M.; Znamirowska, A.; Szajnar, K.; Kalicka, D. The Influence of the Dose of Calcium Bisglycinate on Physicochemical Properties, Sensory Analysis, and Texture Profile of Kefirs during 21 Days of Cold Storage. Acta Sci. Pol. Technol. Aliment. 2016, 15, 37–45. [Google Scholar] [CrossRef]
- Singh, G.; Muthukumarappan, K. Influence of Calcium Fortification on Sensory, Physical, and Rheological Characteristics of Fruit Yogurt. LWT - Food Sci. Technol. 2008, 41, 1145–1152. [Google Scholar] [CrossRef]
- Dertli, E.; Çon, A.H. Microbial Diversity of Traditional Kefir Grains and Their Role in Kefir Aroma. LWT - Food Sci. Technol. 2017, 85, 151–157. [Google Scholar] [CrossRef]
- Tzavaras, D.; Papadelli, M.; Ntaikou, I. From Milk Kefir to Water Kefir: Assessment of Fermentation Processes, Microbial Changes and Evaluation of the Produced Beverages. Fermentation 2022, 8, 135. [Google Scholar] [CrossRef]
- Bazán, D.L.; G. del Río, P.; Domínguez, J.M.; Cortés-Diéguez, S.; Mejuto, J.C.; Pérez-Guerra, N. The Chemical, Microbiological and Volatile Composition of Kefir-like Beverages Produced from Red Table Grape Juice in Repeated 24-h Fed-Batch Subcultures. Foods 2022, 11, 3117. [Google Scholar] [CrossRef]
- Randazzo, W.; Corona, O.; Guarcello, R.; Francesca, N.; Germanà, M.A.; Erten, H.; Moschetti, G.; Settanni, L. Development of New Non-Dairy Beverages from Mediterranean Fruit Juices fermented with Water Kefir Microorganisms. Food Microbiol. 2016, 54, 40–51. [Google Scholar] [CrossRef]
- Clarke, H.J.; Mannion, D.T.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Development of a Headspace Solid-Phase Microextraction Gas Chromatography Mass Spectrometry Method for the Quantification of Volatiles Associated with Lipid Oxidation in Whole Milk Powder using Response Surface Methodology. Food Chem. 2019, 292, 75–80. [Google Scholar] [CrossRef]
- García, M.; Esteve-Zarzoso, B.; Crespo, J.; Cabellos, J.M.; Arroyo, T. Influence of Native Saccharomyces cerevisiae Strains from D.O. “Vinos de Madrid” in the Volatile Profile of White Wines. Fermentation 2019, 5, 94. [Google Scholar] [CrossRef]
- Thomsen, M.; Martin, C.; Mercier, F.; Tournayre, P.; Berdagué, J.L.; Thomas-Danguin, T.; Guichard, E. Investigating Semi-Hard Cheese Aroma: Relationship between Sensory Profiles and Gas Chromatography-Olfactometry Data. Int. Dairy J. 2012, 26, 41–49. [Google Scholar] [CrossRef]
- Sánchez-Palomo, E.; Trujillo, M.; García Ruiz, A.; González Viñas, M.A. Aroma Profile of Malbec Red Wines from La Mancha Region: Chemical and Sensory Characterization. Food Res. Int. 2017, 100, 201–208. [Google Scholar] [CrossRef]
- Costas, M.; Alonso, E.; Guerra, N.P. Nisin Production in Realkalized Fed-Batch Cultures in Whey with Feeding with Lactose- or Glucose-Containing Substrates. Appl. Microbiol. Biotechnol. 2016, 100, 7899–7908. [Google Scholar] [CrossRef]
- Costas, M.; Alonso, E.; Bazán, D.L.; Bendaña, R.J.; Guerra, N.P. Batch and Fed-Batch Production of Probiotic Biomass and Nisin in Nutrient-Supplemented Whey Media. Braz. J. Microbiol. 2019, 50, 915–925. [Google Scholar]
- Costas, M.; Alonso, E.; Bendaña, R.J.; Guerra, N.P. The Joint Effect of pH Gradient and Glucose Feeding on the Growth Kinetics of Lactococcus lactis CECT 539 in Glucose-Limited Fed-Batch Cultures. Polish J. Microbiol. 2019, 68, 269–280. [Google Scholar]
- Valli, M.; Sauer, M.; Branduardi, P.; Borth, N.; Porro, D.; Mattanovich, D. Improvement of Lactic Acid Production in Saccharomyces cerevisiae by Cell Sorting for High Intracellular pH. Appl. Environ. Microbiol. 2006, 72, 5492–549. [Google Scholar] [CrossRef]
- Yalçin, S.K.; Özbaş, Z.Y. Determination of Growth and Glycerol Production Kinetics of a Wine Yeast Strain Saccharomyces cerevisiae Kalecik 1 in Different Substrate Media. World J. Microbiol. Biotechnol. 2005, 21, 1303–1310. [Google Scholar] [CrossRef]
- Azhar, S.H.M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Faik, A.A.M.; Rodrigues, K.F. Yeasts in Sustainable Bioethanol Production: A Review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B. B.; Lonvaud, AA.; Darriet, P.; Towey, J. Handbook of Enology. 3rd ed.; John Wiley & Sons Ltd: Chichester, UK, 2021; Volume 1: The Microbiology of Wine and Vinifications; pp. 229–241.
- Neysens, P.; Messens, W.; Gevers, D.; Swings, J.; De Vuyst, L. Biphasic Kinetics of Growth and Bacteriocin Production with Lactobacillus amylovorus DCE 471 Occur Under Stress Conditions. Microbiol. 2003, 149, 1073–1082. [Google Scholar] [CrossRef]
- Yang, R.; Ray, B. Factors Influencing Production of Bacteriocins by Lactic Acid Bacteria. Food Microbiol. 1994, 11, 281–291. [Google Scholar] [CrossRef]
- Moradi, Z.; Kalanpour, N. Kefiran, a Branched Polysaccharide: Preparation, Properties, and Applications: A Review. Carbohydr. Polym. 2019, 223, 115100. [Google Scholar]
- Sayes, C.; Leyton, Y.; Riquelme, C. Probiotic Bacteria as a Healthy Alternative for Fish Aquaculture. In Antibiotic Use in Animals, 1st ed.; Savic, S., Ed.; InTechOpen Limited, London, UK, 2018; Chapter 7, pp. 115–132.
