Submitted:
13 June 2024
Posted:
14 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Mosquito Sampling and Processing
2.2. Viral Enrichment, Amplification, Library Construction and Sequencing
2.3. Identification and Annotation Eukaryotic Sequences Retrieved from Sequenced Aedes Mosquito Pools
2.4. Virus Identification and Phylogenetic Analysis
2.5. Aedes Mosquito Identification and Quantification of Selected Viruses
3. Results
3.1. Each Sampling Site Is Dominated by a Single Aedes Species
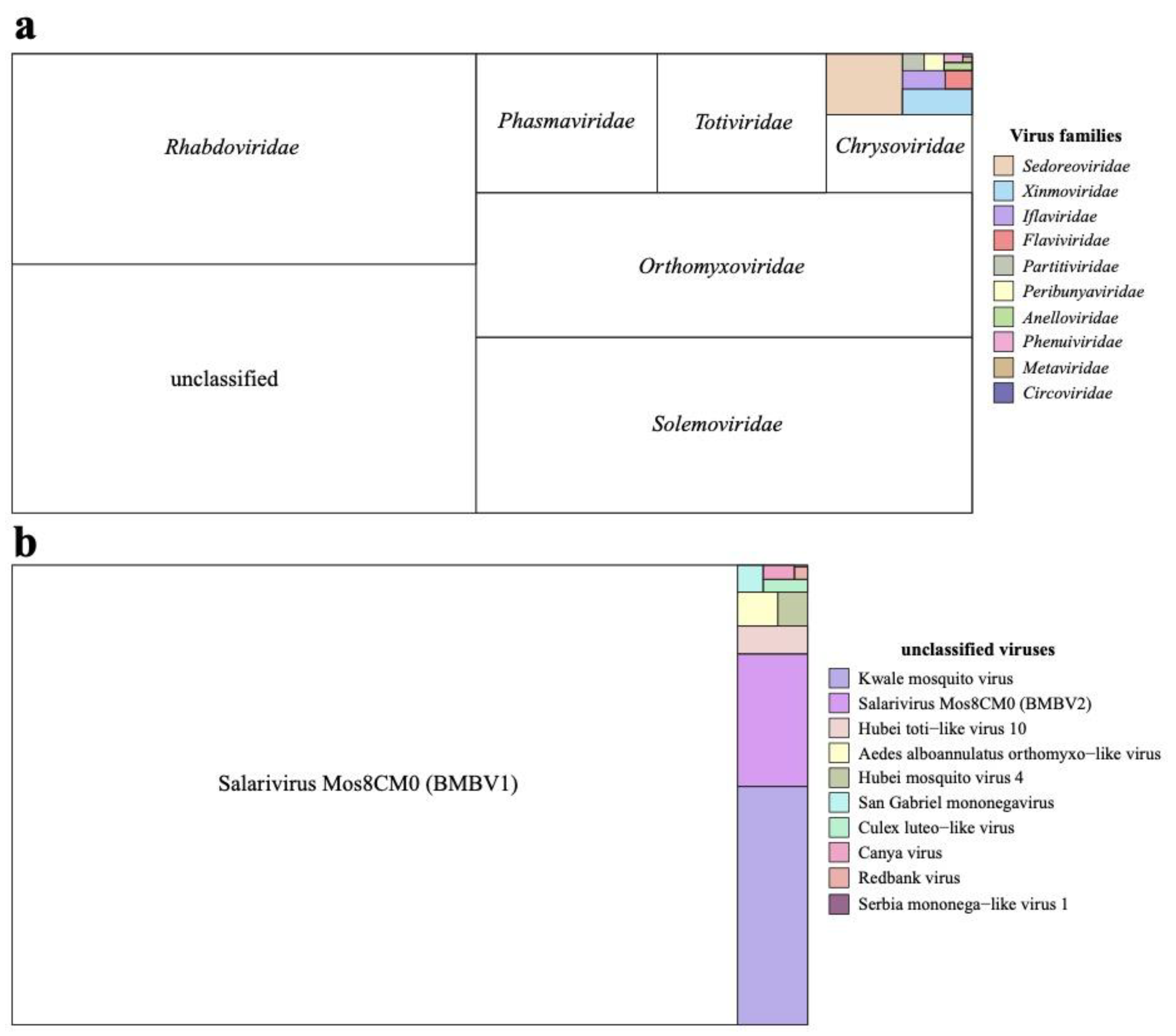
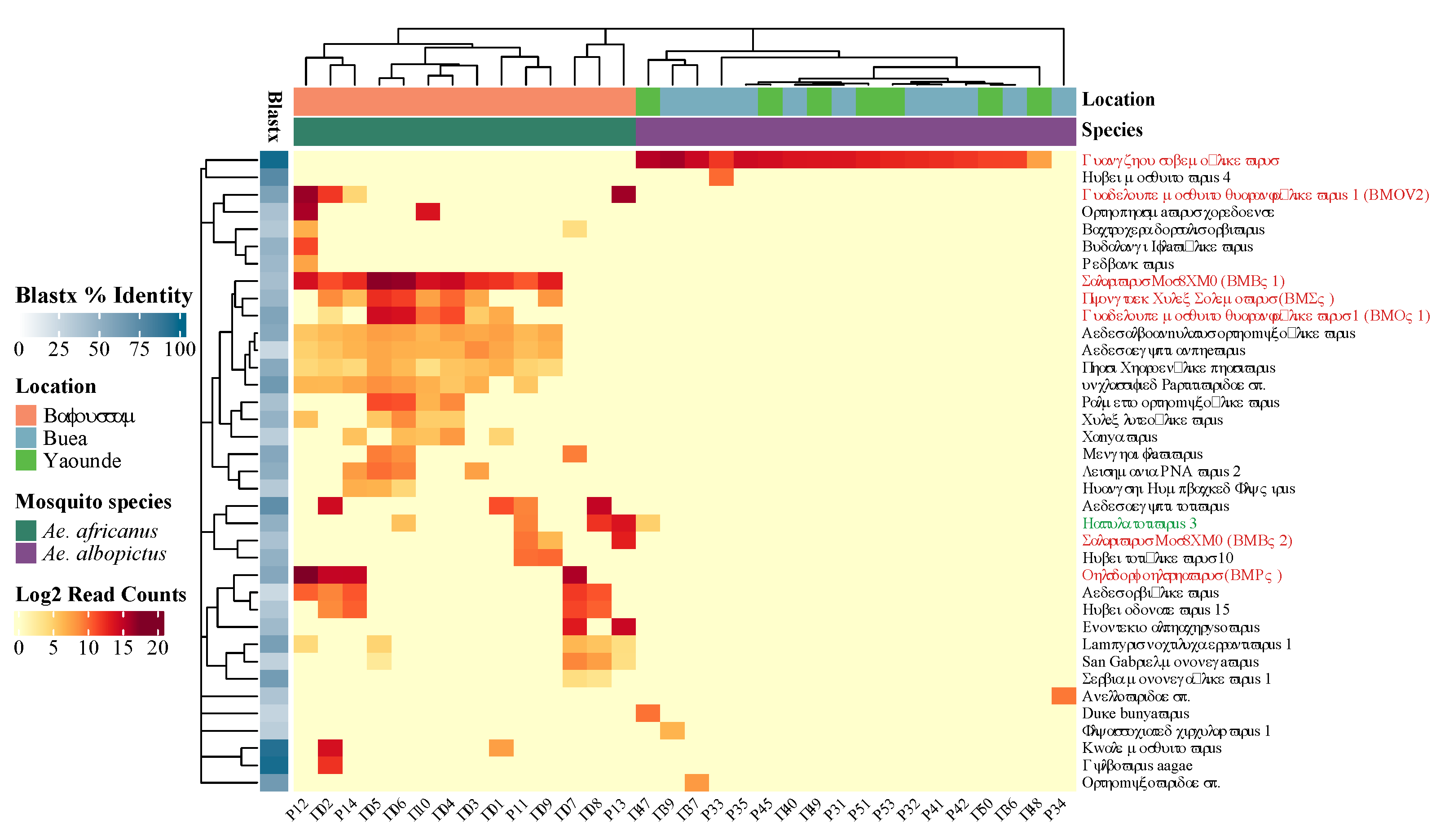
3.2. Distinct Virus Families Identified within Aedes Mosquito Species from Different Sampling Sites
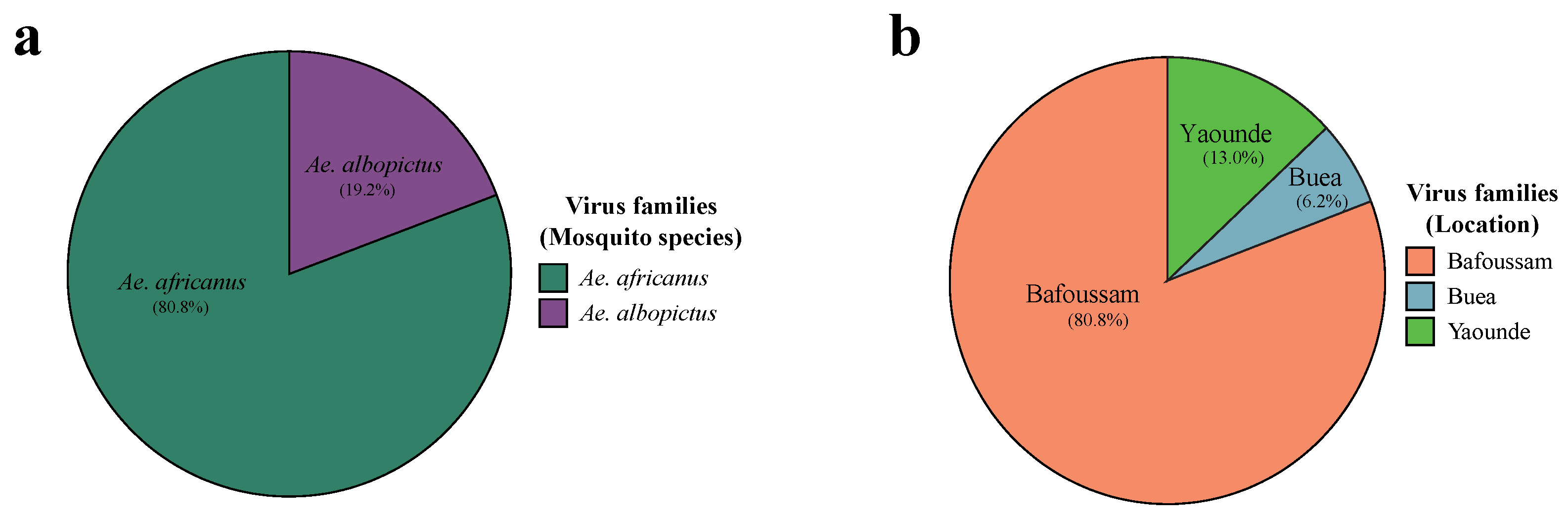
3.3. The Majority of Eukaryotic Viral Genomes Are Found in Aedes Africanus Species Collected in Bafoussam
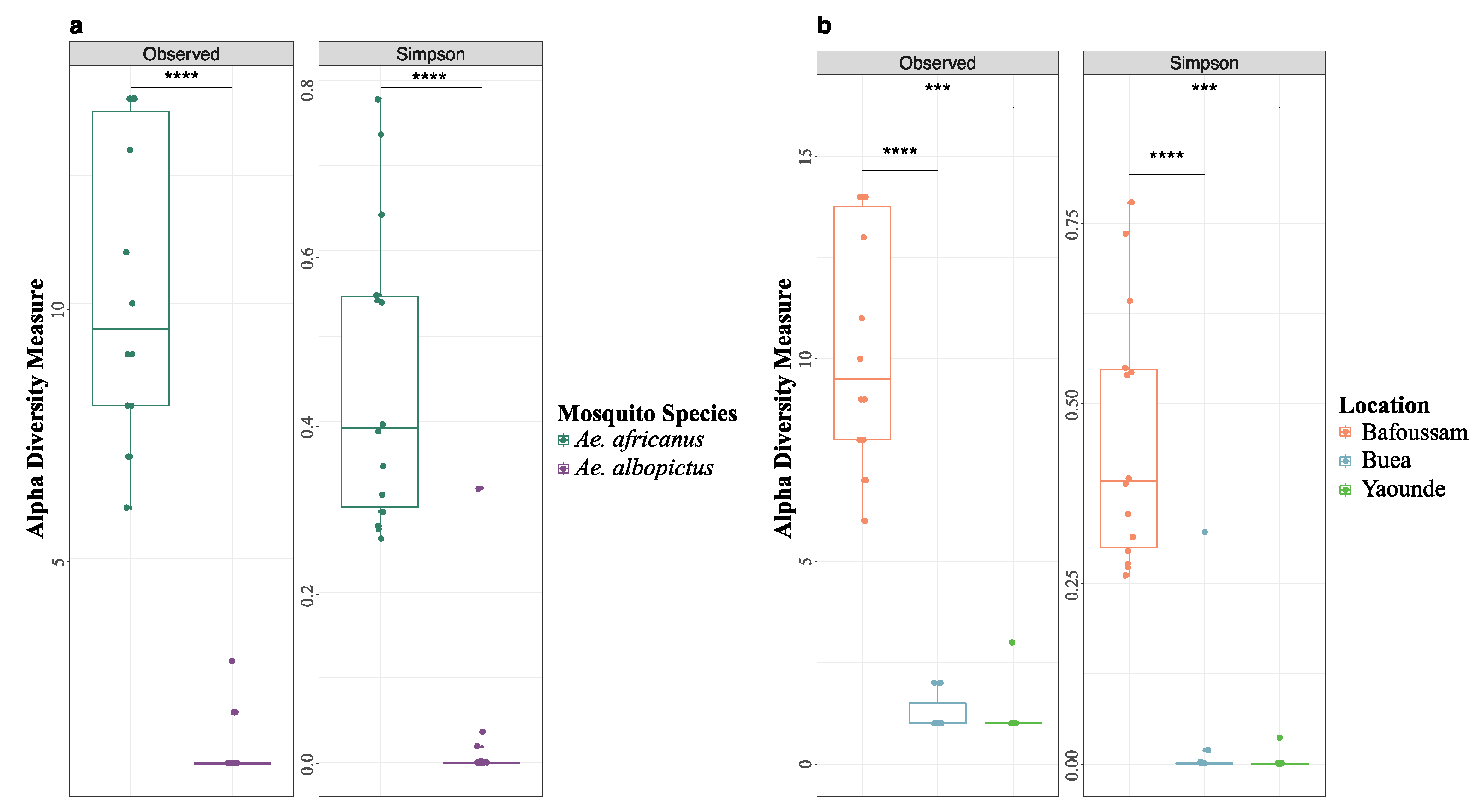
3.4. Significant Difference in Eukaryotic Virome Richness and Diversity between Aedes Mosquito Species
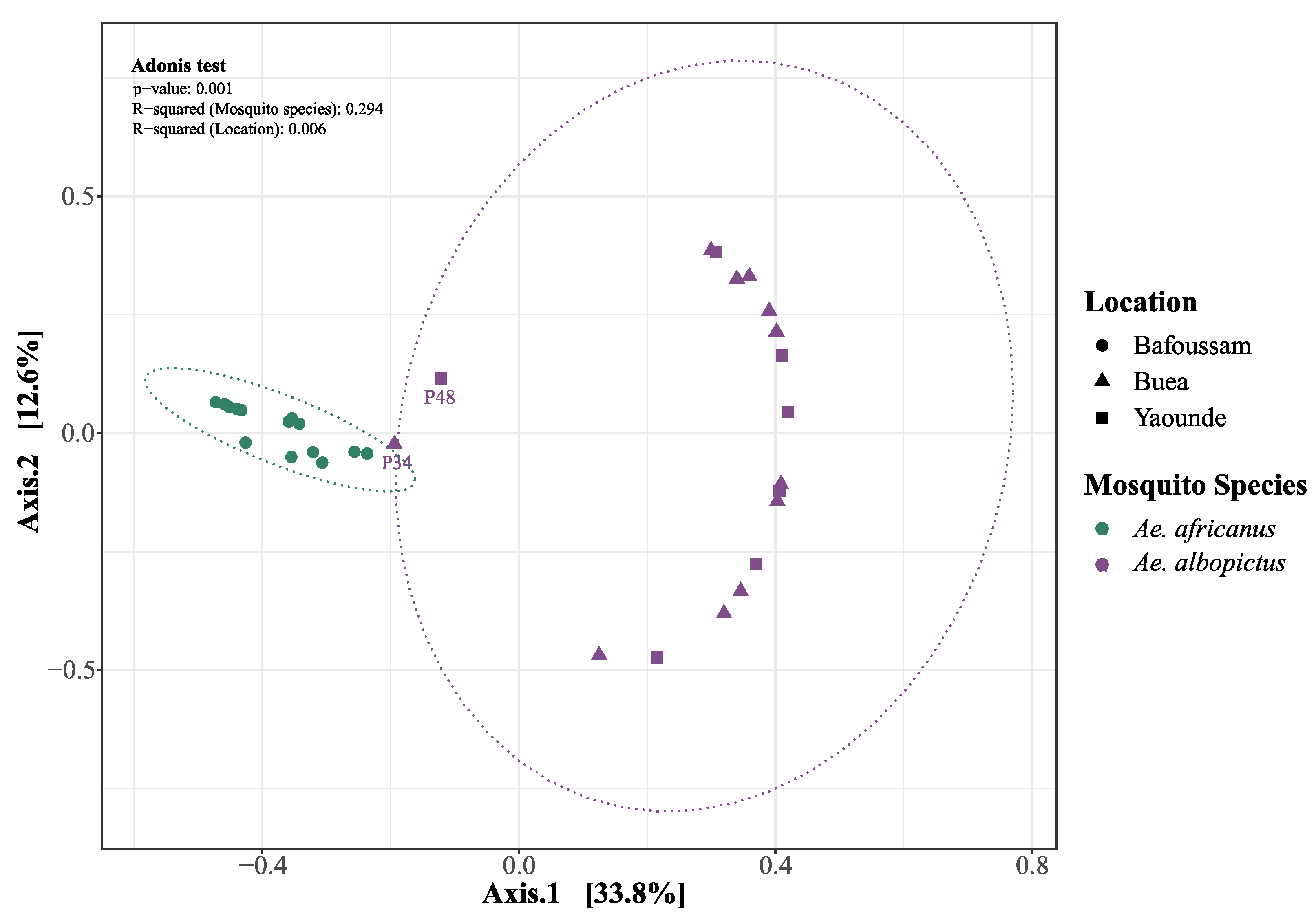
3.5. Significant Difference in Eukaryotic Virome Composition and Distribution of Aedes Mosquito Species from Different Sampling Sites
3.6. Diverse and Abundant Eukaryotic Virome in Aedes Africanus, Compared to Aedes Albopictus Mosquito Pools
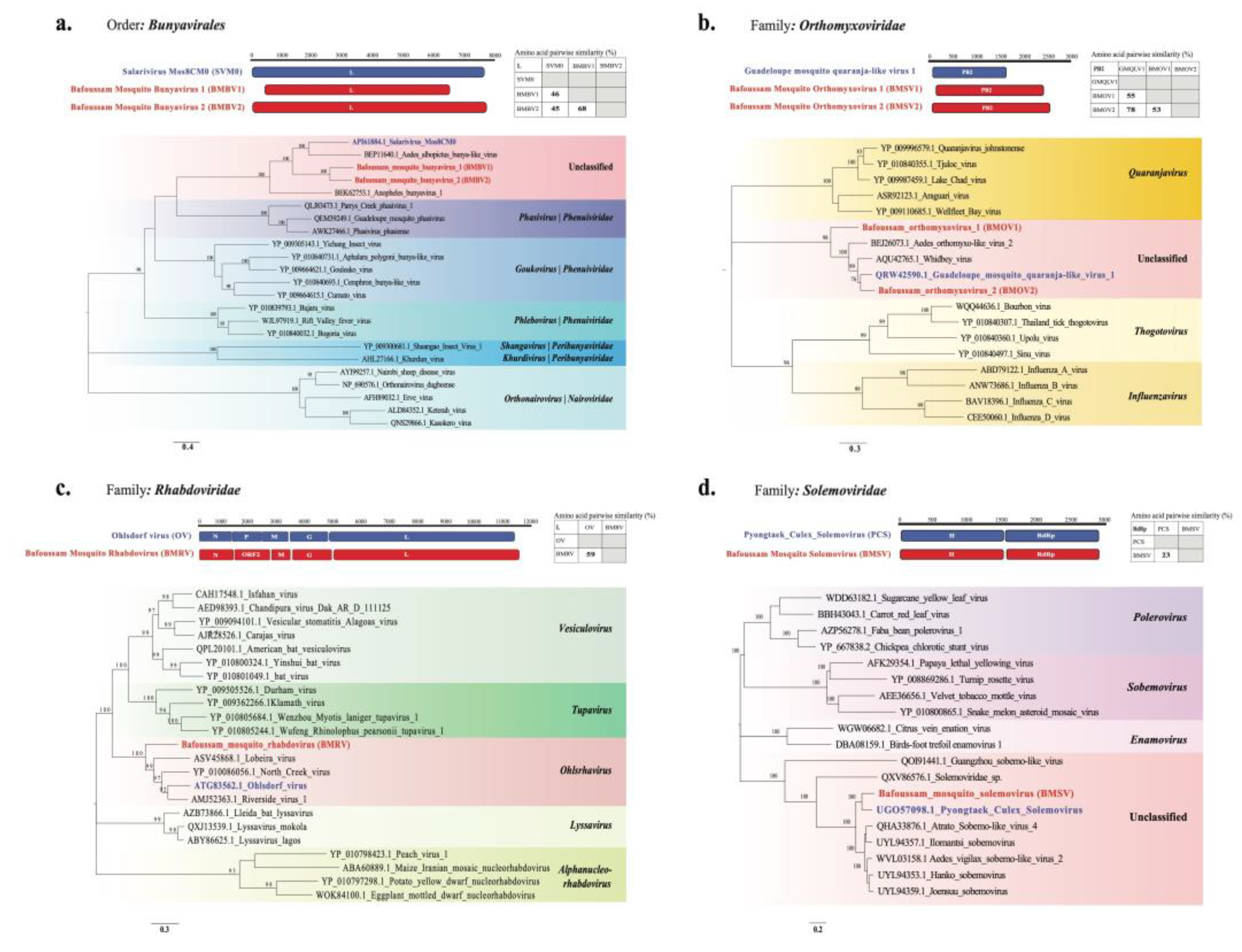
3.7. Phylogenetic Analysis of Six Novel Viruses Identified in Aedes Mosquito Pools
3.7.1. Bunyavirales
3.7.2. Orthomyxoviridae
3.7.3. Rhabdoviridae
3.7.4. Solemoviridae
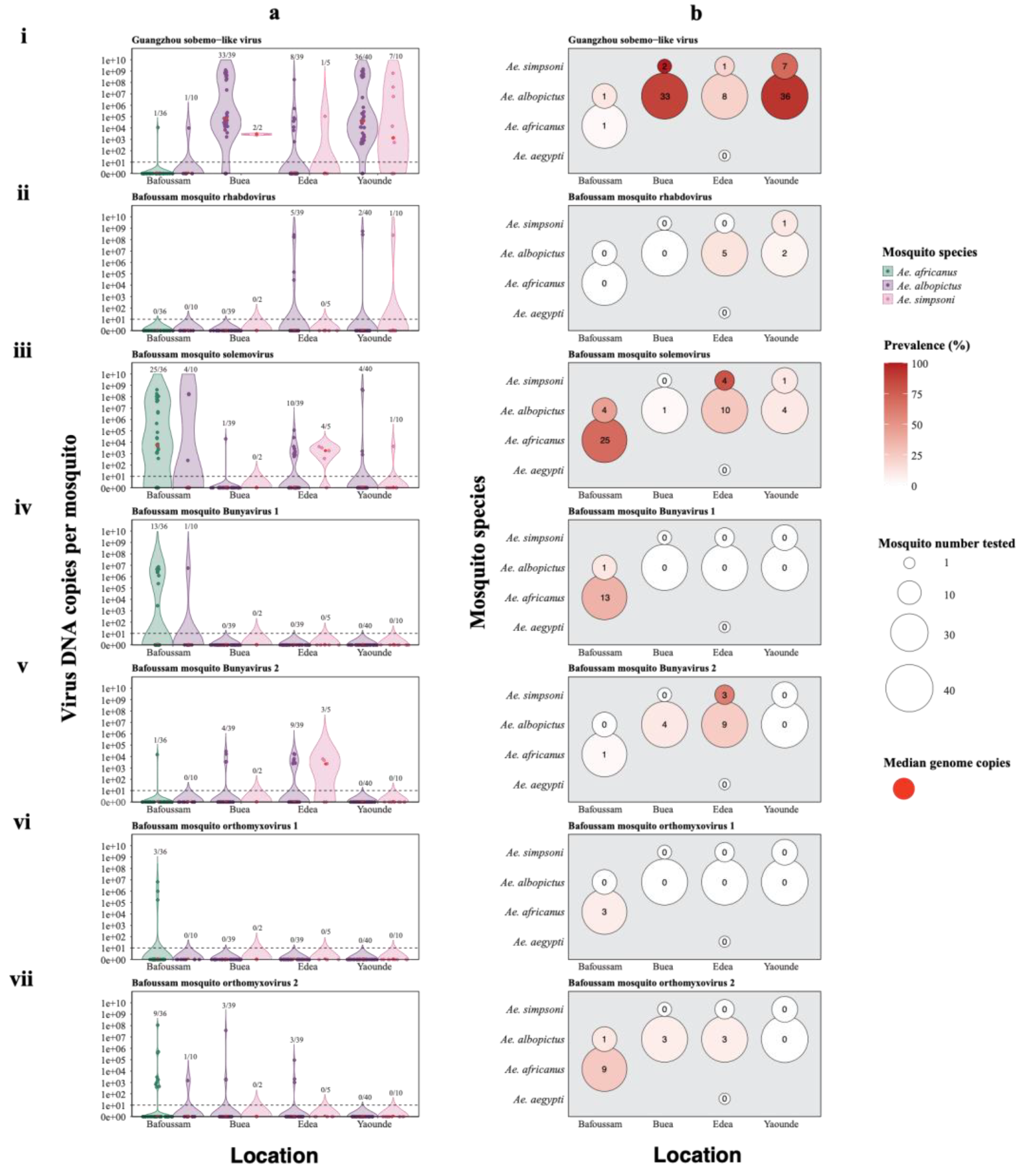