- Gallina, D.A.; Barbosa, P.P.M.; Ormenese, R.C.S.C.; Garcia, A.O. Development and Characterization of Probiotic Fermented Smoothie Beverage. Rev. Ciênc. Agron. 2019, 50, 378–386. [Google Scholar]
- Poolman, B.; Konings, W.N. Relation of Growth of Streptococcus lactis and Streptococcus cremoris to Amino Acid Transport. J. Bacteriol. 1988, 170, 700–707. [Google Scholar] [CrossRef]
- Fajardo, P.; Rodríguez, I.; Pastrana, L.; Guerra, N.P. Production of a Potentially Probiotic Culture of Lactobacillus casei subsp. casei CECT 4043 in Whey. Int. Dairy J. 2008, 18, 1057–1065. [Google Scholar]
- Membré, J.M.; Kubaczka, M.; Chéné, C. Combined Effects of pH and Sugar on Growth Rate of Zygosaccharomyces rouxii, a Bakery Product Spoilage Yeast. Appl. Environ. Microbiol. 1999, 65, 4921–4925. [Google Scholar] [CrossRef]
- Arroyo, F.N.; Orlić, S.; Querol, A.; Barrio, E. Effects of Temperature, pH, and Sugar Concentration on the Growth Parameters of Saccharomyces cerevisiae, S. kudriavzevii and Their Interspecific Hybrid. Int. J. Food Microbiol. 2009, 131, 120–127. [Google Scholar] [CrossRef]
- Oude, S.J.W.H.; Krooneman, J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic Conversion of Lactic Acid to Acetic Acid and 1,2-Propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef]
- Outeiriño, D.; Costa-Trigo, I.; Ochogavias, A.; Pinheiro de Souza Oliveira, R.; Guerra, N.P.; Salgado, J.M.; Domínguez, J.M. Biorefinery of Brewery Spent Grain to obtain Bioproducts with High Value-added in the Market. New Biotechnol. 2024, 79, 111–119. [Google Scholar] [CrossRef]
- Guerra, N.P.; Pastrana, L. Enhancement of Nisin Production by Lactococcus lactis in Periodically Re-Alkalized Cultures. Proc. Biochem. 2002, 37, 1005–1015. [Google Scholar] [CrossRef]
- Tada, S.; Katakura, Y.; Ninomiya, K.; Shioya, S. Fed-Batch Coculture of Lactobacillus kefiranofaciens with Saccharomyces cerevisiae for Effective Production of Kefiran. J. Biosci. Bioeng. 2007, 103, 557–562. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Radchabut, S. Use of Whey Lactose from Dairy Industry for Economical Kefiran Production by Lactobacillus kefiranofaciens in Mixed Cultures with Yeasts. New Biotechnol. 2011, 28, 574–580. [Google Scholar] [CrossRef]
- Felipe, M.G.; Vieira, D.C.; Vitolo, M.; Silva, S.S.; Roberto, I.C.; Manchilha, I.M. Effect of Acetic Acid on Xylose Fermentation to Xylitol by Candida guilliermondii. J. Basic Microbiol. 1995, 35, 171–177. [Google Scholar] [CrossRef]
- Kirtadze, E.; Nutsubidze, N. Metabolic Potential of Alcoholic Fermentation Yeasts. Bull. Georgian Natl. Acad. Sci. 2009, 3, 110–116. [Google Scholar]
- Gomes, R.J.; Borges, M.F.; Rosa, M.F.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic Acid Bacteria in the Food Industry: Systematics, Characteristics and Applications. Food Technol. Biotechnol. 2018, 56, 139–151. [Google Scholar] [CrossRef]
- Garai-Ibabe, G.; Ibarburu, I.; Berregi, I.; Claisse, O.; Lonvaud-Funel, A.; Irastorza, A.; Dueñas, M.T. Glycerol Metabolism and Bitterness Producing Lactic Acid Bacteria in Cidermaking. Int. J. Food Microbiol. 2008, 121, 253–261. [Google Scholar] [CrossRef]
- Mamlouk, D.; Gullo, M. Acetic Acid Bacteria: Physiology and Carbon Sources Oxidation. Indian J. Microbiol., 2013, 53, 377–384. [Google Scholar] [CrossRef]
- van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and other Media. 2nd ed.; Oliemans Punter & Partners BV, The Netherlands, 2011.
- Escudero, A.; Gogorza, B.; Melús, M.A.; Ortín, N.; Cacho, J.A.; Ferreira, V. Characterization of the Aroma of a Wine from Maccabeo. Key Role played by Compounds with Low Odor Activity Values. J. Agric. Food Chem. 2004, 52, 3516–3524. [Google Scholar] [CrossRef]
- Siebert, T.E.; Smyth, H.E.; Capone, D.L.; Neuwöhner, C.; Pardon, K.H.; Skouroumounis, G.K.; Herderich, M.J.; Sefton, M.A.; Pollnitz, A.P. Stable Isotope Dilution Analysis of Wine Fermentation Products by HS-SPME-GC-MS. Anal. Bioanal. Chem. 2005, 381, 937–947. [Google Scholar] [CrossRef]
- Pereira, R.; Resende, D.; Alencar, A.C.; de Abreu, L.R.; Ferreira, W. Survival of Kluyveromyces lactis and Torulaspora delbrueckii to Simulated Gastrointestinal Conditions and their Use as Single and Mixed Inoculum for Cheese Production. Food Res. Int. 2019, 125, 108620. [Google Scholar]
- Czerny, M.; Schieberle, P. Important Aroma Compounds in Freshly Ground Wholemeal and White Wheat Flour Identification and Quantitative Changes during Sourdough Fermentation. J. Agric. Food Chem. 2002, 50, 6835–6840. [Google Scholar] [CrossRef]
- Li, B.; Gao, X.; Li, N.; Mei, J. Fermentation Process of Mulberry Juice-Whey based Tibetan Kefir Beverage Production. Czech J. Food Sci. 2018, 36, 494–501. [Google Scholar] [CrossRef]
- Meilgaard, M.C. Flavor Chemistry of Beer: Part II: Flavor and Threshold of 239 Aroma Volatiles. Tech. Q. Master Brew. Assoc. Am. 1975, 12, 151–168. [Google Scholar]
- Dertli, E.; Çon, A.H. Microbial Diversity of Traditional Kefir Grains and their Role on Kefir Aroma. LWT-Food Sci. Technol. 2017, 85, 151–157. [Google Scholar] [CrossRef]
- Vinh, T.; Schwartz, G.; Moll, M. Identification of 2-Ethyl-Hexanoic (2-Ethyl-Caproic) Acid in Beer. J. Am. Soc. Brew. Chem. 1981, 39, 2–5. [Google Scholar] [CrossRef]
- Oliveira, D.R.; Lopes, A.C.A.; Pereira, R.A.; Cardoso, P.G.; Duarte, W.F. Selection of Potentially Probiotic Kluyveromyces lactis for the Fermentation of Cheese Whey–based Beverage. Ann. Microbiol. 2019, 69, 1361–1372. [Google Scholar] [CrossRef]
- Walsh, A.M.; Crispie, F.; Kilcawley, K.; O’Sullivan, O.; O’Sullivan, M.G.; Claesson, M.J.; Cotter, P.D. Microbial Succession and Flavor Production in the Fermented Dairy Beverage Kefir. MSystems 2016, 1, e00052–16. [Google Scholar] [CrossRef]
- Tian, H.; Shi, Y.; Zhang, Y.; Yu, H.; Mu, H.; Chen, C. Screening of Aroma–Producing Lactic Acid Bacteria and their Application in improving the Aromatic Profile of Yogurt. J. Food Biochem. 2019, 43, e12837. [Google Scholar] [CrossRef]
- Clarke, H.J.; Mannion, D.T.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Development of a Headspace Solid-Phase Microextraction Gas Chromatography Mass Spectrometry Method for the Quantification of Volatiles associated with Lipid Oxidation in Whole Milk Powder using Response Surface Methodology. Food Chem. 2019, 292, 75–80. [Google Scholar] [CrossRef]
- Hayaloglu, A.A.; Brechany, E.Y.; Deegan, K.C.; McSweeney, P.L.H. Characterization of the Chemistry, Biochemistry and Volatile Profile of Kuflu Cheese, a Mould-ripened Variety. LWT-Food Sci. Technol. 2008, 41, 1323–1334. [Google Scholar] [CrossRef]
- Zheng, X.; Li, K.; Shi, X.; Ni, Y.; Li, B.; Zhuge, B. Potential Characterization of Yeasts isolated from Kazak Artisanal Cheese to produce Flavoring Compounds. MicrobiologyOpen. 2018, 7, e00533. [Google Scholar] [CrossRef]
- Salgado, J.M.; González, C.; Rodríguez, R.; Simal, J.; Domínguez, J.M.; Cortés, S. Study of the Volatile Compounds produced by Debaryomyces hansenii NRRL Y-7426 during the Fermentation of Detoxified Concentrated Distilled Grape Marc Hemicellulosic Hydrolysates. World J. Microbiol. Biotechnol. 2012, 28, 3123–3134. [Google Scholar] [CrossRef]
- Pico, J.; Martínez, M.M.; Bernal, J.; Gómez, M. Evolution of Volatile Compounds in Gluten-free Bread: From Dough to Crumb. Food Chem. 2017, 227, 179–186. [Google Scholar] [CrossRef]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Re-investigation on Odour Thresholds of Key Food Aroma Compounds and Development of an Aroma Language based on Odour Qualities of defined Aqueous Odorant Solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, W.; Yu, W.; Zhao, L.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, L.; Wang, S. Study on the Volatile Composition of Table Grapes of Three Aroma Types. LWT-Food Sci. Technol. 2019, 115, 108450. [Google Scholar] [CrossRef]
- Chastrette, M. , Cretin, D., El Aïdi, C. El. Structure-Odor Relationships: Using Neural Networks in the Estimation of Camphoraceous or Fruity Odors and Olfactory Thresholds of Aliphatic Alcohols. J. Chem. Inf. Comput. Sci. 1996, 36, 108–113. [Google Scholar] [CrossRef]
- Komes, D.; Ulrich, D.; Lovric, T. Characterization of Odor-Active Compounds in Croatian Rhine Riesling Wine, Subregion Zagorje. Eur. Food Res. Technol. 2006, 222, 1–7. [Google Scholar] [CrossRef]
- Pionnier, E.; Hugelshofer, D. Characterisation of Key Odorant Compounds in Creams from Different Origins with Distinct Flavours. Dev. Food Sci. 2006, 43, 233–236. [Google Scholar]
- Sarhir, S.T.; Amanpour, A.; Bouseta, A.; Selli, S. Key Odorants of a Moroccan fermented Milk Product “Lben” using Aroma Extract Dilution Analysis. J. Food Sci. Technol. 2019, 56, 3836–3845. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. A Paradigm of Volatile Aroma Compounds in Rice and their Product with Extraction and Identification Methods: A Comprehensive Review. Food Res. Int. 2020, 130, 108924. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz, C. Quantitative Analysis of Headspace Volatile Compounds using Comprehensive Two-dimensional Gas Chromatography and their Contribution to the Aroma of Chardonnay Wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Lević, S.; Petrović, T.; Ivanović, S.; Nedović, V.; Kourkoutas, Y. Encapsulation of Lactobacillus casei ATCC 393 in Alginate Capsules for Probiotic fermented Milk Production. LWT-Food Sci. Technol. 2019, 116, 108501. [Google Scholar] [CrossRef]
- Bingham, E.; Cohrssen, B.; Powell, C.H. Patty's Toxicology. Volume 6, 5th ed.; John Wiley & Sons. Inc. New York, USA, 2001; pp. 318.
- Dan, T.; Chen, H.; Li, T.; Tian, J.; Ren, W.; Zhang, H.; Sun, T. Influence of Lactobacillus plantarum P-8 on Fermented Milk Flavor and Storage Stability. Front. Microbiol. 2019, 9, 3133. [Google Scholar] [CrossRef]
- Montgomery, J.H. Groundwater Chemicals, 4th ed.; Taylor & Francis Group. Boca Raton, Florida, USA, 2007; pp. 718.
- Liu, S.Q.; Holland, R.; Crow, V.L. Esters and their Biosynthesis in Fermented Dairy Products: A Review. Int. Dairy J. 2004, 14, 923–945. [Google Scholar] [CrossRef]