3.8. In Search for an Aedes Mosquito Core Virome Using qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tandina, F.; Doumbo, O.; Yaro, A.S.; Traoré, S.F.; Parola, P.; Robert, V. Mosquitoes (Diptera: Culicidae) and Mosquito-Borne Diseases in Mali, West Africa. Parasit Vectors 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Nebbak, A.; Almeras, L.; Parola, P.; Bitam, I. Mosquito Vectors (Diptera: Culicidae) and Mosquito-Borne Diseases in North Africa. Insects 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.S.; Higgs, S.; Vanlandingham, D.L. Biological Control Strategies for Mosquito Vectors of Arboviruses. Insects 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Dahmana, H.; Mediannikov, O. Mosquito-Borne Diseases Emergence/Resurgence and How to Effectively Control It Biologically. Pathogens 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Mosquitoes of Public Health Importance and Their Control.
- Bamou, R.; Mayi, M.P.A.; Djiappi-Tchamen, B.; Nana-Ndjangwo, S.M.; Nchoutpouen, E.; Cornel, A.J.; Awono-Ambene, P.; Parola, P.; Tchuinkam, T.; Antonio-Nkondjio, C. An Update on the Mosquito Fauna and Mosquito-Borne Diseases Distribution in Cameroon. Parasit Vectors 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Tajudeen, Y.A.; Oladipo, H.J.; Oladunjoye, I.O.; Yusuf, R.O.; Sodiq, H.; Omotosho, A.O.; Adesuyi, D.S.; Yusuff, S.I.; El-Sherbini, M.S. Emerging Arboviruses of Public Health Concern in Africa: Priorities for Future Research and Control Strategies. Challenges 2022, 13, 60. [Google Scholar] [CrossRef]
- Fokam, E.B.; Levai, L.D.; Guzman, H.; Amelia, P.A.; Titanji, V.P.; Tesh, R.B. , &; Weaver, S.C. Silent Circulation of Arboviruses in Cameroon. East Afr Med J 2010, 87, 262–268. [Google Scholar]
- Alenou, L.D.; Nwane, P.; Mbakop, L.R.; Piameu, M.; Ekoko, W.; Mandeng, S.; Bikoy, E.N.; Toto, J.C.; Onguina, H.; Etang, J. Burden of Mosquito-Borne Diseases across Rural versus Urban Areas in Cameroon between 2002 and 2021: Prospective for Community-Oriented Vector Management Approaches. Parasit Vectors 2023, 16. [Google Scholar] [CrossRef]
- Sadeuh-Mba, S.A.; Yonga Wansi, G.M.; Demanou, M.; Gessain, A.; Njouom, R. Serological Evidence of Rift Valley Fever Phlebovirus and Crimean-Congo Hemorrhagic Fever Orthonairovirus Infections among Pygmies in the East Region of Cameroon. Virol J 2018, 15. [Google Scholar] [CrossRef]
- Shi, C.; Beller, L.; Deboutte, W.; Yinda, K.C.; Delang, L.; Vega-Rúa, A.; Failloux, A.B.; Matthijnssens, J. Stable Distinct Core Eukaryotic Viromes in Different Mosquito Species from Guadeloupe, Using Single Mosquito Viral Metagenomics. Microbiome 2019, 7. [Google Scholar] [CrossRef]
- Ramírez, A.L.; Colmant, A.M.G.; Warrilow, D.; Huang, B.; Pyke, A.T.; McMahon, J.L.; Meyer, D.B.; Graham, R.M.A.; Jennison, A. V.; Ritchie, S.A.; et al. Metagenomic Analysis of the Virome of Mosquito Excreta. mSphere 2020, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- L’Ambert, G.; Gendrot, M.; Briolant, S.; Nguyen, A.; Pages, S.; Bosio, L.; Palomo, V.; Gomez, N.; Benoit, N.; Savini, H.; et al. Analysis of Trapped Mosquito Excreta as a Noninvasive Method