- Gil, M.; Cabellos, J.M.; Arroyo, T.; Prodanov, M. Characterization of the Volatile Fraction of Young Wines from the Denomination of Origin “Vinos de Madrid” (Spain). Anal. Chim. Acta. 2006, 563, 145–153. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative Study of Aromatic Compounds in Two Young White Wines subjected to Prefermentative Cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R.; Cacho, J.F. Quantitative Determination of the Odorants of Young Red Wines from Different Grape Varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli´s Handbook of Flavour Ingredients, 6th ed.; CRC Press. Taylor & Francis Group LLC, Boca Raton, Florida, USA, 2010.
- Nikolaou, A.; Tsakiris, A.; Kanellaki, M.; Bezirtzoglou, E.; Akrida-Demertzi, K.; Kourkoutas, Y. Wine Production using Free and Immobilized Kefir Culture on Natural Supports. Food Chem. 2019, 272, 39–48. [Google Scholar] [CrossRef]
- Cheng, H. Volatile Flavor Compounds in Yogurt: A Review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef]
- Curioni, P.M.G.; Bosset, J.O. Key Odorants in Various Cheese Types as determined by Gas Chromatography-Olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Bugaud, C.; Alter, P. Volatile and Non-Volatile Compounds as Odour and Aroma Predictors in Dessert Banana (Musa spp.). Postharvest Biol. Technol. 2016, 112, 14–23. [Google Scholar] [CrossRef]
- López, M.L.; Villatoro, C.; Fuentes, T.; Graell, J.; Lara, I.; Echeverría, G. Volatile Compounds, Quality Parameters and Consumer Acceptance of ‘Pink Lady®’ Apples stored in Different Conditions. Postharvest Biol. Technol. 2007, 43, 55–66. [Google Scholar] [CrossRef]
- Zepka, L.Q.; Garruti, D.S.; Sampaio, K.L.; Mercadante, A.Z.; Da Silva, M.A.A.P. Aroma Compounds derived from the Thermal Degradation of Carotenoids in a Cashew Apple Juice Model. Food Res. Int. 2014, 56, 108–114. [Google Scholar] [CrossRef]
- Barron, L.; Redondo, Y.; Aramburu, M.; Pérez-Elortondo, F.J.; Albisu, M.; Nájera, A.I.; de Renobales, M. Variations in Volatile Compounds and Flavour in Idiazabal Cheese Manufactured from Ewe’s Milk in Farmhouse and Factory. J. Sci. Food Agric. 2005, 85, 1660–1671. [Google Scholar] [CrossRef]
- Hinge, V.R.; Patil, H.B.; Nadaf, A.B. Aroma Volatile Analyses and 2AP Characterization at Various Developmental Stages in Basmati and Non-Basmati Scented Rice (Oryza sativa L.) cultivars. Rice. 2016, 9, 38. [Google Scholar] [CrossRef]
- Dan, T.; Wang, D.; Jin, R.L.; Zhang, H.P.; Zhou, T.T.; Sun, T.S. Characterization of Volatile Compounds in Fermented Milk using Solid-Phase Microextraction Methods coupled with Gas Chromatography-Mass Spectrometry. J. Dairy Sci. 2017, 100, 2488–2500. [Google Scholar] [CrossRef]
- Dan, T.; Wang, D.; Wu, S.; Jin, R.; Ren, W.; Sun, T. Profiles of Volatile Flavor Compounds in Milk Fermented with Different Proportional Combinations of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus. Molecules. 2017, 22, 1633. [Google Scholar]
- Pan, D.D.; Wu, Z.; Peng, T.; Zeng, X.Q.; Li, H. Volatile Organic Compounds Profile during Milk Fermentation by Lactobacillus pentosus and Correlations between Volatiles Flavor and Carbohydrate Metabolism. J. Dairy Sci. 2014, 97, 624–631. [Google Scholar] [CrossRef]
- Kesler, M.K.; González-Orozco, B.D.; Barringer, S.A.; Alvarez, V.B. Mitigation of Undesirable Volatile Aroma Compounds in Kefir by Freeze Drying and Vacuum Evaporation. J. Food Sci. 2023, 88, 3216–3227. [Google Scholar] [CrossRef]
- Aghlara, A.; Mustafa, S.; Manap, Y.A.; Mohamad, R. Characterization of Headspace Volatile Flavor Compounds formed During Kefir Production: Application of Solid Phase Microextraction. Int. J. Food Prop. 2009, 12, 808–818. [Google Scholar] [CrossRef]
- Valero, E.; Villamiel, M.; Miralles, B.; Sanz, J.; Martı́nez-Castro, I. Changes in Flavour and Volatile Components during Storage of Whole and Skimmed UHT Milk. Food Chem. 2001, 72, 51–58. [Google Scholar] [CrossRef]
- Fang, X.; Guo, L.W.; Chen, H.; Ke, W.C.; Guo, W.; Guo, X.S.; Zhang, Y. Characteristics of Volatile Flavor Components in Traditional Fermented Yak Milk Produced in Different Ecoregions of the Qinghai-Tibetan Plateau. J. Dairy Sci. 2020, 103, 191–200. [Google Scholar] [CrossRef]
- Delgado, F.J.; González-Crespo, J.; Cava, R.; Ramírez, R. Formation of the Aroma of a Raw Goat Milk Cheese during Maturation analysed by SPME–GC–MS. Food Chem. 2011, 129, 1156–1163. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Chen, W.; Zhong, Q.; Zhang, G.; Chen, W. Beneficial Effects of Tomato Juice Fermented by Lactobacillus plantarum and Lactobacillus casei: Antioxidation, Antimicrobial Effect, and Volatile Profiles. Molecules. 2018, 23, 2366. [Google Scholar] [CrossRef]
- Zareba, D.; Ziarno, M.; Obiedzinski, M. Volatile Profile of Non-Fermented Milk and Milk Fermented by Bifidobacterium animalis subsp. lactis. Int. J. Food Prop. 2012, 15, 1010–1021. [Google Scholar] [CrossRef]
- Molimard, P.; Spinnler, H.E. Review: Compounds involved in the Flavor of Surface Mold-Ripened Cheeses: Origins and Properties. J. Dairy Sci. 1996, 79, 169–184. [Google Scholar] [CrossRef]
- Arslan, S.; Mutlu, C.; Candal, C.; Erbaş, M. Microbiological and Chemical Properties of Wet Tarhana produced by Different Dairy Products. J. Food Sci. Technol. 2018, 55, 4770–4781. [Google Scholar] [CrossRef]