to Reveal Biodiversity and Arbovirus Circulation. Mol Ecol Resour 2023, 23, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Hamel, R.; Narpon, Q.; Serrato-Pomar, I.; Gauliard, C.; Berthomieu, A.; Wichit, S.; Missé, D.; Sofonea, M.T.; Pompon, J. West Nile Virus Is Transmitted within Mosquito Populations through Infectious Mosquito Excreta 1 2 Short Title: Mosquito Excreta-Mediated Transmission 3 4. [CrossRef]
- Shi, C.; Beller, L.; Deboutte, W.; Yinda, K.C.; Delang, L.; Vega-Rúa, A.; Failloux, A.B.; Matthijnssens, J. Stable Distinct Core Eukaryotic Viromes in Different Mosquito Species from Guadeloupe, Using Single Mosquito Viral Metagenomics. Microbiome 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.I.L.; Suzuki, Y.; Carvajal, T.; Muñoz, M.N.M.; Watanabe, K. Intracellular Interactions Between Arboviruses and Wolbachia in Aedes Aegypti. Front Cell Infect Microbiol 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Leitner, M.; Bishop, C.; Asgari, S. Transcriptional Response of Wolbachia to Dengue Virus Infection in Cells of the Mosquito Aedes Aegypti. mSphere 2021, 6. [Google Scholar] [CrossRef]
- Hall-Mendelin, S.; McLean, B.J.; Bielefeldt-Ohmann, H.; Hobson-Peters, J.; Hall, R.A.; Van Den Hurk, A.F. The Insect-Specific Palm Creek Virus Modulates West Nile Virus Infection in and Transmission by Australian Mosquitoes. Parasit Vectors 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Hobson-Peters, J.; Yam, A.W.Y.; Lu, J.W.F.; Setoh, Y.X.; May, F.J.; Kurucz, N.; Walsh, S.; Prow, N.A.; Davis, S.S.; Weir, R.; et al. A New Insect-Specific Flavivirus from Northern Australia Suppresses Replication of West Nile Virus and Murray Valley Encephalitis Virus in Co-Infected Mosquito Cells. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Ren, Q.; Luo, J.; Tian, Z.; Liu, W.; Zhao, B.; Li, J.; Diao, P.; Tan, Y.; Qiu, X.; et al. Analysis of Microorganism Diversity in Haemaphysalis Longicornis From Shaanxi, China, Based on Metagenomic Sequencing. Front Genet 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Batson, J.; Dudas, G.; Haas-Stapleton, E.; Kistler, A.L.; Li, L.M.; Logan, P.; Ratnasiri, K.; Retallack, H. Single Mosquito Metatranscriptomics Identifies Vectors, Emerging Pathogens and Reservoirs in One Assay. Elife 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Tedjou, A.N.; Kamgang, B.; Yougang, A.P.; Njiokou, F.; Wondji, C.S. Update on the Geographical Distribution and Prevalence of Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae), Two Major Arbovirus Vectors in Cameroon. PLoS Negl Trop Dis 2018, 13. [Google Scholar] [CrossRef]
- Dé Ric Simard, F.; Nchoutpouen, E.E.; Claude Toto, J.; Fontenille, D. Geographic Distribution and Breeding Site Preference of Aedes Albopictus and Aedes Aegypti (Diptera: Culicidae) in Cameroon, Central Africa; 2005; Vol. 42;
- Tedjou, A.N.; Kamgang, B.; Yougang, A.P.; Njiokou, F.; Wondji, C.S. Update on the Geographical Distribution and Prevalence of Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae), Two Major Arbovirus Vectors in Cameroon. PLoS Negl Trop Dis 