- Tu, C.; Azi, F.; Huang, J.; Xu, X.; Xing, G.; Dong, M. Quality and Metagenomics Evaluation of a Novel Functional Beverage produced from Soy Whey using Water Kefir Grains. LWT-Food Sci. Technol. 2019, 113, 108258. [Google Scholar] [CrossRef]
- Rotsatchakul, P.; Visesanguan, W.; Smitinont, T.; Chaiseri, S. Changes in Volatile Compounds during Fermentation of Nham (Thai Fermented Sausage). Int. Food Res. J. 2009, 16, 391–414. [Google Scholar]
- Chen, Y.; Xu, H.; Ding, S.; Zhou, H.; Qin, D.; Deng, F.; Wang, R. Changes in Volatile Compounds of Fermented Minced Pepper during Natural and Inoculated Fermentation Process based on Headspace–Gas Chromatography–Ion Mobility Spectrometry. Food Sci. Nut. 2020, 8, 3362–3379. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, X. , Li, Y.; Chen, J.; Chen, X. Microbial Interactions and Dynamic Changes of Volatile Flavor Compounds during the Fermentation of Traditional Kombucha. Food Chem. 2024, 430, 137060. [Google Scholar] [CrossRef]
- Liang, J.; Yoo, M.J.Y.; Seale, B.; Grazioli, G. Nutritional and Volatile Characterisation of Milk Inoculated with Thermo-Tolerant Lactobacillus bulgaricus through Adaptive Laboratory Evolution. Foods 2021, 10, 2944. [Google Scholar] [CrossRef]
- Buran, I.; Akal, C.; Ozturkoglu-Budak, S.; Yetisemiyen, A. Rheological, Sensorial and Volatile Profiles of Synbiotic Kefirs produced from Cow and Goat Milk Containing Varied Probiotics in Combination with Fructooligosaccharide. LWT-Food Sci. Technol. 2021, 148, 111591. [Google Scholar] [CrossRef]
- Machado, T.A.D.G.; de Oliveria, M.E.G.; Campos, M.I.F.; de Assis, P.O.A.; de Souza, E.L.B.; Madruga, M.S.; Pacheco, M.T.B.; Pintado, M.M.E.-, Queiroga, R.C.R.E. Impact of Honey on Quality Characteristics of Goat Yogurt Containing Probiotic Lactobacillus acidophilus. LWT-Food Sci. Technol. 2017, 80, 221–229. [CrossRef]
- Irigoyen, A.; Arana, I.; Castiella, M.; Torre, P.; Ibañez, F.C. Microbiological, Physicochemical, and Sensory Characteristics of Kefir during Storage. Food Chem. 2005, 90, 613–620. [Google Scholar] [CrossRef]
- Perez, R.; Alonso-Gonzalez, E.; Mejuto, J.C.; Guerra, N. Production of a Potentially Product for Animal Feed and Evaluation of Some of its Probiotic Properties. Int. J. Mol. Sci. 2021, 22, 10004. [Google Scholar] [CrossRef]


| Fermentation I | Fermentation II | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Compound | UWM | 0-24 h | 24-48 h | 48-72 h | 72-96 h | 0-48 h | 48-96 h | 96-144 h | 144-192 h |
| Organic Acids | ||||||||||
| 1 | Acetic acid | N.d. | N.d. | 1.90 ± 0.00 | 2.69 ± 0.83 | 2.35 ± 1.48 | 0.61 ± 0.06 | 3.07 ± 0.53 | 3.38 ± 0.23 | 2.30 ± 1.10 |
| 2 | Butanoic acid | N.d. | 0.68 ± 0.00 | N.d. | 0.80 ± 0.00 | N.d. | 0.21 ± 0.00 | 0.20 ± 0.00 | 0.34 ± 0.01 | 0.31 ± 0.08 |
| 3 | Pentanoic acid | N.d. | N.d. | N.d. | 0.76 ± 0.00 | 0.87 ± 0.00 | N.d. | N.d. | N.d. | N.d. |
| 4 | Hexanoic acid | N.d. | 7.63 ± 5.98 | 23.45 ± 9.71 | 13.53 ± 0.62 | 11.08 ± 2.80 | 3.85 ± 0.17 | 4.44 ± 0.73 | 7.81 ± 0.07 | 7.82 ± 2.82 |
| 5 | 2-Ethyl hexanoic acid | N.d. | 0.52 ± 0.00 | 0.78 ± 0.45 | 1.01 ± 0.26 | 0.99 ± 0.96 | 0.30 ± 0.20 | 1.58 ± 0.03 | 2.61 ± 0.63 | 1.31 ± 0.37 |
| 6 | 2-Methyl-propanoic acid | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 0.64 ± 0.01 | 2.02 ± 0.19 | 1.32 ± 0.24 |
| 7 | Heptanoic acid | N.d. | N.d. | 0.92 ± 0.25 | 0.87 ± 0.00 | 0.78 ± 0.00 | 0.20 ± 0.10 | 0.23 ± 0.00 | 0.31 ± 0.01 | N.d. |
| 8 | Octanoic acid | N.d. | N.d. | N.d. | N.d. | 1.69 ± 0.00 | N.d. | N.d. | N.d. | N.d. |
| 9 | Nonanoic acid | 4.89 ± 0.00 | N.d. | 0.72 ± 0.05 | 1.89 ± 0.00 | N.d. | N.d. | 0.41 ± 0.21 | 0.66 ± 0.03 | 0.26 ± 0.07 |
| 10 | Ethyl boronic acid | N.d. | N.d. | N.d. | 0.59 ± 0.14 | N.d. | N.d. | N.d. | N.d. | N.d. |
| 11 | 8-Methyl-6-nonenoic acid | N.d. | N.d. | N.d. | 0.71 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. |
| 12 | Cyclopentane-undecanoic acid | 7.55 ± 0.19 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 13 | Cis-11-eicosenoic acid | 28.38 ± 1.43 | 8.02 ± 0.00 | 11.46 ± 1.96 | 14.39 ± 1.43 | 11.07 ± 6.88 | 2.97 ± 1.10 | 4.37 ± 0.56 | 4.42 ± 0.18 | 3.20 ± 0.83 |
| 14 | Trans-2-undecenoic acid | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 1.54 ± 0.00 | N.d. | N.d. |
| Number of Organic Acids | 3 | 4 | 6 | 10 | 7 | 6 | 9 | 8 | 7 | |
| Total concentration | 40.83 ± 0.01A | 16.85 ± 5.98B | 39.24 ± 11.27C,A | 37.25 ± 2.63D,C | 28.84 ± 10.86E,D | 8.14 ± 1.07F | 16.48 ± 1.21G,B,E | 21.55 ± 0.63B,E | 16.52 ± 2.53B,G | |
| Alcohols | ||||||||||
| 15 | 1-Pentanol | N.d. | 44.08 ± 1.22 | 40.86 ± 3.86 | 36.06 ± 11.08 | 9.78 ± 0.00 | 10.28 ± 5.49 | 4.16 ± 0.14 | 2.67 ± 0.00 | 1.68 ± 0.81 |
| 16 | 2-Phenylethanol | 8.86 ± 0.00 | 12.36 ± 5.54 | 23.09 ± 9.51 | 22.90 ± 3.73 | 11.41 ± 1.74 | 10.70 ± 4.84 | 9.06 ± 0.39 | 5.34 ± 1.00 | 3.20 ± 0.87 |
| 17 | 2-Methyl-1-propanol | N.d. | N.d. | N.d. | 0.90 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. |
| 18 | 1,3-Octanediol | N.d. | 0.88 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 19 | 1,5-Hexanediol | N.d. | 0.42 ± 0.00 | 1.59 ± 0.16 | N.d. | 0.65 ± 0.37 | 0.62 ± 0.26 | 0.57 ± 0.04 | 0.52 ± 0.17 | N.d. |