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Djiappi-Tchamen, B.; Nana-Ndjangwo, M.S.; Tchuinkam, T.; Makoudjou, I.; Nchoutpouen, E.; Kopya, E.; Talipouo, A.; Bamou, R.; Mayi, M.P.A.; Awono-Ambene, P.; et al. Aedes Mosquito Distribution along a Transect from Rural to Urban Settings in Yaoundé, Cameroon. Insects 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Mercant Osuna, A.; Gidley, A.; Mayi, M.P.A.; Bamou, R.; Dhokiya, V.; Antonio-Nkondjio, C.; Jeffries, C.L.; Walker, T. Diverse Novel Wolbachia Bacteria Strains and Widespread Co-Infections with Asaia Bacteria in Culicine Mosquitoes from Ecologically Diverse Regions of Cameroon. Wellcome Open Res 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Djeunang Dongho, G.B.; Venturi, G.; Fortuna, C.; Paganotti, G.M.; Severini, C.; L’Episcopia, M.; Tsapi, A.T.; Benedetti, E.; Marsili, G.; Amendola, A.; et al. Dengue and Chikungunya Virus Circulation in Cameroon and Gabon: Molecular Evidence among Symptomatic Individuals. Access Microbiol 2022, 4. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, M.C.; Liang, S.; Kracalik, I.T.; Morris, L.; Blackburn, J.K.; Mbam, L.M.; Ba Pouth, S.F.B.; Teboh, A.; Yang, Y.; Arabi, M.; et al. Cholera in Cameroon, 2000-2012: Spatial and Temporal Analysis at the Operational (Health District) and Sub Climate Levels. PLoS Negl Trop Dis 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Rueda, L.M. Pictorial Keys for the Identification of Mosquitoes (Diptera:Culicidae) Associated with Dengue Virus Transmission; Magnolia Press, 2004; ISBN 1877354465.