| 20 | 1-Heptanol | N.d. | N.d. | N.d. | 1.54 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. |
| 21 | 2,10-Dimethyl-9-undecen-1-ol | N.d. | N.d. | 5.83 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 22 | 2,4-Dimethyl-3-pentanol | N.d. | N.d. | N.d. | N.d. | 0.82 ± 0.00 | 0.16 ± 0.00 | N.d. | 0.27 ± 0.00 | N.d. |
| 23 | 2,7-Dimethyl-1-octanol | N.d. | N.d. | 9.60 ± 0.00 | 0.39 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. |
| 24 | 2-Butyl-1-octanol | N.d. | N.d. | N.d. | 3.47 ± 0.00 | 0.93 ± 0.00 | 0.56 ± 0.00 | N.d. | 0.60 ± 0.00 | 0.25 ± 0.00 |
| 25 | 2-Butyl-2,7-octadien-1-ol | N.d. | N.d. | 2.64 ± 0.19 | 3.07 ± 0.69 | N.d. | 0.15 ± 0.00 | 0.85 ± 0.00 | 0.68 ± 0.02 | 0.56 ± 0.15 |
| 26 | 2-Ethyl-hexanol | 6.27 ± 0.00 | 12.00 ± 0.59 | 19.23 ± 3.27 | 17.59 ± 0.83 | 8.03 ± 7.34 | 2.43 ± 0.90 | 3.20 ± 0.03 | 3.37 ± 0.18 | 3.05 ± 0.13 |
| 27 | 2-Heptanol | N.d. | 0.64 ± 0.00 | 0.80 ± 0.00 | N.d. | N.d. | 0.31 ± 0.19 | 0.18 ± 0.00 | 0.37 ± 0.00 | N.d. |
| 28 | 2-Methyl-5-(1- methylethenyl)-cyclohexanol | N.d. | N.d. | N.d. | 2.84 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. |
| 29 | 3,6-Dimethyl-3-heptanol | N.d. | N.d. | N.d. | 0.68 ± 0.12 | N.d. | N.d. | N.d. | N.d. | N.d. |
| 30 | 3-Methyl-1,5-pentanediol | N.d. | 0.50 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 31 | 3-Methyl-1-pentanol | N.d. | 0.78 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 32 | 5,9-Dimethyl-1-decanol | N.d. | N.d. | 1.16 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 33 | 5-Nonadecen-1-ol | N.d. | 11.03 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 34 | Z-10-Pentadecen-1-ol | N.d. | N.d. | 4.08 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 35 | Butane-1,3-diol | N.d. | N.d. | N.d. | N.d. | N.d. | 0.20 ± 0.00 | N.d. | N.d. | N.d. |
| 36 | 1,3,5-Pentanotriol | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 0.60 ± 0.00 |
| 37 | 2-Furanmethanol | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 0.60 ± 0.00 |
| 38 | (E)-2-Tridecen-1-ol | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 1.13 ± 0.76 | N.d. | N.d. |
| 39 | 1-Eicosanol | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 0.21 ± 0.00 | N.d. | N.d. |
| 40 | 2-Hexyl-1-octanol | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 0.33 ± 0.00 |
| 41 | 2-Nonen-1-ol | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 0.48 ± 0.00 | N.d. | N.d. |
| 42 | 3-Tetradecyn-1-ol | N.d. | N.d. | N.d. | N.d. | N.d. | 0.21 ± 0.00 | 0.68 ± 0.00 | N.d. | N.d. |
| 43 | 4-Methyl-2-pentanol | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 0.15 ± 0.00 |
| Number of Alcohols | 2 | 9 | 10 | 10 | 6 | 10 | 10 | 8 | 9 | |
| Total concentration | 15.13 ± 0.00A | 82.71 ± 5.84B | 108.89 ± 16.56C | 89.47 ± 13.96B | 31.63 ± 8.45D | 25.62 ± 6.80E,D | 20.52 ± 0.46E | 13.82 ± 0.86F | 10.42 ± 0.86G | |
| Aldehydes | ||||||||||
| 44 | (E)-2-Nonenal | N.d. | N.d. | 0.84 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 45 | (Z)-14-methyl-8-hexadecenal | N.d. | N.d. | 1.66 ± 0.06 | 2.35 ± 0.49 | 2.61 ± 0.00 | N.d. | N.d. | 0.58 ± 0.01 | 0.67 ± 0.09 |
| 46 | 10-Undecenal | N.d. | 0.83 ± 0.13 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 47 | 2,6-Dimethyl benzaldehyde | N.d. | 0.36 ± 0.11 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 48 | 2-Ethyl hexanal | N.d. | N.d. | 1.21 ± 0.64 | 0.62 ± 0.25 | N.d. | 0.17 ± 0.00 | 0.19 ± 0.11 | 0.24 ± 0.09 | 0.15 ± 0.06 |
| 49 | 2-Methyl undecanal | N.d. | N.d. | 0.81 ± 0.06 | 0.94 ± 0.00 | N.d. | N.d. | 0.14 ± 0.00 | 0.17 ± 0.00 | N.d. |
| 50 | Benzaldehyde | N.d. | 2.26 ± 0.00 | 3.77 ± 0.91 | 2.60 ± 0.37 | N.d. | N.d. | N.d. | N.d. | N.d. |
| 51 | Dodecanal | N.d. | N.d. | N.d. | 0.79 ± 0.00 | 0.51 ± 0.29 | N.d. | N.d. | N.d. | N.d. |
| 52 | Phenylacetaldehyde | N.d. | N.d. | 1.18 ± 0.20 | 0.94 ± 0.25 | N.d. | 0.15 ± 0.05 | 0.17 ± 0.02 | 0.19 ± 0.01 | 0.35 ± 0.15 |
| 53 | Nonanal | N.d. | N.d. | N.d. | 1.61 ± 0.81 | N.d. | N.d. | N.d. | N.d. | N.d. |
| Number of Aldehydes | 0 | 3 | 6 | 7 | 2 | 2 | 3 | 4 | 3 | |
| Total concentration | 0.00 ± 0.00A | 3.45 ± 0.03B | 9.47 ± 0.87C | 9.84 ± 1.40C | 3.12 ± 0.29D | 0.32 ± 0.05E | 0.50 ± 0.18F | 1.18 ± 0.10G | 1.17 ± 0.18G | |
| Ketones | ||||||||||
| 54 | 2,6-Dimethyl-4-heptanone | 2.18 ± 0.00 | 4.98 ± 0.00 | 2.23 ± 1.47 | 2.52 ± 0.00 | 3.19 ± 0.00 | 0.06 ± 0.00 | 0.18 ± 0.00 | 0.42 ± 0.27 | 0.36 ± 0.29 |
| 55 | 2-Heptanone | 28.96 ± 0.00 | 5.52 ± 0.62 | 4.05 ± 0.00 | N.d. | 2.39 ± 0.00 | 0.40 ± 0.24 | 0.59 ± 0.00 | 2.14 ± 0.12 | 0.88 ± 0.70 |
| 56 | 2-Methyl-4-octanone | N.d. | 1.06 ± 0.00 | N.d. | 1.30 ± 0.00 | 1.92 ± 0.00 | N.d. | N.d. | N.d. | N.d. |
| 57 | 2-Nonanone | 14.26 ± 0.00 | 0.40 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 58 | 8-Hydroxy-2-octanone | N.d. | N.d. | N.d. | N.d. | 2.06 ± 0.00 | N.d. | N.d. | N.d. | N.d. |
| 59 | 5-Methyl-3-heptanone | N.d. | N.d. | N.d. | N.d. | N.d. | 0.16 ± 0.00 | N.d. | N.d. | N.d. |
| Number of Ketones | 3 | 4 | 2 | 2 | 4 | 3 | 2 | 2 | 2 | |
| Total concentration | 45.40 ± 0.00A | 11.95 ± 0.62B | 6.28 ± 1.47C | 3.82 ± 0.01D | 9.55 ± 0.01E | 0.62 ± 0.24F | 0.77 ± 0.00G,F | 2.56 ± 0.33H | 1.24 ± 0.76F,G | |
| Esters | ||||||||||
| 60 | 2-Phenylethyl acetate | 1.19 ± 0.00 | 3.27 ± 0.98 | 4.31 ± 2.00 | 4.38 ± 0.92 | 2.70 ± 2.21 | 2.90 ± 1.75 | 1.45 ± 0.40 | 0.75 ± 0.11 | 0.37 ± 0.03 |
| 61 | 2-Methylpentyl acetate | N.d. | N.d. | 0.80 ± 0.20 | N.d. | N.d. | N.d. | N.d. | N.d. | 0.32 ± 0.13 |
| 62 | Ethyl acetate | N.d. | 8.07 ± 0.00 | 5.95 ± 0.94 | 6.19 ± 0.94 | 4.11 ± 0.00 | 2.11 ± 1.11 | 4.62 ± 2.09 | 2.82 ± 0.25 | 1.72 ± 0.53 |
| 63 | Isoamyl acetate | N.d. | 2.58 ± 0.72 | N.d. | 2.02 ± 1.50 | 3.79 ± 2.50 | 0.65 ± 0.00 | 0.81 ± 0.26 | 0.39 ± 0.00 | N.d. |
| 64 | Pentyl acetate | N.d. | N.d. | 2.60 ± 2.11 | N.d. | N.d. | 0.32 ± 0.00 | 2.10 ± 0.00 | 0.21 ± 0.00 | 0.29 ± 0.00 |
| 65 | β-Terpinyl acetate | N.d. | N.d. | 0.98 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 66 | 1-Methylundecyl acrylate | N.d. | 2.12 ± 0.00 | 0.79 ± 0.00 | 1.58 ± 0.32 | N.d. | N.d. | 0.11 ± 0.00 | N.d. | N.d. |
| 67 | 3-Methylbutyl chloroacetate | N.d. | N.d. | N.d. | N.d. | 0.44 ± 0.00 | N.d. | N.d. | N.d. | N.d. |
| 68 | Ethyl 5-methyl nonanoate | N.d. | N.d. | N.d. | N.d. | 0.72 ± 0.00 | N.d. | 0.51 ± 0.00 | N.d. | N.d. |
| 69 | 3-Tridecyl methoxyacetate | N.d. | 1.81 ± 0.00 | N.d. | 1.26 ± 0.00 | N.d. | N.d. | 0.34 ± 0.07 | N.d. | N.d. |
| 70 | 9-Dodecenyl methoxyacetate | N.d. | N.d. | N.d. | N.d. | 0.33 ± 0.00 | N.d. | N.d. | N.d. | N.d. |
| 71 | Allyl pentadecyl oxalate | 2.67 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 72 | Pentyl isohexyl oxalate | N.d. | 0.35 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 73 | Benzyl oleate | N.d. | 1.24 ± 0.00 | N.d. | N.d. | 1.50 ± 0.00 | N.d. | N.d. | N.d. | N.d. |
| 74 | Decyl butanoate | N.d. | 6.16 ± 4.68 | 4.93 ± 1.50 | 2.69 ± 0.00 | 3.53 ± 0.00 | N.d. | N.d. | N.d. | N.d. |
| 75 | Dimethyl ethylboronate | N.d. | N.d. | N.d. | 1.75 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. |
| 76 | Ethyl phenylacetate | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 0.19 ± 0.00 | 0.14 ± 0.03 | N.d. |
| 77 | Ethyl hexanoate | N.d. | 36.47 ± 0.61 | 34.07 ± 1.86 | 19.74 ± 15.87 | 64.05 ± 0.00 | 8.10 ± 3.25 | 2.36 ± 1.75 | 2.52 ± 0.54 | 0.18 ± 0.00 |
| 78 | Ethyl heptanoate | N.d. | 1.43 ± 0.00 | 1.38 ± 0.28 | 0.56 ± 0.08 | 0.31 ± 0.00 | 0.28 ± 0.07 | 0.25 ± 0.00 | N.d. | N.d. |
| 79 | Ethyl octanoate | 3.25 ± 0.00 | 30.70 ± 17.87 | 30.12 ± 2.35 | 12.80 ± 1.49 | 18.92 ± 6.47 | 11.15 ± 3.32 | 2.33 ± 1.46 | 1.92 ± 0.03 | 0.63 ± 0.21 |
| 80 | Ethyl nonanoate | N.d | N.d | N.d | N.d | N.d | 0.12 ± 0.00 | N.d | N.d | N.d |
| 81 | Ethyl decanoate | 2.18 ± 0.01 | 5.53 ± 3.64 | 5.14 ± 1.35 | 2.26 ± 0.01 | 3.63 ± 0.25 | 1.51 ± 0.43 | 0.99 ± 0.00 | 0.61 ± 0.07 | 0.40 ± 0.00 |
| 82 | Ethyl dodecanoate | N.d. | 0.75 ± 0.00 | N.d. | N.d. | N.d. | 0.28 ± 0.10 | N.d. | N.d. | N.d. |
| 83 | Ethyl isobutanoate | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 0.26 ± 0.00 | N.d. | N.d. |
| 84 | Isobutyl 2-methylaminobenzoate | N.d. | 1.43 ± 0.00 | N.d. | N.d. | 0.91 ± 0.00 | 0.27 ± 0.00 | 0.35 ± 0.00 | N.d. | N.d. |
| 85 | Methyl hippurate | 3.67 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 86 | Methyl L-valinate | N.d. | N.d. | N.d. | 2.18 ± 0.00 | 1.75 ± 1.25 | N.d. | 1.97 ± 0.69 | 3.10 ± 1.41 | 2.42 ± 1.47 |
| 87 | Pentyl pentanoate | N.d. | N.d. | N.d. | N.d. | 0.75 ± 0.00 | 0.17 ± 0.05 | N.d. | N.d. | N.d. |
| 88 | Octyl propanoate | N.d. | N.d. | N.d. | 1.65 ± 0.32 | N.d. | N.d. | N.d. | N.d. | N.d. |
| 89 | Octyl pentanoate | N.d. | N.d. | N.d. | N.d. | N.d. | 0.15 ± 0.09 | N.d. | N.d. | N.d. |
| 90 | Octyl butanoate | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 0.45 ± 0.00 | 0.35 ± 0.00 | N.d. |
| 91 | Hexyl propanoate | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 0.28 ± 0.00 |
| 92 | 2-Hidroxy-4-methyl 4-methylpentanoate |
N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 0.76 ± 0.00 | N.d. |
| Number of Esters | 5 | 14 | 11 | 13 | 15 | 13 | 16 | 11 | 9 | |
| Total concentration | 12.96 ± 0.01A | 101.91 ± 19.08B | 91.07 ± 4.22B | 59.06 ± 18.48C | 107.45 ± 8.31B | 28.01 ± 3.02D | 19.09 ± 3.61E | 13.57 ± 1.15A | 6.61 ± 1.16F | |
| Hydrocarbons | ||||||||||
| 93 | (Z)-3-Dodecene | N.d. | N.d. | 1.41 ± 0.00 | 0.63 ± 0.00 | 0.86 ± 0.00 | N.d. | N.d. | N.d. | N.d. |
| 94 | 1,2,4-Trimethylbenzene | N.d. | 23.78 ± 0.00 | N.d. | 7.49 ± 0.00 | 21.30 ± 0.00 | N.d. | N.d. | 5.51 ± 0.00 | 2.56 ± 2.02 |
| 95 | 1,3-Bis(1,1-dimethylethyl)benzene | N.d. | N.d. | 3.64 ± 0.80 | 2.70 ± 0.39 | N.d. | N.d. | N.d. | N.d. | N.d. |
| 96 | 3-Hexadecyne | 27.29 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 1.31 ± 0.00 | N.d. |
| 97 | 5-Butyl-4-nonene | 4.45 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 98 | 9-Eicosyne | N.d. | N.d. | N.d. | N.d. | 7.95 ± 0.00 | N.d. | N.d. | N.d. | N.d. |
| 99 | Decane | 13.37 ± 0.00 | 5.58 ± 4.70 | 3.75 ± 1.90 | 3.72 ± 0.51 | 3.63 ± 0.00 | 0.31 ± 0.11 | 0.50 ± 0.00 | N.d. | N.d. |
| 100 | Dodecane | N.d. | N.d. | N.d. | N.d. | 6.72 ± 0.00 | N.d. | N.d. | N.d. | N.d. |
| 101 | n-Hexane | 11.37 ± 0.00 | 2.70 ± 0.00 | 3.15 ± 2.36 | 2.29 ± 0.30 | 2.05 ± 0.39 | N.d. | N.d. | N.d. | 3.48 ± 2.03 |