- Conceição-Neto, N.; Zeller, M.; Lefrère, H.; De Bruyn, P.; Beller, L.; Deboutte, W.; Yinda, C.K.; Lavigne, R.; Maes, P.; Ranst, M. Van; et al. Modular Approach to Customise Sample Preparation Procedures for Viral Metagenomics: A Reproducible Protocol for Virome Analysis. Sci Rep 2015, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Nurk, S.; Meleshko, D.; Korobeynikov, A.; Pevzner, P.A. MetaSPAdes: A New Versatile Metagenomic Assembler. Genome Res 2017, 27, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Nayfach, S.; Camargo, A.P.; Schulz, F.; Eloe-Fadrosh, E.; Roux, S.; Kyrpides, N.C. CheckV Assesses the Quality and Completeness of Metagenome-Assembled Viral Genomes. Nat Biotechnol 2021, 39, 578–585. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and Sensitive Protein Alignment Using DIAMOND. Nat Methods 2014, 12, 59–60. [Google Scholar] [CrossRef]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive Metagenomic Visualization in a Web Browser. BMC Bioinformatics 2011, 12. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Ren, H. TaxonKit: A Practical and Efficient NCBI Taxonomy Toolkit. Journal of Genetics and Genomics 2021, 48, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Eils, R.; Schlesner, M. Complex Heatmaps Reveal Patterns and Correlations in Multidimensional Genomic Data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. 2009.
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.M.; Valentine, M.J.; Kelly, P.J.; Barua, S.; Murillo, D.F.B.; Wang, C. Modification of the Folmer Primers for the Cytochrome c Oxidase Gene Facilitates Identification of Mosquitoes. Parasit Vectors 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.M.; Tchuenkam, V.P.K.; Colton, M.; Stittleburg, V.; Mitchell, C.; Gaither, C.; Thwai, K.; Espinoza, D.O.; Zhu, Y.; Jamal, H.; et al. Arboviruses as an Unappreciated Cause of Non-Malarial Acute Febrile Illness in the Dschang Health District of Western Cameroon. PLoS Negl Trop Dis 2022, 16. [Google Scholar] [CrossRef] [PubMed]
- Öhlund, P.; Lundén, H.; Blomström, A.L. Insect-Specific Virus Evolution and Potential Effects on Vector Competence. Virus Genes 2019, 55, 127–137. [Google Scholar] [CrossRef]
- Lefeuvre, P.; Martin, D.P.; Elena, S.F.; Shepherd, D.N.; Roumagnac, P.; Varsani, A. Evolution and Ecology of Plant Viruses. Nat Rev Microbiol 2019, 17, 632–644. [Google Scholar] [CrossRef]
- Fermin, G. Host Range, Host-Virus Interactions, and Virus Transmission. In Viruses: Molecular Biology, Host Interactions, and Applications to Biotechnology; Elsevier, 2018; pp. 101–134 ISBN 9780128111949.
- Truong Nguyen, P.T.; Culverwell, C.L.; Suvanto, M.T.; Korhonen, E.M.; Uusitalo, R.; Vapalahti, O.; Smura, T.; Huhtamo, E. Characterisation of the RNA Virome of Nine Ochlerotatus Species in Finland. Viruses 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Jupatanakul, N.; Sim, S.; Dimopoulos, G. Aedes Aegypti ML and Niemann-Pick Type C Family Members Are Agonists of Dengue Virus Infection. Dev Comp Immunol 2014, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yu, X.; Cheng, G. Impact of the Microbiome on Mosquito-Borne Diseases. Protein Cell 2023, 14, 743–761. [Google Scholar] [CrossRef] [PubMed]