| 102 | Tetradecane | 12.47 ± 0.00 | 2.45 ± 0.00 | 4.02 ± 1.02 | 2.44 ± 0.00 | 2.99 ± 1.54 | 0.52 ± 0.13 | 0.83 ± 0.48 | N.d. | 0.71 ± 0.00 |
| Number of Hydrocarbons | 5 | 4 | 5 | 6 | 7 | 2 | 2 | 2 | 3 | |
| Total concentration | 68.95 ± 0.01A | 34.51 ± 4.70B | 15.97 ± 6.01C | 19.27 ± 1.19C | 45.50 ± 1.78D | 0.83 ± 0.10E | 1.33 ± 0.48F | 6.82 ± 0.00G | 6.75 ± 2.31G | |
| Other Compounds | ||||||||||
| 103 | Octyl oxirane | 4.54 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 104 | o-Decyl hydroxylamine | N.d. | 1.04 ± 0.26 | 1.42 ± 0.46 | N.d. | 1.64 ± 0.33 | 0.37 ± 0.08 | N.d. | 0.41 ± 0.03 | N.d. |
| 105 | 3,3,4,4-Tetramethylbutyrolactone | N.d. | 1.68 ± 0.00 | 0.94 ± 0.00 | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. |
| 106 | 2,4,5-Trimethyl-1,3-dioxolane | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | N.d. | 1.52 ± 0.18 | 1.11 ± 0.20 |
| 107 | 3-Trifluoroacetoxy dodecane | N.d. | N.d. | N.d. | N.d. | N.d. | 0.13 ± 0.00 | N.d. | N.d. | N.d. |
| Number of Other Compounds | 1 | 2 | 2 | 0 | 1 | 2 | 0 | 2 | 1 | |
| Total concentration | 4.54 ± 0.00A | 2.72 ± 0.26B | 2.36 ± 0.46C,B | N.d. | 1.64 ± 0.33D | 0.50 ± 0.08E | N.d. | 1.93 ± 0.18C,D | 1.11 ± 0.20F | |
| Total number of volatile compounds | 19 | 40 | 42 | 48 | 42 | 38 | 42 | 37 | 34 | |
| Total concentration of volatile compounds | 187.80 ± 0.01A | 254.11 ± 13.60B | 273.28 ± 29.50C,B | 218.71 ± 24.10D | 227.73 ± 14.73B,D | 64.04 ± 6.63E | 58.69 ± 3.45F,E | 61.43 ± 1.34G,E | 43.82 ± 4.66H | |
| Volatile compound | OPT (µg/L) | Odor descriptors | Series |
|---|---|---|---|
| Organic acids | |||
| Acetic acid | 99000.00 [42] | Vinegar, sour [43,44] | 1 |
| Butanoic acid | 1400.00 [42] | Sweaty, butter, cheese, acid, fermented odor [45] | 1 |
| Pentanoic acid | 3000.00 [42] | Green fruit, vegetables, sweat [14,46] | 1, 4 |
| Hexanoic acid | 3000.00 [42] | Goat cheese, fatty acids, vegetable oil, sweaty, sharp, acidic, green, gammy, dairy sour, dairy, stale, butter, sour, fruity and pungent [43,45,47,48,49] | 1, 2, 4 |
| 2-Ethyl-hexanoic acid | 27000.00 [42] | Taste similar to paint [50] | 5 |
| 2-Methyl-propanoic acid | 6550.50 [42] | Fuits [14] | 2 |
| Heptanoic acid | 640.00 [42] | Cheese [47] | 1 |
| Octanoic acid | 3000.00 [42] | Sweat, cheese, rancid, fatty acids, vegetable oil [47,51] | 1 |
| Nonanoic acid | 8800.00 [42] | Fatty, soapy, waxy, green, goat [52] | 1, 4 |
| Alcohols | |||
| 1-Pentanol | 120.00 [42] | Green fruit, fermented, bread and yeast [47,53,54] | 1, 2 |
| 2-Phenylethanol | 564.20 [42] | Floral, pink, flowery, like honey [47,51,55,56] | 3, 7 |
| 2-Methyl-1-propanol | 550.00 [42] | Wine, solvent, bitter, malty [57,58,59] | 5, 6 |
| 1-Heptanol | 5.40 [42] | Fat [60] | 1 |
| 2-Heptanol | 65.23 [42] | Green [14,47] | 4 |
| 2,4-Dimethyl-3-pentanol | 82.00 [42] | Bitter almond [61] | 2 |
| 2-Ethyl-hexanol | 830.00 [42] | Leather, goat, stable, cow and sheep, cardboard [45,52] | 5, 6 |
| 3-Methyl-1-pentanol | 7.50 [42] | Unpleasant chemical fusel [62] | 5 |
| 2-Furanmethanol | 4500.00 [42] | Floral, sweet [63] |
6 |
| Butane-1,3-diol | 10000.00 [42] | Greasy, floral [64] | 1, 3 |
| Aldehydes | |||
| (E)-2-Nonenal | 0.40 [42] | Fatty, green [59] | 1, 4 |
| 2-Ethyl hexanal | 41.00 [42] | Legume smell [65] | 1 |
| Benzaldehyde | 750.00 [42] | Almond, nut like bitter [47,65] | 2 |
| Dodecanal | 10.00 [42] | Floral, waxy [66] | 1, 3 |
| Phenylacetaldehyde | 6.30 [42] | Honey [47] | 7 |
| Nonanal | 1.10 [42] | Green, fresh, citrus-like, soapy, fruity odor [47,51,67] | 2, 4 |
| Ketones | |||
| 2,6-Dimethyl-4-heptanone | 110.00 [68] | Fruity, sweet [49] | 2 |
| 2-Heptanone | 5.00 [69] | Banana, fruity, floral and musty, fresh cream flavor [47,53,67] | 1, 2, 3 |
| 2-Nonanone | 5.00 [69] | Sweet, fruity, floral, musty [47,67] | 2, 3 |
| 5-Methyl-3-heptanone | 41.00 [42] | Fruit [70] | 2 |
| Esters | |||
| 2-Phenethyl acetate | 249.59 [42] | Floral, rose, honey [71] | 3, 7 |
| Ethyl acetate | 5000.00 [71] | Pineapple, fruit [15,47,72] | 2 |
| Isoamyl acetate | 2.00 [42] | Banana, fruity, sweet [47,73] | 2 |
| Pentyl acetate | 43.00 [42] | Fruit [71] | 2 |
| Decyl butanoate | 250.00 [74] | Fruity, waxy, fatty and slightly citrus [75] | 1, 2 |
| Ethyl decanoate | 23.00 [42] | Apple, floral, fruity and musty [15,71,76] | 2, 3 |
| Ethyl dodecanoate | 5900.00 [42] | Fruity, floral [71] | 2, 3 |
| Ethyl heptanoate | 1.90 [42] | Fruit, overripe [47,71] | 2 |
| Ethyl hexanoate | 1.00 [42] | Banana, apple [71,77] | 2 |
| Ethyl octanoate | 19.30 [42] | Fruity, apple, banana [71,77] | 2 |
| Ethyl phenylacetate | 250.00 [75] | Sweet fragrance of honey [47] | 7 |
| Ethyl nonanoate | 377.00 [42] | Floral, fruit [66,71] | 2 |
| Ethyl isobutanoate | 0.10 [42] | Unripe fruit [78] | 2, 4 |
| Octyl butanoate | 250.00 [42] | Herb, orange, flower [79] | 2, 3, 4 |
| Hexyl propanoate | 8.00 [42] | Apple [80] | 2 |
| Hydrocarbons | |||
| 1,2,4-Trimethyl-benzene | 260.00 [42] | Fruity, citrus, sweet [81] | 2 |
| Decane | 10000.00 [42] | Solvent, sweet-etheric, diffusive [82] | 5 |
| Dodecane | 10000.00 [42] | Alkanes [53] | 5 |
| Tetradecane | 1000.00 [42] | Gasoil [83] | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