- Epelboin, Y.; Talaga, S.; Epelboin, L.; Dusfour, I. Zika Virus: An Updated Review of Competent or Naturally Infected Mosquitoes. PLoS Negl Trop Dis 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Weetman, D.; Kamgang, B.; Badolo, A.; Moyes, C.L.; Shearer, F.M.; Coulibaly, M.; Pinto, J.; Lambrechts, L.; McCall, P.J. Aedes Mosquitoes and Aedes-Borne Arboviruses in Africa: Current and Future Threats. Int J Environ Res Public Health 2018, 15. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.; Zhou, H.; Zhu, W.; Cui, M.; Li, J.; Wang, J.; Liu, J.; Zhu, J.; Li, W.; et al. Metatranscriptomic Sequencing Reveals Host Species as an Important Factor Shaping the Mosquito Virome. Microbiol Spectr 2023, 11. [Google Scholar] [CrossRef]
- Kubacki, J.; Flacio, E.; Qi, W.; Guidi, V.; Tonolla, M.; Fraefel, C. Viral Metagenomic Analysis of Aedes Albopictus Mosquitos from Southern Switzerland. Viruses 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Martinez, D.; Muñoz, M.; Ramírez, J.D. Aedes Aegypti and Ae. Albopictus Microbiome/Virome: New Strategies for Controlling Arboviral Transmission? Parasit Vectors 2022, 15. [Google Scholar] [CrossRef]
- Gangopadhayya, A.; Lole, K.; Ghuge, O.; Ramdasi, A.; Kamble, A.; Roy, D.; Thakar, S.; Nath, A.; Sudeep, A.B.; Cherian, S. Metagenomic Analysis of Viromes of Aedes Mosquitoes across India. Viruses 2024, 16. [Google Scholar] [CrossRef]
- Konstantinidis, K.; Dovrolis, N.; Kouvela, A.; Kassela, K.; Rosa Freitas, M.G.; Nearchou, A.; De Courcy Williams, M.; Veletza, S.; Karakasiliotis, I. Defining Virus-Carrier Networks That Shape the Composition of the Mosquito Core Virome of a Local Ecosystem. Virus Evol 2022, 8. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, L.; Atoni, E.; Zeng, W.; Hu, X.; Matthijnssens, J.; Yuan, Z.; Xia, H. Stability of the Virome in Lab- and Field-Collected Aedes Albopictus Mosquitoes across Different Developmental Stages and Possible Core Viruses in the Publicly Available Virome Data of Aedes Mosquitoes. mSystems 2020, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, N.; Lühken, R.; Jöst, H.; Jansen, S.; Börstler, J.; Rieger, T.; Krüger, A.; Yadouleton, A.; de Mendonça Campos, R.; Cirne-Santos, C.C.; et al. Detection and Characterization of a Novel Rhabdovirus in Aedes Cantans Mosquitoes and Evidence for a Mosquito-Associated New Genus in the Family Rhabdoviridae. Infection, Genetics and Evolution 2017, 55, 260–268. [Google Scholar] [CrossRef] [PubMed]
- De Coninck, L.; Soto, A.; Wang, L.; De Wolf, K.; Smitz, N.; Deblauwe, I.; Mbigha Donfack, K.C.; Müller, R.; Delang, L.; Matthijnssens, J. Lack of Abundant Core Virome in Culex Mosquitoes from a Temperate Climate Region despite a Mosquito Species-Specific Virome. mSystems 2024. [Google Scholar] [CrossRef] [PubMed]
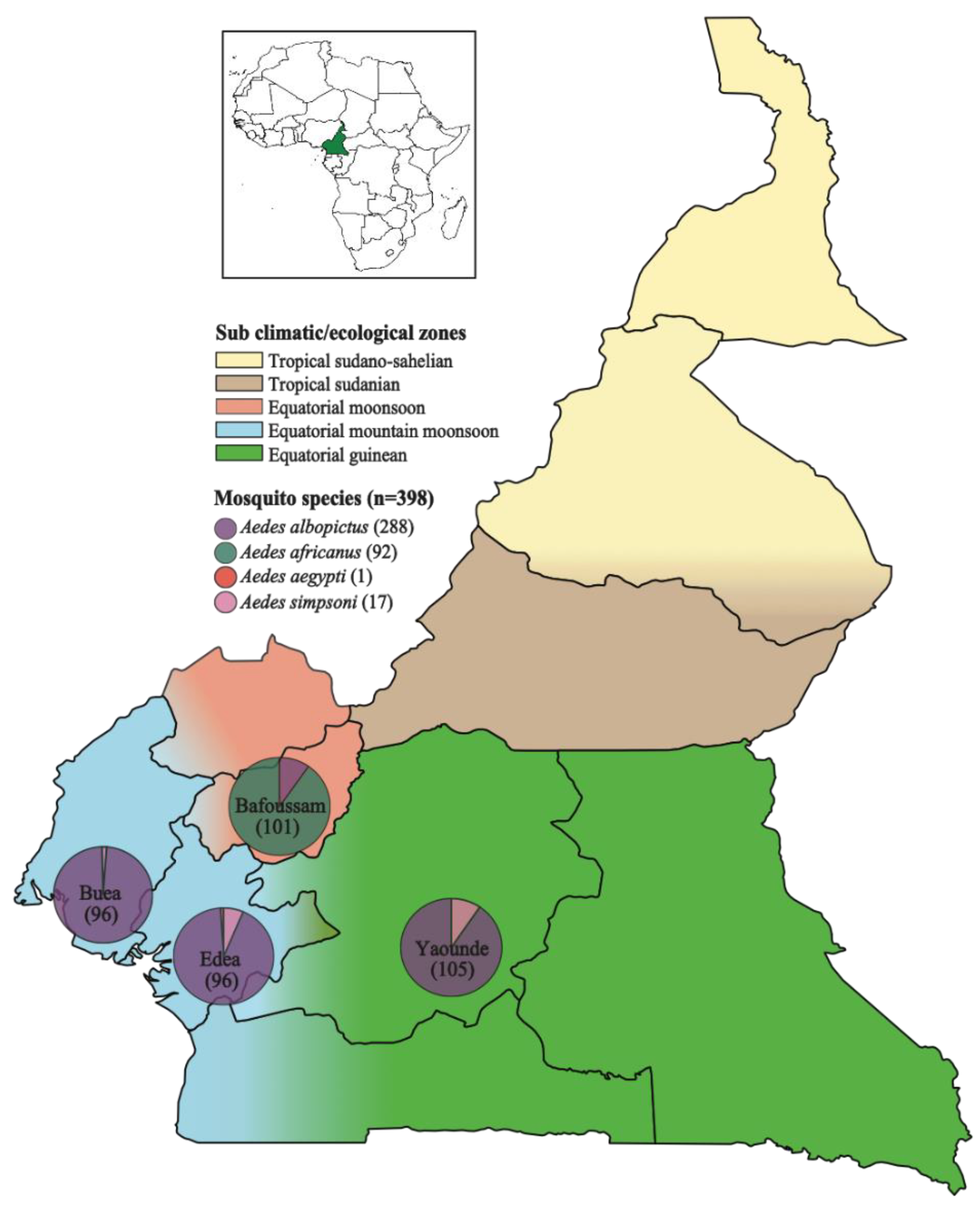
| Location | Mosquito species | Number of Pools of 4 mosquitoes |
|---|---|---|
| Bafoussam | Aedes africanus | 14 |
| Buea | Aedes albopictus | 14 |
| Edea | Aedes albopictus | 12 |
| Yaoundé | Aedes albopictus | 14 |
| Location | Mosquito species | Individual mosquitoes tested |
|---|---|---|
| Bafoussam | Ae. africanus | 36 |
| Ae. albopictus | 10 | |
| Buea | Ae. albopictus | 39 |
| Ae. simpsoni | 2 | |
| Edea | Ae. albopictus | 39 |
| Ae. simpsoni | 5 | |
| Ae. aegypti | 1 | |
| Yaoundé | Ae. albopictus | 40 |
| Ae. simpsoni | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





